1. Introduction
High-temperature proton conductors (HTPCs) have increasingly attracted attention because of their high proton transference number and low activation energy for proton conduction [
1,
2]. Various applications can be targeted for HTPC-based devices. Examples include protonic ceramic fuel cells (PCFCs) for electricity generation [
3,
4,
5], protonic ceramic electrolysis cells (PCECs) for energy storage [
6,
7], membranes for hydrogen separation [
8], membrane reactors for fuels upgrading (e.g., methane dehydroaromatization–MDA) [
9,
10], and hydrogen compression [
11].
The MDA reaction consists of a single-step, non-oxidative route to produce hydrogen and benzene from methane as given in Equation (1):
The reaction is typically carried out around 700 °C using a catalyst consisting of molybdenum carbide nanoparticles supported on shape-selective zeolites such as ZSM-5 and MCM-22 [
12,
13,
14]. However, the methane conversion is thermodynamically limited to about ~12% efficiency at 700 °C. The equilibrium can be shifted toward higher yields if hydrogen is removed from the mixture of products, which can be achieved using a HTPC membrane. The process can be promoted by applying an electric potential to drive the hydrogen across the membrane [
9] and is referred to as a catalytic membrane reactor (CMR). Morejudo et al. [
10] performed CMR measurements using a 20–30-µm-thick, dense layer of BaZr
0.7Ce
0.2Y
0.1O
3-δ (BZCY72) electrolyte on a porous BZCY72-Ni cermet, with a Cu anode for the oxidation of hydrogen to protons. Patki et al. [
15,
16,
17] developed Cu electrodes on BZCY72 substrates by electroless plating (ELP) to overcome the major drawbacks of organometallic copper paste, which include thickness (>15 µm) and delamination problems. One-µm-thick Cu electrodes with excellent adhesion to the BZCY72 substrates were obtained by ELP using three catalysts: Pd, Ru, and Cu. The development of the ELP procedure was initially performed on BZCY72 discs (2 cm in diameter, symmetric cell configuration [
17]) and the process was later transferred to tubes [
15]: BaZr
0.7Ce
0.2Y
0.1O
3-δ (BZCY72) electrolyte on a porous BZCY72-Ni support (referred to as BZCY72/Ni//BZCY72).
ELP Cu electrodes with a Pd catalyst deposited on BZCY72/Ni//BZCY72 tubes were tested in similar conditions as the one reported by Morejudo et al. [
10]. High faradaic efficiency of the BZCY membrane (80–85%) using this electroless-deposited copper layer was observed [
15]. The decrease in faradaic efficiency reported in [
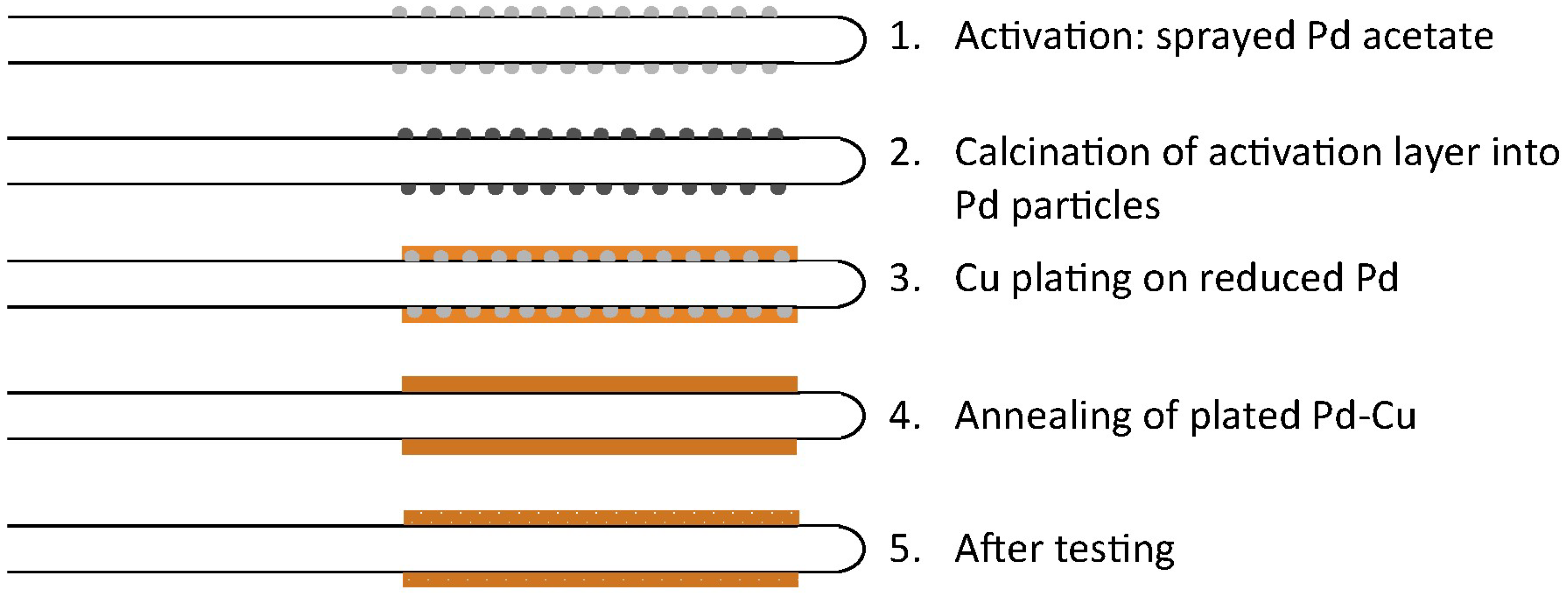
15] with increasing the current density was explained by the history of the BZCY/Ni//BZCY assembly (cerium diffusion from support to electrolyte during sintering) and was not related to the Cu electrode performance. To better understand the role that this electroless copper deposition process plays in the performance, we thoroughly studied the microstructure and composition of the Pd/Cu electrodes at the different stages of the deposition process and after testing (illustrated in
Figure 1) using a variety of characterization techniques including: time-of-flight secondary-ion mass spectrometry (TOF-SIMS), transmission electron microscopy (TEM), scanning spreading-resistance microscopy (SSRM), and atom-probe tomography (APT). By using a multitechnique, multiscale characterization approach, we can best assess the reason for the excellent performance observed with the ELP copper electrodes. The TEM can reveal the atomic-scale structure, TOF-SIMS can provide a nanoscale picture of the lateral distribution of dopants and impurities with 100-nm resolution, correspondingly, SSRM can provide local electronic resistivity at the nanoscale, and APT can elucidate the three-dimensional composition of a material with unparalleled, nanometer-scale resolution.
2. Materials and Methods
Tubular substrates (BZCY72/Ni//BZCY72) were obtained from CoorsTek Inc. (Golden, Colorado, CO, USA) and coated with Pd/Cu ELP electrodes as described by Patki et al. [
15,
16,
17]
. To better understand the role that this electroless copper deposition process plays in the performance, the microstructure and composition of the Pd/Cu electrodes at the different stages of the deposition process and after testing (illustrated in
Figure 1) was thoroughly studied using a variety of characterization techniques outlined in the introduction. Pd acetate in chloroform was sprayed on the tubular substrate (Step 1 in
Figure 1). The calcination procedure of the Pd acetate catalyst (Step 2 in
Figure 1) was slightly different than that reported by Patki et al. [
15,
16,
17]
. The tubes were heated in a dual-atmosphere furnace set-up: dilute hydrogen gas (5% H
2 in Ar) was supplied to the inner part of the tube BZCY72-Ni. The gas supplied to the outside of the tube was first nitrogen, while the tube was heated to 800 °C for 15 min then cooled to 400 °C. Subsequently, the gas was switched to air while the temperature was held at 400 °C for 1 h before cooling to room temperature. All the heating and cooling rates were kept at 5 °C∙min
−1. The activation layer was then reduced and plated (1 h in a heating bath kept at 55 °C) (Step 3 in
Figure 1). The plated sample was then annealed for 4 h at 750 °C in hydrogen (Step 4 in
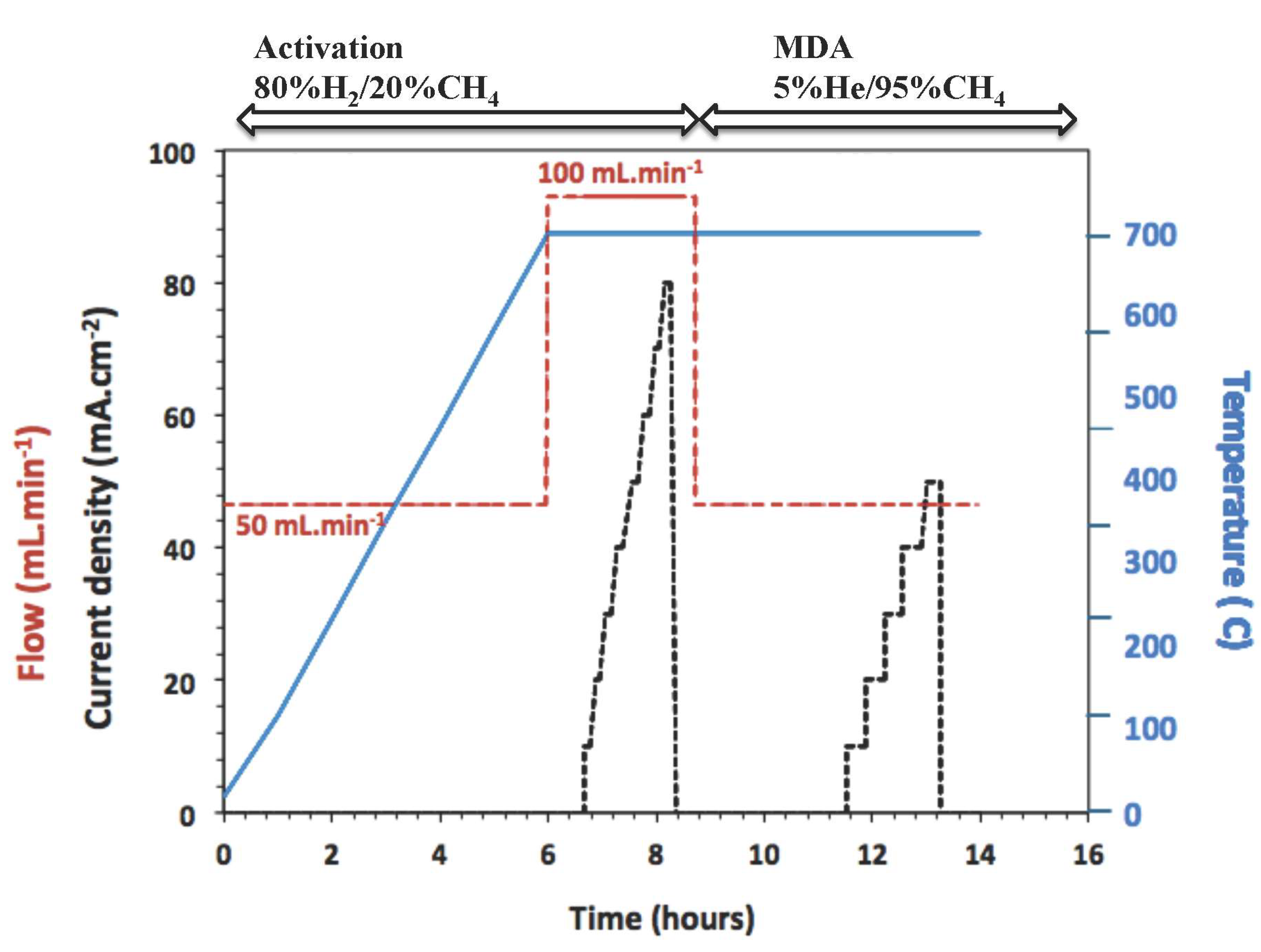
Figure 1) and tested as described in
Figure 2; the sample was heated in a catalytic membrane reactor (i.e., with zeolite catalyst on the outer annulus) under 80% hydrogen/20% methane up to 700 °C. The atmosphere was then switched to 95% methane/5% helium at 700 °C and the hydrogen fluxes were measured under MDA conditions at current densities varying from 0 to 80 mA·cm
−2. After testing, coking was observed between the BZCY72 membrane and the Pd-Cu electrode, discussed in detail later in the manuscript.
Samples were collected at the various stages of the processing as described in
Figure 1 for in-depth characterization. In some cases, the samples were kept as fractured cross-sections; in other cases, the samples were mounted in epoxy and polished down to 1 µm for better finish.
TOF-SIMS measurements were completed using an ION-TOF TOF-SIMS V instrument (Ion-TOF GmbH, Muenster, Germany). SIMS is a powerful analytical technique to determine elemental and isotopic distributions in solids, both organic and inorganic [
18,
19,
20,
21]. TOF-SIMS imaging, spectroscopy-mode profiling, and 3-D tomography were used to investigate the role that the palladium activation layer plays in the performance of the membranes which used the electroless plated copper electrode. Secondary ions for analysis were created by a 3-lens 30-keV BiMn ion gun. 3-D tomography was completed using a Bi
3++ primary ion-beam cluster (100-ns pulse width, 0.1-pA pulsed beam current); this measurement mode is capable of better than 100-nm lateral-resolution chemical imaging. Standard depth profiling was completed using a Bi
+ primary ion beam operated in bunched mode (10-ns pulse width, 1-pA pulsed beam current). An oxygen ion beam with a variable energy from 600 eV to 3 keV was used as the sputtering beam (sputtering current 3–30 nA). After measurements were completed, the sputter craters were measured using an interference light microscope to correlate the sputter time scale to sputter depth.
Secondary-electron micrographs were collected on cross-sections with a JEOL JSM-7000F (JEOL, Tokyo, Japan) scanning electron microscope (SEM). TEM cross-section specimens were prepared using a lift-out technique in an FEI Helios NanoLab 600i. Most of the TEM analyses and energy-dispersive X-ray spectroscopy (EDS) mapping were performed on an FEI Talos F200X instrument (Thermo Fisher Scientific, Hillsboro, Oregon, USA) operated at a 200-kV accelerating voltage. TEM imaging of the APT specimens was performed on a Philips CM200 operated at 200 keV.
SSRM local resistivity imaging is based on the contact-mode atomic force microscope (AFM, Veeco D5000 with Nanoscope V controller). A bias voltage is applied between the probe and sample, and the current flowing through the probe is measured by a logarithm-scale amplifier [
22,
23]. The probe-sample contact resistance was suppressed by pressing the highly doped diamond-coated tip (Bruker Nano, Santa Barbara, CA, USA) into the sample with a large force (~μN) and applying a high bias voltage (10 V) between the probe and sample. This creates a localized area below the probe that is highly strained and creates dangling bonds, so that the contact resistance is sufficiently lowered. The local spreading resistance just below the probe then dominates the overall measured resistance; the local resistivity of the sample is measured with a resolution comparable to the probe size (~40 nm in this case).
Specimens for APT were milled with a focused ion beam (FIB) in an FEI Helios Nanolab 600i [
24]. These specimens were prepared such that consecutive TEM imaging and APT analysis of the tips could be performed [
25]. The APT analyses were conducted using laser pulsing in a Cameca LEAP 4000X Si instrument with a 90-mm flight path length at a temperature of 51.4 K, pulse rates of 500–625 kHz, laser energies of 10–55 pJ, a voltage range of 2–8 kV, and detection rate of 5–8 ions per 1000 laser pulses.
3. Results and Discussion
To investigate the root-cause mechanisms for the observed near-doubling of the faradaic efficiency in the ELP coated copper electrode samples compared to standard painted electrodes, samples from all steps of the ELP process outlined in
Figure 1 were investigated with a variety of characterization techniques. We focus the discussion on those results which are most revealing to elucidate the mechanism for the observed performance increase.
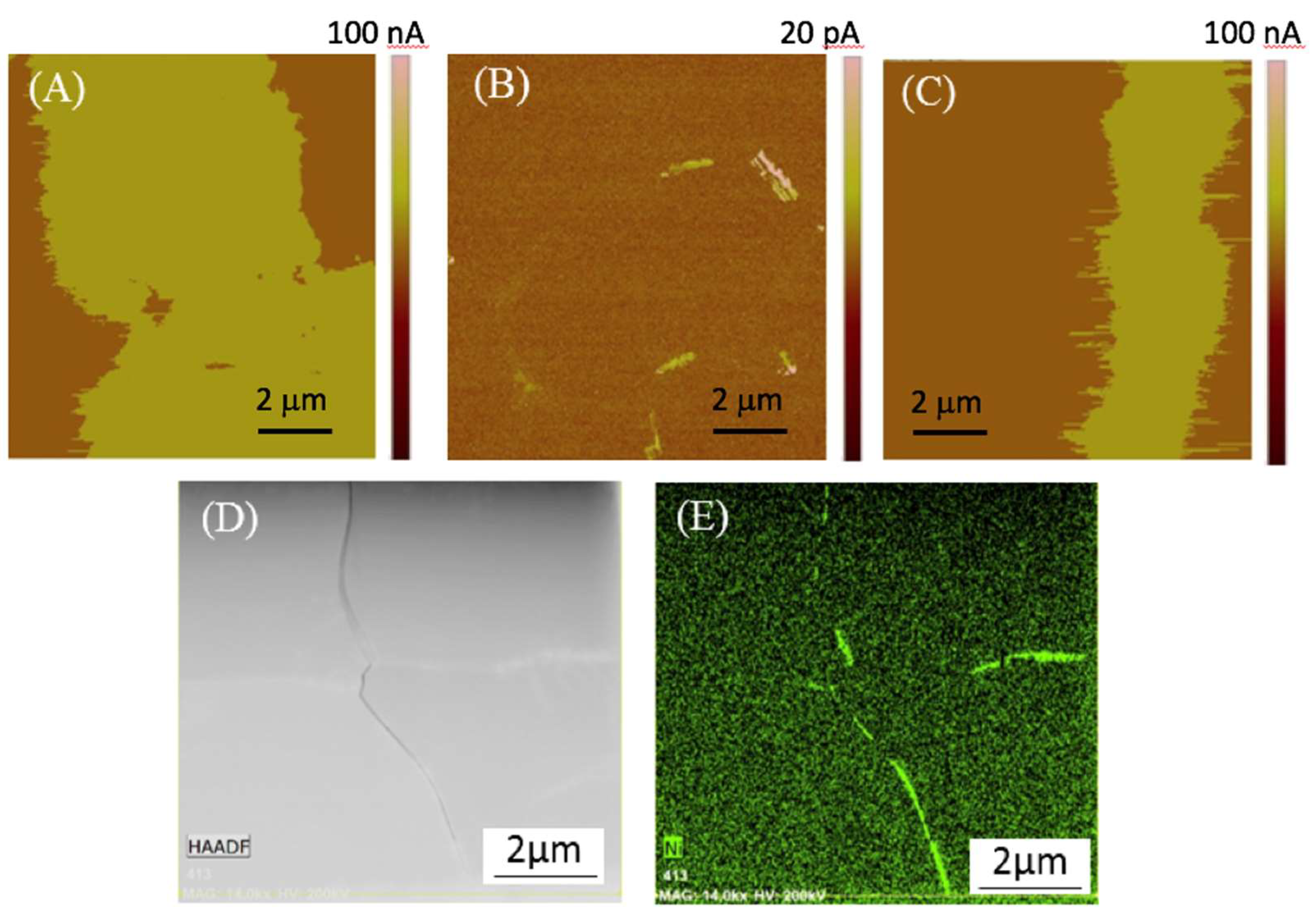
Figure 3 summarizes a series of SSRM maps taken on a polished cross-section of a membrane ready for testing (Step 4 in
Figure 1), as well as some accompanying TEM results of similar areas. The SSRM maps (
Figure 3A–C) show areas of high conductivity as bright (Ni phase) and areas of low conductivity as darker (BZCY phase).
Figure 3A–C represent the two-phase composite scaffold, the dense BZCY electrolyte, and the BZCY/copper electrode interface, respectively. In
Figure 3B, a bright, high-conductivity phase can be seen surrounding a less-conductive BZCY grain.
Figure 3D,E are the high-angle annular dark-field (HAADF) scanning TEM image and resulting EDS map for a similar area of the TEM EDS map; results confirm that the high-conductivity phase is nickel-rich at the grain boundaries. These results are consistent with prior observations on samples prepared by solid-state reactive sintering using nickel oxide as a sintering aid: nickel precipitates at the grain-boundaries upon reduction [
26,
27]. This illustrates the strength of the multitechnique approach taken in this work—that by a combination of techniques, we can obtain nanoscale information about the structure, chemistry, and electrical properties of the material.
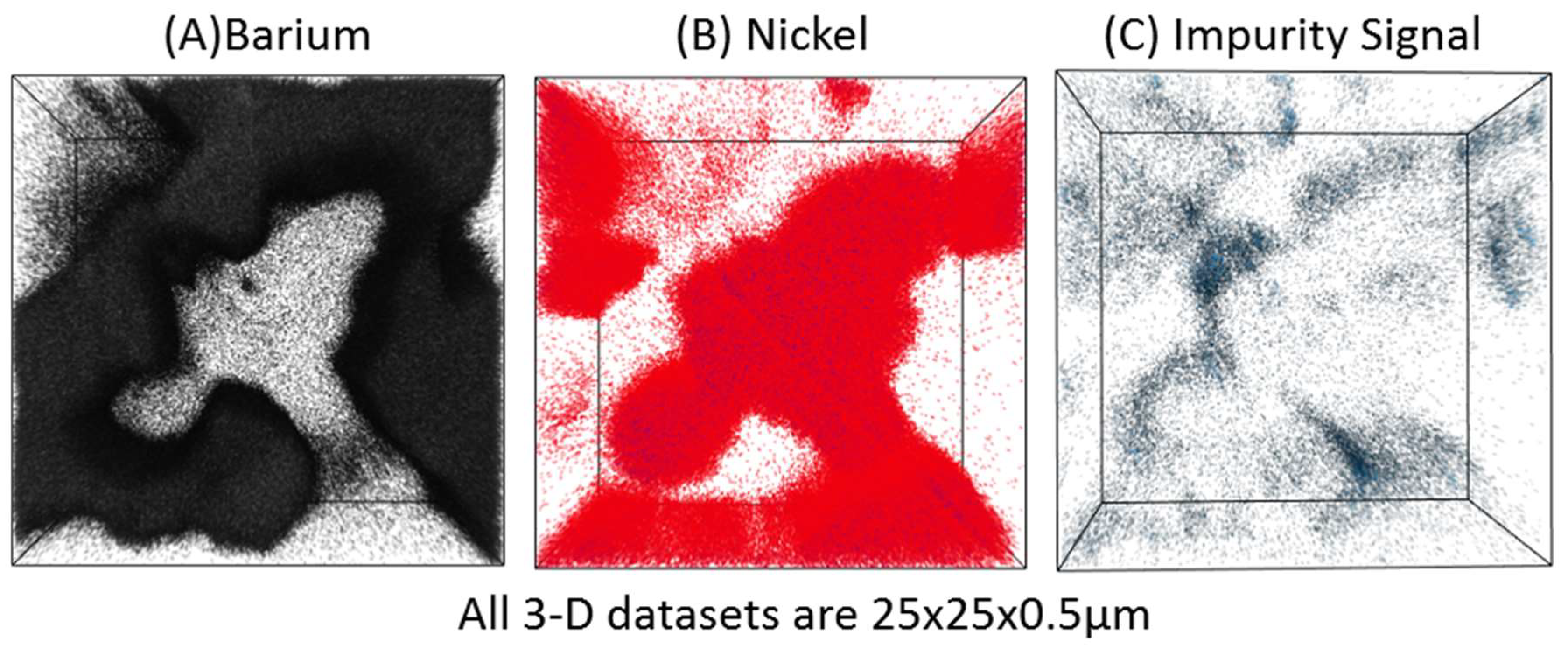
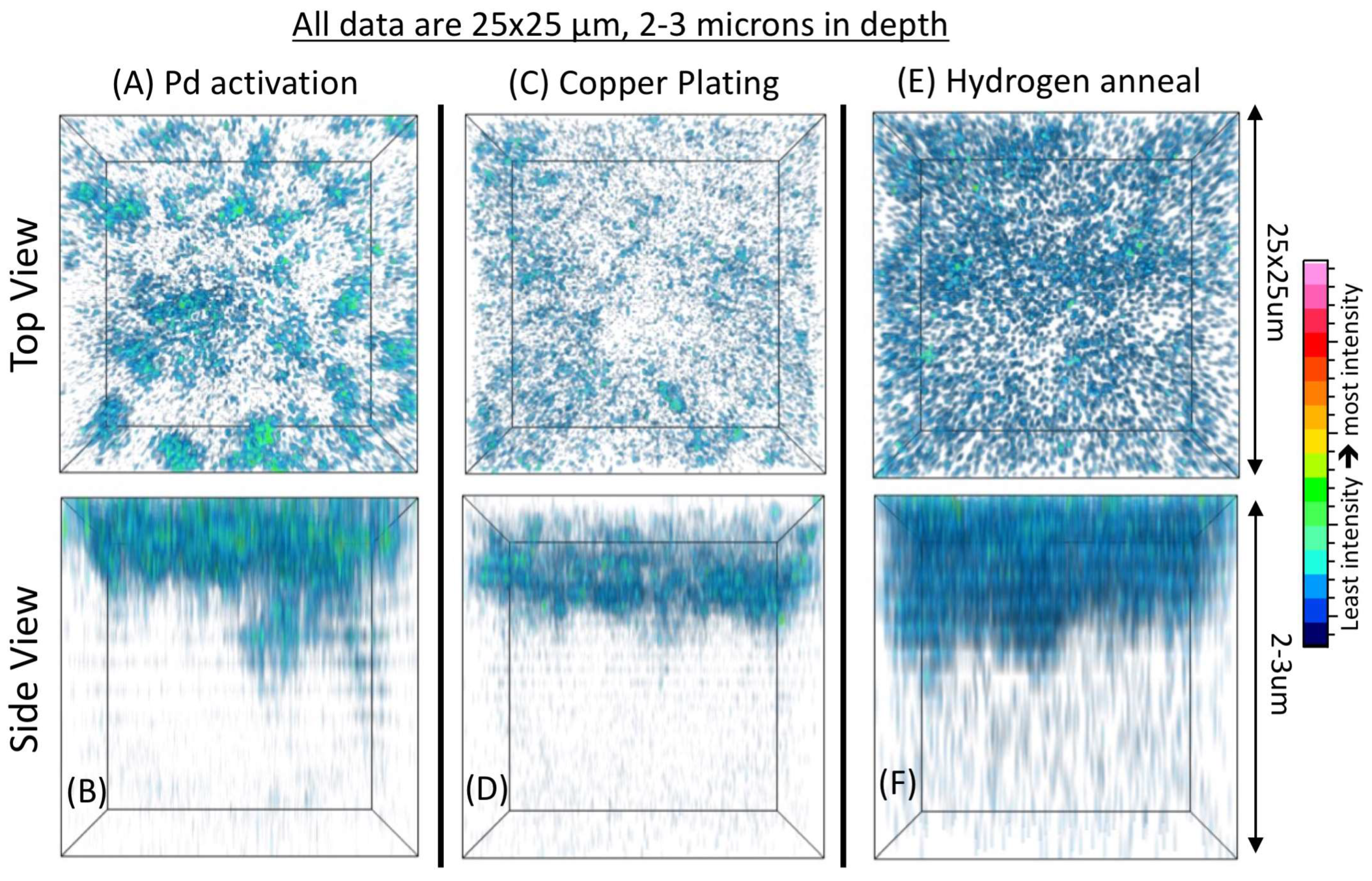
TOF-SIMS 3-D tomography is accomplished by collecting a high-resolution chemical image of the sample, then sputtering away some tens to hundreds of nanometers of material, then imaging again. Through these subsequent imaging and sputtering steps, one can build up a 3-D chemical reconstruction of a small area of the sample. As an illustration of the strength of TOF-SIMS tomography in revealing the spatial distribution of impurities, the 3-D dataset in
Figure 4A,B shows the distribution of the two phases in the BZCY/NiO scaffold for the dense BZCY membrane.
Figure 4C presents the distribution of an impurity (sodium in this case), which shows that the impurity accumulates at the boundaries between the two phases in the two-phase scaffold.
Due to the known propensity for Ni precipitating at the grain boundaries in BZCY [
26,
27], as illustrated in the SSRM and TEM data in
Figure 3, it was unclear if the palladium catalyst layer (Step 1 in
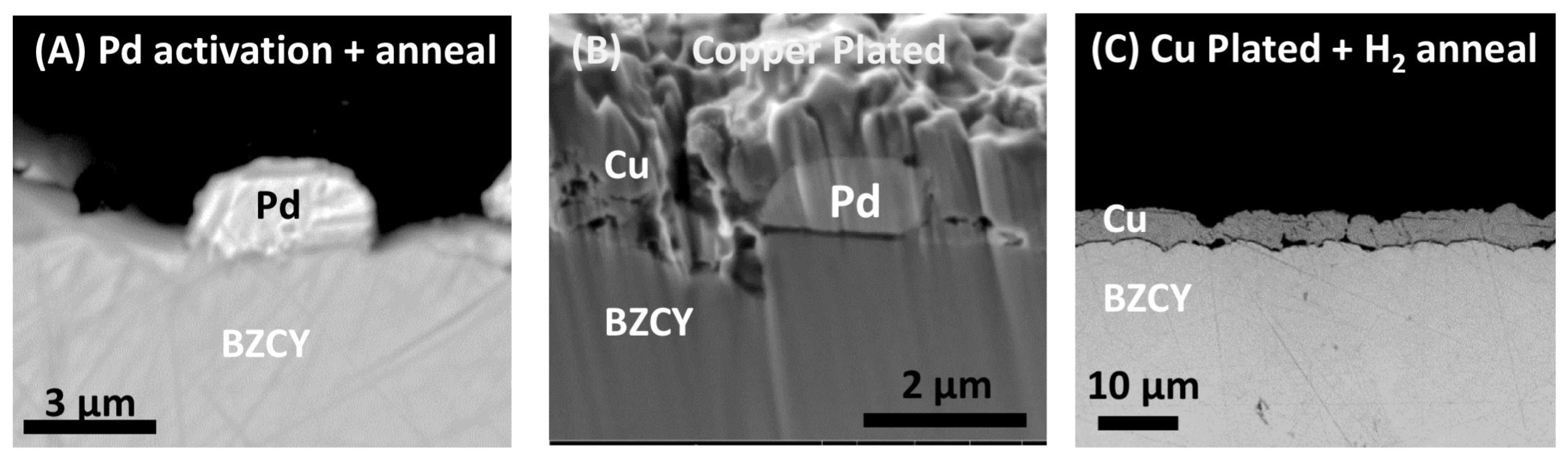
Figure 1) would also migrate to grain boundaries in the BZCY electrolyte after the high-temperature annealing of the copper electrode or during testing. The SEM images in
Figure 5 confirm that the Pd particles, which act as a catalyst for the electroless copper deposition, do not remain as discrete Pd particles after the high-temperature anneal to form the Cu electrode. However, the TEM-EDS data (not shown), with detection limits on the order of one atomic percent, are not able to elucidate the spatial distribution of Pd in the material beyond the copper plating step (Step 3 in
Figure 1). TOF-SIMS, with detection limits on the order of ppm or better, can thus be used to examine the spatial distribution of the palladium through the entire process of the electroless-deposited copper electrode. TOF-SIMS tomography data for palladium during all steps of the electroless-deposited copper electrode deposition process and after testing are shown in
Figure 6A–F. The data in
Figure 6 are presented in two rows: the upper and lower rows are the same dataset, the top row is the 3-D reconstruction viewed from the top, and the bottom row is the same reconstruction viewed from the side. Each reconstruction is 25 µm × 25 µm in size, and 2–3 µm in depth. The data in
Figure 6, and subsequent TOF-SIMS data, are presented on a blue-to-red intensity scale, as shown in the figure on the right-hand side. While white indicates no counts were detected in that area of the dataset, blue represents low intensity measured, while red would be the highest intensity measured for that dataset. The approximate location of the interface between the copper electrode and the BZCY electrolyte is seen in the bottom row of images where the palladium signal begins to decrease. The discrete palladium particles used to catalyze the electroless copper deposition are visible in the copper electrode after the Pd particle deposition step (
Figure 6A,B), and also after the copper electrode deposition (
Figure 6C,D).
Figure 6E,F show that after the high-temperature anneal in a reducing atmosphere, the palladium appears more evenly distributed through the copper electrode, which indicates that the palladium has alloyed with the copper electrode. The palladium alloying is further illustrated in TOF-SIMS data in
Supplementary Figure S1. One potential explanation for the high faradaic efficiency that has been observed with electrodes made via this electroless process is if a Pd-based phase that is proton-conducting but not electronically conductive is formed at the interface BZCY/Cu electrode (preventing potential electronic leakage [
28]). However, the data in
Figure 6 show a uniform Pd/Cu electrode (alloy). Thus, the observed performance is the result of a different mechanism. The SSRM also showed that the average resistance of the copper electrode increases after testing, consistent with a decrease in conductivity expected with copper–palladium alloying [
29].
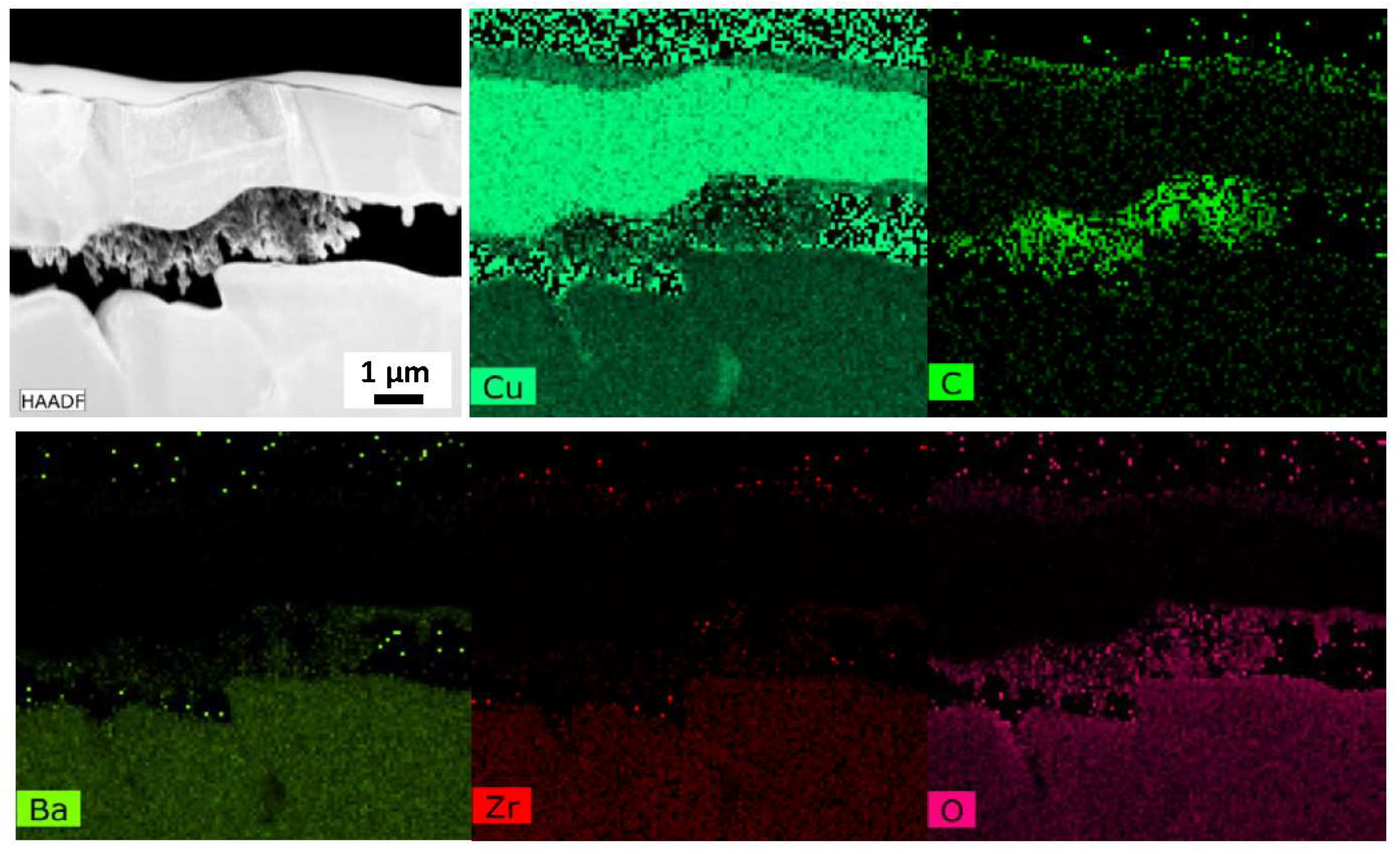
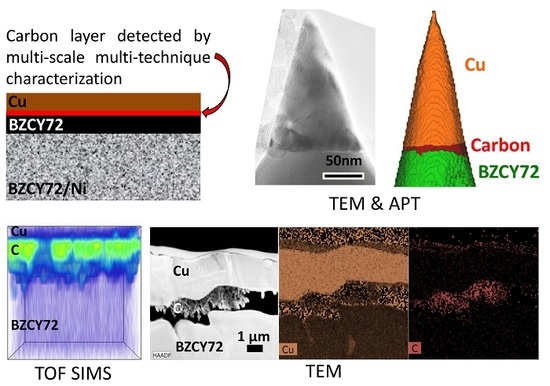
The excellent performance of the electrode is clearly not due to a nonuniform Pd distribution at the Cu/BZCY interface, so the exact mechanism enabling this superior performance required further investigation. The presence of a carbon-rich phase was noted in the samples prepared with the Cu-ELP technique, and we performed additional characterization to investigate this further. APT and TEM data of the sample after the copper plating (Step 3 of
Figure 1) in
Figure 7 show that a carbon-rich phase is already present at the Cu/BZCY interface immediately after the copper plating step. Additional APT data on a second area of the same sample show identical results, presented in the
Supplementary Materials. This suggests that the formation of this interface carbon layer is somehow inherently part of the electrodeposition process. In addition, SSRM did not reveal the presence of a highly electrically conductive phase between the ELP copper electrode and BZCY. This presents another interesting theory for the excellent performance of the observed electrodes: if this carbon-rich phase present at the Cu/BZCY interface blocks electrons but permits proton transport, it could be the reason for the high faradaic efficiency observed experimentally with ELP copper electrode samples.
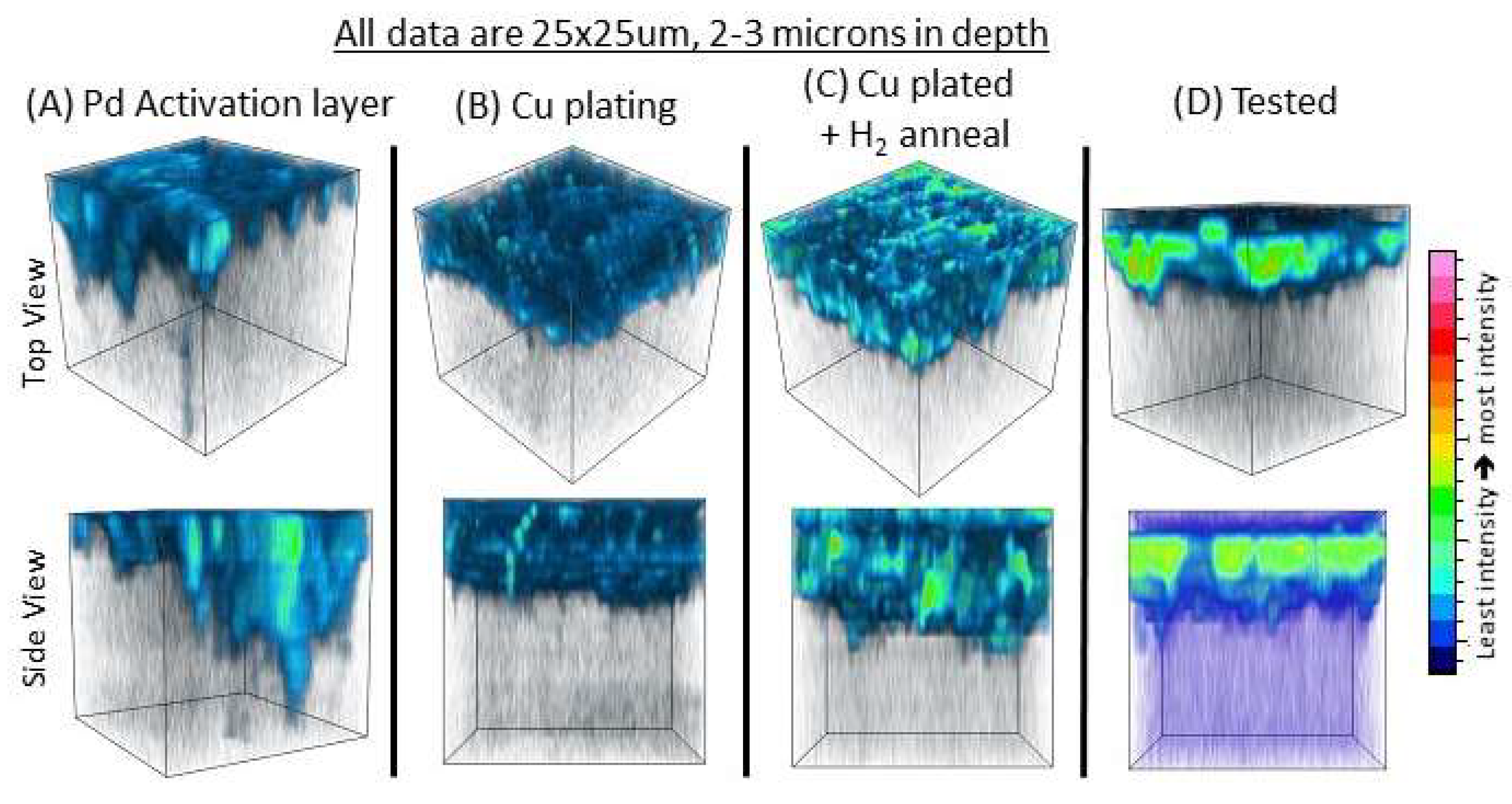
Using TOF-SIMS tomography, we were able to track the distribution and morphology of this carbon-rich phase throughout processing and after testing. These data are presented in
Figure 8, where the carbon-rich phase is detected with TOF-SIMS within the entire copper electrode thickness, as well as at the Cu/BZCY interface. More importantly, as seen in
Figure 8D after testing, the phase becomes more concentrated at the Cu/BZCY interface. The TOF-SIMS data were taken under measurement conditions that would not yield good depth resolution; thus, the exact interface between the copper and BZCY as seen in the APT data is difficult to observe in the data. However, the data on the tested sample shown in
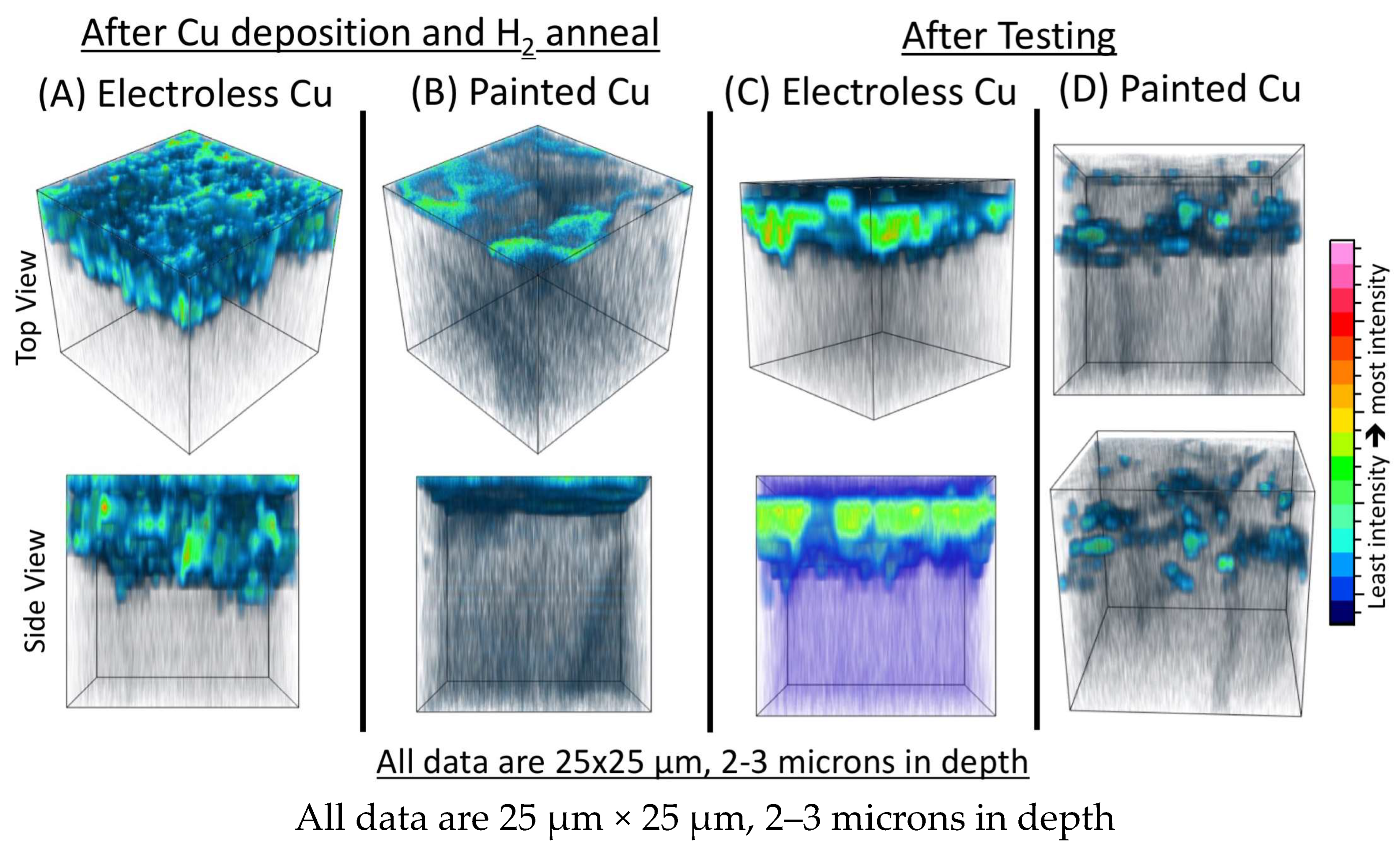
Figure 8D after testing show an apparent concentration of the carbon-rich phase. From these results, it is unclear if this phase that is concentrated at the Cu/BZCY interface after testing is simply a result of coking from the testing environment. To further investigate this, TOF-SIMS and TEM measurements were performed on BZCY samples with the traditional painted + fired copper electrodes before testing and after testing. The TOF-SIMS 3-D reconstruction of the carbon-rich phase for the painted copper electrode samples is shown in
Figure 9, where the data for the electroless plated copper at identical process steps are also shown for direct comparison. It is clear that there is a vastly different distribution of this phase in the electroless plated copper: there is much more carbon throughout the electrode for the electroless copper film prior to testing, and there is a continuous amount of the phase noted at the Cu/BZCY interface after testing in the electroless film compared to the discrete and non-continuous carbon-rich areas at the Cu/BZCY interface noted for the painted copper film tested under similar conditions. TEM results from the same specimen presented in
Figure 10 confirm the presence of this carbon-rich phase at the interface between the electrodeposited copper and the BZCY electrolyte after testing. The TEM results and STEM-EDS maps of the carbon-rich phase at the interface between the copper electrode and the BZCY indicate that this phase is mostly carbon and oxygen. The carbon layer in
Figure 10 appears noncontinuous due to a preferential milling of the carbon during FIB prep of the TEM specimen. One must keep in mind that Pd is more prone to coking than Cu [
30,
31,
32], and previous analysis reported about 12 wt % of Pd in the Cu electrode, indicating that it would be more prone to coking than a pure copper electrode [
16]. However, this increased palladium content is unlikely the explanation for the presence of carbon in the ELP Cu layer. Indeed, the TOF-SIMS data in
Figure 8 and the APT results in
Figure 7 show that carbon is already present in the electroless plated electrode before testing, and the TOF-SIMS data in
Figure 9 show that it is present in much higher amounts than for the copper-paste sample. This is sensible because electroless plating uses organics such as EDTA, glycine, or metal-organic precursors during the deposition process. The TOF-SIMS and TEM data shown in
Figure 9 and
Figure 10 illustrate that this phase becomes more concentrated at the Cu/BZCY interface during testing in a hydrocarbon-rich atmosphere.
Further study is needed to definitively explain the connection between the observed compositional structure and the near-doubling of the faradaic efficiency seen for the Cu electroless plated electrodes when comparing to standard painted electrodes. However, this study proves the presence of carbon in the plated electrodes before testing. In addition, this develops into a carbon-rich phase at the Cu/BZCY interface, which becomes more concentrated with testing. Far less carbon has been observed for the standard painted copper electrodes, suggesting a key link to performance.