Preparation and Structure of Zinc–Calcium Hydroxyapatite Solid Solution Particles and Their Ultraviolet Absorptive Ability
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
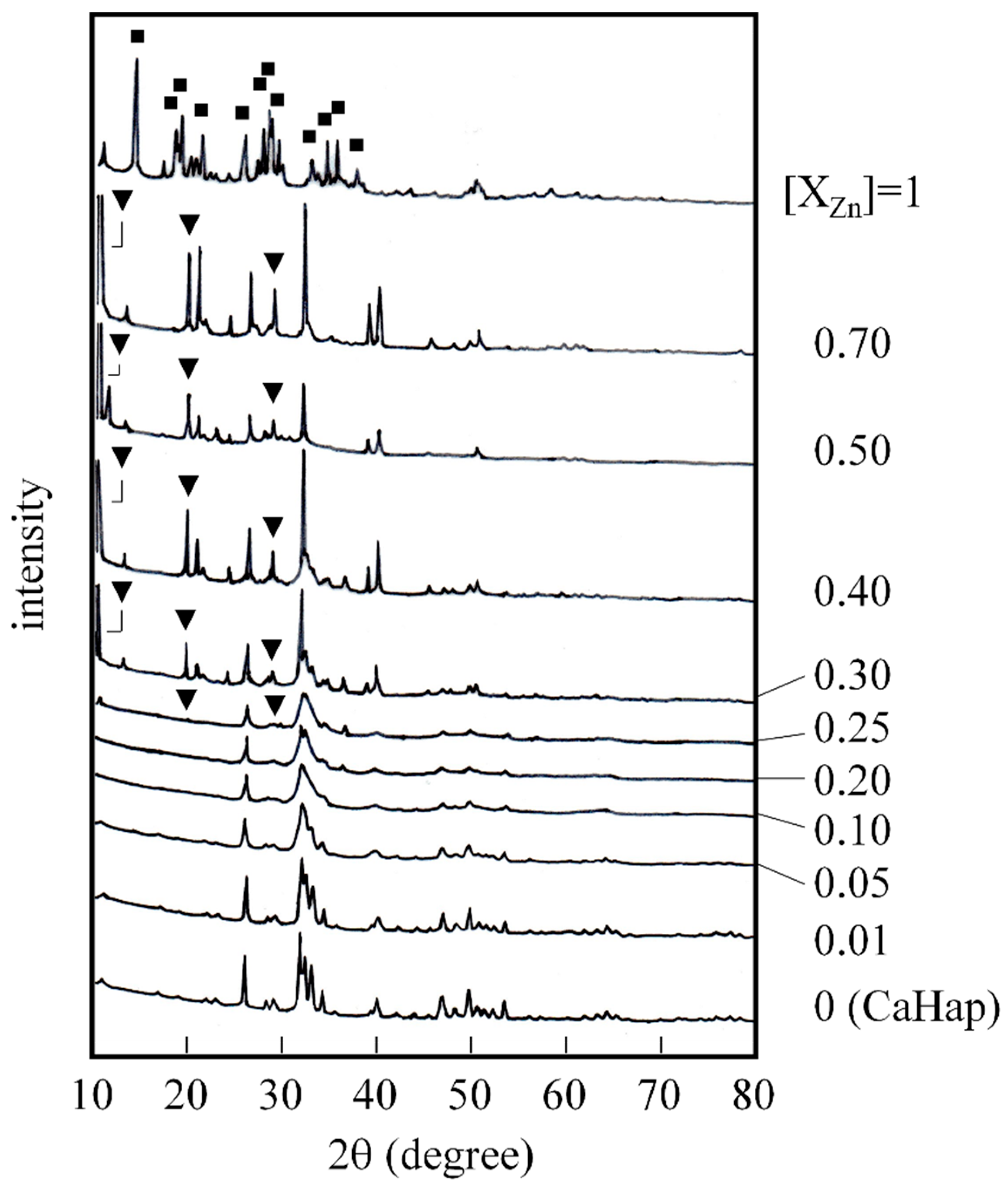
2.2.1. XRD Measurement
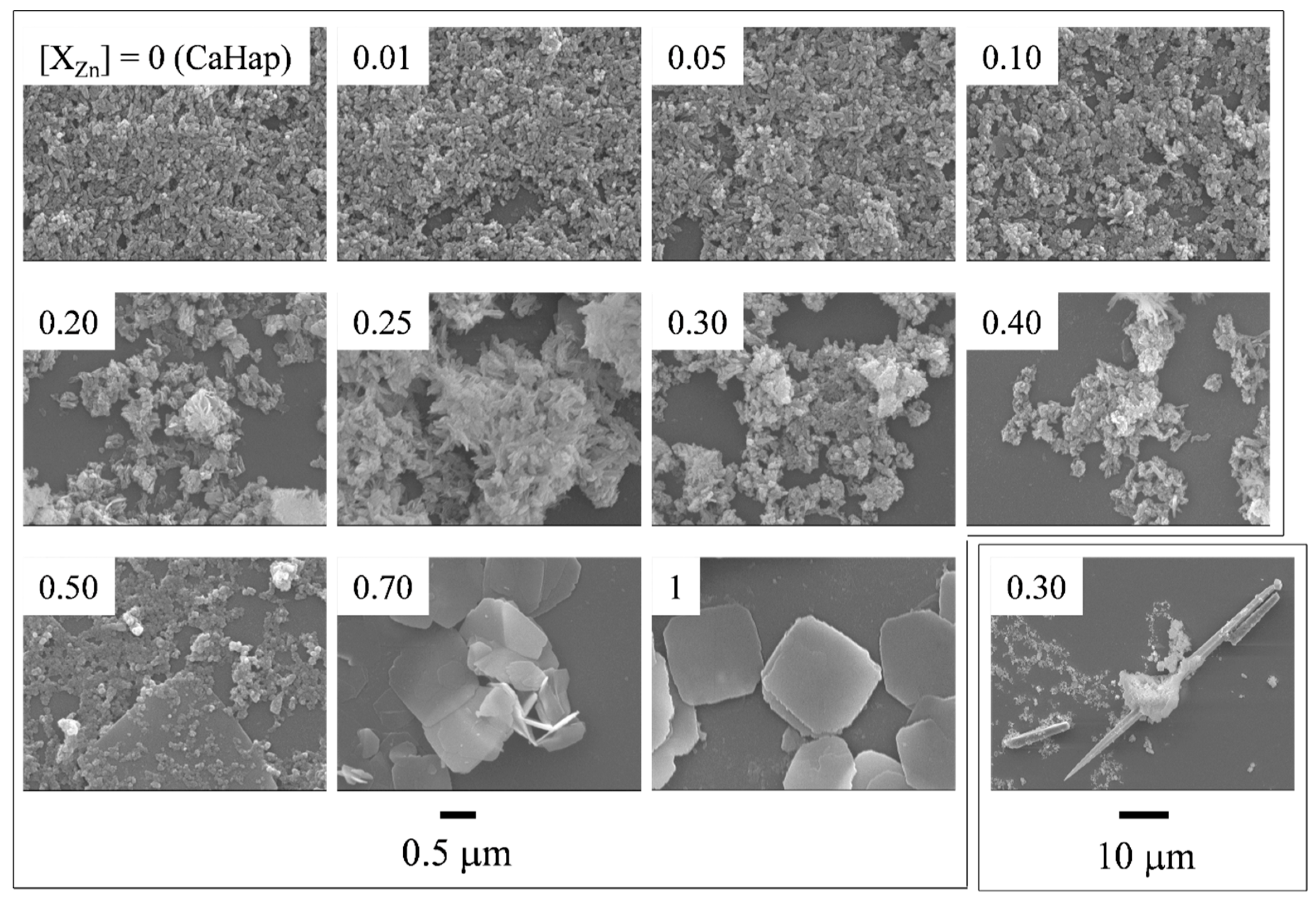
2.2.2. FE-SEM and EDS Analysis
2.2.3. UV—Vis Spectroscopy
3. Results and Discussion
3.1. Crystal Structure and Lattice Parameter
3.2. Morphology
3.3. Particle Sizes and Crystallite Sizes
3.4. Zn2+ and Ca2+ Content
3.5. UV Absorptive Ability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elliott, J.C. Chapter 3 Hydroxyapatite and nonstoichiometric apatites. In Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994; pp. 111–157. [Google Scholar]
- Narasaraju, T.S.B.; Phebe, D.E. Some physico-chemical aspects of hydroxyapatite. J. Mater. Sci. 1996, 31, 1–21. [Google Scholar] [CrossRef]
- Collin, R.L. Strontium-calcium hydroxyapatite solid solutions precipitated from basic, aqueous solutions. J. Am. Chem. Soc. 1960, 82, 5067–5069. [Google Scholar] [CrossRef]
- Joris, S.J.; Amberg, C.H. The nature of deficiency in nonstoichiometric hydroxyapatites. I. Catalytic activity of calcium and strontium hydroxyapatites. J. Phys. Chem. B 1971, 75, 3167–3171. [Google Scholar] [CrossRef]
- Ishikawa, T.; Saito, H.; Yasukawa, A.; Kandori, K. Adsorption of CO2 on non-stoichiometric strontium-calcium hydroxyapatites. J. Chem. Soc. Faraday Trans. 1993, 89, 3821–3825. [Google Scholar] [CrossRef]
- Yasukawa, A.; Higashijima, M.; Kandori, K.; Ishikawa, T. Preparation and characterization of cadmium-calcium hydroxyapatite solid solution particles. Colloids Surf. A Physicochem. 2005, 268, 111–117. [Google Scholar] [CrossRef]
- Narasaraju, T.S.B.; Rao, V.L.N.; Lal, M.; Rai, U.S. Solubility equilibria of solid solutions of calcium & barium hydroxylapatites. Indian J. Chem. 1975, 13, 369–371. [Google Scholar]
- Khudolozhkin, V.O.; Urusov, V.S.; Tobelko, K.I. Distribution of cations between sites in the structure of Ca, Sr, Ba—Apatites. Geochem. Intl. 1973, 10, 266–269. [Google Scholar]
- Yasukawa, A.; Ueda, E.; Kandori, K.; Ishikawa, T. Preparation and characterization of carbonated barium-calcium hydroxyapatite solid solutions. J. Colloid Interface Sci. 2005, 288, 468–474. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphates in oral biology and medicine. Monogr. Oral Sci. 1991, 15, 1–201. [Google Scholar]
- Klement, V.R.; Haselbeck, H. Apatite und Wagnerite zweiwertiger metalle. Z. Anorg. Chem. 1965, 336, 113–128. [Google Scholar] [CrossRef]
- Yasukawa, A.; Ouchi, S.; Kandori, K.; Ishikawa, T. Preparation and characterization of magnesium-calcium hydroxyapatite. J. Mater. Chem. 1996, 6, 1401–1405. [Google Scholar] [CrossRef]
- Engel, G.; Krieg, F.; Reif, G. Mischkristallbildung und kationenordnung im system bleihydroxylapatit-calciumhydroxylapatit. J. Solid State Chem. 1975, 15, 117–126. [Google Scholar] [CrossRef]
- Verbeeck, R.M.H.; Lassuyt, C.J.; Heijligers, H.J.M.; Driessens, F.C.M.; Vrolijk, J.W.G.A. Lattice parameters and cation distribution of solid solutions of calcium and lead hydroxyapatite. Calcif. Tissue Int. 1981, 33, 243–247. [Google Scholar] [CrossRef]
- Yasukawa, A.; Kamiuchi, K.; Yokoyama, T.; Ishikawa, T. Preparation of lead-calcium hydroxyapatite solid solutions by a wet method using acetamide. J. Solid State Chem. 2002, 163, 27–32. [Google Scholar] [CrossRef]
- Yasukawa, A.; Kandori, K.; Tanaka, H.; Gotoh, K. Preparation and structure of carbonated calcium hydroxyapatite substituted with heavy rare earth ions. Mater. Res. Bull. 2012, 47, 1257–1263. [Google Scholar] [CrossRef]
- Yasukawa, A.; Sohma, A. Ultraviolet shielding properties of cotton fabric supported by titanium-calcium hydroxyapatite solid solution particles. Tex. Res. J. 2020, 90, 1581–1589. [Google Scholar] [CrossRef]
- Yasukawa, A.; Gotoh, K.; Tanaka, H.; Kandori, K. Preparation and structure of calcium hydroxyapatite substituted with light rare earth ions. Colloids Surf. A Physicochem. 2012, 393, 53–59. [Google Scholar] [CrossRef]
- Tanizawa, Y.; Sawamura, K. Reaction characteristics of dental and synthetic apatites with Al3+ and La3+ ions in acidic solutions. J. Chem. Soc. Faraday Trans. 1990, 86, 4025–4029. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Z.; Cheng, J. Preparation, characterization and antibacterial property of cerium substituted hydroxyapatite nanoparticles. J. Rare Earths 2007, 25, 452–456. [Google Scholar] [CrossRef]
- Feng, Z.; Liao, Y.; Ye, M. Synthesis and structure of cerium-substituted hydroxyapatite. J. Mater. Sci. Mater. Med. 2005, 16, 417–421. [Google Scholar] [CrossRef]
- Elliott, J.C. Chapter 2 Fluorapatite and chlorapatites. In Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994; pp. 64–95. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Yasukawa, A.; Ruike, S.; Gotoh, K.; Kandori, K. Ultraviolet shielding properties of cotton fabric supported by cerium-calcium hydroxyapatite solid solution particles. Tex. Res. J. 2014, 84, 1578–1585. [Google Scholar] [CrossRef]
- Wakamura, M.; Hashimoto, K.; Watanabe, T. Photocatalysis by Calcium Hydroxyapatite Modified with Ti(IV): Albumin Decomposition and Bactericidal Effect. Langmuir 2003, 19, 3428–3431. [Google Scholar] [CrossRef]
- de Araujo, T.S.; de Souza, S.O.; de Sousa, E.M.B. Effect of Zn2+, Fe3+ and Cr3+ addition to hydroxyapatite for its application as an active constituent of sunscreens. International Conference on Defects in Insulating Materials. J. Phys. Conf. Ser. 2010, 249, 012012. [Google Scholar] [CrossRef]
- Dubrovski, P.D.; Brezocnik, M. Prediction of the ultraviolet protection of cotton woven fabrics dyed with reactive dystuffs. Fibres Text. East Eur. 2009, 17, 55–59. [Google Scholar]
- Popov, A.P.; Priezzhev, A.V.; Lademann, J.; Myllylä, R. TiO2 nanoparticles as an effective UV-B radiation skin-protective compound in sunscreens. J. Phys. D Appl. Phys. 2005, 38, 2564–2570. [Google Scholar] [CrossRef]
- Predoi, D.; Liliana, S.; Mihai, I.; Predoi, V.; Buton, N.; Motelica-Heino, M. Zinc doped hydroxyapatite thin films prepared by sol–gel spin coating procedure. Coatings 2019, 9, 156. [Google Scholar] [CrossRef]
- Mariappan, A.; Pandi, P.; Rani, K.B.; Neyvasagam, K. Study of the photocatalytic and antibacterial effect of Zn and Cu doped hydroxyapatite. Inorg. Chem. Commun. 2021, 136, 109128. [Google Scholar] [CrossRef]
- Song, S.; Wu, S.; Lian, Q.; Peng, Y.; Zheng, X.; Zhang, Z. Synthesis and Characterization of Human Body Trace Elements Substituted Hydroxyapatite for a Bioactive Material. Asian J. Chem. 2013, 25, 6540–6544. [Google Scholar] [CrossRef]
- Dasgupta, S.; Bandyopadhyay, A.; Bose, S. Zn and Mg Doped Hydroxyapatite Nanoparticles for Controlled Release of Protein. Langmuir 2010, 26, 4958–4964. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Prodan, A.M.; Buton, N.; Predoi, D. Structural Characterization and Antifungal Studies of Zinc-Doped Hydroxyapatite Coatings. Molecules 2017, 22, 604. [Google Scholar] [CrossRef]
- Tanji, K.; Navio, J.A.; Chaqroune, A.; Naja, J.; Puga, F.; Hidalgo, M.C.; Kherbeche, A. Fast photodegradation of rhodamine B and caffeine using ZnO-hydroxyapatite composites under UV-light illumination. Catal. Today 2022, 388–389, 176–186. [Google Scholar] [CrossRef]
- Lytkina, D.; Gutsalova, A.; Fedorishin, D.; Korotchenko, N.; Akhmedzhanov, R.; Kozik, V.; Kurzina, I. Synthesis and properties of zinc-modified hydroxyapatite. J. Funct. Biomater. 2020, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Chapter 4 X-ray techniques in catalysis. In Catalysis, Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin, Germany, 1984; Volume 5, pp. 221–273. [Google Scholar]
- Kredleer, E.R.; Hummel, F.A. The crystal chemistry of apatite: Structure fields of fluor- and chlorapatite. Am. Mineral. 1970, 55, 170–184. [Google Scholar]
- Wakamura, M.; Kandori, K.; Ishikawa, T. Surface structure and composition of calcium hydroxyapatites substituted with Al(III), La(III) and Fe(III) ions. Coll. Surf. A 2000, 164, 297–305. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasukawa, A.; Yamada, M. Preparation and Structure of Zinc–Calcium Hydroxyapatite Solid Solution Particles and Their Ultraviolet Absorptive Ability. Colloids Interfaces 2023, 7, 70. https://doi.org/10.3390/colloids7040070
Yasukawa A, Yamada M. Preparation and Structure of Zinc–Calcium Hydroxyapatite Solid Solution Particles and Their Ultraviolet Absorptive Ability. Colloids and Interfaces. 2023; 7(4):70. https://doi.org/10.3390/colloids7040070
Chicago/Turabian StyleYasukawa, Akemi, and Minami Yamada. 2023. "Preparation and Structure of Zinc–Calcium Hydroxyapatite Solid Solution Particles and Their Ultraviolet Absorptive Ability" Colloids and Interfaces 7, no. 4: 70. https://doi.org/10.3390/colloids7040070
APA StyleYasukawa, A., & Yamada, M. (2023). Preparation and Structure of Zinc–Calcium Hydroxyapatite Solid Solution Particles and Their Ultraviolet Absorptive Ability. Colloids and Interfaces, 7(4), 70. https://doi.org/10.3390/colloids7040070






