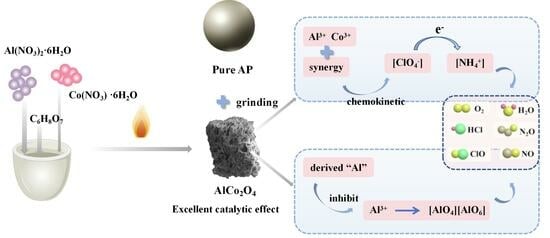
One-Step Synthesis AlCo2O4 and Derived “Al” to Double Optimise the Thermal Decomposition Kinetics and Enthalpy of Ammonium Perchlorate
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of AlCo2O4
2.3. Materials Characterisation
2.4. Catalytic Test
2.5. Combustion Measurements of AP/HTPB-Based CSPs
3. Results and Discussion
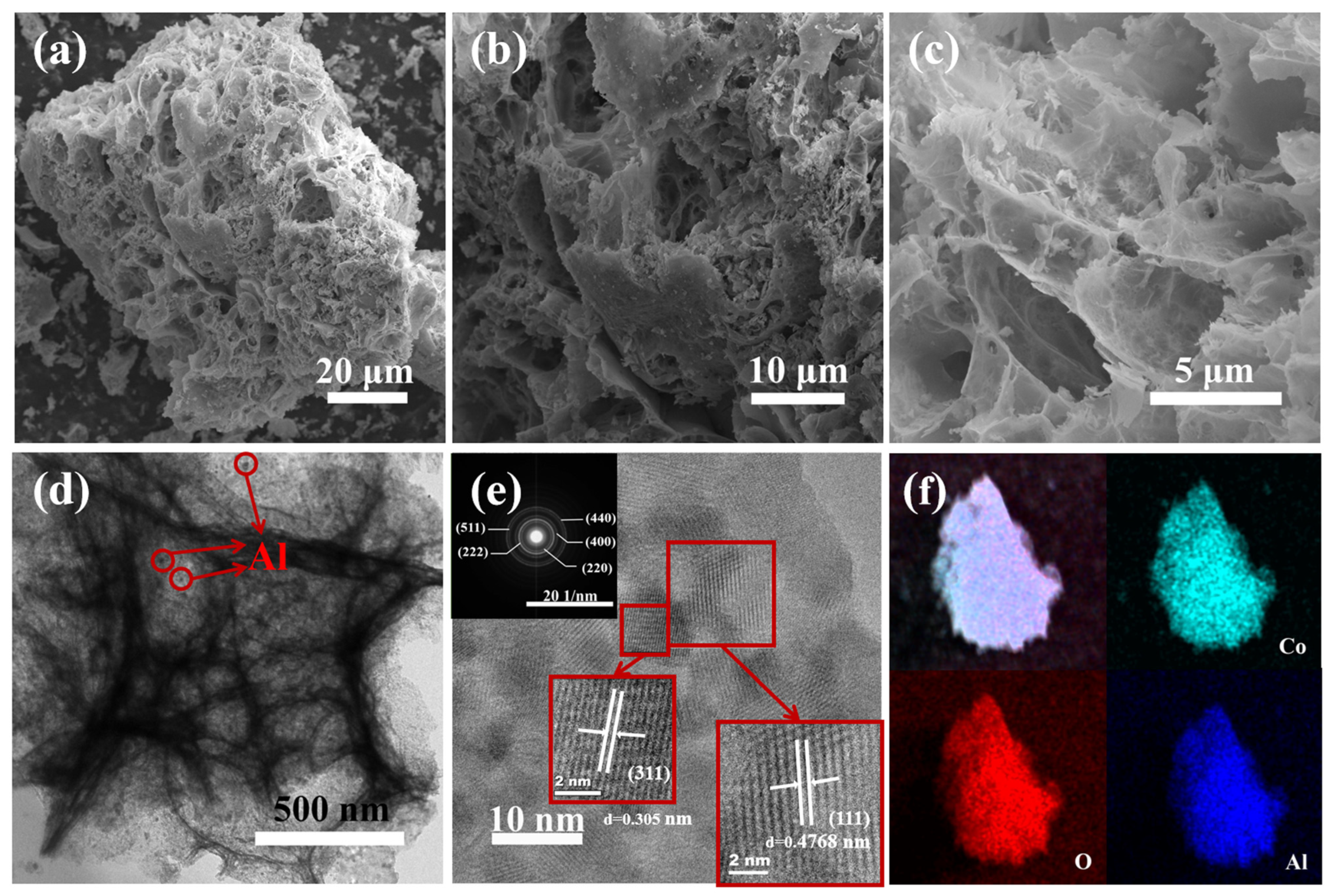
3.1. Characterisation of AlCo2O4
3.2. Catalytic Properties of AlCo2O4 Catalysts for AP Decomposition
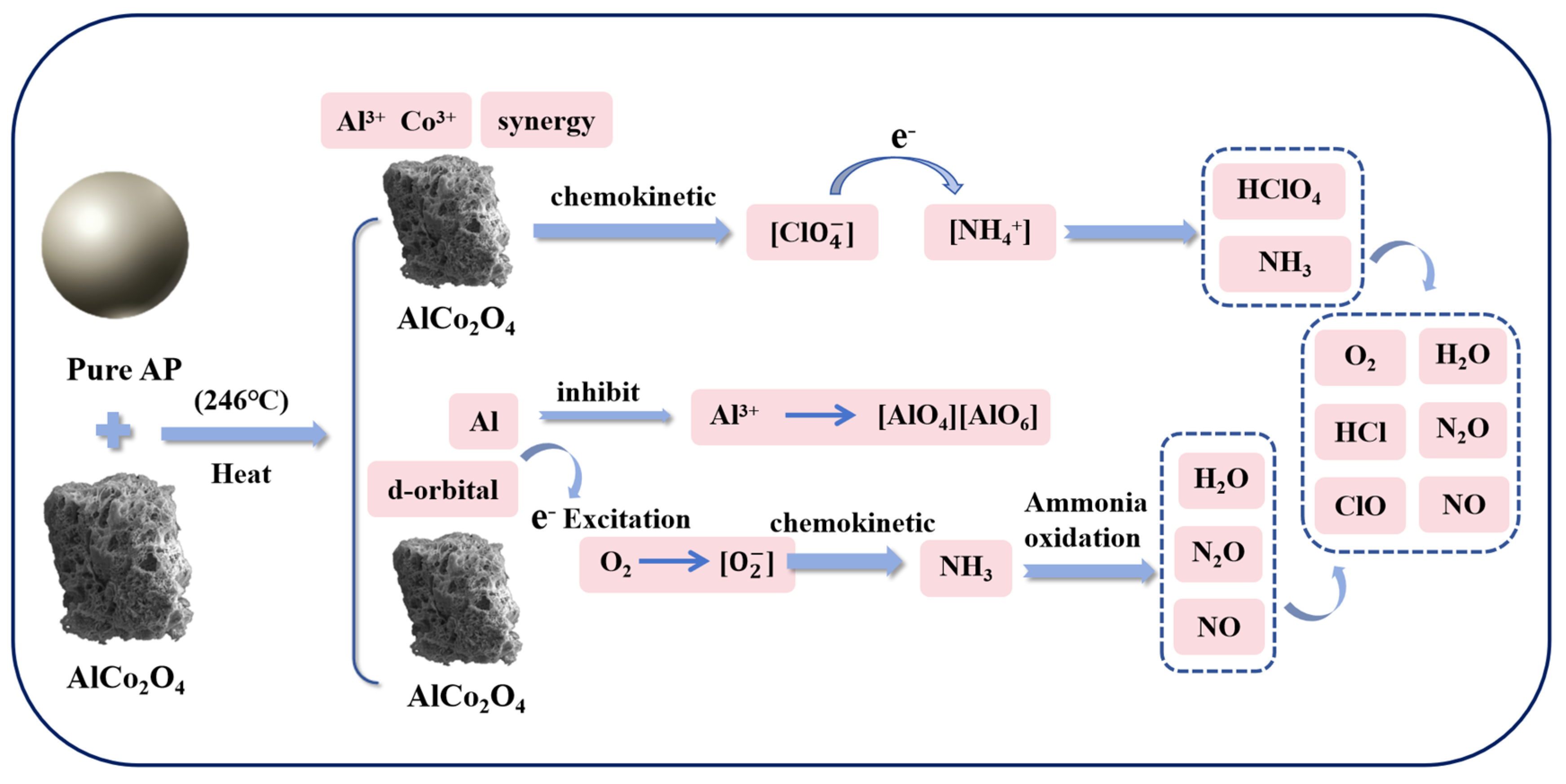
3.3. Catalytic Mechanism of AlCo2O4 Catalysts for AP Decomposition
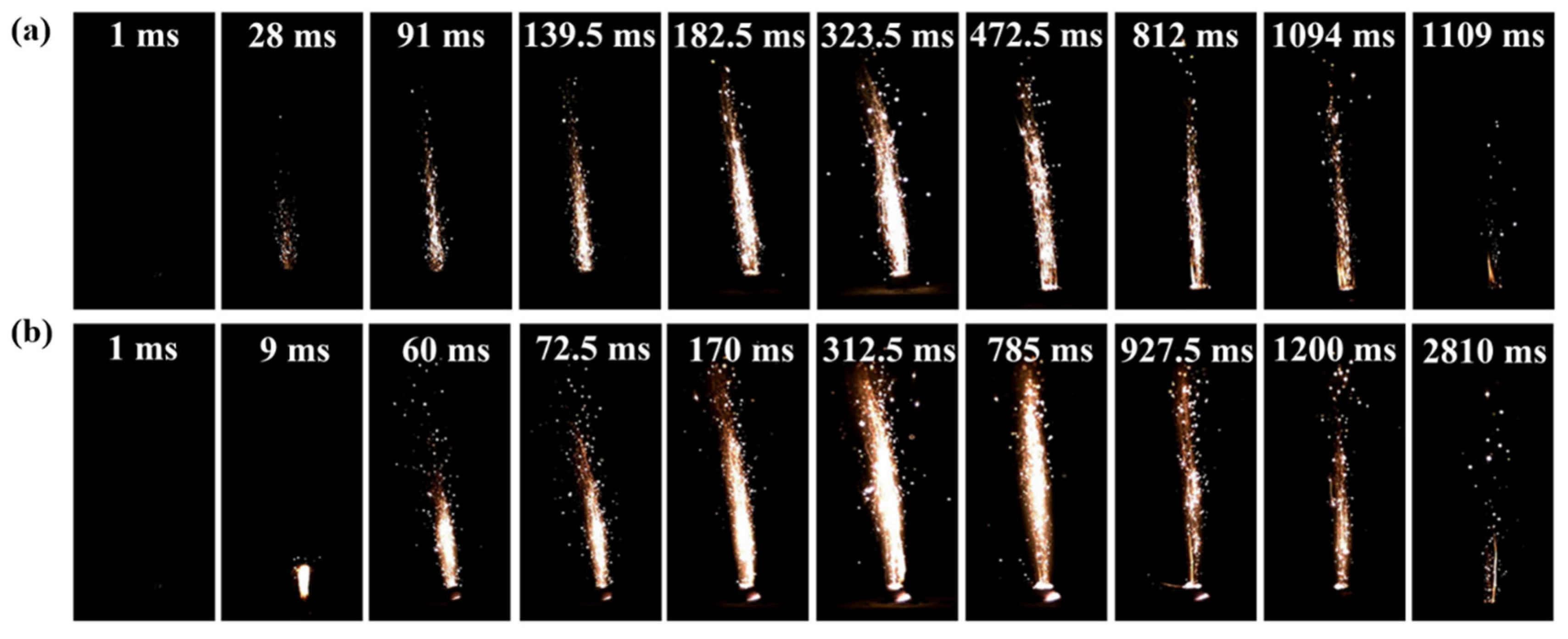
3.4. Application in AP/HTPB-Based CSPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyu, J.-Y.; Yang, S.-L.; Wu, S.; Tang, G.; Yang, W.; Yan, Q.-L. Burning Rate Modulation for Composite Propellants by Interfacial Control of Al@Ap with Precise Catalysis of Cuo. Combust. Flame 2022, 240, 112029. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N.; Patel, N.N. Nano-Alloys: Potential Catalyst for Thermal Decomposition of Ammonium Perchlorate. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 44, 258–262. [Google Scholar] [CrossRef]
- Bai, C.; Yang, D.; Liu, C.; Zhu, F.; Tu, C.; Li, G.; Luo, Y. In Situ Synthesis NiO@TiO2/MXene as a Promoter for Ammonium Perchlorate Based Solid Propellants. Appl. Surf. Sci. 2024, 652, 159228. [Google Scholar] [CrossRef]
- Song, J.; Guo, T.; Yao, M.; Chen, J.; Ding, W.; Liu, X. Effects of Perchlorates on Thermal Properties and Combustion Performance of Al-Mno2 Nanothermite. Chin. J. Energ. Mater. 2020, 28, 953–959. [Google Scholar]
- Liu, L.L.; Li, F.S.; Tan, L.H.; Li, M.; Yang, Y. Effects of Metal and Composite Metal Nanopowders on the Thermal Decomposition of Ammonium Perchlorate (AP) and the Ammonium Perchlorate/Hydroxyterminated Polybutadiene (Ap/HTPB) Composite Solid Propellant. Chin. J. Chem. Eng. 2004, 12, 595–598. [Google Scholar]
- Wang, H.; Yang, H.; Zhu, X.; Yang, Y.; Cheng, Y.; Song, D. Study on the Design and Performance of Double-Base Propellant/Ammonium Perchlorate Based Incendiary Agent. Chin. J. Explos. Propellants 2017, 40, 15. [Google Scholar]
- Yang, S.-L.; Xu, R.; He, W.; Nie, H.; Yan, Q.-L. Enhancing the Thermal Reactivity of AP Crystals by Coating of Al-Based Bi-Metal Nanocomposites. Fuel 2022, 324, 124588. [Google Scholar] [CrossRef]
- Gromov, A. Research on Ignition, Combustion and Catalysis of Energetic Systems with Metal Nanopowders; Russian Science Foundation: Moscow, Russia, 1800. [Google Scholar]
- Wang, X.; Hu, Y.; Zhang, J.; Liang, J.; Yang, Y.; Lin, K.; Pang, A.; Shuai, Y. Capsule Structured Al/FeF3/AP Energetic Microspheres with Enhanced Combustion Performance and Energy Release Efficiency by a Microexplosion Reaction. Fuel 2023, 340, 127546. [Google Scholar] [CrossRef]
- Ma, Z.; Li, F.; Chen, A.; Song, H. Preparation and Characterization of Composite Particles of Al/Ammonium Perchlorate. J. Propuls. Technol. 2004, 25, 373–376. [Google Scholar]
- Chaturvedi, S.; Dave, P.N. Solid Propellants: Ap/Htpb Composite Propellants. Arab. J. Chem. 2019, 12, 2061–2068. [Google Scholar] [CrossRef]
- Lyu, J.-Y.; Xu, G.; Zhang, H.; Yang, W.; Yan, Q.-L. Thermal Decomposition and Combustion Behavior of the Core-Shell Al@AP Composite Embedded with Cuo as a Catalyst. Fuel 2024, 356, 129587. [Google Scholar] [CrossRef]
- Ao, W.; Liu, P.; Yang, W. Agglomerates, Smoke Oxide Particles, and Carbon Inclusions in Condensed Combustion Products of an Aluminized GAP-based Propellant. Acta Astronaut. 2016, 129, 147–153. [Google Scholar] [CrossRef]
- Elbasuney, S.; Fahd, A.; Mostafa, H.E. Combustion Characteristics of Extruded Double Base Propellant Based on Ammonium Perchlorate/Aluminum Binary Mixture. Fuel 2017, 208, 296–304. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, R.; Nie, H.; Yan, Q.; Liu, J.; Sun, Y. Insight into the Precise Catalytic Mechanism of Cuo on the Decomposition and Combustion of Core–Shell Al@Ap Particles. Fuel 2023, 346, 128294. [Google Scholar] [CrossRef]
- Gržetić, J.D.; Mijatov, S.; Fidanovski, B.; Kovacevic, T.; Pavlovic, V.B.; Brzic, S.; Bajic, D. Thermal Decomposition of Ammonium Perchlorate Encapsulated with Copper(Ii)/Iron(Iii) Oxide Nanoparticles. Cent. Eur. J. Energetic Mater. 2023, 20, 114–137. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Thermal Decomposition of Ammonium Perchlorate. Thermochim. Acta 2006, 443, 1–36. [Google Scholar] [CrossRef]
- Deng, P.; Wang, H.; Yang, X.; Ren, H.; Jiao, Q. Thermal Decomposition and Combustion Performance of High-Energy Ammonium Perchlorate-Based Molecular Perovskite. J. Alloys Compd. 2020, 827, 154257. [Google Scholar] [CrossRef]
- Mehilal, S.J.; Nandagopal, S.; Singh, P.P.; Radhakrishnan, K.K.; Bhattacharya, B. Size and Shape of Ammonium Perchlorate and Their Influence on Properties of Composite Propellant. Def. Sci. J. 2009, 59, 294–299. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Kumari, A.; Mehilal; Jain, S.; Jain, M.; Rao, A.S. Preparation, Characterization & Evaluation of Nano-Ammonium Perchlorate. In Proceedings of the 7th International Fall Seminar on Propellants, Explosives and Pyrotechnics, Xi’an, China, 23–26 October 2007; pp. 86–89. [Google Scholar]
- Paulose, S.; Raghavan, R.; George, B.K. Graphite Oxide–Iron Oxide Nanocomposites as a New Class of Catalyst for the Thermal Decomposition of Ammonium Perchlorate. RSC Adv. 2016, 6, 45977–45985. [Google Scholar] [CrossRef]
- Ke, X.; Zhou, X.; Hao, G.Z.; Xiao, L.; Gao, H.; Chen, T.; Jiang, W. Template-Assisted Synthesis of 3d Ordered Macroporous Structured Cuo as Catalyst for Ammonium Perchlorate. Funct. Mater. Lett. 2017, 10, 1700530. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Cui, W.; Liu, C.; Wang, S.; Zheng, S.; Zhang, Y. Synthesis of Nanostructured γ-Alooh and Its Accelerating Behavior on the Thermal Decomposition of Ap. RSC Adv. 2016, 6, 27235–27241. [Google Scholar] [CrossRef]
- XXiao, C.; Peng, B.G.; Cai, L.F.; Zhang, X.M.; Liu, S.R.; Wang, Y.D. The High Efficient Catalytic Properties for Thermal Decomposition of Ammonium Perchlorate Using Mesoporous ZnCo2O4 Rods Synthesized by Oxalate Co-Precipitation Method. Sci. Rep. 2018, 8, 7571. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Dubey, S.; Siril, P.F. Kinetics of Thermal Decomposition of Ammonium Perchlorate with Nanocrystals of Binary Transition Metal Ferrites. Propellants Explos. Pyrotech. 2009, 34, 72–77. [Google Scholar] [CrossRef]
- Li, K.; Liao, J.; Huang, S.; Lei, Y.; Zhang, Y.; Zhu, W. Enhanced Catalytic Properties of Cobaltosic Oxide through Constructing Mxene-Supported Nanocomposites for Ammonium Perchlorate Thermal Decomposition. Appl. Surf. Sci. 2021, 570, 151224. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Zheng, Z.; Gao, Y.; Ma, K.; Ye, J.; Yang, Y. Enhanced Thermal Decomposition Properties of Ammonium Perchlorate through Addition of 3DOM core-shell Fe2O3/Co3O4 Composite. J. Alloys Compd. 2017, 724, 720–727. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, B.; Ma, X.; Wang, J.; Liu, J.; Wu, R.; Zheng, Z.; Liu, J.; Ma, K. Controllable Synthesis of Multi-Shelled Nico2o4hollow Spheres Catalytically for the Thermal Decomposition of Ammonium Perchlorate. RSC Adv. 2019, 9, 23888–23893. [Google Scholar] [CrossRef]
- Gao, X.; Deng, P.; Han, K.; Cao, Y. Facile Synthesis of MgCo2O4 Nanosheets and Its Catalysis Effect on the Decomposition of Ammonium Perchlorate. Can. J. Chem. 2022, 100, 429–433. [Google Scholar] [CrossRef]
- Wang, J.G.; Jin, L.N.; Qian, X.Y.; Dong, M.D. Preparation and Catalytic Activity Comparison of Porous NiO, Co3O4 and NiCo2O4 Superstructures on the Thermal Decomposition of Ammonium Perchlorate. J. Nanosci. Nanotechnol. 2016, 16, 8635–8639. [Google Scholar] [CrossRef]
- Ou, Y.; La, P.; Wei, Y.; Zhu, D. Research Progress in Preparation Methods of Nano Metal Oxide. Mater. Rev. 2012, 26, 36–39. [Google Scholar]
- GLiu, Y.; Wang, B.S.; He, Y.; Guo, J.M. Phase Structures of LiMn1.95Fe0.05O4 Prepared by Solution Combustion Synthesis and Molten-Salt Combustion Synthesis Methods. In Proceedings of the International Conference on Mechanics, Dynamic Systems and Materials Engineering (MDSME 2012), Guangzhou, China, 24–25 November 2012. [Google Scholar]
- González-Cortés, S.L.; Imbert, F.E. Fundamentals, Properties and Applications of Solid Catalysts Prepared by Solution Combustion Synthesis (Scs). Appl. Catal. A Gen. 2013, 452, 117–131. [Google Scholar] [CrossRef]
- Li, F.-T.; Ran, J.; Jaroniec, M.; Qiao, S.Z. Solution Combustion Synthesis of Metal Oxide Nanomaterials for Energy Storage and Conversion. Nanoscale 2015, 7, 17590–17610. [Google Scholar] [CrossRef] [PubMed]
- Vasei, H.V.; Masoudpanah, S.M.; Habibollahzadeh, M. Different Morphologies of ZnO Via Solution Combustion Synthesis: The Role of Fuel. Mater. Res. Bull. 2020, 125, 110784. [Google Scholar] [CrossRef]
- Xiao, X.; Li, Y.; Chen, N.; Xing, X.; Deng, D.; Wang, Y. Combustion Agent Mediated Flash Synthesis of Porous MCo2O4 (M = Zn, Ni, Cu and Fe) Via Self-Sustained Decomposition of Metal-Organic Complexes. Mater. Lett. 2017, 195, 123–126. [Google Scholar] [CrossRef]
- Ayaş, A.O.; Ekicibil, A.; Çetin, S.K.; Coşkun, A.; Er, A.O.; Ufuktepe, Y.; Fırat, T.; Kıymaç, K. The Structural, Superconducting and Transport Properties of the Compounds Y3Ba5Cu8O18 and Y3Ba5Ca2Cu8O18. J. Supercond. Nov. Magn. 2011, 24, 2243–2252. [Google Scholar] [CrossRef]
- Dejene, F.B. Sol-gel Produced Zn2V2O7 Phosphors’ Improved Luminous Properties: The Role of Thermal Treatment. Heliyon 2023, 9, e13878. [Google Scholar] [CrossRef]
- Gonçalves, N.S.; Carvalho, J.A.; Lima, Z.M.; Sasaki, J.M. Size–Strain Study of Nio Nanoparticles by X-Ray Powder Diffraction Line Broadening. Mater. Lett. 2012, 72, 36–38. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Lima, F.M.; Martins, F.M.; Maia, P.H.; Almeida, A.F.L.; Freire, F.N.A. Nanostructured Titanium Dioxide Average Size from Alternative Analysis of Scherrer’s Equation. Matéria 2018, 23, e11965. [Google Scholar] [CrossRef]
- MLee, S.; Kim, S.I.; Lee, M.J.; Ye, B.; Kim, T.; Kim, H.D.; Lee, J.W.; Lee, D.H. Effect of Catalyst Crystallinity on V-Based Selective Catalytic Reduction with Ammonia. Nanomaterials 2021, 11, 1452. [Google Scholar] [CrossRef]
- Shi, J.; Wang, H.; Liu, Y.; Ren, X.; Sun, H.; Lv, B. Rapid Microwave-Assisted Hydrothermal Synthesis of CeO2 Octahedra with Mixed Valence States and Their Catalytic Activity for Thermal Decomposition of Ammonium Perchlorate. Inorg. Chem. Front. 2019, 6, 1735–1743. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chen, H.P.; Hsieh, M.F.; Sun, H.F.; Lai, C.W. Regeneration of Bleaching Clay Waste by Chemical Activation with Chloride Salts. J. Environ. Sci. Health Part A 2003, 38, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, J.; Zhou, Y.; Zhang, Y.; Cen, K. Thermal Decomposition and Combustion Characteristics of Al/AP/HTPB Propellant. J. Therm. Anal. Calorim. 2020, 143, 3935–3944. [Google Scholar] [CrossRef]
- Song, M.; Chen, M.; Zhang, Z. Effect of Zn Powders on the Thermal Decomposition of Ammonium Perchlorate. Propellants Explos. Pyrotech. 2008, 33, 261–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, A.; Ma, D.; Ma, M. The Catalytic Decomposition and Kinetic Analysis of Ammonium Perchlorate on MgO Nanoflakes. J. Phys. Chem. Solids 2021, 157, 110205. [Google Scholar] [CrossRef]
- Hirt, B.D.; Wernex, C.W.; Sehirlioglu, A.; Örnek, M.; Son, S.F. Novel Protonated LiCoO2 as a Catalyst for the Thermal Decomposition of Ammonium Perchlorate. J. Energetic Mater. 2023, 1–20. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Li, H.; Zhao, F.; Yi, J.; Jin, B.; Peng, R. Preparation of Zn–Co Bimetallic Catalysts and Their Catalytic Properties for Thermal Decomposition of Ammonium Perchlorate. Langmuir 2024, 40, 15421–15429. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Fu, X.C.; Deng, N.M. Cu/Carbon Aerogels Derived from Hkust-1 for the Thermal Decomposition of Ammonium Perchlorate. ACS Appl. Nano Mater. 2024, 7, 17373–17378. [Google Scholar] [CrossRef]
- Sa, M.; Yarmohammadi, M.; Pedram, M.Z.; Shahidzadeh, M.; Amini-Fazl, M.S. Evaluation of the Catocene/Graphene Oxide Nanocomposite Catalytic Activity on Ammonium Perchlorate Thermal Decomposition. Int. J. Chem. Kinet. 2019, 51, 337–345. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger Method in Kinetics of Materials: Things to Beware and be Aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Huang, H.; Ren, H.; Jiao, Q.-J. Kinetics of Thermal Decomposition of Ammonium Perchlorate by TG/DSC-MS-Ftir. J. Energetic Mater. 2013, 32, 16–26. [Google Scholar] [CrossRef]
- Babar, Z.U.D.; Malik, A.Q. An Investigation of Thermal Decomposition Kinetics of Nano Zinc Oxide Catalyzed Composite Propellant. Combust. Sci. Technol. 2015, 187, 1295–1315. [Google Scholar] [CrossRef]
- Nakhowong, R.; Kiennork, S.; Wongwanwattana, P.; Seetawan, T.; Chueachot, R. Synthesis, Structural and Optical Properties of Electrospun Magnesium Aluminate Nanofibers. Mater. Lett. 2018, 220, 234–237. [Google Scholar] [CrossRef]
- Ohtsu, N.; Oku, M.; Shishido, T.; Wagatsuma, K. X-Ray Photoelectron Spectroscopic Studies on Phase Identification and Quantification of Nickel Aluminides. Appl. Surf. Sci. 2007, 253, 8713–8717. [Google Scholar] [CrossRef]
- Haasch, R.T.; Abraham, D.P. Lithium-Based Transition-Metal Oxides for Battery Electrodes Analyzed by X-Ray Photoelectron Spectroscopy. V. LiNi0.4Co0.3Mn0.3O2. Surf. Sci. Spectra 2019, 26, 014007. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Thorat, G.M.; Seo, J.G. Facile and Cost-Effective Growth of a Highly Efficient MgCo2O4 Electrocatalyst for Methanol Oxidation. Inorg. Chem. Front. 2018, 5, 1115–1120. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, G.; Wang, Z.; Zhu, Y.; Yan, Z.; Wang, Y. Porous Cobaltate: Structure, Active Sites, Thermocatalytic Properties for Ammonium Perchlorate Decomposition. J. Alloys Compd. 2022, 908, 164624. [Google Scholar] [CrossRef]
- STran, B.T.; Choi, H.; Oh, S.; Park, J.Y. Defective Nb2O5-Supported Pt Catalysts for CO Oxidation: Promoting Catalytic Activity Via Oxygen Vacancy Engineering. J. Catal. 2019, 375, 124–134. [Google Scholar] [CrossRef]
- Lei, J.; Zhang, L.; Li, M.; Liu, W.; Jin, Y.; Li, B. Surface Oxygen Vacancy-Rich Co3o4 Nanowires as an Effective Catalyst of Luminol–H2O2 Chemiluminescence for Sensitive Immunoassay. Anal. Chem. 2023, 95, 17937–17944. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, S.; Zhang, L.; Xiang, G.; Wang, J.; Zeng, X.; Chen, J. Facet Effect of Co3O4 Nanocatalysts on the Catalytic Decomposition of Ammonium Perchlorate. J. Hazard. Mater. 2020, 392, 122358. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, L.; Lu, L.; Yang, X.; Wang, X. Dsc/Tg-Ms Study on in Situ Catalytic Thermal Decomposition of Ammonium Perchlorate over CoC2O4. Chin. J. Catal. 2009, 30, 19–23. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Jin, B.; Zeng, M.; Peng, R. Cu-Mof Derived Cu/Cu2O/C Nanocomposites for the Efficient Thermal Decomposition of Ammonium Perchlorate. J. Solid State Chem. 2021, 297, 122060. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, X.; Wang, Z.; Li, S.; Zhao, Z.; Zhu, Y.; Yan, Z.; Chen, G.; Xiao, X. Double-Modified C–MgCo2O4 as a Highly Active Catalytic Material for the Thermal Decomposition of Ap and Its Application for Csps. Carbon 2023, 215, 118484. [Google Scholar] [CrossRef]






| Catalyst | BJH Pore Size (nm) | BJH Pore Volume (cm3/g) | BET (m2/g) |
|---|---|---|---|
| AlCo2O4-0.5 | 34.024 | 0.264 | 178.825 |
| AlCo2O4-1 | 33.158 | 0.190 | 182.035 |
| AlCo2O4-2 | 32.152 | 0.152 | 201.730 |
| Catalyst | Mass % | Reduction in HTD Temperature (°C) | Increase in Decomposition Heat (J⋅g−1) | Decrease of Ea (kJ⋅mol−1) | Refs. |
|---|---|---|---|---|---|
| MgO | 2.0 | 114.9 | 511.6 | — | [47] |
| LCOP-3 | 2.0 | 54.6 | — | 45.0 | [48] |
| Al/FeF3 | 30.0 | 41.2 | — | 28.93 | [9] |
| Al@AP | — | 32.4 | 381.2 | 48.0 | [12] |
| Al@AP/CuO | — | 87.3 | 159.2 | 55.8 | |
| Zn-Co | 10.0 | 135.4 | — | 82.28 | [49] |
| Cu/C | 5.0 | 60 | — | 82.12 | [50] |
| AlCo2O4-0.5 | 2.0 | 188.08 | 1088.04 | 84.47 | This work |
| AlCo2O4-1 | 2.0 | 179.68 | 1436.14 | — | This work |
| AlCo2O4-2 | 2.0 | 169.18 | 1555.14 | — | This work |
| Conversion (α) | Friedman Method (Ea, kJ·mol−1) | |
|---|---|---|
| Pure AP | 2% AlCo2O4-0.5 + AP | |
| 0.1 | 199.54 | 102.01 |
| 0.2 | 221.38 | 191.91 |
| 0.3 | 228.81 | 202.67 |
| 0.4 | 236.42 | 216.64 |
| 0.5 | 249.76 | 221.41 |
| 0.6 | 272.13 | 226.89 |
| 0.7 | 284.64 | 211.06 |
| 0.8 | 295.87 | 194.71 |
| 0.9 | 300.28 | 160.51 |
| Samples | AlCo2O4-0.5 | AlCo2O4-1 | AlCo2O4-2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co 2p | Co Ion Valence State | +3 | +2 | +3 | +2 | +3 | +2 | ||||||
| Co 2p3/2 peak position (eV) | 780.74 | 781.23 | 780.27 | 781.79 | 780.13 | 781.38 | |||||||
| Co 2p1/2 peak position (eV) | 795.78 | 797.78 | 795.24 | 796.71 | 795.18 | 796.55 | |||||||
| Relative atomic content (%) | 62.11 | 37.89 | 50.77 | 49.23 | 48.72 | 51.28 | |||||||
| Co3+/Co2+ | 1.64 | 1.03 | 0.95 | ||||||||||
| Al 2p | Al elements form | +3 | metallic Al | +3 | metallic Al | +3 | metallic Al | ||||||
| Al 2p Peak position (eV) | 74 | 71.61 | 73.75 | 71.33 | 73.79 | 71.85 | |||||||
| Relative atomic content (%) | 78.82 | 21.12 | 90.16 | 9.84 | 87.42 | 12.58 | |||||||
| Al/Al3+ | 0.27 | 0.11 | 0.14 | ||||||||||
| O 1s | O element existence form | OLatt | OV | -OH | OLatt | OV | -OH | OLatt | OV | -OH | |||
| O 1s Peak position (eV) | 530.22 | 531.13 | 532.46 | 530.18 | 531.34 | 532.39 | 530.27 | 531.34 | 532.68 | ||||
| Relative atomic content (%) | 0.41 | 0.43 | 0.16 | 0.57 | 0.31 | 0.12 | 0.58 | 0.34 | 0.08 | ||||
| O V/(OLatt+-OH) | 0.76 | 0.45 | 0.52 | ||||||||||
| Samples | Ingredient | Content Ratio (wt) | Functionality |
|---|---|---|---|
| AP/HTPB | AP | 74 | Oxidising agent |
| HTPB | 11 | Binding agent | |
| Al | 15 | Metallic fuel agent | |
| AlCo2O4-0.5 -AP/HTPB | AP | 67 | Oxidising agent |
| HTPB | 11 | Binding agent | |
| Al | 15 | Metallic fuel agent | |
| AlCo2O4-0.5 | 7 | Catalyst |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Yang, Y.; Zhao, Z.; Yan, Z.; Xiao, X. One-Step Synthesis AlCo2O4 and Derived “Al” to Double Optimise the Thermal Decomposition Kinetics and Enthalpy of Ammonium Perchlorate. Colloids Interfaces 2025, 9, 28. https://doi.org/10.3390/colloids9030028
He K, Yang Y, Zhao Z, Yan Z, Xiao X. One-Step Synthesis AlCo2O4 and Derived “Al” to Double Optimise the Thermal Decomposition Kinetics and Enthalpy of Ammonium Perchlorate. Colloids and Interfaces. 2025; 9(3):28. https://doi.org/10.3390/colloids9030028
Chicago/Turabian StyleHe, Kaihua, Yanzhi Yang, Zhengyi Zhao, Zhiyong Yan, and Xuechun Xiao. 2025. "One-Step Synthesis AlCo2O4 and Derived “Al” to Double Optimise the Thermal Decomposition Kinetics and Enthalpy of Ammonium Perchlorate" Colloids and Interfaces 9, no. 3: 28. https://doi.org/10.3390/colloids9030028
APA StyleHe, K., Yang, Y., Zhao, Z., Yan, Z., & Xiao, X. (2025). One-Step Synthesis AlCo2O4 and Derived “Al” to Double Optimise the Thermal Decomposition Kinetics and Enthalpy of Ammonium Perchlorate. Colloids and Interfaces, 9(3), 28. https://doi.org/10.3390/colloids9030028