Nutrient-Doped Hydroxyapatite: Structure, Synthesis and Properties
Abstract
1. Introduction
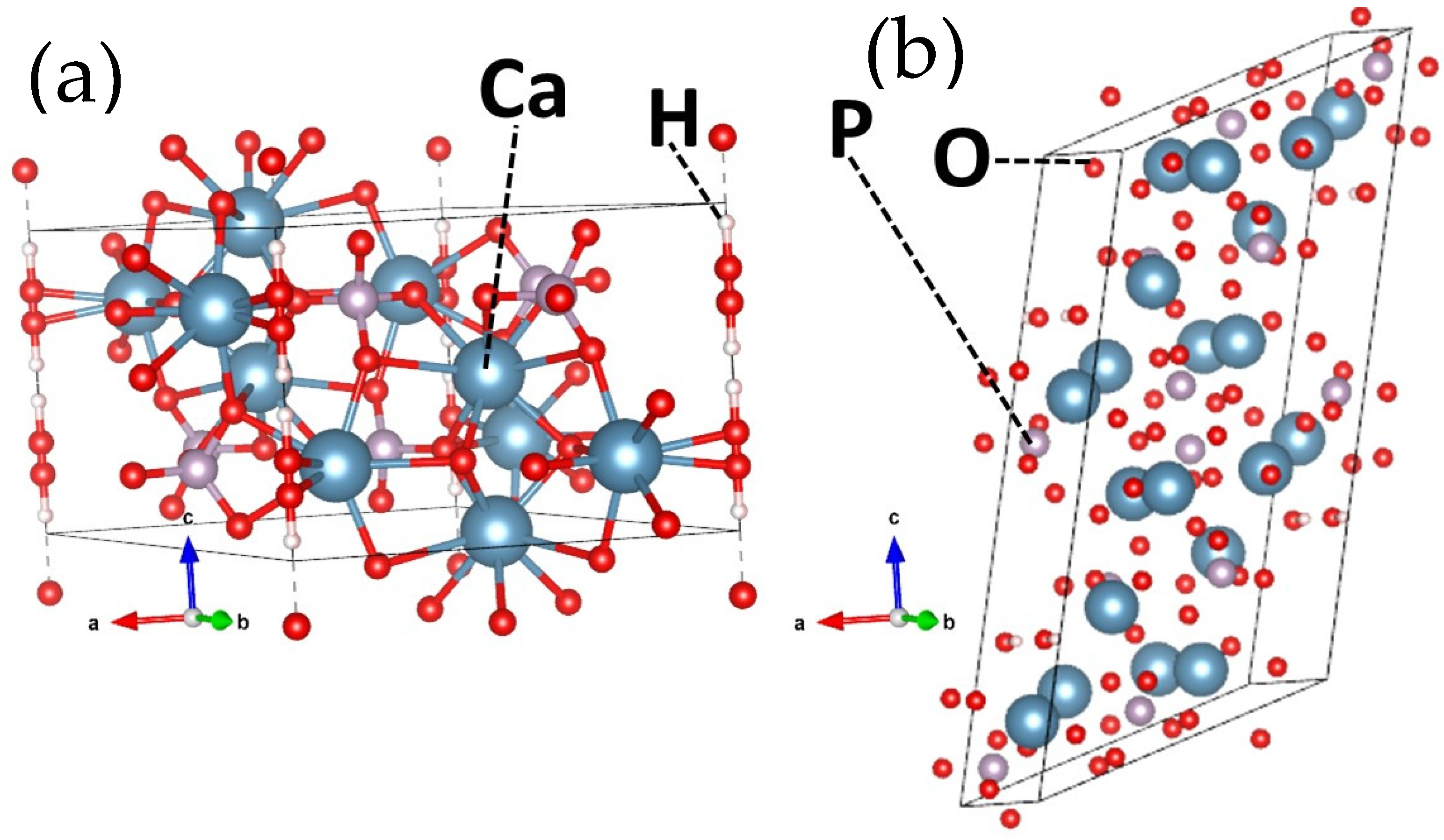
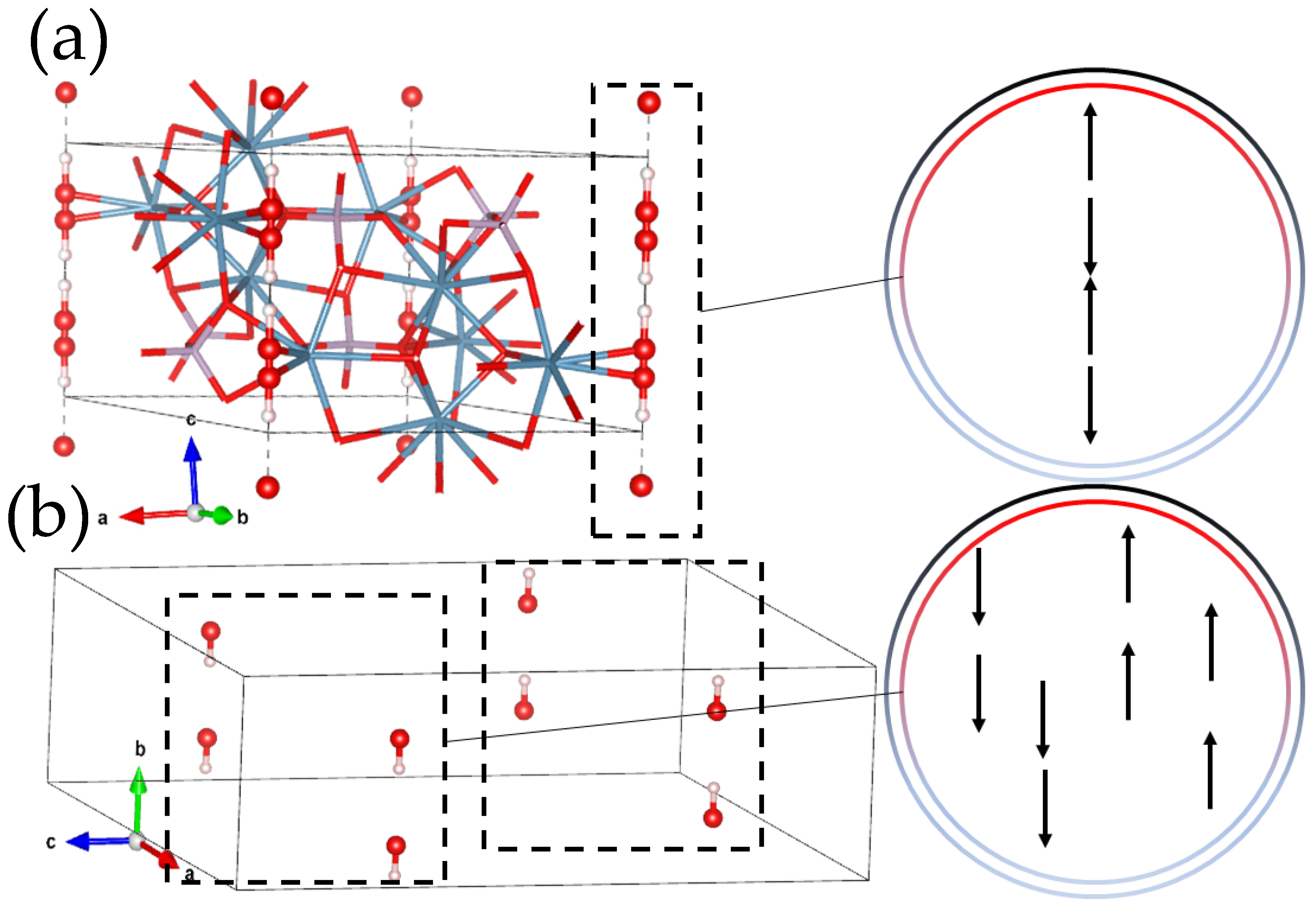
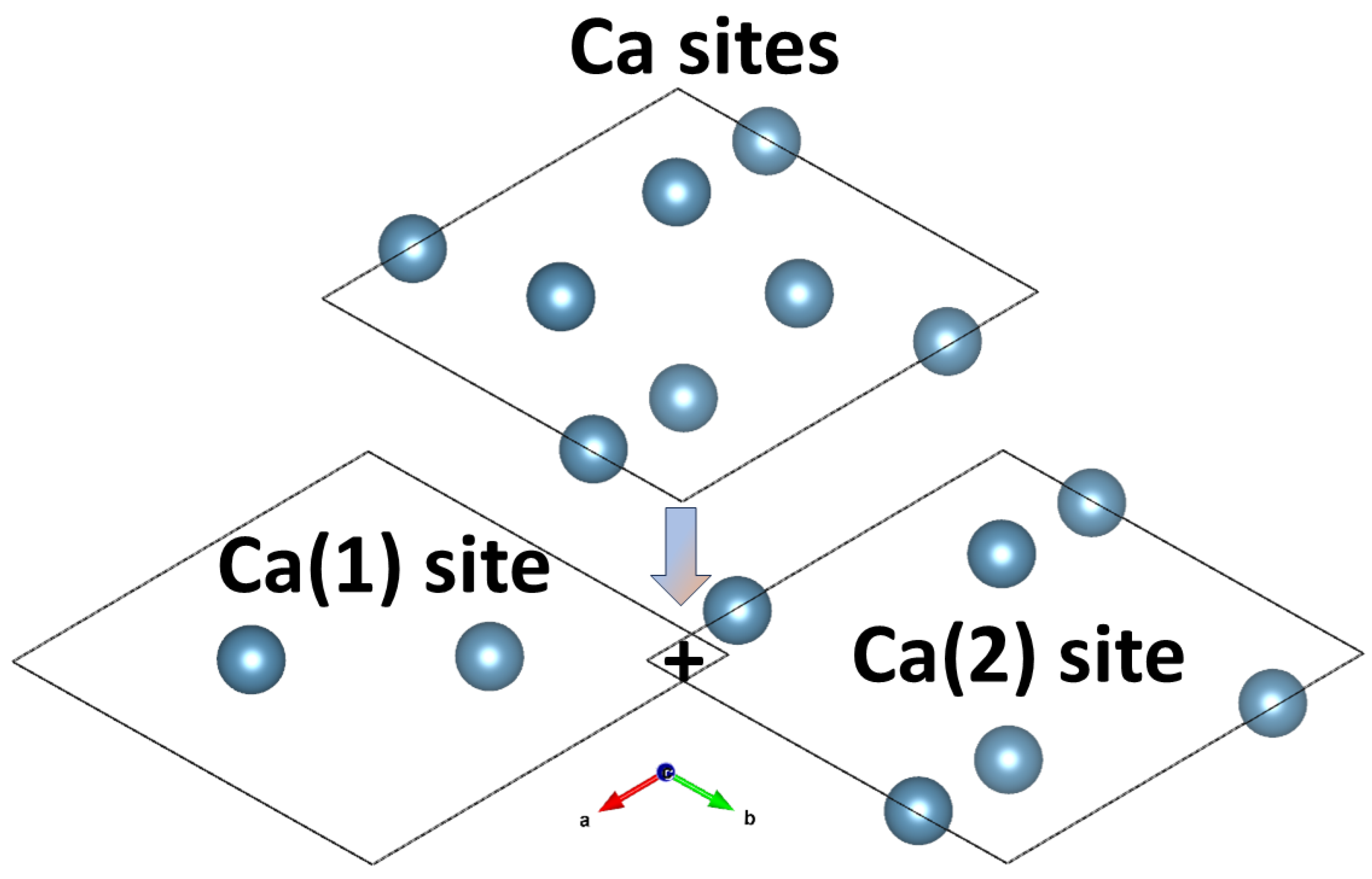
2. HAP Structure
2.1. Overview
2.2. Structure
3. Ionic Doping of HAP
3.1. Carbonate Anion (CO32−)
3.2. Chlorine Anion (Cl−)
3.3. Nitrate Anion (NO3−)
3.4. Sulfate Anion (SO42−)
3.5. Borate Anion (BO33−)
3.6. Iron (Fe2+ and Fe3+)
3.7. Magnesium (Mg2+)
3.8. Zinc (Zn2+)
3.9. Nickel (Ni2+)
3.10. Copper (Cu2+)
3.11. Potassium (K+)
3.12. Molybdenum (Mo2+)
3.13. Co-Doping (Two or More Ions)
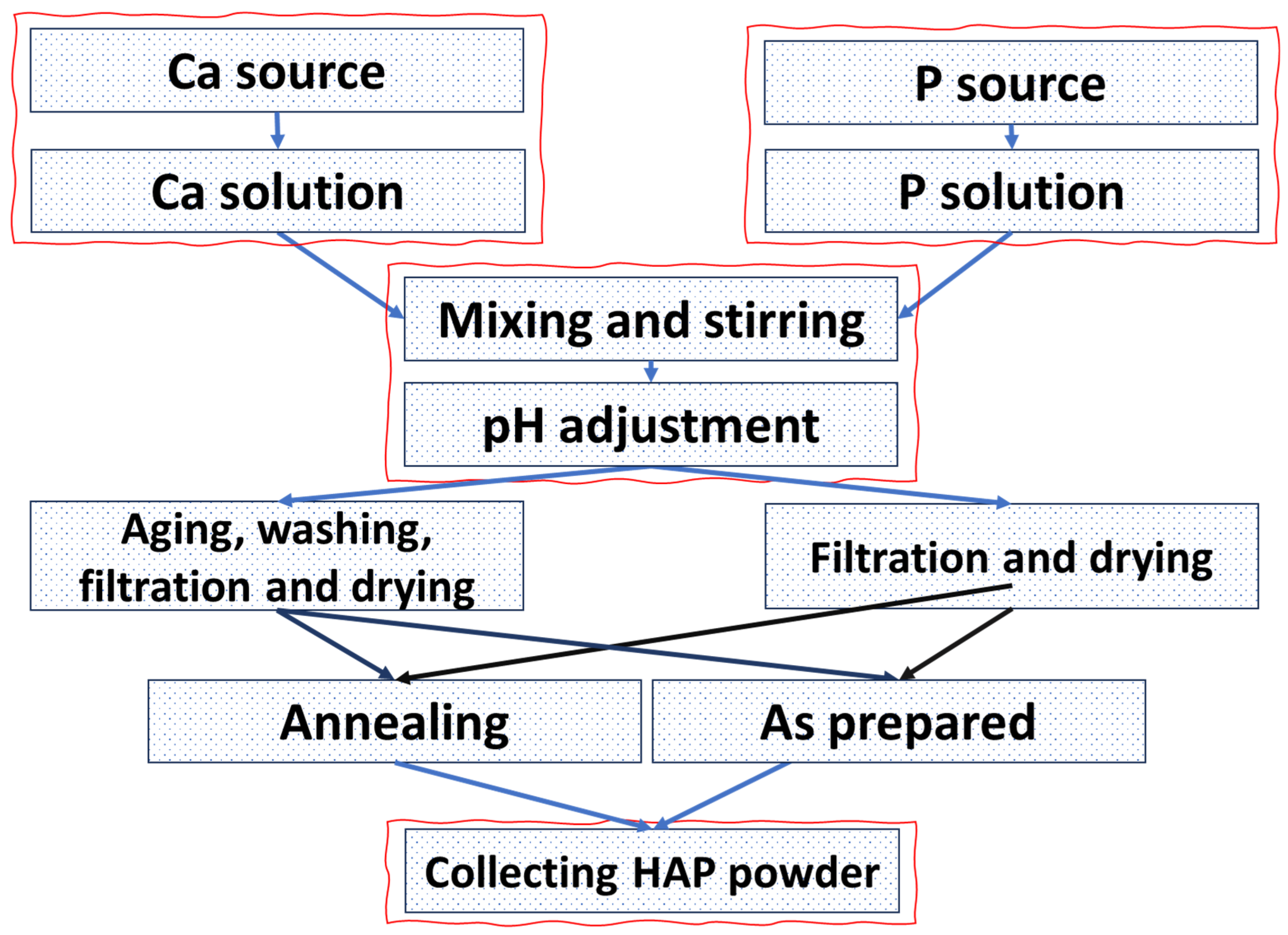
4. Synthesis of HAP
4.1. Wet Chemical Synthesis
4.1.1. Coprecipitation
4.1.2. Hydrothermal Method
4.2. Solid-State Reactions
4.2.1. Solid-State Reaction and/or Thermal Decomposition
4.2.2. Mechanochemical Synthesis
4.3. Economic Feasibility of the Mechanochemical HAP Synthesis Method
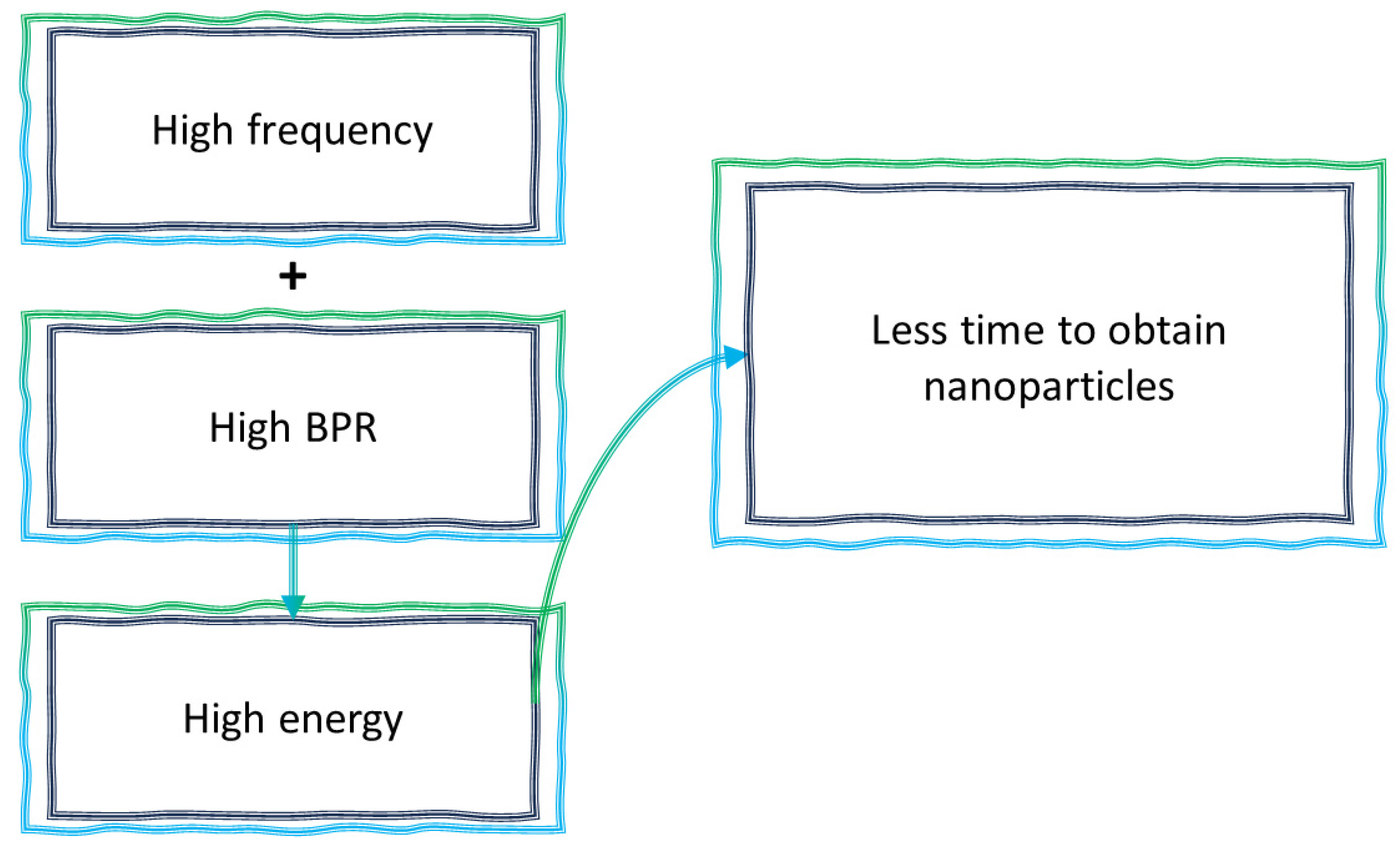
4.4. Parameters Affecting Mechanochemical Synthesis Procedure
4.4.1. Time of Milling
4.4.2. Effect of Ball Milling on Biological Properties
4.4.3. The Potential to Incorporate Dopants into HAP Structure Using Mechanochemical Synthesis
5. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fellet, G.; Pilotto, L.; Marchiol, L.; Braidot, E. Tools for Nano-Enabled Agriculture: Fertilizers Based on Calcium Phosphate, Silicon, and Chitosan Nanostructures. Agronomy 2021, 11, 1239. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, E.A.; Johnston, A.E. Soil and fertilizer phosphorus in relation to crop nutrition. In The Ecophysiology of Plant-Phosphorus Interactions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 177–223. [Google Scholar] [CrossRef]
- Montalvo, D.; McLaughlin, M.J.; Degryse, F. Efficacy of Hydroxyapatite Nanoparticles as Phosphorus Fertilizer in Andisols and Oxisols. Soil Sci. Soc. Am. J. 2015, 79, 551–558. [Google Scholar] [CrossRef]
- Elsayed, A.A.A.; El-Gohary, A.; Taha, Z.K.; Farag, H.M.; Hussein, M.S.; AbouAitah, K. Hydroxyapatite nanoparticles as novel nano-fertilizer for production of rosemary plants. Sci. Hortic. 2022, 295, 110851. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Alhuthal, N.A.; Albukhaty, S. Green synthesis of phosphorous-containing hydroxyapatite nanoparticles (nHAP) as a novel nano-fertilizer: Preliminary assessment on pomegranate (Punica granatum L.). Nanomaterials 2022, 12, 1527. [Google Scholar] [CrossRef]
- Tang, S.; Fei, X. Refractory Calcium Phosphate-Derived Phosphorus Fertilizer Based on Hydroxyapatite Nanoparticles for Nutrient Delivery. Sci. Hortic. 2021, 4, 1364–1376. [Google Scholar] [CrossRef]
- Huang, R.; Mao, P.; Xiong, L.; Qin, G.; Zhou, J.; Zhang, J.; Li, Z.; Wu, J. Negatively charged nano-hydroxyapatite can be used as a phosphorus fertilizer to increase the efficacy of wollastonite for soil cadmium immobilization. J. Hazard. Mater. 2023, 443, 130291. [Google Scholar] [CrossRef]
- Taskin, H.; Gunes, A. Synthetic nano-hydroxyapatite as an alternative phosphorus source for wheat grown under field conditions. J. Plant Nutr. 2023, 46, 3653–3666. [Google Scholar] [CrossRef]
- Sofyane, A.; Lahcini, M.; El Meziane, A.; Khouloud, M.; Dahchour, A.; Caillol, S.; Raihane, M. Properties of Coated Controlled Release Diammonium Phosphate Fertilizers Prepared with the Use of Bio-based Amino Oil. J. Am. Oil Chem. Soc. 2020, 97, 751–763. [Google Scholar] [CrossRef]
- Tiwari, K.; Kumar, Y.; Singh, T.; Nayak, R. Nano technology based P fertilizers for higher efficiency and agriculture sustainability. Ann. Plant Soil Res. 2022, 24, 198–207. [Google Scholar] [CrossRef]
- Fertahi, S.; Bertrand, I.; Ilsouk, M.; Oukarroum, A.; Amjoud, M.B.; Zeroual, Y.; Barakat, A. Impact of plasticizers on lignin–carrageenan formulation properties and on phosphorus release from a coated triple superphosphate fertilizer. Ind. Eng. Chem. Res. 2020, 59, 14172–14179. [Google Scholar] [CrossRef]
- Raniro, H.R.; Bettoni Teles, A.P.; Adam, C.; Pavinato, P.S. Phosphorus solubility and dynamics in a tropical soil under sources derived from wastewater and sewage sludge. J. Environ. Manag. 2022, 302, 113984. [Google Scholar] [CrossRef]
- Cowan, N.; Ferrier, L.; Spears, B.; Drewer, J.; Reay, D.; Skiba, U. CEA systems: The means to achieve future food security and environmental sustainability? Front. Sustain. Food Syst. 2022, 6, 891256. [Google Scholar] [CrossRef]
- Pradel, M.; Lippi, M.; Daumer, M.L.; Aissani, L. Environmental performances of production and land application of sludge-based phosphate fertilizers-a life cycle assessment case study. Environ. Sci. Pollut. Res. Int. 2020, 27, 2054–2070. [Google Scholar] [CrossRef] [PubMed]
- Sakhno, Y.; Ma, C.; Borgatta, J.; Jin, Y.; White, J.C.; Jaisi, D.P. Role of Cation Substitution and Synthesis Condition in a Calcium Phosphate-Based Novel Nanofertilizer on Lettuce (Lactuca sativa) Yield. ACS Sustain. Chem. Eng. 2022, 10, 15414–15422. [Google Scholar] [CrossRef]
- Tosun, U.G.; Sakhno, Y.; Jaisi, D.P. Synthesis of hydroxyapatite nanoparticles from phosphorus recovered from animal wastes. ACS Sustain. Chem. Eng. 2021, 9, 15117–15126. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef]
- Marchiol, L.; Filippi, A.; Adamiano, A.; Degli Esposti, L.; Iafisco, M.; Mattiello, A.; Petrussa, E.; Braidot, E. Influence of hydroxyapatite nanoparticles on germination and plant metabolism of tomato (Solanum lycopersicum L.): Preliminary evidence. Agronomy 2019, 9, 161. [Google Scholar] [CrossRef]
- Pohshna, C.; Mailapalli, D.R. Engineered urea-doped hydroxyapatite nanomaterials as nitrogen and phosphorus fertilizers for rice. ACS Agric. Sci. Technol. 2021, 2, 100–112. [Google Scholar] [CrossRef]
- Pradhan, S.; Durgam, M.; Mailapalli, D.R. Urea loaded hydroxyapatite nanocarrier for efficient delivery of plant nutrients in rice. Arch. Agron. Soil Sci. 2020, 67, 371–382. [Google Scholar] [CrossRef]
- Alrbaihat, M. Mechanochemical Synthesis of Dendrimers as Nanocarriers: A Review. Adv. Mater. Res. 2023, 1175, 37–46. [Google Scholar] [CrossRef]
- Garcia-Espejo, G.; Rodriguez-Padron, D.; Luque, R.; Camacho, L.; de Miguel, G. Mechanochemical synthesis of three double perovskites: Cs2AgBiBr6, (CH3NH3)2TlBiBr6 and Cs2AgSbBr6. Nanoscale 2019, 11, 16650–16657. [Google Scholar] [CrossRef]
- Tsuzuki, T. Mechanochemical synthesis of metal oxide nanoparticles. Commun. Chem. 2021, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.F.; Torresi, R.M.; Emmerling, F.; Camargo, P.H. Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mat. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Colacino, E.; Isoni, V.; Crawford, D.; García, F. Upscaling mechanochemistry: Challenges and opportunities for sustainable industry. Trends Chem. 2021, 3, 335–339. [Google Scholar] [CrossRef]
- Martinez, V.; Stolar, T.; Karadeniz, B.; Brekalo, I.; Uzarevic, K. Advancing mechanochemical synthesis by combining milling with different energy sources. Nat. Rev. Chem. 2023, 7, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Dubadi, R.; Huang, S.D.; Jaroniec, M. Mechanochemical Synthesis of Nanoparticles for Potential Antimicrobial Applications. Materials 2023, 16, 1460. [Google Scholar] [CrossRef]
- Rehmanullah; Muhammad, Z.; Inayat, N.; Majeed, A. Application of nanoparticles in agriculture as fertilizers and pesticides: Challenges and opportunities. In New Frontiers in Stress Management for Durable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 281–293. [Google Scholar] [CrossRef]
- Naaz, H.; Rawat, K.; Saffeullah, P.; Umar, S. Silica nanoparticles synthesis and applications in agriculture for plant fertilization and protection: A review. Environ. Chem. Lett. 2023, 21, 539–559. [Google Scholar] [CrossRef]
- Pu’ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.; Idris, M.; Lee, T. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Baskaran, T.; Mohammad, N.F.; Saleh, S.S.M.; Nasir, N.F.M.; Daud, F.D.M. Synthesis Methods of Doped Hydroxyapatite: A Brief Review. J. Phys. Conf. Ser. 2021, 2071, 012008. [Google Scholar] [CrossRef]
- Xin, Y.; Shirai, T. Noble-metal-free hydroxyapatite activated by facile mechanochemical treatment towards highly-efficient catalytic oxidation of volatile organic compound. Sci. Rep. 2021, 11, 7512. [Google Scholar] [CrossRef] [PubMed]
- Chaikina, M.; Bulina, N.; Prosanov, I.Y.; Vinokurova, O.; Ishchenko, A. Structure formation of zinc-substituted hydroxyapatite during mechanochemical synthesis. Inorg. Mater. 2020, 56, 402–408. [Google Scholar] [CrossRef]
- Bulina, N.V.; Vinokurova, O.B.; Eremina, N.V.; Prosanov, I.Y.; Khusnutdinov, V.R.; Chaikina, M.V. Features of solid-phase mechanochemical synthesis of hydroxyapatite doped by copper and zinc ions. J. Solid State Chem. 2021, 296, 121973. [Google Scholar] [CrossRef]
- Dinda, S.; Bhagavatam, A.; Alrehaili, H.; Dinda, G.P. Mechanochemical synthesis of nanocrystalline hydroxyapatite from Ca (H2PO4)2. H2O, CaO, Ca(OH)2, and P2O5 mixtures. Nanomaterials 2020, 10, 2232. [Google Scholar] [CrossRef]
- Galotta, A.; Agostinacchio, F.; Motta, A.; Dirè, S.; Sglavo, V.M. Mechanochemical synthesis and cold sintering of mussel shell-derived hydroxyapatite nano-powders for bone tissue regeneration. J. Euro. Ceram. Soc. 2023, 43, 639–647. [Google Scholar] [CrossRef]
- Makarova, S.V.; Bulina, N.V.; Golubeva, Y.A.; Klyushova, L.S.; Dumchenko, N.B.; Shatskaya, S.S.; Ishchenko, A.V.; Khvostov, M.V.; Dudina, D.V. Hydroxyapatite Double Substituted with Zinc and Silicate Ions: Possibility of Mechanochemical Synthesis and In Vitro Properties. Materials 2023, 16, 1385. [Google Scholar] [CrossRef]
- Hendronursito, Y.; Sukmana, I.; Risano, A. Study of the potential utilization of local Lampung Province resources in development of dental implant bioceramics. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1173, 012026. [Google Scholar] [CrossRef]
- Kumar, P.N.; Kannan, S.; Ferreira, J.M. Combined occupancy of gadolinium at the lattice sites of β-Ca3 (PO4) 2 and t-ZrO2 crystal structures. Eur. J. Inorg. Chem. 2020, 2020, 1163–1171. [Google Scholar] [CrossRef]
- Albulescu, R.; Popa, A.C.; Enciu, A.M.; Albulescu, L.; Dudau, M.; Popescu, I.D.; Mihai, S.; Codrici, E.; Pop, S.; Lupu, A.R.; et al. Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications. Materials 2019, 12, 3704. [Google Scholar] [CrossRef]
- Qi, C.; Musetti, S.; Fu, L.-H.; Zhu, Y.-J.; Huang, L. Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications. Chem. Soc. Rev. 2019, 48, 2698–2737. [Google Scholar] [CrossRef]
- Braga, R.R. Calcium phosphates as ion-releasing fillers in restorative resin-based materials. Dent. Mater. 2019, 35, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Pierre, C.; Bertrand, G.; Rey, C.; Benhamou, O.; Combes, C. Calcium phosphate coatings elaborated by the soaking process on titanium dental implants: Surface preparation, processing and physical-chemical characterization. Dent. Mater. 2019, 35, e25–e35. [Google Scholar] [CrossRef] [PubMed]
- Demina, V.A.; Krasheninnikov, S.V.; Buzin, A.I.; Kamyshinsky, R.A.; Sadovskaya, N.V.; Goncharov, E.N.; Zhukova, N.A.; Khvostov, M.V.; Pavlova, A.V.; Tolstikova, T.G.; et al. Biodegradable poly(l-lactide)/calcium phosphate composites with improved properties for orthopedics: Effect of filler and polymer crystallinity. Mater. Sci. Eng. C 2020, 112, 110813. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for biomedical applications: A short overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Lemos, A.L.; Tavaria, F.K.; Pintado, M. Chitosan and Hydroxyapatite Based Biomaterials to Circumvent Periprosthetic Joint Infections. Materials 2021, 14, 804. [Google Scholar] [CrossRef] [PubMed]
- Sans, J.; Arnau, M.; Turon, P.; Aleman, C. Permanently polarized hydroxyapatite, an outstanding catalytic material for carbon and nitrogen fixation. Mater. Horiz. 2022, 9, 1566–1576. [Google Scholar] [CrossRef]
- Bystrov, V.; Paramonova, E.; Avakyan, L.; Coutinho, J.; Bulina, N. Simulation and Computer Study of Structures and Physical Properties of Hydroxyapatite with Various Defects. Nanomaterials 2021, 11, 2752. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Stoica, A.E.; Vasile, B.S.; Andronescu, E. Luminescent Hydroxyapatite Doped with Rare Earth Elements for Biomedical Applications. Nanomaterials 2019, 9, 239. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, Y.; Li, D.; Wang, J.; Li, B.; Ma, W. Fabrication of Selenium-Doped Hydroxyapatite Coatings by Suspension Plasma Spraying: Characterization and Improvement of Coating Properties. J. Thermal Spray Technol. 2023, 32, 1893–1905. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Hou, X.-R.; Wang, L.-L.; Zhou, Y.-M.; Liu, X.-X.; Tian, C.-Y. Effect of Different Element Doping on Drug Delivery Properties of Ordered Porous Hydroxyapatite. Russ. J. Phys. Chem. A 2022, 96, 2476–2481. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Z.; Huang, J. Substituted hydroxyapatite: A recent development. Mater. Technol. 2019, 35, 785–796. [Google Scholar] [CrossRef]
- Yook, H.; Hwang, J.; Yeo, W.; Bang, J.; Kim, J.; Kim, T.Y.; Choi, J.S.; Han, J.W. Design Strategies for Hydroxyapatite-Based Materials to Enhance Their Catalytic Performance and Applicability. Adv. Mater. 2022, e2204938. [Google Scholar] [CrossRef] [PubMed]
- Filip, D.G.; Surdu, V.-A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Func. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Basu, B. Doped biphasic calcium phosphate: Synthesis and structure. J. Asian Ceram. Soc. 2019, 7, 265–283. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Lu, X.; Wang, K.; Ren, F. Computer simulation of ions doped hydroxyapatite: A brief review. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 978–987. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited Hydroxyapatite-Based Biocoatings: Recent Progress and Future Challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Nayak, B.; Samant, A.; Misra, P.K.; Saxena, M. Nanocrystalline Hydroxyapatite: A Potent Material for Adsorption, Biological and Catalytic Studies. Mater. Today Proc. 2019, 9, 689–698. [Google Scholar] [CrossRef]
- Pandaa, R.N.; Hsieha, M.F.; Chunga, R.J.; Chin, T.S. FTIR, XRD, SEM and solid state NMR investigations of carbonate-containing hydroxyapatite nano-particles synthesized by hydroxide-gel technique. J. Phy. Chem. Solids 2003, 64, 193–199. [Google Scholar] [CrossRef]
- Bystrov, V.S. Computational studies of the hydroxyapatite nanostructures, peculiarities and properties. Math. Biol. Bioinform. 2017, 12, 14–54. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Baciut, M.; Baciut, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug. Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Broda, E.; Skwarek, E. Clay, Hydroxyapatite and Their Composites—Brief Review. In Nanooptics and Photonics, Nanochemistry and Nanobiotechnology, and Their Applications; Springer: Cham, Switzerland, 2020; pp. 255–272. [Google Scholar]
- Hartati, Y.W.; Irkham, I.; Zulqaidah, S.; Syafira, R.S.; Kurnia, I.; Noviyanti, A.R.; Topkaya, S.N. Recent advances in hydroxyapatite-based electrochemical biosensors: Applications and future perspectives. Sens. Bio-Sens. Res. 2022, 38, 100542. [Google Scholar] [CrossRef]
- Verma, R.; Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. Hydroxyapatite-based composites: Excellent materials for environmental remediation and biomedical applications. Adv. Colloid Interface Sci. 2023, 315, 102890. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Fomin, A.S.; Murzakhanov, F.F.; Makshakova, O.N.; Donskaya, N.O.; Antonova, O.S.; Gnezdilov, O.I.; Mikheev, I.V.; Knotko, A.V.; Kudryavtsev, E.A.; et al. The improved textural properties, thermal stability, and cytocompatibility of mesoporous hydroxyapatite by Mg2+ doping. Mater. Chem. Phys. 2022, 289, 126461. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.M.; Lamonier, J.F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Zilm, M.; Thomson, S.D.; Wei, M. A Comparative Study of the Sintering Behavior of Pure and Manganese-Substituted Hydroxyapatite. Materials 2015, 8, 6419–6436. [Google Scholar] [CrossRef]
- Abdul Halim, N.A.; Hussein, M.Z.; Kandar, M.K. Nanomaterials-Upconverted Hydroxyapatite for Bone Tissue Engineering and a Platform for Drug Delivery. Int. J. Nanomed. 2021, 16, 6477–6496. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744–747, 657–661. [Google Scholar] [CrossRef]
- Pebriani, C.; Sari, Y. Microwave-assisted precipitation of carbonated hydroxyapatite. J. Phys. Conf. Ser. 2019, 1248, 012078. [Google Scholar] [CrossRef]
- Sakhno, Y.; Jaisi, D.P. Novel Route to Enhance the Solubility of Apatite, a Potential Nanofertilizer, through Structural Incorporation of Sodium and Potassium Ions. ACS Agric. Sci. Technol. 2021, 1, 488–498. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W.; Betancourt, T. Synthesis, bioactivity and zeta potential investigations of chlorine and fluorine substituted hydroxyapatite. Mater. Sci. Eng. C 2016, 59, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Naqshbandi, A.; Rahman, A. Synthesis and characterization of chlorinated hydroxyapatite as novel synthetic bone substitute with negative zeta potential. Ceram. Int. 2022, 48, 8112–8117. [Google Scholar] [CrossRef]
- Hsu, C.-S.; Haag, S.L.; Bernards, M.T.; Li, Q. Evaluation of chlorine substituted hydroxyapatite (ClHAP)/polydopamine composite coatings on Ti64. Colloid Surf. B Biointerfaces 2020, 189, 110799. [Google Scholar] [CrossRef]
- Gafurov, M.; Biktagirov, T.; Mamin, G.; Orlinskii, S. A DFT, X-and W-band EPR and ENDOR study of nitrogen-centered species in (nano) hydroxyapatite. Appl. Magn. Reson. 2014, 45, 1189–1203. [Google Scholar] [CrossRef]
- Biktagirov, T.; Gafurov, M.; Mamin, G.; Klimashina, E.; Putlayev, V.; Orlinskii, S. Combination of EPR measurements and DFT calculations to study nitrate impurities in the carbonated nanohydroxyapatite. J. Phys. Chem. A 2014, 118, 1519–1526. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Goh, Y.-F.; Akram, M.; Razali, I.R.; Abdul Kadir, M.R.; Hussain, R. Microwave assisted synthesis of nano sized sulphate doped hydroxyapatite. Mater. Res. Bull. 2013, 48, 2106–2110. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Mesoporous strontium doped nano sized sulphate hydroxyapatite as a novel biomaterial for bone tissue applications. RSC Adv. 2016, 6, 68058–68071. [Google Scholar] [CrossRef]
- Radha, G.; Venkatesan, B.; Jaisankar, S.N.; Rajashree, P.; Balakumar, S. Interplay between surface chemistry and osteogenic behaviour of sulphate substituted nano-hydroxyapatite. Mater. Sci. Eng. C 2021, 120, 111617. [Google Scholar] [CrossRef]
- Atila, D.; Karataş, A.; Evcin, A.; Keskin, D.; Tezcaner, A. Bacterial cellulose-reinforced boron-doped hydroxyapatite/gelatin scaffolds for bone tissue engineering. Cellulose 2019, 26, 9765–9785. [Google Scholar] [CrossRef]
- Tuncay, E.O.; Demirtas, T.T.; Gumusderelioglu, M. Microwave-induced production of boron-doped HAp (B-HAp) and B-HAp coated composite scaffolds. J. Trace Elem. Med. Biol. 2017, 40, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Jodati, H.; Evis, Z.; Tezcaner, A.; Alshemary, A.Z.; Motameni, A. 3D porous bioceramic based boron-doped hydroxyapatite/baghdadite composite scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2023, 140, 105722. [Google Scholar] [CrossRef] [PubMed]
- Makshakova, O.N.; Shurtakova, D.V.; Vakhin, A.V.; Grishin, P.O.; Gafurov, M.R. Incorporation of Iron(II) and (III) in Hydroxyapatite—A Theoretical Study. Crystals 2021, 11, 1219. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.-B.; Kumar, G.S.; Kolesnikov, E.; Janarthanan, G.; Pillai, M.M.; Rajendran, S.; Boobalan, S.; Sudha, K.G.; Rajeshkumar, M.P. Mesoporous Mg-doped hydroxyapatite nanorods prepared from bio-waste blue mussel shells for implant applications. Ceram. Int. 2020, 46, 28514–28527. [Google Scholar] [CrossRef]
- Laurencin, D.; Almora-Barrios, N.; de Leeuw, N.H.; Gervais, C.; Bonhomme, C.; Mauri, F.; Chrzanowski, W.; Knowles, J.C.; Newport, R.J.; Wong, A.; et al. Magnesium incorporation into hydroxyapatite. Biomaterials 2011, 32, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Makshakova, O.N.; Gafurov, M.R.; Goldberg, M.A. The Mutual Incorporation of Mg2+ and CO32−; into Hydroxyapatite: A DFT Study. Materials 2022, 15, 9046. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Buton, N.; Megier, C. Obtaining and Characterizing Thin Layers of Magnesium Doped Hydroxyapatite by Dip Coating Procedure. Coatings 2020, 10, 510. [Google Scholar] [CrossRef]
- Sharma, B.; Afonso, L.O.B.; Singh, M.P.; Soni, U.; Cahill, D.M. Zinc- and magnesium-doped hydroxyapatite-urea nanohybrids enhance wheat growth and nitrogen uptake. Sci. Rep. 2022, 12, 19506. [Google Scholar] [CrossRef]
- Guerra-López, J.R.; Echeverría, G.A.; Güida, J.A.; Viña, R.; Punte, G. Synthetic hydroxyapatites doped with Zn (II) studied by X-ray diffraction, infrared, Raman and thermal analysis. J. Phys. Chem. Solids 2015, 81, 57–65. [Google Scholar] [CrossRef]
- Abeywardana, L.; de Silva, M.; Sandaruwan, C.; Dahanayake, D.; Priyadarshana, G.; Chathurika, S.; Karunaratne, V.; Kottegoda, N. Zinc-doped hydroxyapatite–urea nanoseed coating as an efficient macro–micro plant nutrient delivery agent. ACS Agric. Sci. Technol. 2021, 1, 230–239. [Google Scholar] [CrossRef]
- Abeywardana, L.; Sandaruwan, C.; Chathurika, S.; Karunaratne, V.; Kottegoda, N. Advanced coating on Zea mays seeds using modified hydroxyapatite nanoparticles as a plant nutrient delivery system for enhanced plant growth. CURR. SCI. 2023, 124, 599. [Google Scholar]
- Kurinjinathan, P.; Thanigai Arul, K.; Ramana Ramya, J.; Manikandan, E.; Hegazy, H.H.; Umar, A.; Algarni, H.; Ahmad, N. Effect of Nickel Doping on the Properties of Hydroxyapatite Nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Sebastiammal, S.; Fathima, A.S.L.; Henry, J.; Wadaan, M.A.; Mahboob, S.; Wadaan, A.M.; Manzoor, I.; Gopinath, K.; Rajeswary, M.; Govindarajan, M. Synthesis, Characterization, Antibacterial, Antifungal, Antioxidant, and Anticancer Activities of Nickel-Doped Hydroxyapatite Nanoparticles. Fermentation 2022, 8, 677. [Google Scholar] [CrossRef]
- Bazin, T.; Magnaudeix, A.; Mayet, R.; Carles, P.; Julien, I.; Demourgues, A.; Gaudon, M.; Champion, E. Sintering and biocompatibility of copper-doped hydroxyapatite bioceramics. Ceram. Int. 2021, 47, 13644–13654. [Google Scholar] [CrossRef]
- Unabia, R.B.; Bonebeau, S.; Candidato, R.T.; Jouin, J.; Noguera, O.; Pawłowski, L. Investigation on the structural and microstructural properties of copper-doped hydroxyapatite coatings deposited using solution precursor plasma spraying. J. Eur. Ceram. Soc. 2019, 39, 4255–4263. [Google Scholar] [CrossRef]
- Imrie, F.; Skakle, J.; Gibson, I. Preparation of copper-doped hydroxyapatite with varying x in the composition Ca10 (PO4) 6CuxOyHz. Bioceram. Dev. Appl. 2013, 1, 2013. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Chahkandi, M.; Arami, S.R.S.; Mirzaei, M.; Mahdavi, B.; Hosseini-Tabar, S.M. A new effective nano-adsorbent and antibacterial material of hydroxyapatite. J. Iran. Chem. Soc. 2018, 16, 695–705. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Yahia, I.S. Novel and facile microwave-assisted synthesis of Mo-doped hydroxyapatite nanorods: Characterization, gamma absorption coefficient, and bioactivity. Mater. Sci. Eng. C 2017, 78, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Uggeri, J.; Medri, V.; Guizzardi, S. Sr, Mg cosubstituted HA porous macro-granules: Potentialities as resorbable bone filler with antiosteoporotic functions. J. Biomed. Mater. Res. Part A 2013, 101, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Meng, J.; Pan, W.; Gu, T.; Zhang, Q.; Zhang, J.; Wang, X.; Bu, C.; Piao, G. Effect of hydroxyl and Mo doping on activity and carbon deposition resistance of hydroxyapatite supported NixMoy catalyst for syngas production via DRM reaction. Int. J. Hydrog. Energy 2023, 48, 19033–19045. [Google Scholar] [CrossRef]
- Gu, M.; Li, W.; Jiang, L.; Li, X. Recent progress of rare earth doped hydroxyapatite nanoparticles: Luminescence properties, synthesis and biomedical applications. Acta Biomater. 2022, 148, 22–43. [Google Scholar] [CrossRef]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. Part B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Pandya, H.M.; Anitha, P. Comprehensive Review of preparation methodologies of nano hydroxyapatite. J. Environ. Nanotechnol. 2014, 3, 101–121. [Google Scholar] [CrossRef]
- Arteaga-Díaz, S.J.; Meramo-Hurtado, S.I.; León-Pulido, J.; Zuorro, A.; González-Delgado, A.D. Environmental assessment of large scale production of magnetite (Fe3O4) nanoparticles via coprecipitation. Appl. Sci. 2019, 9, 1682. [Google Scholar] [CrossRef]
- Besenhard, M.O.; LaGrow, A.P.; Hodzic, A.; Kriechbaum, M.; Panariello, L.; Bais, G.; Loizou, K.; Damilos, S.; Cruz, M.M.; Thanh, N.T.K. Co-precipitation synthesis of stable iron oxide nanoparticles with NaOH: New insights and continuous production via flow chemistry. Chem. Eng. J. 2020, 399, 125740. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, L.; Li, J.; Liu, X.; Wang, R.; Wang, L.; Tu, G. Growth mechanism of CsPbBr 3 perovskite nanocrystals by a co-precipitation method in a CSTR system. Nano Res. 2019, 12, 121–127. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Fan, H.; Li, B.; Guo, B.; Liu, M.; Zhang, X. Fabrication, biological effects, and medical applications of calcium phosphate nanoceramics. Mater. Sci. Eng. R Rep. 2010, 70, 225–242. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Soflaee, F. Synthesis and characterization of α-Fe2O3 nanoparticles by simple co-precipitation method. Phys. Chem. Res. 2015, 3, 191–196. [Google Scholar] [CrossRef]
- Jarudilokkul, S.; Tanthapanichakoon, W.; Boonamnuayvittaya, V. Synthesis of hydroxyapatite nanoparticles using an emulsion liquid membrane system. Colloids Surf. A Physicochem. Eng. Asp. 2007, 296, 149–153. [Google Scholar] [CrossRef]
- Bader, N.; Benkhayal, A.A.; Zimmermann, B. Co-precipitation as a sample preparation technique for trace element analysis: An overview. Int. J. Chem. Sci. 2014, 12, 519–525. [Google Scholar]
- Sciena, C.R.; dos Santos, M.F.; Moreira, F.K.V.; Sena Neto, A.R.; Marconcini, J.M.; Correa, D.S.; Paris, E.C. Starch:Pectin Acidic Sachets Development for Hydroxyapatite Nanoparticles Storage to Improve Phosphorus Release. J. Poly. Environment 2019, 27, 794–802. [Google Scholar] [CrossRef]
- Nayak, A.K. Hydroxyapatite synthesis methodologies: An overview. Int. J. ChemTech Res. 2010, 2, 903–907. [Google Scholar]
- Kien, P.T.; Phu, H.D.; Linh, N.V.V.; Quyen, T.N.; Hoa, N.T. Recent Trends in Hydroxyapatite (HA) Synthesis and the Synthesis Report of Nanostructure HA by Hydrothermal Reaction. Adv. Exp. Med. Biol. 2018, 1077, 343–354. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Al-Khafaji, Z.; Adnan, M.; Radhi, N.S.; Musa, S.J.; Hadi, Z.M.; Radhi, S.S.; Mareai, B.M. The Growing Importance of Hydroxyapatite in Modern Biomedicine (HAP): A Review of Recent Advances and Challenges. Eng. Sci. 2023, 1. [Google Scholar] [CrossRef]
- Zastulka, A.; Clichici, S.; Tomoaia-Cotisel, M.; Mocanu, A.; Roman, C.; Olteanu, C.D.; Culic, B.; Mocan, T. Recent Trends in Hydroxyapatite Supplementation for Osteoregenerative Purposes. Materials 2023, 16, 1303. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, M.; Shaaban, A.; Seliman, S. Overview: Process Parameters Forhydrothermal Synthesis OF Hydroxyapatite. J. Adv. Manuf. Technol. 2012, 2022, 3481677. [Google Scholar] [CrossRef]
- Cho, S.J.; Uddin, M.J.; Alaboina, P. Review of Nanotechnology for Cathode Materials in Batteries. In Emerging Nanotechnologies in Rechargeable Energy Storage Systems; Rodriguez-Martinez, L.M., Omar, N., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 83–129. [Google Scholar]
- Fereshteh, Z.; Salavati-Niasari, M. Effect of ligand on particle size and morphology of nanostructures synthesized by thermal decomposition of coordination compounds. Adv. Colloid Interface Sci. 2017, 243, 86–104. [Google Scholar] [CrossRef]
- Khalaji, A.D.; Das, D. Synthesis and characterizations of NiO nanoparticles via solid-state thermal decomposition of nickel (II) Schiff base complexes. Int. Nano Lett. 2014, 4, 117. [Google Scholar] [CrossRef]
- Abdel-Monem, Y.K.; Emam, S.M.; Okda, H.M. Solid state thermal decomposition synthesis of CuO nanoparticles from coordinated pyrazolopyridine as novel precursors. J. Mater. Sci. Mater. Electron. 2017, 28, 2923–2934. [Google Scholar] [CrossRef]
- Zhang, Q.; Saito, F. A review on mechanochemical syntheses of functional materials. Adv. Powder Technol. 2012, 23, 523–531. [Google Scholar] [CrossRef]
- Sakamoto, H.; Matsuda, R.; Kitagawa, S. Systematic mechanochemical preparation of a series of coordination pillared layer frameworks. Dalton Trans. 2012, 41, 3956–3961. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Abdullah, M.M.A.B.; Abd Rahim, S.Z.; Luhar, S.; Sandu, A.V.; Jamil, N.H.; Nabiałek, M. Synthesis methods of hydroxyapatite from natural sources: A review. Ceram. Int. 2022, 48, 14959–14979. [Google Scholar] [CrossRef]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized calcium phosphates as novel macronutrient nano-fertilizers. Nanomaterials 2022, 12, 2709. [Google Scholar] [CrossRef]
- de Silva, M.; Sandaruwan, C.; Hernandez, F.C.R.; Sahin, O.; Ashokkumar, M.; Ajayan, P.M.; Karunaratne, V.; Amaratunga, G.A.; Kottegoda, N. A Greener Mechanochemical Approach to the Synthesis of Urea-Hydroxyapatite Nanohybrids for Slow Release of Plant Nutrients. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Han, G.F.; Li, F.; Chen, Z.W.; Coppex, C.; Kim, S.J.; Noh, H.J.; Fu, Z.; Lu, Y.; Singh, C.V.; Siahrostami, S.; et al. Mechanochemistry for ammonia synthesis under mild conditions. Nat. Nanotechnol. 2021, 16, 325–330. [Google Scholar] [CrossRef] [PubMed]
- AlShamaileh, E.; Al-Rawajfeh, A.E.; Alrbaihat, M. Mechanochemical synthesis of slow-release fertilizers: A review. Open Agric. J. 2018, 12, 11–19. [Google Scholar] [CrossRef]
- Mohamed, M.; Saadallah, H.; Gonzalez-Martinez, I.; Hantusch, M.; Valldor, M.; Büchner, B.; Hampel, S.; Gräßler, N. Mechanochemical synthesis of Li-rich (Li2Fe) SO cathode for Li-ion batteries. Green Chem. 2023, 25, 3878–3887. [Google Scholar] [CrossRef]
- Baláž, P.; Dutková, E. Fine milling in applied mechanochemistry. Miner. Eng. 2009, 22, 681–694. [Google Scholar] [CrossRef]
- Silva, C.C.; Graça, M.P.F.; Valente, M.A.; Sombra, A.S.B. Crystallite size study of nanocrystalline hydroxyapatite and ceramic system with titanium oxide obtained by dry ball milling. J. Mater. Sci. 2007, 42, 3851–3855. [Google Scholar] [CrossRef]
- Nasiri-Tabrizi, B.; Fahami, A.; Ebrahimi-Kahrizsangi, R.; Khazraei, A.; Yazdani, M.R.; Kajbafzadeh, M.J. A study on mechanochemical behavior of CaO–P2O5–CaF2–ZrO2 system to produce fluorapatite–zirconia composite nanopowders. Powder Technol. 2013, 243, 59–70. [Google Scholar] [CrossRef]
- Ebrahimi-Kahrizsangi, R.; Nasiri-Tabrizi, B.; Chami, A. Characterization of single-crystal fluorapatite nanoparticles synthesized via mechanochemical method. Particuology 2011, 9, 537–544. [Google Scholar] [CrossRef]
- Fakharzadeh, A.; Ebrahimi-Kahrizsangi, R.; Nasiri-Tabrizi, B.; Jefrey Basirun, W. Effect of dopant loading on the structural features of silver-doped hydroxyapatite obtained by mechanochemical method. Ceram. Int. 2017, 43, 12588–12598. [Google Scholar] [CrossRef]
- Chaikina, M.V.; Bulina, N.V.; Vinokurova, O.B.; Prosanov, I.Y.; Dudina, D.V. Interaction of calcium phosphates with calcium oxide or calcium hydroxide during the “soft” mechanochemical synthesis of hydroxyapatite. Ceram. Int. 2019, 45, 16927–16933. [Google Scholar] [CrossRef]
- Bulina, N.V.; Chaikina, M.V.; Prosanov, I.Y.; Komarova, E.G.; Sedelnikova, M.B.; Sharkeev, Y.P.; Sheikin, V.V. Lanthanum-silicate-substituted apatite synthesized by fast mechanochemical method: Characterization of powders and biocoatings produced by micro-arc oxidation. Mater. Sci. Eng. C 2018, 92, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.A.; El-Midany, A.A.; El-Shall, H. Mechanochemical–hydrothermal preparation of nano-crystallite hydroxyapatite using statistical design. Mater. Chem. Phys. 2008, 112, 202–207. [Google Scholar] [CrossRef]
- Iwasaki, T.; Takeda, R. Mechanochemically assisted synthesis of La0.7Sr0.3MnO3 nanoparticles and induction heating properties of the composites with hydroxyapatite. Curr. Appl. Phys. 2021, 25, 12–17. [Google Scholar] [CrossRef]
- Fahami, A.; Ebrahimi-Kahrizsangi, R.; Nasiri-Tabrizi, B. Mechanochemical synthesis of hydroxyapatite/titanium nanocomposite. Solid State Sci. 2011, 13, 135–141. [Google Scholar] [CrossRef]
- Ferro, A.C.; Guedes, M. Mechanochemical synthesis of hydroxyapatite using cuttlefish bone and chicken eggshell as calcium precursors. Mater. Sci. Eng. C 2019, 97, 124–140. [Google Scholar] [CrossRef]
- Coreño, A.J.; Coreño, A.O.; Cruz, R.J.; Rodríguez, C.C. Mechanochemical synthesis of nanocrystalline carbonate-substituted hydroxyapatite. Opt. Mater. 2005, 27, 1281–1285. [Google Scholar] [CrossRef]
- Lala, S.; Maity, T.; Singha, M.; Biswas, K.; Pradhan, S. Effect of doping (Mg, Mn, Zn) on the microstructure and mechanical properties of spark plasma sintered hydroxyapatites synthesized by mechanical alloying. Ceram. Int. 2017, 43, 2389–2397. [Google Scholar] [CrossRef]
- Bulina, N.V.; Vinokurova, O.B.; Prosanov, I.Y.; Vorobyev, A.M.; Gerasimov, K.B.; Borodulina, I.A.; Pryadko, A.; Botvin, V.V.; Surmeneva, M.A.; Surmenev, R.A. Mechanochemical synthesis of strontium-and magnesium-substituted and cosubstituted hydroxyapatite powders for a variety of biomedical applications. Ceram. Int. 2022, 48, 35217–35226. [Google Scholar] [CrossRef]
- Youness, R.A.; Taha, M.A.; Elhaes, H.; Ibrahim, M. Molecular modeling, FTIR spectral characterization and mechanical properties of carbonated-hydroxyapatite prepared by mechanochemical synthesis. Mater. Chem. Phys. 2017, 190, 209–218. [Google Scholar] [CrossRef]
- Hussain, S.; Sabiruddin, K. Effect of heat treatment on the synthesis of hydroxyapatite from Indian clam seashell by hydrothermal method. Ceram. Int. 2021, 47, 29660–29669. [Google Scholar] [CrossRef]
- Ravindranadh, K.; Babu, B.; Manjari, V.P.; Rao, G.T.; Rao, M.; Ravikumar, R. Optical and structural properties of undoped and Mn2+ doped Ca–Li hydroxyapatite nanopowders using mechanochemical synthesis. J. Lumin. 2015, 159, 119–127. [Google Scholar] [CrossRef]
- Nasiri-Tabrizi, B.; Fahami, A.; Ebrahimi-Kahrizsangi, R. A comparative study of hydroxyapatite nanostructures produced under different milling conditions and thermal treatment of bovine bone. J. Ind. Eng. Chem. 2014, 20, 245–258. [Google Scholar] [CrossRef]
- Burgio, N.; Iasonna, A.; Magini, M.; Martelli, S.; Padella, F. Mechanical alloying of the Fe−Zr system. Correlation between input energy and end products. Il Nuovo Cim. D 1991, 13, 459–476. [Google Scholar] [CrossRef]
- Angioni, D.; Orrù, R.; Cao, G.; Garroni, S.; Bellucci, D.; Cannillo, V. Bioactivity enhancement by a ball milling treatment in novel bioactive glass-hydroxyapatite composites produced by spark plasma sintering. J. Eur. Ceram. Soc. 2023, 43, 1220–1229. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Esmaeilkhanian, A.; Moradi, M.; Pakseresht, A.; Asl, M.S.; Karimi-Maleh, H.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Biocompatibility and mechanical properties of pigeon bone waste extracted natural nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. B 2021, 264, 114950. [Google Scholar] [CrossRef]
- Lala, S.; Brahmachari, S.; Das, P.K.; Das, D.; Kar, T.; Pradhan, S.K. Biocompatible nanocrystalline natural bonelike carbonated hydroxyapatite synthesized by mechanical alloying in a record minimum time. Mater. Sci. Eng. C 2014, 42, 647–656. [Google Scholar] [CrossRef]
- Fernández, C.; Martínez, C.; Prado, M.O.; Olmedo, D.; Ozols, A. Bone Regeneration with Wharton’s Jelly-Bioceramic-Bioglass Composite. Procedia Mater. Sci. 2015, 9, 205–212. [Google Scholar] [CrossRef][Green Version]
- Lala, S.; Ghosh, M.; Das, P.K.; Das, D.; Kar, T.; Pradhan, S.K. Magnesium substitution in carbonated hydroxyapatite: Structural and microstructural characterization by Rietveld’s refinement. Mater. Chem. Phys. 2016, 170, 319–329. [Google Scholar] [CrossRef]
- Shu, C.; Yanwei, W.; Hong, L.; Zhengzheng, P.; Kangde, Y. Synthesis of carbonated hydroxyapatite nanofibers by mechanochemical methods. Ceram. Int. 2005, 31, 135–138. [Google Scholar] [CrossRef]
- Bulina, N.V.; Chaikina, M.V.; Andreev, A.S.; Lapina, O.B.; Ishchenko, A.V.; Prosanov, I.Y.; Gerasimov, K.B.; Solovyov, L.A. Mechanochemical Synthesis of SiO4–-Substituted Hydroxyapatite, Part II—Reaction Mechanism, Structure, and Substitution Limit. Eur. J. Inorg. Chem. 2014, 2014, 4810–4825. [Google Scholar] [CrossRef]
- Bulina, N.V.; Chaikina, M.V.; Prosanov, I.Y.; Dudina, D.V. Strontium and silicate co-substituted hydroxyapatite: Mechanochemical synthesis and structural characterization. Mater. Sci. Eng. B 2020, 262, 114719. [Google Scholar] [CrossRef]



| Synthesis Method | Advantages | Disadvantages | Refs |
|---|---|---|---|
| Coprecipitation | No need for special devices, simple, low-cost, low-crystalline particles, suitable for massive production. | Particles are easily aggregated, and chemical residuals can affect plants, different steps, a wide range of sizes, and uncontrollable morphology. | [114,115] |
| Hydrothermal | Size and morphology can be well controlled. | Low yield and relatively long time, need a special device, high crystalline particles, unsuitable for large scale production. | [116] |
| Mechanochemical | Simple, low cost, no need for high temperature, appropriate for large production, reliability, safety, no chemical solutions, one-step synthesis. | Needs special devices, nanoparticles tend to aggregate, contamination possibility from milling media, a wide range of particle sizes and shapes (inhomogeneous particulate) | [114,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, M.; Ashraf, S.; Baltrusaitis, J. Nutrient-Doped Hydroxyapatite: Structure, Synthesis and Properties. Ceramics 2023, 6, 1799-1825. https://doi.org/10.3390/ceramics6030110
Ammar M, Ashraf S, Baltrusaitis J. Nutrient-Doped Hydroxyapatite: Structure, Synthesis and Properties. Ceramics. 2023; 6(3):1799-1825. https://doi.org/10.3390/ceramics6030110
Chicago/Turabian StyleAmmar, Mohamed, Sherif Ashraf, and Jonas Baltrusaitis. 2023. "Nutrient-Doped Hydroxyapatite: Structure, Synthesis and Properties" Ceramics 6, no. 3: 1799-1825. https://doi.org/10.3390/ceramics6030110
APA StyleAmmar, M., Ashraf, S., & Baltrusaitis, J. (2023). Nutrient-Doped Hydroxyapatite: Structure, Synthesis and Properties. Ceramics, 6(3), 1799-1825. https://doi.org/10.3390/ceramics6030110







