Preparation of Colloidal Silver Triangular Nanoplates and Their Application in SERS Detection of Trace Levels of Antibiotic Enrofloxacin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
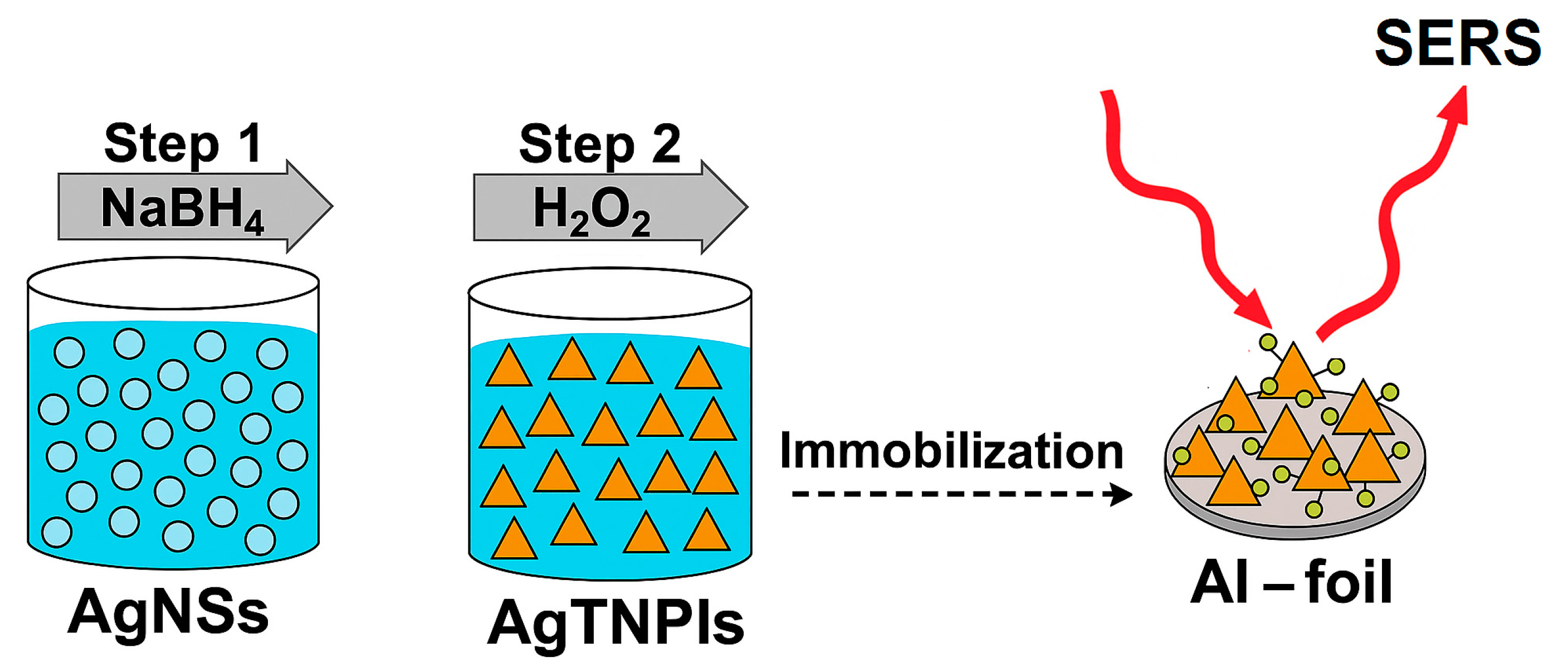
2.2. Prepararion of the Silver Nanospheres (AgNSs) and Silver Triangular Nanoplate (AgTNPl) Colloids
2.3. Preparations for SERS Measurements
2.4. Instrumentations
3. Results
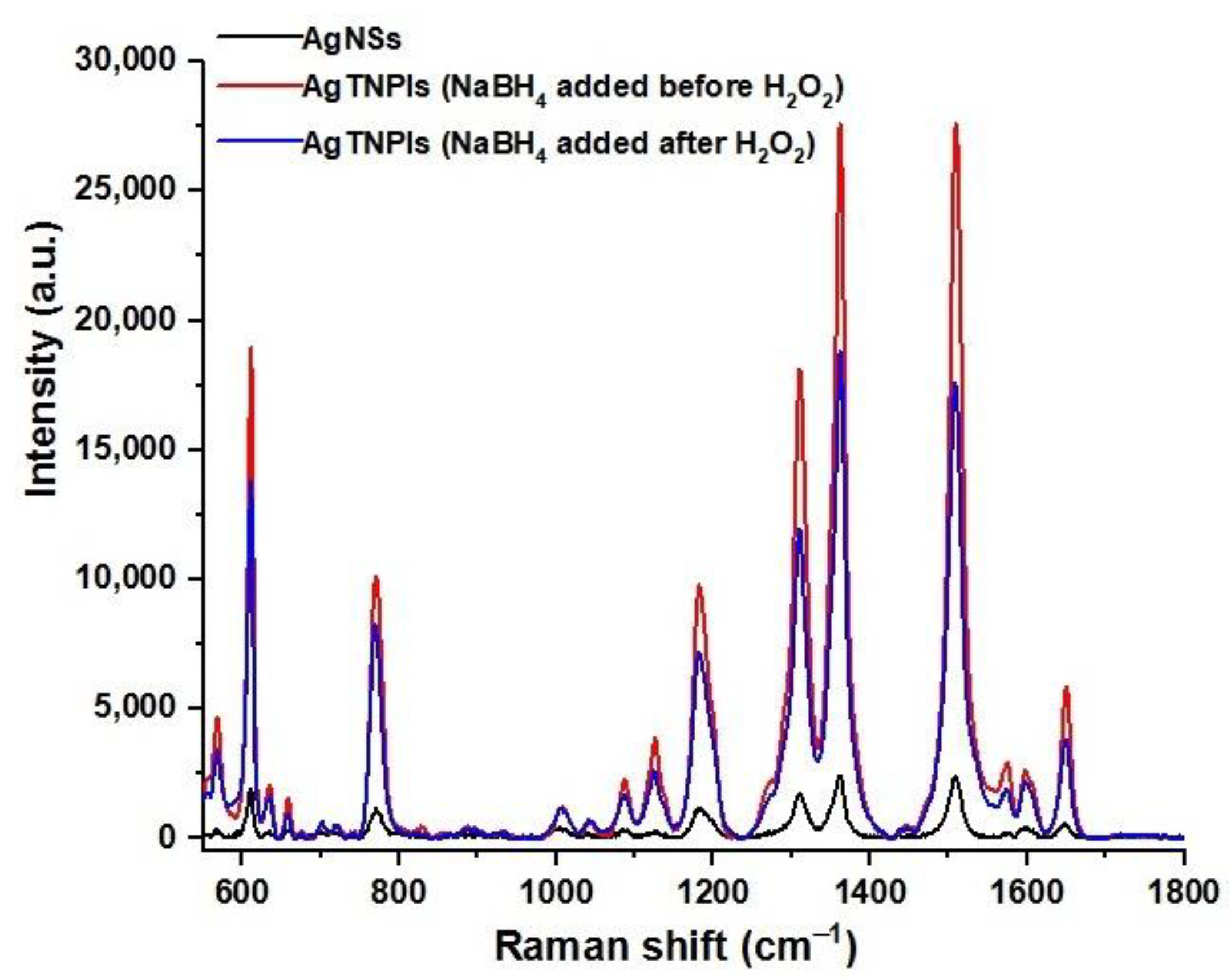
3.1. The AgTNPls Synthesis Process
3.2. Color of Silver Colloids
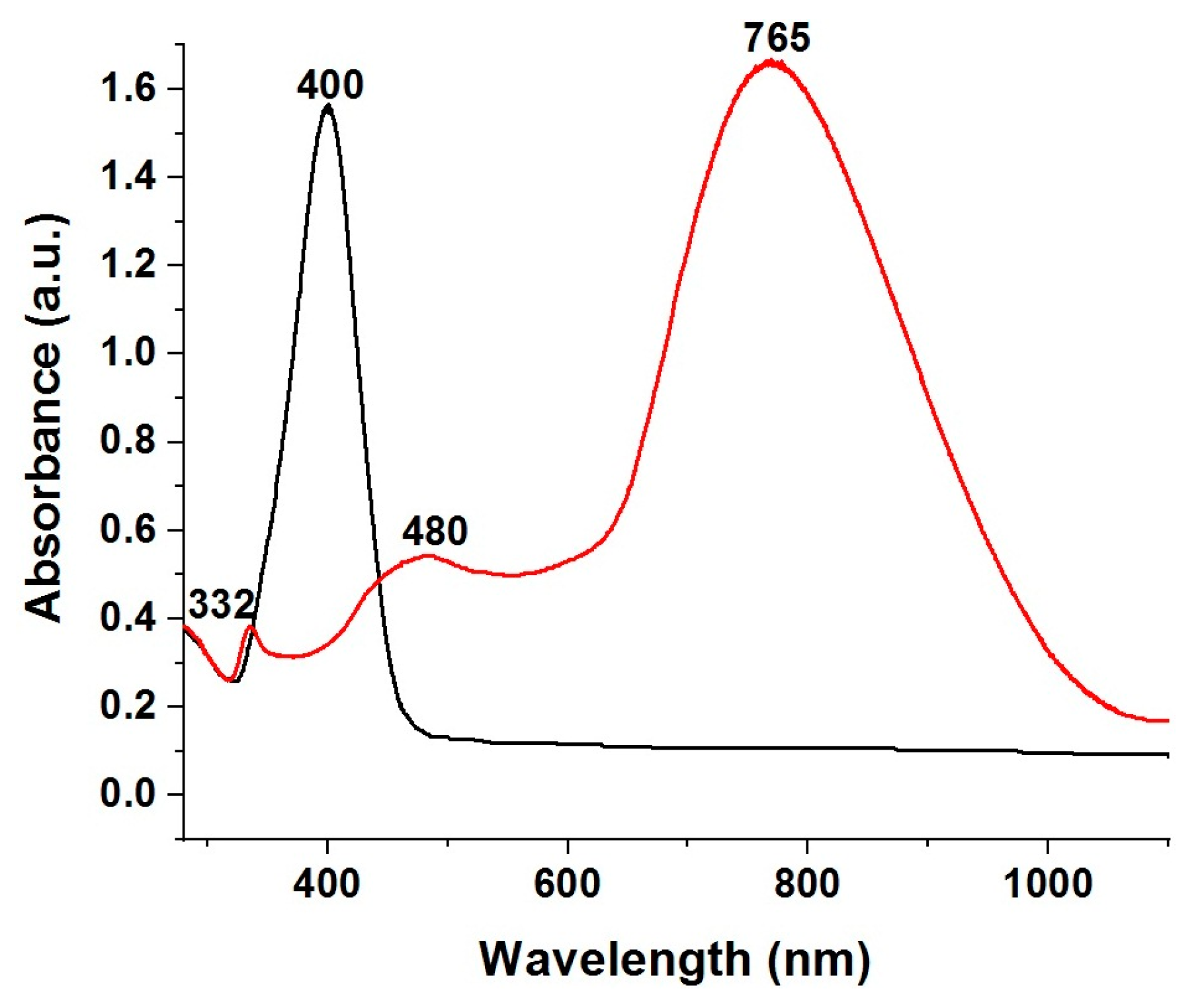
3.3. UV-Vis Spectroscopy of Silver Colloids
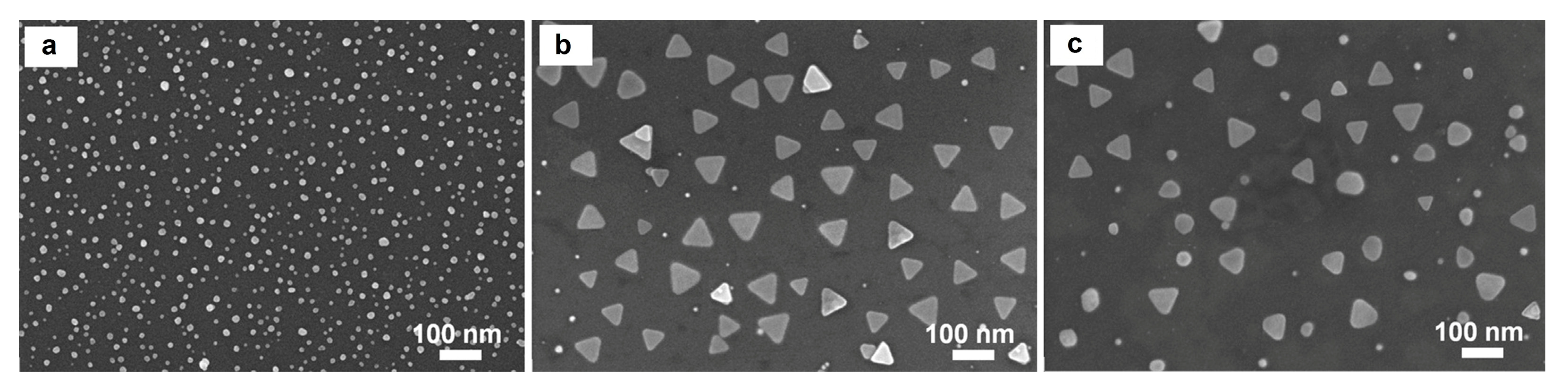
3.4. SEM Images of Synthesized AgNSs and AgTNPLs
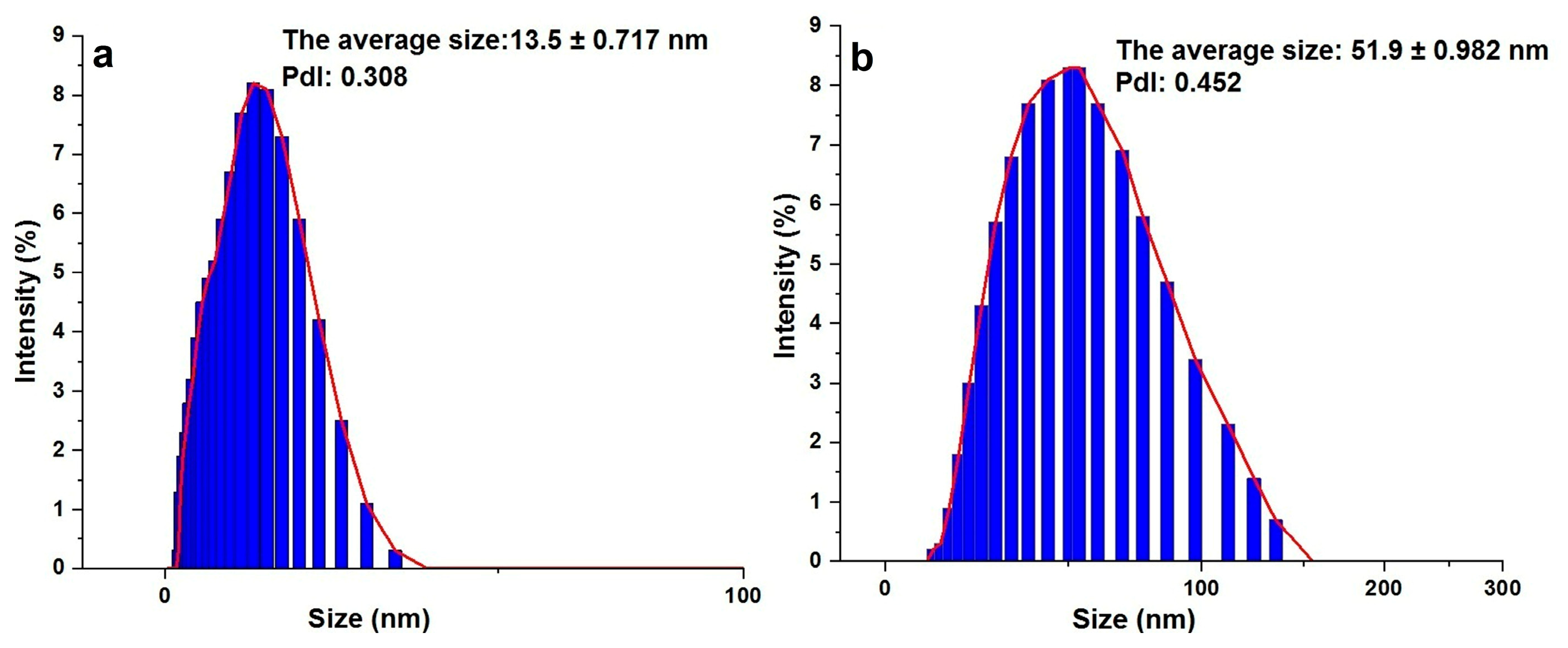
3.5. Size of the Synthesized AgNSs and AgTNPls
3.6. Recording the SERS Spectrum of R6G to Check the Quality of the SERS Substrate
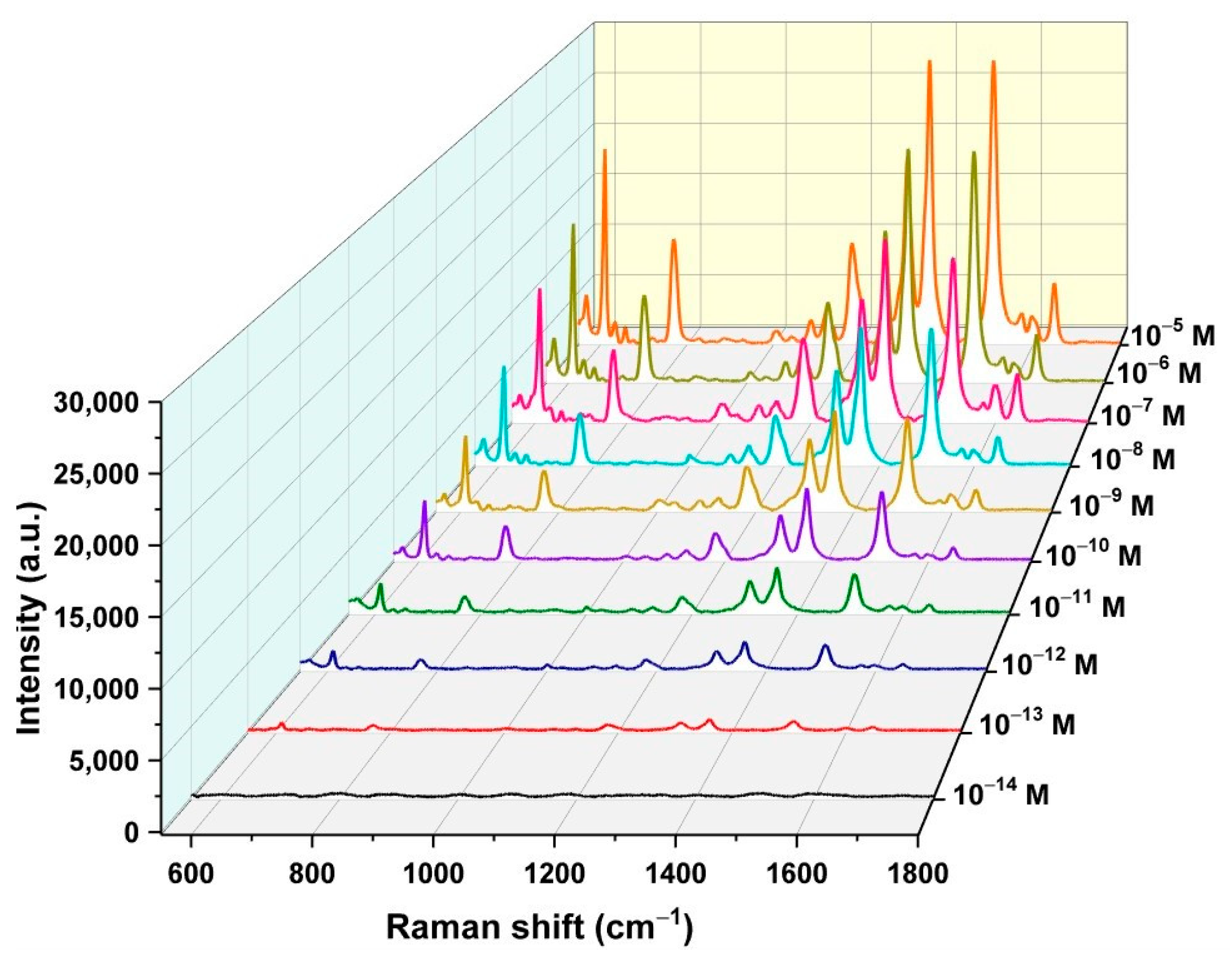
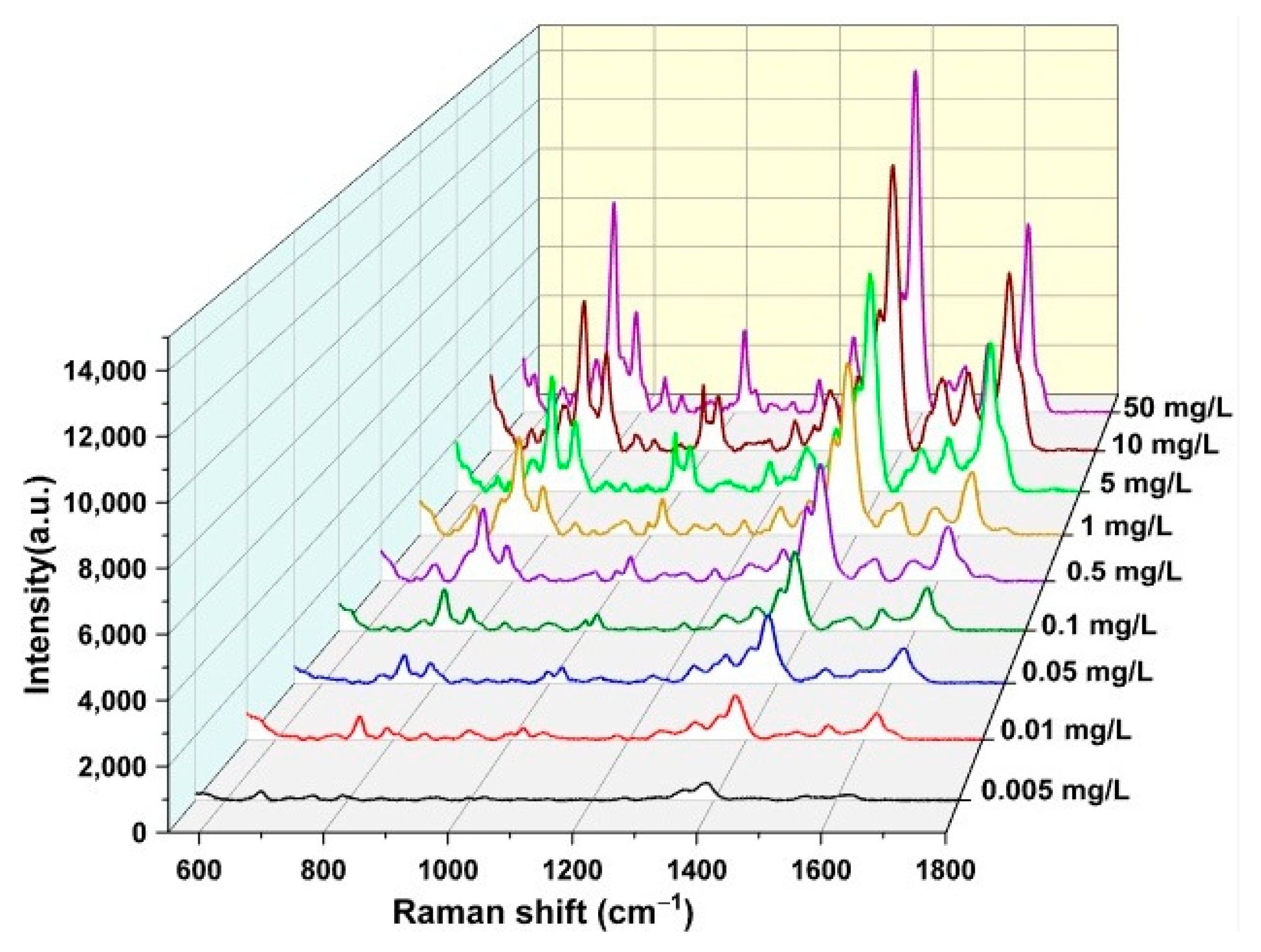
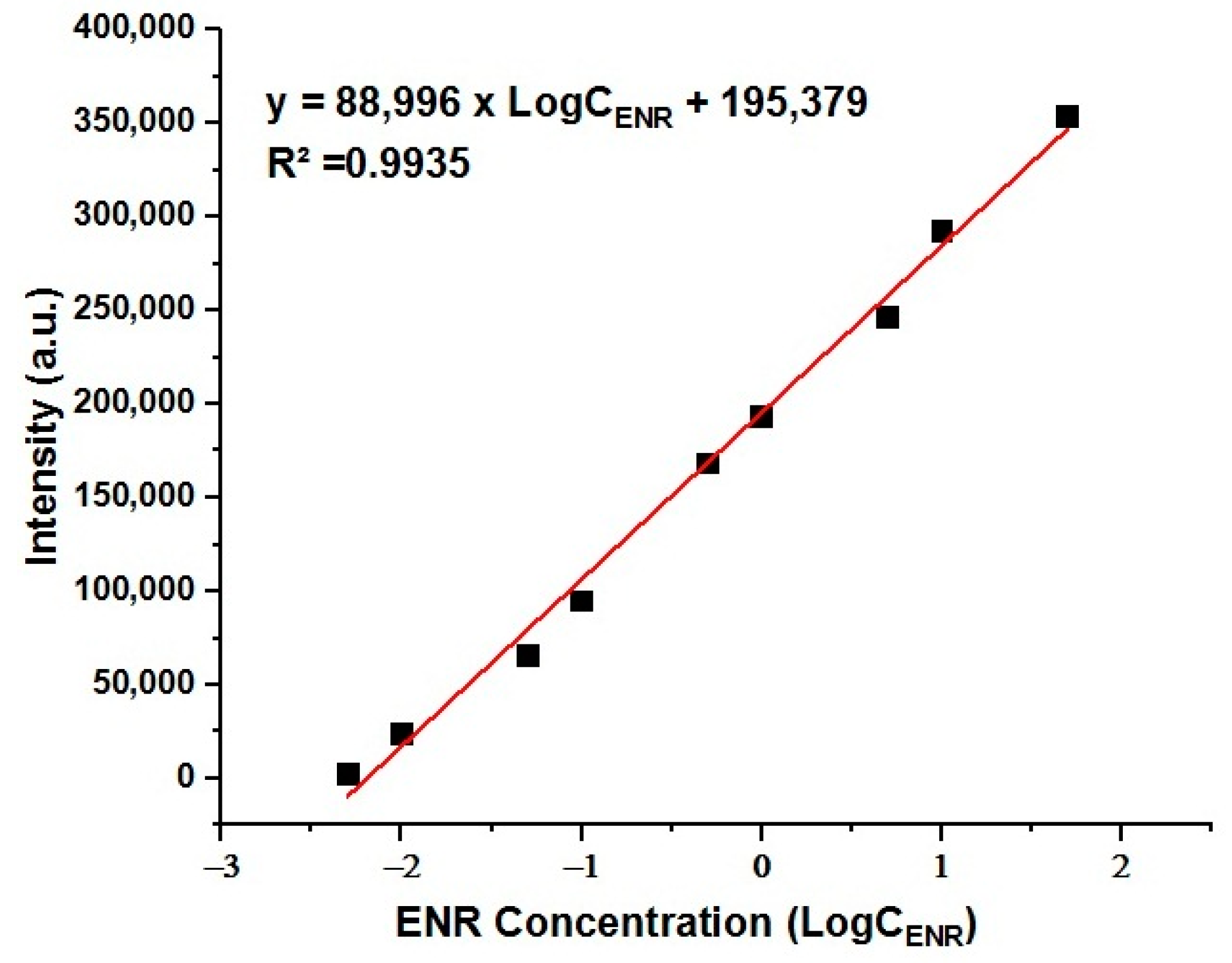
3.7. Recording the SERS Spectrum of ENR
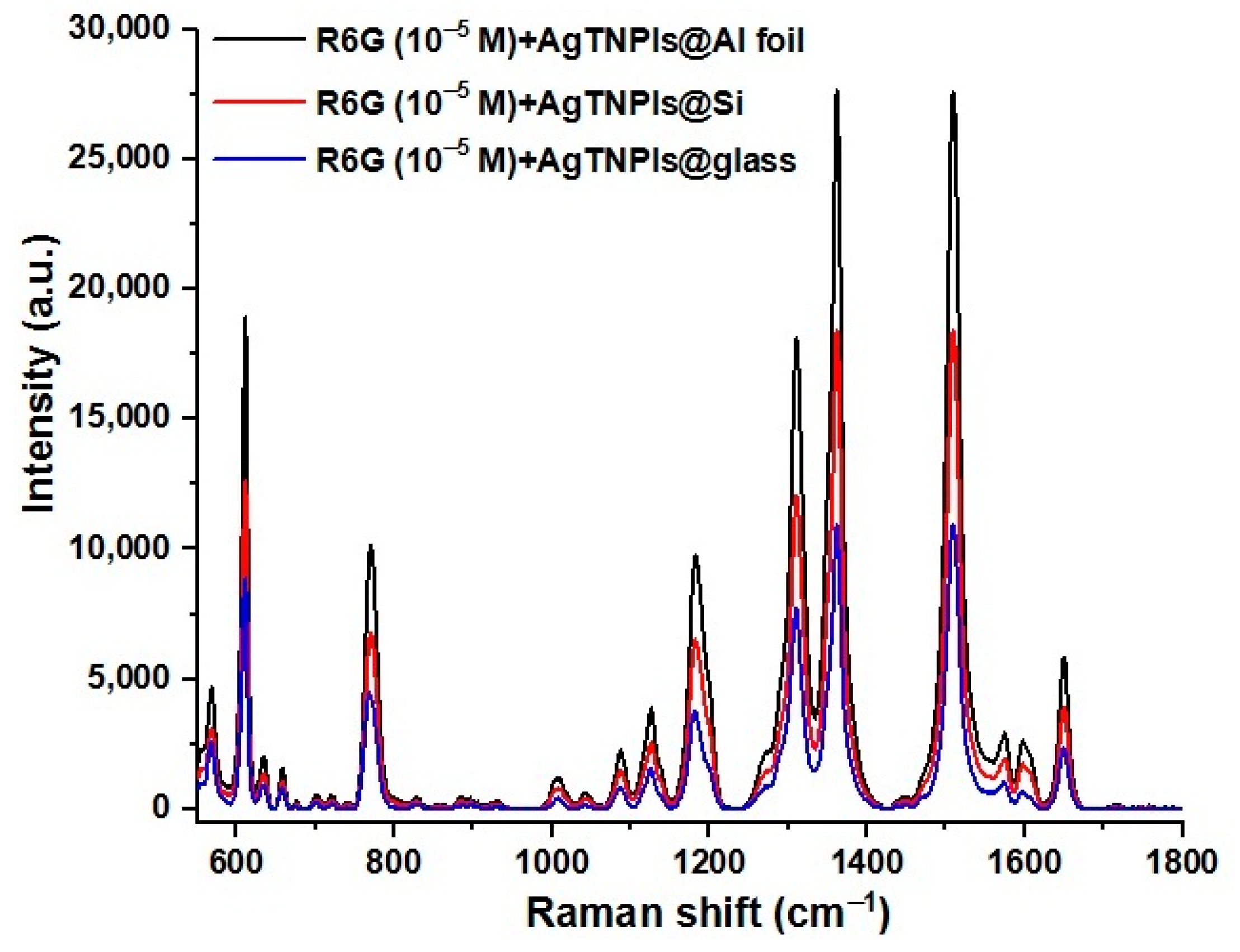
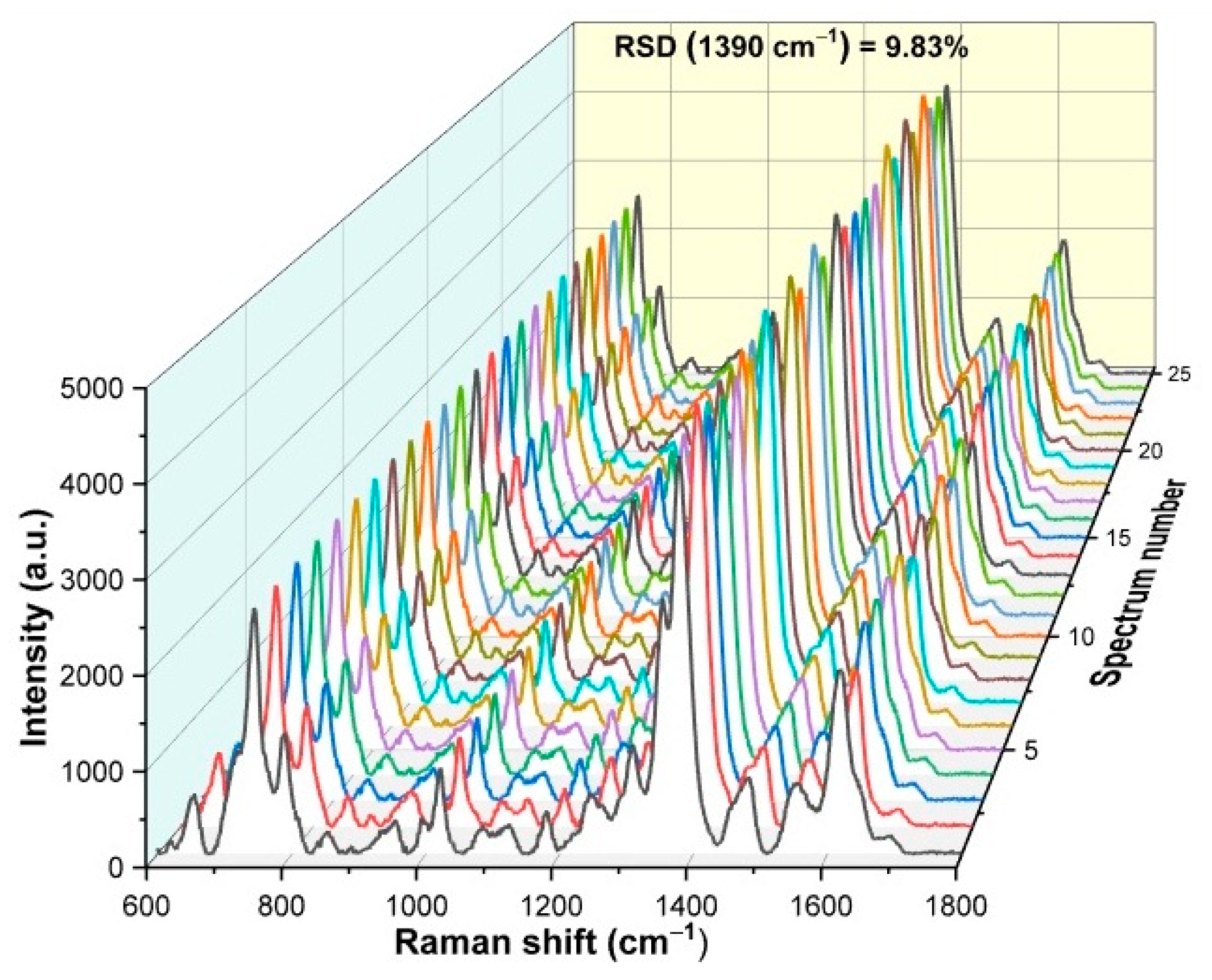
3.8. Uniformity of the SERS Substrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sur, U.K. Surface-enhanced Raman scattering (SERS) spectroscopy: A versatile spectroscopic and analytical technique used in nanoscience and nanotechnology. Adv. Nano Res. 2013, 1, 111–124. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Cao, D.T.; Ngan, L.T.Q.; Anh, C.T.; Minh, K.N. Fabrication of silver nanostructures in the form of particles, dendrites and flowers on silicon for use in SERS substrates. Commun. Phys. 2022, 32, 201–212. [Google Scholar]
- Schwartzberg, A.M.; Grant, C.D.; Wolcott, A.; Talley, C.E.; Huser, T.R.; Bogomolni, R.; Zhang, J.Z. Unique gold nanoparticle aggregates as a highly active surface-enhanced Raman scattering substrate. J. Phys. Chem. B 2004, 108, 19191–19197. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, Q.; Xue, P.; Qi, H.; Wu, Y.; Teng, Y.; Zhang, Y.; Liu, Y.; Zhao, X.; Liu, X. A highly sensitive and stable SERS sensor for malachite green detection based on Ag nanoparticles in situ generated on 3D MoS2 nanoflowers. Chem. Sel. 2020, 5, 354–359. [Google Scholar] [CrossRef]
- Zavaleta, C.L.; Smith, B.R.; Walton, I.; Doering, W.; Davis, G.; Shojaei, B.; Natan, M.J.; Gambhir, S.S. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2009, 106, 13511–13516. [Google Scholar] [CrossRef]
- Furletov, A.A.; Apyari, V.V.; Zaytsev, V.D.; Sarkisyan, A.O.; Dmitrienko, S.G. Silver triangular nanoplates: Synthesis and application as an analytical reagent in optical molecular spectroscopy. A review. TrAC-Trends Anal. Chem. 2023, 166, 117202. [Google Scholar] [CrossRef]
- Cheng, D.; He, M.; Ran, J.; Cai, G.; Wu, J.; Wang, X. Depositing a flexible substrate of triangular silver nanoplates onto cotton fabrics for sensitive SERS detection. Sens. Actuators B Chem. 2018, 270, 508–517. [Google Scholar] [CrossRef]
- Xu, R.; Yan, L. Preparation and SERS Study of Triangular Silver Nanoplates. IOP Conf. Ser. Mater. Sci. Eng. 2019, 678, 012007. [Google Scholar] [CrossRef]
- Zannotti, M.; Rossi, A.; Giovannetti, R. SERS activity of silver nanosphere, triangular nanoplates, hexagonal nanoplates and quasi-spherical nanoparticles: Effect of shape and morphology. Coatings 2020, 10, 288. [Google Scholar] [CrossRef]
- Weng, G.; Feng, Y.; Zhao, J.; Li, J.; Zhu, J.; Zhao, J. Size dependent SERS activity of Ag triangular nanoplates on different substrates: Glass vs. paper. Appl. Sur. Sci. 2019, 478, 275–283. [Google Scholar] [CrossRef]
- Kumar, G.; Soni, R.K. Trace-Level Detection of Explosive Molecules with Triangular Silver Nanoplates-Based SERS Substrates. Plasmonics 2022, 17, 559–573. [Google Scholar] [CrossRef]
- Fan, R.; Yang, Y.; Xue, J.; Zhang, F.; Sun, J.; Xu, H.; Jiang, T. Synthesis of Photothermal Stable Triangular Silver Nanoplates for SERS Application and Photokilling of Bacteria. Chem. Nano Mat. 2020, 6, 148–153. [Google Scholar]
- Xue, C.; Métraux, G.S.; Millstone, J.E.; Mirkin, C.A. Mechanistic study of photomediated triangular silver nanoprism growth. J. Am. Chem. Soc. 2008, 130, 8337–8344. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ji, X.; Jing, J.; Li, M.; Li, J.; Yang, W. Synthesis of Triangular Silver Nanoprisms by Stepwise Reduction of Sodium Borohydride and Trisodium Citrate. J. Phys. Chem. C. 2010, 114, 2070–2074. [Google Scholar] [CrossRef]
- Metraux, G.S.; Mirkin, C.A. Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv. Mater. 2005, 17, 412–415. [Google Scholar] [CrossRef]
- Torres, V.; Popa, M.; Crespo, D.; Moreno, J.M.C. Silver nanoprism coatings on optical glass substrates. Microelectron. Eng. 2007, 84, 1665–1668. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Goebl, J.; Lu, Z.; Yin, Y. A systematic study of the synthesis of silver nanoplates: Is citrate a “magic” reagent? J. Am. Chem. Soc. 2011, 133, 18931–18939. [Google Scholar] [CrossRef]
- Pham, V.D.; Chu, H.H.; Le, T.B.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar]
- Bakar, N.A.; Shapter, J.G.; Salleh, M.M.; Umar, A.A. Self-Assembly of High Density of Triangular Silver Nanoplate Films Promoted by 3-Aminopropyltrimethoxysilane. Appl. Sci. 2015, 5, 209–221. [Google Scholar] [CrossRef]
- Velgosova, O.; Macák, L.; Lisnichuk, M.; Vojtko, M. Synthesis and Analysis of Polymorphic Silver Nanoparticles and Their Incorporation into the Polymer Matrix. Polymers 2022, 14, 2666. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Q.; Quinlivan, S.; Goebl, J.; Gan, Y.; Yin, Y. H2O2-Aided Seed-Mediated Synthesis of Silver Nanoplates with Improved Yield and Efficiency. Chem. Phys. Chem. 2012, 13, 2526–2530. [Google Scholar] [CrossRef] [PubMed]
- Parnklang, T.; Lertvachirapaiboon, C.; Pienpinijtham, P.; Wongravee, K.; Thammacharoen, C.; Ekgasit, S. H2O2-triggered shape transformation of silver nanospheres to nanoprisms with controllable longitudinal LSPR wavelengths. RSC Adv. 2013, 3, 12886–12894. [Google Scholar] [CrossRef]
- Parnklang, T.; Lamlua, B.; Gatemala, H.; Thammacharoen, C.; Kuimalee, S.; Lohwongwatana, B.; Ekgasit, S. Shape transformation of silver nanospheres to silver nanoplates induced by redox reaction of hydrogen peroxide. Mater. Chem. Phys. 2015, 153, 127–134. [Google Scholar] [CrossRef]
- Musino, D.; Rivard, C.; Novales, B.; Landrot, G.; Capron, I. Tuning of Ag Nanoparticle Properties in Cellulose Nanocrystals/Ag Nanoparticle Hybrid Suspensions by H2O2 Redox Post-Treatment: The Role of the H2O2/AgNP Ratio. Nanomaterials 2020, 10, 1559. [Google Scholar] [CrossRef]
- Hoppmann, E.P.; Yu, W.W.; White, I.M. Inkjet-Printed Fluidic Paper Devices for Chemical and Biological Analytics Using Surface Enhanced Raman spectroscopy. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 195–204. [Google Scholar]
- Pham, T.T.H.; Dien, N.D.; Vu, X.H.; Tran, T.T.; Ca, N.X.; Nguyen, V.T.; Tan, P.M.; Van, H.T.; Phan, V.D. Synthesis and In-Depth Study of the Mechanism of Silver Nanoplate and Nanodecahedra Growth by LED Irradiation for SERS Application. J. Electron. Mater. 2020, 49, 5009–5027. [Google Scholar] [CrossRef]
- Lai, C.-H.; Wang, G.-A.; Ling, T.-K.; Wang, T.-J.; Chiu, P.-K.; Chou Chau, Y.-F.; Huang, C.-C.; Chiang, H.-P. Near infrared surface-enhanced Raman scattering based on starshaped gold/silver nanoparticles and hyperbolic metamaterial. Sci. Rep. 2017, 7, 5446. [Google Scholar] [CrossRef]
- Thu, V.T.; Cuong, N.M.; Cao, D.T.; Hung, L.T.; Ngan, L.T.-Q. Trace detection of ciprofloxacin antibiotic using surface-enhanced Raman scattering coupled with silver nanostars. Optik 2022, 260, 169043. [Google Scholar] [CrossRef]
- Martínez-Torres, P.G.; Martínez-García, M.M.; Cardoso-Ávila, P.E.; Pichardo-Molina, J.L. Facile Nanostructured Substrate Preparation Using Gold Nanocuboids for SERS. Nanomater. Nanotechnol. 2015, 5, 12. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Chang, Q.; Zhou, J.; Zhang, Y.; Wang, Z. Grooved Nanoplate Assembly for Rapidly Surface Enhanced Raman Scattering Detecting. Nanoscale 2017, 9, 15390–15396. [Google Scholar] [CrossRef] [PubMed]
- Gudun, K.; Elemessova, Z.; Khamkhash, L.; Ralchenko, E.; Bukasov, R. Commercial Gold Nanoparticles on Untreated Aluminum Foil: Versatile, Sensitive, and Cost-Effective SERS Substrate. J. Nanomater. 2017, 2017, 9182025. [Google Scholar] [CrossRef]
- Sergiienko, S.; Moor, K.; Gudun, K.; Yelemessova, Z.; Bukasov, R. Nanoparticle–nanoparticle vs. nanoparticle–substrate hot spot contributions to the SERS signal: Studying Raman labelled monomers, dimers and trimers. Phys. Chem. Chem. Phys. 2017, 19, 4478–4487. [Google Scholar] [CrossRef]
- Mukanova, Z.; Gudun, K.; Elemessova, Z.; Khamkhash, L.; Ralchenko, E.; Bukasov, R. Detection of Paracetamol in Water and Urea in Artificial Urine with Gold Nanoparticle@Al Foil Cost-efficient SERS Substrate. Anal. Sci. 2018, 34, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.S.; Chung, M.S.; Bae, D.H. Simple Detection of Residual Enrofloxacin in Meat Products Using Microparticles and Biochips. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 817–823. [Google Scholar] [CrossRef]
- Aymard, C.; Kanso, H.; Serrano, M.J.; Pagán, R.; Noguer, T.; Istamboulie, G. Development of a New Dual Electrochemical Immunosensor for a Rapid and Sensitive Detection of Enrofloxacin in Meat Samples. Food Chem. 2022, 370, 131016. [Google Scholar] [CrossRef]
- Aslam, B.; Kousar, N.; Javed, I.; Raza, A.; Ali, A.; Khaliq, T.; Muhammad, F.; Khan, J.A. Determination of Enrofloxacin Residues in Commercial Broilers Using High Performance Liquid Chromatography. Int. J. Food Prop. 2016, 19, 2463–2470. [Google Scholar] [CrossRef]
- Fernandez-Torres, R.; Lopez, M.A.B.; Consentino, M.O.; Mochon, M.C. Simultaneous determination of selected veterinary antibiotics and their main metabolites in fish and mussel samples by high-performance liquid chromatography with diode array-fluorescence (HPLC-DAD-FLD) Detection. , Anal. Lett. 2011, 44, 2357–2372. [Google Scholar] [CrossRef]
- Xu, H.; Chen, L.; Sun, L.; Sun, X.; Du, X.; Wang, J.; Wang, T.; Zeng, Q.; Wang, H.; Xu, Y.; et al. Microwave-Assisted Extraction and in Situ Clean-up for the Determination of Fluoroquinolone Antibiotics in Chicken Breast Muscle by LC-MS/MS. J. Sep. Sci. 2011, 34, 142–149. [Google Scholar] [CrossRef]
- Mou, S.A.; Islam, R.; Shoeb, M.; Nahar, N. Determination of chloramphenicol in meat samples using liquid chromatography–tandem mass spectrometry. Food Sci. Nutr. 2021, 9, 5670–5675. [Google Scholar]
- Tang, Y.; Chen, X.; Lv, Y.; Wu, Z.; Chen, F.; Chen, Z. Excellent Surface Enhanced Raman Scattering of SiO2 Fiber Membrane Embedded with Ag Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2018, 28, 251–257. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Z.; Ren, Z.; Qin, Y.; Pan, Z.; Shao, K.; She, Y.; Huang, W. Interface-Induced Ag Monolayer Film for SurfaceEnhanced Raman Scattering Detection of Water-Insoluble Enrofloxacin. Plasmonics 2021, 16, 349–358. [Google Scholar] [CrossRef]
- Chen, J.; Huang, M.; Kong, L. Flexible Ag/Nanocellulose Fibers SERS Substrate and Its Applications for in-Situ Hazardous Residues Detection on Food. Appl. Surf. Sci. 2020, 533, 147454. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Z.; Li, J.; Ma, S.; Fu, G.; Jin, W.; Bi, W.; Dong, Y. Microcavity Fiber SERS Probe Coated With Ag Nanoparticles For Detecting Antibiotic in Milk. IEEE Photonics J. 2021, 13, 6800513. [Google Scholar] [CrossRef]
- Neng, J.; Wang, Y.; Zhang, Y.; Chen, P.; Yang, K. MIPs–SERS Sensor Based on Ag NPs Film for Selective Detection of Enrofloxacin in Food. Biosensors 2023, 13, 330. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Zhao, Z.; Luan, D.; Bian, X.; Lai, K.; Yan, J. Colloidal SERS measurement of enrofloxacin with petaloid nanostructure clusters formed by terminal deoxynucleotidyl transferase catalyzed cytosine-constituted ssDNA. Food Chem. 2023, 429, 136954. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Macák, L.; Cižmárová, E.; Mára, V. Influence of Reagents on the Synthesis Process and Shape of Silver Nanoparticles. Materials 2022, 15, 6829. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Huang, T.; Xu, X.-H.N. Synthesis and Characterization of Tunable Rainbow Colored Colloidal Silver Nanoparticles Using Single-Nanoparticle Plasmonic Microscopy and Spectroscopy. Mater. Chem. 2010, 20, 9867–9876. [Google Scholar] [CrossRef]
- Zannotti, M.; Vicomandi, V.; Rossi, A.; Minicucci, M.; Ferraro, S.; Petetta, L.; Giovannetti, R. Tuning of hydrogen peroxide etching during the synthesis of silver nanoparticles. An application of triangular nanoplates as plasmon sensors for Hg2+ in aqueous solution. J. Mol. Liq. 2020, 309, 113238. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Wang, H.; Su, H.; Mao, X.; Jiang, L.; Liu, M.; Sun, D.; Hou, S. Synthesis of triangular silver nanoprisms and studies on the interactions with human serum albumin. J. Mol. Liq. 2016, 220, 14–20. [Google Scholar] [CrossRef]
- Wijaya, Y.N.; Kim, J.; Choi, W.M.; Park, S.H.; Kim, M.H. A systematic study of triangular silver nanoplates: One-pot green synthesis, chemical stability, and sensing application. Nanoscale 2017, 9, 11705–11712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Teng, Y.; Qin, Y.; Ren, Z.; Wang, Z. Determination of Ciprofloxacin in Fish by Surface-Enhanced Raman Scattering Using a Liquid-Liquid Self-Assembled Gold Nanofilm. Anal. Lett. 2020, 53, 660–670. [Google Scholar] [CrossRef]
- Dies, H.; Siampani, M.; Escobedo, C.; Docoslis, A. Direct Detection of Toxic Contaminants in Minimally Processed Food Products Using Dendritic Surface-Enhanced Raman Scattering Substrates. Sensors 2018, 18, 2726. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anh, C.T.; Cao, D.T.; Ngan, L.T.-Q. Preparation of Colloidal Silver Triangular Nanoplates and Their Application in SERS Detection of Trace Levels of Antibiotic Enrofloxacin. Colloids Interfaces 2025, 9, 31. https://doi.org/10.3390/colloids9030031
Anh CT, Cao DT, Ngan LT-Q. Preparation of Colloidal Silver Triangular Nanoplates and Their Application in SERS Detection of Trace Levels of Antibiotic Enrofloxacin. Colloids and Interfaces. 2025; 9(3):31. https://doi.org/10.3390/colloids9030031
Chicago/Turabian StyleAnh, Cao Tuan, Dao Tran Cao, and Luong Truc-Quynh Ngan. 2025. "Preparation of Colloidal Silver Triangular Nanoplates and Their Application in SERS Detection of Trace Levels of Antibiotic Enrofloxacin" Colloids and Interfaces 9, no. 3: 31. https://doi.org/10.3390/colloids9030031
APA StyleAnh, C. T., Cao, D. T., & Ngan, L. T.-Q. (2025). Preparation of Colloidal Silver Triangular Nanoplates and Their Application in SERS Detection of Trace Levels of Antibiotic Enrofloxacin. Colloids and Interfaces, 9(3), 31. https://doi.org/10.3390/colloids9030031







