Barium Silicate Glasses and Glass–Ceramic Seals for YSZ-Based Electrochemical Devices
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirera, A.; Lpez-Gándara, C.; Ramos, F.M. YSZ-based oxygen sensors and the use of nanomaterials: A review from classical models to current trends. J. Sens. 2009, 2009, 15. [Google Scholar] [CrossRef]
- Muroyama, H.; Okuda, S.; Matsui, T.; Hashigami, S.; Kawano, M.; Inagaki, T.; Eguchi, K. Gas composition analysis using yttria-stabilized zirconia oxygen sensor during dry reforming and partial oxidation of methane. J. Jpn. Pet. Inst. 2018, 61, 72–79. [Google Scholar] [CrossRef]
- Liu, M.; Dong, D.; Peng, R.; Gao, J.; Diwu, J.; Liu, X.; Meng, G. YSZ-based SOFC with modified electrode/electrolyte interfaces for operating at temperature lower than 650 °C. J. Power Sources 2008, 180, 215–220. [Google Scholar] [CrossRef]
- Hauch, A.; Brodersen, K.; Chen, M.; Mogensen, M.B. Ni/YSZ electrodes structures optimized for increased electrolysis performance and durability. Solid State Ion. 2016, 293, 27–36. [Google Scholar] [CrossRef]
- Jiang, L.; Lv, S.; Tang, W.; Zhao, L.; Wang, C.; Wang, J.; Wang, T.; Guo, X.; Liu, F.; Wang, C.; et al. YSZ-based acetone sensor using a Cd2SnO4 sensing electrode for exhaled breath detection in medical diagnosis. Sens. Actuators B Chem. 2021, 345, 130321. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef]
- Tho, N.D.; Van Huong, D.; Ngan, P.Q.; Thai, G.H.; Thu, D.T.A.; Thu, D.T.; Tuoi, N.T.M.; Toan, N.N.; Giang, H.T. Effect of sintering temperature of mixed potential sensor Pt/YSZ/LaFeO3 on gas sensing performance. Sens. Actuators B Chem. 2016, 224, 747–754. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Liu, A.; You, R.; Liu, F.; Li, S.; Zhao, L.; Jin, R.; He, J.; Yang, Z.; et al. High-response mixed-potential type planar YSZ-based NO2 sensor coupled with CoTiO3 sensing electrode. Sens. Actuators B Chem. 2019, 287, 185–190. [Google Scholar] [CrossRef]
- Schubert, F.; Wollenhaupt, S.; Kita, J.; Hagen, G.; Moos, R. Platform to develop exhaust gas sensors manufactured by glass-solder-supported joining of sintered yttria-stabilized zirconia. J. Sens. Sens. Syst. 2016, 5, 25–32. [Google Scholar] [CrossRef]
- Zhang, X.D.; Li, J.J.; Guo, X. Oxygen pump based on stabilized zirconia. Rev. Sci. Instrum. 2015, 86, 115103. [Google Scholar] [CrossRef]
- Tao, M.; Feng, J.; Li, R.; Guan, C.; Wang, J.; Chi, B.; Pu, J. Interfacial compatibility and thermal cycle stability for glass-sealed oxygen sensors. Ceram. Int. 2023, 49, 23180–23188. [Google Scholar] [CrossRef]
- Spirin, A.; Lipilin, A.; Ivanov, V.; Paranin, S.; Nikonov, A.; Khrustov, V.; Portnov, D.; Gavrilov, N.; Mamaev, A. Solid Oxide Electrolyte Based Oxygen Pump. In Proceedings of the 12th International Ceramics Congress Part D, Montecatini Terme, Italy, 6–11 June 2010; Volume 65, pp. 257–262. [Google Scholar]
- Spirin, A.V.; Nikonov, A.V.; Lipilin, A.S.; Paranin, S.N.; Ivanov, V.V.; Khrustov, V.R.; Valentsev, A.V.; Krutikov, V.I. Electrochemical cell with solid oxide electrolyte and oxygen pump thereof. Russ. J. Electrochem. 2011, 47, 569–578. [Google Scholar] [CrossRef]
- Pham, A.Q.; Glass, R.S. Oxygen pumping characteristics of yttria-stabilized-zirconia. Electrochim. Acta. 1998, 43, 2699–2708. [Google Scholar] [CrossRef]
- Khodimchuk, A.V.; Anan’ev, M.V.; Eremin, V.A.; Tropin, E.S.; Farlenkov, A.S.; Porotnikova, N.M.; Kurumchin, E.K.; Bronin, D.I. Oxygen isotope exchange between the gas-phase and the electrochemical cell O2, Pt | YSZ | Pt, O2 under conditions of applied potential difference. Russ. J. Electrochem. 2017, 53, 838–845. [Google Scholar] [CrossRef]
- Gunduz, S.; Dogu, D.; Deka, D.J.; Meyer, K.E.; Fuller, A.; Co, A.C.; Ozkan, U.S. Application of solid electrolyte cells in ion pump and electrolyzer modes to promote catalytic reactions: An overview. Catal. Today 2019, 323, 3–13. [Google Scholar] [CrossRef]
- Sohn, S.B.; Choi, S.Y.; Kim, G.H.; Song, H.S.; Kim, G.D. Suitable Glass-Ceramic Sealant for Planar Solid-Oxide Fuel Cells. J. Am. Ceram. Soc. 2004, 87, 254–260. [Google Scholar] [CrossRef]
- Batfalsky, P.; Haanappel, V.A.C.; Malzbender, J.; Menzler, N.H.; Shemet, V.; Vinke, I.C.; Steinbrech, R.W. Chemical interaction between glass-ceramic sealants and interconnect steels in SOFC stacks. J. Power Sources 2006, 155, 128–137. [Google Scholar] [CrossRef]
- Wang, X.; Yan, D.; Fang, D.; Luo, J.; Pu, J.; Chi, B.; Jian, L. Optimization of Al2O3-glass composite seals for planar intermediate-temperature solid oxide fuel cells. J. Power Sources 2013, 226, 127–133. [Google Scholar] [CrossRef]
- Singh, K.; Walia, T. Review on silicate and borosilicate-based glass sealants and their interaction with components of solid oxide fuel cell. Int. J. Energy Res. 2021, 45, 20559–20582. [Google Scholar] [CrossRef]
- Meinhardt, K.D.; Kim, D.S.; Chou, Y.S.; Weil, K.S. Synthesis and properties of a barium aluminosilicate solid oxide fuel cell glass-ceramic sealant. J. Power Sources 2008, 182, 188–196. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K. Glass-based seals for solid oxide fuel and electrolyzer cells—A review. Mater. Sci. Eng. R Reports 2010, 67, 65–85. [Google Scholar] [CrossRef]
- Kosiorek, M.; Żurawska, A.; Ajdys, L.; Kolasa, A.; Naumovich, Y.; Wiecińska, P.; Yaremchenko, A.; Kupecki, J. Glass–Zirconia Composites as Seals for Solid Oxide Cells: Preparation, Properties, and Stability over Repeated Thermal Cycles. Materials 2023, 16, 1634. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, R.; Yang, J.; Gu, D.; Yan, D.; Pu, J.; Chi, B.; Li, J. Effect of YSZ addition on the performance of glass-ceramic seals for intermediate temperature solid oxide fuel cell application. Int. J. Hydrog. Energy 2018, 43, 8040–8047. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, G.; Pandey, O.P.; Singh, K.; Lu, K. Effect of Thermal Treatment on Chemical Interaction Between Yttrium Borosilicate Glass Sealants and YSZ for Planar Solid Oxide Fuel Cells. Int. J. Appl. Glas. Sci. 2014, 5, 410–420. [Google Scholar] [CrossRef]
- Kaur, G.; Pandey, O.P.; Singh, K. Chemical compatibility between MgO-SiO2-B2O3-La2O3 glass sealant and low, high temperature electrolytes for solid oxide fuel cell applications. Int. J. Hydrog. Energy 2012, 37, 17235–17244. [Google Scholar] [CrossRef]
- Smeacetto, F.; Salvo, M.; Ferraris, M.; Cho, J.; Boccaccini, A.R. Glass-ceramic seal to join Crofer 22 APU alloy to YSZ ceramic in planar SOFCs. J. Eur. Ceram. Soc. 2008, 28, 61–68. [Google Scholar] [CrossRef]
- Sabato, A.G.; Rost, A.; Schilm, J.; Kusnezoff, M.; Salvo, M.; Chrysanthou, A.; Smeacetto, F. Effect of electric load and dual atmosphere on the properties of an alkali containing diopside-based glass sealant for solid oxide cells. J. Power Sources 2019, 415, 15–24. [Google Scholar] [CrossRef]
- Ferraris, M.; De la Pierre, S.; Sabato, A.G.; Smeacetto, F.; Javed, H.; Walter, C.; Malzbender, J. Torsional shear strength behavior of advanced glass-ceramic sealants for SOFC/SOEC applications. J. Eur. Ceram. Soc. 2020, 40, 4067–4075. [Google Scholar] [CrossRef]
- Li, R.; Liang, X.; Wang, X.; Zeng, W.; Yang, J.; Yan, D.; Pu, J.; Chi, B.; Li, J. Improvement of sealing performance for Al2O3 fiber-reinforced compressive seals for intermediate temperature solid oxide fuel cell. Ceram. Int. 2019, 45, 21953–21959. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Dong, Y.; Yang, J.J.; Pu, J.; Chi, B.; Jian, L. Development of flexible ceramic-glass seals for intermediate temperature planar solid oxide fuel cell. Int. J. Hydrog. Energy 2016, 41, 6036–6044. [Google Scholar] [CrossRef]
- Krainova, D.A.; Saetova, N.S.; Kuzmin, A.V.; Raskovalov, A.A.; Eremin, V.A.; Ananyev, M.V.; Steinberger-Wilckens, R. Non-crystallising glass sealants for SOFC: Effect of Y2O3 addition. Ceram. Int. 2020, 46, 5193–5200. [Google Scholar] [CrossRef]
- Krainova, D.A.; Saetova, N.S.; Polyakova, I.G.; Farlenkov, A.S.; Zamyatin, D.A.; Kuzmin, A.V. Behaviour of 54.4SiO2-13.7Na2O-1.7K2O-5.0CaO-12.4MgO-0.6Y2O3-11.3Al2O3-0.9B2O3 HT-SOFC glass sealant under oxidising and reducing atmospheres. Ceram. Int. 2022, 48, 6124–6130. [Google Scholar] [CrossRef]
- Krainova, D.A.; Saetova, N.S.; Farlenkov, A.S.; Khodimchuk, A.V.; Polyakova, I.G.; Kuzmin, A.V. Long-term stability of SOFC glass sealant under oxidising and reducing atmospheres. Ceram. Int. 2021, 47, 8973–8979. [Google Scholar] [CrossRef]
- Saetova, N.S.; Krainova, D.A.; Kuzmin, A.V.; Raskovalov, A.A.; Zharkinova, S.T.; Porotnikova, N.M.; Farlenkov, A.S.; Moskalenko, N.I.; Ananyev, M.V.; Dyadenko, M.V.; et al. Alumina–silica glass–ceramic sealants for tubular solid oxide fuel cells. J. Mater. Sci. 2019, 54, 4532–4545. [Google Scholar] [CrossRef]
- Kaur, G. Solid Oxide Fuel Cell Components: Seal Glass for Solid Oxide Fuel Cells; Springer: Berlin/Heidelberg, Germany, 2016; Volume 58, ISBN 978-3-319-25596-5. [Google Scholar]
- Walia, T.; Singh, K. Mixed alkaline earth modifiers effect on thermal, optical and structural properties of SrO-BaO-SiO2-B2O3-ZrO2 glass sealants. J. Non. Cryst. Solids 2021, 564, 120812. [Google Scholar] [CrossRef]
- Kaur, G.; Pandey, O.P.; Singh, K. Effect of modifiers field strength on optical, structural and mechanical properties of lanthanum borosilicate glasses. J. Non. Cryst. Solids 2012, 358, 2589–2596. [Google Scholar] [CrossRef]
- Rodríguez-López, S.; Wei, J.; Laurenti, K.C.; Mathias, I.; Justo, V.M.; Serbena, F.C.; Baudín, C.; Malzbender, J.; Pascual, M.J. Mechanical properties of solid oxide fuel cell glass-ceramic sealants in the system BaO/SrO-MgO-B2O3-SiO2. J. Eur. Ceram. Soc. 2017, 37, 3579–3594. [Google Scholar] [CrossRef]
- Lim, E.S.; Kim, B.S.; Lee, J.H.; Kim, J.J. Effect of BaO content on the sintering and physical properties of BaO-B2O3-SiO2 glasses. J. Non. Cryst. Solids 2006, 352, 821–826. [Google Scholar] [CrossRef]
- QI, S.; Portnikova, N.M.; Ananyev, M.V.; Kuzmin, A.V.; Eremin, V.A. High-temperature glassy-ceramic sealants SiO2—Al2O3—BaO—MgO and SiO2—Al2O3—ZrO2—CaO—Na2O for solid oxide electrochemical devices. Trans. Nonferrous Met. Soc. China 2016, 26, 2916–2924. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, G.; Kumar, D.; Singh, K. Mechanical and thermal properties of SrO/BaO modified Y2O3-Al2O3-B2O3-SiO2 glasses and their compatibility with solid oxide fuel cell components. J. Phys. Chem. Solids 2018, 118, 248–254. [Google Scholar] [CrossRef]
- Rezazadeh, L.; Hamnabard, Z.; Baghshahi, S.; Golikand, A.N. Adhesion and interfacial interactions of BaO–SiO2–B2O3-based glass-ceramic seals and AISI430 interconnect for solid oxide fuel cell applications. Ionics 2016, 22, 1899–1908. [Google Scholar] [CrossRef]
- Navarro-Pardo, F.; Martínez-Barrera, G.; Martínez-Hernández, A.L.; Castaño, V.M.; Rivera-Armenta, J.L.; Medellín-Rodríguez, F.; Velasco-Santos, C. Effects on the thermo-mechanical and crystallinity properties of nylon 6,6 electrospun fibres reinforced with one dimensional (1D) and two dimensional (2D) carbon. Materials 2013, 6, 3494–3513. [Google Scholar] [CrossRef] [PubMed]
- Kerstan, M.; Müller, M.; Rüssel, C. Thermal expansion of Ba2ZnSi2O7, BaZnSiO4 and the solid solution series BaZn2-xMgxSi2O7 (0 ≤ x ≤ 2) studied by high-temperature X-ray diffraction and dilatometry. J. Solid State Chem. 2012, 188, 84–91. [Google Scholar] [CrossRef]
- Saetova, N.S.; Krainova, D.A.; Kuzmin, A.V. Effect of rare-earth oxides on thermal behavior of alumina-silica glass sealants. J. Phys. Conf. Ser. 2021, 1967, 012006. [Google Scholar] [CrossRef]
- Smiljanić, S.V.; Grujić, S.R.; Tošić, M.B.; Živanović, V.D.; Stojanović, J.N.; Matijašević, S.D.; Nikolić, J.D. Crystallization and sinterability of glass-ceramics in the system La2O3-SrO-B2O3. Ceram. Int. 2014, 40, 297–305. [Google Scholar] [CrossRef]
- Puig, J.; Ansart, F.; Lenormand, P.; Conradt, R.; Gross-Barsnick, S.M. Development of barium boron aluminosilicate glass sealants using a sol–gel route for solid oxide fuel cell applications. J. Mater. Sci. 2016, 51, 979–988. [Google Scholar] [CrossRef]
- Goel, A.; Reddy, A.A.; Pascual, M.J.; Gremillard, L.; Malchere, A.; Ferreira, J.M.F. Sintering behavior of lanthanide-containing glass-ceramic sealants for solid oxide fuel cells. J. Mater. Chem. 2012, 22, 10042–10054. [Google Scholar] [CrossRef]
- Saetova, N.S.; Shirokova, E.S.; Krainova, D.A.; Chebykin, N.S.; Ananchenko, B.A.; Tolstobrov, I.V.; Belozerov, K.S.; Kuzmin, A.V. The development of 3D technology for the creation of glass sealants for tubular oxide fuel cells. Int. J. Appl. Glas. Sci. 2022, 13, 684–694. [Google Scholar] [CrossRef]
- Ghosh, S.; Kundu, P.; Das Sharma, A.; Basu, R.N.; Maiti, H.S. Microstructure and property evaluation of barium aluminosilicate glass-ceramic sealant for anode-supported solid oxide fuel cell. J. Eur. Ceram. Soc. 2008, 28, 69–76. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, Q.S. Thermal cycle stability of BaO-B2O3-SiO2 sealing glass. J. Power Sources 2009, 194, 880–885. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Bartolomé, J.F.; De Aza, A.H.; Mello-Castanho, S. Glass ceramic sealants belonging to BAS (BaO-Al2O3-SiO2) ternary system modified with B2O3 addition: A different approach to access the SOFC seal issue. J. Eur. Ceram. Soc. 2016, 36, 631–644. [Google Scholar] [CrossRef]
- Li, X.; Yazhenskikh, E.; Groß-Barsnick, S.M.; Baumann, S.; Behr, P.; Deibert, W.; Koppitz, T.; Müller, M.; Meulenberg, W.A.; Natour, G. Crystallization behavior of BaO–CaO–SiO2–B2O3 glass sealant and adjusting its thermal properties for oxygen transport membrane joining application. J. Eur. Ceram. Soc. 2023, 43, 2541–2552. [Google Scholar] [CrossRef]
- Craievich, A.F.; Zanotto, E.E.; James, P.F. Kinetics of sub-liquidus phase separation in silicate and borate glasses. A review. Bull. Mineral. 1983, 106, 169–184. [Google Scholar] [CrossRef]
- Abdel-Hameed, S.A.M.; Abo-Naf, S.M.; Hamdy, Y.M. The effect of heat treatment on photoluminescence and magnetic properties of new yellow phosphor based on sanbornite (BaSi2O5) glass ceramic doped with Gd3+ and Mn2+. J. Non. Cryst. Solids 2019, 517, 106–113. [Google Scholar] [CrossRef]
- Saxena, S.K.; Chatterjee, N.; Fei, Y.; Shen, G. Thermodynamic Data on Oxides and Silicates: An Assessed Data Set Based on Thermochemistry and High Pressure Phase Equilibrium; Springer: Berlin/Heidelberg, Germany, 1993; ISBN 3642783325. [Google Scholar]
- Decterov, S.A.; Jung, I.-H.; Pelton, A.D. Thermodynamic Modeling of the FeO-Fe2O3-MgO-SiO2 System. J. Am. Ceram. Soc. 2002, 85, 2903–2910. [Google Scholar] [CrossRef]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D.; Kim, H.M.; Kang, Y.B. Thermodynamic evaluation and modeling of the Fe-Co-O system. Acta. Mater. 2004, 52, 507–519. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, K.; Pandey, O.P. Investigations on Interfacial Interaction of Glass Sealants with Electrolytes and Interconnect for Solid Oxide Fuel Cells. Ph.D. Thesis, Thapar University, Punjab, India, 2012. [Google Scholar]
- Brochu, M.; Gauntt, B.D.; Shah, R.; Miyake, G.; Loehman, R.E. Comparison between barium and strontium-glass composites for sealing SOFCs. J. Eur. Ceram. Soc. 2006, 26, 3307–3313. [Google Scholar] [CrossRef]
- Chukhlantsev, V.G.; Yu, M. Galkin Study of the BaO-ZrO2-SiO2 System at Subsolidus temperatures. Dokl. Akad. Nauk. SSSR 1968, 168–169, 128. [Google Scholar]
- Li, B.; Tang, B.; Xu, M. Influences of CaO on Crystallization, Microstructures, and Properties of BaO-Al2O3-B2O3-SiO2 Glass–Ceramics. J. Electron. Mater. 2015, 44, 3849–3854. [Google Scholar] [CrossRef]
- Li, B.; Xu, M.; Tang, B. Effects of ZnO on crystallization, microstructures and properties of BaO–Al2O3–B2O3–SiO2 glass–ceramics. J. Mater. Sci. Mater. Electron. 2016, 27, 70–76. [Google Scholar] [CrossRef]
- Kermani, P.S.; Ghatee, M.; Sirr Irvine, J.T. Characterization of a barium-calcium-aluminosilicate glass/fiber glass composite seal for intermediate temperature solid oxide fuel cells. Bol. La Soc. Esp. Ceram. Y Vidr. 2022, in press. [Google Scholar] [CrossRef]
- Lu, X.; Deng, L.; Kerisit, S.; Du, J. Structural role of ZrO2 and its impact on properties of boroaluminosilicate nuclear waste glasses. Npj Mater. Degrad. 2018, 2, 19. [Google Scholar] [CrossRef]
- Khan, S.; Kaur, G.; Singh, K. Effect of ZrO2 on dielectric, optical and structural properties of yttrium calcium borosilicate glasses. Ceram. Int. 2017, 43, 722–727. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Z.; Zhang, Q.; Wang, J.; Zhang, T.; Wang, J. Reducing the interfacial reaction between borosilicate sealant and yttria-stabilized zirconia electrolyte by addition of HfO2. J. Eur. Ceram. Soc. 2015, 35, 2–6. [Google Scholar] [CrossRef]
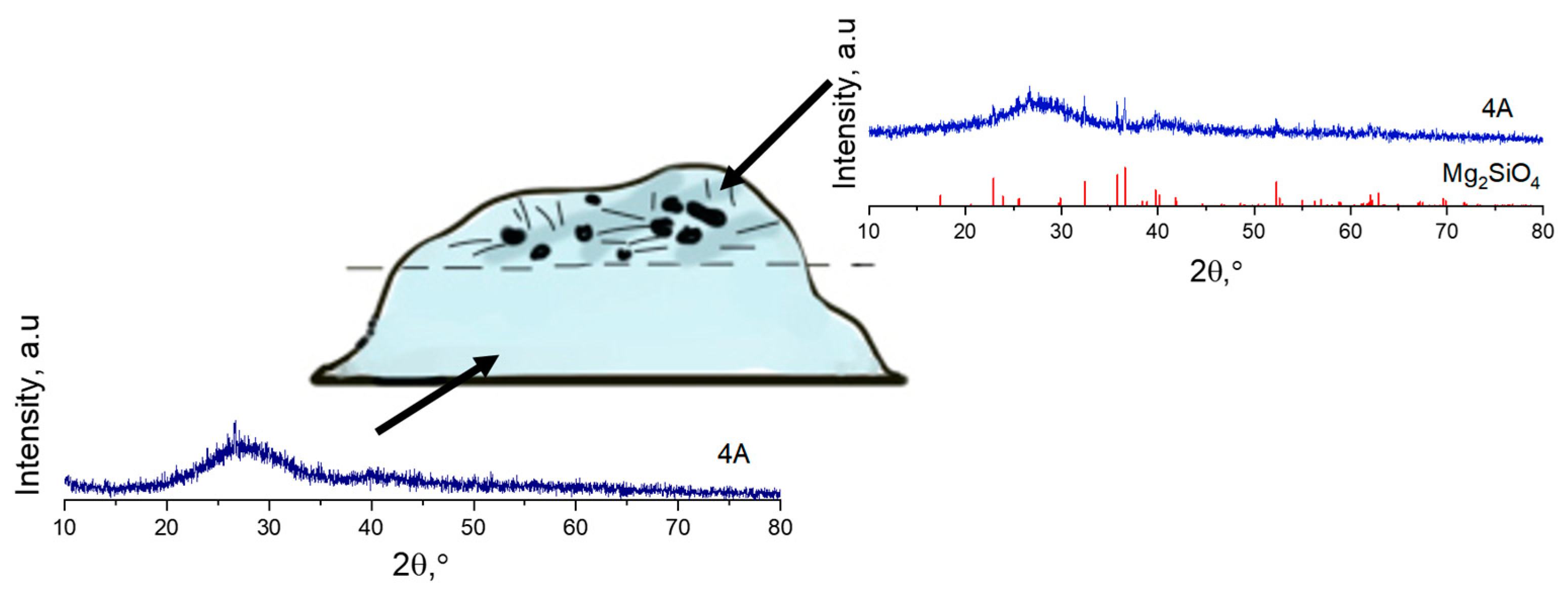
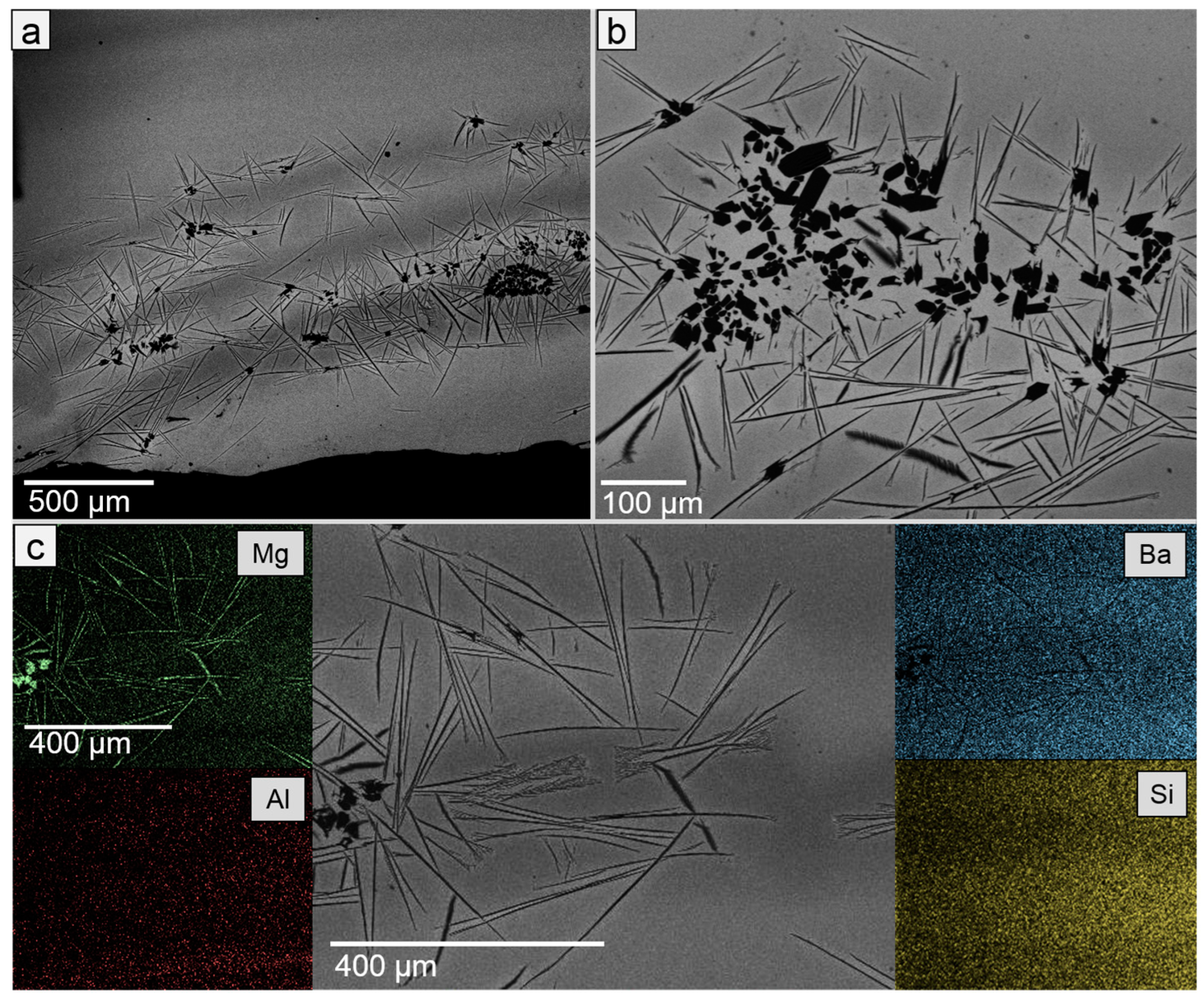
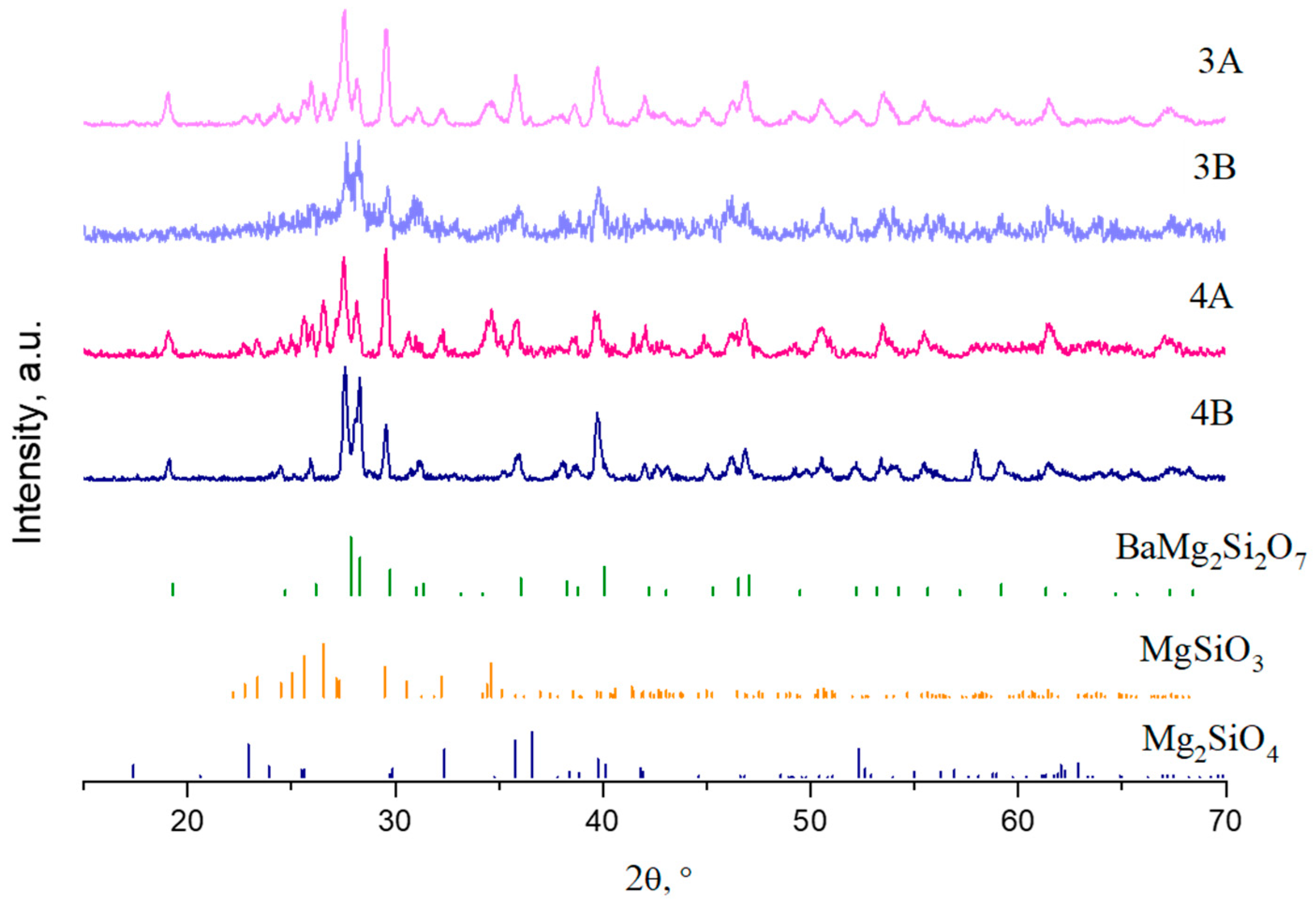
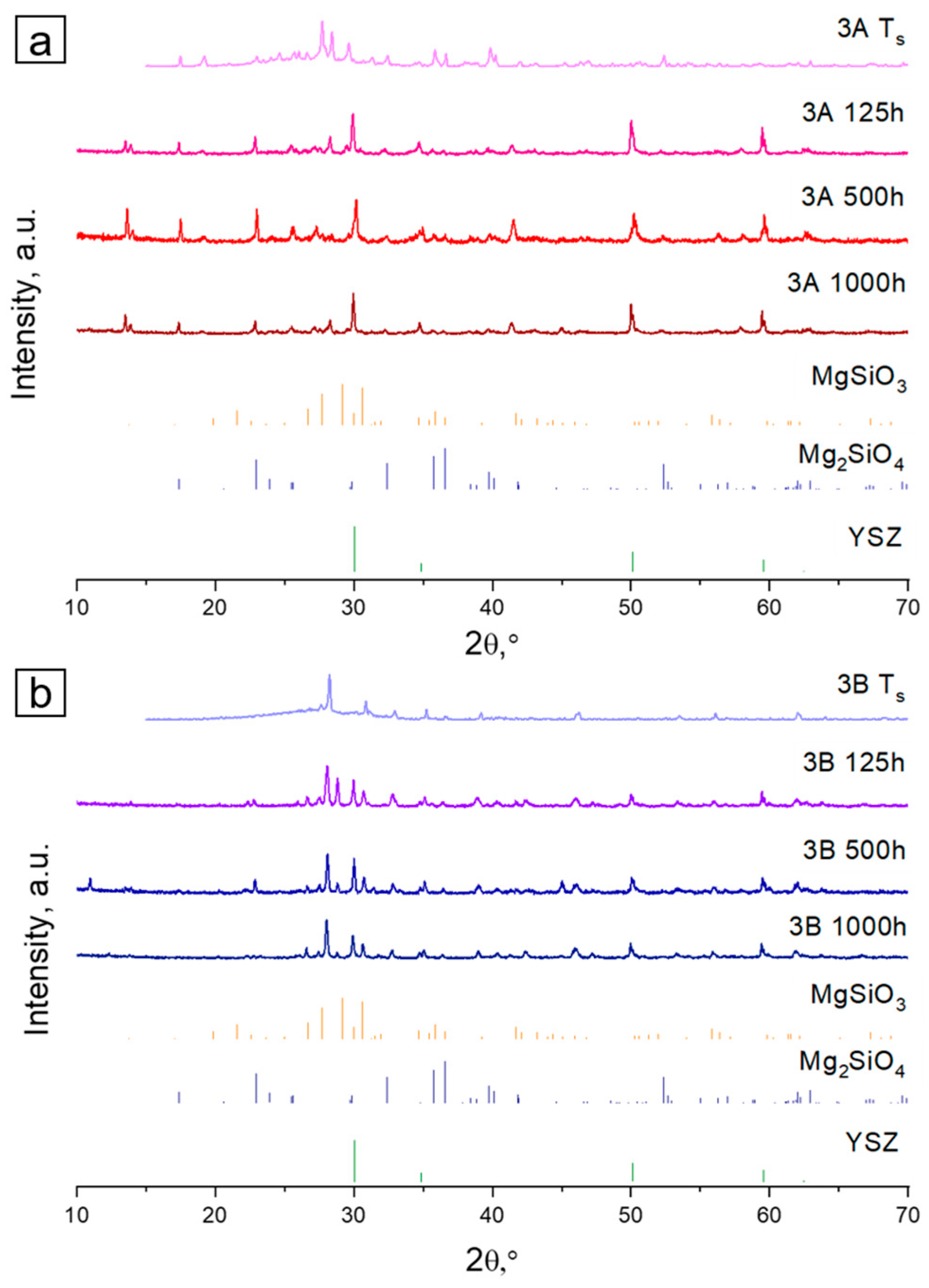



| Sample | SiO2 | MgO | BaO | Al2O3 | B2O3 |
|---|---|---|---|---|---|
| 3B | 47.0 | 20.0 | 30.0 | - | 3.0 |
| 3B AES | 46.3 | 17.9 | 31.4 | 1.8 | 2.6 |
| 3A | 47.0 | 20.0 | 30.0 | 3.0 | - |
| 3A AES | 43.9 | 20.2 | 32.1 | 3.8 | - |
| 4B | 46.0 | 20.0 | 30.0 | - | 4.0 |
| 4B AES | 45.7 | 21.7 | 27.4 | 1.6 | 3.6 |
| 4A | 46.0 | 20.0 | 30.0 | 4.0 | - |
| 4A AES | 44.9 | 18.4 | 31.8 | 4.9 | - |
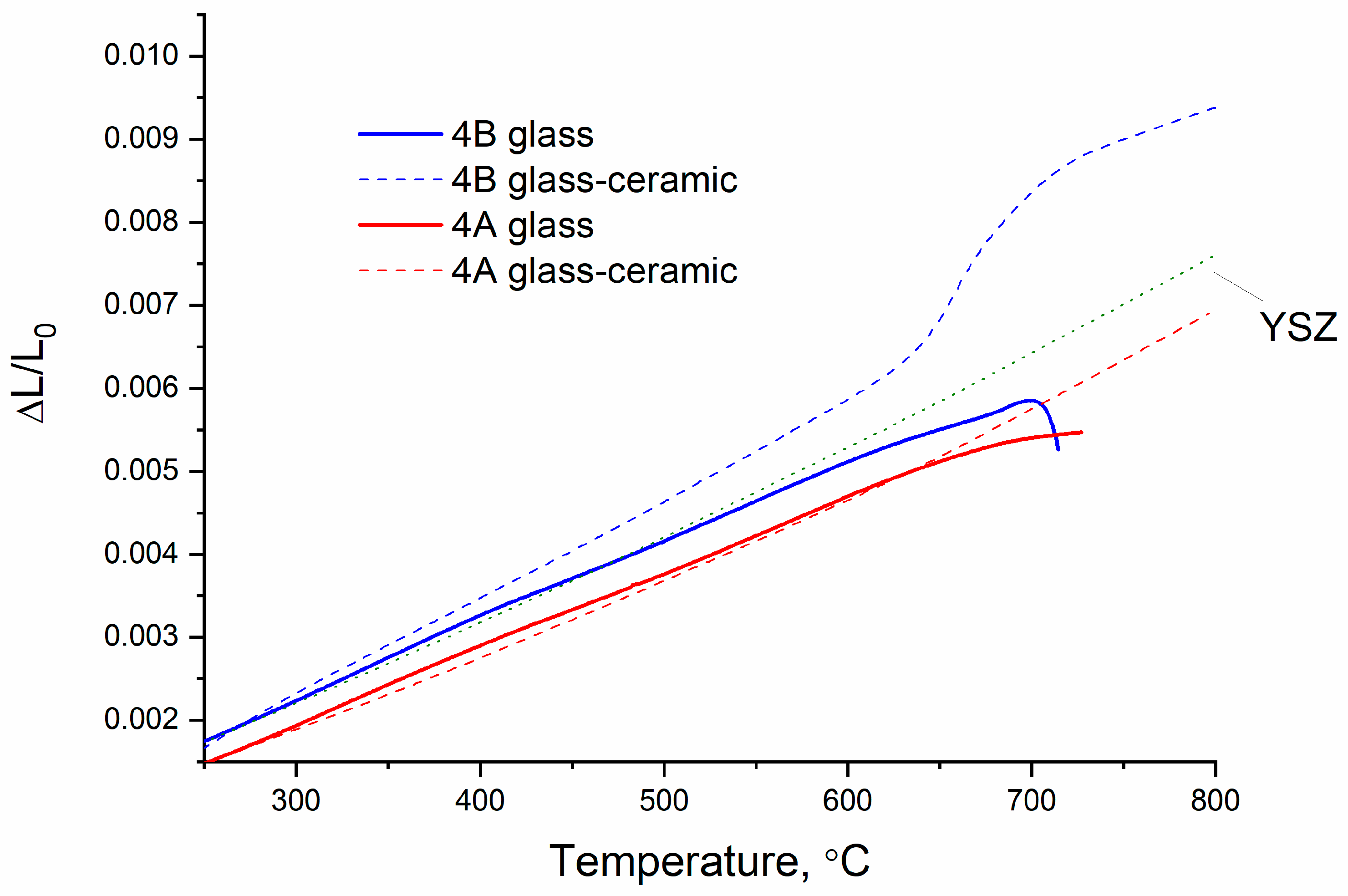
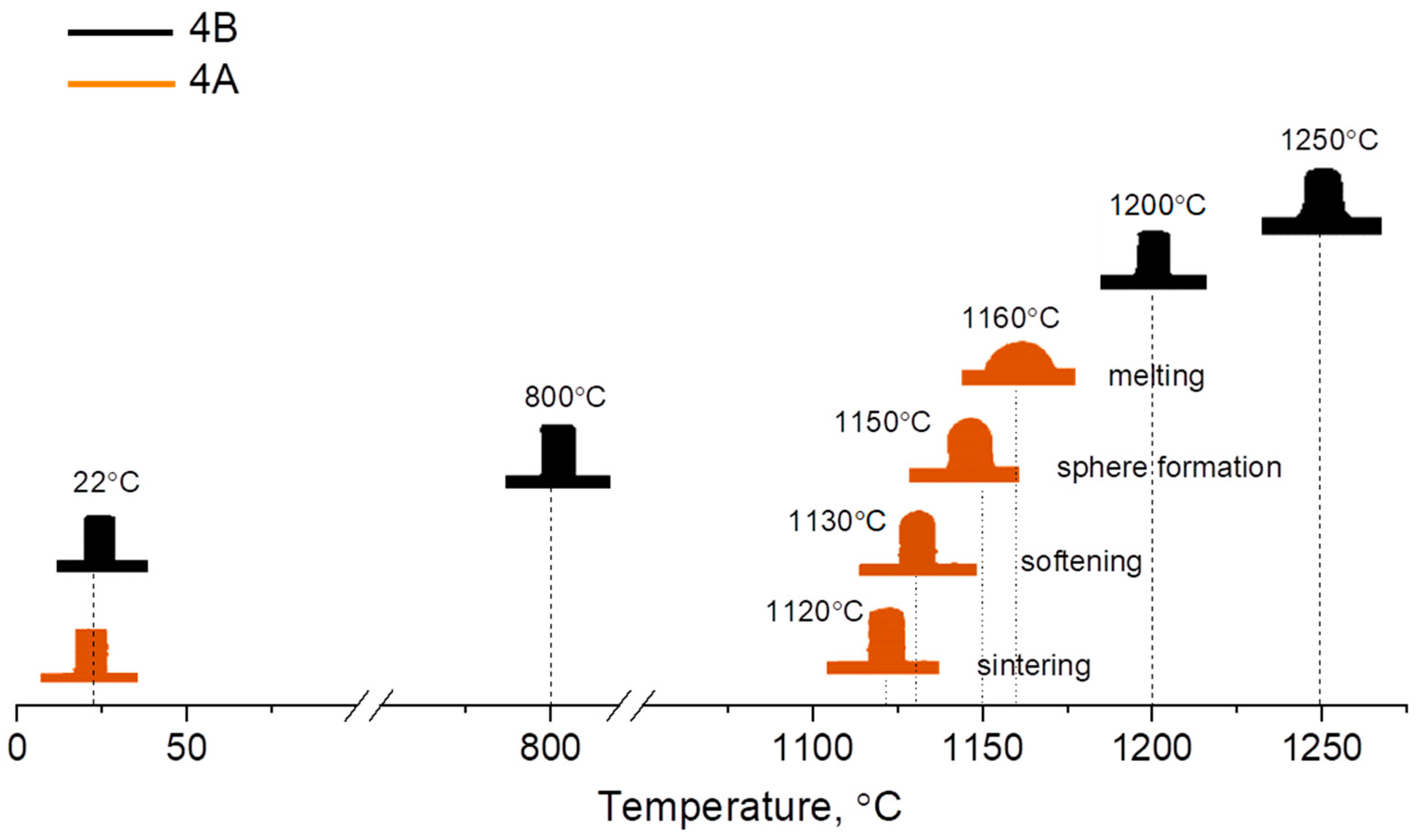
| Sample | HSM, °C (±10 °C) | DSC, °C (±2 °C) | CTE × 10−6 K−1 (±0.1) | ||
|---|---|---|---|---|---|
| TS | Tg | Tc | Bulk | Pressed | |
| 3A | 1170 | 740 | 915 | 8.4 | 9.4 |
| 4A | 1130 | 740 | 930 | 8.7 | 8.2 |
| 3B | 1150 | 725 | 910 | 8.3 | 9.5 |
| 4B | 1200 | 720 | 925 | 9.5 | 9.9 |
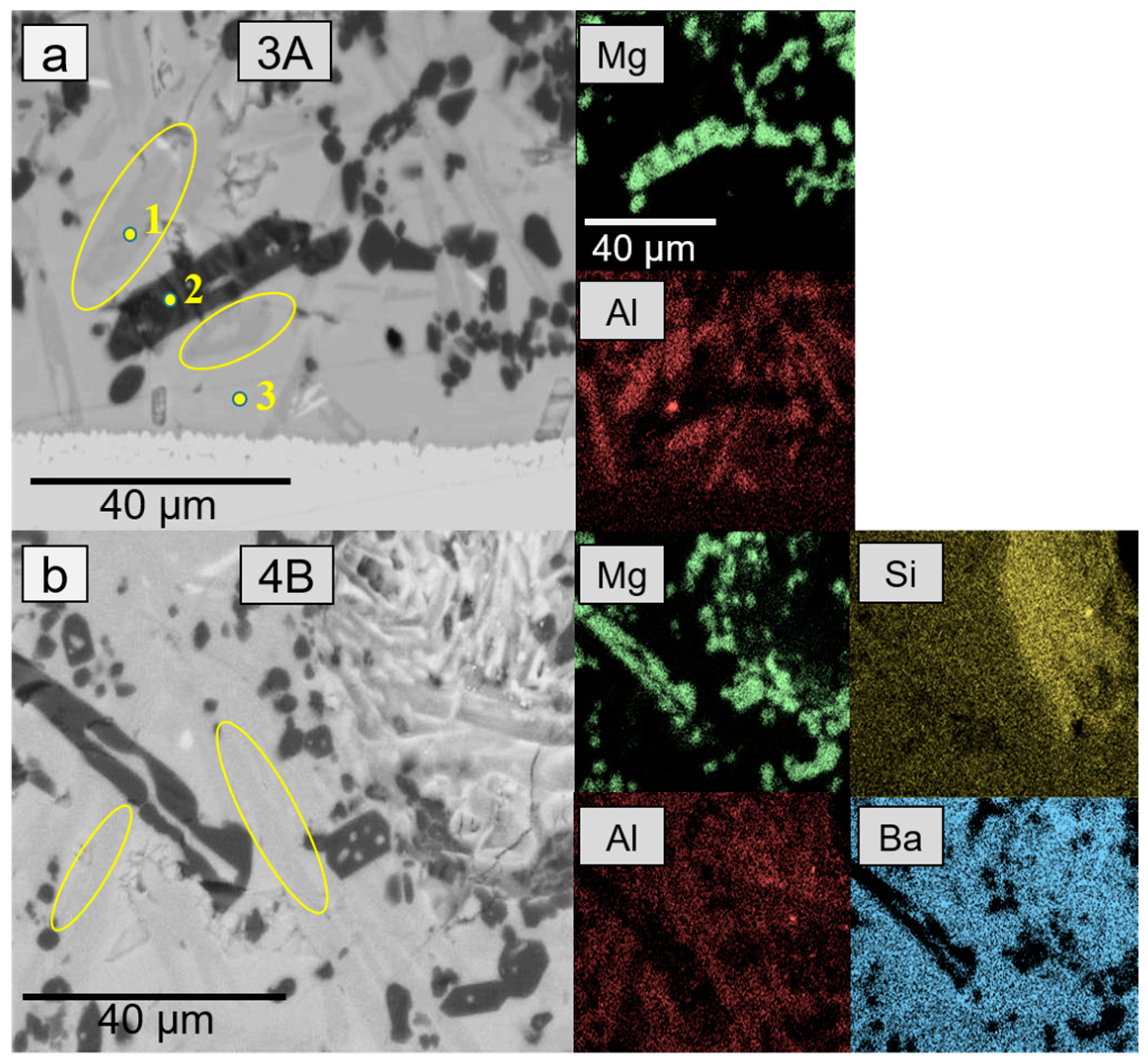
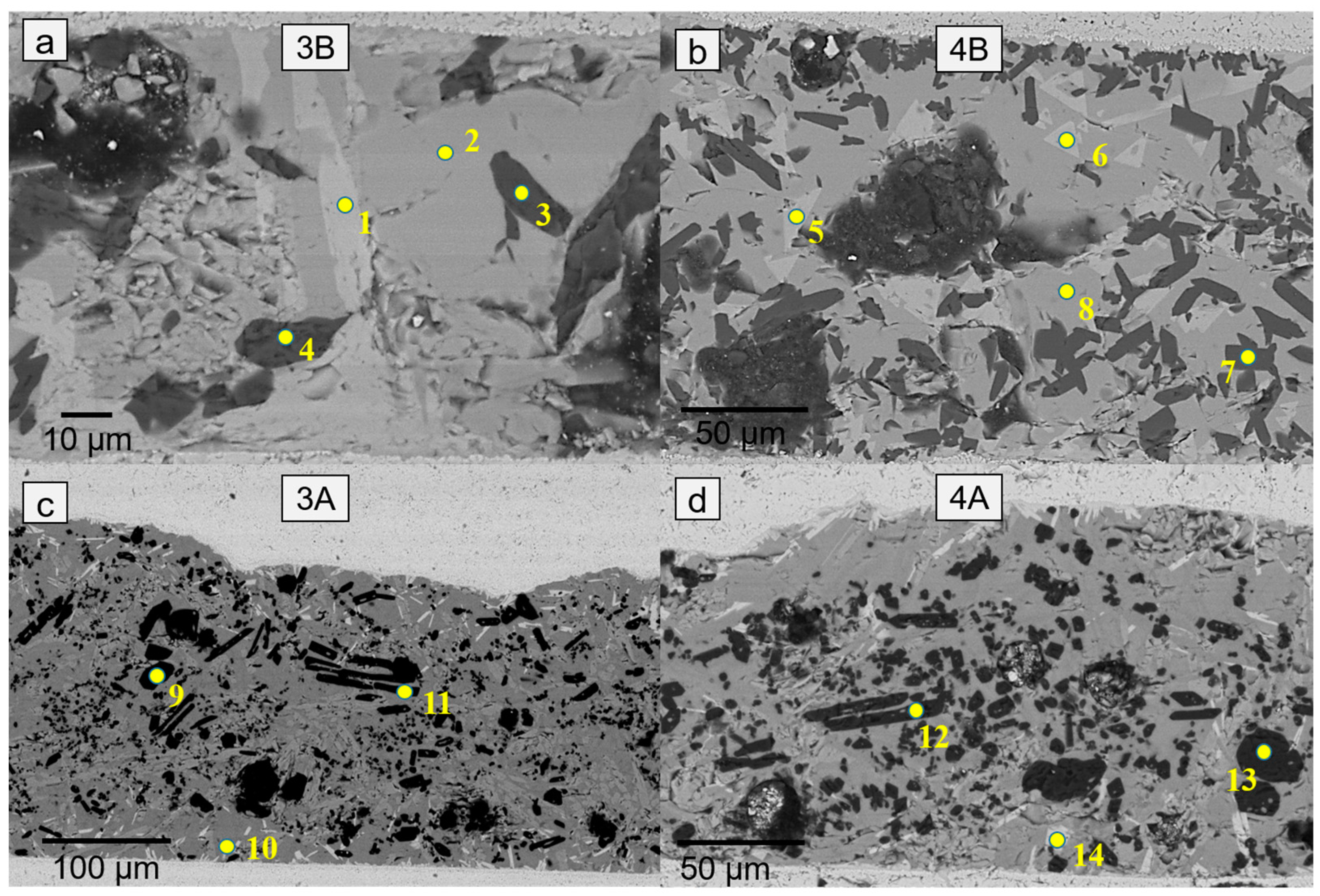
| Element (wt %) | Ba | Mg | Si | O | Al | Zr | Y |
|---|---|---|---|---|---|---|---|
| 1 | 34.4 | 0.3 | 22.7 | 42.2 | 0.3 | 20.4 | 0.4 |
| 2 | 32.2 | 3.1 | 24.6 | 37.2 | 1.1 | 1.3 | 3.5 |
| 3 | 0.1 | 32.6 | 19.2 | 48.1 | - | - | - |
| 4 | 0.8 | 33.8 | 20.7 | 44.7 | - | - | - |
| 5 | 38.2 | 3.1 | 19.9 | 33.5 | 5.4 | - | - |
| 6 | 37.5 | 3.1 | 24.1 | 34.2 | 1.2 | 4.1 | 3.2 |
| 7 | 41.6 | 2.6 | 22.0 | 32.9 | 0.9 | 8.2 | 2.3 |
| Mg2SiO4 ** | - | 34.6 | 20.0 | 45.5 | - | - | - |
| BaZrSi3O9 ** | 30.1 | - | 18.4 | 31.5 | - | 20.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vepreva, A.; Dubovtsev, D.; Krainova, D.; Chetvertnykh, Y.; Belyakov, S.; Saetova, N.; Kuzmin, A. Barium Silicate Glasses and Glass–Ceramic Seals for YSZ-Based Electrochemical Devices. Ceramics 2023, 6, 1314-1329. https://doi.org/10.3390/ceramics6030081
Vepreva A, Dubovtsev D, Krainova D, Chetvertnykh Y, Belyakov S, Saetova N, Kuzmin A. Barium Silicate Glasses and Glass–Ceramic Seals for YSZ-Based Electrochemical Devices. Ceramics. 2023; 6(3):1314-1329. https://doi.org/10.3390/ceramics6030081
Chicago/Turabian StyleVepreva, Alyona, Dmitry Dubovtsev, Daria Krainova, Yulia Chetvertnykh, Semyon Belyakov, Nailya Saetova, and Anton Kuzmin. 2023. "Barium Silicate Glasses and Glass–Ceramic Seals for YSZ-Based Electrochemical Devices" Ceramics 6, no. 3: 1314-1329. https://doi.org/10.3390/ceramics6030081
APA StyleVepreva, A., Dubovtsev, D., Krainova, D., Chetvertnykh, Y., Belyakov, S., Saetova, N., & Kuzmin, A. (2023). Barium Silicate Glasses and Glass–Ceramic Seals for YSZ-Based Electrochemical Devices. Ceramics, 6(3), 1314-1329. https://doi.org/10.3390/ceramics6030081







