New Glasses in the PbCl2–PbO–B2O3 System: Structure and Optical Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
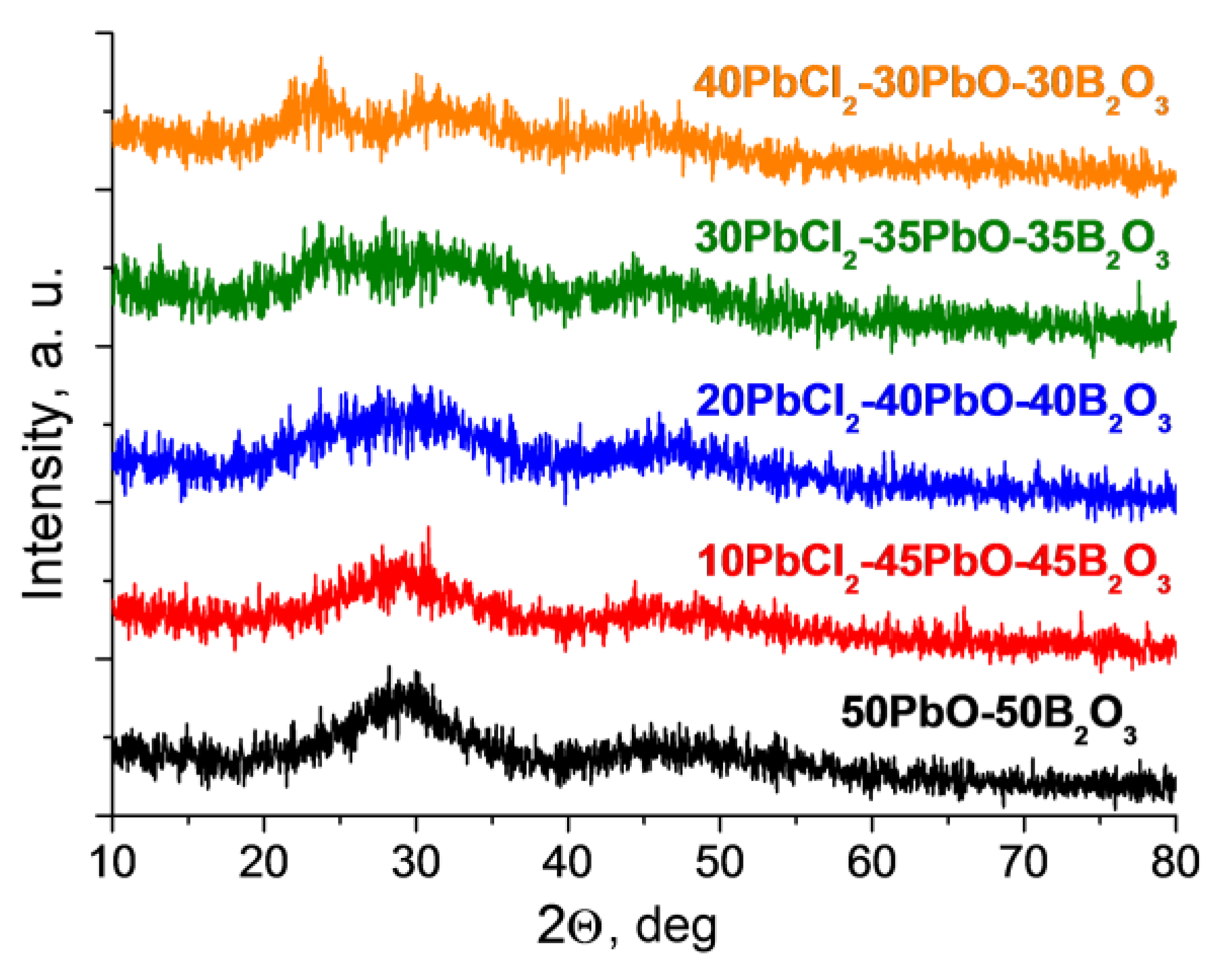
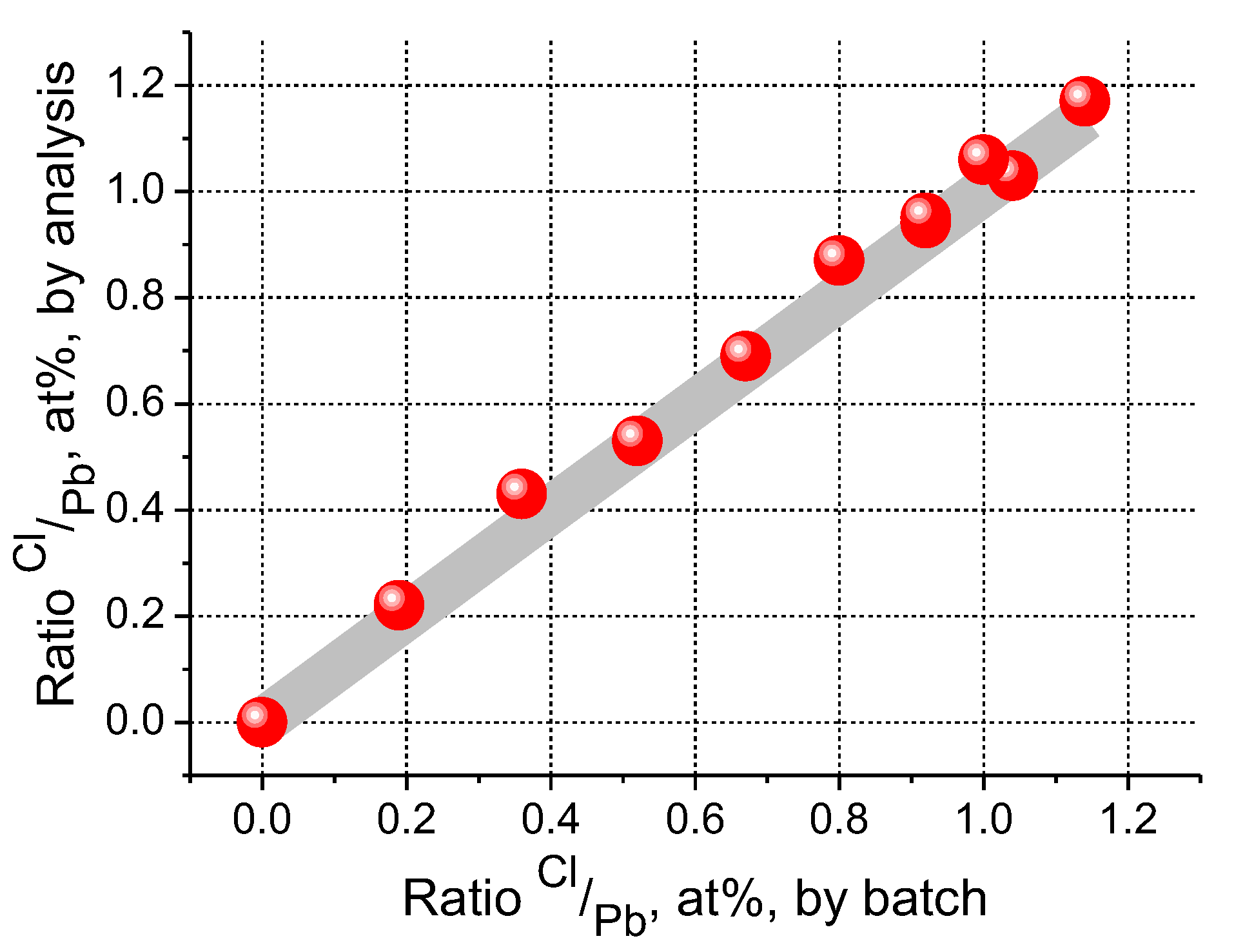
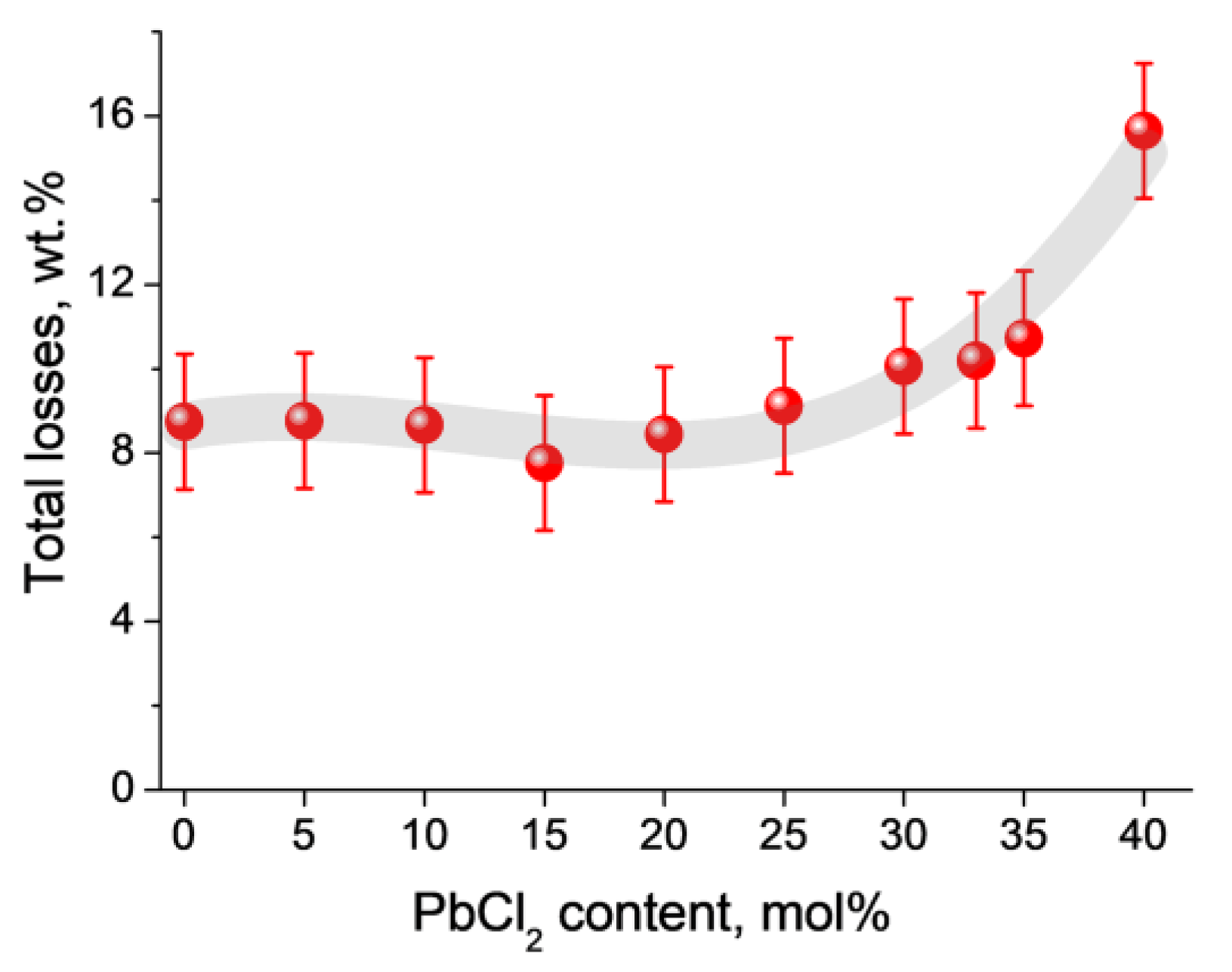
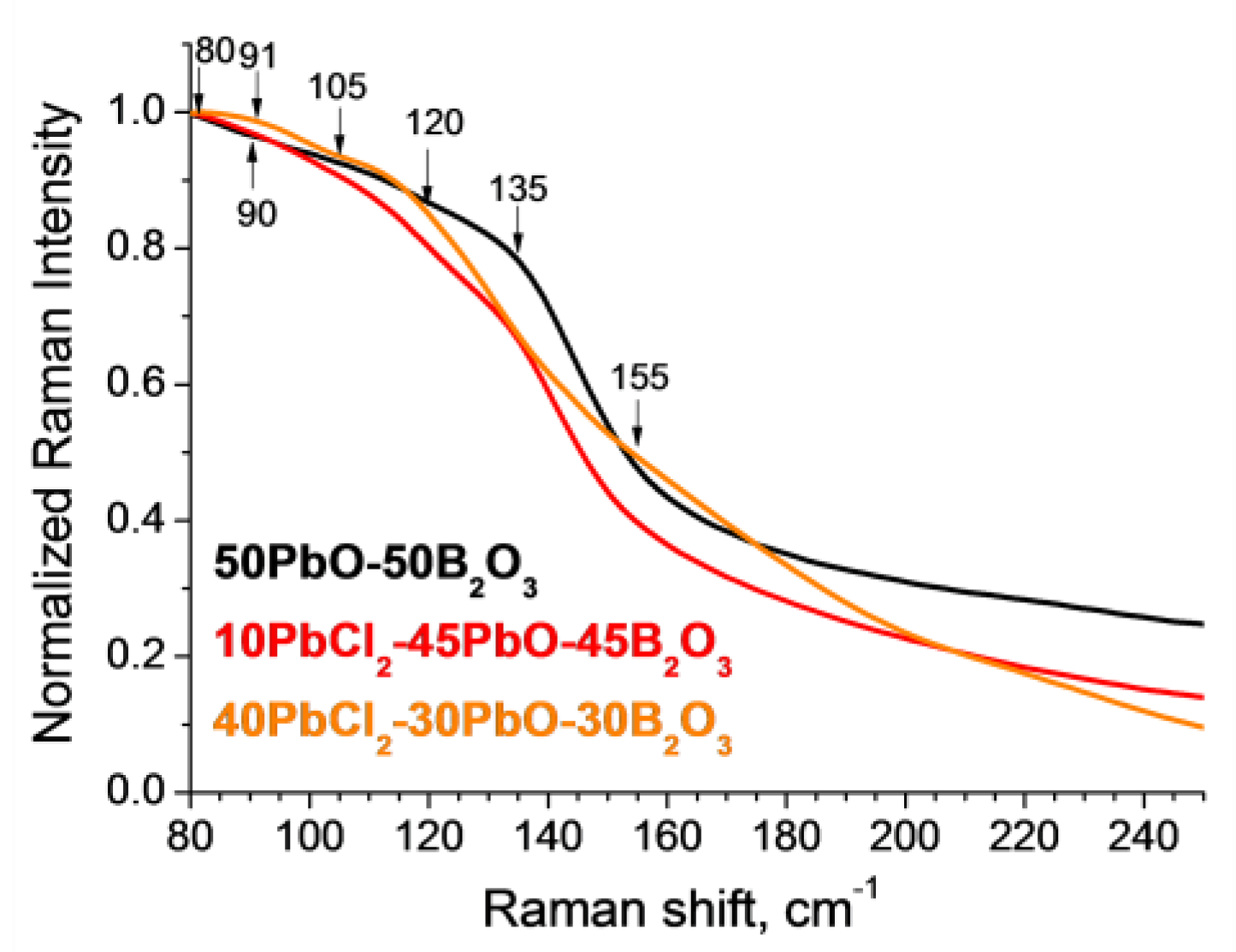
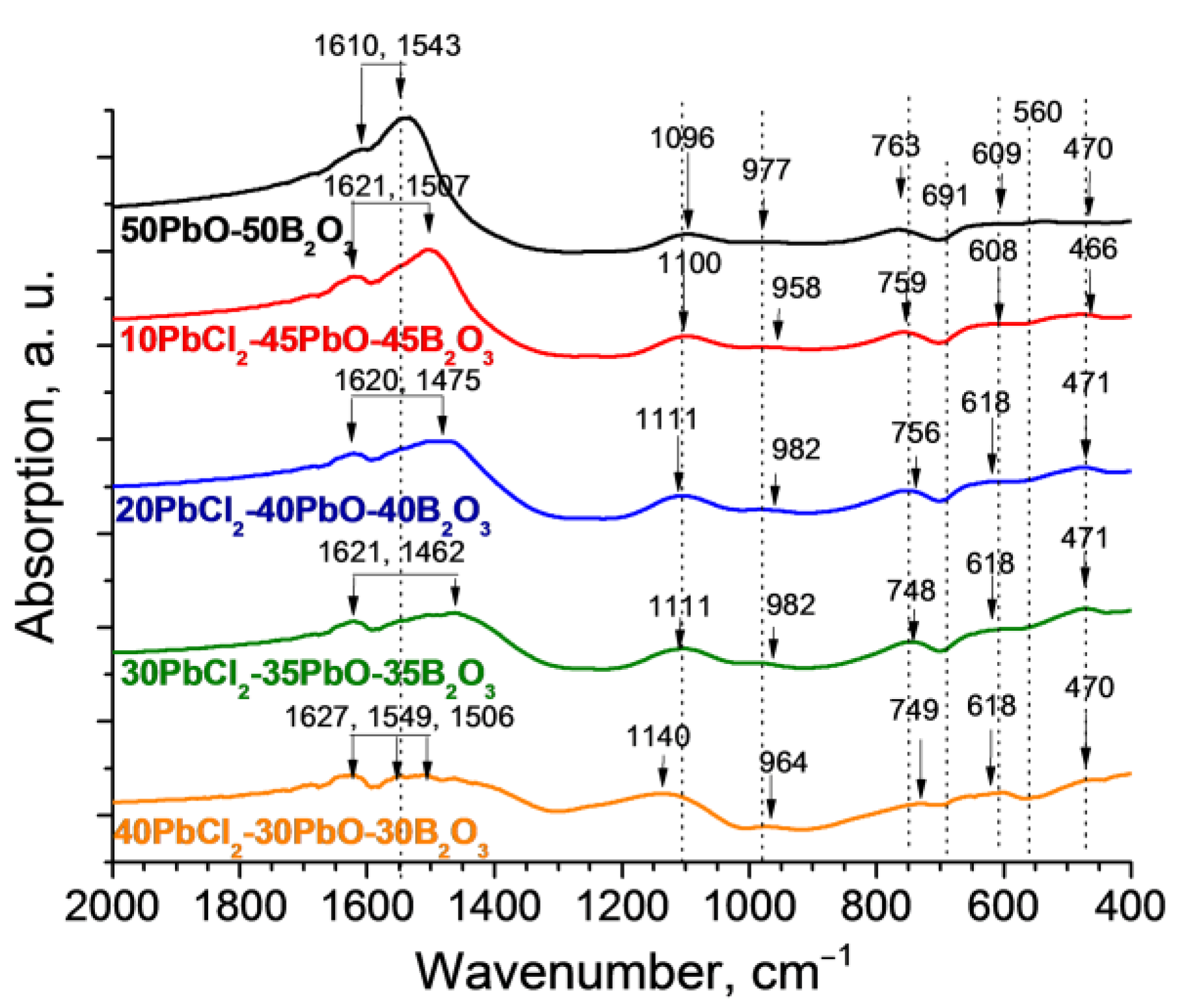
3.1. Glass Composition and Structure Characterization
| Range of 80–250 cm−1 | Range of 250–1750 cm−1 | ||||
|---|---|---|---|---|---|
| Wavenumber, cm−1 | Interpretation | Reference | Wavenumber, cm−1 | Interpretation | Reference |
| 80 | Bending vibrations in structural units of PbO4 | [23,30] | 310 | Vibrations of [PbO4]2− units | [23,31] |
| 90 | Stretching of predominantly ionic Pb–O bonds in polyhedral PbOn units | [23,24] | 476 | Pb–O bond vibrations | [32,33] |
| 570 | Oscillations of borate tetrahedrons BØ4ˉ | [23,25] | |||
| 91 | Vibrations associated with PbCl2, presumably Cl− | [21,22,27,34,35] | 620 | Deformation modes of BO3 metaborate chains | [23,26] |
| 710 | BO3 deformation modes in ring metaborate groups | [23,26] | |||
| 105 | Cations Pb2+ | [25,28,36] | 870 | Oscillations BØ4ˉ in pentaborate groups | [26,37] |
| 120 | Pb-Cl stretching vibration | [22,29], | 910 | Oscillations BØ4ˉ in ortho- and pentaborate groups | [26,37] |
| 135 | Symmetric Pb–O stretching in the [PbO4]2− pyramid configuration | [25,26,32,38] | 980 | Oscillations BØ4ˉ of diborate groups | [26,39,40] |
| 150–180 | Valence vibrations of Pb–Cl, bending vibrations of the Pb–Cl bond | [22,27,32,34,35,41] | 1050 | Oscillations BØ4ˉ of diborate groups | [23,39,40] |
| 1075 | Oscillations BØ4ˉ of diborate groups | [23,39] | |||
| 1285 | B-Oˉ stretches in metaborate triangles (BØ2Oˉ), mostly forming chain structures. A minor fraction: BØ3 extensions | [23,25,33] | |||
| Wavenumber, cm−1 | Corresponding Vibrational Mode | Reference |
|---|---|---|
| ~466 | Lead cation oscillations Pb2+ | [42,43] |
| ~560 | Oscillations of free BO4 groups | [44] |
| ~610 | bending vibrations of the B–O–B bonds in the BO3 group | [45] |
| ~691 | B-O oscillations associated with the PbO—BO3 bridge in the lead borate network | [45,46] |
| ~760 | Bending vibrations of the O3B–O–BO4 bond | [5] |
| ~800–1200 | Tensile vibrations of the B–O bonds of the tetrahedral block BO4 in ortho-, pyro-, and metaborate groups | [5,45,46] |
| 970 | Tensile vibrations of B–O bonds in BO4 groups | [45,46] |
| ~1050–1150 | Tensile B-O oscillations in BO4 units from tri-, tetra-, and pentaborate groups | [4,5] |
| 1300–1700 | Bond fluctuations in BO3 groups | [4,5,45,46] |
| ~1505, 1559, 1611 | Asymmetric relaxations of B–O bond stretching of BO3 trigonal blocks | [44] |
| ~2350 | Asymmetric modes of CO2 stretching | [47] |
| ~3445 | Stretching vibrations of OH− groups | [4,44,47] |
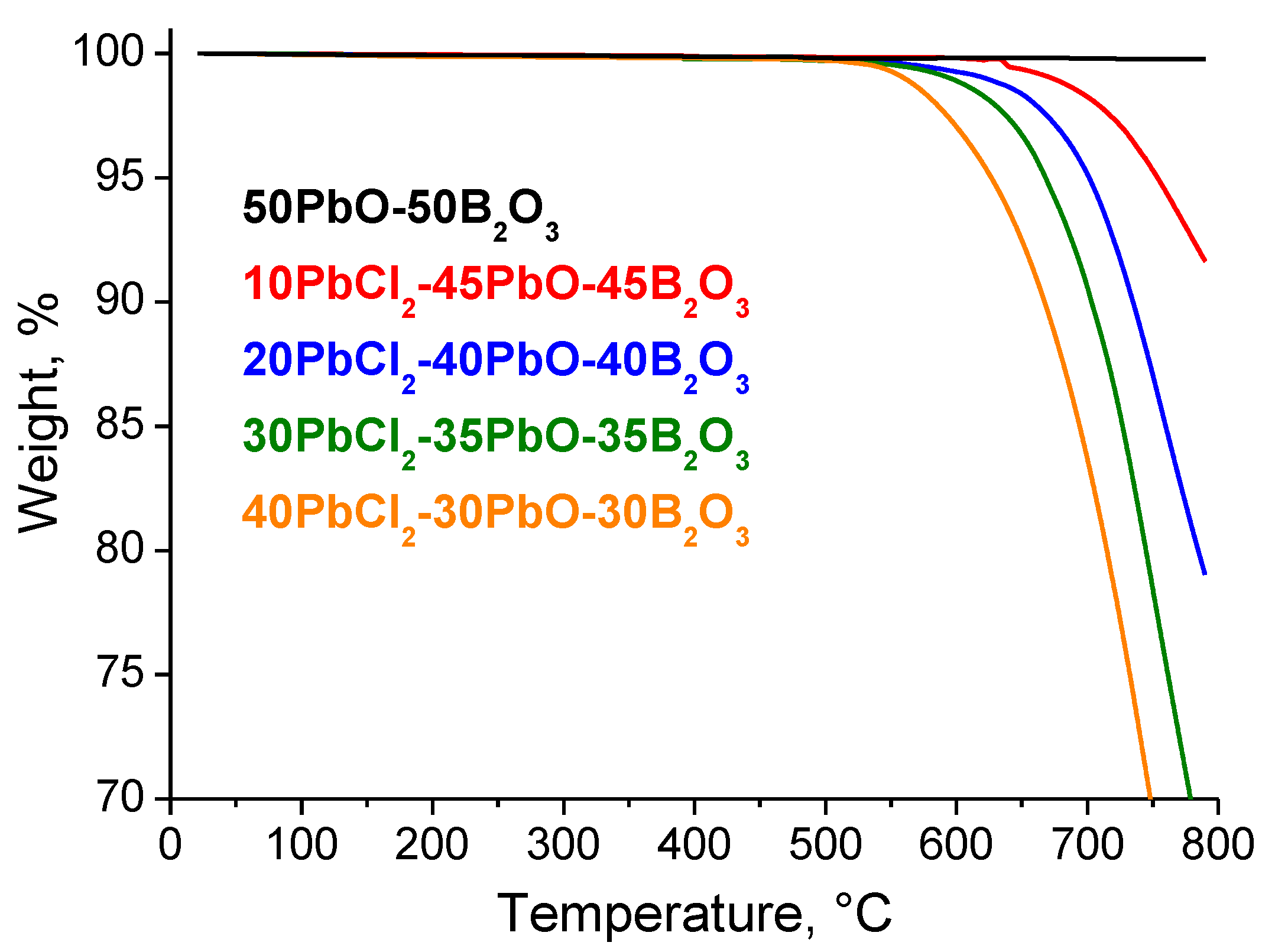
3.2. DSC Characterization and Physical Properties
3.3. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bengisu, M. Borate glasses for scientific and industrial applications: A review. J. Mater. Sci. 2016, 51, 2199–2242. [Google Scholar] [CrossRef]
- Sokolov, I.A.; Murin, I.V.; Mel’Nikova, N.A.; Pronkin, A.A. A Study of Ionic Conductivity of Glasses in the PbCl2–PbO · B2O3 and PbCl2–2PbO · B2O3 Systems. Glas. Phys. Chem. 2003, 29, 291–299. [Google Scholar] [CrossRef]
- Singh, G.P.; Singh, J.; Kaur, P.; Singh, T.; Kaur, R.; Singh, D.P. The Role of Lead Oxide in PbO-B2O3 Glasses for Solid State Ionic Devices. Mater. Phys. Mech. 2021, 47, 951–961. [Google Scholar] [CrossRef]
- Pisarska, J. Novel oxychloroborate glasses containing neodymium ions: Synthesis, structure and luminescent properties. J. Mol. Struct. 2008, 887, 201–204. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Hameed, A.; Sathe, V.G.; Chary, M.N.; Shareefuddin, M. Physical, optical and structural studies of copper-doped lead oxychloro borate glasses. Bull. Mater. Sci. 2018, 41, 79. [Google Scholar] [CrossRef]
- Saddeek, Y.B. Structural and acoustical studies of lead sodium borate glasses. J. Alloys Compd. 2009, 467, 14–21. [Google Scholar] [CrossRef]
- Yousef, E.S.S. Thermal and optical properties of zinc halotellurite glasses. J. Mater. Sci. 2007, 42, 4502–4507. [Google Scholar] [CrossRef]
- Brown, E.; Hömmerich, U.; Bluiett, A.G.; Trivedi, S.B.; Zavada, J.M. Synthesis and spectroscopic properties of neodymium doped lead chloride. J. Appl. Phys. 2007, 101, 113103. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Hameed, A.; Ramadevudu, G.; Chary, M.N. Shareefuddin Physical and spectroscopic studies on manganese ions in lead halo borate glasses. Mod. Phys. Lett. B 2017, 31, 1750180. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Pisarska, J.; Lisiecki, R.; Grobelny, Ł.; Dominiak-Dzik, G.; Ryba-Romanowski, W. Erbium-doped oxide and oxyhalide lead borate glasses for near-infrared broadband optical amplifiers. Chem. Phys. Lett. 2009, 472, 217–219. [Google Scholar] [CrossRef]
- Pisarska, J. Borate glasses with PbO and PbCl2 containing Dy3+ ions. Opt. Appl. 2010, 40, 367–374. [Google Scholar]
- El-Damrawi, G. Influence of PbCl2 on physical properties of lead chloroborate glasses. J. Non-Cryst. Solids 1994, 176, 91–97. [Google Scholar] [CrossRef]
- Sebastian, S.; Khadar, M.A. Optical properties of 60B2O3-(40-x)PbO-xMCl2 and 50B2O3-(50-x) PbO-xMCl2 (M = Pb, Cd) glasses. Bull. Mater. Sci. 2004, 27, 207–212. [Google Scholar] [CrossRef]
- Sebastian, S.; Khadar, M.A. Microhardness indentation size effect studies in 60B2O3-(40-x)PbO-xMCl2 and 50B2O3(50-x)PbO-xMCl2 (M = Pb, Cd) glasses. J. Mater. Sci. 2005, 40, 1655–1659. [Google Scholar] [CrossRef]
- Menezes, D.; Bannwart, E.; De Souza, J.; Rojas, S. Thermoluminescence emission on lead oxychloroborate glasses under UV exposure. Luminescence 2019, 34, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Silim, H.A.; El-Damrawi, G.; Moustafa, Y.M.; Hassan, A.K. Electrical and elastic properties of binary lead chloroborate glasses. J. Phys. Condens. Matter 1994, 6, 6189–6196. [Google Scholar] [CrossRef]
- Nunes, C.; Mahendrasingam, A.; Suryanarayanan, R. Quantification of Crystallinity in Substantially Amorphous Materials by Synchrotron X-ray Powder Diffractometry. Pharm. Res. 2005, 22, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.; Zografi, G.; Engers, D.; Morris, K.; Crowley, K.; Newman, A. Analysis of Amorphous and Nanocrystalline Solids from Their X-ray Diffraction Patterns. Pharm. Res. 2006, 23, 2333–2349. [Google Scholar] [CrossRef]
- Cattaneo, A.S.; Lima, R.P.; Tambelli, C.E.; Magon, C.J.; Mastelaro, V.R.; Garcia, A.; de Souza, J.E.; de Camargo, A.S.S.; de Araujo, C.C.; Schneider, J.F.; et al. Structural Role of Fluoride in the Ion-Conducting Glass System B2O3–PbO–LiF Studied by Single- and Double-Resonance NMR. J. Phys. Chem. C 2008, 112, 10462–10471. [Google Scholar] [CrossRef]
- Singh, G.P.; Kaur, P.; Kaur, S.; Singh, D. Role of WO3 in structural and optical properties of WO3–Al2O3–PbO–B2O3 glasses. Phys. B Condens. Matter 2011, 406, 4652–4656. [Google Scholar] [CrossRef]
- Tsai, P.; Cooney, R.P. Raman spectra of polynuclear hydroxo-compounds of lead(II) chloride. J. Chem. Soc. Dalton Trans. 1976, 1631–1634. [Google Scholar] [CrossRef]
- Melo, F.E.A.; Filho, J.M.; Moreira, J.E.; Lemos, V.; Cerdeira, F. Anharmonic effects in Raman-active modes of PbCl2. J. Raman Spectrosc. 1984, 15, 128–131. [Google Scholar] [CrossRef]
- Chatzipanagis, K.I.; Tagiara, N.S.; Kamitsos, E.I.; Barrow, N.; Slagle, I.; Wilson, R.; Greiner, T.; Jesuit, M.; Leonard, N.; Phillips, A.; et al. Structure of lead borate glasses by Raman, 11B MAS, and 207Pb NMR spectroscopies. J. Non-Cryst. Solids 2022, 589, 121660. [Google Scholar] [CrossRef]
- Takaishi, T.; Jin, J.; Uchino, T.; Yoko, T. Structural Study of PbO-B2O3 Glasses by X-ray Diffraction and 11B MAS NMR Techniques. J. Am. Ceram. Soc. 2004, 83, 2543–2548. [Google Scholar] [CrossRef]
- Pan, Z.; Henderson, D.O.; Morgan, S.H. A Raman investigation of lead haloborate glasses. J. Chem. Phys. 1994, 101, 1767–1774. [Google Scholar] [CrossRef]
- Filho, A.S.; Filho, J.M.; Melo, F.; Custódio, M.; Lebullenger, R.; Hernandes, A. Optical properties of Sm3+ doped lead fluoroborate glasses. J. Phys. Chem. Solids 2000, 61, 1535–1542. [Google Scholar] [CrossRef]
- Ozin, G.A. The single crystal Raman spectrum of orthorhombic PbCl2. Can. J. Chem. 1970, 48, 2931–2933. [Google Scholar] [CrossRef]
- Mendes-Filho, J.; Melo, F.E.; Moreira, J.E. Raman spectra of lead chloride single crystals. J. Raman Spectrosc. 1979, 8, 199–202. [Google Scholar] [CrossRef]
- Sun, H.-T.; Wen, L.; Duan, Z.-C.; Hu, L.-L.; Zhang, J.-J.; Jiang, Z.-H. Intense frequency upconversion fluorescence emission of Er3+/Yb3+-codoped oxychloride germanate glass. J. Alloys Compd. 2006, 414, 142–145. [Google Scholar] [CrossRef]
- Witke, K.; Hübert, T.; Reich, P.; Splett, C. Quantitative Raman investigations of the structure of glasses in the system B2O3-PbO. Phys. Chem. Glas. 1994, 35, 28–33. [Google Scholar]
- Adams, D.M.; Stevens, D.C. Single-crystal vibrational spectra of tetragonal and orthorhombic lead monoxide. J. Chem. Soc. Dalton Trans. 1977, 1096–1103. [Google Scholar] [CrossRef]
- Frost, R.L.; Williams, P.A. Raman spectroscopy of some basic chloride containing minerals of lead and copper. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2004, 60, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.C.; Rao, G.V.; Chary, B.S.; Phani, A.V.L.; Sathe, V.G.; Narsimlu, N. Shareefuddin Raman study on lead halo borate glasses: Effect of PbO as modifier. In Proceedings of the International Conference on Advanced Materials: ICAM 2019, Kerala, India, 12–14 June 2019; p. 020144. [Google Scholar] [CrossRef]
- Zakir’yanov, D.O.; Chernyshev, V.; Zakir’yanova, I.D. Phonon spectrum of lead oxychloride Pb3O2Cl2: Ab initio calculation and experiment. Phys. Solid State 2016, 58, 325–332. [Google Scholar] [CrossRef]
- Maroni, V.A.; Cunningham, P.T. Laser-Raman spectra of gaseous BiBr3 and PbCl2. Appl. Spectrosc. 1973, 27, 428–430. [Google Scholar] [CrossRef]
- Faulques, E.; Zubkowski, J.D.; Perry, D.L. Infrared and Raman Spectra of bis-Thiourea Lead(II) Chloride. Spectrosc. Lett. 1996, 29, 1275–1284. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Liu, L.; Zhang, Z. Modification of the Structure of Ti-Bearing Mold Flux by the Simultaneous Addition of B2O3 and Na2O. Met. Mater. Trans. E 2016, 3, 28–36. [Google Scholar] [CrossRef]
- Kurushkin, M.; Semencha, A.; Blinov, L.N.; Mikhail, M. Lead-containing oxyhalide glass. Glas. Phys. Chem. 2014, 40, 421–427. [Google Scholar] [CrossRef]
- Górny, A.; Kuwik, M.; Pisarski, W.A.; Pisarska, J. Lead Borate Glasses and Glass-Ceramics Singly Doped with Dy3+ for White LEDs. Materials 2020, 13, 5022. [Google Scholar] [CrossRef]
- Meera, B.; Sood, A.; Chandrabhas, N.; Ramakrishna, J. Raman study of lead borate glasses. J. Non-Cryst. Solids 1990, 126, 224–230. [Google Scholar] [CrossRef]
- Coleman, F.; Feng, G.; Murphy, R.W.; Nockemann, P.; Seddon, K.R.; Swadźba-Kwaśny, M. Lead(ii) chloride ionic liquids and organic/inorganic hybrid materials—A study of chloroplumbate(ii) speciation. Dalton Trans. 2013, 42, 5025–5035. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer Mineralogy; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Muthuselvi, C.; Anbuselvi, T.; Pandiarajan, S. Synthesis and Characterization of Lead (II) Chloride and Lead (II) Carbonate Nano. Part. Rev. Res. 2016, 5, 1–13. [Google Scholar]
- Abdelghany, A.M. Combined DFT, Deconvolution Analysis for Structural Investigation of Copper–doped Lead Borate Glasses. Open Spectrosc. J. 2012, 6, 9–14. [Google Scholar] [CrossRef]
- Abdullah, M.; Shafieza, S.; Kasim, A.; Hashim, A. The Effect of PbO on the Physical and Structural Properties of Borate Glass System. Mater. Sci. Forum 2016, 846, 177–182. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Pisarska, J.; Ryba-Romanowski, W. Structural role of rare earth ions in lead borate glasses evidenced by infrared spectroscopy: BO3↔BO4 conversion. J. Mol. Struct. 2005, 744–747, 515–520. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, W.; Sun, Q.; Liu, X. Raman and infrared spectroscopic quantification of the carbonate concentration in K2CO3 aqueous solutions with water as an internal standard. Geosci. Front. 2021, 12, 1018–1030. [Google Scholar] [CrossRef]
- Rao, K.J.; Rao, B.G.; Elliott, S.R. Glass formation in the system PbO-PbCl2. J. Mater. Sci. 1985, 20, 1678–1682. [Google Scholar] [CrossRef]
- El-Damrawi, G. PbCl2 conducting glasses with mixed glass formers. J. Phys. Condens. Matter 1995, 7, 1557–1563. [Google Scholar] [CrossRef]
- Gressler, C.A.; Shelby, J.E. Lead fluoroborate glasses. J. Appl. Phys. 1988, 64, 4450–4453. [Google Scholar] [CrossRef]
- Coon, J.; Horton, M.; Shelby, J.E. Stability and crystallization of lead halosilicate glasses. J. Non-Cryst. Solids 1988, 102, 143–147. [Google Scholar] [CrossRef]
- Pisarski, W.; Pisarska, J.; Goryczka, T.; Dominiak-Dzik, G.; Ryba-Romanowski, W. Influence of thermal treatment on spectroscopic properties of Er3+ ions in multicomponent InF3-based glasses. J. Alloys Compd. 2005, 398, 272–275. [Google Scholar] [CrossRef]
- Gressler, C.A.; Shelby, J.E. Properties and structure of PbO-PbF2-B2O3 glasses. J. Appl. Phys. 1989, 66, 1127–1131. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiao, H.; Guo, W. Structure and crystallization kinetics of PbO–B2O3 glasses. Ceram. Int. 2007, 33, 1341–1347. [Google Scholar] [CrossRef]
- Petrova, O.; Velichkina, D.; Zykova, M.; Khomyakov, A.; Uslamina, M.; Nischev, K.; Pynenkov, A.; Avetisov, R.; Avetissov, I.C. Nd/La, Nd/Lu-co-doped transparent lead fluoroborate glass-ceramics. J. Non-Cryst. Solids 2020, 531, 119858. [Google Scholar] [CrossRef]
- Greenblatt, S.; Bray, P.J. Structural Aspects of B-O Network in Glassy Borates. Glas. Phys. Chem. 1967, 8, 312. [Google Scholar]
- Konijnendijk, W.; Stevels, J. The structure of borate glasses studied by Raman scattering. J. Non-Cryst. Solids 1975, 18, 307–331. [Google Scholar] [CrossRef]
- Abdullah, M.; Mohd, W.; Husin, H.W.; Kassim, A.; Yahya, N. The physical properties of lead borate (PbO-B2O3) glass. KONAKA Konf. Akad. 2015, 2015, 380–386. [Google Scholar]
- Hogarth, C.A.; Assadzadeh-Kashani, E. Some studies of the optical properties of tungsten-calcium-tellurite glasses. J. Mater. Sci. 1983, 18, 1255–1263. [Google Scholar] [CrossRef]
- Duffy, J.A. Ultraviolet transparency of glass: A chemical approach in terms of band theory, polarisability and electronegativity. Glas. Phys. Chem. 2001, 42, 151–157. [Google Scholar]
- Butenkov, D.A.; Runina, K.I.; Petrova, O.B. Synthesis and Properties of Nd-Doped Chlorofluorosilicate Lead Glasses. Glas. Ceram. 2021, 78, 135–139. [Google Scholar] [CrossRef]
- Urbach, F. The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Lezal, D.; Pedlikova, J.; Kostka, P.; Bludska, J.; Poulain, M.; Zavadil, J. Heavy metal oxide glasses: Preparation and physical properties. J. Non-Cryst. Solids 2001, 284, 288–295. [Google Scholar] [CrossRef]
- Avetissov, I.C.; Petrova, O.B.; Anurova, M.O.; Khomyakov, A.V.; Akkuzina, A.A.; Taidakov, I.V. Mechanical and optical properties of hybrid materials based on inorganic glass matrix and organic metal complex phosphors. J. Phys. Conf. Ser. 2018, 1045, 012006. [Google Scholar] [CrossRef]
- Richards, B.D.O.; Teddy-Fernandez, T.; Jose, G.; Binks, D.; Jha, A. Mid-IR (3–4 μm) fluorescence and ASE studies in Dy3+ doped tellurite and germanate glasses and a fs laser inscribed waveguide. Laser Phys. Lett. 2013, 10, 085802. [Google Scholar] [CrossRef]
- French, R.H.; Müllejans, H.; Jones, D.J. Optical Properties of Aluminum Oxide: Determined from Vacuum Ultraviolet and Electron Energy-Loss Spectroscopies. J. Am. Ceram. Soc. 2005, 81, 2549–2557. [Google Scholar] [CrossRef]


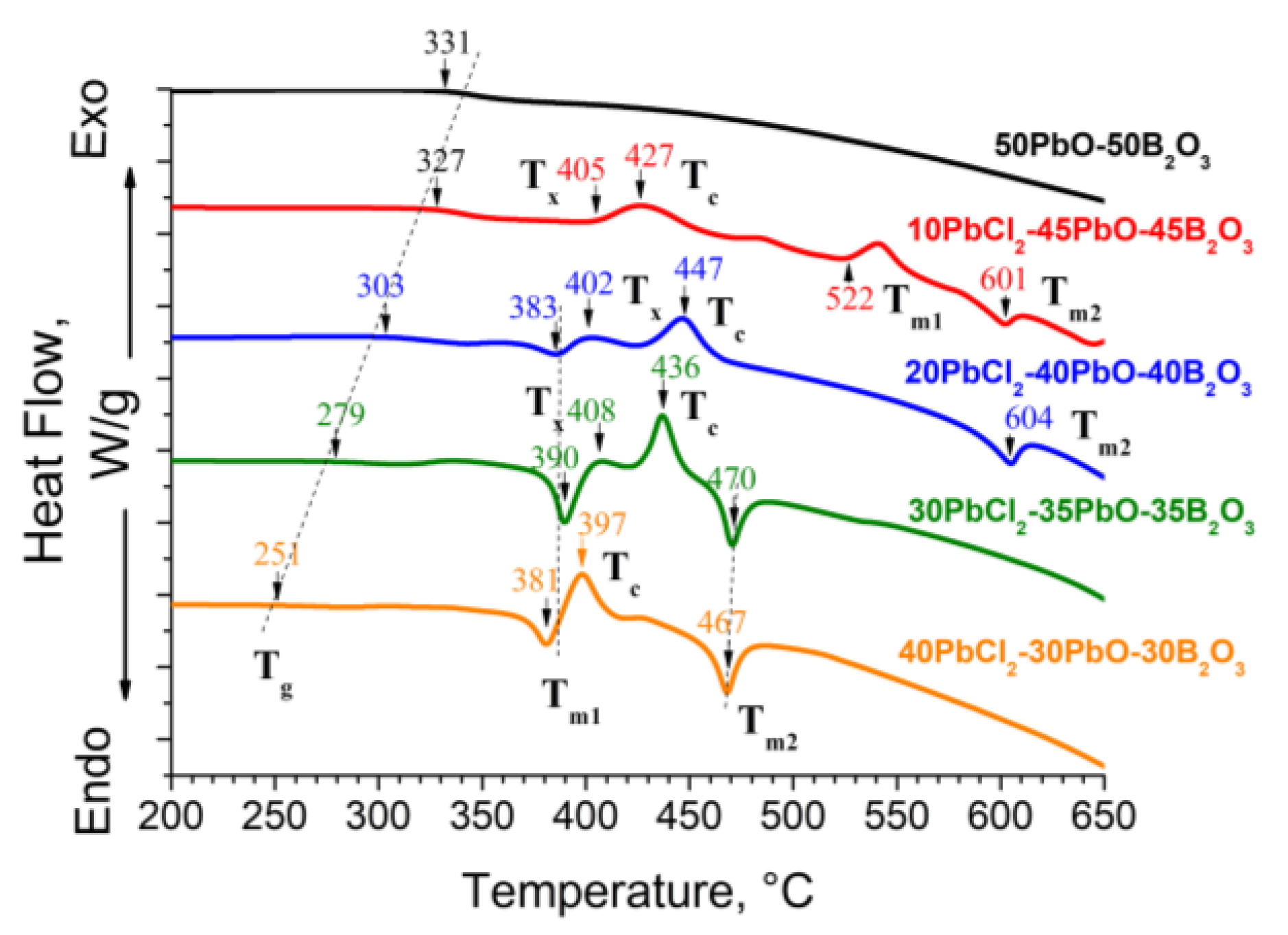
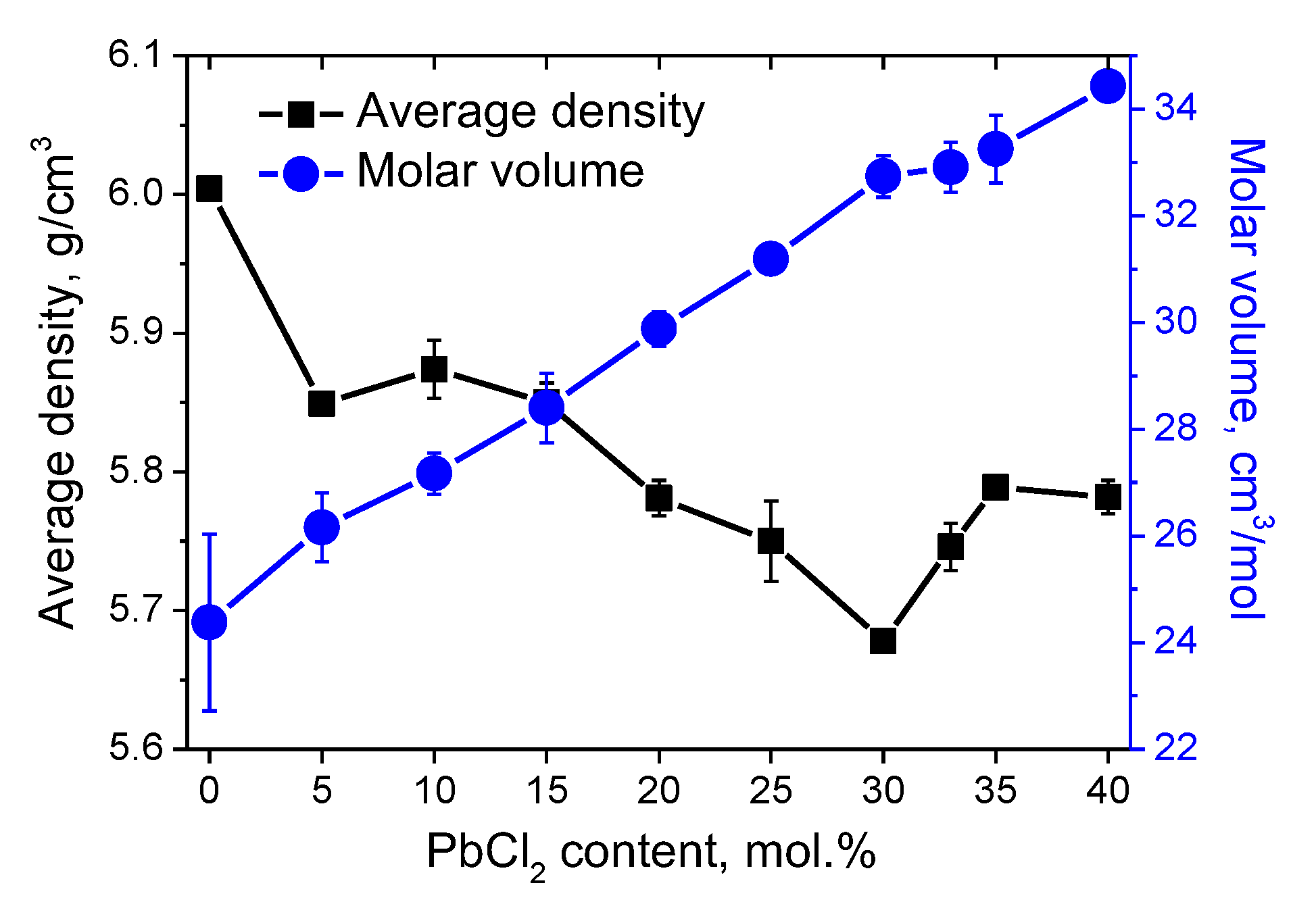
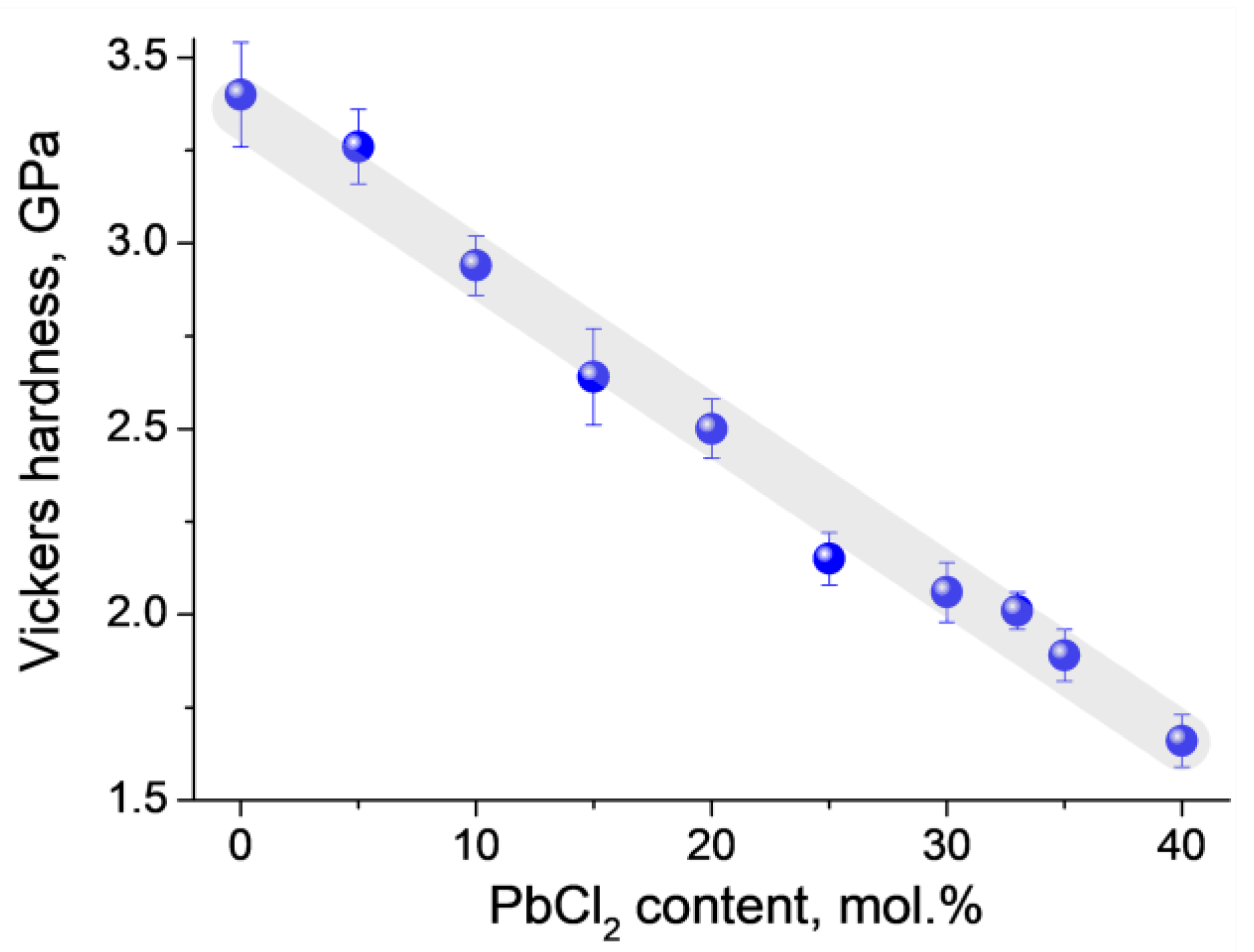

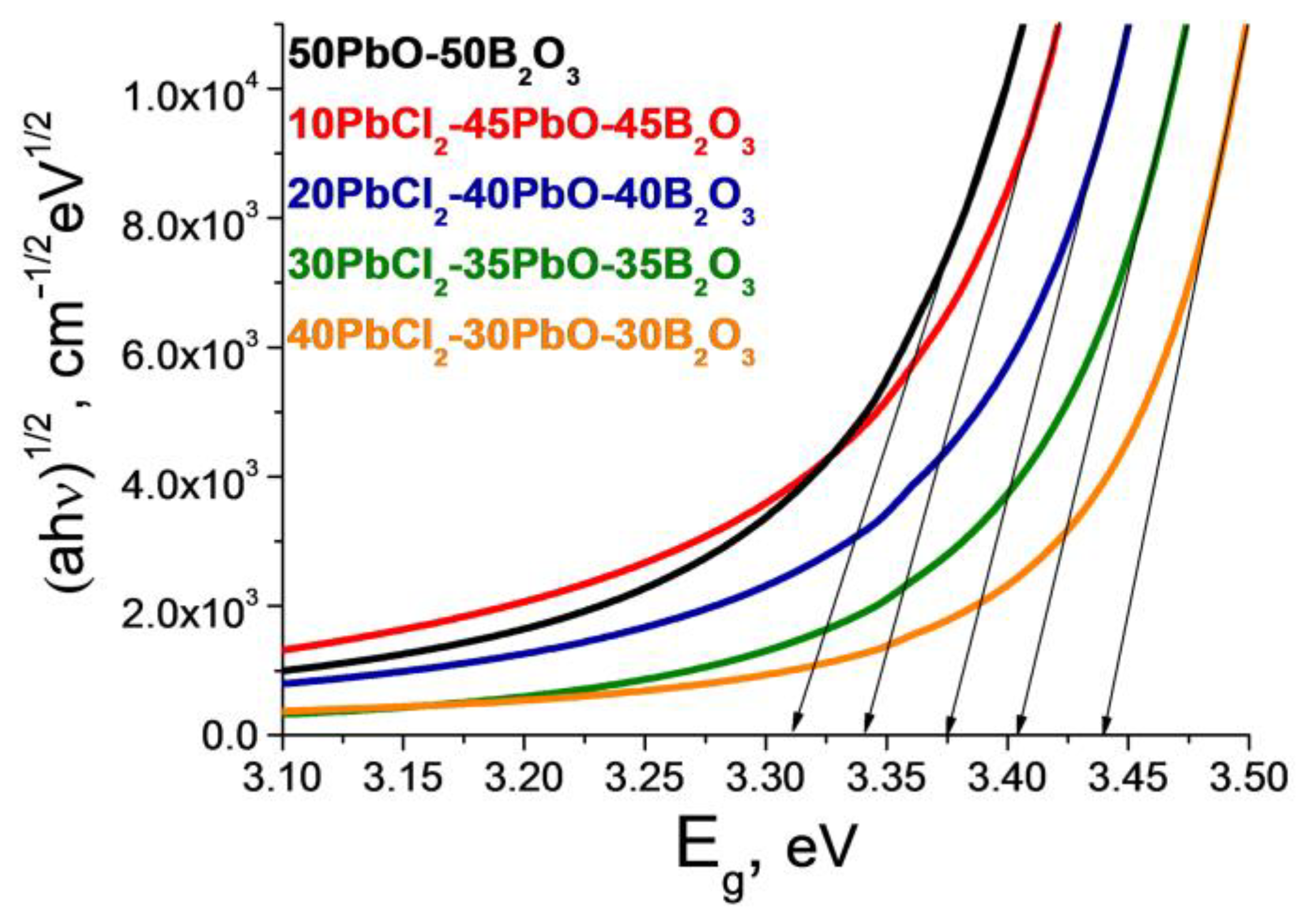
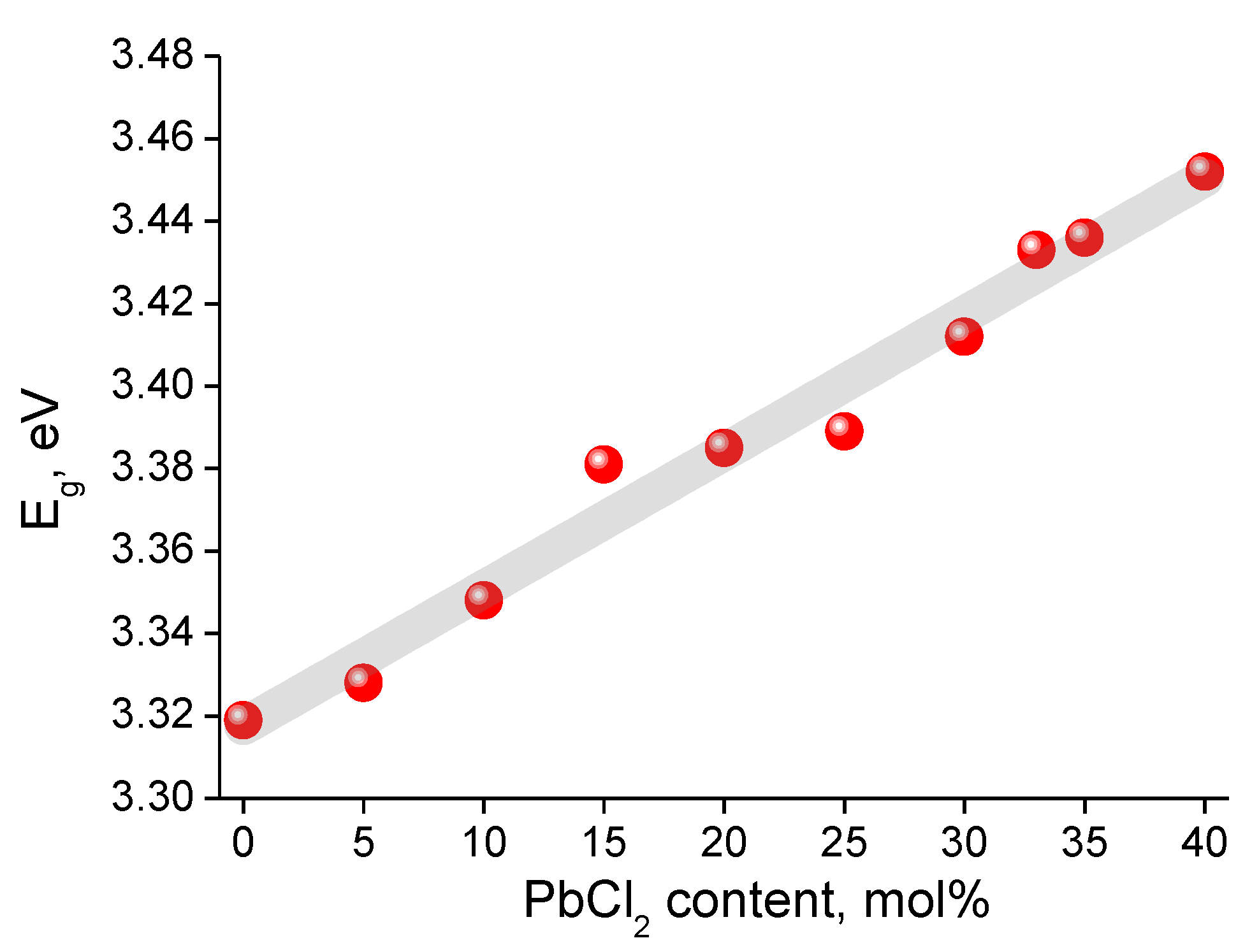
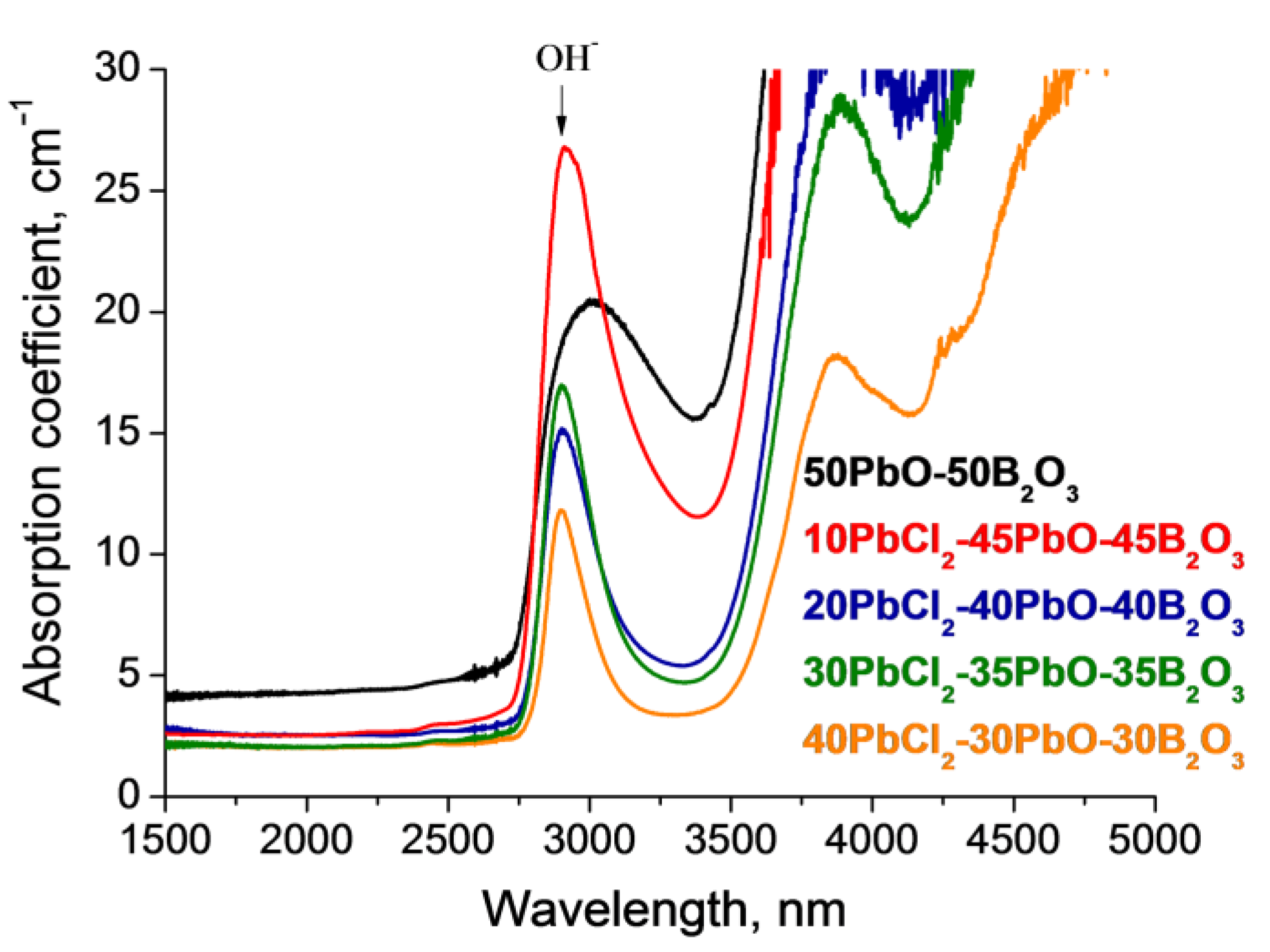
| Glass Composition, mol% | Tg, °C | Tx1, °C | Tc1, °C | Tx2, °C | Tc2, °C | Tm1, °C | Tm2, °C | ΔT = (Tx1 − Tg), °C |
|---|---|---|---|---|---|---|---|---|
| 50PbO–50B2O3 | 331 | - | - | - | - | - | - | - |
| 10PbCl2-–5PbO–45B2O3 | 327 | 405 | 427 | 485 | 540 | 522 | 601 | 78 |
| 20PbCl2–40PbO–40B2O3 | 303 | 402 | 447 | - | - | 383 | 604 | 99 |
| 30PbCl2–35PbO–35B2O3 | 279 | 408 | 436 | - | - | 390 | 470 | 129 |
| 40PbCl2–30PbO–30B2O3 | 251 | 381 | 397 | 428 | - | 381 | 467 | 130 |
| Glass Composition, mol% | Short- Wavelength Absorption Edge λ, nm | Tauc Energy Eg, eV * | Urbach Energy Ee, eV * | Average Absorption Coefficient, cm−1 | Long- Wavelength Absorption Edge λ, nm | Absorption of the OH− Group Band, cm−1 |
|---|---|---|---|---|---|---|
| 50PbO–50B2O3 | 365 | 3.307 | 0.468 | 5.7 | 3611 | 15.5 |
| 5PbCl2–47.5PbO–47.5B2O3 | 365 | 3.320 | 0.393 | 3.6 | 3626 | 22.0 |
| 10PbCl2–45PbO–45B2O3 | 363 | 3.327 | 0.504 | 4.7 | 3664 | 22.4 |
| 15PbCl2–42.5PbO–42.5B2O3 | 360 | 3.378 | 0.432 | 4.4 | 3690 | 20.3 |
| 20PbCl2–40PbO–40B2O3 | 360 | 3.376 | 0.435 | 5.0 | 3739 | 12.4 |
| 25PbCl2–37.5PbO–37.5B2O3 | 359 | 3.389 | 0.505 | 5.2 | 3788 | 11.9 |
| 30PbCl2–35PbO–35B2O3 | 357 | 3.406 | 0.377 | 2.4 | 4349 | 14.6 |
| 33PbCl2–33PbO–34B2O3 | 356 | 3.414 | 0.453 | 4.6 | 4370 | 10.9 |
| 35PbCl2–32.5PbO–32.5B2O3 | 356 | 3.423 | 0.434 | 4.4 | 4462 | 9.5 |
| 40PbCl2–30PbO–30B2O3 | 355 | 3.440 | 0.427 | 3.8 | 4710 | 9.4 |
| Glass Composition, mol% | Refractive Indices (n) | |||
|---|---|---|---|---|
| Estimated, ±0.01 | Experimental, ±0.001 | |||
| 633 nm | 969 nm | 1539 nm | ||
| 50PbO–50B2O3 | 2.04 | 1.914 | 1.895 | 1.890 |
| 5PbCl2–47.5PbO–47.5B2O3 | 2.05 | 1.969 | 1.930 | 1.903 |
| 10PbCl2–45PbO–45B2O3 | 2.06 | 1.989 | 1.949 | 1.932 |
| 15PbCl2–42.5PbO–42.5B2O3 | 2.06 | 1.991 | 1.963 | 1.940 |
| 20PbCl2–40PbO–40B2O3 | 2.07 | 2.009 | 1.969 | 1.954 |
| 25PbCl2–37.5PbO–37.5B2O3 | 2,08 | 2.017 | 1.978 | 1.960 |
| 30PbCl2–35PbO–35B2O3 | 2.09 | 2.040 | 2.000 | 1.981 |
| 33PbCl2–33PbO–34B2O3 | 2.09 | 2.051 | 2.010 | 1.991 |
| 35PbCl2–32.5PbO–32.5B2O3 | 2.10 | 2.058 | 2.016 | 1.997 |
| 40PbCl2–30PbO–30B2O3 | 2.10 | 2.076 | 2.032 | 2.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butenkov, D.; Bakaeva, A.; Runina, K.; Krol, I.; Uslamina, M.; Pynenkov, A.; Petrova, O.; Avetissov, I. New Glasses in the PbCl2–PbO–B2O3 System: Structure and Optical Properties. Ceramics 2023, 6, 1348-1364. https://doi.org/10.3390/ceramics6030083
Butenkov D, Bakaeva A, Runina K, Krol I, Uslamina M, Pynenkov A, Petrova O, Avetissov I. New Glasses in the PbCl2–PbO–B2O3 System: Structure and Optical Properties. Ceramics. 2023; 6(3):1348-1364. https://doi.org/10.3390/ceramics6030083
Chicago/Turabian StyleButenkov, Dmitry, Anna Bakaeva, Kristina Runina, Igor Krol, Maria Uslamina, Aleksandr Pynenkov, Olga Petrova, and Igor Avetissov. 2023. "New Glasses in the PbCl2–PbO–B2O3 System: Structure and Optical Properties" Ceramics 6, no. 3: 1348-1364. https://doi.org/10.3390/ceramics6030083
APA StyleButenkov, D., Bakaeva, A., Runina, K., Krol, I., Uslamina, M., Pynenkov, A., Petrova, O., & Avetissov, I. (2023). New Glasses in the PbCl2–PbO–B2O3 System: Structure and Optical Properties. Ceramics, 6(3), 1348-1364. https://doi.org/10.3390/ceramics6030083