Impact of Hydrophobic and Electrostatic Forces on the Adsorption of Acacia Gum on Oxide Surfaces Revealed by QCM-D
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
2.3. Surface Properties
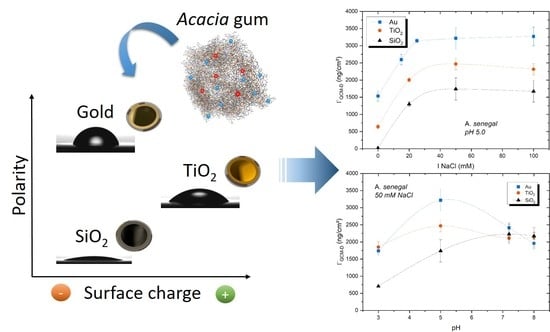
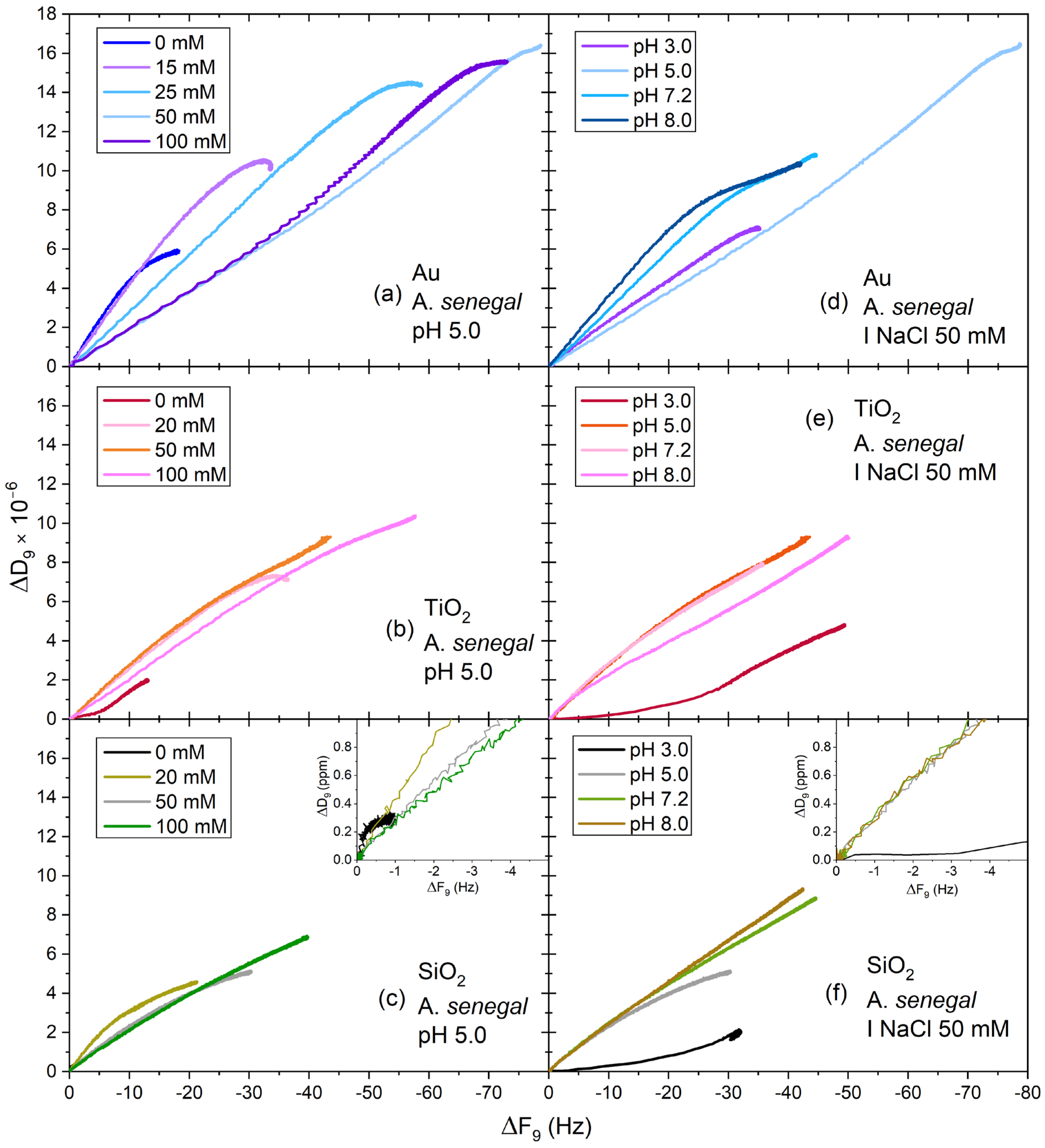
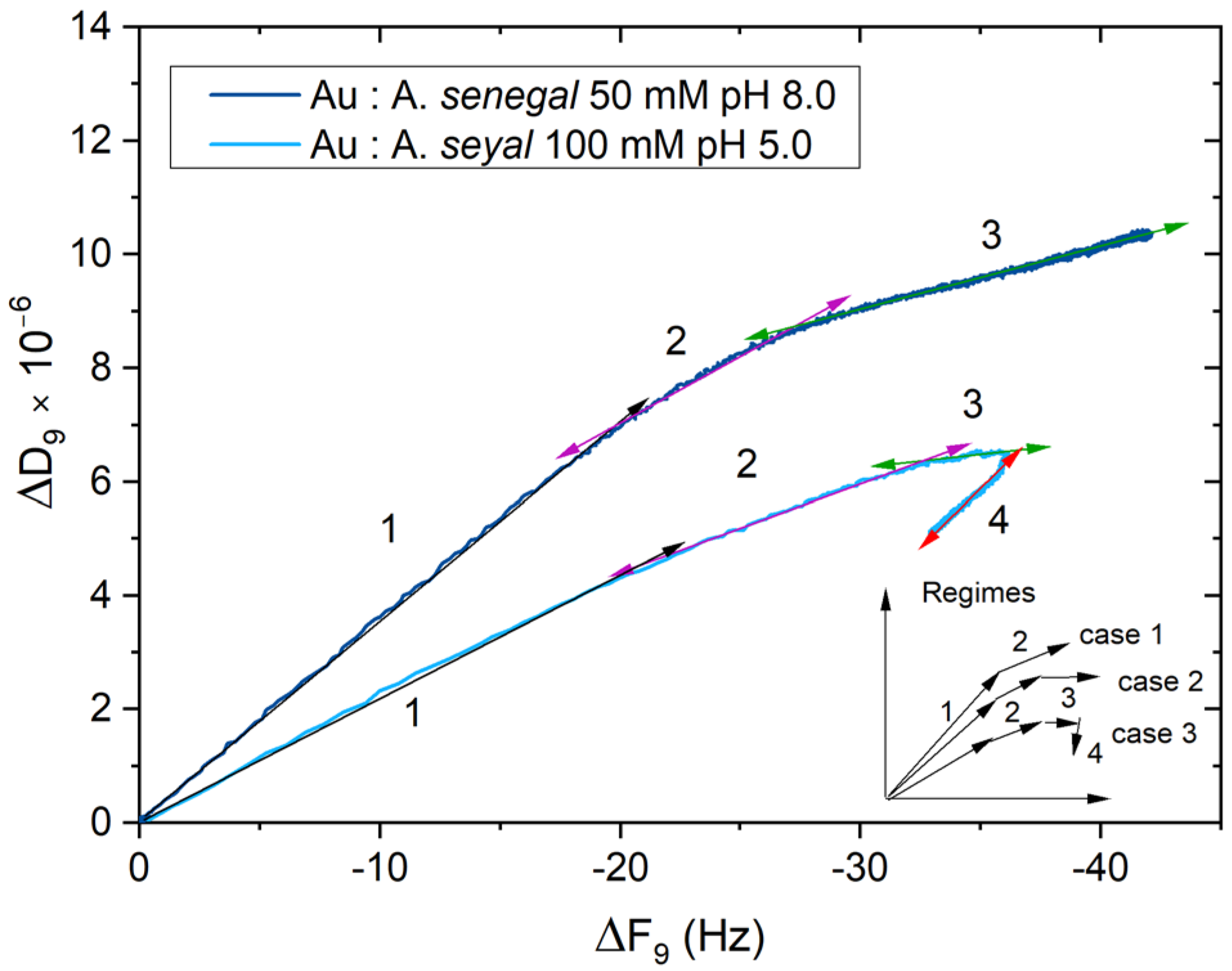
3. Results and Discussion
3.1. Characterization of Quartz Sensors
3.2. Acacia Gum Adsorption
3.3. Viscoelastic Properties and Conformational Change of Acacia Gum Layers—D-f Plots
3.4. Interfacial Rheological Behavior of Acacia Gum Layers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nussinovitch, A. Plant Gum Exudates of the World: Sources, Distribution, Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-15046-3. [Google Scholar]
- Sanchez, C.; Nigen, M.; Mejia Tamayo, V.; Doco, T.; Williams, P.; Amine, C.; Renard, D. Acacia Gum: History of the Future. Food Hydrocoll. 2018, 78, 140–160. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of Natural Polymer Gum Arabic: A Review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Sivan, S.K.; Shankar, S.S.; Kandambath Padinjareveetil, A.; Pilankatta, R.; Kumar, V.B.S.; Mathew, B.; George, B.; Makvandi, P.; Černík, M.; Padil, V.V.; et al. Fabrication of a Greener TiO2@Gum Arabic-Carbon Paste Electrode for the Electrochemical Detection of Pb2+ Ions in Plastic Toys. ACS Omega 2020, 5, 25390–25399. [Google Scholar] [CrossRef] [PubMed]
- Huseen, R.H.; Taha, A.A.; Ali, I.Q.; Abdulhusein, O.M.; Al-Jawad, S.M.H. Biological Activity of Gum Arabic-Coated Ferrous Oxide Nanoparticles. Mod. Phys. Lett. B 2021, 35, 2150411. [Google Scholar] [CrossRef]
- Williams, D.N.; Gold, K.A.; Holoman, T.R.P.; Ehrman, S.H.; Wilson, O.C. Surface Modification of Magnetic Nanoparticles Using Gum Arabic. J. Nanopart. Res. 2006, 8, 749–753. [Google Scholar] [CrossRef]
- Leong, Y.K.; Seah, U.; Chu, S.Y.; Ong, B.C. Effects of Gum Arabic Macromolecules on Surface Forces in Oxide Dispersions. Colloids Surf. A 2001, 182, 263–268. [Google Scholar] [CrossRef]
- Gamal-Eldeen, A.M.; Moustafa, D.; El-Daly, S.M.; Abo-Zeid, M.A.M.; Saleh, S.; Khoobchandani, M.; Katti, K.; Shukla, R.; Katti, K.V. Gum Arabic-Encapsulated Gold Nanoparticles for a Non-Invasive Photothermal Ablation of Lung Tumor in Mice. Biomed. Pharmacother. 2017, 89, 1045–1054. [Google Scholar] [CrossRef]
- Thipe, V.C.; Amiri, K.P.; Bloebaum, P.; Karikachery, A.R.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of Resveratrol-Conjugated Gold Nanoparticles: Interrelationship of Increased Resveratrol Corona on Anti-Tumor Efficacy against Breast, Pancreatic and Prostate Cancers. Int. J. Nanomed. 2019, 14, 4413–4428. [Google Scholar] [CrossRef]
- Gonçalves, J.P.; da Cruz, A.F.; Nunes, Á.M.; Meneghetti, M.R.; de Barros, H.R.; Borges, B.S.; de Medeiros, L.C.A.S.; Soares, M.J.; dos Santos, M.P.; Grassi, M.T.; et al. Biocompatible Gum Arabic-Gold Nanorod Composite as an Effective Therapy for Mistreated Melanomas. Int. J. Biol. Macromol. 2021, 185, 551–561. [Google Scholar] [CrossRef]
- de Barros, H.R.; Cardoso, M.B.; de Oliveira, C.C.; Franco, C.R.C.; de Lima Belan, D.; Vidotti, M.; Riegel-Vidotti, I.C. Stability of Gum Arabic-Gold Nanoparticles in Physiological Simulated PHs and Their Selective Effect on Cell Lines. RSC Adv. 2016, 6, 9411–9420. [Google Scholar] [CrossRef]
- Şişmanoğlu, T.; Karakuş, S.; Birer, Ö.; Soylu, G.S.P.; Kolan, A.; Tan, E.; Ürk, Ö.; Akdut, G.; Kilislioglu, A. Preparation and Characterization of Antibacterial Senegalia (Acacia) Senegal/Iron–Silica Bio-Nanocomposites. Appl. Surf. Sci. 2015, 354, 250–255. [Google Scholar] [CrossRef]
- Renard, D.; Lavenant-Gourgeon, L.; Ralet, M.-C.; Sanchez, C. Acacia Senegal Gum: Continuum of Molecular Species Differing by Their Protein to Sugar Ratio, Molecular Weight, and Charges. Biomacromolecules 2006, 7, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Apolinar-Valiente, R.; Williams, P.; Nigen, M.; Tamayo, V.M.; Doco, T.; Sanchez, C. Recovery, Structure and Physicochemical Properties of an Aggregate-Rich Fraction from Acacia Senegal Gum. Food Hydrocoll. 2019, 89, 864–873. [Google Scholar] [CrossRef]
- Lopez-Torrez, L.; Nigen, M.; Williams, P.; Doco, T.; Sanchez, C. Acacia Senegal vs. Acacia Seyal Gums—Part 1: Composition and Structure of Hyperbranched Plant Exudates. Food Hydrocoll. 2015, 51, 41–53. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on Acacia Exudate Gums. Part I: The Molecular Weight of Acacia Senegal Gum Exudate. Food Hydrocoll. 2005, 19, 647–660. [Google Scholar] [CrossRef]
- Davantès, A.; Nigen, M.; Sanchez, C.; d’Orlando, A.; Renard, D. Adsorption of Hyperbranched Arabinogalactan-Proteins from Plant Exudate at the Solid–Liquid Interface. Colloids Interfaces 2019, 3, 49. [Google Scholar] [CrossRef]
- Davantès, A.; Nigen, M.; Sanchez, C.; Renard, D. Adsorption Behavior of Arabinogalactan-Proteins (AGPs) from Acacia Senegal Gum at a Solid–Liquid Interface. Langmuir 2021, 37, 10547–10559. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011; ISBN 978-0-12-391933-5. [Google Scholar]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent Progress in Understanding Hydrophobic Interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef]
- Tabor, R.F.; Grieser, F.; Dagastine, R.R.; Chan, D.Y.C. The Hydrophobic Force: Measurements and Methods. Phys. Chem. Chem. Phys. 2014, 16, 18065–18075. [Google Scholar] [CrossRef]
- Zeng, H.; Shi, C.; Huang, J.; Li, L.; Liu, G.; Zhong, H. Recent Experimental Advances on Hydrophobic Interactions at Solid/Water and Fluid/Water Interfaces. Biointerphases 2015, 11, 018903. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the Driving Force of Hydrophobic Assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Christenson, H.K.; Claesson, P.M. Direct Measurements of the Force between Hydrophobic Surfaces in Water. Adv. Colloid Interface Sci. 2001, 91, 391–436. [Google Scholar] [CrossRef]
- Ishida, N.; Kusaka, Y.; Ushijima, H. Hydrophobic Attraction between Silanated Silica Surfaces in the Absence of Bridging Bubbles. Langmuir 2012, 28, 13952–13959. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Cui, X.; Gong, L.; Chen, J.; Zeng, H. Recent Advances in the Quantification and Modulation of Hydrophobic Interactions for Interfacial Applications. Langmuir 2020, 36, 2985–3003. [Google Scholar] [CrossRef]
- Höök, F.; Rodahl, M.; Brzezinski, P.; Kasemo, B. Energy Dissipation Kinetics for Protein and Antibody–Antigen Adsorption under Shear Oscillation on a Quartz Crystal Microbalance. Langmuir 1998, 14, 729–734. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Krozer, A.; Brzezinski, P.; Kasemo, B. Quartz Crystal Microbalance Setup for Frequency and Q-factor Measurements in Gaseous and Liquid Environments. Rev. Sci. Instrum. 1995, 66, 3924–3930. [Google Scholar] [CrossRef]
- Voinova, M.V.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic Acoustic Response of Layered Polymer Films at Fluid-Solid Interfaces: Continuum Mechanics Approach. Phys. Scr. 1999, 59, 391. [Google Scholar] [CrossRef]
- Reviakine, I.; Johannsmann, D.; Richter, R.P. Hearing What You Cannot See and Visualizing What You Hear: Interpreting Quartz Crystal Microbalance Data from Solvated Interfaces. Anal. Chem. 2011, 83, 8838–8848. [Google Scholar] [CrossRef]
- Johannsmann, D. Viscoelastic, Mechanical, and Dielectric Measurements on Complex Samples with the Quartz Crystal Microbalance. Phys. Chem. Chem. Phys. 2008, 10, 4516–4534. [Google Scholar] [CrossRef]
- Johannsmann, D. The Quartz Crystal Microbalance in Soft Matter Research; Soft and Biological Matter; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-07835-9. [Google Scholar]
- Sadman, K.; Wiener, C.G.; Weiss, R.A.; White, C.C.; Shull, K.R.; Vogt, B.D. Quantitative Rheometry of Thin Soft Materials Using the Quartz Crystal Microbalance with Dissipation. Anal. Chem. 2018, 90, 4079–4088. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B.; Nylander, T.; Fant, C.; Sott, K.; Elwing, H. Variations in Coupled Water, Viscoelastic Properties, and Film Thickness of a Mefp-1 Protein Film during Adsorption and Cross-Linking: A Quartz Crystal Microbalance with Dissipation Monitoring, Ellipsometry, and Surface Plasmon Resonance Study. Anal. Chem. 2001, 73, 5796–5804. [Google Scholar] [CrossRef] [PubMed]
- Mejia Tamayo, V.; Nigen, M.; Apolinar-Valiente, R.; Doco, T.; Williams, P.; Renard, D.; Sanchez, C. Flexibility and Hydration of Amphiphilic Hyperbranched Arabinogalactan-Protein from Plant Exudate: A Volumetric Perspective. Colloids Interfaces 2018, 2, 11. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Williams, P.; Nigen, M.; Tamayo, V.M.; Doco, T.; Sanchez, C. Fractionation of Acacia Seyal Gum by Ion Exchange Chromatography. Food Hydrocoll. 2020, 98, 105283. [Google Scholar] [CrossRef]
- Ramón-Torregrosa, P.J.; Rodríguez-Valverde, M.A.; Amirfazli, A.; Cabrerizo-Vílchez, M.A. Factors Affecting the Measurement of Roughness Factor of Surfaces and Its Implications for Wetting Studies. Colloids Surf. A Physicochem. Eng. Asp. 2008, 323, 83–93. [Google Scholar] [CrossRef]
- Kim, I.; Yang, Y.; Park, G. Effect of Titanium Surface Roughness on Oxygen Catalytic Recombination in a Shock Tube. Acta Astronaut. 2020, 166, 260–269. [Google Scholar] [CrossRef]
- Tsapikouni, T.S.; Missirlis, Y.F. Protein–Material Interactions: From Micro-to-Nano Scale. Mater. Sci. Eng. B 2008, 152, 2–7. [Google Scholar] [CrossRef]
- Norde, W. Driving Forces for Protein Adsorption at Solid Surfaces. Macromol. Symp. 1996, 103, 5–18. [Google Scholar] [CrossRef]
- Hlady, V.; Buijs, J. Protein Adsorption on Solid Surfaces. Curr. Opin. Biotechnol. 1996, 7, 72–77. [Google Scholar] [CrossRef]
- Haynes, C.A.; Norde, W. Globular Proteins at Solid/Liquid Interfaces. Colloids Surf. B Biointerfaces 1994, 2, 517–566. [Google Scholar] [CrossRef]
- Kubiak, K.; Adamczyk, Z.; Cieśla, M. Fibrinogen Adsorption Mechanisms at the Gold Substrate Revealed by QCM-D Measurements and RSA Modeling. Colloids Surf. B Biointerfaces 2016, 139, 123–131. [Google Scholar] [CrossRef]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Lee, M.-K.; Park, Y.-C. Contact Angle Relaxation on Amorphous, Mixed-Phase (Anatase + Rutile), and Anatase TiO2 Films and Its Mechanism. Langmuir 2021, 37, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Vrakatseli, V.; Farsari, E.; Mataras, D. Wetting Properties of Transparent Anatase/Rutile Mixed Phase Glancing Angle Magnetron Sputtered Nano-TiO2 Films. Micromachines 2020, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Park, Y.-C. Contact Angle Relaxation and Long-Lasting Hydrophilicity of Sputtered Anatase TiO2 Thin Films by Novel Quantitative XPS Analysis. Langmuir 2019, 35, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Jachimska, B.; Świątek, S.; Loch, J.I.; Lewiński, K.; Luxbacher, T. Adsorption Effectiveness of β-Lactoglobulin onto Gold Surface Determined by Quartz Crystal Microbalance. Bioelectrochemistry 2018, 121, 95–104. [Google Scholar] [CrossRef]
- Schrems, A.; Kibrom, A.; Küpcü, S.; Kiene, E.; Sleytr, U.B.; Schuster, B. Bilayer Lipid Membrane Formation on a Chemically Modified S-Layer Lattice. Langmuir 2011, 27, 3731–3738. [Google Scholar] [CrossRef]
- Kosmulski, M. Compilation of PZC and IEP of Sparingly Soluble Metal Oxides and Hydroxides from Literature. Adv. Colloid Interface Sci. 2009, 152, 14–25. [Google Scholar] [CrossRef]
- Bousse, L.; Mostarshed, S.; Van Der Shoot, B.; de Rooij, N.F.; Gimmel, P.; Göpel, W. Zeta Potential Measurements of Ta2O5 and SiO2 Thin Films. J. Colloid Interface Sci. 1991, 147, 22–32. [Google Scholar] [CrossRef]
- Nezu, T.; Masuyama, T.; Sasaki, K.; Saitoh, S.; Taira, M.; Araki, Y. Effect of PH and Addition of Salt on the Adsorption Behavior of Lysozyme on Gold, Silica, and Titania Surfaces Observed by Quartz Crystal Microbalance with Dissipation Monitoring. Dent. Mater. J. 2008, 27, 573–580. [Google Scholar] [CrossRef]
- Kanno, K.; Sase, G.; McNamee, C.E. Use of Mixed Langmuir Films of Nanoparticles to Form Metal Oxide Materials with the Optimal Surface Charge. Langmuir 2021, 37, 7643–7654. [Google Scholar] [CrossRef]
- McNamee, C.E.; Tsujii, Y.; Matsumoto, M. Physicochemical Characterization of an Anatase TiO2 Surface and the Adsorption of a Nonionic Surfactant: An Atomic Force Microscopy Study. Langmuir 2005, 21, 11283–11288. [Google Scholar] [CrossRef] [PubMed]
- Larson, I.; Drummond, C.J.; Chan, D.Y.C.; Grieser, F. Direct Force Measurements between Titanium Dioxide Surfaces. J. Am. Chem. Soc. 1993, 115, 11885–11890. [Google Scholar] [CrossRef]
- Yates, D.E.; Healy, T.W. Titanium Dioxide–Electrolyte Interface. Part 2.—Surface Charge (Titration) Studies. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1980, 76, 9–18. [Google Scholar] [CrossRef]
- Kosmulski, M. The Significance of the Difference in the Point of Zero Charge between Rutile and Anatase. Adv. Colloid Interface Sci. 2002, 99, 255–264. [Google Scholar] [CrossRef]
- Bullard, J.W.; Cima, M.J. Orientation Dependence of the Isoelectric Point of TiO2 (Rutile) Surfaces. Langmuir 2006, 22, 10264–10271. [Google Scholar] [CrossRef]
- Chau, L.-K.; Porter, M.D. Surface Isoelectric Point of Evaporated Silver Films: Determination by Contact Angle Titration. J. Colloid Interface Sci. 1991, 145, 283–286. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the Surface Isoelectric Point of Oxide Films on Metals by Contact Angle Titration. J. Colloid Interface Sci. 1997, 194, 344–355. [Google Scholar] [CrossRef]
- Trevino, K.J.; Shearer, J.C.; McCurdy, P.R.; Pease-Dodson, S.E.; Okegbe, C.C.; Fisher, E.R. Isoelectric Points of Plasma-Modified and Aged Silicon Oxynitride Surfaces Measured Using Contact Angle Titrations. Surf. Interface Anal. 2011, 43, 1257–1270. [Google Scholar] [CrossRef]
- Trevino, K.J.; Shearer, J.C.; Tompkins, B.D.; Fisher, E.R. Comparing Isoelectric Point and Surface Composition of Plasma Modified Native and Deposited SiO2 Films Using Contact Angle Titrations and X-Ray Photoelectron Spectroscopy. Plasma Process. Polym. 2011, 8, 951–964. [Google Scholar] [CrossRef]
- Hanly, G.; Fornasiero, D.; Ralston, J.; Sedev, R. Electrostatics and Metal Oxide Wettability. J. Phys. Chem. C 2011, 115, 14914–14921. [Google Scholar] [CrossRef]
- van Oss, C.J. Chapter Twelve—Influence of the PH and the Ionic Strength of Water on Contact Angles Measured with Drops of Aqueous Solutions on Electrically Charged, Amphoteric and Uncharged Surfaces. In Interface Science and Technology; van Oss, C.J., Ed.; The Properties of Water and their Role in Colloidal and Biological Systems; Elsevier: Amsterdam, The Netherlands, 2008; Volume 16, pp. 161–166. [Google Scholar]
- Virga, E.; Spruijt, E.; de Vos, W.M.; Biesheuvel, P.M. Wettability of Amphoteric Surfaces: The Effect of PH and Ionic Strength on Surface Ionization and Wetting. Langmuir 2018, 34, 15174–15180. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Pomorska, A.; Nattich-Rak, M.; Wytrwal-Sarna, M.; Bernasik, A. Protein Adsorption Mechanisms at Rough Surfaces: Serum Albumin at a Gold Substrate. J. Colloid Interface Sci. 2018, 530, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Molino, P.J.; Higgins, M.J.; Innis, P.C.; Kapsa, R.M.I.; Wallace, G.G. Fibronectin and Bovine Serum Albumin Adsorption and Conformational Dynamics on Inherently Conducting Polymers: A QCM-D Study. Langmuir 2012, 28, 8433–8445. [Google Scholar] [CrossRef]
- Attwood, S.J.; Kershaw, R.; Uddin, S.; Bishop, S.M.; Welland, M.E. Understanding How Charge and Hydrophobicity Influence Globular Protein Adsorption to Alkanethiol and Material Surfaces. J. Mater. Chem. B 2019, 7, 2349–2361. [Google Scholar] [CrossRef]
- Grein-Iankovski, A.; Ferreira, J.G.L.; Orth, E.S.; Sierakowski, M.-R.; Cardoso, M.B.; Simas, F.F.; Riegel-Vidotti, I.C. A Comprehensive Study of the Relation between Structural and Physical Chemical Properties of Acacia Gums. Food Hydrocoll. 2018, 85, 167–175. [Google Scholar] [CrossRef]
- Renard, D.; Davantès, A.; D’orlando, A.; Cahier, K.; Molinari, M.; Nigen, M.; Chalier, P.; Sanchez, C. Adsorption of Arabinogalactan-Proteins from Acacia Gums (Senegal and Seyal) and Its Molecular Fractions onto Latex Particles. Food Hydrocoll. 2022, 125, 107360. [Google Scholar] [CrossRef]
- Ma, F.; Bell, A.E.; Davis, F.J. Effects of High-Hydrostatic Pressure and PH Treatments on the Emulsification Properties of Gum Arabic. Food Chem. 2015, 184, 114–121. [Google Scholar] [CrossRef]
- Höök, F.; Rodahl, M.; Kasemo, B.; Brzezinski, P. Structural Changes in Hemoglobin during Adsorption to Solid Surfaces: Effects of PH, Ionic Strength, and Ligand Binding. Proc. Natl. Acad. Sci. USA 1998, 95, 12271–12276. [Google Scholar] [CrossRef]
- Guo, S.; Pranantyo, D.; Kang, E.-T.; Loh, X.J.; Zhu, X.; Jańczewski, D.; Neoh, K.G. Dominant Albumin–Surface Interactions under Independent Control of Surface Charge and Wettability. Langmuir 2018, 34, 1953–1966. [Google Scholar] [CrossRef]
- Anand, G.; Sharma, S.; Dutta, A.K.; Kumar, S.K.; Belfort, G. Conformational Transitions of Adsorbed Proteins on Surfaces of Varying Polarity. Langmuir 2010, 26, 10803–10811. [Google Scholar] [CrossRef]
- Jia, P.; He, M.; Gong, Y.; Chu, X.; Yang, J.; Zhao, J. Probing the Adjustments of Macromolecules during Their Surface Adsorption. ACS Appl. Mater. Interfaces 2015, 7, 6422–6429. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of Protein Adsorption: Surface-Induced Conformational Changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Martin, E.J.; Mathew, M.T.; Shull, K.R. Viscoelastic Properties of Electrochemically Deposited Protein/Metal Complexes. Langmuir 2015, 31, 4008–4017. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, D. Integrated Study of Water Sorption/Desorption Behavior of Weak Polyelectrolyte Layer-by-Layer Films. Macromolecules 2013, 46, 2793–2799. [Google Scholar] [CrossRef]
- Saarinen, T.; Österberg, M.; Laine, J. Properties of Cationic Polyelectrolyte Layers Adsorbed on Silica and Cellulose Surfaces Studied by QCM-D—Effect of Polyelectrolyte Charge Density and Molecular Weight. J. Dispers. Sci. Technol. 2009, 30, 969–979. [Google Scholar] [CrossRef]
- de Kerchove, A.J.; Elimelech, M. Formation of Polysaccharide Gel Layers in the Presence of Ca2+ and K+ Ions: Measurements and Mechanisms. Biomacromolecules 2007, 8, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Llamas, S.; Guzmán, E.; Ortega, F.; Baghdadli, N.; Cazeneuve, C.; Rubio, R.G.; Luengo, G.S. Adsorption of Polyelectrolytes and Polyelectrolytes-Surfactant Mixtures at Surfaces: A Physico-Chemical Approach to a Cosmetic Challenge. Adv. Colloid Interface Sci. 2015, 222, 461–487. [Google Scholar] [CrossRef]
- Hodges, C.S.; Harbottle, D.; Biggs, S. Investigating Adsorbing Viscoelastic Fluids Using the Quartz Crystal Microbalance. ACS Omega 2020, 5, 22081–22090. [Google Scholar] [CrossRef]
- Murata, H. Rheology—Theory and Application to Biomaterials. In Polymerization; De Souza Gomes, A., Ed.; InTech: Vienna, Austria, 2012; ISBN 978-953-51-0745-3. [Google Scholar]
- Jin, Q.; Li, X.; Cai, Z.; Zhang, F.; Yadav, M.P.; Zhang, H. A Comparison of Corn Fiber Gum, Hydrophobically Modified Starch, Gum Arabic and Soybean Soluble Polysaccharide: Interfacial Dynamics, Viscoelastic Response at Oil/Water Interfaces and Emulsion Stabilization Mechanisms. Food Hydrocoll. 2017, 70, 329–344. [Google Scholar] [CrossRef]
- Erni, P.; Windhab, E.J.; Gunde, R.; Graber, M.; Pfister, B.; Parker, A.; Fischer, P. Interfacial Rheology of Surface-Active Biopolymers: Acacia Senegal Gum versus Hydrophobically Modifed Starch. Biomacromolecules 2007, 8, 3458–3466. [Google Scholar] [CrossRef]
- Erni, P.; Parker, A. Nonlinear Viscoelasticity and Shear Localization at Complex Fluid Interfaces. Langmuir 2012, 28, 7757–7767. [Google Scholar] [CrossRef] [PubMed]
- Erni, P.; Jerri, H.A.; Wong, K.; Parker, A. Interfacial Viscoelasticity Controls Buckling, Wrinkling and Arrest in Emulsion Drops Undergoing Mass Transfer. Soft Matter 2012, 8, 6958–6967. [Google Scholar] [CrossRef]
- Sanchez, C.; Renard, D.; Robert, P.; Schmitt, C.; Lefebvre, J. Structure and Rheological Properties of Acacia Gum Dispersions. Food Hydrocoll. 2002, 16, 257–267. [Google Scholar] [CrossRef]
- Elmanan, M.; Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on Acacia Exudate Gums: Part VI. Interfacial Rheology of Acacia Senegal and Acacia Seyal. Food Hydrocoll. 2008, 22, 682–689. [Google Scholar] [CrossRef]




| Quartz | Au | SiO2 | TiO2 |
|---|---|---|---|
| Contact Angle θ ° | 77.0 ± 1.2 | 11.1 ± 3.1 | 20–60 |
| Isoelectric Point | 2.9–3.4 | 2 | 5.5–6 |
| RMS (nm) | 1.76 ± 0.08 | 1.26 ± 0.09 | 1.06 ± 0.04 |
| r | 1.006 ± 0.004 | 1.006 ± 0.005 | 1.005 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davantès, A.; Nigen, M.; Sanchez, C.; Renard, D. Impact of Hydrophobic and Electrostatic Forces on the Adsorption of Acacia Gum on Oxide Surfaces Revealed by QCM-D. Colloids Interfaces 2023, 7, 26. https://doi.org/10.3390/colloids7020026
Davantès A, Nigen M, Sanchez C, Renard D. Impact of Hydrophobic and Electrostatic Forces on the Adsorption of Acacia Gum on Oxide Surfaces Revealed by QCM-D. Colloids and Interfaces. 2023; 7(2):26. https://doi.org/10.3390/colloids7020026
Chicago/Turabian StyleDavantès, Athénaïs, Michaël Nigen, Christian Sanchez, and Denis Renard. 2023. "Impact of Hydrophobic and Electrostatic Forces on the Adsorption of Acacia Gum on Oxide Surfaces Revealed by QCM-D" Colloids and Interfaces 7, no. 2: 26. https://doi.org/10.3390/colloids7020026
APA StyleDavantès, A., Nigen, M., Sanchez, C., & Renard, D. (2023). Impact of Hydrophobic and Electrostatic Forces on the Adsorption of Acacia Gum on Oxide Surfaces Revealed by QCM-D. Colloids and Interfaces, 7(2), 26. https://doi.org/10.3390/colloids7020026