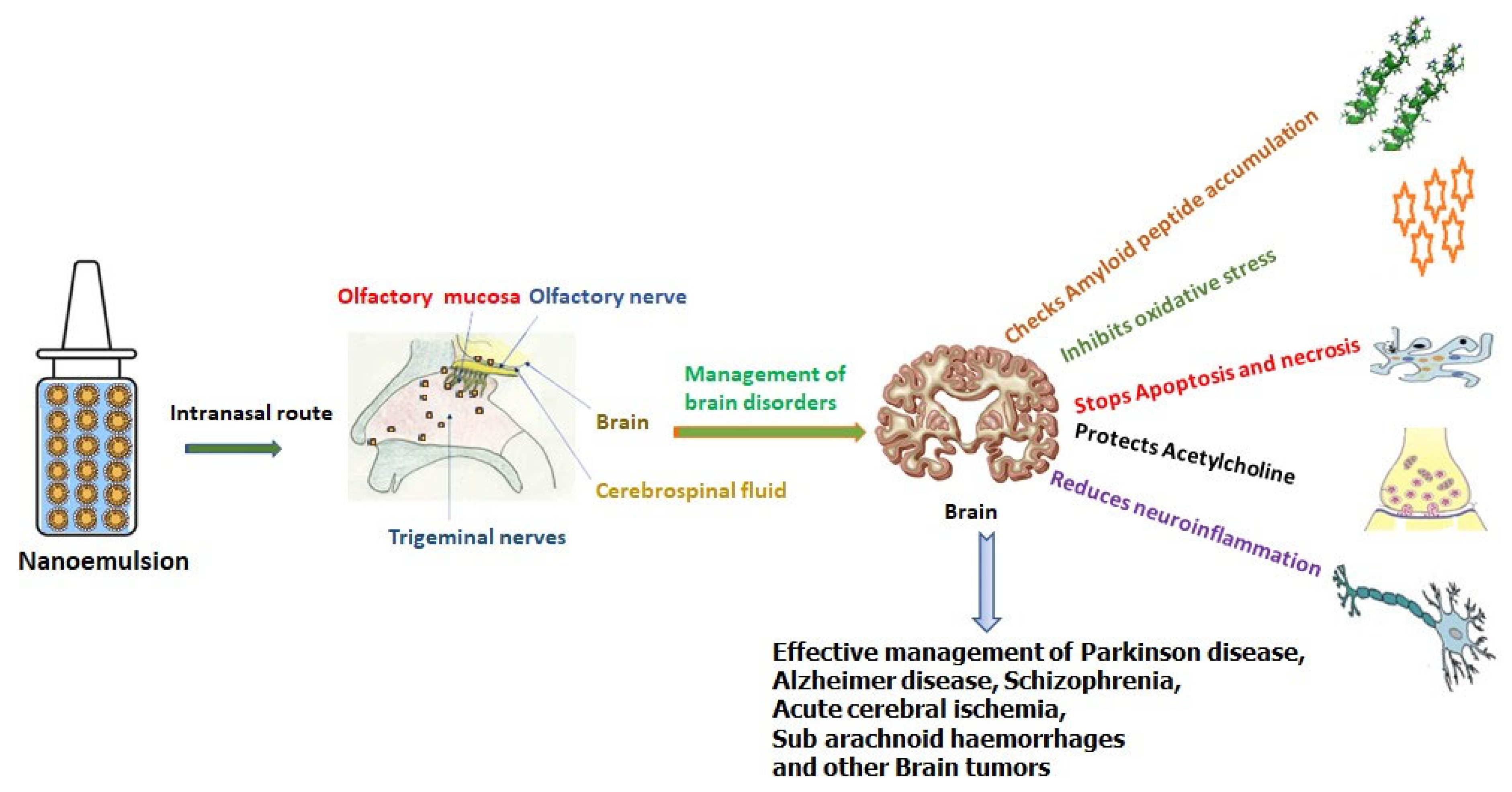
Nose-to-Brain Targeting via Nanoemulsion: Significance and Evidence
Abstract
1. Introduction
Complexity of Nose-to-Brain Drug Delivery Path
2. Nanoemulsions
2.1. Oil and Globule Size
2.2. Surfactant and Stability
3. Intranasal Nanoemulsions for the Management of Brain Diseases
3.1. Nanoemulsions for Neurological Disorders
3.2. Nanoemulsions for Brain Tumour
3.3. Nanoemulsions in Cerebral Ischemia
3.4. Nanoemulsions in Brain Infections
3.5. Nanoemulsions in Migraine and Cerebral Vasospasm
4. Future Prospects and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P.; Jiang, C. Brain-targeting drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1818. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug. Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Kaushik, A.; Jayant, R.D.; Bhardwaj, V.; Nair, M. Personalized nano medicine for CNS diseases. Drug. Discov. Today 2018, 23, 1007–1015. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.M.; Bleier, B.S. The blood-brain barrier and nasal drug delivery to the central nervous system. Am. J. Rhinol. Allergy 2015, 29, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Jones, N. The nose and paranasal sinuses physiology and anatomy. Adv. Drug. Deliv. Rev. 2001, 51, 5–19. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways by passing the blood-brainbarrier: An excellent platform for brain targeting. Expert. Opin. Drug. Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef]
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release 2015, 201, 68–77. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nose-to-brain delivery: Investigation of the transport of nanoparticles with different surface characteristics and sizes in excised porcine olfactory epithelium. Mol. Pharm. 2015, 12, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Guastella, A.J.; Westlye, L.T.; Andreassen, O.A. The promise and pitfalls of intranasally administering psychopharmacological agents for the treatment of psychiatric disorders. Mol. Psychiatry 2016, 21, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; McClements, D.J. Application of advanced emulsion technology in the food industry: A review and critical evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Siasios, I.; Kapsalaki, E.Z.; Fountas, K.N. Cerebral vasospasm pharmacological treatment: An update. Neurol. Res. Int. 2013, 2013, 571328. [Google Scholar] [CrossRef]
- Shen, R.; Yang, X.; Lin, D. pH sensitive double-layered emulsions stabilized by bacterial cellulose nanofibers/soy protein isolate/chitosan complex enhanced the bio accessibility of curcumin: In vitro study. Food Chem. 2023, 402, 134262. [Google Scholar] [CrossRef]
- Piacentini, E.; Figoli, A.; Giorno, L.; Drioli, E. Membrane Emulsification. In Comprehensive Membrane Science and Engineering; Drioli, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 47–78. [Google Scholar]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Pickering Emulsions: A Novel Tool for Cosmetic Formulators. Cosmetics 2022, 9, 68. [Google Scholar] [CrossRef]
- Gao, H.; Ma, L.; Cheng, C.; Liu, J.; Liang, R.; Zou, L.; Liu, W.; McClements, D.J. Review of recent advances in the preparation, properties, and applications of high internal phase emulsions. Trends Food Sci. Technol. 2021, 112, 36–49. [Google Scholar] [CrossRef]
- Costa, C.P.; Moreira, J.N.; Sousa, L.J.M.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Karmakar, S.; Biswas, E.; Dey, G.; Barik, R.; Mandal, M.; Pal, T.K. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int. J. Pharm. 2014, 460, 131–143. [Google Scholar] [CrossRef]
- Edmond, J. Essential polyunsaturated fatty acids and the barrier to the brain: The components of a model for transport. J. Mol. Neurosci. 2001, 16, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Virmani, T.; Pathak, K.; Kamaly, O.A.; Saleh, A. Central Composite Design Implemented Azilsartan Medoxomil Loaded Nanoemulsion to Improve Its Aqueous Solubility and Intestinal Permeability: In Vitro and Ex Vivo Evaluation. Pharmaceuticals 2022, 15, 1343. [Google Scholar] [CrossRef]
- Morrison, E.E.; Costanzo, R.M. Morphology of olfactory epithelium in humans and other vertebrates. Microsc. Res. Tech. 1992, 23, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Phukan, K.; Nandy, M.; Sharma, R.B.; Sharma, H.K. Nanosized Drug Delivery Systems for Direct Nose to Brain Targeting: A Review. Recent. Pat. Drug. Deliv. Formul. 2016, 10, 156–164. [Google Scholar] [CrossRef]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef]
- Hosny, K.M.; Banjar, Z.M. The formulation of a nasal nanoemulsion zaleplon in situ gel for the treatment of insomnia. Expert Opin. Drug. Deliv. 2013, 10, 1033–1041. [Google Scholar] [CrossRef]
- Chatterjee, B.; Gorain, B.; Mohananaidu, K.; Sengupta, P.; Mandal, U.K.; Choudhury, H. Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Int. J. Pharm. 2019, 565, 258–268. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for "Nose-to-Brain" Drug Delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, C.; Garcia, M.J.; Azemar, N.; Solans, C. Formation and stability of nanoemulsions prepared using the phase inversion temperature method. Langmuir 2002, 18, 26–30. [Google Scholar] [CrossRef]
- Sood, S.; Jain, K.; Gowthamarajan, K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surf. B Bio. Interfaces 2014, 113, 330–337. [Google Scholar] [CrossRef]
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal nanoemulsions for direct nose-to-brain delivery of actives for CNS disorders. Pharmaceutics 2020, 12, 1230. [Google Scholar] [CrossRef]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery—The potential of nano technology. Bioorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef] [PubMed]
- Dalpiaz, A.; Fogagnolo, M.; Ferraro, L.; Capuzzo, A.; Pavan, B.; Rassu, G.; Salis, A.; Giunchedi, P.; Gavini, E. Nasal chitosan microparticles target a zidovudine prodrug to brain HIV sanctuaries. Antiviral. Res. 2015, 123, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, C.; Camargo, Z.I.; Schineider, M.C.; Taise, F.M.; Maissar, K.N.; Badea, I.; Mara, M.R. Mucoadhesive nanoemulsion enhances brain bioavailability of luteolin after intranasal administration and induces apoptosis to SH-SY5Y neuroblastoma cells. Int. J. Pharm. 2022, 626, 122142. [Google Scholar] [CrossRef]
- Rassu, G.; Porcu, E.P.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal delivery of genistein-loaded nanoparticles as a potential preventive system against neurodegenerative disorders. Pharmaceutics 2018, 11, 8. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Prakash, R.T.; Thiagarajan, P. Nanoemulsions for drug delivery through different routes. Res. Biotechnol. 2011, 2, 1–13. [Google Scholar]
- Mehta, M.; Adem, A.; Sabbagh, M.N. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Y.; Ding, Z.; Yu, Q. Investigation of the "Nose-to-Brain" pathways in intranasal Hup Anan emulsions and evaluation of their in vivo pharmacokinetics and brain-targeting ability. Int. J. Nanomed. 2022, 17, 3443–3456. [Google Scholar] [CrossRef] [PubMed]
- Parikh, H.R.; Patel, J.R. Nanoemulsions for intranasal delivery of riluzole to improve brain bioavailability: Formulation development and pharmacokinetic studies. Curr. Drug. Deliv. 2016, 13, 1130–1143. [Google Scholar] [CrossRef]
- Usama, A.M.; Vyas, P.; Vohora, D.; Kumar, S.P.; Nigam, K.; Dang, S.; Ali, J.; Baboota, S. Amelioration of oxidative stress utilizing nanoemulsion loaded with bromocriptine and glutathione for the management of Parkinson’s disease. Int. J. Pharm. 2022, 618, 121683. [Google Scholar] [CrossRef]
- Patel, R.J.; Parikh, R.H. Intranasal delivery of topiramate nanoemulsion: Pharmacodynamic, pharmacokinetic and brain uptake studies. Int. J. Pharm. 2020, 585, 119486. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Nigam, K.; Srivastava, S.; Tyagi, A.; Dang, S. Memantine nanoemulsion: A new approach to treat alzheimer’s disease. J. Microencapsul. 2020, 37, 355–365. [Google Scholar] [CrossRef]
- Kaur, A.; Nigam, K.; Bhatnagar, I.; Sukhpal, H.; Awasthy, S.; Shanka r, S.; Tyagi, A.; Dang, S. Treatment of Alzheimer’s disease using donepezil nanoemulsion: An intranasal approach. Drug. Deliv. Transl. Res. 2020, 10, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, C.; Zhai, W.; Zhuang, N.; Han, T.; Ding, Z. The optimization design of lactoferrin loaded hup A nanoemulsion for targeted drug transport via intranasal route. Int. J. Nanomed. 2019, 14, 9217–9234. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Mubarak, A.H.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S. Coconut oil-based resveratrol nanoemulsion: Optimization using response surface methodology, stability assessment and pharmacokinetic evaluation. Food Chem. 2021, 357, 129721. [Google Scholar] [CrossRef]
- Das, S.S.; Sarkar, A.; Chabattula, S.C.; Verma, P.R.P.; Nazir, A.; Gupta, P.K.; Ruokolainen, J.; Kesari, K.K.; Singh, S.K. Food-grade quercetin-loaded nanoemulsion ameliorates effects associated with Parkinson’s disease and cancer: Studies employing atransgenic C. elegans model and human cancer cell lines. Antioxidants 2022, 11, 1378. [Google Scholar] [CrossRef]
- Gaba, B.; Khan, T.; Haider, M.F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin Eloaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-OHDA Parkinson’s disease model. Biomed Res. Int. 2019, 2019, 2382563. [Google Scholar] [CrossRef]
- Kumar, S.; Ali, J.; Baboota, S. Design Expert(®) Supported optimization and predictive analysis of selegiline nanoemulsion via the olfactory region with enhanced behavioural performance in Parkinson’s disease. Nanotechnology 2016, 27, 435101. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M. Development of An optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug. Deliv. 2016, 23, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alam, M.A.; Ahmad, F.J.; Amir, M. Impact of ultrasonication techniques on the preparation of novel amiloride-nanoemulsion used for intranasal delivery in the treatment of epilepsy. Artif. Cells Nanomed. Biotechnol. 2018, 46, S192–S207. [Google Scholar] [CrossRef]
- Iqbal, R.; Ahmed, S.; Jain, G.K.; Vohora, D. Design and development of letrozolen an emulsion: A comparative evaluation of brain targeted nanoemulsion with free letrozole against status epilepticus and neurodegeneration in mice. Int. J. Pharm. 2019, 565, 20–32. [Google Scholar] [CrossRef] [PubMed]
- El-Zaafarany, G.M.; Soliman, M.E.; Mansour, S.; Cespi, M.; Palmieri, G.F.; Illum, L.; Casettari, L.; Awad, G.A.S. A Tailored Thermosensitive PLGA-PEG-PLGA/emulsomes composite for enhanced oxcarbazepine brain delivery via the nasal route. Pharmaceutics 2018, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against β-amyloid toxicity through the regulation of amyloid genesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef]
- Ramires Júnior, O.V.; Alves, B.D.S.; Barros, P.A.B.; Rodrigues, J.L.; Ferreira, S.P.; Monteiro, L.K.S.; Araújo, G.M.S.; Fernandes, S.S.; Vaz, G.R.; Dora, C.L.; et al. Nanoemulsion improves the neuroprotective effects of curcumin in an experimental model of Parkinson’s disease. Neurotox. Res. 2021, 39, 787–799. [Google Scholar] [CrossRef]
- Geetha, K.M.; Shankar, J.; Wilson, B. Neuroprotective effect of chia seed oil nanoemulsion against rotenone induced motor impairment and oxidative stress in mice model of Parkinson’s disease. Adv. Tradit. Med. 2022. [Google Scholar] [CrossRef]
- Pandey, Y.R.; Kumar, S.; Gupta, B.K.; Ali, J.; Baboota, S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: Formulation, behavioural and biochemical estimation. Nanotechnology 2016, 27, 025102. [Google Scholar] [CrossRef]
- Samiun, W.S.; Ashari, S.E.; Salim, N.; Ahmad, S. Optimization of processing parameters of nanoemulsion containing aripiprazole using response surface methodology. Int. J. Nanomed. 2020, 15, 1585–1594. [Google Scholar] [CrossRef]
- Kumbhar, S.A.; Kokare, C.R.; Shrivastava, B.; Gorain, B.; Choudhury, H. Preparation, characterization, and optimization of asenapine maleate mucoadhesive nanoemulsion using Box-Behnken design: In vitro and in vivo studies for brain targeting. Int. J. Pharm. 2020, 586, 119499. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Patil, K.; Yeole, P.; Gaikwad, R. Brain targeting studies on buspirone hydrochloride after intranasal administration of mucoadhesive formulation in rats. J. Pharm. Pharmacol. 2009, 61, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Khalid, M.S.; Al Ramadhan, A.M.; Alaradi, M.Z.; Al Hammad, M.R.; Ansari, K.; Alqurashi, Y.D.; Khan, M.F.; Albassam, A.A.; Ansari, M.J.; et al. Preparation of melatonin novel-mucoadhesive nanoemulsion used in the treatment of depression. Polym. Bull. 2022, 1–40. [Google Scholar] [CrossRef]
- Saenzdel Burgo, L.; Hernández, R.M.; Orive, G.; Pedraz, J.L. Nanotherapeutic approaches for brain cancer management. Nanomedicine 2014, 10, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Wang, A.; Ni, L.; Yan, X.; Song, Y.; Zhao, M.; Sun, K.; Mu, H.; Liu, S.; Wu, Z.; et al. Nose-to-brain delivery of temozolomide-loaded PLGA nano particles functionalized with anti-EPHA3 for glioblastoma targeting. Drug. Deliv. 2018, 25, 1634–1641. [Google Scholar] [CrossRef]
- Yan, A.; Joachims, M.L.; Thompson, L.F.; Miller, A.D.; Canoll, P.D.; Bynoe, M.S. CD73 promotes glioblastoma pathogenesis and enhances its chemoresistance via A2B adenosine receptor signaling. J. Neurosci. 2019, 39, 4387–4402. [Google Scholar] [CrossRef]
- Chaskis, E.; Luce, S.; Goldman, S.; Sadeghi, N.; Melot, C.; De Witte, O.; Devriendt, D.; Lefranc, F. Early postsurgical temozolomide treatment in newly diagnosed bad prognosis glioblastoma patients: Feasibility study. Bull. Cancer 2018, 105, 664–670. [Google Scholar] [CrossRef]
- Ban, M.M.; Chakote, V.R.; Dhembre, G.N.; Rajguru, J.R.; Joshi, D.A. In-situ gel for nasal drug delivery. Int. J. Dev. Res. 2013, 8, 18763–18769. [Google Scholar]
- Bayanati, M.; Khosroshahi, A.G.; Alvandi, M.; Mahboobian, M.M. Fabrication of a Thermosensitive In Situ Gel Nanoemulsion for Nose to Brain Delivery of Temozolomide. J. Nanomater. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Colombo, M.; Figueiró, F.; de Fraga, D.A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Patil, N.D. Nanoemulsion containing a synergistic combination of curcumin and quercetin for nose-to-brain delivery: In vitro and in vivo studies. Asian Pac. J. Trop. Biomed. 2021, 11, 510–518. [Google Scholar] [CrossRef]
- Azambuja, J.H.; Schuh, R.S.; Michels, L.R.; Gelsleichter, N.E.; Beckenkamp, L.R.; Iser, I.C.; Lenz, G.S.; de Oliveira, F.H.; Venturin, G.; Greggio, S.; et al. Nasal Administration of cationic nanoemulsions as CD73-siRNA delivery system for glioblastoma to treatment: A new therapeutical approach. Mol. Neurobiol. 2020, 57, 635–649. [Google Scholar] [CrossRef]
- Qu, Y.; Li, A.; Ma, L.; Iqbal, S.; Sun, X.; Ma, W.; Li, C.; Zheng, D.; Xu, Z.; Zhao, Z.; et al. Nose-to-brain delivery of disulfiram nanoemulsion in situ gel formulation for glioblastoma targeting therapy. Int. J. Pharm. 2021, 597, 120250. [Google Scholar] [CrossRef]
- Savale, S.K. Formulation and evaluation of quercetin nanoemulsions for treatment of brain tumor via intranasal pathway. Asian J. Biomater. Res. 2017, 3, 28–32. [Google Scholar]
- Desai, A.; Vyas, T.; Amiji, M. Cytotoxicity and apoptosis enhancement in brain tumor cells upon coadministration of paclitaxel and ceramide in nanoemulsion formulations. J. Pharm. Sci. 2008, 97, 2745–2756. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, X.; Ma, L.; Li, C.; Xu, Z.; Ma, W.; Zhou, Y.; Zhao, Z.; Ma, D. Therapeutic effect of disulfiram inclusion complex embedded in hydroxypropyl-β-cyclodextrin on intracranial glioma-bearing male rats via intranasal route. Eur. J. Pharm. Sci. 2021, 156, 105590. [Google Scholar] [CrossRef]
- Martini, S.R.; Kent, T.A. Ischemic Stroke. In Cardiology Secrets, Ved.; Glenn, N.L., Ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 493–504. [Google Scholar]
- Kumar, M.; Nishad, D.K.; Kumar, A.; Bhatnagar, A.; Karwasra, R.; Khanna, K.S.K.; Sharma, D.; Dua, K.; Mudaliyar, V.; Sharma, N. Enhancement in brain uptake of vitamin D3 nanoemulsion for treatment of cerebral ischemia: Formulation, gamma scintigraphy andefficacy study in transient middle cerebral artery occlusion rat models. J. Microencapsul. 2020, 37, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Sadeghnia, H.R.; Shaterzadeh, H.; Forouzanfar, F.; Hosseinzadeh, H. Neuroprotective effect of safranal, an active ingredient of Crocus sativus, in a rat model of transient cerebral ischemia. Folia Neuropathol. 2017, 55, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alam, M.A.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Quantification and evaluation of thymoquinone loadedmucoadhesivenanoemulsionfortreatmentofcerebralischemia. Int. J. Biol. Macromol. 2016, 88, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Abbas, N.A.; Ashafaq, M.; Alam, M.A.; Ahmad, F.J.; Al-Ghamdi, M.S. The effect of safranal loaded mucoadhesive nanoemulsion on oxidative stress markers in cerebral ischemia. Artif. Cells Nanomed. Biotechnol. 2017, 45, 775–787. [Google Scholar] [CrossRef]
- Huang, C.; Wang, C.; Zhang, W.; Yang, T.; Xia, M.; Lei, X.; Peng, Y.; Wu, Y.; Feng, J.; Li, D.; et al. Preparation, In vitro and in vivo evaluation of nanoemulsion in situ gel for transnasal delivery of traditional Chinese medicine volatile oil from Ligusticumsinense Oliv.cv. Chaxiong. Molecules 2022, 27, 7644. [Google Scholar] [CrossRef]
- Niyaz, A.; Rizwan, A.; Mohd, A.; Md, A.; Alam, M.Z.A.; Abuzer, A.; Ahmad, A.; Ashraf, K. Ischemic brain treated with 6-gingerol loaded mucoadhesive nanoemulsion via intranasal delivery and their comparative pharmacokinetic effect in brain. J. Drug. Deliv. Sci. Technol. 2021, 61, 102130. [Google Scholar]
- Nemade, S.M.; Kakad, S.P.; Kshirsagar, S.J.; Padole, T.R. Development of nanoemulsion of antiviral drug for brain targeting in the treatment of neuro-AIDS. Beni-Suef Univ. J. Basic. Appl. Sci. 2022, 11, 138. [Google Scholar] [CrossRef]
- Solomon, I.H. Molecular and Histologic Diagnosis of Central Nervous System Infections. Surg. Pathol. Clin. 2020, 13, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Oliva, A.; Sabatino, M.; Imbriano, A.; Hanieh, P.N.; Garzoli, S.; Mastroianni, C.M.; DeAngelis, M.; Miele, M.C.; Arnaut, M.; et al. Antimicrobial essential oil formulation: Chitosan coated nanoemulsions for nose to brain delivery. Pharmaceutics 2020, 12, 678. [Google Scholar] [CrossRef] [PubMed]
- Franklyne, J.S.; Gopinath, P.M.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsions: The rising star of antiviral therapeutics and nano delivery system-current status and prospects. Curr. Opin. Colloid. Interface Sci. 2021, 54, 101458. [Google Scholar] [CrossRef]
- Giovane, R.A.; Lavender, P.D. Central Nervous System Infections. Prim. Care 2018, 45, 505–518. [Google Scholar] [CrossRef]
- Hitendra, S.; Mahajan, M.S.; Mahajan, P.P.; Nerkar Agrawal, A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug. Deliv. 2014, 21, 148–154. [Google Scholar]
- Vos, T.; Abajobir, A.A.; Abate, K.A. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries,1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Chen, P.K.; Wang, S.J. Non-headache symptoms in migraine patients. F1000Research 2018, 7, 188. [Google Scholar] [CrossRef]
- Tanna, V.; Sawarkar, S.P.; Ravikumar, P. Exploring nose to brain nano delivery for effective management of migraine. Curr. Drug. Deliv. 2023, 20, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, R.S.; Gatne, M.M.; Gaikwad, R.V.; Bajaj, A.N.; Morde, M.A. Nanoemulsion based intranasal delivery of antimigraine drugs for nose to brain targeting. Indian J. Pharm. Sci. 2009, 71, 707–709. [Google Scholar]
- Abdou, E.M.; Kandil, S.M.; Miniawy, H.M.F.E. Brain targeting efficiency of antimigraine drug loaded mucoadhesive intranasal nanoemulsion. Int. J. Pharm. 2017, 529, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; Rodrigues da Silva, G.H.; Couto, V.M.; Castro, S.R.; Breitkreitz, M.C.; Martinez, C.S.; Igartúa, D.E.; Prieto, M.J.; de Paula, E. Functional hybrid nanoemulsions for sumatriptan intranasal delivery. Front. Chem. 2020, 8, 589503. [Google Scholar] [CrossRef]
- Chan, A.; Choi, E.; Yuki, I.; Suzuki, S.; Golshani, K.; Chen, J.; Hsu, F. Cerebral vasospasm after subarachnoid hemorrhage: Developing treatments. Brain Hemorrhages 2021, 2, 15–23. [Google Scholar] [CrossRef]
- Huang, S.; Huang, Z.; Fu, Z.; Shi, Y.; Dai, Q.; Tang, S.; Gu, Y.; Xu, Y.; Chen, J.; Wu, X.; et al. A novel drug delivery carrier comprised of nimodipine drug solution and a nanoemulsion: Preparation, characterization, invitro, and in vivo studies. Int. J. Nanomed. 2020, 15, 1161–1172. [Google Scholar] [CrossRef]
- Dhobale, A. Formulation and evaluation of carbamazepine nanoemulsion for brain targeted drug delivery via intranasal route. Indo Am. J. Pharm. Sci. 2018, 8, 1437–1452. [Google Scholar]
- Maruhashi, T.; Higashi, Y. An overview of pharmacotherapy for cerebral vasospasm and delayed cerebral ischemia after subarachnoid hemorrhage. Expert Opin. Pharmacother. 2021, 22, 1601–1614. [Google Scholar] [CrossRef]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Gudkov, S.V.; Plotnikov, E.Y.; Turovsky, E.A. Size-Dependent Cytoprotective Effects of Selenium Nanoparticles during Oxygen-Glucose Deprivation in Brain Cortical Cells. Int. J. Mol. Sci. 2022, 23, 7464. [Google Scholar] [CrossRef]



| Advanced Emulsion | Structure | Salient Features | Applications | Ref. |
|---|---|---|---|---|
| Nanoemulsion |  | Nanoscaled droplets provide thermodynamically (kinetically stable) colloidal dispersions. Controlled and effective lipophilic bioactive agents can be transported. | Widely explored in food industry for its superb stability and shelf life of encapsulated colouring/flavouring components/nutraceutical and bioactive agents | [16] |
| Multilayer emulsion |  | It contains hydrated lipophilic multi-molecular layers around oil (dispersed phase). Formed multilayer film provides adequate viscosity; hence, stable formulation is obtained. | Delivery and bio-accessibility of fat-soluble curcumin and other pH-sensitive bio-active agents | [17] |
| Multiple emulsion |  | These are emulsions within emulsion: i.e., o/w/o and w/o/w. Multiple emulsion limits bioactive degradation and is suitable for drug delivery via diverse routes such as ocular, oral, parenteral, nasal and pulmonary. | Delivery of low caloric food products, designing of easily spreadable creams, controlled and targeted dosages | [18] |
| Pickering emulsion |  | Instead of surface-active agents, solid particles confine at the interface of both phases and provide stability. These emulsions are more stable, tunable, feasible, stimuli-responsive and less toxic compared to the traditional ones. | Photocatalysis, protein recognition, purification of water, cosmeceutical products | [19] |
| High internal phase emulsion |  | HIPEs have high internal phase volume and are used to swap hydrogenated fats added in food stuffs. Hence, they improve stability and functionality and stabilize the system. The volume fraction is more than 0.74, ensuing formation of solid dispersed phase or polyhedral in the form of thin films. | Preparation of food stuffs, fuel and cosmetics | [20] |
| A. Alzheimer Disease | ||||
|---|---|---|---|---|
| Therapeutic Agent | Objective | Study Model | Outcome | Ref. |
| Memantine | To bypass BBB for effective management of Alzheimer’s disease | Particle size, in vitro release and radiolabelling with technetiumpertechnetate (99mTc). | Average globular size~11 nm. 80% drug release in nasal simulated fluid. ~98% cell viability. Higher uptake of drug (3.6% radioactivity) within 1.5 h in brain of rat. | [46] |
| Donepezil | To enhance drug delivery in brain | Particle size In vitro release radio labelling with Technetium pertechnetate | ~65 nm average globular size, 0.084 poly dispersity index (PDI) and −10.7 mV Zeta potential | [47] |
| 99% drug release in phosphatebuffer and 98% in simulated cerebrospinal mediawithin 4 h and 2 h, respectively. Dose-dependent radical scavenging and toxicity | ||||
| Huperzine A | Selectively Acetylcholinesterase inhibition via huperzine loaded nanoemulsion modified with lactoferrin | Particle size and zeta potential, in vitromodel (hCMEC/D3cells), drug-targeting index | 15.24 average particle size, 0.12 PDI and negative 4.48 mV zetapotential Drug-targetingindexwas 3.2 ± 0.75 Nanoemulsion was uptake by transcytosis and transported via specific multidrug resistance associated proteins transporters | [48] |
| Resveratrol | Coconut oil-based resveratrol nanoemulsion was targeted for the alleviation of Alzheimer’s disease | Effect of process variables, in vitro and in vivo permeation study in goat | Average particle size ~110 nm, −21.13 mV and 88.54% drug release in 8 h. Added coconut oil enhanced permeation efficiency. Optimum drugconcentrationwas 2.64 mg/mL. Higher resveratrol concentration in blood and brain (~1234.87 ng/mL and~5762.30 ng/mL in the brain, respectively, at 2 mg/kg dose. Superior permeation of prepared nanoemulsion compared to suspension in Goat nasal mucosa. | [49] |
| B. Parkinson disease | ||||
| Quercetin | Nanoemulsion comprising food-grade quercetin and oil (Capmul MCM NF) was aimed at neuroprotective action | HR-TEM, area electron diffraction studies and in vivo studies (cytotoxic study) and oxidative stress in wild-type Caenorhabditis elegans N2 strain | Spherical and ~50 nm particle size nanoemulsion potentially enhanced mitochondrial (3.080 ± 1.1) and fat content (2.64 ± 0.1) and reduced aggregation. Downregulated reactive Oxygen species content in | [50] |
| C elegans N2 strain. Dose and time-dependent toxicity. i.e., for A549 cells 300 µg/mL in 48 h. No toxicity against lymphocytes. | ||||
| Naringenin | Vitamin E adorned naringenin nanoemulsion was aimed at combating Parkinson’s disease | Structural property, refractive index and transmittance. Multiple behavioural analysis and oxidative study | Average smaller droplet size (~38.7 nm), 0.14 PDI with optimum zetapotential (−27.4 mV) and 19.6 Pas dynamic viscosity. 1.43 Refractive index and 98.1% transmittance indicated isotropic nature and stability of prepared nanoemulsion Three times high permeation coefficient and flux of nanoemulsion compared to suspension AUC0–48 hfor NE in brain 5345.1 and blood 3777.6 ng/mL X h Intranasally administered NE reversed Parkinson’s in 6-OHDA-persuaded rats while given with levodopa | [51] |
| Selegiline | Intranasal delivery monoamine oxidase B inhibitor (selegiline) nanoemulsion for better treatment of neuro degenerative disease | Quality by design approach for optimization of variables and behavioural study in rats | Optimized nanoemulsion (~61 nm) was quite stable (−34 mV). PDI (~0.203) Transmittance (~99.8%) and refractive index (1.30 ± 0.01) Almost 3.7-fold selegiline drug permeation observed compared to its oral suspension. Significantly improved locomotor activity and muscle coordination in experimental rat model on comparing with oral route. | [52] |
| Hyaluronic acid coloaded with resveratrol and curcumin) | Mucoadhesive polyphenols containing nanoemulsion were targeted for brain delivery | Antioxidant potential, in vitro and ex vivo and in vivo assessment of polyphenols in rat brain | Average particle size ~115 nm with negative zeta potential (23.9 mV). Higher nasal mucosa adhesive potential of the developed nanoemulsion Preserved polyphenolic antioxidant efficacy and protected from their degradation. Controlled diffusion was achieved for 6 h and showed ex vivo permeation efflux for resveratrol (2.86 µg/cm2) and curcumin (2.09 µg/cm2) across sheep nasal mucosa. Increased amount of two polyphenols in brain | [53] |
| C. Epilepsy | ||||
| Amiloride | To enhance brain bioavailability of nanoemulsion containing diuretic amiloride | 33 factorial central composite design, Physicochemical parameters, in vitro drug release and antiepileptic effect in mice | Concentration of olei cacid (2.5%), Tween 20 and Carbitol (10%) and sonication time (45 s) were optimised, which resulted in ~89 nm hydrodynamic diameter. ~1.38 refractive index, 6.4 ± 0.2 pH and ~41 cp viscosity of nanoemulsion ~99% percentage transmittance and ~80% cumulative drug release. Drastically increased nose-to-brain transport (~586%) and efficiency (~1992%) in mice model Improved seizure threshold in epileptic rodent model and induced seizure in mice. | [54] |
| Letrozole | Nanoemulsion contained aromatase inhibitor letrozole designed for brain delivery while avoiding peripheral responses | Structural analysis, in vitro and ex vivo drug diffusion study and behavioural study | Spherical and smaller particles of mean diameter. i.e., 96 nm and 0.162 PDI. Negative −7.12 zetapotential Prolonged drug release during intranasal administration that results in high concentration letrozole in brain region. Enhanced neuroprotective and antiepileptic effects. Inhibition and diversion of aromatomization and metabolic pathways of testosterone into 17-beta estradiol observed. | [55] |
| Oxcarbazepine | PLGA tri-block polymer admixed oxcarbazepine emulsome (emulsion +liposome) and its thermogel were developed to amplify drug concentration in brain | In vitro study, histopathological analysis, and pharmacokinetic parameters | Concentration dependent rheological behaviour. The emulsome exhibited sustained effect of 81.1% for 24 h. Higher drug transport in brain tissue uptake in rat model compared to drug solution and suspension (Trileptal®). 3818.8 ng/mL and 5699.9 ng/mL Cmax of nanoemul gels in plasma and brain, respectively. Histopathology study of nasal tissues revealed mild anti-inflammatory response and vascular congestion without toxicity. | [56] |
| D. Neuroprotection | ||||
| Naringenin | To explore bioflavonoid naringenin for its neuroprotective effect owing to its antioxidant and anti-inflammatory virtues | Particle size and zeta potential, neuroprotective assay (Aβ triggered ROS, total tau, amyloid precursor protein) against neuroblastoma cells, i.e., SH-SY5Y | Average droplet size 113.8 nm with narrow PDI~0.312. Percentage transmittance ~97.01% and +12.4 mV zeta potential. Enhanced neurotoxic effects SH-SY5Y cells due to down-regulation of amyloid precursor protein. Nanoemulsion checked | [57] |
| amyloid-genesis and reduced level of phosphorylated tau at low concentration of 0.125 µM in comparison with bare drug 25 µM. | ||||
| Curcumin | Improvement of solubility and bioavailability issues of curcumin added in nanoemulsion for the novel neuroprotective action | Oxidative stress and mitochondrial complex I activity | Curcumin-loaded nanoemulsion significantly enhanced motor activity at 25 mg/kg dose Lessened lipoperoxidation and improved antioxidant activity in mitochondria while compared to free curcumin. Curcumin-loaded nanoemulsion inhibited Mitochondrial complex 1 activity | [58] |
| Chia seed oil | Developed nanoemulsion containing Chia seed oil had potential to overcome issues of Parkinson’s disease and neuroprotective actions | Motor and behavioural evaluation. i.e., rotarod and locomotor tests and biochemical evaluation | Solubility, bioavailability, and stability of Chia seed oil contained nanoemulsion was escalated Potential application of developed nanoemulsion is suggested for neuroprotective effect in neurodegenerative disorders | [59] |
| E. Depression and Schizophrenia | ||||
| Paroxetine | Selective serotonin reuptake inhibitor ‘paroxetine ‘embedded intranasal nanoemulsion was explored for treatment of depression that also avoided first-pass metabolism | Average particle size, PDI, zetapotential, permeation, behavioural studies, Forced swimming test in Wistar rat and antidepressant studies | Spherical droplets of size ~58 nm with 0.339 PDI and −33 mV zeta potential. Good% transmittance and refractive index. i.e., 100.6% and 1.41, respectively 2.57 times higher permeation of nanoemulsion compared to paroxetine suspension. Improved anti-depression action due to enhancement of reduced level of glutathione on intranasal delivery. Decreased level of | [60] |
| Thiobarbituric acid reactive Substance (TBARS). | ||||
| Aripiprazole | Quinolinone derivative antidepressant aripiprazole nanoemulsion was designed for treatment of schizophrenia and bipolar disorder | Response surface method and central composite rotatable design for optimization | Optimized overhead stirring time (120 min), high shear homogenization (15 min) and rpm (4400) resulted in nanosized (~62 nm) droplets and 3.72 mPa viscosity. At pH 7.4, osmolality of nanoemulsion was ~297 mOsm/kg Stable for three months at variable temperatures. i.e., 4 °C, 25 °C and 45 °C. | [61] |
| Asenapine maleate | Mucoadhesive nanoemulsion of antipsychotic asenapine maleate prepared for improved nasomucosal adhesion, efficient brain-targeting and safety | Sigle-dose pharmacokinetic study, animal behaviour study, ex vivo ciliotoxicity in sheep nasal mucosa | Spherical particles with average diameter of 21.2 nm with narrow PDI (0.355). Increased drug concentration in Wistar rat brain (within 1 h) after intranasal delivery compared to intravenous route (3 h). Enhanced brain targeting capacity (284.33 ng/mL). Improved locomotor activity with no extrapyramidal symptoms in sheep nasal mucosa. | [62] |
| Therapeutic Agent | Objective | Evaluated Parameters | Result | Application | Ref. |
|---|---|---|---|---|---|
| Kaempferol | Investigation of glioma cells inhibition efficacy with and without chitosan-loaded kaempferol nanoemulsion and compared mucoadhesive properties after intranasal delivery | Average droplet size (180 nm) pH (5.56 ± 0.02) Viscosity (11.48 ± 0.13 cp25 °C) Drug content (96.37 ± 2.67%) Association efficiency (99.35 ± 0.10%) Permeation Efficiency across nasal mucosa (13.04 µg/cm2) | Chitosan improved residing properties in the nasal mucosa owing to its mucoadhesive property, hence enhancing permeation and reducing nasal clearance of drug. Further, chitosan-KPF nanoemulsion inhibited C6 glioma cells viability via rapidly triggering apoptosis compared to without chitosan-loaded kaempferol nanoemulsion. | Treatment of glioma | [71] |
| Curcumin(CUR) and quercetin(QUE) | Two phytoconstituents containing nanoemulsions were designed for synergistically inhibiting growth of glioblastoma U373MG cells | Average droplet size 93 nm PDI 0.149 Drug content 42.4% for CUR and 55.1% for QUE Zetapotential –14.8 mV Drug release in pH6.4 For CUR, 95.84% For QUE, 94.0% | Optimized nanoemulsion displayed significantly high percentage of brain targeting efficiency, i.e., ~178% for curcumin and ~170% for quercetin. Successful nose-to-brain delivery of both curcumin and quercetin (~44% and ~38%, respectively) indicated potential CNS targeting through intranasal pathway. Further, the nanoemulsion exhibited synergistically site-specific targeting of human glioblastoma cells compared to doxorubicin drug. | Treatment of human glioblastoma | [72] |
| CD73SiRNA | Cationic nanoemulsion composed of si-RNACD73R was successfully developed and targeted to inhibit brain tumour growth | Cell viability 30–50% Glioma cells inhibition 60–80% Tumour growth reduction 60% decreased adenosine level 95% | Developed si-RNA-CD73R Nanoemulsion enabled silencing of surface enzyme CD73 and overexpression of adenosine in the brain of rats after nasal delivery. Administered nanoemulsion reduced growth of glioblastoma cells and adenosine level in cancerous cells. | Treatment of brain tumour | [73] |
| Disulfiram inclusion complex with copper ion | Ion-sensitive disulfiram nanoemulsion was explored for the management of glioblastoma | Average particlesize ~63.4 nm. Zeta potential (−23.5 mV) Prolonged drug release 50% at 4 h and and 75% at 12 h | Performed in vitro studies indicated effective inhibition of proliferation of glioblastoma cells. i.e., C6 andU87. Further, formulated in situ gel nanoemulsion showed excellent uptake and brain target capability by producing highest signal fluorescence in the brain of rats. | Glioblastoma targeting therapy | [74] |
| Polyphenolic flavonoid quercetin | Enhancement of bioavailability and permeability of quercetin-loaded nanoemulsion across blood–brain barrier | Mean droplet size 125.5 nm PDI 0.251 Entrapment efficiency ~87.0%. Cmax 5962.7 ng/mL after 4 h Mean resident time 46.13 h. AUC is 5.32 times higher than pure drug | Intranasal-administered quercetin nanoemulsion improved solubility, therapeutic index and permeability in the brain region. | Brain cancer | [75] |
| Therapeutic Agent | Purpose | Evaluated Parameters | Outcomes | Application | Ref. |
|---|---|---|---|---|---|
| Safranal | Nanoemulsion containing antioxidant safranal was prepared to reduce oxidative stress in brain injury | Optimized mean size 89.64 nm Zetapotential −11.39 mV Drug content 98.47% Viscosity 124 cp | Improved locomotor activity, grip strength and antioxidant activity observed. Significantly decrease in glutathione reductase, superoxide dismutase and lipid peroxidation in model rat brain. | Treatment for cerebral ischemia-reperfusion injury | [82] |
| Chaxiong volatile oil | Thermosensitive intranasal in situ nanoemulsion gel was developed for brain targeting | Mean particle size ~21.02 nm, PDI 0.14 Negative zeta potential −20.4 mV pH 4.52 Viscosity 32.5 mV, gelling strength 42–47 s Mucoadhesive strength 5.2 × 102 dy/cm2 Release kinetics Ritger–Peppas model. | Both nanoemulsion and in situ gel had potential to lessen neurological deficit score in ischemic rat model. Cerebral infarction size was reduced, which improved ischemic stroke. | Cerebral ischemic stroke | [83] |
| 6-Gingerol | Mucoadhesive intranasal nanoemulsion was designed to improve brain bioavailability and Neuro-protectiveness effect of 6-gingerol | Average particle size ~94.89 nm, Narrow PDI 0.129 Zetapotential +1.892 mV Retention time 1.27 min | Nanoemulsion preparedwith antioxidant 6-gingerol and lauroglycol 90 was then converted in to mucoadhesive with chitosan. Improved Cmax and AUC after intranasal administration exhibited and histopathological assay displayed reduction in infarction volume in induced Ischemic model. | Treatment of cerebral ischemia | [84] |
| Tenofovir disoproxil fumarate | Stable nucleotide reductase inhibitor tenofovir disproxil fumerate-loaded nanoemulsion was developed and optimized by phase diagram to target neuro-AIDS in brain. | Phase diagram for optimization of surfactant and co-surfactant (45%). Zeta potential (−18.7 mV) Average globular size (156.2 nm) PDI (0.463) Drug diffusion (egg membrane) 74.98% and sheep nasal mucosa (75.98%) after 3 h. | Developed nanoemulsion increased surface area and provided lipophilicity to the formulation. This alternate strategy provided quick onset of action against viral infection for all age groups sufferers. | Neuro-AIDS treatment | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misra, S.K.; Pathak, K. Nose-to-Brain Targeting via Nanoemulsion: Significance and Evidence. Colloids Interfaces 2023, 7, 23. https://doi.org/10.3390/colloids7010023
Misra SK, Pathak K. Nose-to-Brain Targeting via Nanoemulsion: Significance and Evidence. Colloids and Interfaces. 2023; 7(1):23. https://doi.org/10.3390/colloids7010023
Chicago/Turabian StyleMisra, Shashi Kiran, and Kamla Pathak. 2023. "Nose-to-Brain Targeting via Nanoemulsion: Significance and Evidence" Colloids and Interfaces 7, no. 1: 23. https://doi.org/10.3390/colloids7010023
APA StyleMisra, S. K., & Pathak, K. (2023). Nose-to-Brain Targeting via Nanoemulsion: Significance and Evidence. Colloids and Interfaces, 7(1), 23. https://doi.org/10.3390/colloids7010023










