Abstract
In recent years, emulsions stabilized by solid particles (known as Pickering emulsions) have gained considerable attention due to their excellent stability and for being environmentally friendly compared to the emulsions stabilized by synthetic surfactants. In this context, edible Pickering stabilizers from agri-food byproducts have attracted much interest because of their noteworthy benefits, such as easy preparation, excellent biocompatibility, and unique interfacial properties. Consequently, different food-grade particles have been reported in recent publications with distinct raw materials and preparation methods. Moreover, emulsions stabilized by solid particles can be applied in a wide range of industrial fields, such as food, biomedicine, cosmetics, and fine chemical synthesis. Therefore, this review aims to provide a comprehensive overview of Pickering emulsions stabilized by a diverse range of edible solid particles, specifically agri-food byproducts, including legumes, oil seeds, and fruit byproducts. Moreover, this review summarizes some aspects related to the factors that influence the stabilization and physicochemical properties of Pickering emulsions. In addition, the current research trends in applications of edible Pickering emulsions are documented. Consequently, this review will detail the latest progress and new trends in the field of edible Pickering emulsions for readers.
1. Introduction
Many raw materials of plant origin such as soy, almond, rice, coconut, oat, and lupin, among others, are used to produce plant-based foods, which include meat and dairy analogues. However, the production of plant-based foods produces large amounts of valuable byproducts, which commonly are discarded by industry [1]. In this regard, there is a great opportunity to add value to these byproducts, thus contributing to the circular economy and environmental protection. Therefore, it is necessary to find specific and innovative applications of these food waste/byproducts. From this perspective, a new and relevant aspect is the use of agro-industrial byproducts as an alternative, renewable, and inexpensive source of “natural stabilizers” (solid particles) for various industrial applications [2]. Accordingly, it should be mentioned that development and research in this field are still very limited. Therefore, the utilization of novel particles obtained from “plant sources” could satisfy consumer demands to replace synthetic surfactants with ingredients with a lower environmental impact.
Emulsions are liquid–liquid (oil–water) colloidal systems that generally are obtained in the presence of surface-active molecules such as surfactants, amphiphilic polymers, or natural polymers (proteins) [3]. However, the use of surfactants in large-scale industrial applications and personal care products is not cost-effective, and in some cases, may cause adverse effects such as irritation and hemolytic behavior [4]. Therefore, the use of natural surface-active particles may be a promising alternative for use as surfactants. In this context, S. Pickering [5] observed that solid amphiphilic particles can also be used to stabilize emulsions, which were called Pickering emulsions. This type of system refers to emulsions that are not physically stabilized by conventional emulsifier molecules, but instead by solid colloidal particles [6]. The stabilization mechanism of this type of emulsion involves the partial adsorption of solid particles at the interface between oil and water (i.e., dual wettability) [4]. The adsorption of a particle at the oil–water interface is strongly influenced by its wettability, which depends on the oil–water interface contact angle [7].
In recent years, the study and use of Pickering emulsions have attracted a lot of interest in the field of food and pharmaceutical research [8]. Pickering emulsions have several advantages over traditional surfactant-stabilized emulsions, such as high stability against coalescence (even with large droplets), Ostwald ripening, and the emulsion does not contain surfactants, among others. Furthermore, in comparison to traditional emulsions, Pickering emulsions seem to be more appropriate in the development of encapsulation and delivery systems for bioactive compounds, for instance, antioxidants, probiotics, polyphenols, carotenoids, and tocotrienols, among others [8,9,10].
Many studies have been addressed to studying particle-stabilized Pickering emulsions. However, very few studies have been focused on food applications, mainly because many Pickering particles are of inorganic origin, which are not food-grade [6]. Therefore, to date, there is still a limited variety of food-grade inorganic particles due to their low biocompatibility and biodegradability. In addition, many of these particles require time-consuming, expensive, and environmentally unfriendly processes to be synthetized [11].
On the other hand, most emulsions are stabilized by using inorganic particles, restricting their used in food-grade formulations [12]. According to Jiang et al. [4], the first colloidal particles used to stabilize Pickering emulsions were inorganic particles, which have been extensively studied for this purpose. Among them, silica (as a colloidal particle) has been extensively used to stabilize Pickering emulsions owing to its ability to resist acidic and basic conditions, its easily modified surface, and the ability to control its size and structure [4]. Since most inorganic particles are not food-grade, it is necessary to explore new and effective Pickering stabilizers as an alternative to inorganic particles so that they are suitable for human consumption. In this context, the use of byproducts derived from natural sources, such as oil seeds, fruits, and legumes, has attracted great interest due to their techno-functional properties and low cost. However, studies on this area remain practically unexplored or are still very limited.
Based on the above considerations, this review provides a comprehensive overview of the recent advances in the stabilization of Pickering emulsions using diverse agri-food byproduct particles. Thus, several types of solid particles will be listed and discussed in detail. These novel approaches will provide relevant information on the behavior of solid plant-based particles in the development of new emulsion systems for food applications. In addition, this knowledge will help to valorize different byproducts/wastes as potential surface-active sources, thereby contributing to the circular economy.
2. Factors That Influence Pickering Emulsion Stabilization
2.1. Particle Wettability
Pickering emulsions have a stabilization mechanism different from emulsions stabilized by traditional emulsifiers and biopolymers with two distinct hydrophilic and hydrophobic regions. Pickering emulsions are stabilized through adsorption of solid particles at the oil–water interface and, therefore, they does not need to be amphiphilic [13]. Thus, the wettability of particles is a crucial parameter factor in particles adsorbing at the surface of the droplets and in the final stability of Pickering emulsions [7]. In particle-stabilized emulsions, whether the emulsion is oil/water (O/W) or water/oil (W/O) type is dependent on the wettability of the particle, which is determined by the contact angle in water at the oil–particle–water interface [14]. Likewise, the adsorption of particles at the oil–water interface is strongly influenced by its hydrophobicity. According to the contact angles of solid particles (Figure 1), hydrophilic particles (i.e., with a contact angle of <90° measured through the water phase) should stabilize O/W emulsions. Conversely, hydrophobic particles (i.e., with a contact angle of >90°) should better stabilize W/O emulsions [7]. However, particles that are completely wetted by water or oil remain dispersed in that phase and cannot form an emulsion.
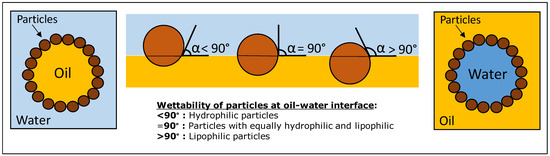
Figure 1.
Schematic representation of wettability of Pickering stabilizers. The solid particles adsorbed at the oil–water interface to stabilize the oil droplets of the emulsion.
In the majority of cases, the effectiveness of Pickering stabilizers is associated with their wettability, which is highly influenced by the particles’ hydrophobic nature and consequently can directly impact the type of Pickering emulsion formed (O/W or W/O). Thus, a strong adsorption at the oil–water interface occurs due to the partial wettability of spherical solid particles, resulting in robust steric hindrance. This can prevent droplet coalescence and flocculation in the emulsion by the steric mechanism [13]. Apart from the wettability of particles, other external factors can also play a crucial role in emulsion stability, including the particle size, pH, particle concentration, ionic strength, the droplet size of emulsion, particle type, and proportion of the oil phase [7]. It is important to mention that although a contact angle of around 90° is theoretically considered optimal for stabilizing Pickering emulsions [1], various other factors, such as particle size, electrical potential, and particle shape, among others [1], can also impact the formation of Pickering emulsions.
2.2. Particle Concentration
Another factor that can influence the stability of the Pickering emulsion is the concentration of solid particles. The stability of the emulsion and droplet size are significantly influenced by the concentration of particles [2,15]. This is because solid particles need to be adsorbed at the oil–water interface of droplets to perform as emulsifiers, and thus the emulsion stability tends to increase proportionally with particle concentration [7]. Burgos-Díaz et al. [6,8] observed that when the concentration of food-grade particles increased, the emulsion was stable against creaming for 45 days. This behavior was attributed to the greater amount of particles that could cover the oil–water interface and thus improve the emulsion stability (Figure 2). Likewise, the particle concentration had an impact on the emulsion droplet size.
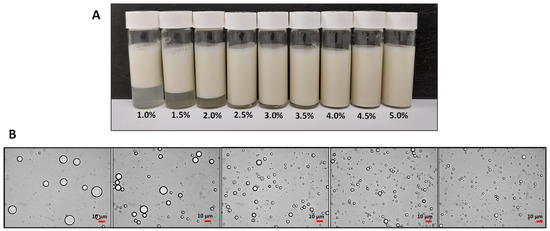
Figure 2.
(A) Images of O/W emulsions stabilized by lupin hull at different concentrations (1–5%, w/w) after storage for 45 days. (B) Optical micrographs of the O/W Pickering emulsions stabilized at different byproduct concentrations (1.0–5.0%, w/w). The images were acquired at 40× magnification. The emulsions were prepared at a fixed oil concentration (20%, w/w) (image adapted from Burgos-Díaz et al. [1]).
According to Li et al. [13], if the particle concentration is insufficient to cover the newly formed droplets during emulsion preparation, these droplets will only be partly covered by particles (Figure 3). Thus, emulsion droplets can progressively merge, leading to fast coalescence and large droplets. On the contrary, with the increase in the concentration, more particles can adsorb at the interface (oil–water) to form a single or multi-layer structure, which avoids the coalescence of emulsion droplets and thus stabilizes the emulsion (Figure 3).
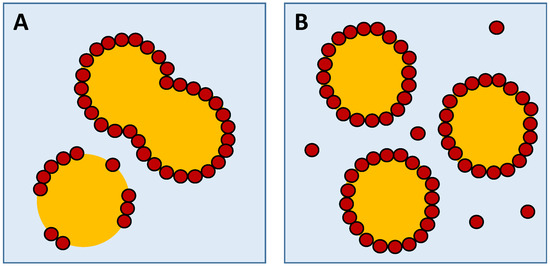
Figure 3.
Schematic representation of the oil droplet distribution. (A) Droplets partly covered by solid particles; (B) droplets totally covered by solid particles.
2.3. Morphology of Solid Particles
Food-grade particles of different shapes have attracted great interest from the scientific community because particle shape plays a vital role in emulsion stability. Particle shapes can be classified as regular spherical or irregular shapes with anisotropic morphology [16]. The factors that influence the distinct shapes of solid particles are associated with their origin or source, their structure, their properties, and the methods used for their preparation [13]. In this regard, non-spherical particles such as fibers, rods, and cubes have been utilized to stabilize Pickering emulsions [17]. Li et al. [13] reported that the properties of Pickering emulsions can be influenced by the type and shape of solid particles. In particular, the shape of the particles governs their behavior at the interface (oil–water) and their ability to stabilize the emulsion. Particles (as a Pickering stabilizer) with different shapes may have different densities, desorption energy, and capillary forces between adjacent particles, which can significantly impact the stabilization principles and applications of the emulsions. Additionally, the particle shape can play a critical role in the stabilization of emulsions by altering the wettability behavior of particles and the interactions between adjacent particles. According to Burgos-Díaz et al. [1], the stabilization of a Pickering emulsion can be influenced not only by the particle wettability but also by the particle shape, size, and electrical potential. Figure 4 illustrates the use of Pickering stabilizers obtained from different agri-food byproducts, which have different shapes and sizes, to stabilize O/W emulsions.
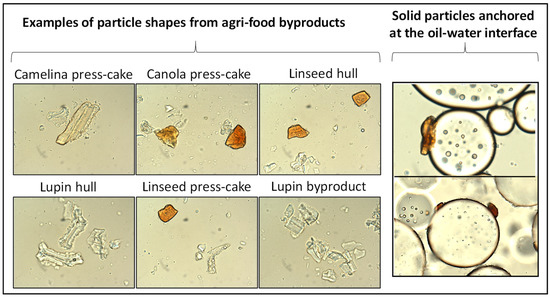
Figure 4.
Micrographs corresponding to the morphology of particles obtained from different byproducts and used to stabilize Pickering emulsions (image adapted from Burgos-Díaz et al. [1]).
2.4. Oil Volume Fraction
The emulsion stability and type (O/W or W/O) can be also influenced by the dispersed phase volume. In this regard, the stability and type of emulsion can be influenced by the oil fraction present between the disperse and continuous phases. Burgos-Díaz et al. [6] showed that when the oil volume fraction increased from 5% to 20%, the emulsion droplet size varied in all emulsions stabilized with Pickering particles at different concentrations. The authors observed that at a fixed Pickering stabilizer concentration, the size of the oil droplets increased as the oil fraction increased. This behavior is expected, since when the particle concentration is kept relatively low and constant, the number of particles present may not be enough to provide complete stabilization for oil droplets as the oil volume fraction is raised. As a result, the number of particles may not be enough to completely cover the surface of freshly formed oil droplets during the emulsification process, leading to droplet coalescence and oiling off. Figure 5 shows a schematic representation of the effect of particle and oil concentration on the droplet size of the emulsion.
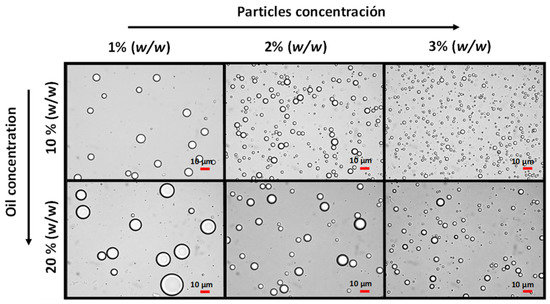
Figure 5.
Micrographs of the effect of particle and oil concentration on droplet size of an O/W Pickering emulsion.
3. Preparation of Pickering Emulsions
Various methods or techniques have been used for the preparation of both O/W and W/O Pickering emulsions. Among them, rotor-stator homogenization, high-pressure homogenization, and sonication are the most commonly employed to formulate Pickering emulsions [18,19]. Nevertheless, monodisperse Pickering emulsions can also be produced through membrane emulsification and microfluidics [19]. In the majority of research, the common method for preparing Pickering emulsions is the “rotor–stator homogenization method” (UltraTurrax), which consists of a homogenizer with a rotor and a stator with openings. In this technique, the rotation speed and the homogenization time are the primary parameters that affect the emulsion droplet size with a rotor–stator homogenizer [18]. On the other hand, the “high-pressure homogenization method” is a continuous emulsification process in which a pre-emulsification is required to obtain a coarse emulsion (primary emulsion), which is then passed through the slits of a high-pressure homogenizer, and its cavitation, turbulence, and shear are utilized to form a fine emulsion [20]. Conversely, the “ultrasonic method” also uses cavitation, turbulence, and shear stress to prepare emulsions, promoting adoption of the Pickering stabilizer on a two-phase interface. During emulsion preparation, cavitation produces localized high temperatures, high pressures, and stress, which is beneficial to the formation of Pickering emulsions [20].
Recently, techniques such as membrane emulsification and microfluidic emulsification have also been applied to prepare Pickering emulsions. Regarding the “membrane emulsification method”, this method presses a pure dispersed phase or primary emulsion into a microporous membrane and controls the injection rate and shearing conditions to prepare a Pickering emulsion [20]. The two main types of membrane emulsification techniques are “direct membrane emulsification” and “premix membrane emulsification”. In direct membrane emulsification, the dispersed phase is pressed or injected through a microporous membrane into the continuous phase. The same principle is applied in “premix membrane emulsification”, except that it is a pre-emulsified mixture that is pressed through the membrane [18]. Apparently, the pore size of the membrane, the viscosities of the continuous phase and the dispersed phase, and the magnitude of the surface tension are important factors affecting the droplet size of the Pickering emulsion [18]. Finally, “microfluidic technology” is a drop-by-drop technology used for preparing Pickering emulsions. In this method, the dispersed phase flows horizontally and the continuous phase flows vertically, and when they intersect, the dispersed phase forms spherical droplets under the influence of the continuous phase drag [20].
4. Agri-Food Byproducts as a Source of Pickering Stabilizers
Different functional ingredients, such as proteins, polysaccharides, fibers, flavor molecules, and phytochemicals, can be generally found in agri-food byproducts [3,21]. In addition, this type of byproduct is an excellent source of natural emulsifier molecules and therefore this makes them good candidates to develop new solid amphiphilic particles or Pickering stabilizers. The great potential of Pickering particles is that these natural stabilizers can be an effective alternative to synthetic surfactants commonly used for stabilizing emulsions. In general, Pickering stabilizers can be classified as non-food-grade Pickering particles, e.g., calcium carbonate, barium sulfate, silica, clays (montmorillonite and laponite), and particles based on synthetic polymers (polystyrene, poly(N-isopropylacrylamide), and PS-polybutadiene block (PB)-block-PMMA) [22], and food-grade Pickering particles, e.g., polysaccharide particles (starch, chitosan, and cellulose), fat crystals, complex particles, flavonoid particles, food-grade wax, protein-based particles proteins (zein, whey, soy, and lupin), and byproducts (apple peel pomace, pomace, cocoa, and rapeseed press-cake) [1]. Most food-grade particles such as starch, cellulose, chitin, and proteins must be physically or chemically modified to improve their interfacial functionality and stability in Pickering emulsions [20].
In recent years, various byproducts have been investigated as potential natural Pickering stabilizers (Figure 6), including apple pomace [23], citrus fibers [24], cocoa, rapeseed press-cake [25], protein from moringa seed residues [26], tea residues [27], perilla protein isolate extracted from oilseed residues [28], polysaccharide extract from peanut oil residues [29], lupine hull, lupine byproducts, camelina press-cake, linseed hull, and linseed press-cake [1], among others. Hence, it is necessary to give immediate attention to managing agri-food byproducts and valorize them as edible techno-functional ingredients for the food industry.
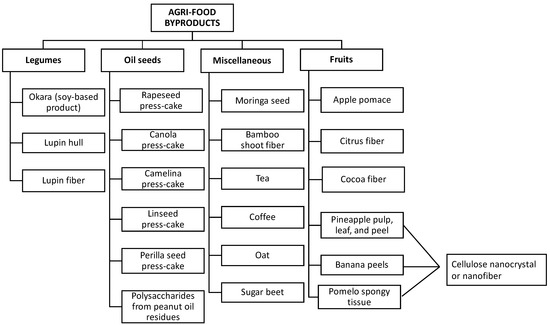
Figure 6.
Classification of agri-food byproducts as a source of Pickering stabilizers.
4.1. Legume Byproducts
Legume byproducts have a high content of insoluble proteins and fibers, which make them good candidates to obtain novel solid particles to stabilize Pickering emulsions. In addition, these byproducts possess other bioactive molecules of interest to the food industry (phenols, carotenoids, phytosterols, and fibers), which also can provide benefits to emulsion stability and human health [30,31,32].
For instance, okara is a byproduct of the processing of soy-based products (milk, tofu, or protein isolate) composed of insoluble dietary fiber (IDF), protein [33], and polysaccharides [34,35]. As a result of its nutritional composition (mainly proteins, fiber, and carbohydrates), the effectiveness of the okara solid particles as Pickering stabilizers has been explored. For example, Yang et al. [36] reported the extraction of nanoparticles from insoluble okara soybean polysaccharides and their utilization as Pickering stabilizers. Bao et al. [37] modified okara insoluble dietary fiber (OIDF) by fermentation using Kluyveromyces marxianus. The modified OIDF exhibited a porous structure with a honeycomb shape, emulsification properties, and a significant increase in water and oil holding capacity.
Burgos-Díaz et al. [1] reported that lupin byproducts (a byproduct based on insoluble fiber–protein compounds and lupin hull) can be used effectively as particle-based emulsifiers to prepare stable O/W Pickering emulsions. Their study showed that all samples contained surface-active agents, including proteins (30.80–17.90 g/100 g) and dietary fiber (60.59–67.10 g/100 g). In addition, the authors also determined that the presence of proteins, both soluble and insoluble, is relevant because they are the main surface-active compounds found in raw plant materials.
4.2. Oil Seed Byproducts
Oilseed cakes (e.g., from canola seed, camelina seed, rapeseed, and linseed) are byproducts obtained during the pressing process to obtain oils [38]. After the pressing process, a 60% byproduct (press-cake) is obtained, which contains a high protein content (25–35%) [39], 8–10% residual oil (triglycerides), and different amounts of cellulose, lignin [40], glucosinolates, phenolic compounds, phytic acid, and other compounds [41,42].
The use of press-cakes as Pickering stabilizers has been previously reported in a study performed by Joseph and coworkers [3,25], who evaluated the utilization of rapeseed press-cake powders as Pickering stabilizers, determining that this byproduct can effectively stabilize oil-in-water Pickering emulsions. The authors attributed this ability to the higher amount of amphiphilic species such as insoluble protein (34–38 g/100 g), polyphenols (0.8 eq gallic acid/100 g), insoluble polysaccharide–polyphenol, and protein–polyphenol complexes. Burgos-Díaz and coworkers [1] evaluated the emulsifying properties of different oilseed byproducts, such as canola, camelina, and linseed press-cake. In this study, the authors reported that camelina press-cakes showed the best results as Pickering stabilizers, which could be attributed to the presence of macronutrients mainly associated with the content of protein (46.71 g/100 g) and fibers (38.58 g/100 g) since these macronutrients can perform as natural emulsifiers.
Another type of byproduct used for stabilizing emulsions is perilla seed press-cakes, which are obtained during the processing of perilla oilseeds to obtain oil. This obtained residue has a high content of proteins (35–45%), fibers (55–65%), phytic acid, polysaccharides, and phenolic compounds [28]. Zhao et al. [43] determined that perilla seed protein has a high surface hydrophobicity (119.29), solubility (78.71%), and a small particle size (318 nm), and therefore it can be considered as a natural emulsifier.
On the other hand, the peanut oil extraction process produces three fractions: (i) an oil-rich cream fraction, (ii) a protein-rich water fraction, and (iii) a sediment fraction (neutral and acidic polysaccharides) [44]. Acidic polysaccharides have been found to have a high particle aggregation ability, while alkaline-extracted polysaccharides reduce the oil–water surface tension [29,45]. Based on the antecedents described previously, Ye et al. [29] obtained polysaccharides from peanut oil processing residues by the alkaline method to be used as a Pickering stabilizer. The obtained complexes (PEC) comprised polysaccharides (56–68%) and proteins (13–18%). The presence of protein improved the hydrophobicity of the PECs. Furthermore, as the extraction pH value increased to 10.0, the protein in the PECs covalently bound to the polysaccharide and the polysaccharide conformation unfolded simultaneously, leading to the particle size increasing from 264 ± 5 nm to 360 ± 13 nm, which in turn resulted in the higher emulsifying capacity of the PECs [29]. Therefore, oilseed byproducts have great potential to develop edible Pickering stabilizers.
4.3. Fruit Byproducts
Fruit and vegetable industries generate 14.8 Mt of waste and byproducts in the European Union [46]. These types of byproducts can be considered hazardous from an environmental point of view, since they can affect the deterioration of drinking water quality, contaminate aqueous media, inhibit seed germination, and cause intestinal disorders in animals. Nevertheless, with appropriate treatment, they can represent a cost-effective raw material which is abundant in valuable functional compounds [47], which make them good ingredients as Pickering stabilizers. For this reason, various studies have shown the potential of apple pomace to be used as a natural stabilizer due to its good emulsifying properties. The emulsifying properties of apple pomace powders were attributed to their insoluble fraction content (90.5% wt over dry matter) and they are responsible for the stability and formation of a network in Pickering emulsions [23,48,49]. Soluble components alone may not be enough to stabilize oil-in-water emulsions, since they could provide a synergistic or antagonistic effect at the interface with insoluble particles [50]. Another byproduct is the fiber obtained from citrus peel, which is a residue from the pectin industry and orange juice production. Citrus fibers are mainly composed of proteins (~8%), pectins (~35–42%), cellulose (>45%), hemicellulose, and lignin, which can vary in concentration depending on the origin. The fibers have a high water retention capacity and apparent viscosity, which is dependent on their structure [51,52,53]. For instance, Qi et al. [24] developed a Pickering emulsion using dietary fibers fragmented by ultra-high-pressure homogenization. However, in contrast to other particles, the fibers form a network, which swells and can bind oil droplets to the surface and absorb water into the network.
The chocolate industry produces mainly two byproducts: cocoa fiber resulting from the grinding of the husk and cocoa powder obtained from the grinding of the cocoa press-cake. Typically, the cocoa powder contains different functional components, such as phenolic compounds, hydrocolloids, sugars, proteins, fibers, theobromine, and lipids, which account for approximately 10–25% of its content [54]. Gould et al. [55] reported the use of cocoa mass, cocoa fibers, cocoa particles, and cocoa with different fat contents to obtain Pickering emulsions. In addition, Joseph et al. [25,54] produced Pickering emulsions using cocoa powder defatting with hexane as a stabilizer. They reported that the cocoa powder had a soluble fraction of 23.6 g/100 g and a protein content of 25 g/100 g, which could explain its ability to stabilize emulsions.
Fruit byproducts can be used to obtain particles with improved characteristics (nanocrystals or cellulose nanofibers) using different techniques such as enzymatic hydrolysis, hydrothermal treatment, acid or basic hydrolysis, high-pressure homogenization, and ultrasound, among others [56,57,58].
In this area, cellulose nanocrystals (CNC) stand out as Pickering stabilizers since they have a low density, high surface area, high crystallinity, amphiphilicity, low toxicity, and excellent functionality [59]. Tang et al. [60] compared the efficiency of Pickering stabilizers of cellulose nanocrystals (CNCs) obtained by acid hydrolysis from pineapple pulp (PPu), pineapple leaf (PL), and pineapple peel (PPe) residues. PPu nanocrystals showed the best emulsifying properties, attributed to their high cellulose content (96.10%), a CNC yield of 23.26%, and an average length of 141.5 nm. Foo et al. [61] obtained CNCs by acid hydrolysis of empty fruit bunches (EFB) available from the palm oil industry, in which they obtained a yield of 53.23%, a crystallinity of 76.55%, and a hydrodynamic diameter of 69.22 nm, while the width and length of the CNCs were 16 nm and 257 nm, respectively.
Another Pickering stabilizer is cellulose nanofiber (CNF), used for its low crystallinity and high compatibility with proteins and lipids. In addition, its hydrophilic nature, together with its large size (long), causes the CNF to overlap and join disordered networks and macroscopic gels, acting as a viscosity modifier. CNFs from banana peels, which are produced by chemical and enzymatic treatments, show a potential to be used as natural stabilizers [62]. Costa et al. [63] evaluated the influence of the processes of ultrasound and high-pressure homogenization on the CNF from banana peels. The study determined that the ultrasonication power and pressure led to shortening of the length, modifications in the crystallinity index, expansion of hydrophobic domains, and enhancement in zeta potential values (from −16.1 to −44.1 mV) of CNFs, which favored the stabilization of emulsions. Pomelo spongy tissue has been used to obtain cellulose nanofibers (CNFs) by its high dietary fiber content. Wen et al. [64] obtained CNFs by a chemical method (acidic and basic) and using a high-pressure homogenizer. They obtained low lignin values (0.70%), high cellulose contents (79.68%), and particle sizes of >3 μm in length and 33–64 nm in width.
4.4. Other Agri-Food Byproducts
Moringa seed residue protein is one of the main byproducts of Moringa oil extraction. Huang et al. [26] extracted the protein to be used as a Pickering stabilizer, achieving a Moringa seed residue protein (MSRP) yield of 14.24%. The particle diameter of the positively charged MSRP was greater than 233 nm. MSRP was observed to be soluble at a pH of 5 (206.89 mg/g) and in the presence of 0.2 M NaCl (202.55 mg/g). Tea residues are another resource used to extract plant proteins. This byproduct is obtained from the production of tea beverages and the extraction of bioactive components [65]. Tea residues are 90% insoluble protein, which can be extracted by alkaline or enzymatic methods [66]. Ren et al. [67] fabricated water-insoluble tea protein nanoparticles (TWIPNs). TWIPNs were obtained by the nanoprecipitation method and determined to be irregular colloidal particles with a hydrodynamic diameter greater than 300 nm and a zeta potential greater than −30 mV at ionic strengths of 0–400 mM and a fixed concentration of TWIPNs (2.0%); these properties indicate the potential of these nanoparticles to stabilize emulsions [67].
He et al. [68] studied the use of bamboo shoot fibers as plant food particle stabilizers for O/W Pickering emulsions. In this study, the bamboo shoot fibers were treated by high-pressure homogenization, which produced a filamentous morphology of fibers. A rheological analysis showed that the fibers present a shear thinning behavior. The findings suggest that the dietary fibers derived from bamboo shoots possess a soft nature and appropriate shape for producing stable edible Pickering emulsions with potential applications in the food industry.
Huc-Mathis et al. [48] analyzed the properties of oat and sugar beet residues for potential application as Pickering stabilizers. They obtained a particle size of 6.60 ± 0.01 μm for oats and 5.80 ± 0.02 μm for sugar beet using only a micronizing process. The insoluble contents of sugar beet and oats were 93.7 ± 0.8% and 94.0 ± 0.1%, respectively, and the Zeta potentials were −31 ± 5 mV and −22.6 ± 1.2 mV, respectively. The results obtained, in addition the fact that sugar beets present emulsifying properties associated with their pectins and fibers [69] and the saponins present in oat extract possess emulsifying properties [70], indicated propertied that made it possible to use these byproducts as Pickering stabilizers.
Coffee residues have a lignin content of between 20 and 27% (wt/wt) [71], which is indicative of a hydrophobic molecule. However, it has been demonstrated that lignin may exhibit hydrophilic, hydrophobic, and amphiphilic characteristics depending on the botanical source and the methods used for extraction. Gould et al. [72] performed a hydrothermal treatment to remove lignin from coffee particles. This treatment produced an increase in the hydrophobicity of the particle surface, which improved their emulsifying properties.
5. O/W Pickering Emulsions Stabilized by Different Agri-Food Byproduct Particles
This section provides an overview of the recent studies on the stabilization of Pickering emulsions (O/W) using edible natural particles. Thus, different works on Pickering emulsions stabilized by different agri-food byproducts are described below. For instance, Yang et al. [36] showed that it was possible to stabilize O/W Pickering emulsions by using nanoparticles from insoluble soybean polysaccharides of okara. The results showed that nanoparticles have high emulsifying and gelling properties, which favored the stabilization of Pickering emulsions. It should be noted that the concentration of particles and the oil content in the formation of the emulsion favored their stability [36]. Bao et al. [37] showed that fermentation-modified okara insoluble dietary fiber (OIDF) exhibits excellent properties to be considered as a Pickering stabilizer. These particles had a strong electrostatic interaction, smaller droplets, and a higher encapsulation efficiency (95%) and yield than unmodified OIDF.
Huc-Mathis et al. [48] evaluated the ability of apple pomace as a Pickering stabilizer. Their first investigation showed that apple powder had better emulsifying properties than oat bran. The insoluble fibers contributed to emulsion stability through the Pickering mechanism for 15 days, which was favored by the stabilization provided by proteins and pectin in the soluble fraction [50]. Then, the same authors designed an experiment to analyze the influence of apple powder, microcrystalline cellulose, and oil content on the properties of Pickering emulsions. They determined that the higher the apple powder content, the lower the oil droplet size. Likewise, the highest concentrations of fat and polymer resulted in an increase in the values of the elastic modulus, G’, and viscosity, with a simultaneous decrease in the tan(δ) value [23]. In their latest research, they compared apple powder to sugar beet powder and oat bran. These byproducts were able to produce stable emulsions that were resistant to coalescence. Of these, apple powder provided supplementary stabilization by preventing drainage of the continuous phase. The smallest emulsion oil droplets were obtained with sugar beet, followed by apple then oat, which was associated with the emulsifying properties of the byproducts [48]. On the other hand, Lu et al. [49] studied micronized apple pomace as a novel food-grade emulsifier for stabilizing O/W Pickering emulsions. These emulsions exhibited a smaller droplet size and improved gelation and antioxidant properties when the particle size was reduced.
Qi et al. [24] studied the use of citrus fibers for the stabilization of O/W emulsions, evaluating the Pickering mechanism and the fiber-based network effect. This study showed that the O/W emulsions were more stable using a fiber concentration of 2% (wt/v) and 25% (v/v) oil. These emulsions had a storage stability of ≥60 days and they were not obviously influenced by changes in the pH, ionic strength of NaCl, or temperature. He et al. [68] used water-insoluble dietary fibers from bamboo shoots to stabilize O/W emulsions. The emulsions remained stable against coalescence for at least 4 weeks, and their stability was not affected by changes in pH, ionic strength, or pasteurization conditions.
Gould et al. [55] showed the ability to form coalescence-stable O/W emulsions using cocoa particles. They also determined that increasing the cocoa particle concentration reduced the average droplet size. On the other hand, Joseph et al. [54] prepared O/W Pickering emulsions stabilized by defatted cocoa powder. For this, the emulsions were studied using three techniques, i.e., rotor–stator, sonication, and microfluidization, where the microfluidization technique was the most efficient emulsification technique. The emulsion obtained with this technique showed the highest anchorage rate, the smallest droplets (4.2 µm), and the emulsion was stable after 90 days. In addition, Joseph et al. [3] investigated the production of powdered re-dispersible Pickering stabilizers based on cocoa powder and rapeseed press-cakes. The rehydrated O/W Pickering emulsions showed an average droplet size of <100 μm, which was larger compared to their parent emulsions. The authors stated that this behavior could be attributed to the coalescence phenomena occurring during the drying process. The formulations based on rapeseed press-cakes provided excellent results, since after spray drying, the re-dispersed emulsion exhibited almost the same characteristics as the original emulsion in terms of size distribution. Finally, Joseph et al. [25] obtained O/W Pickering emulsions stabilized by cocoa, rapeseed, and lupin solid particles. The authors also tested three emulsification techniques and found that the thinnest emulsions and the highest anchorage ratios were obtained using microfluidization, independently of the nature of the particles. The emulsions showed narrow droplet size distributions, especially in the presence of rapeseed and cocoa powder. Finally, the authors concluded that rapeseed powder derived from defatted press-cakes was the most effective in terms of interfacial coverage.
Gould et al. [72] showed that ground coffee residue particles can be used as a Pickering stabilizer to prepare O/W emulsions. These emulsions exhibited a droplet size of around 100 µm and no change in microstructure during a 12 week storage period. The droplet size was also unaffected by pH changes. They also determined their stability against coalescence under shear and their stability under pasteurization conditions (10 min at 80 °C) [72].
Burgos-Díaz et al. [1] characterized and compared six food-grade Pickering stabilizers obtained from various sources of agri-food byproducts (canola press-cake, camelina press-cake, linseed hull, linseed press-cake, lupin byproduct, and lupin hull). The study showed that emulsions stabilized with camelina press-cake, lupin hull, and lupin byproduct at concentrations of ≥3.5% (wt/wt) exhibited remarkable stability against creaming for at least 45 days of storage. The size of the droplets was significantly influenced by the concentration of particles and the type of raw material utilized. Microscopy studies showed that the solid particles were anchored to the surfaces of the oil droplets, which is clear evidence of the formation of a Pickering emulsion stabilized by solid particles [1].
Foo et al. [73] obtained Pickering nanoemulsions with nanocrystalline cellulose from an empty oil palm fruit bunch. The main results found in this study were based on the role of lignocellulosic residues in the formation and stability of Pickering nanoemulsions, in which nanocrystalline cellulose significantly contributed to the stabilization of the nanoemulsion, as well as to the size of the particles obtained. The nanoemulsion exhibited a droplet size of approximately 400 nm, and a high stability for 6 months against the formation of creaming and coalescence. These results are promising, since the stabilization obtained by nanocrystalline cellulose could help to obtain improved products in the food and personal care industries [73]. In addition, pomelo spongy tissue cellulose nanofibers (PCNFs) have been used to stabilize Pickering emulsions [64]. The emulsions obtained showed an excellent stability and a strong concentration-dependent effect of PCNFs; at higher concentrations of PCNFs, increasingly stable emulsions were observed. Tang et al. [60] reported that the emulsions stabilized with pineapple cellulose nanocrystals (PCNCs) obtained from pineapple peel were stable after storage for 50 days, which was attributed to the microstructure of the PCNCs. On the other hand, Costas et al. [63] investigated the impact of emulsification conditions using ultrasound and a high-pressure homogenizer on the physicochemical characteristics of emulsions stabilized by cellulose nanofibers (CNFs) sourced from banana peel. The authors determined that both emulsification processes presented oil droplet creaming. The ultrasonic emulsification process resulted in a reduction in the length and aspect ratio of the CNFs, thus forming smaller emulsion droplets due to the obtained particles achieved to cover the droplet–emulsion interface, which prevented coalescence of the emulsion.
Huang et al. [26] evaluated the stability of Pickering emulsions using moringa seed residue proteins through the effect of pH and ionic strength. The results showed that the emulsion presented high zeta potential values and excellent morphological characteristics. Concerning the emulsion stability, a cream layer appeared rapidly within 2 h; however, after 30 days of storage, the emulsions showed no apparent changes, indicating a good stability against creaming. On the other hand, at pH 5 and 0.2 M NaCl, elastic networks can be obtained in Pickering emulsions stabilized with moringa seed residues, so stabilization with this type of residue could play an important role in emulsifying and stabilizing processes in the food and beverage industry [26]. In addition, Ren et al. [67] obtained Pickering emulsions stabilized with nanoparticles of tea-water-insoluble proteins (TWIPs). The main results showed that the Pickering emulsions generated firm and thick surface layers as the concentration of nanoparticles increased, which helped to reduce the size of the emulsion droplets at the amounts of oil and used water (4:6). According to an analysis of creaming stability, a cream layer was visible on top of the emulsions after 6 h of emulsion preparation. However, during 3–40 days of storage, no evident change was observed in the creaming behavior. This indicated the excellent anti-creaming ability of the TWIPs [67].
According to Zhao et al. [43], perilla protein isolate extracted from cold pressing residues displayed excellent functional properties, including a high foaming ability (90.67%), emulsification capacity (3390.09 m2/g), and water holding capacity (2.17 g/g). Therefore, this byproduct exhibited an excellent capacity to stabilize Pickering emulsions based on its small droplet size, high ζ-potential, and low creaming index of emulsion droplets. Liu et al. [28] showed that increasing the perilla seed protein (PSP) concentration (0.25 at 1.0 wt %) was favorable to avoid the aggregation/flocculation of emulsions. They found that PSP-stabilized emulsions were stable at NaCl concentrations of less than 150 mmol/L and at pHs between 3.0 and 9.0. Moreover, the stability of the emulsion was enhanced against aggregation and creaming when it was subjected to a temperature of 70 °C. Finally, polysaccharide–protein complexes (PECs) of peanut sediment from aqueous extraction processes were used as Pickering stabilizers by Ye et al. [29]. They determined that a 4% wt concentration of PEC10.0 (pH = 10) stabilized Pickering emulsions with a superior creaming stability, which was mainly attributed to the fact that the relatively high viscosity limited droplet movement. These emulsions were stable for 20 days, with an average particle size of 16.96 µm for an oil fraction of 0.6.
Recent studies using agri-food byproducts as Pickering stabilizers in emulsions are summarized in Table 1.

Table 1.
Pickering emulsions based on agri-food byproducts.
6. Applications and Future Trends
In recent years, the superior performance of Pickering emulsions has been reported in various fields such as cosmetics, pharmacy, biomedicine, and food [13]. Compared with conventional emulsions, food-grade Pickering emulsions have several advantages, such as increased safety, good stability, environmental friendliness, and excellent biocompatibility [20]. According to Xia et al. [74], this type of emulsion has been studied and characterized and has a wide spectrum of applications in the food industry. For instance, Pickering emulsions have been used as fat substitutes [75,76], delivery systems for nutraceuticals [77,78], and even in the manufacturing of food-grade cleaning agents [79], as is detailed in Table 2. Regarding fat substitutes, Pickering emulsions have been demonstrated as a butter substitute. A study showed that the use of Pickering emulsions (stabilized by ethyl cellulose and camellia seed oil) could replace cream in the production of frozen yoghurt and ice cream [74]. In terms of nutraceutical delivery, Pickering emulsions are suitable delivery systems to enhance the physical stability, the compatibility with food matrices, the oxidative stability, and the protection of labile bioactive compounds. Thus, Pickering solid particles adsorbed on the oil–water interface can form a physical barrier and prevent the degradation of nutrients [74].
On the other hand, Pickering emulsions have been also applied in other fields indirectly related to food science; they have been applied in biomedicine in order to improve the solubility of poorly soluble drugs [80]. In biomedicine, the utilization of Pickering emulsions as a drug carrier or delivery system is due to their excellent biocompatibility, since these emulsions are prepared using non-toxic raw materials [81]. Another feature that has enhanced the applications of these types of emulsions is their greater stability compared with conventional emulsions. For instance, Pickering emulsions are not easily affected by environmental stresses, such as temperature, ionic strength, and pH.

Table 2.
Current applications of food-grade Pickering emulsions.
Table 2.
Current applications of food-grade Pickering emulsions.
| Application | Main Products | Purpose | References |
|---|---|---|---|
| Fat substitutes | Butter Yoghurt Ice cream |
| [75,76,82,83] |
| Delivery systems for bioactive compounds | Curcumin Hesperidin β-carotene |
| [10,77,78] |
| Cleaning agents | Green detergent from corncob | Cleaning oil stains in an eco-friendly and safe way. | [79] |
Food-grade Pickering emulsions have evolved with the new trends that have emerged in recent years. These evolutions include Pickering double emulsions, nutraceutical co-delivery, multilayer Pickering emulsions, Pickering emulsions fixed in gels, preparation of porous materials, and responsive Pickering emulsions [74]. Briefly, “Pickering double emulsions” can be classified as water-in-oil-in-water (W/O/W) or oil-in-water-in-oil (O/W/O) emulsions. Regarding O/W/O, this emulsion consists of a continuous oil system containing water droplets with smaller oil droplets inside them, whereas W/O/W emulsions are water-continuous systems containing oil droplets within which smaller water droplets are dispersed [74]. One potential application of this type of colloidal system is in the production of a reduced-fat emulsion product that has a lower oil content but with a similar texture perceived in the mouth. Moreover, Pickering double emulsions can encapsulate and protect bioactive compounds in the inner phase of Pickering double emulsions, which are then subsequently released during the digestive process [74].
Nutraceutical co-delivery: Other emerging trends within the functional food industry include products with bioactive compounds or nutraceutical micro/nano encapsulated products. In this context, Pickering emulsions as a delivery system have been widely reported. However, most of these studies describe the encapsulation of a single molecule in the disperse phase emulsion and there have been few reports on the co-delivery of bioactive compounds in Pickering emulsions at the same time [74]. Therefore, the current applications in this area are focused on the development of encapsulation systems that include two or more bioactive compounds to develop new food products. Some studies have shown that the combination of some compounds in the preparation of nutraceuticals, such as curcumin and resveratrol, increases antioxidant effects, and even synergistic effects have been reported [84].
Multilayer Pickering emulsion: This colloidal system refers to emulsions which are stabilized by multiple layers of insoluble particles. Multilayer emulsions have been applied to protect different labile lipophilic compounds and have shown better physical stability against environmental stresses, such as heat treatment, freeze-drying, and ionic strength, among others [85]. Despite the potential applications of multilayer Pickering emulsions, this type of multilayer emulsion has not been investigated before [74].
Pickering emulsions fixed in gels: “Pickering emulsion gel” refers to colloidal systems based on oleogels and hydrogels. Oleogel-based Pickering emulsions have shown greater stability in comparison to other emulsion systems. For example, Pickering emulsions stabilized by ovotransferrin fibrils and based on oleogels demonstrated a remarkable stability during storage and a high level of stability during freeze–thaw cycles [78]. In addition, oleogels have been used to replace saturated fats and trans fats in food.
Preparation of porous materials: This new trend has great advantages because it presents particles that can facilitate the fabrication of porous materials. This could allow the introduction of some functional groups within particles or even as an absorbent of heavy metals due to the porous characteristics they present [74,86].
Responsive Pickering emulsions: This type of emulsion, also known as “stimuli-response Pickering emulsions”, has attracted attention because of their potential applications for emulsion polymerization and target delivery of bioactive compounds. In this context, pH-responsive emulsions are the most common stimuli-responsive Pickering emulsions. These emulsion systems (pH-responsive) are characterized by their simplicity and diversity of materials available for use because variations in the pH of these systems result in changes in the surface behavior of the materials [74,87].
Dairy products: The physicochemical characteristics of dairy products could be improved by the use of Pickering emulsions, as they transfer their stability to these products. As a consequence, the shelf-life of milk and its derivatives could be prolonged. Therefore, there is a wide range of potential applications in the dairy industry [74,75,88,89,90].
On the other hand, edible Pickering emulsions are commonly liquids. However, in some cases, the liquid formulation presents complications when it is incorporated into food matrix systems, which complicates food processing [75]. One of the strategies used to avoid this problem is the use of hydrogels, which immobilizes Pickering emulsions. For instance, it has been observed that hydrogels stabilized with alginates improve oil retention, inhibit lipid oxidation, and also help the controlled release of compounds of interest [91]. Finally, it is important to mention that Pickering emulsions are still considered a novel type of colloidal system, which is an indicator of the need for new perspectives and future trends in the application of these emulsion systems [81].
7. Conclusions
Pickering emulsions have not only gained ground in the food industry but have also attracted the interest of the pharmaceutical industry in recent years. However, new food trends are pushing scientists to pay more attention to the sustainability of the bio-based particles they are using to stabilize emulsions. Thereby, the use of agri-food byproducts represents an excellent opportunity to explore new particle sources to be used as natural stabilizers. Until now, the advantageous stability of Pickering emulsions has been demonstrated, which is largely attributed to the diverse range of stabilization mechanisms in comparison to traditional emulsifiers. Therefore, exploring new, more natural, food-grade stabilizers seems to be a logical step in this field. Thus, this review briefly described a variety of natural particles from different agri-food byproducts used as Pickering stabilizers. Owing to the recent information exposed, the use of agri-food byproducts from legumes, oil seeds, and fruits is proven to be an alternative, renewable, and inexpensive source of solid amphiphilic particles to be used as “natural stabilizers” in different industrial applications. In addition, the exposed information shows that Pickering emulsions have great potential in fields other than food, such as interfacial catalysis, biomedicine, drug delivery, functional materials, and others.
Author Contributions
Conceptualization, C.B.-D. and K.A.G.-M.; resources, C.B.-D. and K.A.G.-M.; writing—original draft preparation, C.B.-D., K.A.G.-M., D.A.P., M.O.-N., M.C.-F. and M.B.; review and editing, supervision, C.B.-D. and K.A.G.-M. The authors have contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID through FONDECYT-REGULAR project N° 1210136 and FONDECYT-POSTDOCTORADO N° 3220459.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the Chilean Agency for Research and Development (ANID), FONDECYT-REGULAR project N° 1210136 and FONDECYT-POSTDOCTORADO N° 3220459.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burgos-Díaz, C.; Mosi-Roa, Y.; Opazo-Navarrete, M.; Bustamante, M.; Garrido-Miranda, K. Comparative Study of Food-Grade Pickering Stabilizers Obtained from Agri-Food Byproducts: Chemical Characterization and Emulsifying Capacity. Foods 2022, 11, 2514. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Savoire, R.; Harscoat-Schiavo, C.; Pintori, D.; Monteil, J.; Faure, C.; Leal-Calderon, F. Redispersible Dry Emulsions Stabilized by Plant Material: Rapeseed Press-Cake or Cocoa Powder. LWT-Food Sci. Technol. 2019, 113, 108311. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering Emulsions: Versatility of Colloidal Particles and Recent Applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Wandersleben, T.; Olivos, M.; Lichtin, N.; Bustamante, M.; Solans, C. Food-Grade Pickering Stabilizers Obtained from a Protein-Rich Lupin Cultivar (AluProt-CGNA®): Chemical Characterization and Emulsifying Properties. Food Hydrocoll. 2019, 87, 847–857. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderón, F.; Bustamante, M. Food-Grade Pickering Emulsion as a Novel Astaxanthin Encapsulation System for Making Powder-Based Products: Evaluation of Astaxanthin Stability during Processing, Storage, and Its Bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef] [PubMed]
- Haji, F.; Cheon, J.; Baek, J.; Wang, Q.; Tam, K.C. Application of Pickering Emulsions in Probiotic Encapsulation—A Review. Curr. Res. Food Sci. 2022, 5, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-Grade Pickering Emulsions for Encapsulation and Delivery of Bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Ho, K.W.; Ooi, C.W.; Tey, B.T.; Chan, E.S. Facile Method for Forming Ionically Cross-Linked Chitosan Microcapsules from Pickering Emulsion Templates. Food Hydrocoll. 2016, 55, 26–33. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Wang, Y.; Boesch, C.; Zhao, Y.; Sarkar, A. Pickering Emulsions Stabilized by Colloidal Gel Particles Complexed or Conjugated with Biopolymers to Enhance Bioaccessibility and Cellular Uptake of Curcuminal. Curr. Res. Food Sci. 2020, 3, 178–188. [Google Scholar] [CrossRef]
- Li, W.; Jiao, B.; Li, S.; Faisal, S.; Shi, A.; Fu, W.; Chen, Y.; Wang, Q. Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications. Front. Nutr. 2022, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Gillet, G.; Gressin, L.; Desobry, S. Interest of Pickering Emulsions for Sustainable Micro/Nanocellulose in Food and Cosmetic Applications. Polymers. 2020, 12, 2385. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Bowen, B.D.; Partridge, S.J. Stabilization of Emulsions by Fine Particles II. Capillary and van Der Waals Forces between Particles. Colloids Surf. 1989, 38, 345–364. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent Advances of Characterization Techniques for the Formation, Physical Properties and Stability of Pickering Emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef]
- Ming, Y.; Xia, Y.; Ma, G. Aggregating Particles on the O/W Interface: Tuning Pickering Emulsion for the Enhanced Drug Delivery Systems. Aggregate 2022, 3, e162. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering Emulsions: Preparation Processes, Key Parameters Governing Their Properties and Potential for Pharmaceutical Applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Kempin, M.V.; Kraume, M.; Drews, A. W/O Pickering Emulsion Preparation Using a Batch Rotor-Stator Mixer—Influence on Rheology, Drop Size Distribution and Filtration Behavior. J. Colloid Interface Sci. 2020, 573, 135–149. [Google Scholar] [CrossRef]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Schrade, A.; Landfester, K.; Ziener, U. Pickering-Type Stabilized Nanoparticles by Heterophase Polymerization. Chem. Soc. Rev. 2013, 42, 6823–6839. [Google Scholar] [CrossRef] [PubMed]
- Huc-Mathis, D.; Guilbaud, A.; Fayolle, N.; Bosc, V.; Blumenthal, D. Valorizing Apple By-Products as Emulsion Stabilizers: Experimental Design for Modeling the Structure-Texture Relationships. J. Food Eng. 2020, 287, 110115. [Google Scholar] [CrossRef]
- Qi, J.R.; Song, L.W.; Zeng, W.Q.; Liao, J.S. Citrus Fiber for the Stabilization of O/W Emulsion through Combination of Pickering Effect and Fiber-Based Network. Food Chem. 2021, 343, 128523. [Google Scholar] [CrossRef]
- Joseph, C.; Savoire, R.; Harscoat-Schiavo, C.; Pintori, D.; Monteil, J.; Faure, C.; Leal-Calderon, F. Pickering Emulsions Stabilized by Various Plant Materials: Cocoa, Rapeseed Press Cake and Lupin Hulls. LWT-Food Sci. Technol. 2020, 130, 109621. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, X.; Zhou, W.; Zhang, L.; Liu, F.; Li, J.; Peng, S.; Cao, Y.; Li, Y.; Li, R.; et al. Fabrication and Stability of Pickering Emulsions Using Moringa Seed Residue Protein: Effect of PH and Ionic Strength. Int. J. Food Sci. Technol. 2021, 56, 3484–3494. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Weng, W.; Li, B. Pickering Emulsions Stabilized by Tea Water-Insoluble Protein Nanoparticles From Tea Residues: Responsiveness to Ionic Strength. Front. Nutr. 2022, 9, 840. [Google Scholar] [CrossRef]
- Liu, N.; Chen, Q.; Li, G.; Zhu, Z.; Yi, J.; Li, C.; Chen, X.; Wang, Y. Properties and Stability of Perilla Seed Protein-Stabilized Oil-in-Water Emulsions: Influence of Protein Concentration, PH, NaCl Concentration and Thermal Treatment. Molecules 2018, 23, 1533. [Google Scholar] [CrossRef]
- Ye, J.; Hua, X.; Zhao, Q.; Dong, Z.; Li, Z.; Zhang, W.; Yang, R. Characteristics of Alkali-Extracted Peanut Polysaccharide-Protein Complexes and Their Ability as Pickering Emulsifiers. Int. J. Biol. Macromol. 2020, 162, 1178–1186. [Google Scholar] [CrossRef]
- Tassoni, A.; Tedeschi, T.; Zurlini, C.; Cigognini, I.M.; Petrusan, J.I.; Rodríguez, Ó.; Neri, S.; Celli, A.; Sisti, L.; Cinelli, P.; et al. State-of-the-Art Production Chains for Peas, Beans and Chickpeas—Valorization of Agro-Industrial Residues and Applications of Derived Extracts. Molecules 2020, 25, 1383. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suárez, M.J.; Zapata-Revilla, M.A.; Tenorio-Sanz, M.D. Pea Pod, Broad Bean Pod and Okara, Potential Sources of Functional Compounds. LWT-Food Sci. Technol. 2010, 43, 1467–1470. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suárez, M.J. Broad Bean and Pea By-Products as Sources of Fibre-Rich Ingredients: Potential Antioxidant Activity Measured in Vitro. J. Sci. Food Agric. 2012, 92, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Vucelic-Radovic, B.V. Composition of Proteins in Okara as a Byproduct in Hydrothermal Processing of Soy Milk. J. Agric. Food Chem. 2012, 60, 9221–9228. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Yin, T.; Xiong, S.; Zhang, J.; Din, Z.-U.; Zhang, M. Structural Characteristics and Physicochemical Properties of Okara (Soybean residue) Insoluble Dietary Fiber Modified by High-Energy Wet Media Milling. LWT-Food Sci. Technol. 2017, 82, 15–22. [Google Scholar] [CrossRef]
- Porfiri, M.C.; Vaccaro, J.; Stortz, C.A.; Navarro, D.A.; Wagner, J.R.; Cabezas, D.M. Insoluble Soybean Polysaccharides: Obtaining and Evaluation of Their O/W Emulsifying Properties. Food Hydrocoll. 2017, 73, 262–273. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.X.; Li, X.T.; Tang, C.H. Novel Nanoparticles from Insoluble Soybean Polysaccharides of Okara as Unique Pickering Stabilizers for Oil-in-Water Emulsions. Food Hydrocoll. 2019, 94, 255–267. [Google Scholar] [CrossRef]
- Bao, Y.; Xue, H.; Yue, Y.; Wang, X.; Yu, H.; Piao, C. Preparation and Characterization of Pickering Emulsions with Modified Okara Insoluble Dietary Fiber. Foods 2021, 10, 2982. [Google Scholar] [CrossRef]
- Moreno-González, M.; Girish, V.; Keulen, D.; Wijngaard, H.; Lauteslager, X.; Ferreira, G.; Ottens, M. Recovery of Sinapic Acid from Canola/Rapeseed Meal Extracts by Adsorption. Food Bioprod. Process. 2020, 120, 69–79. [Google Scholar] [CrossRef]
- Arntfield, S.D. Proteins from Oil-Producing Plants. In Proteins in Food Processing, 2nd ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 187–221. [Google Scholar]
- Parodi, E.; La Nasa, J.; Ribechini, E.; Petri, A.; Piccolo, O. Extraction of Proteins and Residual Oil from Flax (Linum usitatissimum), Camelina (Camelina sativa), and Sunflower (Helianthus annuus) Oilseed Press Cakes. Biomass Convers. Biorefinery 2021, 13, 1915–1926. [Google Scholar] [CrossRef]
- Fetzer, A.; Herfellner, T.; Stäbler, A.; Menner, M.; Eisner, P. Influence of Process Conditions during Aqueous Protein Extraction upon Yield from Pre-Pressed and Cold-Pressed Rapeseed Press Cake. Ind. Crops Prod. 2018, 112, 236–246. [Google Scholar] [CrossRef]
- Li, T.; Dai, T.; Ahlström, C.; Thuvander, J.; Rayner, M.; Matos, M.; Gutiérrez, G.; Östbring, K. The Effect of Precipitation PH on Protein Recovery Yield and Emulsifying Properties in the Extraction of Protein from Cold-Pressed Rapeseed Press Cake. Molecules 2022, 27, 2957. [Google Scholar]
- Zhao, Q.; Wang, L.; Hong, X.; Liu, Y.; Li, J. Structural and Functional Properties of Perilla Protein Isolate Extracted from Oilseed Residues and Its Utilization in Pickering Emulsions. Food Hydrocoll. 2021, 113, 106412. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Han, X.; Liu, J.; Liu, Y.; Gasmalla, M.A.A.; Yang, R. Demulsification of Oil-Rich Emulsion and Characterization of Protein Hydrolysates from Peanut Cream Emulsion of Aqueous Extraction Processing. J. Food Eng. 2017, 204, 64–72. [Google Scholar] [CrossRef]
- Ye, J.; Hua, X.; Zhao, Q.; Zhao, W.; Chu, G.; Zhang, W.; Yang, R. Chain Conformation and Rheological Properties of an Acid-Extracted Polysaccharide from Peanut Sediment of Aqueous Extraction Process. Carbohydr. Polym. 2020, 228, 115410. [Google Scholar] [CrossRef] [PubMed]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products—A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef]
- Huc-Mathis, D.; Almeida, G.; Michon, C. Pickering Emulsions Based on Food Byproducts: A Comprehensive Study of Soluble and Insoluble Contents. J. Colloid Interface Sci. 2021, 581, 226–237. [Google Scholar] [CrossRef]
- Lu, Z.; Ye, F.; Zhou, G.; Gao, R.; Qin, D.; Zhao, G. Micronized Apple Pomace as a Novel Emulsifier for Food O/W Pickering Emulsion. Food Chem. 2020, 330, 127325. [Google Scholar] [CrossRef]
- Huc-Mathis, D.; Journet, C.; Fayolle, N.; Bosc, V. Emulsifying Properties of Food By-Products: Valorizing Apple Pomace and Oat Bran. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 84–91. [Google Scholar] [CrossRef]
- Chatsisvili, N.T.; Amvrosiadis, I.; Kiosseoglou, V. Physicochemical Properties of a Dressing-Type o/w Emulsion as Influenced by Orange Pulp Fiber Incorporation. LWT-Food Sci. Technol. 2012, 46, 335–340. [Google Scholar] [CrossRef]
- Lundberg, B.; Pan, X.; White, A.; Chau, H.; Hotchkiss, A. Rheology and Composition of Citrus Fiber. J. Food Eng. 2014, 125, 97–104. [Google Scholar] [CrossRef]
- Wallecan, J.; McCrae, C.; Debon, S.J.J.; Dong, J.; Mazoyer, J. Emulsifying and Stabilizing Properties of Functionalized Orange Pulp Fibers. Food Hydrocoll. 2015, 47, 115–123. [Google Scholar] [CrossRef]
- Joseph, C.; Savoire, R.; Harscoat-Schiavo, C.; Pintori, D.; Monteil, J.; Leal-Calderon, F.; Faure, C. O/W Pickering Emulsions Stabilized by Cocoa Powder: Role of the Emulsification Process and of Composition Parameters. Food Res. Int. 2019, 116, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.; Vieira, J.; Wolf, B. Cocoa Particles for Food Emulsion Stabilisation. Food Funct. 2013, 4, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, M.C.N.; Emmambux, M.N. Isolation, Characterization, and Application of Nanocellulose from Agro-Industrial By-Products: A Review. Food Rev. Int. 2021, 1–29. [Google Scholar] [CrossRef]
- Kazmi, M.Z.H.; Karmakar, A.; Michaelis, V.K.; Williams, F.J. Separation of Cellulose/Hemicellulose from Lignin in White Pine Sawdust Using Boron Trihalide Reagents. Tetrahedron 2019, 75, 1465–1470. [Google Scholar] [CrossRef]
- Baksi, S.; Saha, S.; Birgen, C.; Sarkar, U.; Preisig, H.A.; Markussen, S.; Wittgens, B.; Wentzel, A. Valorization of Lignocellulosic Waste (Crotalaria Juncea) Using Alkaline Peroxide Pretreatment under Different Process Conditions: An Optimization Study on Separation of Lignin, Cellulose, and Hemicellulose. J. Nat. Fibers 2019, 16, 662–676. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent Advances on Cellulose Nanocrystals for Pickering Emulsions: Development and Challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Tang, L.; Liao, J.; Dai, H.; Liu, Y.; Huang, H. Comparison of Cellulose Nanocrystals from Pineapple Residues and Its Preliminary Application for Pickering Emulsions. Nanotechnology 2021, 32, 495708. [Google Scholar] [CrossRef]
- Foo, M.L.; Ooi, C.W.; Tan, K.W.; Chew, I.M.L. A Step Closer to Sustainable Industrial Production: Tailor the Properties of Nanocrystalline Cellulose from Oil Palm Empty Fruit Bunch. J. Environ. Chem. Eng. 2020, 8, 104058. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Rodrigues, M.I.; Menegalli, F.C. Cellulose Nanofibers Produced from Banana Peel by Enzymatic Treatment: Study of Process Conditions. Ind. Crops Prod. 2017, 95, 664–674. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Tibolla, H.; Menegalli, F.C.; Cunha, R.L. Cellulose Nanofibers from Banana Peels as a Pickering Emulsifier: High-Energy Emulsification Processes. Carbohydr. Polym. 2018, 194, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhang, W.; Xu, Y.; Yu, Y.; Lin, X.; Fu, M.; Liu, H.; Peng, J.; Zhao, Z. Cellulose Nanofiber from Pomelo Spongy Tissue as a Novel Particle Stabilizer for Pickering Emulsion. Int. J. Biol. Macromol. 2022, 224, 1439–1449. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M. Recycling Coffee Grounds and Tea Leaf Wastes to Improve the Yield and Mineral Content of Grains of Paddy Rice. J. Sci. Food Agric. 2011, 91, 2108–2111. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, Z.; Zhang, Y.; Zhao, T.; Ye, X.; Gao, X.; Lin, X.; Li, B. Functional Properties and Structural Profiles of Water-Insoluble Proteins from Three Types of Tea Residues. LWT-Food Sci. Technol. 2019, 110, 324–331. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Li, B. Novel Food-Grade Pickering Emulsions Stabilized by Tea Water-Insoluble Protein Nanoparticles from Tea Residues. Food Hydrocoll. 2019, 96, 322–330. [Google Scholar] [CrossRef]
- He, K.; Li, Q.; Li, Y.; Li, B.; Liu, S. Water-Insoluble Dietary Fibers from Bamboo Shoot Used as Plant Food Particles for the Stabilization of O/W Pickering Emulsion. Food Chem. 2020, 310, 125925. [Google Scholar] [CrossRef] [PubMed]
- Maravić, N.; Šereš, Z.; Nikolić, I.; Dokić, P.; Kertész, S.; Dokić, L. Emulsion Stabilizing Capacity of Sugar Beet Fibers Compared to Sugar Beet Pectin and Octenyl Succinate Modified Maltodextrin in the Production of O/W Emulsions: Individual and Combined Impact. LWT-Food Sci. Technol. 2019, 108, 392–399. [Google Scholar] [CrossRef]
- Ralla, T.; Salminen, H.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Oat Bran Extract (Avena Sativa L.) from Food by-Product Streams as New Natural Emulsifier. Food Hydrocoll. 2018, 81, 253–262. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The Chemical Composition of Exhausted Coffee Waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Gould, J.; Garcia-Garcia, G.; Wolf, B. Pickering Particles Prepared from Food Waste. Materials 2016, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Foo, M.L.; Ooi, C.W.; Tan, K.W.; Chew, I.M.L. Preparation of Black Cumin Seed Oil Pickering Nanoemulsion with Enhanced Stability and Antioxidant Potential Using Nanocrystalline Cellulose from Oil Palm Empty Fruit Bunch. Chemosphere 2022, 287, 132108. [Google Scholar] [CrossRef]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical Characteristics, Applications and Research Trends of Edible Pickering Emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Edible Pickering Emulsions Stabilized by Ovotransferrin–Gum Arabic Particles. Food Hydrocoll. 2019, 89, 590–601. [Google Scholar] [CrossRef]
- Kargar, M.; Fayazmanesh, K.; Alavi, M.; Spyropoulos, F.; Norton, I.T. Investigation into the Potential Ability of Pickering Emulsions (Food-Grade Particles) to Enhance the Oxidative Stability of Oil-in-Water Emulsions. J. Colloid Interface Sci. 2012, 366, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-Carotene in Wheat Gluten Nanoparticle-Xanthan Gum-Stabilized Pickering Emulsions: Enhancement of Carotenoid Stability and Bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, J.; Huang, Q. Food-Grade Pickering Emulsions Stabilized by Ovotransferrin Fibrils. Food Hydrocoll. 2019, 94, 592–602. [Google Scholar] [CrossRef]
- Liu, B.; Li, T.; Wang, W.; Sagis, L.M.C.; Yuan, Q.; Lei, X.; Cohen Stuart, M.A.; Li, D.; Bao, C.; Bai, J.; et al. Corncob Cellulose Nanosphere as an Eco-Friendly Detergent. Nat. Sustain. 2020, 3, 448–458. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, C.; Zou, S.; Liu, H.; Tong, Z. Chitosan Nanoparticles as Particular Emulsifier for Preparation of Novel PH-Responsive Pickering Emulsions and PLGA Microcapsules. Polymer 2012, 53, 1229–1235. [Google Scholar] [CrossRef]
- Guo, Q. Progress in the Preparation, Stability and Functional Applications of Pickering Emulsion. IOP Conf. Ser. Earth Environ. Sci. 2021, 639, 012028. [Google Scholar] [CrossRef]
- Feng, X.; Sun, Y.; Yang, Y.; Zhou, X.; Cen, K.; Yu, C.; Xu, T.; Tang, X. Zein Nanoparticle Stabilized Pickering Emulsion Enriched with Cinnamon Oil and Its Effects on Pound Cakes. LWT-Food Sci. Technol. 2020, 122, 109025. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.; Yuan, J.; Wu, Y.; Li, F.; Li, D.; Huang, Q. Effects of Pectin Polydispersity on Zein/Pectin Composite Nanoparticles (ZAPs)as High Internal-Phase Pickering Emulsion Stabilizers. Carbohydr. Polym. 2019, 219, 77–86. [Google Scholar] [CrossRef]
- Guo, C.; Yin, J.; Chen, D. Co-Encapsulation of Curcumin and Resveratrol into Novel Nutraceutical Hyalurosomes Nano-Food Delivery System Based on Oligo-Hyaluronic Acid-Curcumin Polymer. Carbohydr. Polym. 2018, 181, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Gao, Y. Physicochemical Properties of β-Carotene Bilayer Emulsions Coated by Milk Proteins and Chitosan-EGCG Conjugates. Food Hydrocoll. 2016, 52, 590–599. [Google Scholar] [CrossRef]
- Zhou, F.Z.; Yu, X.H.; Zeng, T.; Yin, S.W.; Tang, C.H.; Yang, X.Q. Fabrication and Characterization of Novel Water-Insoluble Protein Porous Materials Derived from Pickering High Internal-Phase Emulsions Stabilized by Gliadin-Chitosan-Complex Particles. J. Agric. Food Chem. 2019, 67, 3423–3431. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Guo, J.; Wan, Z.; Ren, J.; Yang, X. PH Switchable Pickering Emulsion Based on Soy Peptides Functionalized Calcium Phosphate Particles. Food Hydrocoll. 2017, 70, 219–228. [Google Scholar] [CrossRef]
- Zhu, Y.; McClements, D.J.; Zhou, W.; Peng, S.; Zhou, L.; Zou, L.; Liu, W. Influence of Ionic Strength and Thermal Pretreatment on the Freeze-Thaw Stability of Pickering Emulsion Gels. Food Chem. 2020, 303, 125401. [Google Scholar] [CrossRef]
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel Carboxymethyl Cellulose-Polyvinyl Alcohol Blend Films Stabilized by Pickering Emulsion Incorporation Method. Carbohydr. Polym. 2017, 167, 79–89. [Google Scholar] [CrossRef]
- Fasihi, H.; Noshirvani, N.; Hashemi, M.; Fazilati, M.; Salavati, H.; Coma, V. Antioxidant and Antimicrobial Properties of Carbohydrate-Based Films Enriched with Cinnamon Essential Oil by Pickering Emulsion Method. Food Packag. Shelf Life 2019, 19, 147–154. [Google Scholar] [CrossRef]
- Lim, H.P.; Ho, K.W.; Surjit Singh, C.K.; Ooi, C.W.; Tey, B.T.; Chan, E.S. Pickering Emulsion Hydrogel as a Promising Food Delivery System: Synergistic Effects of Chitosan Pickering Emulsifier and Alginate Matrix on Hydrogel Stability and Emulsion Delivery. Food Hydrocoll. 2020, 103, 105659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).