Effect of a Phosphorus Additive on Luminescent and Scintillation Properties of Ceramics GYAGG:Ce
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Initial Powders
2.2. Characterization of Initial Powders
2.3. Ceramics Fabrication
2.4. Characterization of Ceramic Samples
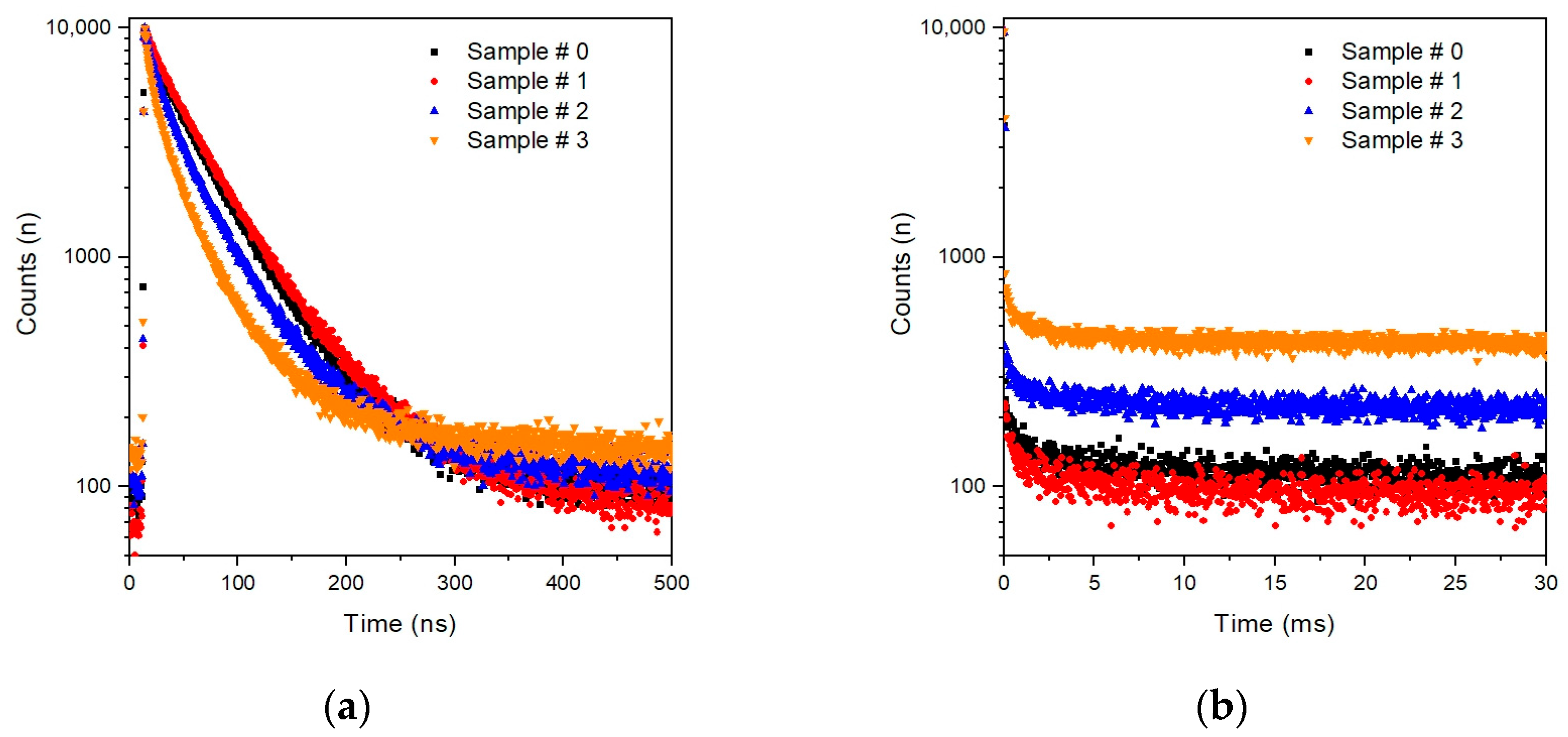
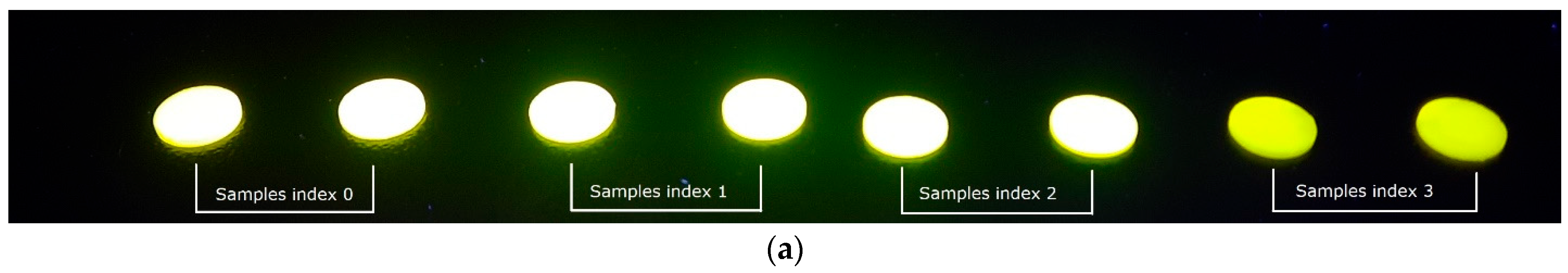
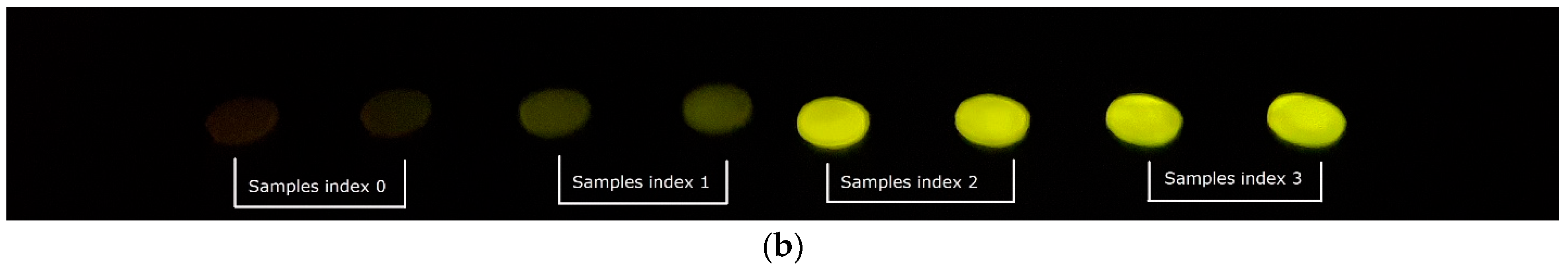
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ueda, J.; Tanabe, S. Review of luminescent properties of Ce3+-doped garnet phosphors: New insight into the effect of crystal and electronic structure. Opt. Mater. X 2019, 1, 100018. [Google Scholar] [CrossRef]
- Xia, Z.; Meijerink, A. Ce3+-Doped garnet phosphors: Composition modification, luminescence properties and applications. Chem. Soc. Rev. 2017, 46, 275–299. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Gundiah, G.; Cheetham, A.K. Structure–property correlations in Ce-doped garnet phosphors for use in solid state lighting. Chem. Phys. Lett. 2007, 441, 250–254. [Google Scholar] [CrossRef]
- Palashov, O.V.; Starobor, A.V.; Perevezentsev, E.A.; Snetkov, I.L.; Mironov, E.A.; Yakovlev, A.I.; Balabanov, S.S.; Permin, D.A.; Belyaev, A.V. Thermo-optical studies of laser ceramics. Materials 2021, 14, 3944. [Google Scholar] [CrossRef] [PubMed]
- Korjik, M.; Bondarau, A.; Dosovitskiy, G.; Dubov, V.; Gordienko, K.; Karpuk, P.; Komendo, I.; Kuznetsova, D.; Mechinsky, V.; Pustovarov, V.; et al. Lanthanoid-doped quaternary garnets as phosphors for high brightness cathodoluminescence-based light sources. Heliyon 2022, 8, E10193. [Google Scholar] [CrossRef] [PubMed]
- Korzhik, M.; Abashev, R.; Fedorov, A.; Dosovitskiy, G.; Gordienko, E.; Kamenskikh, I.; Kazlou, D.; Kuznecova, D.; Mechinsky, V.; Pustovarov, V.; et al. Towards effective indirect radioisotope energy converters with bright and radiation hard scintillators of (Gd,Y)3Al2Ga3O12 family. Nucl. Eng. Technol. 2022, 54, 2579–2585. [Google Scholar] [CrossRef]
- Korzhik, M.; Borisevich, A.; Fedorov, A.; Gordienko, E.; Karpyuk, P.; Dubov, V.; Sokolov, P.; Mikhlin, A.; Dosovitskiy, G.; Mechinsky, V.; et al. The scintillation mechanisms in Ce and Tb doped (GdxY1-x)Al2Ga3O12 quaternary garnet structure crystalline ceramics. J. Lumin. 2021, 234, 117933. [Google Scholar] [CrossRef]
- Retivov, V.; Dubov, V.; Komendo, I.; Karpyuk, P.; Kuznetsova, D.; Sokolov, P.; Talochka, Y.; Korzhik, M. Compositionally disordered crystalline compounds for next generation of radiation detectors. Nanomaterials 2022, 12, 4295. [Google Scholar] [CrossRef]
- Martinazzoli, L.; Nargelas, S.; Bohacek, P.; Cala, R.; Dusek, M.; Rohlicek, J.; Tamulaitis, G.; Auffray, E.; Nikl, M. Compositional engineering of multicomponent garnet scintillators: Towards an ultra-accelerated scintillation response. Mater. Adv. 2022, 3, 6842–6852. [Google Scholar] [CrossRef]
- Zhu, D.; Nikl, M.; Chewpraditkul, W.; Li, J. Development and prospects of garnet ceramic scintillators: A review. J. Adv.Ceram. 2022, 11, 1825–1848. [Google Scholar] [CrossRef]
- Korzhik, M.; Retivov, V.; Dosovitskiy, G.; Dubov, V.; Kamenskikh, I.; Karpuk, P.; Komendo, I.; Kuznetsova, D.; Smyslova, V.; Mechinsky, V.; et al. First Observation of the Scintillation Cascade in Tb3+-Doped Quaternary Garnet Ceramics. Phys. Status Solidi R 2023, 17, 2200368. [Google Scholar] [CrossRef]
- Karpyuk, P.; Korzhik, M.; Fedorov, A.; Kamenskikh, I.; Komendo, I.; Kuznetsova, D.; Leksina, E.; Mechinsky, V.; Pustovarov, V.; Smyslova, V.; et al. The Saturation of the Response to an Electron Beam of Ce- and Tb-Doped GYAGG Phosphors for Indirect β-Voltaics. Appl. Sci. 2023, 13, 3323. [Google Scholar] [CrossRef]
- Dubov, V.; Gogoleva, M.; Saifutyarov, R.; Kucherov, O.; Korzhik, M.; Kuznetsova, D.; Komendo, I.; Sokolov, P. Micro-Nonuniformity of the Luminescence Parameters in Compositionally Disordered GYAGG:Ce Ceramics. Photonics 2023, 10, 54. [Google Scholar] [CrossRef]
- Retivov, V.; Dubov, V.; Kuznetsova, D.; Ismagulov, A.; Korzhik, M. Gd3+ content optimization for mastering high light yield and fast GdxAl2Ga3O12:Ce3+ scintillation ceramics. J. Rare Earths 2023, in press. [Google Scholar] [CrossRef]
- Kuznetsova, D.; Dubov, V.; Bondarev, A.; Dosovitskiy, G.; Mechinsky, V.; Retivov, V.; Kucherov, O.; Saifutyarov, R.; Korzhik, M. Tailoring of the Gd–Y–Lu ratio in quintuple (Gd, Lu, Y)3Al2Ga3O12:Ce ceramics for better scintillation properties. J. Appl. Phys. 2022, 132, 203104. [Google Scholar] [CrossRef]
- Dantelle, G.; Boulon, G.; Guyot, Y.; Testemale, D.; Guzik, M.; Kurosawa, S.; Kamada, K.; Yoshikawa, A. Research on Efficient Fast Scintillators: Evidence and X-Ray Absorption Near Edge Spectroscopy Characterization of Ce4+ in Ce3+, Mg2+-Co-Doped Gd3Al2Ga3O12 Garnet Crystal. Phys. Status Solidi B 2020, 257, 1900510. [Google Scholar] [CrossRef]
- Korzhik, M.; Alenkov, V.; Buzanov, O.; Dosovitskiy, G.; Fedorov, A.; Kozlov, D.; Mechinsky, V.; Nargelas, S.; Tamulaitis, G.; Vaitkevicius, A. Engineering of a new single-crystal multi-ionic fast and high-light-yield scintillation material (Gd0.5–Y0.5)3Al2Ga3O12:Ce,Mg. CrystEngComm 2020, 22, 2502–2506. [Google Scholar] [CrossRef]
- Spassky, D.; Kozlova, N.; Zabelina, E.; Kasimova, V.; Krutyak, N.; Ukhanova, A.; Morozov, V.A.; Morozov, A.V.; Buzanov, O.; Chernenko, K.; et al. Influence of the Sc cation substituent on the structural properties and energy transfer processes in GAGG:Ce crystals. CrystEngComm 2020, 22, 2621–2631. [Google Scholar] [CrossRef]
- Shi, Y.; Shichalin, O.; Xiong, Y.; Kosyanov, D.; Wu, T.; Zhang, Q.; Wang, L.; Zhou, Z.; Wang, H.; Fang, J.; et al. Ce3+ doped Lu3Al5O12 ceramics prepared by spark plasma sintering technology using micrometre powders: Microstructure, luminescence, and scintillation properties. J. Eur. Ceram. Soc. 2022, 42, 6663–6670. [Google Scholar] [CrossRef]
- Kuznetsov, S.V.; Sedov, V.S.; Martyanov, A.K.; Batygov, S.C.; Vakalov, D.S.; Boldyrev, K.N.; Tiazhelov, I.A.; Popovich, A.F.; Pasternak, D.G.; Bland, H.; et al. Cerium-doped gadolinium-scandium-aluminum garnet powders: Synthesis and use in X-ray luminescent diamond composites. Ceram. Int. 2022, 48, 12962–12970. [Google Scholar] [CrossRef]
- Fedorov, A.; Komendo, I.; Amelina, A.; Gordienko, E.; Gurinovich, V.; Guzov, V.; Dosovitskiy, G.; Kozhemyakin, V.; Kozlov, D.; Lopatik, A.; et al. GYAGG/6LiF composite scintillation screen for neutron detection. Nucl. Eng. Technol. 2022, 54, 1024–1029. [Google Scholar] [CrossRef]
- Rudzik, T.J.; Seeley, Z.M.; Drobshoff, A.D.; Cherepy, N.J.; Wang, Y.; Onorato, S.P.; Squillante, M.R.; Payne, S.A. Additively manufactured transparent ceramic thin disk gain medium Opt. Mater. Express. 2022, 12, 3648–3657. [Google Scholar] [CrossRef]
- Osborne, R.A.; Wineger, T.J.; Yee, T.D.; Cherepy, N.J.; Seeley, Z.M.; Gaume, R.; Dubinskii, M.; Payne, S.A. Fabrication of engineered dopant profiles in Er/Lu:YAG transparent laser ceramics via additive manufacturing. Opt. Mater. Express. 2023, 13, 526–537. [Google Scholar] [CrossRef]
- Ermakova, L.V.; Dubov, V.V.; Saifutyarov, R.R.; Kuznetsova, D.E.; Malozovskaya, M.S.; Karpyuk, P.V.; Dosovitskiy, G.A.; Sokolov, P.S. Influence of Luminescent Properties of Powders on the Fabrication of Scintillation Ceramics by Stereolithography 3D Printing. Ceramics 2023, 6, 43–57. [Google Scholar] [CrossRef]
- Fedorov, A.A.; Dubov, V.V.; Ermakova, L.V.; Bondarev, A.G.; Karpyuk, P.V.; Korzhik, M.V.; Kuznetsova, D.E.; Mechinsky, V.A.; Smyslova, V.G.; Dosovitskiy, G.A.; et al. Gd3Al2Ga3O12:Ce Scintillation Ceramic Elements for Measuring Ionizing Radiation in Gases and Liquids. Instrum. Exp. Tech. 2023, 66, 234–238. [Google Scholar] [CrossRef]
- Karipbayev, Z.T.; Lisitsyn, V.M.; Mussakhanov, D.A.; Alpyssova, G.K.; Popov, A.I.; Polisadova, E.F.; Elsts, E.; Akilbekov, A.T.; Kukenova, A.B.; Kemere, M.; et al. Time-resolved luminescence of YAG:Ce and YAGG:Ce ceramics prepared by electron beam assisted synthesis. Nucl. Instrum. Meth. B 2020, 479, 222–228. [Google Scholar] [CrossRef]
- Abd, H.R.; Hassan, Z.; Ahmed, N.M.; Omar, A.F.; Thahab, S.M.; Lau, K.S. Rapid synthesis of Ce3+:YAG via CO2 laser irradiation combustion method: Influence of Ce doping and thickness of phosphor ceramic on the performance of a white LED device. J. Solid State Chem. 2021, 294, 121866. [Google Scholar] [CrossRef]
- Loiko, P.; Basyrova, L.; Maksimov, R.; Shitov, V.; Baranov, M.; Starecki, F.; Mateos, X.; Camy, P. Comparative study of Ho:Y2O3 and Ho:Y3Al5O12 transparent ceramics produced from laser-ablated nanoparticles. J. Lumin. 2021, 240, 118460. [Google Scholar] [CrossRef]
- Timoshenko, A.D.; Matvienko, O.O.; Doroshenko, A.G.; Parkhomenko, S.V.; Voronova, I.O.; Kryzhanovska, O.S.; Safronova, N.A.; Vovk, O.O.; Tolmachev, A.V.; Baumer, V.N.; et al. Highly-doped YAG:Sm3+ transparent ceramics: Effect of Sm3+ ions concentration. Ceram. Int. 2023, 49, 7524–7533. [Google Scholar] [CrossRef]
- Wagner, A.; Ratzker, B.; Kalabukhov, S.; Kolusheva, S.; Sokol, M.; Frage, N. Highly-doped Nd:YAG ceramics fabricated by conventional and high pressure SPS. Ceram. Int. 2019, 45, 12279–12284. [Google Scholar] [CrossRef]
- Derdzyan, M.V.; Hovhannesyan, K.L.; Santos, S.N.C.; Dujardin, C.; Petrosyan, A.G. Influence of Air Annealing on Optical and Scintillation Properties of YAG:Pr,Ca. Phys. Status Solidi A 2022, 220, 2200571. [Google Scholar] [CrossRef]
- Kuznetsova, D.E.; Volkov, P.A.; Dosovitskiy, G.A.; Mikhlin, A.L.; Bogatov, K.B.; Retivov, V.M.; Dosovitskiy, A.E. Influence of alkali metal impurities on properties of yttrium aluminum garnet doped with cerium. Russ. Chem. Bull. 2016, 65, 1734–1738. [Google Scholar] [CrossRef]
- Karpyuk, P.; Shurkina, A.; Kuznetsova, D.; Smyslova, V.; Dubov, V.; Dosovitskiy, G.; Korzhik, M.; Retivov, V.; Bondarev, A. Effect of Sintering Additives on the Sintering and Spectral-Luminescent Characteristics of Quaternary GYAGG:Ce Scintillation Ceramics. J. Electron. Mater. 2022, 51, 6481–6491. [Google Scholar] [CrossRef]
- Vorona, I.O.; Yavetskiy, R.P.; Parkhomenko, S.V.; Doroshenko, A.G.; Kryzhanovska, O.S.; Safronova, N.A.; Timoshenko, A.D.; Balabanov, A.E.; Tolmachev, A.V.; Baumer, V.N. Effect of complex Si4++Mg2+ additive on sintering and properties of undoped YAG ceramics. J. Eur. Ceram. Soc. 2022, 42, 6104–6109. [Google Scholar] [CrossRef]
- Setlur, A.A.; Heward, W.J.; Hannah, M.E.; Happek, U. Incorporation of Si4+–N3− into Ce3+ -Doped Garnets for Warm White LED Phosphors. Chem. Mater. 2008, 20, 6277–6283. [Google Scholar] [CrossRef]
- Dosovitskii, G.A.; Bogatov, K.B.; Volkov, P.A.; Mikhlin, A.L.; Dosovitskii, A.E. Effect of adding boron on morphological and functional properties of aluminum-yttrium garnet activated with europium. Refract. Ind. Ceram. 2013, 54, 69–73. [Google Scholar] [CrossRef]
- Yan, Y.; Meshbah, A.; Kheouz, L.; Bouillet, C.; Lorentz, C.; Blanchard, N.; Berends, A.C.; van der Haar, M.A.; Lerouge, F.; Krames, M.R.; et al. Ultra-Small YPO4-YAG:Ce Composite Nanophosphors with a Photoluminescence Quantum Yield Exceeding 50%. Small 2023, 19, 2208055. [Google Scholar] [CrossRef]
- Pirrung, F.O.H.; Noordam, A.; Harbers, P.J.; Loen, E.M.; Munneke, A.E. Phosphoric Acid Esters and Their Use as Wetting and Dispersing Agent. US Patent 7595416B2, 28 February 2005. Available online: https://patents.google.com/patent/US7595416B2/ (accessed on 5 July 2023).
- Gobelt, B.; Nagelsdiek, R.; Omeis, J.; Piestert, F.; Pritschins, W.; Meznaric, N.; Schroder, D.; Tiegs, W. Dispersing Additives Based on Phosphoric Acid Ester Derivatives. US Patent 9518146, 4 May 2012. Available online: https://patents.google.com/patent/US9518146B2 (accessed on 5 July 2023).
- Gordienko, E.; Fedorov, A.; Radiuk, E.; Mechinsky, V.; Dosovitskiy, G.; Vashchenkova, E.; Kuznetsova, D.; Retivov, V.; Dosovitskiy, A.; Korjik, M.; et al. Synthesis of crystalline Ce-activated garnet phosphor powders and technique to characterize their scintillation light yield. Opt. Mater. 2018, 78, 312–318. [Google Scholar] [CrossRef]
- Pardo, A.; Romero, J.; Ortiz, E. High-temperature behaviour of ammonium dihydrogen phosphate. J. Phys. Conf. Ser. 2017, 935, 012050. [Google Scholar] [CrossRef]
- Su, C.H.; Chen, C.C.; Liaw, H.J.; Wang, S.C. The Assessment of Fire Suppression Capability for the Ammonium Dihydrogen Phosphate Dry Powder of Commercial Fire Extinguishers. Procedia Eng. 2014, 84, 485–490. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Guan, L.; Fu, Y.; Guo, Z.; Yuan, K.; Tie, L.; Yang, Z.; Teng, F. Influence of pH value on properties of YPO4:Tb3+ phosphor by co-precipitation method. J. Rare Earths 2015, 33, 346–349. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Li, P.; Nan, C.; Zhang, Y. Roles of (NH4)2HPO4 and NH4H2PO4 in the phase transition and luminescence enhancement of YPO4:Eu. J. Phys. Chem. Solids 2021, 150, 109821. [Google Scholar] [CrossRef]
- Agrawal, D.; Hummel, F.A. The Systems Y2O3-P2O5 and Gd2O3-P2O5. J. Electrochem. Soc. 1980, 127, 1550. [Google Scholar] [CrossRef]
- Szuszkiewicz, W.; Znamierowska, T. Y2O3-P2O5 Phase Diagram. Pol. J. Chem. 1989, 63, 381–391. [Google Scholar]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Halenius, U. Nomenclature of the garnet supergroup. Am. Miner. 2013, 88, 785–811. [Google Scholar] [CrossRef]
- Thompson, R.N. Is upper-mantle phosphorus contained in sodic garnet? Earth Planet Sci. Lett. 1975, 26, 417–424. [Google Scholar] [CrossRef]
- Brunet, F.; Bonneau, V.; Irifune, T. Complete solid-solution between Na3Al2(PO4)3 and Mg3Al2(SiO4)3 garnets at high pressure. Am. Mineral. 2006, 91, 211–215. [Google Scholar] [CrossRef]

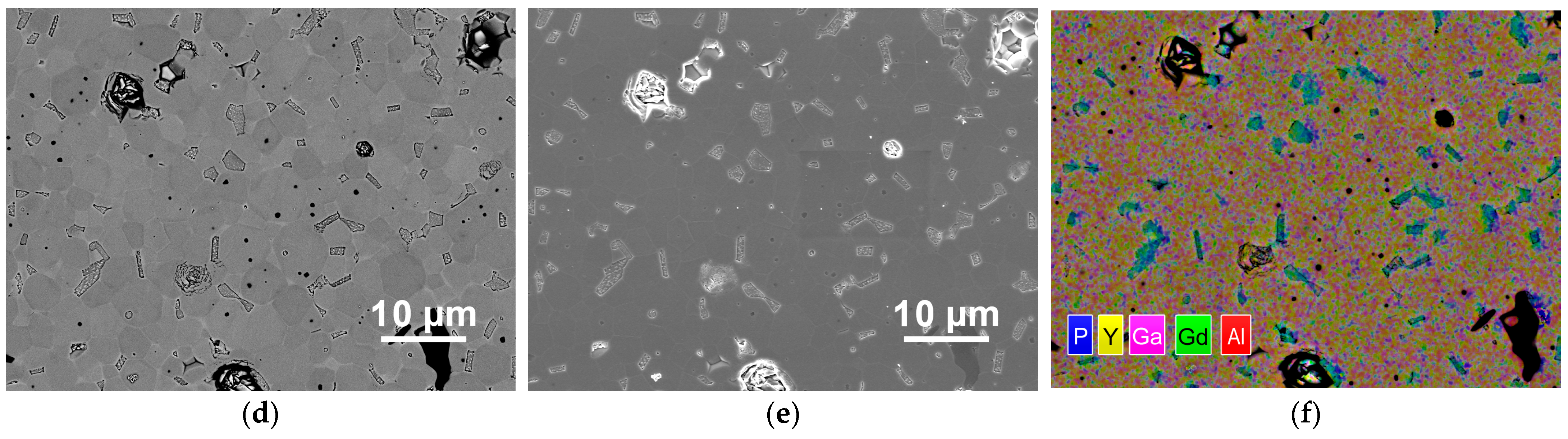
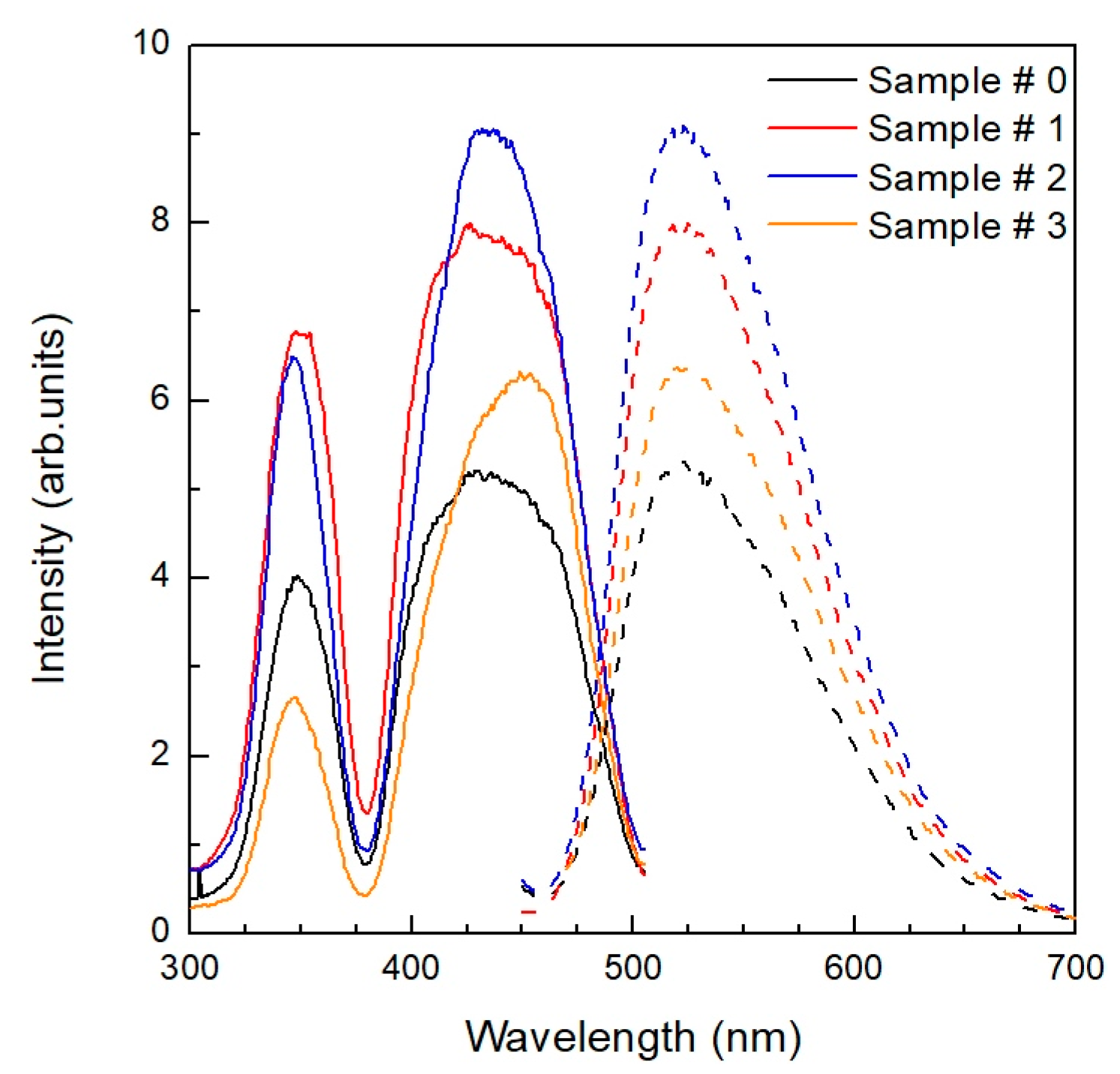
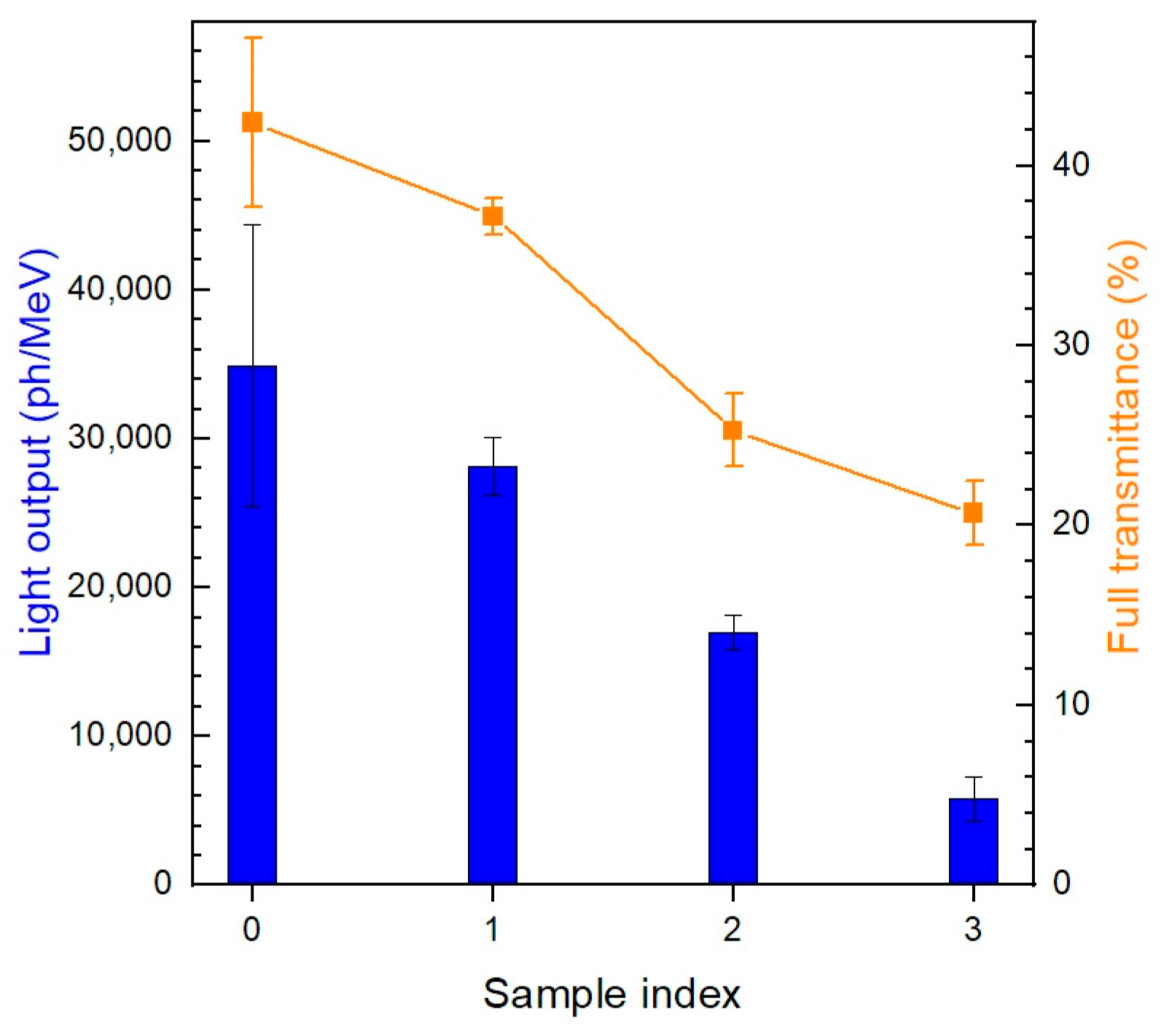
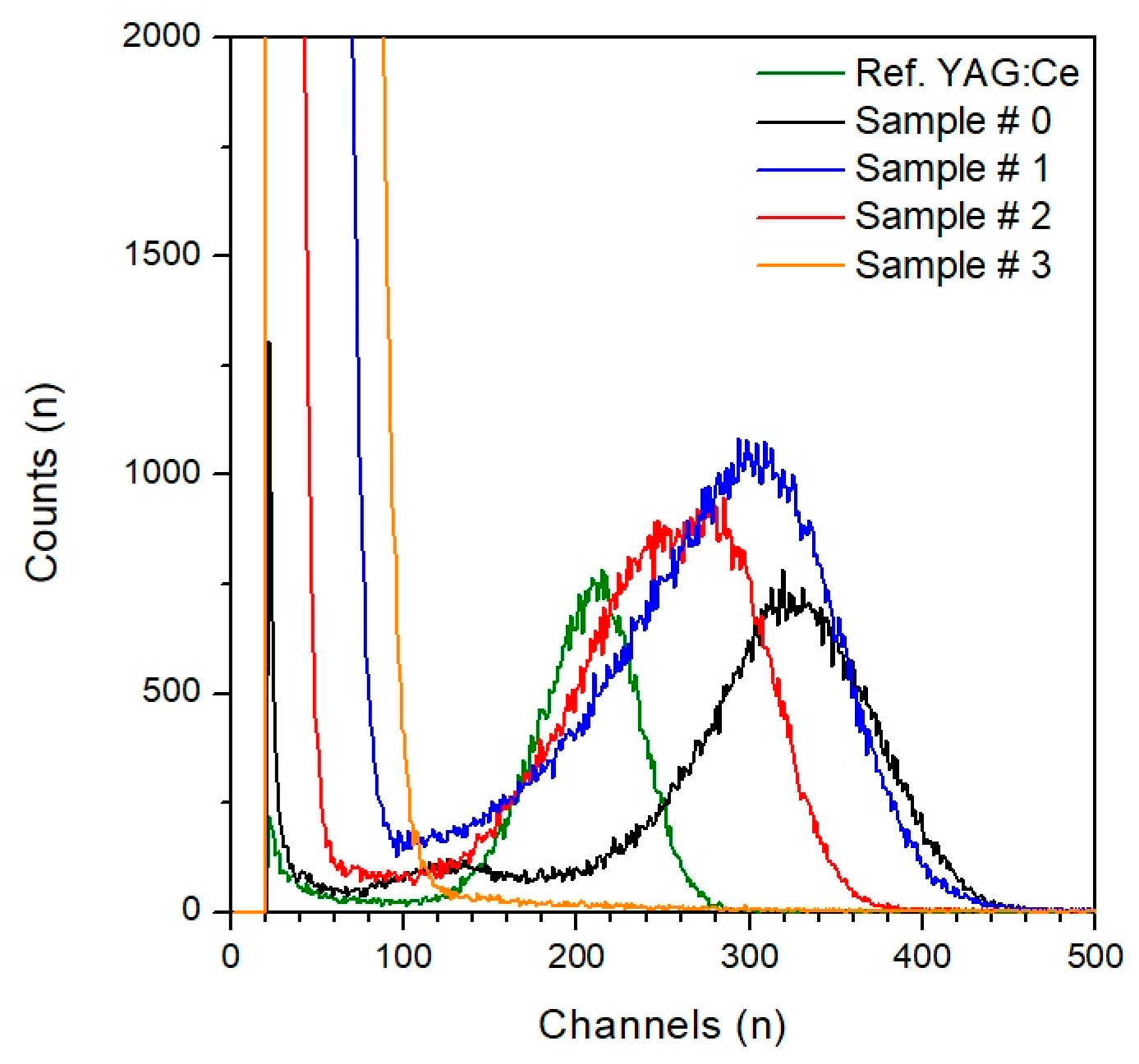
| Sample # | Hydroxocarbonate Precipitates | Oxide Powders Calcined at 850 °C |
|---|---|---|
| 0 | - | - |
| 1 | 0.027 | 0.040 |
| 2 | 0.114 | 0.156 |
| 3 | 0.456 | 0.623 |
| Sample # | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Relative density | 100 | 99.7 | 98.8 | 97.5 |
| Sample # | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Average grain size (μm) | 1.90 (1) | 3.2 (2) | 6.9 (2) | 2.8 (2) |
| Fraction of inclusions + pores (%) | 0.1 (1) | 0.9 (5) | 3.5 (5) | 16.3 (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermakova, L.V.; Smyslova, V.G.; Dubov, V.V.; Kuznetsova, D.E.; Malozovskaya, M.S.; Saifutyarov, R.R.; Karpyuk, P.V.; Sokolov, P.S.; Komendo, I.Y.; Bondarau, A.G.; et al. Effect of a Phosphorus Additive on Luminescent and Scintillation Properties of Ceramics GYAGG:Ce. Ceramics 2023, 6, 1478-1489. https://doi.org/10.3390/ceramics6030091
Ermakova LV, Smyslova VG, Dubov VV, Kuznetsova DE, Malozovskaya MS, Saifutyarov RR, Karpyuk PV, Sokolov PS, Komendo IY, Bondarau AG, et al. Effect of a Phosphorus Additive on Luminescent and Scintillation Properties of Ceramics GYAGG:Ce. Ceramics. 2023; 6(3):1478-1489. https://doi.org/10.3390/ceramics6030091
Chicago/Turabian StyleErmakova, Lydia V., Valentina G. Smyslova, Valery V. Dubov, Daria E. Kuznetsova, Maria S. Malozovskaya, Rasim R. Saifutyarov, Petr V. Karpyuk, Petr S. Sokolov, Ilia Yu. Komendo, Aliaksei G. Bondarau, and et al. 2023. "Effect of a Phosphorus Additive on Luminescent and Scintillation Properties of Ceramics GYAGG:Ce" Ceramics 6, no. 3: 1478-1489. https://doi.org/10.3390/ceramics6030091
APA StyleErmakova, L. V., Smyslova, V. G., Dubov, V. V., Kuznetsova, D. E., Malozovskaya, M. S., Saifutyarov, R. R., Karpyuk, P. V., Sokolov, P. S., Komendo, I. Y., Bondarau, A. G., Mechinsky, V. A., & Korzhik, M. V. (2023). Effect of a Phosphorus Additive on Luminescent and Scintillation Properties of Ceramics GYAGG:Ce. Ceramics, 6(3), 1478-1489. https://doi.org/10.3390/ceramics6030091









