Abstract
99Tc and 129I are two long-lived, highly soluble and mobile fission products that pose a long-term hazard. A proposed wasteform for the disposal of radio-iodine is iodovanadinite (Pb5(VO4)3I), an apatite-structured vanadate. In this investigation, a suite of potential iodovanadinite wasteforms designed for the co-disposal of Tc and I or the sole disposal of I were synthesised via hot isostatic pressing (with Mo as a surrogate for Tc). It was found that direct synthesis from oxide and iodide precursors was possible using hot isostatic pressing (HIPing). Increasing overpressure during HIPing was found to improve the density of the final product. X-ray diffraction (XRD) and scanning electron microscopy (SEM) analyses indicated that the use of AgI as the source of iodine affected the formation of the target iodovanadinite phase and produced unfavourable phase assemblages. Here, we report the direct synthesis of Pb5(VO4)3I in a single step by hot isostatic pressing.
1. Introduction
Radioactive iodine isotopes are produced as by-products of the nuclear fission of uranium fuel during nuclear power production. During the reprocessing of spent nuclear fuel (SNF), iodine is liberated. Although many iodine isotopes are relatively short-lived (e.g., 131I, t1/2 = 8 days) 129I has a half-life of 15.7 million years and poses a significant long-term hazard. Consequently, sequestering and immobilising radio-iodine to reduce its long-term environmental hazards are of particular importance. Volatilised iodine is removed using various off-gas technologies, including aqueous scrubs or silver-impregnated solid sorbents that react to form AgI [1,2,3,4,5]. Although it is possible to subsequently isolate iodine from these extractants, any wasteform that can directly handle AgI would be advantageous [6,7]. Further, 99Tc, like 129I, is a long-lived (t1/2 = 211,000 years) and highly soluble mobile fission product [8]. Technetium is a problematic element with regards to vitrification but may be extracted from SNF during reprocessing by careful modification of PUREX/UREX flowsheets or by head-end processes such as voloxidation [9,10,11]. This raises the possibility of producing a tailored wasteform for its safe disposal. Given the long half-lives of 99Tc and 129I, co-immobilisation of these two isotopes in a single, tailored wasteform would be conceptually advantageous.
Iodovanadinite (Pb5(VO4)3I), sometimes referred to as lead iodoapatite, has been identified as a potential host matrix for the immobilisation of 129I [12]. Iodovanadinite is closely related to the mineral vanadinite (Pb5(VO4)3Cl) and its counterparts pyromorphite (Pb5(PO4)3Cl) and mimetite (Pb5(AsO4)3Cl), all members of the larger apatite mineral family, crystallising in the hexagonal spacegroup P63/m [13]. The corner-sharing PO4 tetrahedra found in archetypal apatites may be replaced with larger structural units, such as VO4 or AsO4 tetrahedra. Substituting the PO4 unit with larger structural units increases the aperture of the tunnel sites and subsequently improves the incorporation of larger anions, such as iodide, within the wasteform [12,13,14,15]. Apatites containing the ReO5 structural unit have also successfully been synthesised. Because Re exhibits similar chemistry as its lighter congener, Tc, and is frequently used as an inactive surrogate, such apatite phases demonstrate potential for the co-immobilisation of both iodine and technetium in a single matrix [14,16].
Although iodovanadinite has previously been successfully synthesised by a variety of methods, including spark plasma sintering (SPS) and microwave synthesis [12,17,18], the volatile nature of iodine greatly complicates the synthesis and consolidation of these compounds. High-temperature heat treatment in an open atmosphere results in considerable loss of the initial iodine inventory, and consequently, methods of sample preparation or consolidation that minimise volatilisation, e.g., hot isostatic pressing (HIP) or mechanochemical processing, are of great interest [19,20,21,22,23].
The existence of the closely related mineral phases vanadinite, pyromorphite and mimetite provides an indication that the framework structure is relatively durable in the environment and therefore worthy of consideration as a possible host phase for the geological disposal of radionuclides. Studies of synthetic iodovanadinite have found that the release of iodine from the structure may be affected by the pH of the leaching solution. Maddrell and Abraitis (2004) reported that, over the course of a 14-day leach test in alkaline media (pH 11, SA/V = 1 cm−1), the normalised release rate of iodine from samples of Pb5(VO4)3I was 1.5 g·m−2d−1 and that the release of iodine was incongruent with the dissolution of the matrix [23]. Dissolution of a partially phosphate substituted composition (Pb10(VO4)4.8(PO4)1.2I2), reported by Audubert and Guy (2002), found that the initial release rate in deionised water at 90 °C was 2.4 × 10−3 g·m−2d−1 and increased markedly when moving to increasingly acidic media [24]. It was again found that iodine release was incongruent with matrix dissolution.
In this investigation, iodovanadinite compositions in the Pb5−xAgx(VO4)3−x(MoO4)xI and Pb4.5−xBaxAg0.5(VO4)3I0.5 series were synthesised using HIP with the aim of producing wasteforms suitable for both the sole disposal of AgI and the co-disposal of iodine and technetium. The resulting materials were characterised by powder X-ray diffraction, scanning electron microscopy and thermal analysis techniques. Materials similar to the Pb4.5−xBaxAg0.5(VO4)3I0.5 series have previously been described but not fully characterised by Uno et al. (2004) [25]. Subsequently, Johnstone et al. reported unsuccessful synthesis attempts of Pb9Ag(VO4)6I and Ba9Ag(VO4)6I endmembers via reaction in sealed quartz tubes [26]. Our study of the Pb4.5−xBaxAg0.5(VO4)3I0.5 series was pursued with the aim of determining whether a combination of Pb/Ba in conjunction with HIPing would prove more successful. Incorporation of AgI in the Pb5−xAgx(VO4)3−x(MoO4)xI series was hypothesised to be charge balanced by the incorporation of Mo as the MoO42− anion, with Mo effectively acting as a surrogate for Tc. Mo was selected for this purpose due to the similarities between Tc and Mo in chemistry and ionic radius (adjacent 5d transition metals, ionic radius Tc7+ = 0.41 Å and ionic radius of Mo6+ = 0.37 Å in four-fold co-ordination). The MoO42− anion is of a different charge to the pertechnetate anion (TcO4−), which is evidently a limitation on the use of Mo as a Tc surrogate in this speciation; however, the counterpart TcO42, tetraoxotechnetate (VI), species is known [10]. It was recognised that Re, as a congener of Tc, is more commonly used as a surrogate for Tc. However, for the purpose of this exploratory study, Mo was selected as a more cost-effective surrogate since the chemistry and ionic size of Mo and Tc were considered sufficiently similar to provide useful insight for the intended purpose. Nevertheless, the results presented here should be considered insightful only in the context of the hypothesised solid solution behaviour and methodology, and appropriate caution should be exercised in extrapolating the behaviour of Mo to Tc.
2. Materials and Methods
Iodovanadinites were prepared by a solid-state reaction between PbO, V2O5, MoO3, PbI2, AgI and Ba3(VO4)2 precursors using a hot isostatic press (HIP). Ba3(VO4)2 was used to provide a suitable Ba precursor that would not evolve gas during the HIPing process. In the Pb5−xAgx(VO4)3−x(MoO4)xI series (x = 0, 0.2, 0.4, 0.6, 0.8, 1.0), PbI2 was incrementally replaced as the iodine source by AgI. In the Pb4.5−xBaxAg0.5(VO4)3I0.5 series (x = 0, 0.5, 1.0, 1.5, 2.0, 2.5), AgI was the sole iodine source. Precursors were mixed with cyclohexane to form a slurry and ball milled in yttria-stabilised zirconia pots with yttria-stabilised zirconia media using a Fritsch Pulverisette 6 for five periods of 3 min at 500 rpm, changing the direction of milling with each period. Consolidated samples were produced by HIP (American Isostatic Presses–630H HIP, Columbus, OH, USA) in C106 copper tubing (~1.5 g sample, 6.5 mm OD, 2 mm ID) for 4 h at a temperature of 800 °C with an overpressure of 100 MPa. A preliminary set of baseline Pb5(VO4)3I samples was produced to study the effect of overpressure on consolidation by HIPing at overpressures of 5, 10, 25 and 100 MPa and was used as a basis for the processing conditions for subsequent syntheses. Our choice of synthesis conditions was guided by consideration of the appropriate temperature to achieve a solid-state reaction within the temperature stability field of Pb5(VO4)3I [19] and the expected range of overpressure necessary to achieve densification and the elimination of inter-granular porosity.
Reacted materials were ground to a fine powder and characterised by powder X-ray diffraction (XRD). XRD was performed using a Bruker D2 Phaser diffractometer (Bruker, Coventry, UK) utilising Cu Kα radiation, a Ni foil Kβ filter and a position-sensitive detector. Measurements were made at 10° < 2θ < 70° with a step size of 0.02 increments and a scan rate of 1 s per step. Phase analysis was completed using Diffrac.Suite Eva V.3 (Bruker) software.
Consolidated materials were sectioned using a Buehler Isomet Low Speed Saw, and the microstructures were characterised by scanning electron microscopy (SEM) in combination with energy dispersive X-ray spectroscopy (EDX) using a Hitachi TM3030 SEM (Hitachi, Warrington, UK) equipped with a Bruker Quantax EDX. An accelerating voltage of 15 kV was used for imaging. EDX data was processed using Bruker Quantax software. Sectioned materials were prepared for SEM analysis by mounting in cold setting resin and polishing with SiC paper and progressively finer diamond pastes to an optical finish (1 µm). Samples were sputter-coated with carbon to reduce surface charging effects.
To determine the behaviour of precursor powders during heating, samples were investigated by thermogravimetric analysis-mass spectrometry using a Netzsch TG449 F3 Jupiter with an integrated QMS 403 D Aëolos mass spectrometer (Netzsch, Wolverhampton, UK) Samples were heated at a rate of 20 °C min−1 in an argon atmosphere up to a temperature of 800 °C. The mass loss of samples was recorded, and evolved species were analysed using a 64 channel QMS 403 D Aëolos mass spectrometer. We optimised the sensitivity of our analysis for iodine detection, and, therefore, it was not possible to make an accurate assessment of weight loss attribution to other evolved gases.
3. Results and Discussion
3.1. HIPing of Baseline Pb5(VO4)3I and the Effect of Overpressure during Consolidation
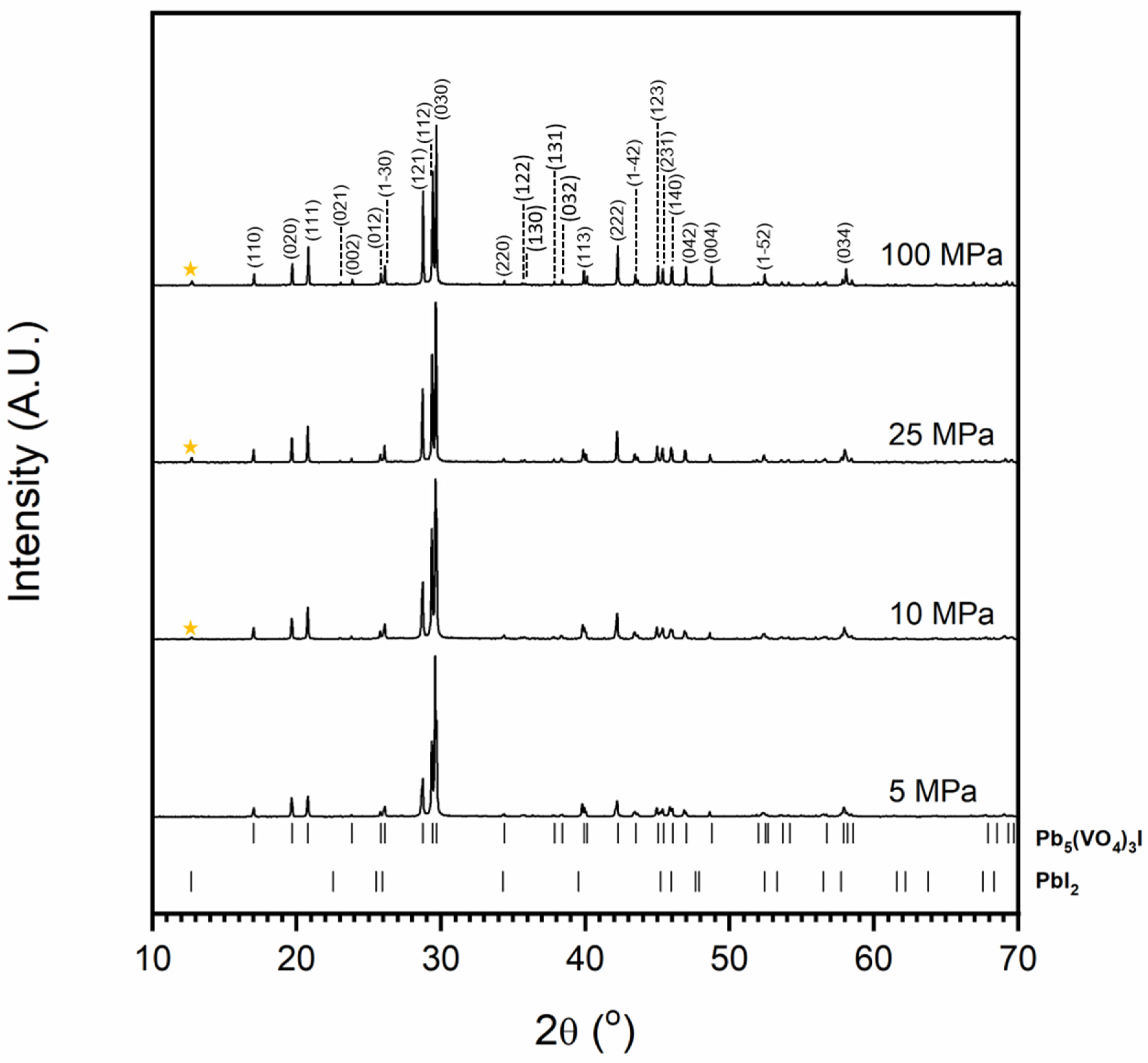
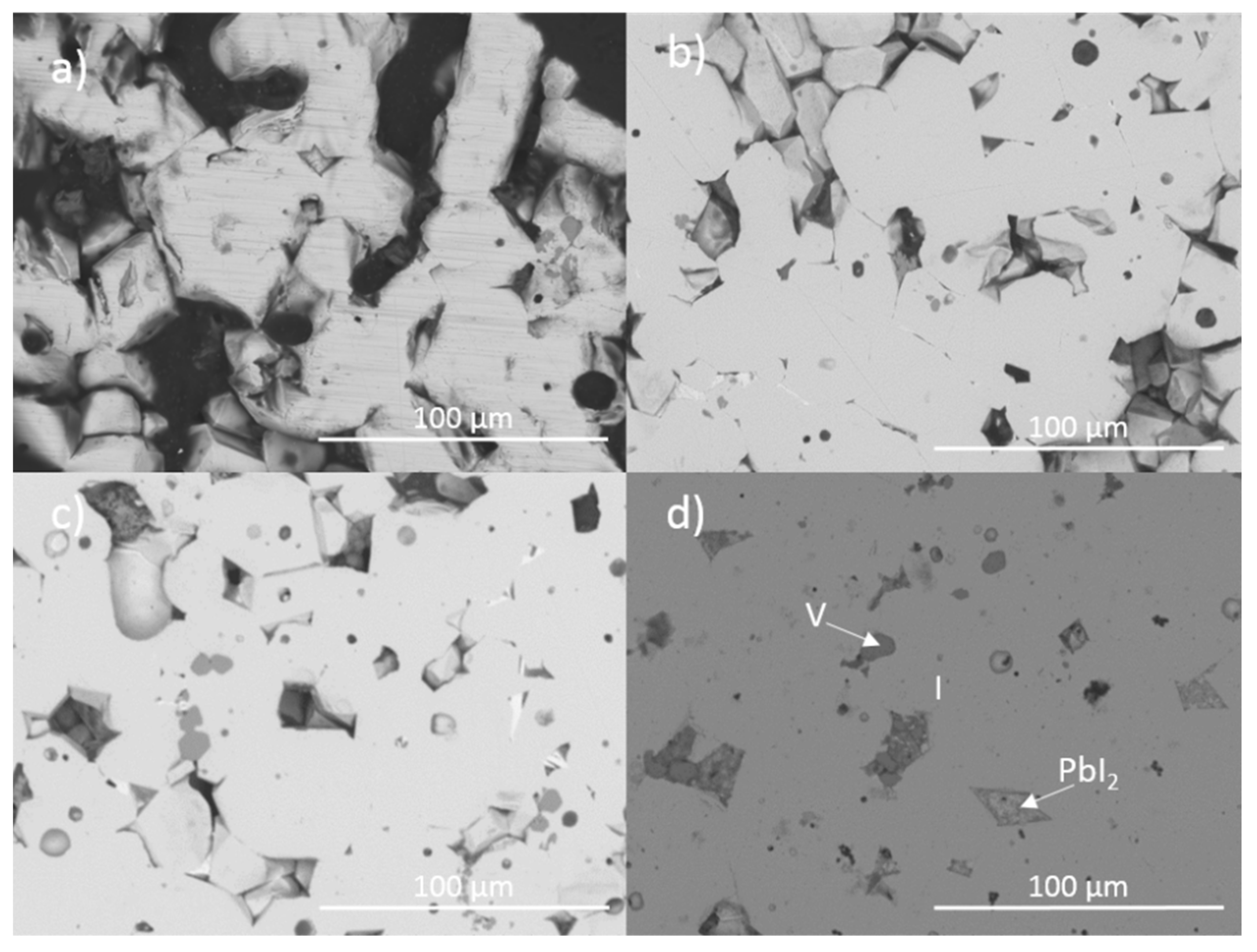
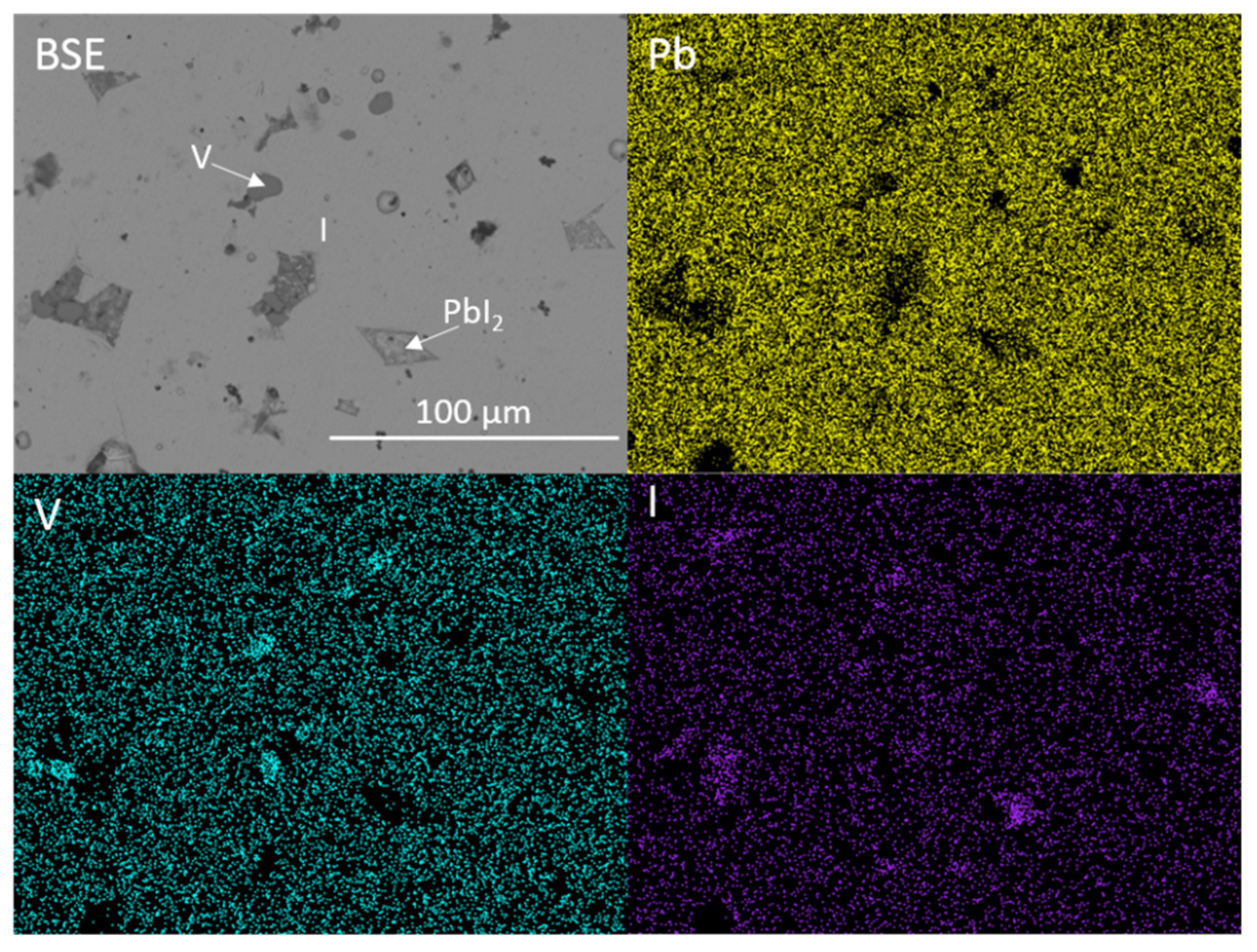
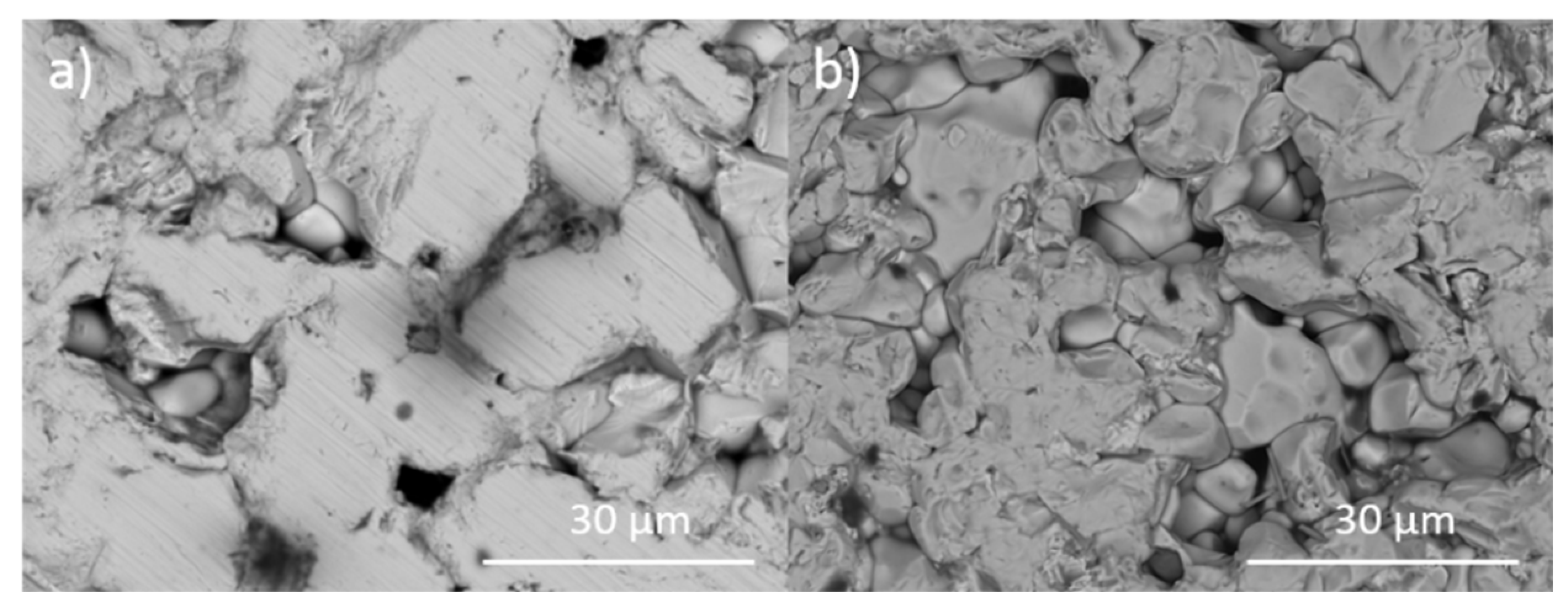
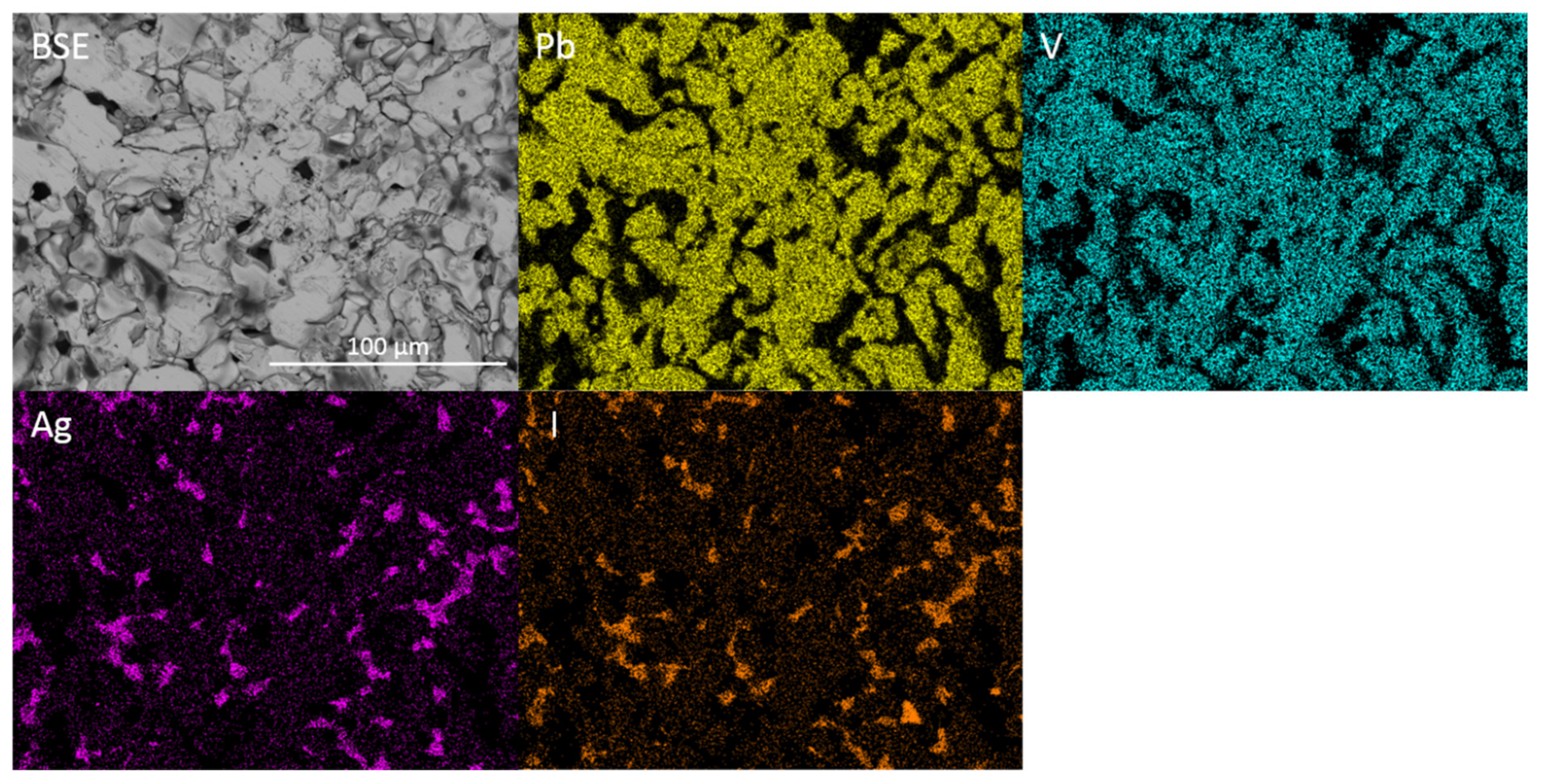
Figure 1 and Figure 2 show the results of X-ray diffraction and representative micrographs for baseline iodovanadinite (Pb5(VO4)3I) consolidated at overpressures of 5, 10, 25 and 100 MPa, respectively. As can be seen in Figure 1, the iodovanadinite phase is formed under all overpressures; there is also evidence of the retention of a small amount of PbI2. Although the applied overpressure does not have a major effect on the observed phase assemblage, it does have an effect on the final microstructure. Increasing overpressure results in improved densification and lower inter-granular porosity, yielding a superior wasteform relative to those produced with lower overpressures. An overpressure of 100 MPa was required to eliminate inter-granular porosity. It was not possible to make a more quantitative determination of porosity due to the preferential pull-out of PbI2 grains during specimen preparation for SEM analysis. It is also apparent from SEM (Figure 2) that, while not evident by XRD, a small proportion of V2O5 was retained (phases were determined by SEM-EDX mapping, see Figure 3; iodine-rich regions were assumed to be PbI2 based on XRD results, thus differentiating them from iodovanadinite). Although iodovanadinite has previously been synthesised directly from oxides by mechanochemical processing followed by thermal annealing or spark plasma sintering [21,27], this represents the first reported instance of direct synthesis by hot isostatic pressing. The removal of the intermediate Pb3(VO4)2 synthesis step reduces the number of necessary operations and is therefore a considerable simplification of the conceptual manufacturing process.
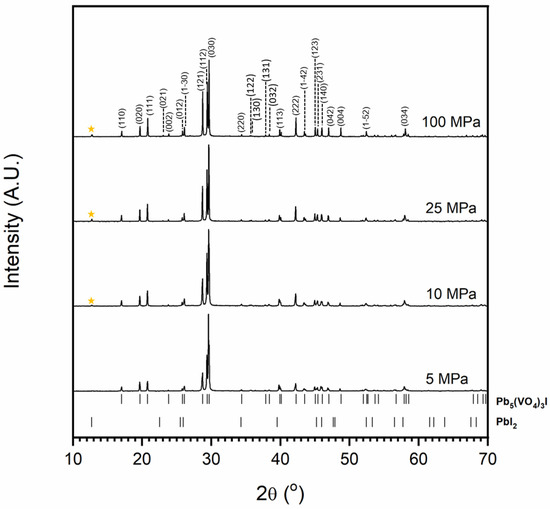
Figure 1.
Powder X-ray diffraction profiles of iodovanadinite (labelled (hkl) Miller indices) synthesised with varying overpressure. The orange star indicates major PbI2 peak. Tick marks show major reflections of Pb5(VO4)3I (ICSD 280065) and PbI2 (ICSD 42013).
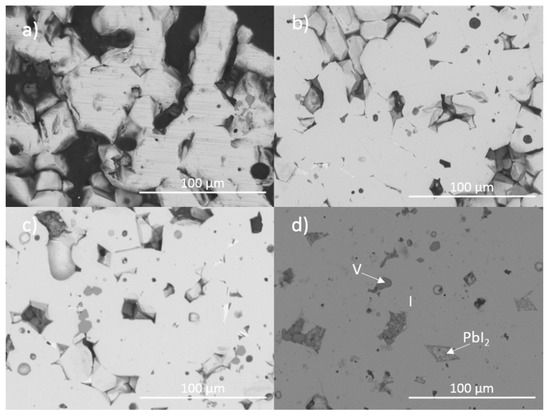
Figure 2.
Representative backscattered electron micrographs of Pb5(VO4)3I consolidated with differing overpressures: (a) 5 MPa, (b) 10 MPa, (c) 25 MPa and (d) 100 MPa. I = iodovanadinite, V = V2O5.
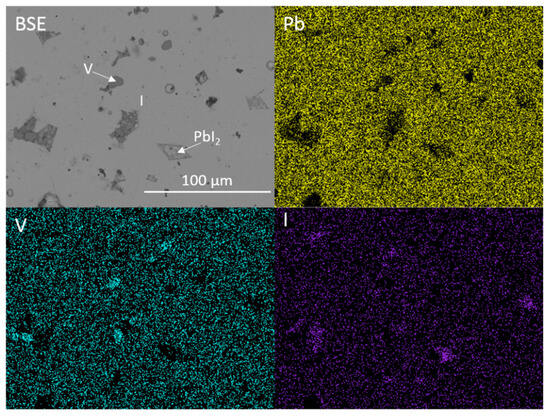
Figure 3.
BSE micrograph and accompanying EDX maps displaying the microstructure and elemental distribution of Pb, V and I in baseline Pb5(VO4)3I. I = iodovanadinite, V = V2O5.
3.2. Pb5−xAgx(VO4)3−x(MoO4)xI Series
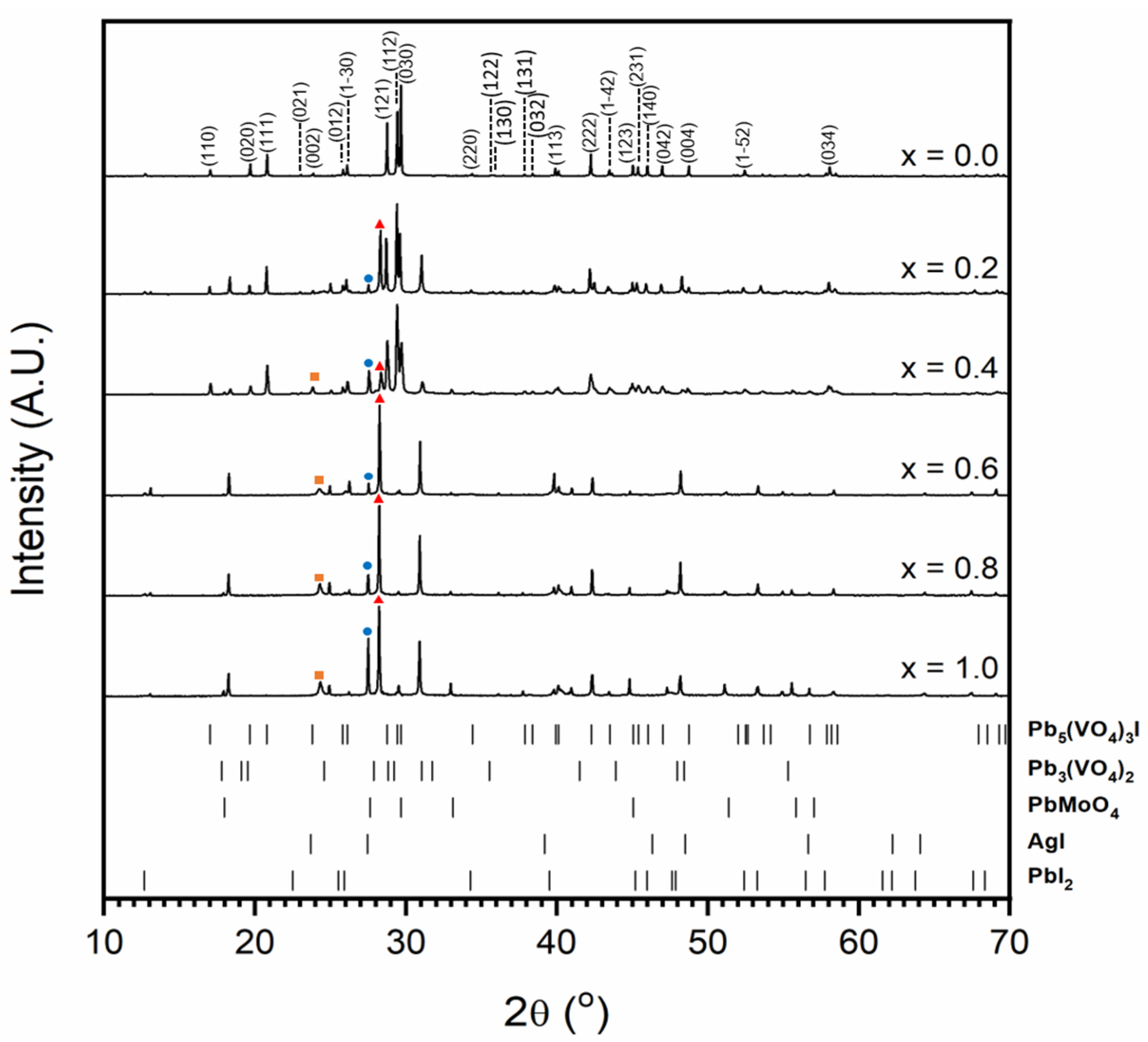
Figure 4 shows the results of X-ray diffraction for attempted syntheses in the Pb5−xAgx(VO4)3−x(MoO4)xI series; the unit cell parameters of the synthesised iodovanadinite phase are given in Table 1. As can be seen in Figure 4, even low levels of substitution, e.g., x = 0.2, resulted in the formation of secondary phases and the absence of the target iodovanadinite phase above x = 0.6. Observed secondary phases were PbI2, PbMoO4, Pb3(VO4)2 and AgI. It is evident that increasing substitution of Mo led to increased formation of the PbMoO4 phase. The incorporation of AgI was also limited, as can be seen by the increasing intensity of the associated reflections in the XRD profile.
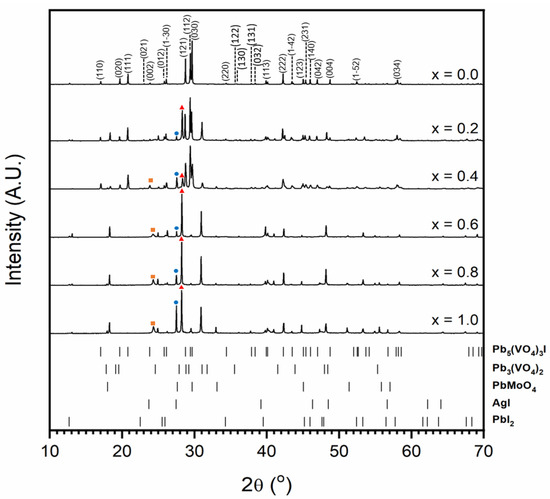
Figure 4.
Powder X-ray diffraction profiles of synthesised iodovanadinite (labelled (hkl) Miller indices) and attempted syntheses in the Pb5−xAgx(VO4)3−x(MoO4)xI series. The orange square, blue circle and red triangle indicate major AgI, PbMoO4 and Pb3(VO4)2 peaks, respectively. Tick marks show major reflections for Pb5(VO4)3I (ICSD 280065), Pb3(VO4)2 (ICSD 29360), PbMoO4 (ICSD 164725), AgI (ICSD 164959) and PbI2 (ICSD 42013). The minimum intensity of tick marks shown is 2% of their maximum intensity.

Table 1.
Unit cell parameters of iodovanadinite phase in the Pb5−xAgx(VO4)3−x(MoO4)xI series. The iodovanadinite phase did not form for x ≥ 0.6.
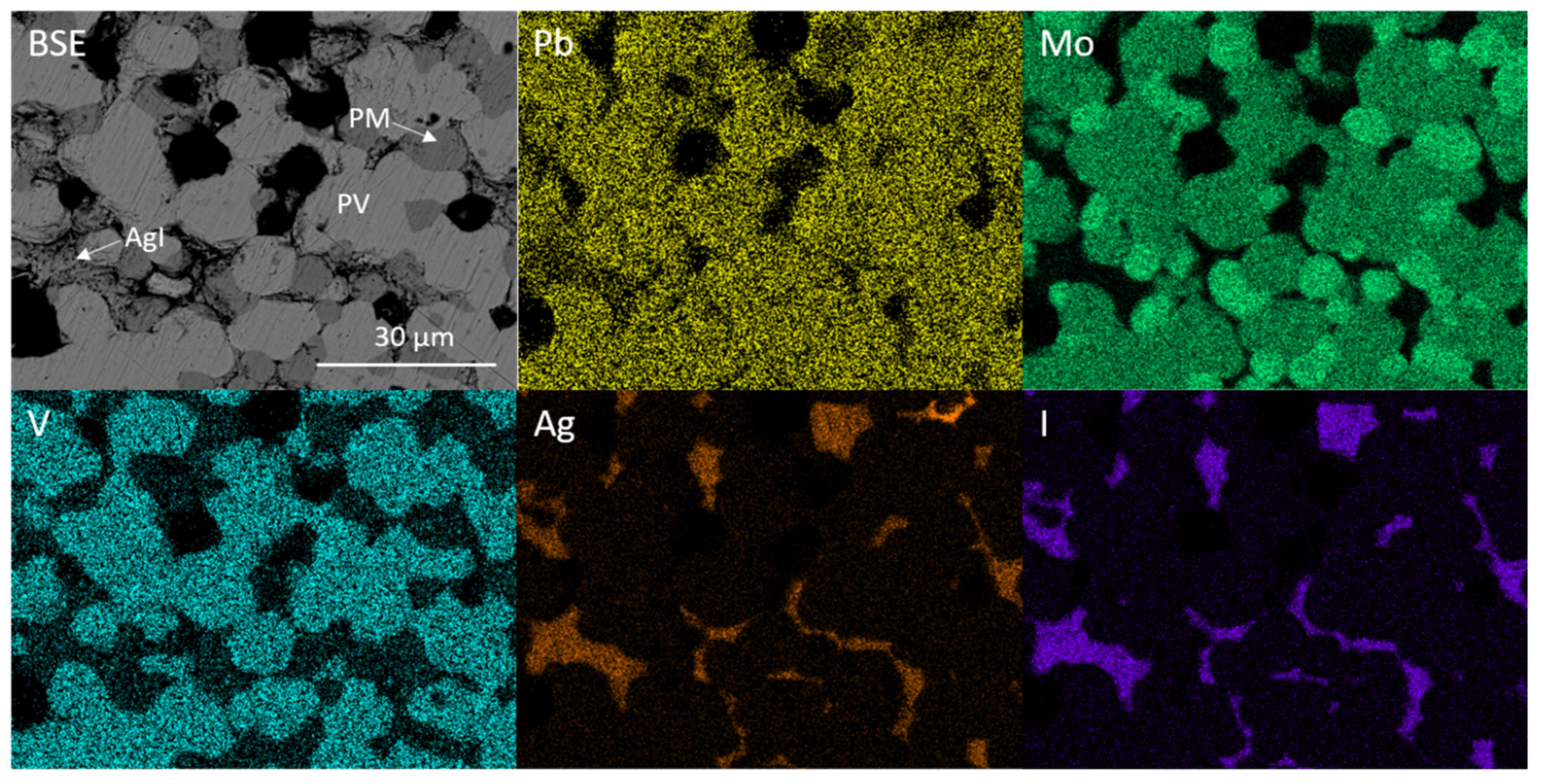
Figure 5 shows the backscattered electron (BSE) image of the targeted iodovanadinite composition Pb4Ag(VO4)2(MoO4)I. Comparing Figure 3 and Figure 5, the coupled Pb/Ag and V/Mo substitution led to the retention of AgI and the formation of PbMoO4 and Pb3(VO4)2 instead of the desired iodovanadinite.
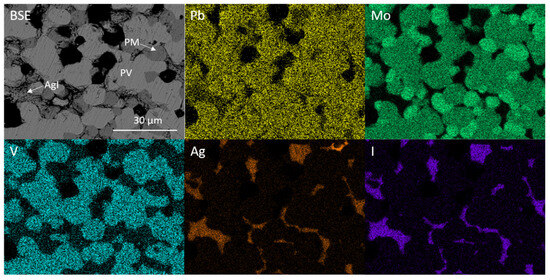
Figure 5.
BSE micrograph and corresponding EDX maps displaying elemental distribution of Pb, Mo, V, Ag and I for targeted Pb4Ag(VO4)2(MoO4)I. PV = Pb3(VO4)2, PM = PbMoO4.
When PbI2 was used as the major iodine source, although small inclusions of unreacted PbI2 were identified in the final sample, iodine incorporation into the iodovanadinite phase was observed. Conversely, when AgI was used as the major iodine source, the iodovanadinite phase was not observed, and instead, the iodine remained as AgI inclusions found between grains of the PbMoO4 and Pb3(VO4)2 phases. It is noted that Klement and Harth (1962) found that Pb3(VO4)2 did not react with NaI to form an iodovanadinite, whereas reactions with NaCl and NaBr yielded the corresponding chloro- and bromovanadinite phases [28]. This would suggest that halovanadinite formation is sensitive to which precursor is used, and therefore the immobilisation of I sequestered in AgI may benefit from an intermediate processing step to produce a more suitable precursor (such as PbI2).
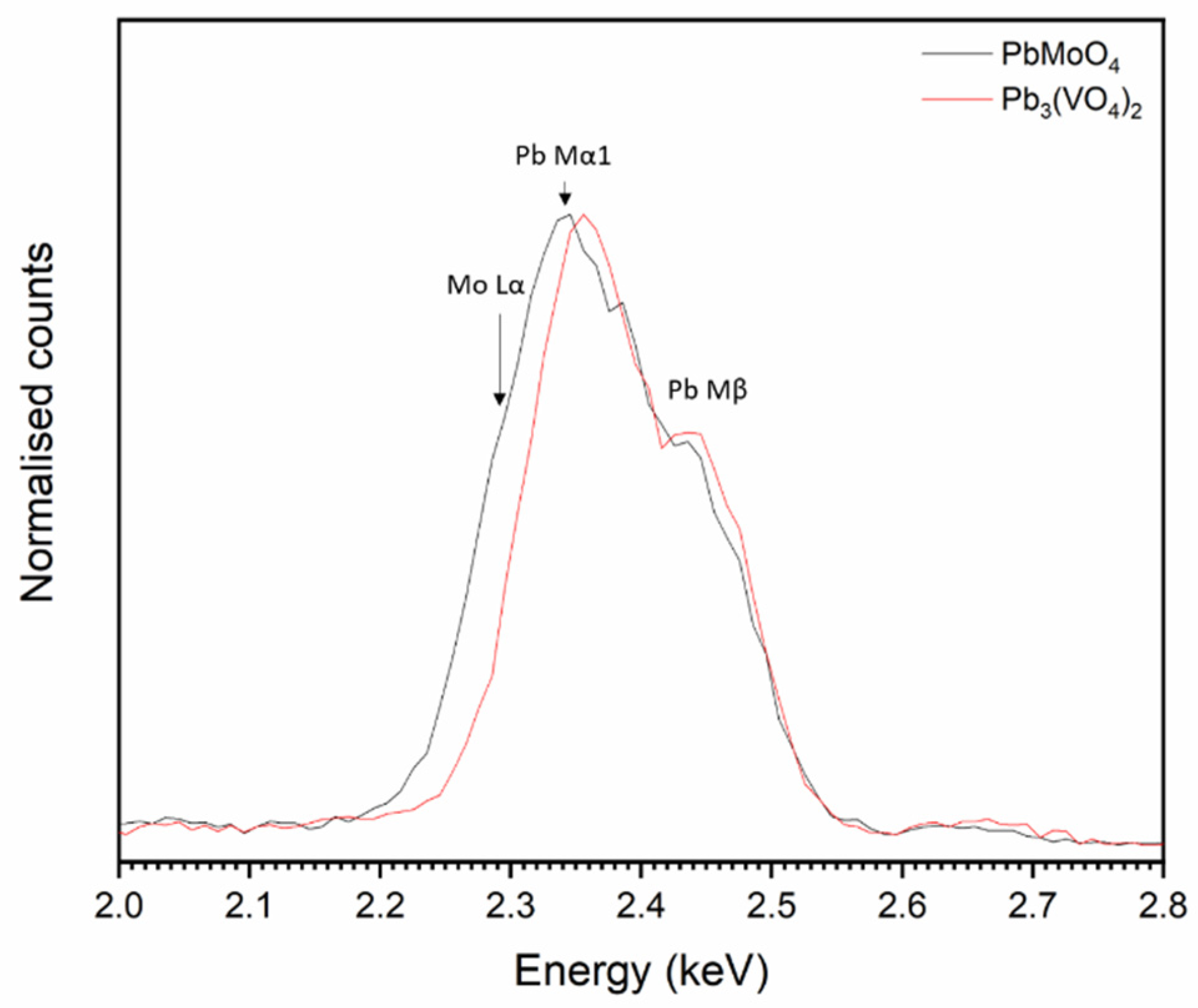
The overlap of the Pb Mα and Mo Lα emission lines (2.3423 and 2.2921 keV, respectively) makes it difficult to discern whether Mo is incorporated into both Pb3(VO4)2 and PbMoO4 by study of elemental maps (Figure 5). However, it is apparent from the contrast in backscattered electron images that two Pb-bearing phases exist, and closer inspection of EDX point spectra reveals that a negligible amount of Mo was incorporated in the Pb3(VO4)2 phase, due to the absence of characteristic X-ray emission below ~2.3 keV (see Figure 6). Regarding the formation of secondary PbMoO4, Porter et al. (2004) studied the durability of several Pb mineral phases over a wide pH range and found that the solubility of PbMoO4 was comparable to pyromorphite Pb5(PO4)3Cl, an apatite phase isostructural to the target iodovanadinite phase, at pH > 7 and superior at pH < 7 [29]. This may indicate that, although PbMoO4 is formed as an accessory phase, it could, hypothetically, act as a suitable host phase for technetium in a multiphase wasteform with suitable charge compensation. Indeed, we note that Tc-99m may be isolated from solution by quantitative co-precipitation with PbMoO4 [30].
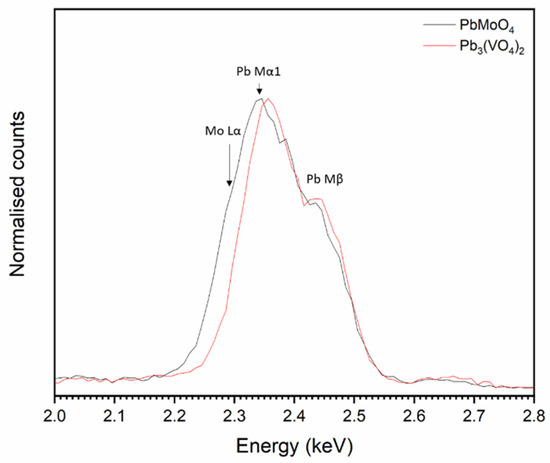
Figure 6.
Normalised, summed EDX data taken from point spectra of PbMoO4 and Pb3(VO4)2 grains.
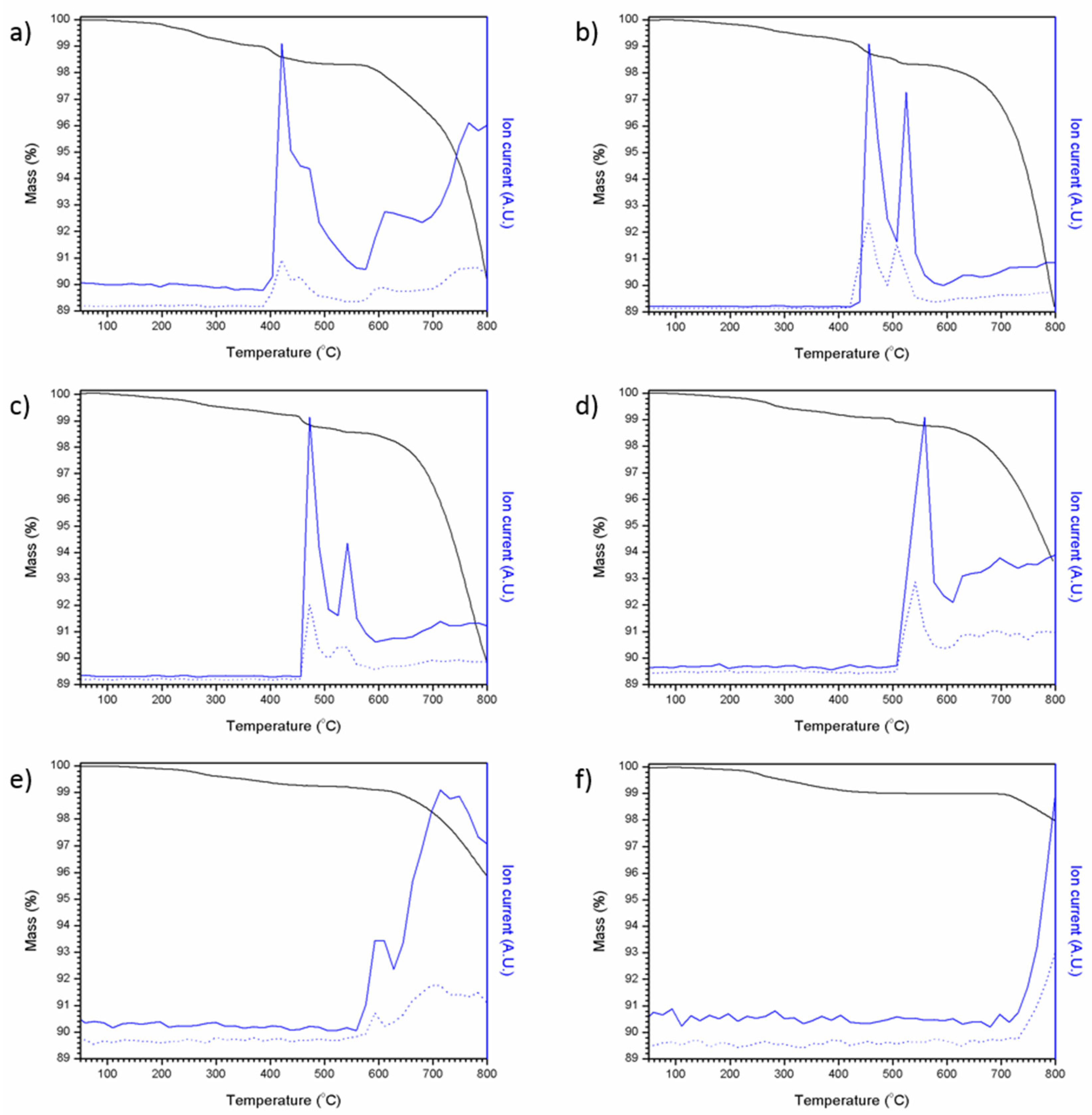
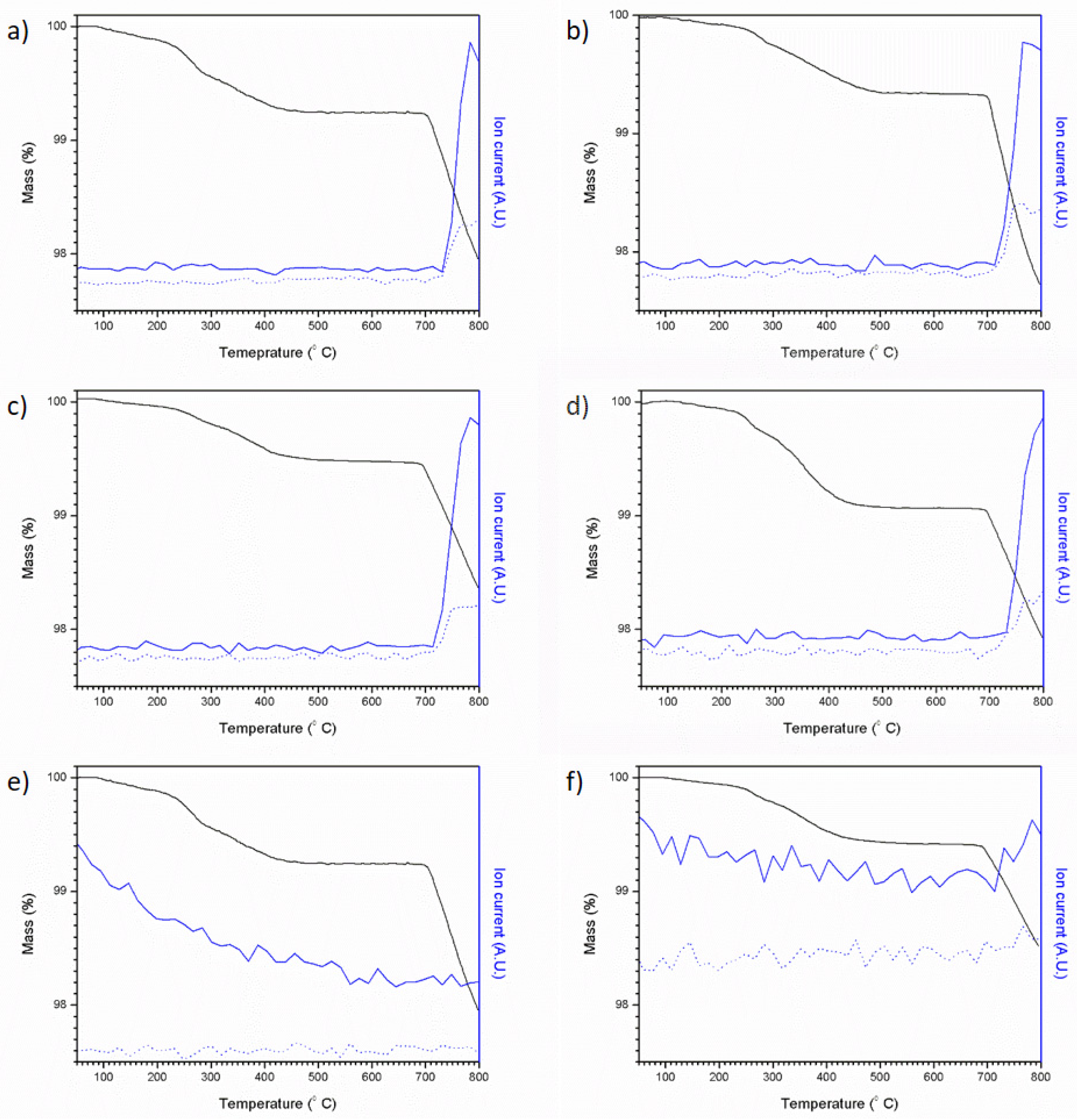
Figure 7 shows the results of coupled thermogravimetric analysis and mass spectrometry for the Pb5−xAgx(VO4)3−x(MoO4)xI series. For all compositions, an initial weight loss was observed, followed by a larger weight loss, which can be associated with the loss of iodine; an intermediate weight loss step was also observed in samples containing greater amounts of PbI2 (x = 0–0.6). Mass spectrometry of evolved gases was consistent with both monatomic and diatomic iodine (127 and 254 atomic mass units, respectively) being evolved with increasing temperature. It is apparent that, when PbI2 was used as the iodine source, mass loss was far greater under these conditions, as can be seen in Figure 7a between 390 and 800 °C in the mass spectrum. This mass loss equates to ~8.9 wt%, a value comparable with the theoretical maximum iodine loading of 8.4 wt%, indicating complete iodine loss. These results are comparable to those reported by Stennett et al. (2011) and Lu et al. (2014) [18,21]. Samples containing a greater proportion of AgI were found to lose less iodine as a result of the greater thermal stability of AgI (m.p. = 558 °C) relative to PbI2 (m.p. = 402 °C) e.g., mass loss observed for Pb4Ag(VO4)2(MoO4)I (made using AgI as the sole iodine source) was only 1.19 wt% over the same temperature range. Although PbI2 and AgI are both molten at temperatures greater than 558 °C, a proportion of iodine is retained within the melt and is liberated at higher temperatures, as indicated by the release of iodine at temperatures above the melting point of either precursor. From these results, it is clear that any processing of iodovanadinite wasteforms would benefit from the use of a closed system such as a HIP, especially those using a PbI2 precursor.
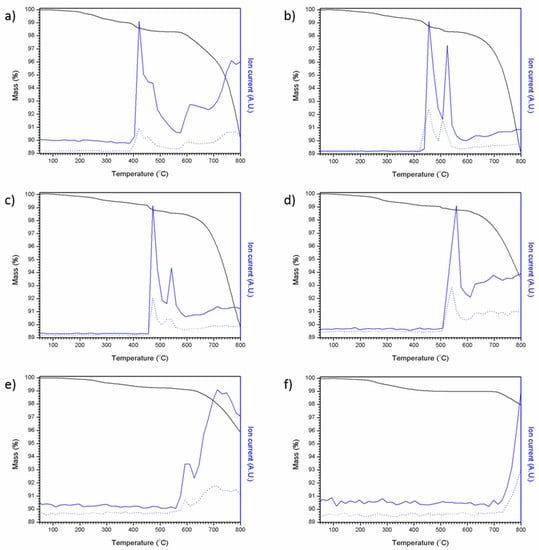
Figure 7.
TGA-MS results for attempted Pb5−xAgx(VO4)3−x(MoO4)xI syntheses: (a) x = 0.0, (b) x = 0.2, (c) x = 0.4, (d) x = 0.6, (e) x = 0.8 and (f) x = 1.0. Ion current values are normalised, solid blue line = 127 AMU and dotted blue line = 254 AMU, black line = mass %.
3.3. Pb4.5−xBaxAg0.5(VO4)3I0.5 Series
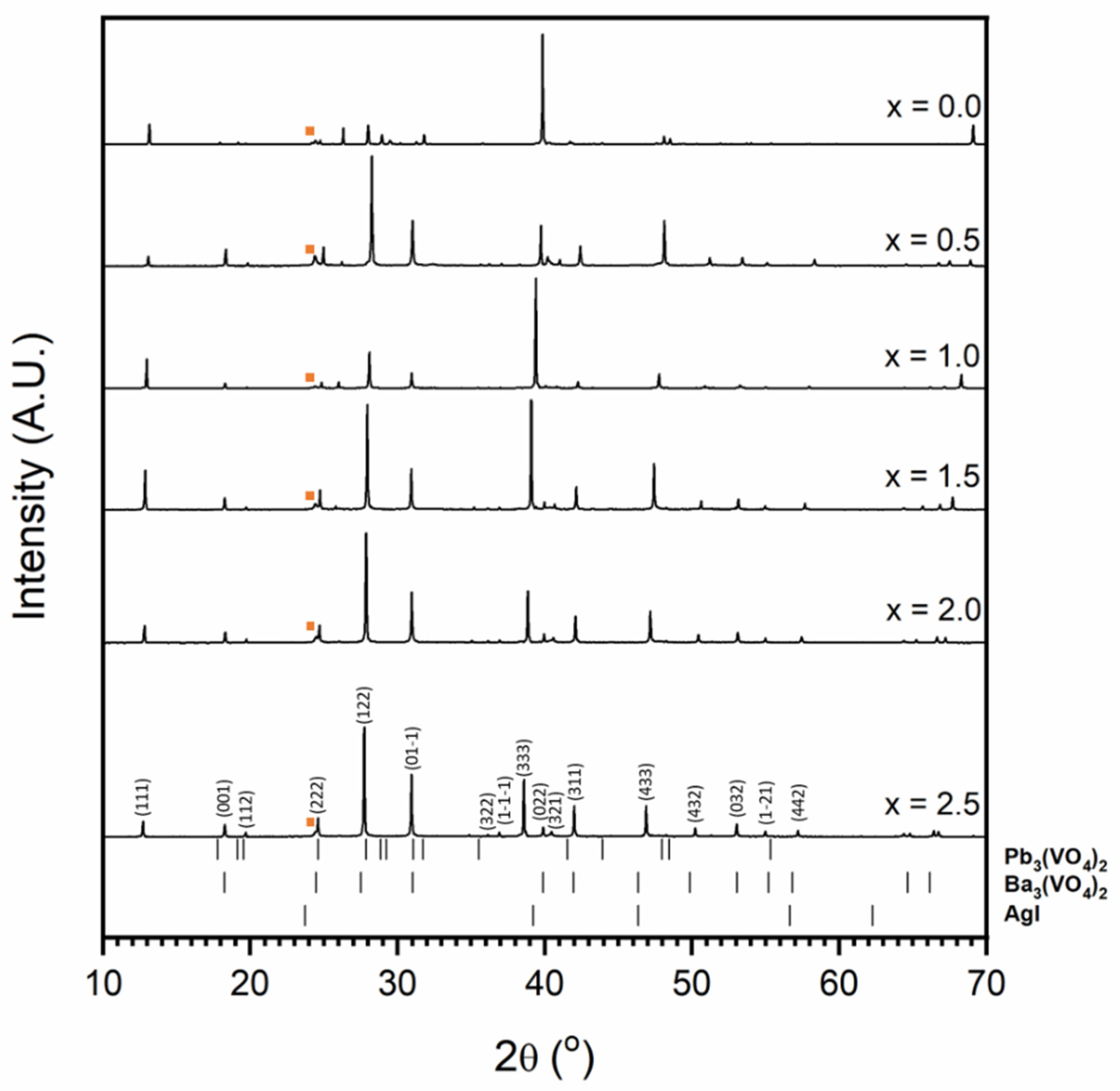
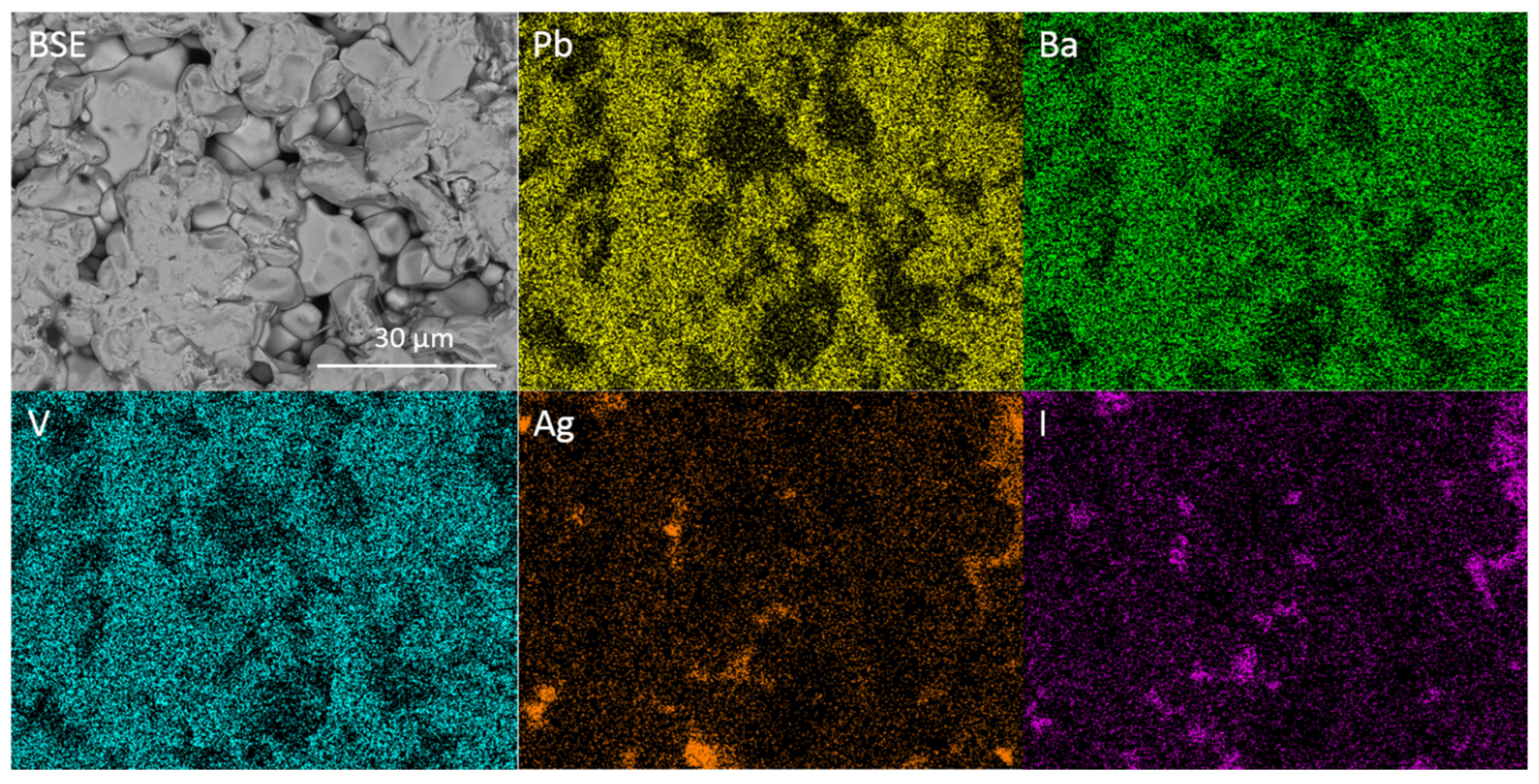
Figure 8 shows the X-ray diffraction patterns observed for the results of attempted syntheses of the Pb4.5−xBaxAg0.5(VO4)3I0.5 series. Phase-pure iodovanadinite is not formed for any composition in the series. Instead, substitution of Ba for Pb results in the formation of Ba3(VO4)2 structured material at levels as low as x = 0.5 formula units. The shift in the position of major XRD reflections with increasing Ba substitution is indicative of solid solution behaviour between Pb and Ba in the Ba3(VO4)2 structure. This is supported by the refined unit cell parameters and EDX spot analyses (Table 2) that show linear variation in the Pb:Ba ratio and that, as Ba content increases, unit cell size increases. This is as expected, given the larger ionic radius of Ba relative to Pb (rBa = 1.52 Å and 1.61 Å, and rPb = 1.4 Å and 1.49 Å, in 10 and 12-fold co-ordination, respectively). Further SEM-EDX analyses of sectioned HIP samples supported the results of XRD. Grains of reacted vanadate material separated by unreacted AgI were observed (Figure 9, Figure 10 and Figure 11). The interconnected AgI affected the sintering of the vanadate material, resulting in poorly consolidated material that is prone to pulling-out during polishing, as can be seen in the BSE images.
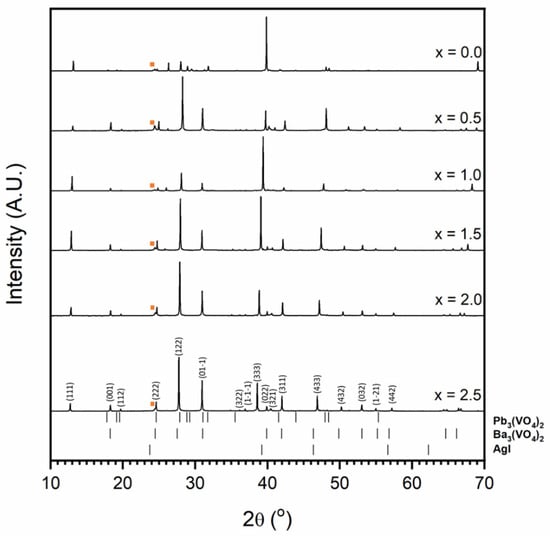
Figure 8.
Powder diffraction patterns for materials resulting from attempted syntheses in the Pb4.5−xBaxAg0.5(VO4)3I0.5 series. The orange square indicates major AgI peak. Tick marks show allowed major reflections for Pb3(VO4)2 (ICSD 29360), Ba3(VO4)2 (labelled (hkl) Miller indices; ICSD 14237) and AgI (ICSD 164959). The minimum intensity of tick marks shown is 2% of their maximum intensity.

Table 2.
Refined unit cell parameters of Ba3(VO4)2 structured phase.
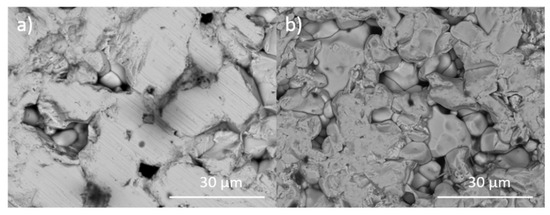
Figure 9.
Representative backscattered electron images of targeted iodovanadinites in the Pb4.5−xBaxAg0.5(VO4)3I0.5 series: (a) Pb4.5Ag0.5(VO4)3I0.5 and (b) Pb2Ba2.5Ag0.5(VO4)3I0.5.
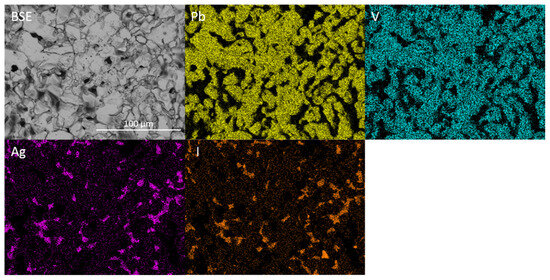
Figure 10.
BSE image and corresponding EDX maps displaying elemental distribution of Pb, V, Ag and I for target stoichiometry Pb4.5Ag0.5(VO4)3I0.5.

Figure 11.
BSE image of microstructure and corresponding EDX maps displaying elemental distribution of Pb, Ba, V, Ag and I for target stoichiometry Pb2Ba2.5Ag0.5(VO4)3I0.5.
These results are similar to those of Klement and Harth, who found that barium vanadate did not react with molten NaI or NaBr and remained as Ba3(VO4)2, whereas reaction with molten NaCl yielded the vanadinite phase [31]. Johnstone et al. attempted the synthesis of AgPb9(VO4)6I and the AgBa9(VO4)6I phase described by Uno et al. using AgI, Pb3(VO4)2 and Ba3(VO4)2 precursors [26], but showed that these compounds cannot be isolated; synthesis under the reported conditions yields a mixture of AgI and Ba3(VO4)2 or Pb3(VO4)2. Similarly, in the present study, AgI was found to not react with vanadate phases irrespective of vanadate phase stoichiometry, and variation in Pb/Ba stoichiometry did not improve the final phase assemblage. Maddrell and Abraitis found it was possible to synthesise single-phase Pb4.5Ba0.5(VO4)3I, indicating that it is likely that the use of AgI as the iodine source has affected the formation of the iodovanadinite phase, and it may be possible to synthesise Ba-containing iodovanadinite if a different precursor is used [23]. In this context, it is worth noting that the synthesis of Pb5(VO4)3I was successfully achieved using PdI2 as a sacrificial iodine source, relevant to the direct immobilisation of undissolved PdI2 residues formed in nuclear fuel reprocessing [32].
Figure 12 shows the results of coupled thermogravimetric analysis and mass spectrometry for the Pb4.5−xBaxAg0.5(VO4)3I0.5 series. The results of TGA-MS were very similar to those observed for Pb4Ag(VO4)2(MoO4)I (AgI used as the sole iodine source). An initial weight loss was followed by a plateau from ~500–700 °C and then a second weight loss in association with the evolution of monatomic and diatomic iodine. In the case of target compositions x = 2.0 and x = 2.5, the corresponding MS data, Figure 12e,f, respectively, did not evidence a signal associated with the evolution of monatomic and diatomic iodine but are consistent with the background signal observed for compositions x = 0.0, 0.5 and 1.0 (Figure 12a–c). This was due to iodine plating out in the TGA-MS transfer line in the case of compositions x = 1.5 and x = 2.0, as a result of a drop in temperature.
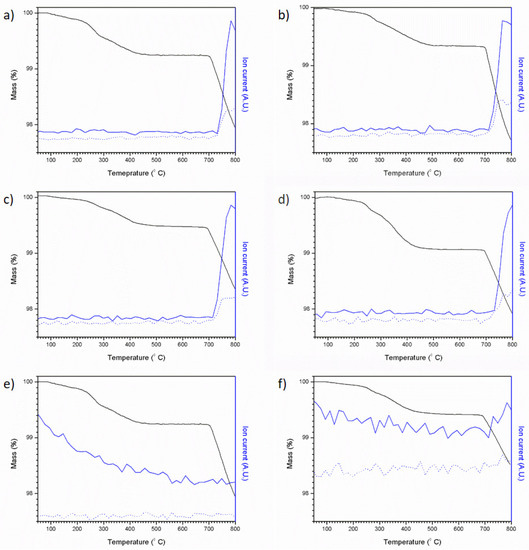
Figure 12.
TGA-MS results for attempted Pb4.5-xBaxAg0.5(VO4)3I0.5 syntheses: (a) x = 0.0, (b) x = 0.5, (c) x = 1.0, (d) x = 1.5, (e) x = 2.0 and (f) x = 2.5. Ion current values are normalised, solid blue line = 127 AMU and dotted blue line = 254 AMU, black line = mass %.
4. Conclusions
The potential for co-immobilisation of I and Tc in iodovanadinites derived from Pb5(VO4)3I was investigated in the hypothetical systems Pb5−xAgx(VO4)3-x(MoO4)xI and Pb4.5−xBaxAg0.5(VO4)3I0.5, using Mo as a Tc surrogate. Solid-state synthesis utilised a closed system and hot isostatic pressing to prevent iodine volatilisation. Importantly, the direct synthesis of Pb5(VO4)3I by hot isostatic pressing was proven successfully for the first time, utilising PbI2 as an iodine source. The HIP overpressure does not strongly influence the phase assemblage, but a higher pressure of 100 MPa proved optimal for achieving a ceramic microstructure with negligible inter-granular porosity and near single-phase Pb5(VO4)3I. Synthesis of Pb5−xAgx(VO4)3−x(MoO4)xI iodovanadinites proved unsuccessful, with even a low level of substitution (x = 0.2) resulting in the formation of secondary phases PbI2, PbMoO4, Pb3(VO4)2 and AgI. Likewise, the synthesis of the Pb4.5−xBaxAg0.5(VO4)3I0.5 iodovanadinites proved unsuccessful; the products comprised an apparently novel (Ba,Pb)3(VO4)2 solid solution plus AgI. The use of AgI as an iodine source was found to be problematic, with negligible incorporation into the target iodovanadinite phase observed. Although this investigation did not successfully demonstrate co-immobilisation of I and Tc in the iodovanadinite structure, with Mo as a Tc surrogate, the approach may have some merit for further development, targeting a composite Pb5(VO4)3I–PbMoO4 ceramic, given that PbMoO4 has been shown to incorporate Tc. Nevertheless, this work has taken an important step forward in demonstrating the first direct synthesis of Pb5(VO4)3I as a wasteform for I-129 by hot isostatic pressing.
Author Contributions
Conceptualization, D.J.B. and N.C.H.; Methodology, D.J.B. and N.C.H.; Formal analysis, D.J.B., E.V.J., M.C.S. and N.C.H.; Investigation, D.J.B., E.V.J., M.C.S. and C.L.C.; Writing—original draft, D.J.B.; Writing—review and editing, D.J.B., E.V.J. and N.C.H.; Supervision, E.V.J., M.C.S., C.L.C. and N.C.H.; Funding acquisition, N.C.H. and C.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to EPSRC (Engineering and Physical Sciences Research Council) for sponsoring this research under grant references EP/M026566/1, EP/S032959/1, EP/S011935/1 and EP/S01019X/1. NCH is grateful to the Royal Academy of Engineering and the Nuclear Decommissioning Authority for the partial sponsorship of this research. CLC is grateful to EPSRC for the award of an Early Career Fellowship under grant reference EP/N017374/1. The research reported here was performed at the MIDAS/HADES Facility at the University of Sheffield, which was established with support from the DECC/BEIS and EPSRC under grant reference EP/T011424/1 [33].
Data Availability Statement
The raw data that supports these findings cannot be shared at this time as the data also form part of an ongoing study.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wilson, P.D. The Nuclear Fuel Cycle: From Ore to Waste; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Riley, B.J.; Vienna, J.D.; Strachan, D.M.; McCloy, J.S.; Jerden, J.L., Jr. Materials and processes for the effective capture and immobilization of radioiodine: A review. J. Nucl. Mater. 2016, 470, 307–326. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Montoya, E.; Sun, S.K.; George, J.; Kirk, C.; Dixon Wilkins, M.C.; Weck, P.F.; Kim, E.; Knight, K.S.; Hyatt, N.C. Crystal and electronic structures of A2NaIO6 periodate double perovskites (A = Sr, Ca, Ba): Candidate wasteforms for I-129 immobilization. Inorg. Chem. 2020, 59, 18407–18419. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Turner, J.; Chong, S.; Riley, B.J. Review of recent developments in iodine wasteform production. Front. Chem. 2022, 10, 1043653. [Google Scholar] [CrossRef] [PubMed]
- Umadevi, K.; Mandal, D. Performance of radio-iodine discharge control methods of nuclear reprocessing plants. J. Environ. Radioact. 2021, 234, 106623. [Google Scholar] [CrossRef] [PubMed]
- Buck, E.C.; Mausolf, E.J.; McNamara, B.K.; Soderquist, C.Z.; Schwantes, J.M. Sequestration of radioactive iodine in silver-palladium phases in commercial spent nuclear fuel. J. Nucl. Mater. 2016, 482, 229–235. [Google Scholar] [CrossRef]
- Chapman, K.W.; Chupas, P.J.; Nenoff, T.M. Radioactive iodine capture in silver-containing mordenites through nanoscale silver iodide formation. J. Am. Chem. Soc. 2010, 132, 8897–8899. [Google Scholar] [CrossRef]
- Olsen, Y.S.; i Batlle, J.V. A model for the bioaccumulation of 99Tc in lobsters (Homarus gammarus) from the West Cumbrian coast. J. Environ. Radioact. 2003, 67, 219–233. [Google Scholar] [CrossRef]
- Donald, I.W. Waste Immobilization in Glass and Ceramic Based Hosts: Radioactive, Toxic and Hazardous Wastes; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Schwochau, K. Technetium: Chemistry and Radiopharmaceutical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Poineau, F.; Du Mazaubrun, J.; Ford, D.; Fortner, J.; Kropf, J.; Silva, G.C.; Smith, N.; Long, K.; Jarvinen, G.; Czerwinski, K.R. Uranium/technetium separation for the UREX process–synthesis and characterization of solid reprocessing forms. Radiochim. Acta 2008, 96, 527–533. [Google Scholar] [CrossRef]
- Audubert, F.; Carpena, J.; Lacout, J.L.; Tetard, F. Elaboration of an iodine-bearing apatite iodine diffusion into a Pb3(VO4)2 matrix. Solid State Ionics 1997, 95, 113–119. [Google Scholar] [CrossRef]
- White, T.J.; d Dong, Z. Structural derivation and crystal chemistry of apatites. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2003, 59, 1–16. [Google Scholar] [CrossRef]
- Schriewer, M.S.; Jeitschko, W. Preparation and crystal structure of the isotypic orthorhombic strontium perrhenate halides Sr5(ReO5)3X (X = Cl, Br, I) and structure refinement of the related hexagonal apatite-like compound Ba5(ReO5)3Cl. J. Solid State Chem. 1993, 107, 1–11. [Google Scholar] [CrossRef]
- Audubert, F.; Savariault, J.M.; Lacout, J.L. Pentalead tris (vanadate) iodide, a defect vanadinite-type compound. Acta Crystallogr. C Struct. Chem. 1999, 55, 271–273. [Google Scholar] [CrossRef]
- Baud, G.; Besse, J.P.; Sueur, G.; Chevalier, R. Structure de nouvelles apatites au rhenium contenant des anions volumineux: Ba10(ReO5)6X2 (X = Br, I). Mater. Res. Bull. 1979, 14, 675–682. [Google Scholar] [CrossRef]
- Le Gallet, S.; Campayo, L.; Courtois, E.; Hoffmann, S.; Grin, Y.; Bernard, F.; Bart, F. Spark plasma sintering of iodine-bearing apatite. J. Nucl. Mater. 2010, 400, 251–256. [Google Scholar] [CrossRef]
- Stennett, M.C.; Pinnock, I.J.; Hyatt, N.C. Rapid synthesis of Pb5(VO4)3I, for the immobilisation of iodine radioisotopes, by microwave dielectric heating. J. Nucl. Mater. 2011, 414, 352–359. [Google Scholar] [CrossRef]
- Redfern, S.A.T.; Smith, S.E.; Maddrell, E.R. High-temperature breakdown of the synthetic iodine analogue of vanadinite, Pb5 (VO4)3I: An apatite-related compound for iodine radioisotope immobilization? Miner. Mag. 2012, 76, 997–1003. [Google Scholar] [CrossRef]
- Suetsugu, Y. Synthesis of lead vanadate iodoapatite utilizing dry mechanochemical process. J. Nucl. Mater. 2014, 454, 223–229. [Google Scholar] [CrossRef]
- Lu, F.; Yao, T.; Xu, J.; Wang, J.; Scott, S.; Dong, Z.; Ewing, R.C.; Lian, J. Facile low temperature solid state synthesis of iodoapatite by high-energy ball milling. RSC Adv. 2014, 4, 38718–38725. [Google Scholar] [CrossRef]
- Zhang, M.; Maddrell, E.R.; Abraitis, P.K.; Salje, E.K.H. Impact of leach on lead vanado-iodoapatite [Pb5(VO4)3I]: An infrared and Raman spectroscopic study. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2007, 137, 149–155. [Google Scholar] [CrossRef]
- Maddrell, E.R.; Abraitis, P.K. A comparison of wasteforms and processes for the immobilisation of iodine-129. MRS Proc. 2003, 807, 565–570. [Google Scholar] [CrossRef]
- Guy, C.; Audubert, F.; Lartigue, J.E.; Latrille, C.; Advocat, T.; Fillet, C. New conditionings for separated long-lived radionuclides. Comptes Rendus Phys. 2002, 3, 827–837. [Google Scholar] [CrossRef]
- Uno, M.; Kosuga, A.; Masuo, S.; Imamura, M.; Yamanaka, S. Thermal and mechanical properties of AgPb9(VO4)6I and AgBa9(VO4)6I. J. Alloys Compd. 2004, 384, 300–302. [Google Scholar] [CrossRef]
- Johnstone, E.V.; Bailey, D.J.; Stennett, M.C.; Heo, J.; Hyatt, N.C. On the existence of AgM9(VO4)6I (M = Ba, Pb). RSC Adv. 2017, 7, 49004–49009. [Google Scholar] [CrossRef]
- Yao, T.; Lu, F.; Sun, H.; Wang, J.; Ewing, R.C.; Lian, J. Bulk iodoapatite ceramic densified by spark plasma sintering with exceptional thermal stability. J. Am. Ceram. Soc. 2014, 97, 2409–2412. [Google Scholar] [CrossRef]
- Klement, R.; Harth, R. Über Bleiapatite und tertiäres Bleiphosphat. Z. Für Anorg. Allg. Chem. 1962, 314, 238–244. [Google Scholar] [CrossRef]
- Porter, S.K.; Scheckel, K.G.; Impellitteri, C.A.; Ryan, J.A. Toxic metals in the environment: Thermodynamic considerations for possible immobilization strategies for Pb, Cd, As, and Hg. Crit. Rev. Environ. Sci. Technol. 2004, 34, 495–604. [Google Scholar] [CrossRef]
- Ichikawa, F. The isolation of technetium by coprecipitation or anion exchange. Bull. Chem. Soc. Jpn. 1959, 32, 1126–1129. [Google Scholar] [CrossRef]
- Klement, R.; Harth, R. Das Verhalten von tertiären Erdalkaliphosphaten, -arsenaten und -vanadaten in geschmolzenen Halogeniden. Chem. Ber. 1961, 94, 1452–1456. [Google Scholar] [CrossRef]
- Johnstone, E.V.; Bailey, D.J.; Lawson, S.; Stennett, M.C.; Corkhill, C.L.; Kim, M.; Heo, J.; Matsumura, D.; Hyatt, N.C. Synthesis and characterization of iodovanadinite using PdI2, an iodine source for the immobilisation of radioiodine. RSC Adv. 2020, 10, 25116–25124. [Google Scholar] [CrossRef]
- Hyatt, N.C.; Corkhill, C.L.; Stennett, M.C.; Hand, R.J.; Gardner, L.J.; Thorpe, C.L. The HADES facility for high activity decommissioning engineering & science: Part of the UK national nuclear user facility. IOP Conf. Ser. Mater. Sci. 2020, 818, 012022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).