Impact of Carrageenan-Based Encapsulation on the Physicochemical, Structural, and Antioxidant Properties of Freshwater Snail (Bellamya bengalensis) Protein Hydrolysates
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
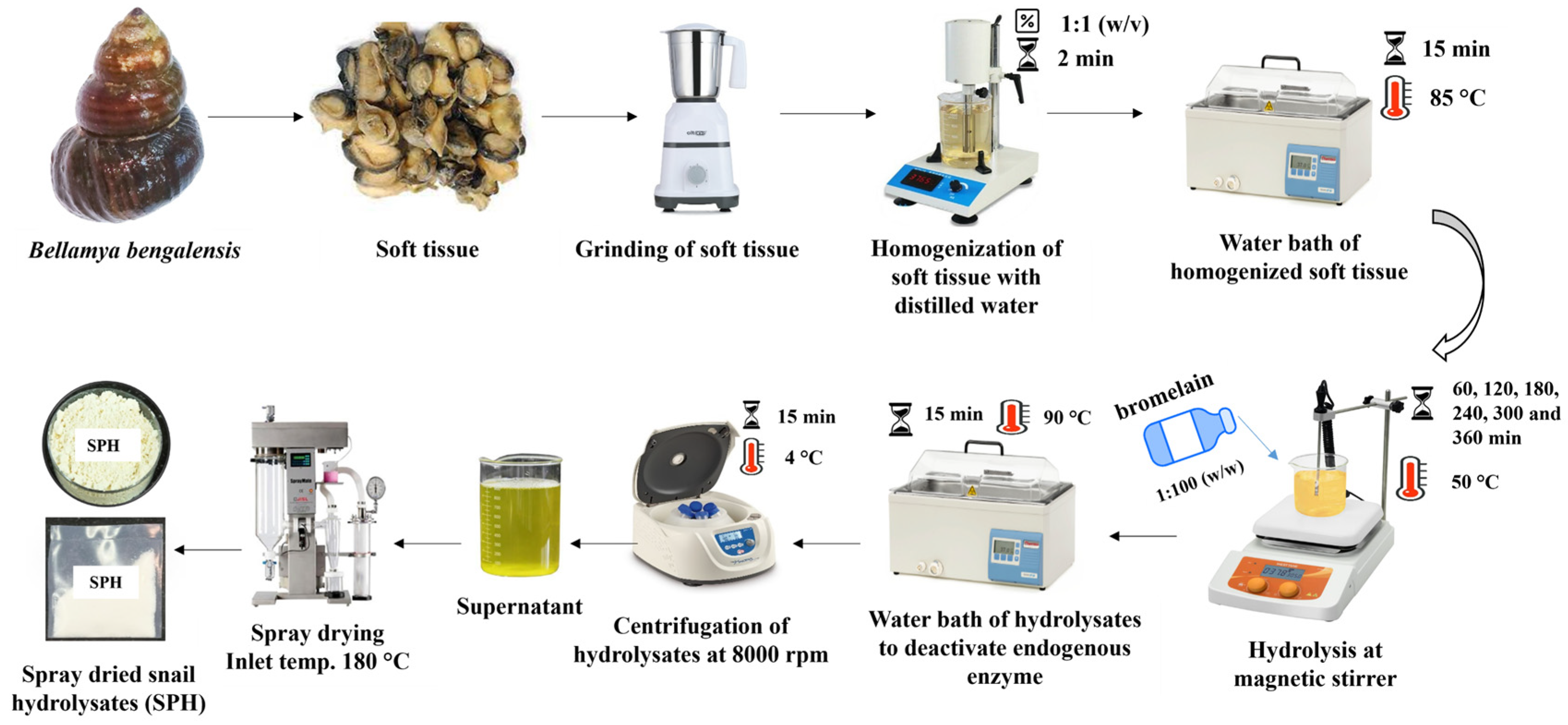
2.2. Preparation of Protein Hydrolysate
2.3. Degree of Hydrolysis (DH)
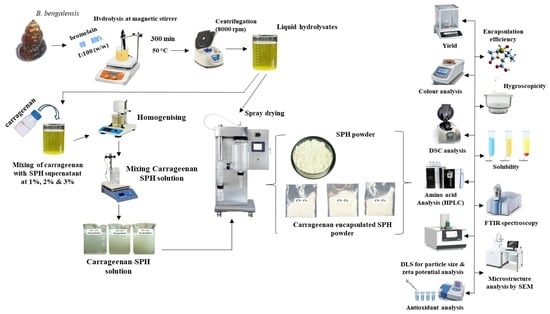
2.4. Encapsulation of Hydrolysates
2.5. Yield
2.6. Encapsulation Efficiency (EE)
2.7. Hygroscopicity
2.8. Color Analysis
2.9. Amino Acid Profiling
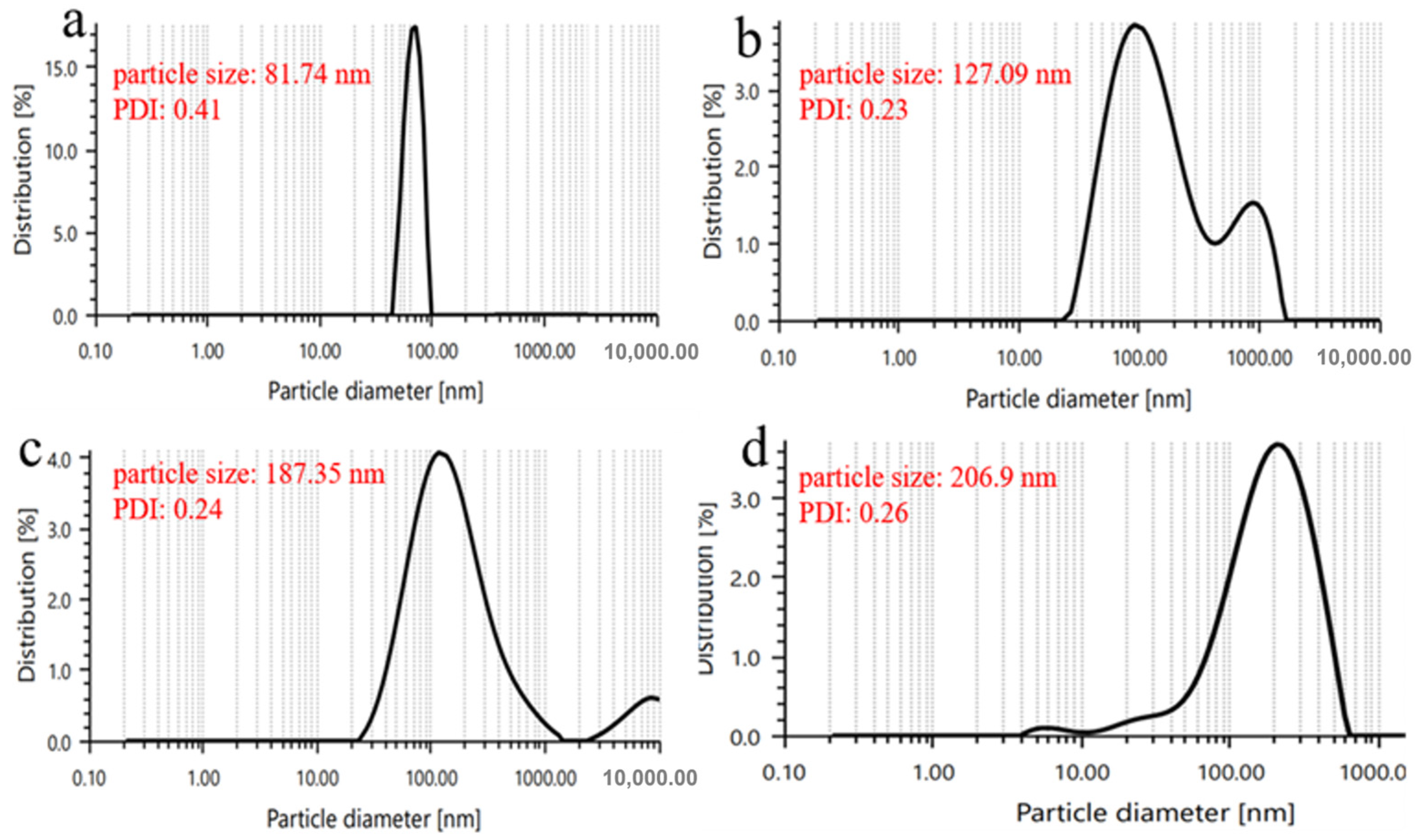
2.10. Particle Size Distribution
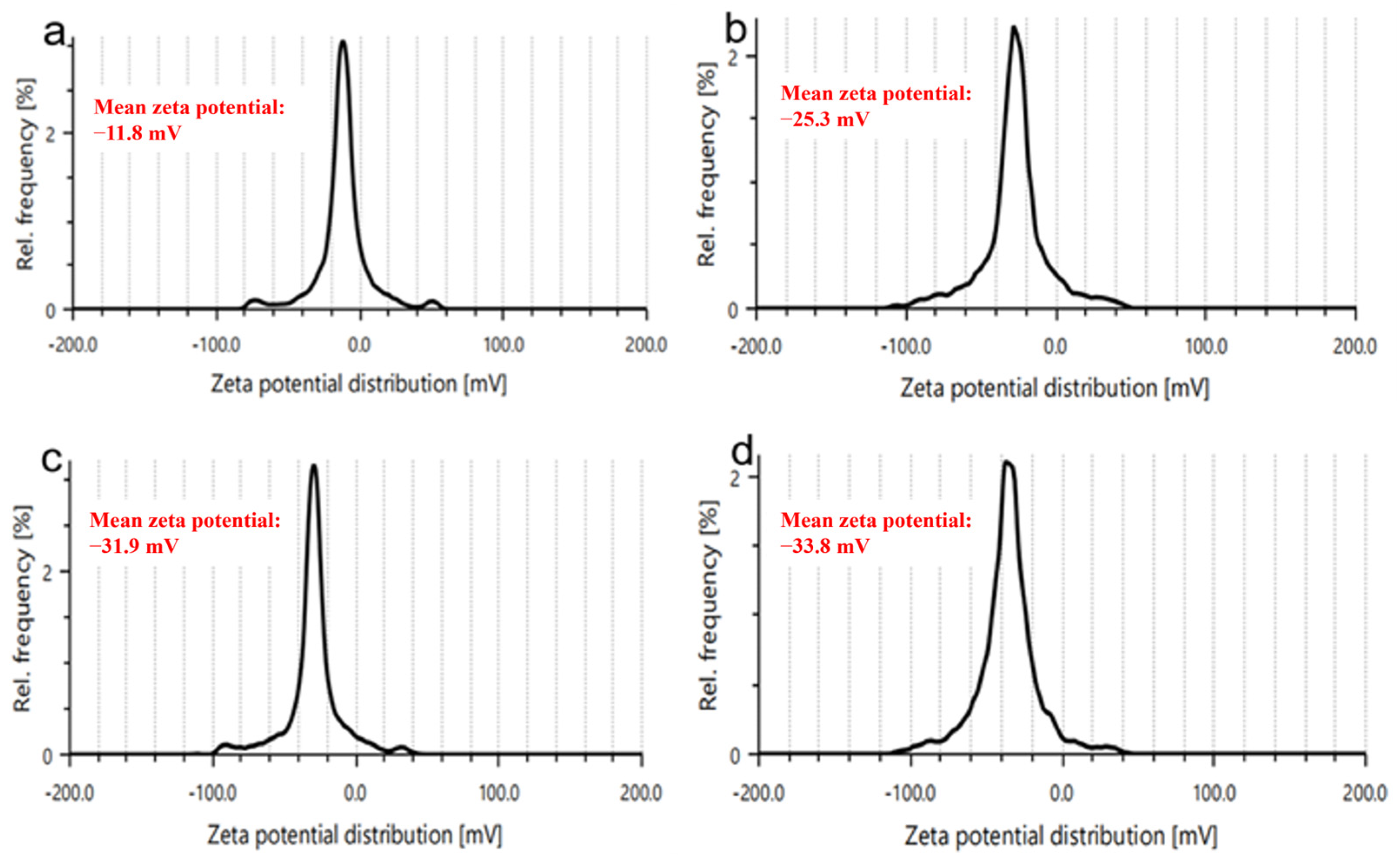
2.11. Zeta Potential Measurements
2.12. Solubility
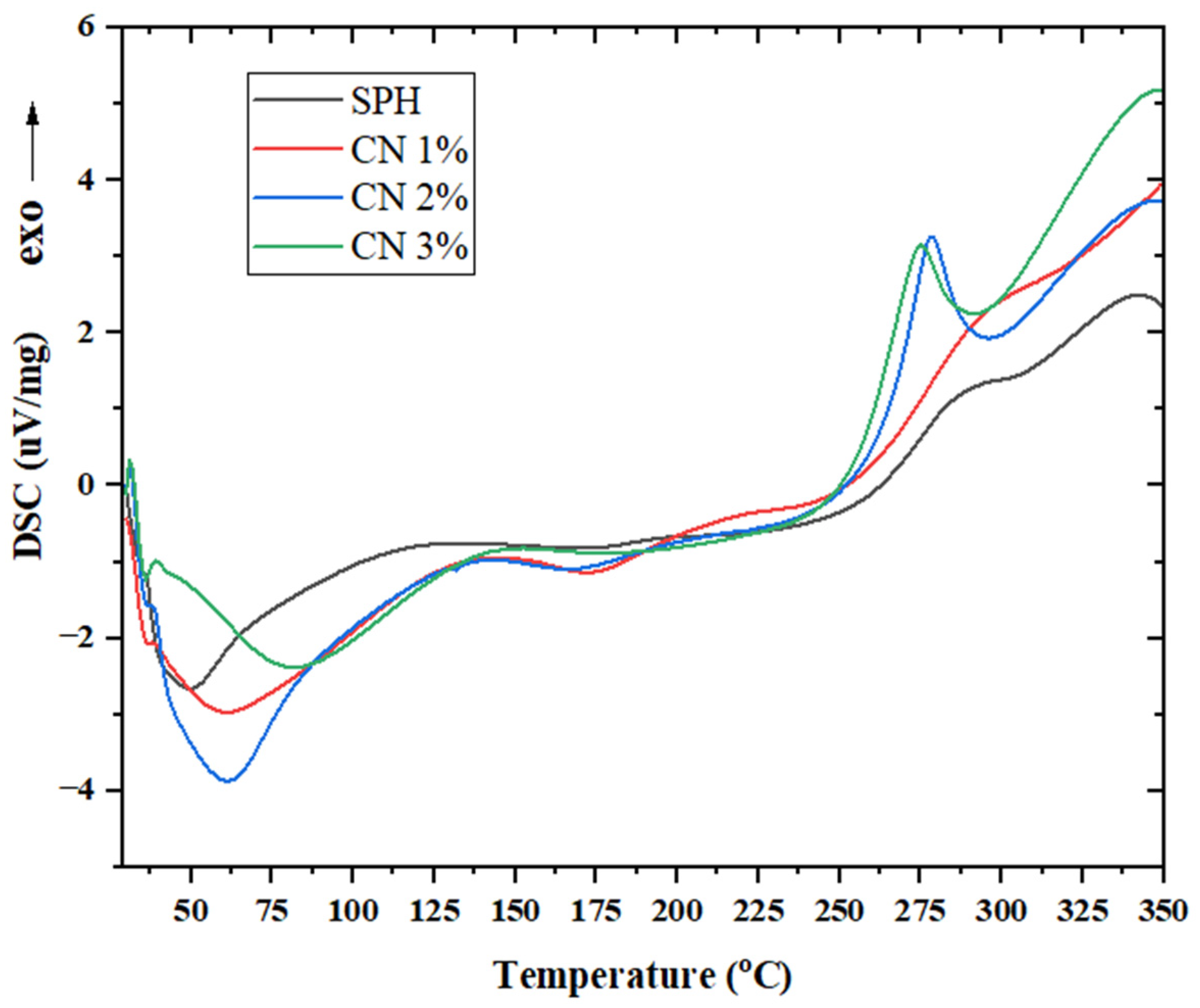
2.13. Differential Scanning Calorimetry (DSC)
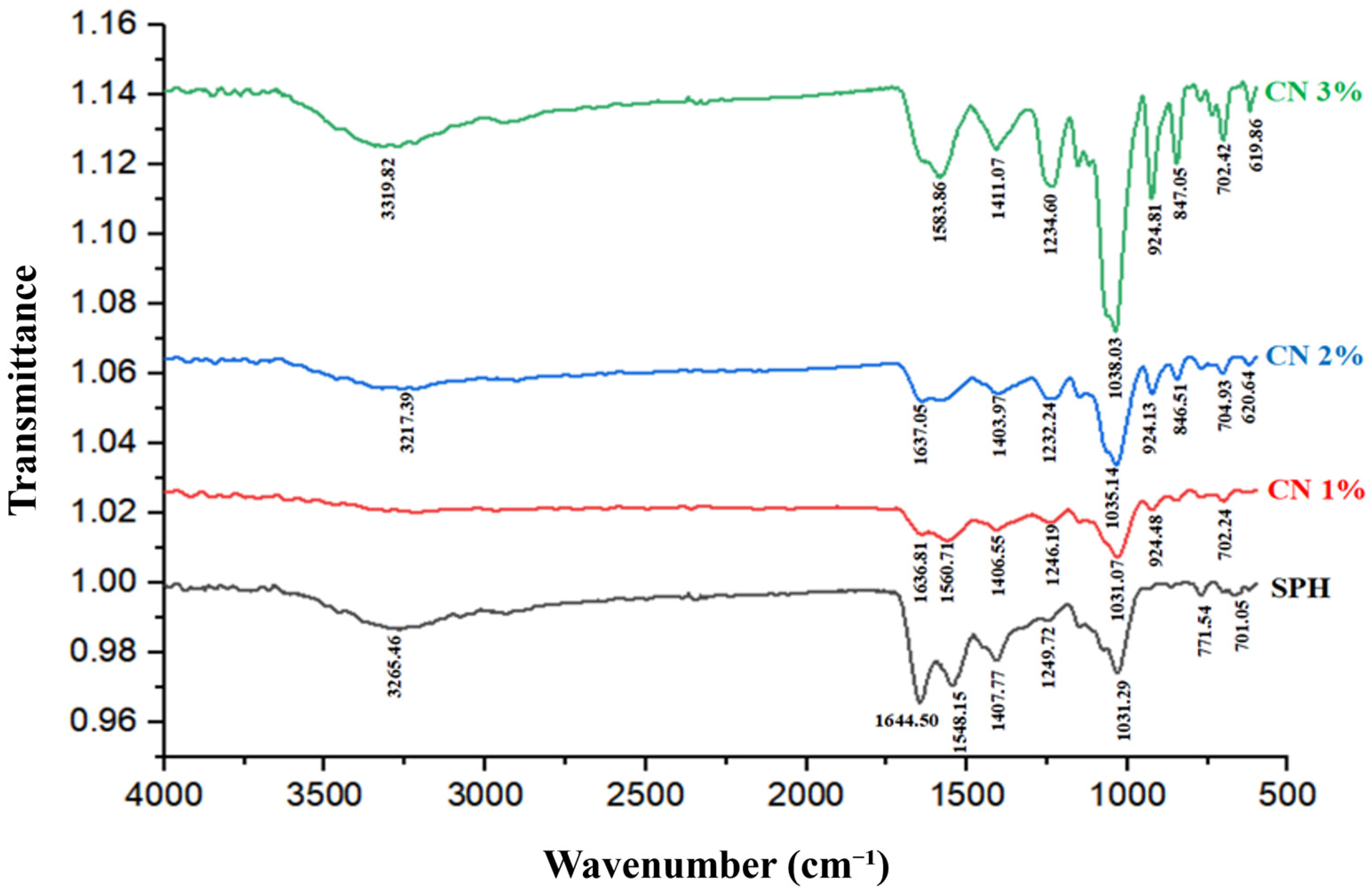
2.14. Fourier Transform Infrared (FTIR) Spectroscopy
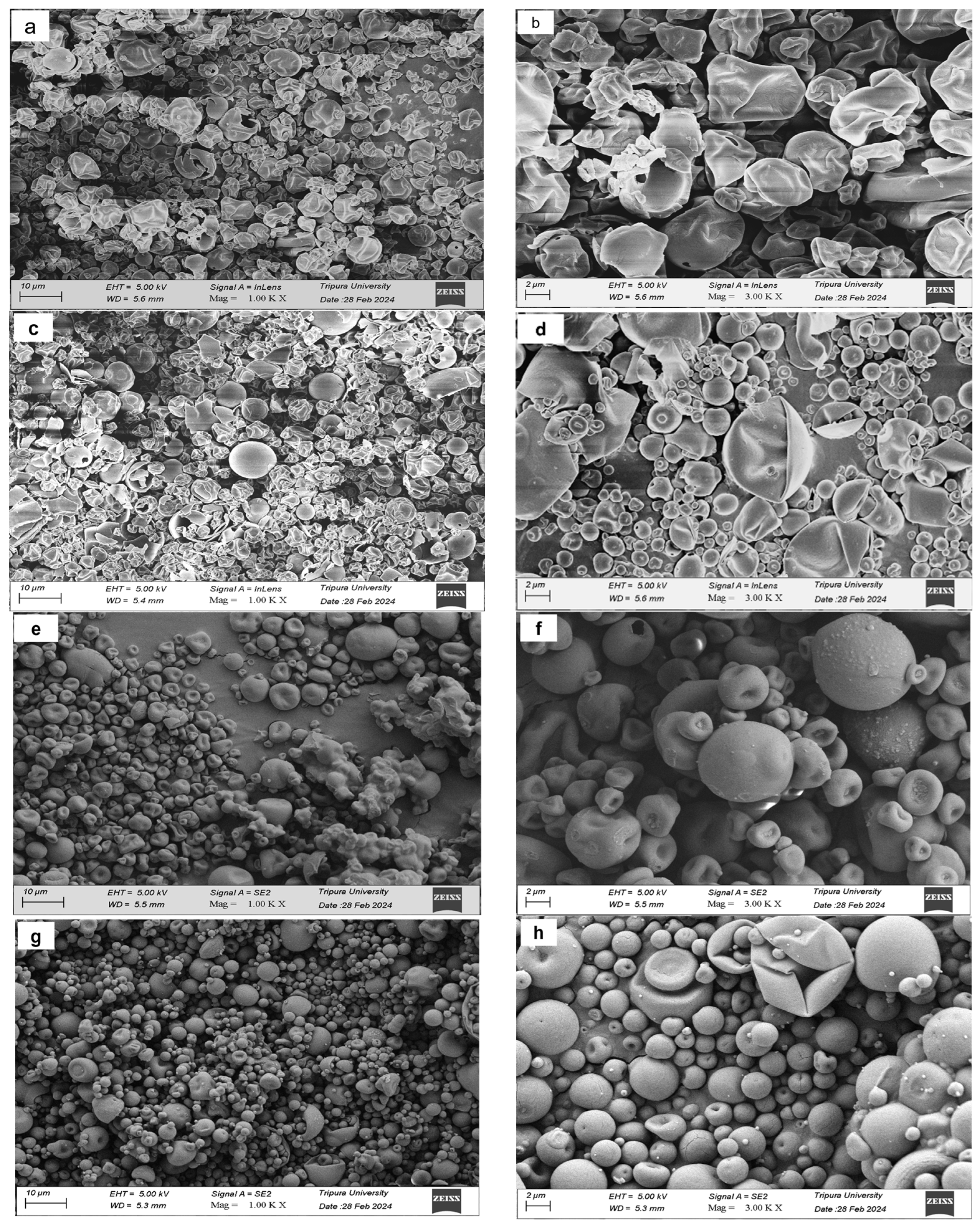
2.15. Scanning Electron Microscopy (SEM)
2.16. Bioactivity Property Analysis
2.16.1. DPPH (1,1-diphenyl-2-picrylhydrazyl) Radical-Scavenging Activity
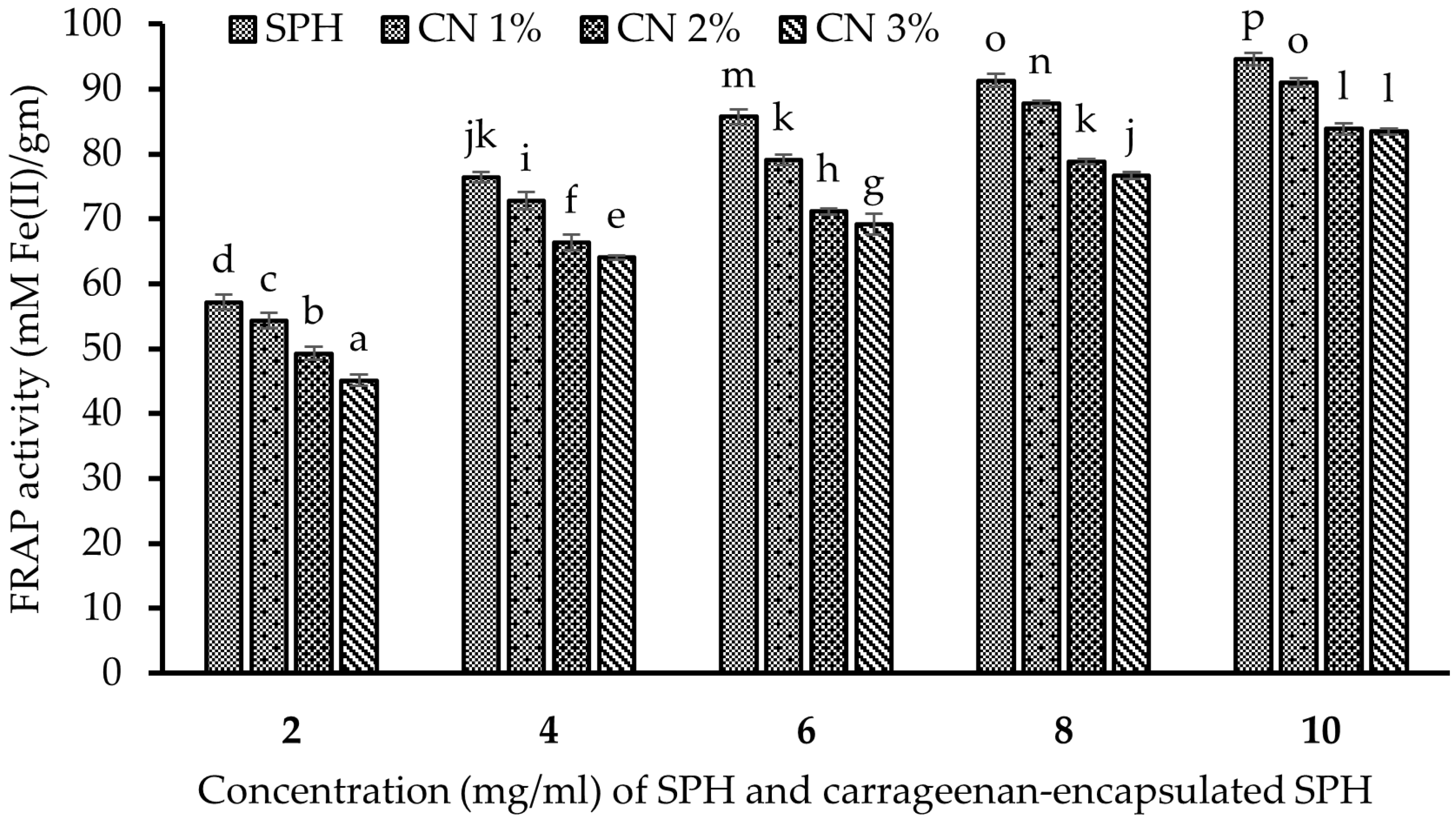
2.16.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.16.3. Hydroxyl Radical-Scavenging Activity (HRSA)
2.16.4. ABTS (2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)) Radical-Scavenging Activity
2.17. Statistical Analysis
3. Results and Discussion
3.1. Degree of Hydrolysis (DH)
3.2. Yield
3.3. Encapsulation Efficiency (EE)
3.4. Hygroscopicity
3.5. Color Analysis
3.6. Amino Acid Analysis
3.7. Particle Size Distribution and Polydispersity Index (PDI)
3.8. Zeta Potential Measurements
3.9. Solubility
3.10. Differential Scanning Calorimetry (DSC)
3.11. Fourier Transform Infrared (FTIR) Spectroscopy
3.12. Scanning Electron Microscopy (SEM) Analysis
3.13. Bioactivity Property Analysis
3.13.1. DPPH (1,1-diphenyl-2-picrylhydrazyl) Radical-Scavenging Activity
3.13.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.13.3. Hydroxyl Radical-Scavenging Activity
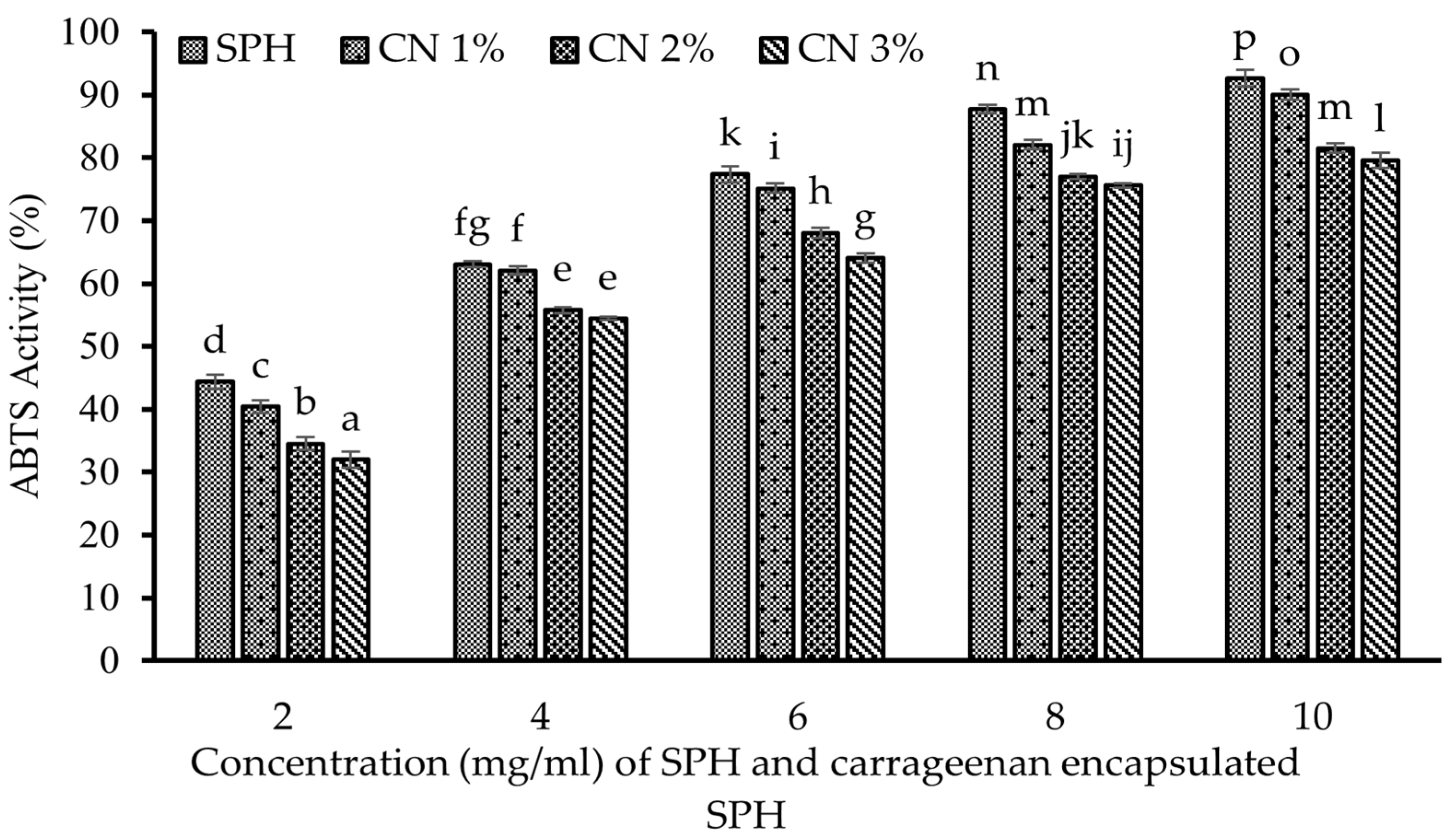
3.13.4. ABTS (2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)) Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasri, R.; Hamdi, M.; Touir, S.; Li, S.; Karra-Chaâbouni, M.; Nasri, M. Development of delivery system based on marine chitosan: Encapsulation and release kinetic study of antioxidant peptides from chitosan microparticle. Int. J. Biol. Macromol. 2021, 167, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, T.R.; Hossain, A.; Shahidi, F. Marine bioactives and their application in the food industry: A review. Appl. Sci. 2023, 13, 12088. [Google Scholar] [CrossRef]
- Vásquez, P.; Cian, R.E.; Drago, S.R. Marine Bioactive Peptides (Fishes, Algae, Cephalopods, Molluscs, and Crustaceans). In Handbook of Food Bioactive Ingredients; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer: Cham, Switzerland, 2023; pp. 1–16. [Google Scholar] [CrossRef]
- Bar, A. Bellamya bengalensis: A review on its Ecological importance, Nutritional values & Ethnomedicinal importance. Eur. J. Pharm. Med. Res. 2020, 7, 315–319. [Google Scholar]
- Chakraborty, D.; Mukherjee, M.; Maity, J. Ethnomedicinal importance of viviparous gastropod, Bellamya bengalensis (Lamarck, 1822) in terms of estimation of its proximate protein and amino acid composition. Int. J. Sci. Res. 2015, 3, 245–248. [Google Scholar]
- Gupta, A.; Khanal, P. The potential of snails as a source of food and feed. J. Agric. Food Res. 2024, 18, 101330. [Google Scholar] [CrossRef]
- Pissia, M.A.; Matsakidou, A.; Kiosseoglou, V. Raw materials from snails for food preparation. Future Foods 2021, 3, 100034. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Morellon-Sterling, R.; Tavano, O.; Berenguer-Murcia, Á.; Vela-Gutiérrez, G.; Fernandez-Lafuente, R. Bioactive peptides from fisheries residues: A review of use of papain in proteolysis reactions. Int. J. Biol. Macromol. 2021, 184, 415–428. [Google Scholar] [CrossRef]
- Khalua, R.K.; Tripathy, S.; Paul, B.; Bairy, D. Seasonal variation of carbohydrate, protein and lipid of common freshwater edible gastropod (Bellamya bengalensis) of Medinipur district, West Bengal. Res. J. Biol. 2014, 2, 49–52. [Google Scholar]
- Debnath, C.; Sahoo, L.; Haldar, A.; Datta, M.; Yadav, G.S.; Singha, A.; Bhattacharjee, J. Proximate and mineral composition of freshwater snails of Tripura, North–East India. Fish. Technol. 2016, 53, 307–312. [Google Scholar]
- Chatterjee, R.; Dey, T.K.; Roychoudhury, A.; Paul, D.; Dhar, P. Enzymatically excised oligopeptides from Bellamya bengalensis show potent antioxidative and anti-hypertensive activity. J. Food Sci. Technol. 2020, 57, 2586–2601. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.M. Bio-Chemistry and Nutritional health benefits of Bellamya bengalensis (Edible Gastropod): A Review. Afr. J. Bio. Sci. 2023, 5, 112–118. [Google Scholar]
- Baghele, M.; Mishra, S.; Meyer-Rochow, V.B.; Jung, C.; Ghosh, S. A review of the nutritional potential of edible snails: A sustainable underutilized food resource. Int. J. Nat. Prod. Res. 2022, 13, 47930. [Google Scholar] [CrossRef]
- Hayes, M.; Mora, L. Alternative Proteins as a Source of Bioactive Peptides: The Edible Snail and Generation of Hydrolysates Containing Peptides with Bioactive Potential for Use as Functional Foods. Foods 2021, 10, 276. [Google Scholar] [CrossRef]
- Ortizo, R.G.G.; Sharma, V.; Tsai, M.L.; Wang, J.X.; Sun, P.P.; Nargotra, P.; Dong, C.D. Extraction of novel bioactive peptides from fish protein hydrolysates by enzymatic reactions. Appl. Sci. 2023, 13, 5768. [Google Scholar] [CrossRef]
- Wu, Y.H.S.; Chen, Y.C. Trends and applications of food protein-origin hydrolysates and bioactive peptides. J. Food Drug Anal. 2022, 30, 172. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current evidence on the bioavailability of food bioactive peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of bioactive peptides: A strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Karami, Z.; Pateiro, M.; Lorenzo, J.M. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules 2021, 11, 631. [Google Scholar] [CrossRef]
- Mazloomi, N.; Safari, B.; Karaca, A.C.; Karimzadeh, L.; Moghadasi, S.; Ghanbari, M.; Assadpour, E.; Sarabandi, K.; Jafari, S.M. Loading bioactive peptides within different nanocarriers to enhance their functionality and bioavailability; in vitro and in vivo studies. Adv. Colloid Interface Sci. 2024, 334, 103318. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray drying encapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef] [PubMed]
- Culas, M.S.; Popovich, D.G.; Rashidinejad, A. Recent advances in encapsulation techniques for cinnamon bioactive compounds: A review on stability, effectiveness, and potential applications. Food Biosci. 2023, 57, 103470. [Google Scholar] [CrossRef]
- Vaishnav, A.; Lal, J.; Mehta, N.K.; Mohanty, S.; Yadav, K.K.; Priyadarshini, M.B.; Singh, S.K. Unlocking the Potential of Fishery Waste: Exploring Diverse Applications of Fish Protein Hydrolysates in Food and Nonfood Sectors. Environ. Sci. Pollut. Res. 2025, 22, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Askari Vaselabadi, S.; Gharibzahedi, S.M.T.; Greiner, R.; Vale, J.M.; Ovenseri, A.C.; Rashidinejad, A.; Roohinejad, S. Advancements in Spray-Drying for the Microencapsulation of Fat-Soluble Vitamins: Stability, Bioavailability, and Applications. J. Food Biochem. 2025, 2025, 9974476. [Google Scholar] [CrossRef]
- Baltrusch, K.L.; Torres, M.D.; Domínguez, H.; Flórez-Fernández, N. Spray-drying microencapsulation of tea extracts using green starch, alginate or carrageenan as carrier materials. Int. J. Biol. Macromol. 2022, 203, 417–429. [Google Scholar] [CrossRef]
- Furuta, T.; Neoh, T.L. Microencapsulation of food bioactive components by spray drying: A review. Dry. Technol. 2021, 39, 1800–1831. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: Cambridge, MA, USA, 2011; Volume 65, pp. 115–217. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M. Novel technologies for seaweed polysaccharides extraction and their use in food with therapeutically applications—A review. Foods 2022, 11, 2654. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Song, Z.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Addressing clean label trends in commercial meat processing: Strategies, challenges and insights from consumer perspectives. Foods 2023, 12, 2062. [Google Scholar] [CrossRef]
- Jafari, S.; Jafari, S.M.; Ebrahimi, M.; Kijpatanasilp, I.; Assatarakul, K. A decade overview and prospect of spray drying encapsulation of bioactives from fruit products: Characterization, food application and in vitro gastrointestinal digestion. Food Hydrocoll. 2023, 134, 108068. [Google Scholar] [CrossRef]
- Chakraborty, S. Carrageenan for encapsulation and immobilization of flavour, fragrance, probiotics, and enzymes: A review. J. Carbohydr. Chem. 2017, 36, 1–19. [Google Scholar] [CrossRef]
- Mardani, M.; Siahtiri, S.; Besati, M.; Baghani, M.; Baniassadi, M.; Nejad, A.M. Microencapsulation of natural products using spray drying; an overview. J. Microencapsul. 2024, 41, 649–678. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, A.; Mehta, N.K.; Hussain, S.A.; Acharya, P.C.; Biswal, S.; Nath, H.; Priyadarshini, M.B.; Thangarani, A.J.; Pal, P.; Singh, S.K.; et al. Bromelain excised hydrolysates with potent bioactivity from Bellamya bengalensis soft tissues: Process optimization and characterization. J. Agric. Food Res. 2025, 17, 101595. [Google Scholar] [CrossRef]
- Hoyle, N.T.; Merritt, J.H. Quality of fish protein hydrolysates from herring (Clupea harengus). J. Food Sci. 1994, 59, 76–79. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis of the AOAC International, 20th ed.; Association of Official and Analytical Chemists International: Washington, VA, USA, 2016. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Elavarasan, K.; Naveen Kumar, V.; Shamasundar, B.A. Antioxidant and functional properties of fish protein hydrolysates from fresh water carp (Catla catla) as influenced by the Nature of Enzyme. J. Food Process. Preserv. 2014, 38, 1207–1214. [Google Scholar] [CrossRef]
- Mendanha, D.V.; Ortiz, S.E.M.; Favaro-Trindade, C.S.; Mauri, A.; Monterrey-Quintero, E.S.; Thomazini, M. Microencapsulation of casein hydrolysate by complex coacervation with SPI/pectin. Food Res. Int. 2009, 42, 1099–1104. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Kizhakkethil, B.P.; George, J.C.; Aliyamveetil Abubacker, Z.; Ninan, G.; Nagarajarao, R.C. Antioxidant peptides from dark meat of yellowfin tuna (Thunnus albacares): Process optimization and characterization. Waste Biomass Valorization 2021, 12, 1845–1860. [Google Scholar] [CrossRef]
- Zaitoun, B.J.; Palmer, N.; Amamcharla, J.K. Characterization of a Commercial Whey Protein Hydrolysate and Its Use as a Binding Agent in the Whey Protein Isolate Agglomeration Process. Foods 2022, 11, 1797. [Google Scholar] [CrossRef]
- Yin, B.; Li, T.; Zhang, S.; Li, Z.; He, P. Sensitive analysis of 33 free amino acids in serum, milk, and muscle by ultrahigh-performance liquid chromatography-quadrupole-orbitrap high resolution mass spectrometry. Food Anal. Methods 2016, 9, 2814–2823. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Gaurav, K.; Mehta, N.K.; Priyadarshini, M.B.; Pal, P.; Nirmal, N.P.; Sharma, S.; Vaishnav, A. Fat Uptake Reduction During Deep-Fat Frying of Fish Fillets Coated with Soy Protein Isolate Edible Coating. J. Aquat. Food Prod. Technol. 2024, 33, 573–588. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hamishehkar, H.; McClements, D.J.; Shahvalizadeh, R.; Barri, A. Encapsulation of Spirulina protein hydrolysates in liposomes: Impact on antioxidant activity and gastrointestinal behavior. Food Chem. 2023, 400, 133973. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhao, M.; Liu, R.H.; Regenstein, J.M. Antioxidant and antiproliferative activities of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. J. Agric. Food Chem. 2011, 59, 7948–7953. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials 2021, 11, 61439. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zarei, M.; Abdul-Hamid, A.; Saari, N. Optimization of bromelain-aided production of angiotensin I-converting enzyme inhibitory hydrolysates from stone fish using response surface methodology. Mar. Drugs 2017, 15, 104. [Google Scholar] [CrossRef]
- Yi, D.; Lin, Q.; Johns, P.W. Estimation of degree of hydrolysis of protein hydrolysates by size exclusion chromatography. Food Anal. Methods 2021, 14, 805–813. [Google Scholar] [CrossRef]
- Megrous, S.; Zhao, X.; Al-Dalali, S.; Yang, Z. Response surface methodology and optimization of hydrolysis conditions for the in vitro calcium-chelating and hypoglycemic activities of casein protein hydrolysates prepared using microbial proteases. J. Food Meas. Charact. 2024, 18, 3069–3084. [Google Scholar] [CrossRef]
- da Rosa Zavareze, E.; Telles, A.C.; El Halal, S.L.M.; da Rocha, M.; Colussi, R.; de Assis, L.M.; Prentice-Hernández, C. Production and characterization of encapsulated antioxidative protein hydrolysates from Whitemouth croaker (Micropogonias furnieri) muscle and byproduct. LWT-Food Sci. Technol. 2014, 59, 841–848. [Google Scholar] [CrossRef]
- Vioque, J.; Sánchez-Vioque, R.; Clemente, A.; Pedroche, J.; Millán, F. Partially hydrolysed rapeseed protein isolates with improved functional properties. J. Am. Oil Chem. Soc. 2000, 77, 447–450. [Google Scholar] [CrossRef]
- Fenton, T.; Kanyuck, K.; Mills, T.; Pelan, E. Formulation and characterization of kappa-carrageenan gels with nonionic surfactant for melting-triggered controlled release. Carbohydr. Polym. Technol. Appl. 2021, 2, 100060. [Google Scholar] [CrossRef]
- Udo, T.; Mummaleti, G.; Mohan, A.; Singh, R.K.; Kong, F. Current and emerging applications of carrageenan in the food industry. Food Res. Int. 2023, 173, 113369. [Google Scholar] [CrossRef]
- Gu, L.; McClements, D.J.; Li, J.; Su, Y.; Yang, Y.; Li, J. Formulation of alginate/carrageenan microgels to encapsulate, protect and release immunoglobulins: Egg Yolk IgY. Food Hydrocoll. 2021, 112, 106349. [Google Scholar] [CrossRef]
- Chuaychan, S.; Benjakul, S. Effect of maltodextrin on characteristics and antioxidative activity of spray-dried powder of gelatin and gelatin hydrolysate from scales of spotted golden goatfish. J. Food Sci. Technol. 2016, 53, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, B.; Qiao, D.; Yan, X.; Zhao, S.; Jia, C.; Xu, Y. Addition of κ-carrageenan increases the strength and chewiness of gelatin-based composite gel. Food Hydrocoll. 2022, 128, 107565. [Google Scholar] [CrossRef]
- Kokkuvayil Ramadas, B.; Rhim, J.W.; Roy, S. Recent progress of carrageenan-based composite films in active and intelligent food packaging applications. Polymers 2024, 16, 1001. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Kaveh, S.; Mahoonak, A.S.; Erfani-Moghadam, V.; Ghorbani, M.; Gholamhosseinpour, A.; Raeisi, M. Protein Hydrolysates of Niosome-Encapsulated Skipjack (Katsuwonus pelamis) Viscera: Antioxidant, Anticancer, and Release Properties. Appl. Food Res. 2025, 5, 100733. [Google Scholar] [CrossRef]
- Sablania, V.; Bosco, S.J.D.; Rohilla, S.; Shah, M.A. Microencapsulation of Murraya koenigii L. leaf extract using spray drying. J. Food Meas. Charact. 2018, 12, 892–901. [Google Scholar] [CrossRef]
- Permanadewi, I.; Kumoro, A.C.; Wardhani, D.H.; Aryanti, N. Effect of viscosity on iron encapsulation using alginate as a carrying agent in a controlled spray drying process. Food Res. 2022, 6, 56–67. [Google Scholar] [CrossRef]
- Ren, D.; Xue, G.; Zheng, H.; Yang, W.; Cao, W.; Lin, H.; Gao, J.; Qin, X.; Zhang, C. Thermal stabilization effects of κ-Carrageenan on water-soluble protein extracted from Pinctada martensii meat. J. Food Meas. Charact. 2022, 16, 4985–4995. [Google Scholar] [CrossRef]
- Annamalai, J.; Aliyamveetil Abubacker, Z.; Lakshmi, N.M.; Unnikrishnan, P. Microencapsulation of Fish Oil Using Fish Protein Hydrolysate, Maltodextrin, and Gum Arabic: Effect on Structural and Oxidative Stability. J. Aquat. Food Prod. Technol. 2020, 29, 293–306. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Park, K.J.; Hubinger, M.D. Effect of Carrier Agents on the Physicochemical Properties of a Spray Dried Chicken Meat Protein Hydrolysate. J. Food Eng. 2009, 94, 326–333. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Mavelil-Sam, R.; Ouseph, E.M.; Morreale, M.; Scaffaro, R.; Thomas, S. Recent developments and formulations for hydrophobic modification of carrageenan bionanocomposites. Polymers 2023, 15, 1650. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Xue, C. Recent advances in carrageenan-based delivery systems for bioactive ingredients: A review. Trends Food Sci. Technol. 2021, 112, 348–361. [Google Scholar] [CrossRef]
- Marín-Peñalver, D.; Alemán, A.; Montero, M.P.; Gómez-Guillén, M.C. Entrapment of natural compounds in spray-dried and heat-dried iota-carrageenan matrices as functional ingredients in surimi gels. Food Funct. 2021, 12, 2137–2147. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Llavata-Cabrero, B.; Martínez-Sanz, M.; Fabra, M.J.; López-Rubio, A. Self-assembled gelatin-ι-carrageenan encapsulation structures for intestinal-targeted release applications. J. Colloid Interface Sci. 2018, 517, 113–123. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, S.; Su, J.; Zhu, M.; Zhou, M.; Chen, T.; Han, Y. Recent advances in carrageenan-based films for food packaging applications. Front. Nutr. 2022, 9, 1004588. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Guidea, A.; Zăgrean-Tuza, C.; Moț, A.C.; Sârbu, C. Comprehensive evaluation of radical scavenging, reducing power, and chelating capacity of free proteinogenic amino acids using spectroscopic assays and multivariate exploratory techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 233, 118158. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H. Metabolism of sulfur-containing amino acids: How the body copes with excess methionine, cysteine, and sulfide. J. Nutr. 2020, 150, 2494S–2505S. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and byproducts: A review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Dimina, L.; Rémond, D.; Huneau, J.F.; Mariotti, F. Combining plant proteins to achieve amino acid profiles adapted to various nutritional objectives—An exploratory analysis using linear programming. Front. Nutr. 2022, 8, 809685. [Google Scholar] [CrossRef]
- Feng, L.; Wu, Y.; Han, Y.; Yao, X.; Li, Q.; Liu, M.; Cao, Y. Structural characteristics, functional properties and nutritional value of walnut protein by limited enzymatic hydrolysis. LWT 2024, 197, 115923. [Google Scholar] [CrossRef]
- Bielecka, M.; Cichosz, G.; Czeczot, H. Antioxidant, antimicrobial, and anticarcinogenic activities of bovine milk proteins and their hydrolysates—A review. Int. Dairy J. 2022, 127, 105208. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoscale nutrient delivery systems for food applications: Improving bioactive dispersibility, stability, and bioavailability. J. Food Sci. 2015, 80, N1602–N1611. [Google Scholar] [CrossRef]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Juntrapirom, S.; Kanjanakawinkul, W.; Müllertz, A.; Chaiyana, W. Chitosan Alginate Nanoparticles of Protein Hydrolysate from Acheta domesticus with Enhanced Stability for Skin Delivery. Pharmaceutics 2024, 16, 724. [Google Scholar] [CrossRef]
- Krempel, M.; Griffin, K.; Khouryieh, H. 13-Hydrocolloids as Emulsifiers and Stabilizers in Beverage Preservation. In Preservatives and Preservation Approaches in Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 427–465. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Sun, H.; Liu, X.; Leng, X. Physicochemical stability and functional properties of selenium nanoparticles stabilized by chitosan, carrageenan, and gum Arabic. Carbohydr. Polym. 2021, 255, 117379. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Cheng, H. Impact of κ-Carrageenan on the Cold-Set Pea Protein Isolate Emulsion-Filled Gels: Mechanical Property, Microstructure, and In Vitro Digestive Behavior. Foods 2024, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, F. Whey protein hydrolysates and infant formulas: Effects on physicochemical and biological properties. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13337. [Google Scholar] [CrossRef] [PubMed]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 67, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.R.M.M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of bioactive compounds for food and agricultural applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, J.M.; Wan, Z.L.; Yang, X.Q.; Chen, X.W. Corn protein hydrolysate as a new structural modifier for soybean protein isolate based O/W emulsions. LWT 2020, 118, 108763. [Google Scholar] [CrossRef]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro and nanotechnological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ramezanzade, L.; Nikkhah, M. Nano-Liposomal Entrapment of Bioactive Peptidic Fraction from Fish Gelatin Hydrolysate. Int. J. Biol. Macromol. 2017, 105, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Kilker, S.R.; Lee, Y. Surface charge (zeta-potential) of nanoencapsulated food ingredients. In Characterization of Nanoencapsulated Food Ingredients; Academic Press: Cambridge, MA, USA, 2020; pp. 213–241. [Google Scholar] [CrossRef]
- Yousefi, N.; Abbasi, S. Food proteins: Solubility & thermal stability improvement techniques. Food Chem. Adv. 2022, 1, 100090. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alrosan, M.; Gammoh, S.; Tranchant, C.C.; Alhamad, M.N.; Rababah, T.; Zghoul, R.; Alzoubi, H.; Ghatasheh, S.; Ghozlan, K.; et al. Encapsulation-based technologies for bioactive compounds and their application in the food industry: A roadmap for food-derived functional and health-promoting ingredients. Food Biosci. 2022, 50, 101971. [Google Scholar] [CrossRef]
- Rivera-Jiménez, J.; Berraquero-García, C.; Pérez-Gálvez, R.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Peptides and protein hydrolysates exhibiting anti-inflammatory activity: Sources, structural features, and modulation mechanisms. Food Funct. 2022, 13, 12510–12540. [Google Scholar] [CrossRef]
- Berraquero-García, C.; Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M.; García-Moreno, P.J. Encapsulation of bioactive peptides by spray-drying and electrospraying. Foods 2023, 12, 2005. [Google Scholar] [CrossRef]
- Gharanjig, H.; Gharanjig, K.; Hosseinnezhad, M.; Jafari, S.M. Differential scanning calorimetry (DSC) of nanoencapsulated food ingredients. In Characterization of Nanoencapsulated Food Ingredients; Academic Press: Cambridge, MA, USA, 2020; pp. 295–346. [Google Scholar] [CrossRef]
- Sooram, B.; Gupta, N.; Chethireddy, V.R.; Tripathi, T.; Saudagar, P. Applications of Differential Scanning Calorimetry in Studying Folding and Stability of Proteins. In Protein Folding Dynamics and Stability: Experimental and Computational Methods; Springer Nature: Singapore, 2023; pp. 37–60. [Google Scholar] [CrossRef]
- TIP, T. Interpreting DSC Curves Part 1: Dynamic Measurements. Available online: https://www.eng.uc.edu/~beaucag/Classes/Characterization/DSCParts/Artifacts%20in%20DSC%20Usercom_11.pdf (accessed on 20 February 2025).
- Zhou, H.X.; Pang, X. Electrostatic interactions in protein structure, folding, binding, and condensation. Chem. Rev. 2018, 118, 1691–1741. [Google Scholar] [CrossRef]
- Yang, X.; Lv, Z.; Han, C.; Zhang, J.; Duan, Y.; Guo, Q. Stability and encapsulation properties of daidzein in zein/carrageenan/sodium alginate nanoparticles with ultrasound treatment. Int. J. Biol. Macromol. 2024, 262, 130070. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Vaidya, S.; Sheshappa, M.B. Functional properties of protein hydrolyzate from ribbon fish (Leptura canthus savala) as-prepared by enzymatic hydrolysis. Int. J. Food Prop. 2022, 25, 187–203. [Google Scholar] [CrossRef]
- Patil, U.; Gulzar, S.; Ma, L.; Zhang, B.; Benjakul, S. Pickering emulsion stabilized by fish myofibrillar proteins modified with tannic acid, as influenced by different drying methods. Foods 2023, 12, 1556. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, F.; Zhao, M.; Yang, B.; Cui, C. Physicochemical changes of myofibrillar proteins during processing of Cantonese sausage in relation to their aggregation behavior and in vitro digestibility. Food Chem. 2011, 129, 472–478. [Google Scholar] [CrossRef]
- Wachirattanapongmetee, K.; Katekaew, S.; Weerapreeyakul, N.; Thawornchinsombut, S. Differentiation of protein types extracted from tilapia byproducts by FTIR spectroscopy combined with chemometric analysis and their antioxidant protein hydrolysates. Food Chem. 2024, 437, 137862. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid-soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Webber, V.; de Carvalho, S.M.; Ogliari, P.J.; Hayashi, L.; Barreto, P.L.M. Optimization of the extraction of carrageenan from Kappaphycus alvarezii using response surface methodology. Food Sci. Technol. 2012, 32, 812–818. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak fitting applied to Fourier transform infrared and Raman spectroscopic analysis of proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Sarabandi, K.; Gharehbeglou, P.; Jafari, S.M. Spray-drying encapsulation of protein hydrolysates and bioactive peptides: Opportunities and challenges. Dry. Technol. 2020, 38, 577–595. [Google Scholar] [CrossRef]
- Vicente, J.; Pinto, J.; Menezes, J.; Gaspar, F. Fundamental analysis of particle formation in spray drying. Powder Technol. 2013, 247, 1–7. [Google Scholar] [CrossRef]
- Gomes, M.H.G.; Kurozawa, L.E. Surface composition and microstructure of spray-dried microparticles: A review about their effect on functional properties. Dry. Technol. 2024, 42, 1665–1682. [Google Scholar] [CrossRef]
- Bakry, A.M.; Huang, J.; Zhai, Y.; Huang, Q. Myofibrillar protein with κ- or λ-carrageenans as novel shell materials for microencapsulation of tuna oil through complex coacervation. Food Hydrocoll. 2019, 96, 43–53. [Google Scholar] [CrossRef]
- Garzón, A.G.; Cian, R.E.; Drago, S.R. Effects of agar-carrageenan wall materials and core-to-wall material ratio on physicochemical properties and in vitro bioaccessibility of microencapsulated bioactive peptides. Food Hydrocoll. 2023, 139, 108570. [Google Scholar] [CrossRef]
- McClements, D.J. Recent developments in encapsulation and release of functional food ingredients: Delivery by design. Curr. Opin. Food Sci. 2018, 23, 80–84. [Google Scholar] [CrossRef]
- Klojdová, I.; Milota, T.; Smetanová, J.; Stathopoulos, C. Encapsulation: A strategy to deliver therapeutics and bioactive compounds? Pharmaceutics 2023, 16, 362. [Google Scholar] [CrossRef] [PubMed]
- Slizyte, R.; Rommi, K.; Mozuraityte, R.; Eck, P.; Five, K.; Rustad, T. Bioactivities of fish protein hydrolysates from defatted salmon backbones. Biotechnol. Rep. 2016, 11, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Klomklao, S.; Benjakul, S. Protein hydrolysates prepared from the viscera of skipjack tuna (Katsuwonus pelamis): Antioxidative activity and functional properties. Turk. J. Fish. Aquat. Sci. 2018, 18, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Kishimura, H. Antioxidant and sensory properties of protein hydrolysate derived from Nile tilapia (Oreochromis niloticus) by one-and two-step hydrolysis. J. Food Sci. Technol. 2015, 52, 3336–3349. [Google Scholar] [CrossRef][Green Version]
- Xue, J.; Luo, Y. Protein-polysaccharide nanocomplexes as nanocarriers for delivery of curcumin: A comprehensive review on preparation methods and encapsulation mechanisms. J. Future Foods 2023, 3, 99–114. [Google Scholar] [CrossRef]
- Pourashouri, P.; Shabanpour, B.; Heydari, S.; Raeisi, S. Encapsulation of fish oil by carrageenan and gum tragacanth as wall materials and its application to the enrichment of chicken nuggets. LWT 2021, 137, 110334. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Kumar, B.D. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J. Food Sci. Technol. 2015, 52, 5817–5825. [Google Scholar] [CrossRef]
- Tadesse, S.A.; Emire, S.A.; Barea, P.; Illera, A.E.; Melgosa, R.; Beltrán, S.; Sanz, M.T. Valorization of low-valued ray-finned fish (Labeo barbus nedgia) by enzymatic hydrolysis to obtain fish-discarded protein hydrolysates as functional foods. Food Bioprod. Process. 2023, 141, 167–184. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Machado, M.; Cermeño, M.; Kleekayai, T.; Machado, S.; Rego, A.M.; FitzGerald, R.J. Enzyme-assisted release of antioxidant peptides from Porphyra dioica conchocelis. Antioxidants 2021, 10, 249. [Google Scholar] [CrossRef]
- Slizyte, R.; Mozuraityte, R.; Remman, T.; Rustad, T. Two-stage processing of salmon backbones to obtain high-quality oil and proteins. Int. J. Food Sci. Technol. 2018, 53, 2378–2385. [Google Scholar] [CrossRef]
- Nemati, M.; Shahosseini, S.R.; Ariaii, P. Review of fish protein hydrolysates: Production methods, antioxidant and antimicrobial activity and nanoencapsulation. Food Sci. Biotechnol. 2024, 33, 1789–1803. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Ou, A.; Liu, S.; Elango, J.; Wang, S.; Henriques da Silva, T.; Bao, B. Effects of ion concentrations on the hydroxyl radical scavenging rate and reducing power of fish collagen peptides. J. Food Biochem. 2019, 43, e12789. [Google Scholar] [CrossRef]
- Xu, C.; Cai, C.; Liu, T.; Kang, J.; Li, S.; Zhang, J.; Wang, H. Enhancing the antioxidant activity of fish scale collagen hydrolysates through Plastein reaction. Food Bioprocess Technol. 2024, 17, 3693–3703. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Ramos-González, R.; Prado-Barragán, L.A.; Aguilar, C.N.; Rodríguez-Herrera, R.; Iliná, A.; Esparza-González, S.C.; Flores-Gallegos, A.C. Antihypertensive amaranth protein hydrolysates encapsulation in alginate/pectin beads: Influence on bioactive properties upon in vitro digestion. Polysaccharides 2024, 5, 450–462. [Google Scholar] [CrossRef]
- Rezagholizade-Shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive compound encapsulation: Characteristics, applications in food systems, and implications for human health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Jemil, I.; Jridi, M.; Nasri, R.; Ktari, N.; Salem, R.B.S.B.; Mehiri, M.; Hajji, M.; Nasri, M. Functional, antioxidant, and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochem. 2014, 49, 963–972. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Evaluation of the Bioactive Potential of Foods Fortified with Fish Protein Hydrolysates. Food Res. Int. 2020, 137, 109572. [Google Scholar] [CrossRef]
- Xia, W.; Gao, Y.; Fang, X.; Jin, L.; Liu, R.; Wang, L.S.; Deng, Y.; Gao, J.; Yang, H.; Wu, W.; et al. Simulated Gastrointestinal Digestion of Walnut Protein Yields Anti-Inflammatory Peptides. Food Chem. 2024, 445, 138646. [Google Scholar] [CrossRef]




| Parameters | SPH Without Encapsulation | 1% Carrageenan Encapsulated SPH (CN 1%) | 2% Carrageenan Encapsulated SPH (CN 2%) | 3% Carrageenan Encapsulated SPH (CN 3%) |
|---|---|---|---|---|
| Yield (%) | 8.92 ± 0.45 a | 19.74 ± 0.65 b | 33.46 ± 0.17 c | 35.28 ± 0.61 d |
| EE (%) | - | 73.31 ± 1.18 a | 82.51 ± 0.10 b | 84.96 ± 0.84 c |
| Hygroscopicity (%) | 26.08 ± 0.40 d | 16.88 ± 0.39 c | 8.72 ± 0.64 b | 7.24 ± 0.21 a |
| Lightness | 89.33 ± 0.54 a | 90.67 ± 0.81 b | 92.54 ± 0.21 c | 93.58 ± 0.30 d |
| Redness | 0.93 ± 0.005 d | 0.64 ± 0.015 c | 0.38 ± 0.010 b | 0.27 ± 0.005 a |
| Yellowness | 13.78 ± 0.56 d | 12.55 ± 0.38 c | 11.46 ± 0.28 b | 10.55 ± 0.30 a |
| Chroma | 13.81 ± 0.56 d | 12.57 ± 0.39 c | 11.47 ± 0.28 b | 10.56 ± 0.30 a |
| Whiteness | 82.54 ± 0.77 a | 84.33 ± 0.54 b | 86.31 ± 0.23 c | 87.64 ± 0.14 d |
| Amino Acids | Percentage (%) |
|---|---|
| Histidine ** | 23.22 |
| Serine | 1.07 |
| Arginine | 21.93 |
| Glycine | 1.11 |
| Aspartic acid | 0.94 |
| Glutamic acid | 0.35 |
| Threonine | 3.07 |
| Alanine * | 2.13 |
| Proline * | 13.48 |
| Cysteine ** | 1.91 |
| Lysine | 1.97 |
| Tyrosine ** | 20.11 |
| Methionine *,** | 7.30 |
| Valine * | 0.51 |
| Isoleucine * | 0.33 |
| Leucine * | 0.11 |
| Phenylalanine * | 0.45 |
| Solubility (%) | |||||
|---|---|---|---|---|---|
| pH | 2 | 4 | 6 | 8 | 10 |
| Snail protein hydrolysates | 97.31 ± 0.81 bD | 99.96 ± 0.020 dD | 99.26 ± 0.18 cdD | 98.35 ± 0.48 cD | 94.61 ± 0.65 aD |
| Carrageenan-encapsulated SPH (1%) | 72.13 ± 0.40 bC | 79.83 ± 0.58 eC | 77.84 ± 0.65 dC | 74.10 ± 0.98 cC | 70.87 ± 0.42 aC |
| Carrageenan-encapsulated SPH (2%) | 58.31 ± 0.90 bB | 63.32 ± 0.96 dB | 60.39 ± 0.55 cB | 57.06 ± 0.74 bB | 51.75 ± 0.84 aB |
| Carrageenan-encapsulated SPH (3%) | 43.28 ± 0.56 bA | 45.73 ± 0.63 cA | 46.89 ± 0.76 cA | 43.77 ± 0.62 bA | 41.10 ± 0.78 aA |
| Band Position (cm−1) | Functional Group/Vibration Type | SPH Without Encapsulation | 1% Carrageenan-Encapsulated SPH | 2% Carrageenan-Encapsulated SPH | 3% Carrageenan-Encapsulated SPH |
|---|---|---|---|---|---|
| 3319.92–3265.46 | O–H and N–H stretching (hydrogen bonding) | 3265.46 | 3217.39 | 3285.15 | 3319.92 |
| 1583.86–1644.50 | Amide I band (C=O stretching of peptide bonds) | 1644.50 | 1635.81 | 1637.05 | 1583.86 |
| 1548.15–1411.07 | Amide II band (N–H bending, C–N stretching) | 1548.15 | 1560.71 | 1403.97 | 1411.07 |
| 1407.77–1403.97 | CH2 bending vibration | 1407.77 | 1506.57 | 1403.97 | Nil |
| 1249.72–1223.24 | Amide III band (N–H bending, C–N stretching) | 1249.72 | 1246.60 | 1223.24 | 1234.60 |
| 1038.63–1031.29 | C–O stretching (carbohydrate/protein side chains) | 1031.29 | 1036.14 | 1038.63 | 1038.63 |
| 924.81–924.48 | C–O–S or C–O–C stretching (polysaccharide/carrageenan) | Nil | 924.48 | 924.45 | 924.81 |
| 847.05 | Sulfate group (S=O symmetric stretching) | Nil | Nil | Nil | 847.05 |
| 702.42–702.24 | Sulfate group (S=O stretching in carrageenan) | 701.05 | 702.24 | 702.64 | 702.42 |
| 771.54–771.45 | C–H out-of-plane bending or secondary protein structures | 771.45 | Nil | Nil | Nil |
| 620.64–619.88 | Sulfate group (possible S–O vibration) | Nil | Nil | 620.64 | 619.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaishnav, A.; Mehta, N.K.; Priyadarshini, M.B.; Singh, S.K.; Acharya, P.C.; Biswal, S.; Nath, H.; Hussain, S.A.; Pal, P.; Lal, J.; et al. Impact of Carrageenan-Based Encapsulation on the Physicochemical, Structural, and Antioxidant Properties of Freshwater Snail (Bellamya bengalensis) Protein Hydrolysates. Colloids Interfaces 2025, 9, 29. https://doi.org/10.3390/colloids9030029
Vaishnav A, Mehta NK, Priyadarshini MB, Singh SK, Acharya PC, Biswal S, Nath H, Hussain SA, Pal P, Lal J, et al. Impact of Carrageenan-Based Encapsulation on the Physicochemical, Structural, and Antioxidant Properties of Freshwater Snail (Bellamya bengalensis) Protein Hydrolysates. Colloids and Interfaces. 2025; 9(3):29. https://doi.org/10.3390/colloids9030029
Chicago/Turabian StyleVaishnav, Anand, Naresh Kumar Mehta, Mocherla Bhargavi Priyadarshini, Soibam Khogen Singh, Pratap Chandra Acharya, Satyajeet Biswal, Harjeet Nath, Syed Arshad Hussain, Prasenjit Pal, Jham Lal, and et al. 2025. "Impact of Carrageenan-Based Encapsulation on the Physicochemical, Structural, and Antioxidant Properties of Freshwater Snail (Bellamya bengalensis) Protein Hydrolysates" Colloids and Interfaces 9, no. 3: 29. https://doi.org/10.3390/colloids9030029
APA StyleVaishnav, A., Mehta, N. K., Priyadarshini, M. B., Singh, S. K., Acharya, P. C., Biswal, S., Nath, H., Hussain, S. A., Pal, P., Lal, J., Singh, N. S., & Pati, B. K. (2025). Impact of Carrageenan-Based Encapsulation on the Physicochemical, Structural, and Antioxidant Properties of Freshwater Snail (Bellamya bengalensis) Protein Hydrolysates. Colloids and Interfaces, 9(3), 29. https://doi.org/10.3390/colloids9030029