Structural Modification of Jackfruit Leaf Protein Concentrate by Enzymatic Hydrolysis and Their Effect on the Emulsifier Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Substances
2.3. Extraction and Hydrolysis of the LPC of Jackfruit
2.4. Amino Acid Profile
2.5. Fourier Transform Infrared Spectroscopy
2.6. Thermal Properties
2.7. Preparation of Emulsions with LPC and Protein Hydrolysates
2.8. Particle Size Distribution of Emulsions
2.9. Statistical Analysis
3. Results and Discussion
3.1. Amino Acid Composition
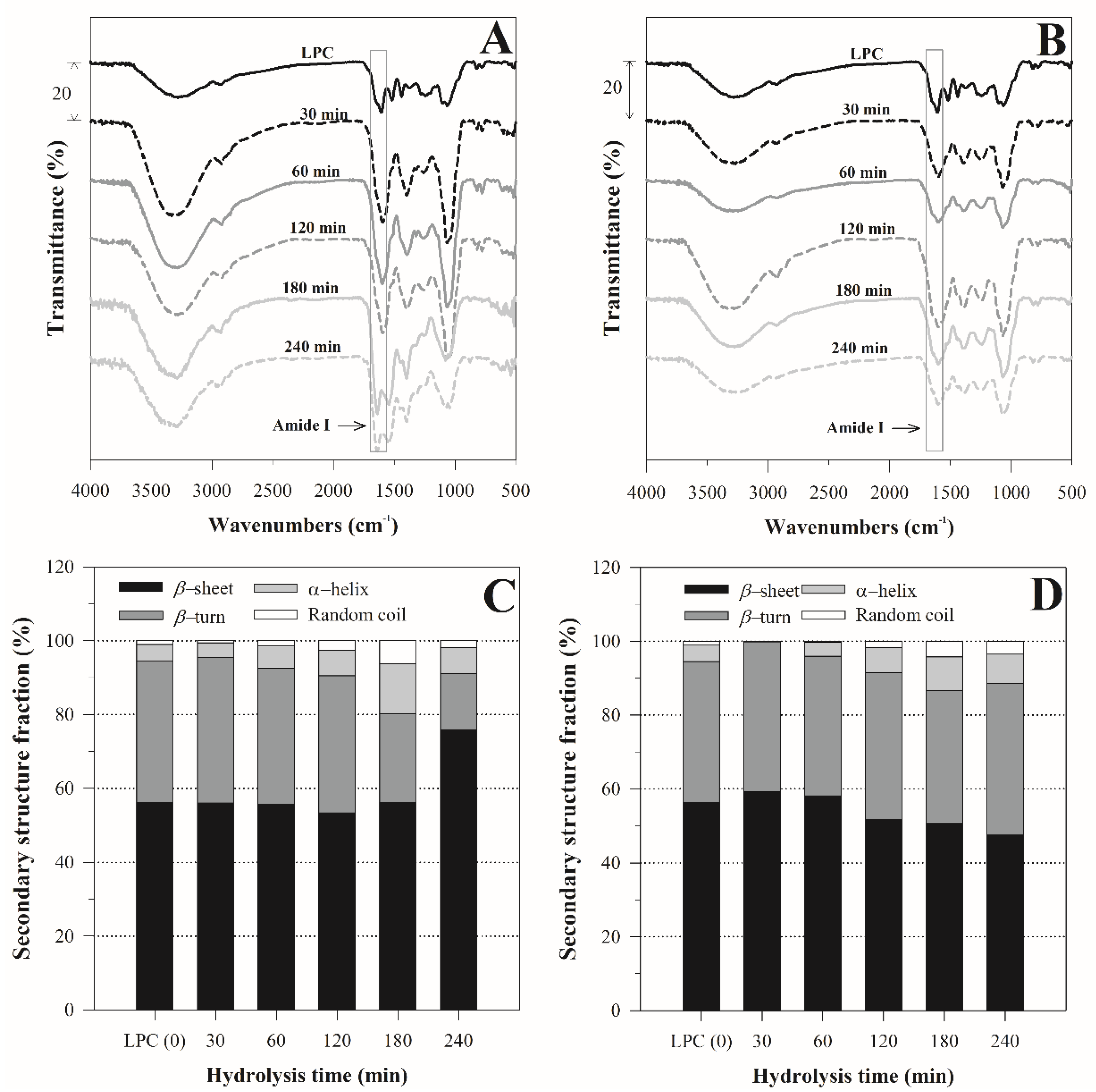
3.2. FTIR Spectra Analysis
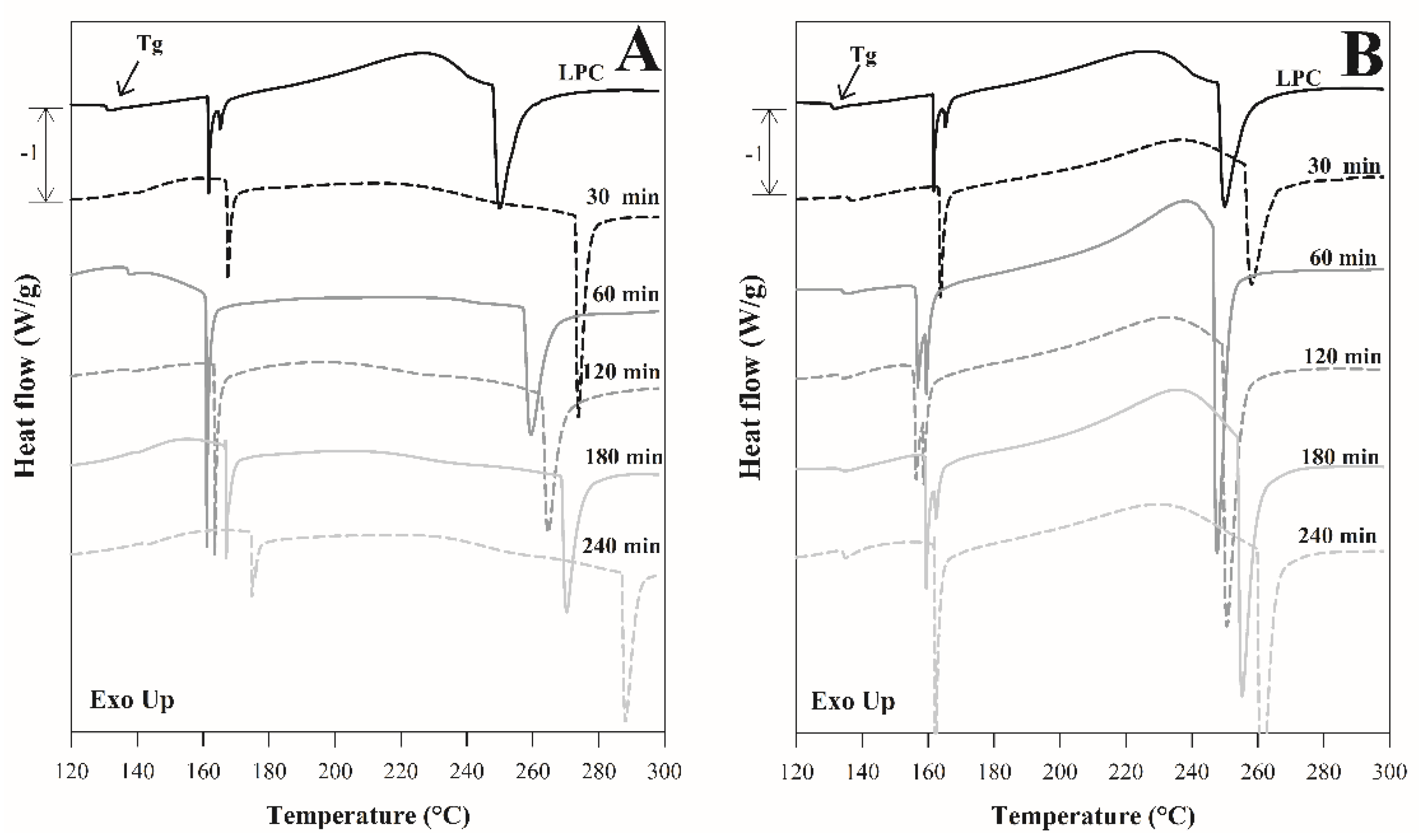
3.3. Thermal Properties
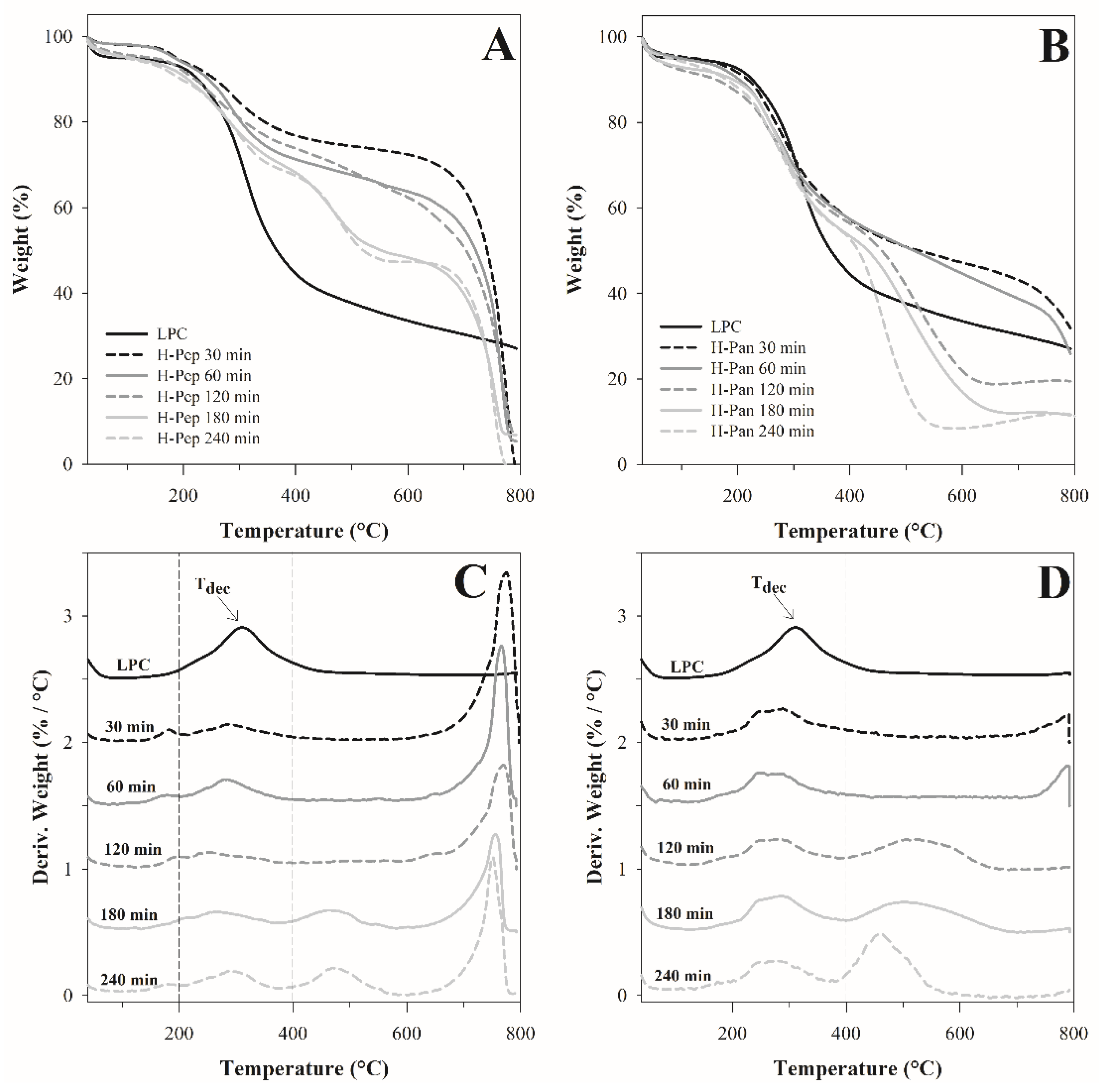
3.3.1. Thermogravimetric Analysis
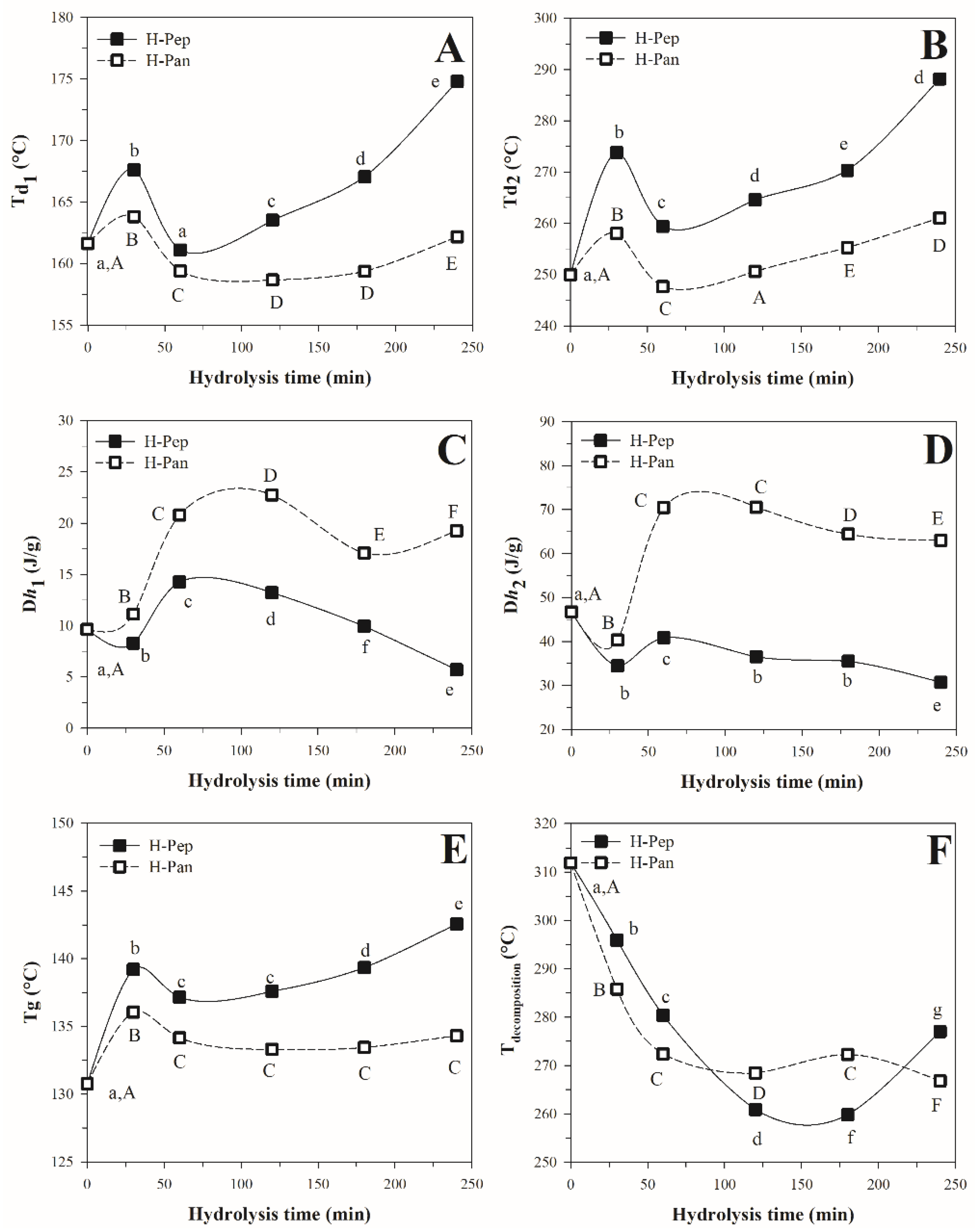
3.3.2. Differential Scanning Calorimetry Analysis
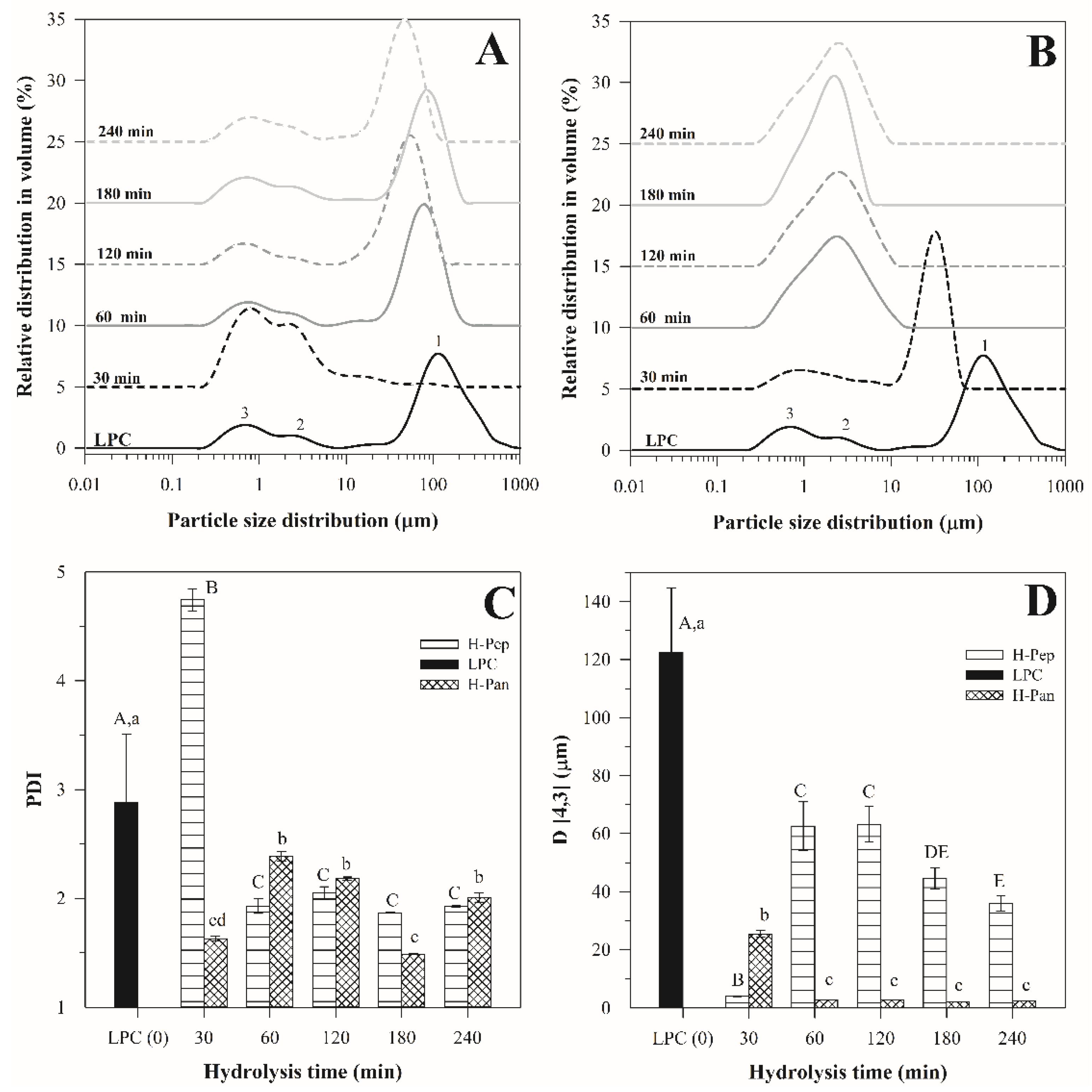
3.4. Particle Size Distribution of Emulsions Stabilized with LPC and Hydrolysates
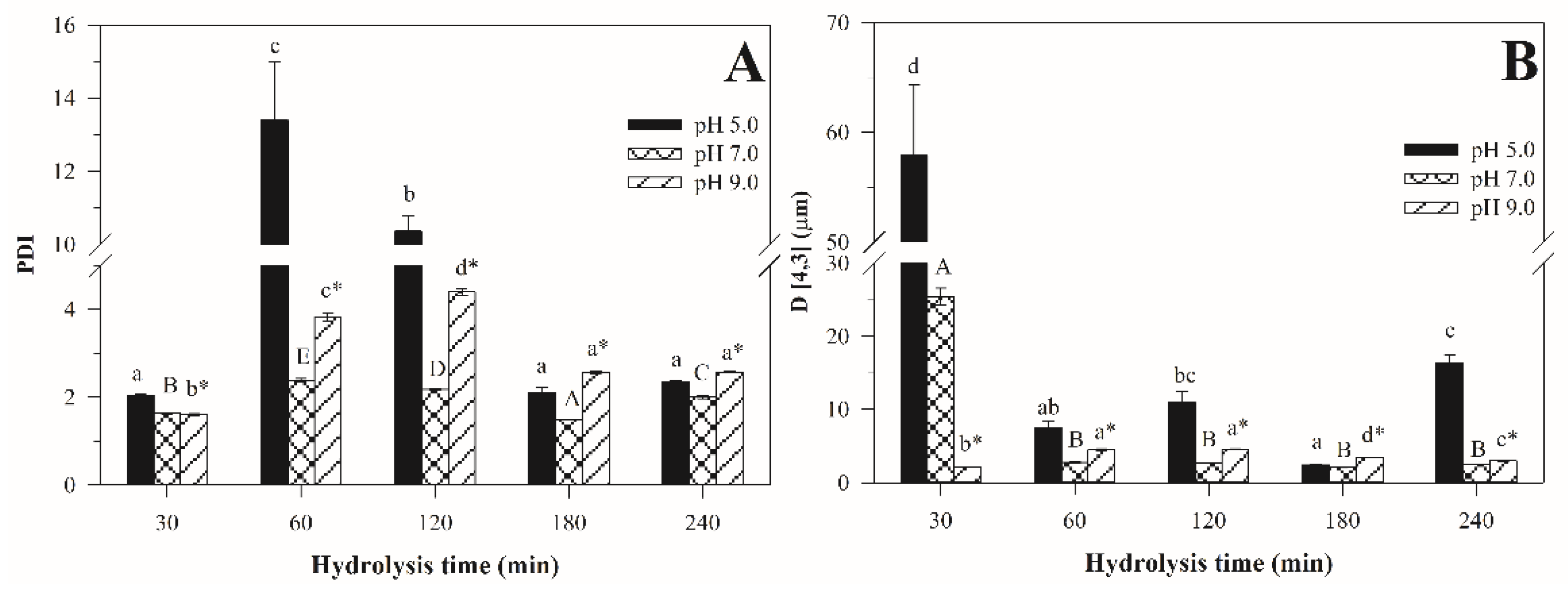
Effect of pH on the Particle Size Distribution of Emulsions Stabilized with H–Pan
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drapala, K.P.; Mulvihill, D.M.; O’Mahony, J.A. A Review of the Analytical Approaches Used for Studying the Structure, Interactions and Stability of Emulsions in Nutritional Beverage Systems. Food Struct. 2018, 16, 27–42. [Google Scholar] [CrossRef]
- Dapčević-Hadnađev, T.; Dizdar, M.; Pojić, M.; Krstonošić, V.; Zychowski, L.M.; Hadnađev, M. Emulsifying Properties of Hemp Proteins: Effect of Isolation Technique. Food Hydrocoll. 2019, 89, 912–920. [Google Scholar] [CrossRef]
- Burger, T.G.; Zhang, Y. Recent Progress in the Utilization of Pea Protein as an Emulsifier for Food Applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-Innovative Technologies for Extraction of Proteins for Human Consumption from Renewable Protein Sources of Plant Origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Galves, C.; Galli, G.; Miranda, C.G.; Kurozawa, L.E. Improving the Emulsifying Property of Potato Protein by Hydrolysis: An Application as Encapsulating Agent with Maltodextrin. Innov. Food Sci. Emerg. Technol. 2021, 70, 102696. [Google Scholar] [CrossRef]
- Chang, C.; Nickerson, M.T. Encapsulation of Omega 3-6-9 Fatty Acids-Rich Oils Using Protein-Based Emulsions with Spray Drying. J. Food Sci. Technol. 2018, 55, 2850–2861. [Google Scholar] [CrossRef]
- Grasberger, K.F.; Gregersen, S.B.; Jensen, H.B.; Sanggaard, K.W.; Corredig, M. Plant-Dairy Protein Blends: Gelation Behaviour in a Filled Particle Matrix. Food Struct. 2021, 29, 100198. [Google Scholar] [CrossRef]
- Fernández-Sosa, E.I.; Chaves, M.G.; Henao Ossa, J.S.; Quiroga, A.V.; Avanza, M.V. Protein Isolates from Cajanus cajan L. as Surfactant for o:W Emulsions: PH and Ionic Strength Influence on Protein Structure and Emulsion Stability. Food Biosci. 2021, 42, 101159. [Google Scholar] [CrossRef]
- Ozturk, B.; McClements, D.J. Progress in Natural Emulsifiers for Utilization in Food Emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Gafsi, I.M.; Sila, A.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Effects of Enzymatic Hydrolysis on Conformational and Functional Properties of Chickpea Protein Isolate. Food Chem. 2015, 187, 322–330. [Google Scholar] [CrossRef]
- Tamm, F.; Herbst, S.; Brodkorb, A.; Drusch, S. Functional Properties of Pea Protein Hydrolysates in Emulsions and Spray-Dried Microcapsules. Food Hydrocoll. 2016, 58, 204–214. [Google Scholar] [CrossRef]
- Liu, C.; Bhattarai, M.; Mikkonen, K.S.; Heinonen, M. Effects of Enzymatic Hydrolysis of Fava Bean Protein Isolate by Alcalase on the Physical and Oxidative Stability of Oil-in-Water Emulsions. J. Agric. Food Chem. 2019, 67, 6625–6632. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Yue, C.; Wang, Y.; Shao, M.; Yu, G. Effect of Limited Enzymatic Hydrolysis on the Structure and Emulsifying Properties of Rice Bran Protein. J. Cereal Sci. 2019, 85, 168–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Romero, H.M. Exploring the Structure-Function Relationship of Great Northern and Navy Bean (Phaseolus vulgaris L.) Protein Hydrolysates: A Study on the Effect of Enzymatic Hydrolysis. Int. J. Biol. Macromol. 2020, 162, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Herman-Lara, E.; Ragazzo-Sánchez, J.A. Jackfruit (Artocarpus heterophyllus Lam) Leaf as a New Source to Obtain Protein Hydrolysates: Physicochemical Characterization, Techno-Functional Properties and Antioxidant Capacity. Food Hydrocoll. 2021, 112, 106319. [Google Scholar] [CrossRef]
- Brion-Espinoza, I.A.; Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Calderón-Chiu, C.; Calderón-Santoyo, M. Edible Pectin Film Added with Peptides from Jackfruit Leaves Obtained by High-Hydrostatic Pressure and Pepsin Hydrolysis. Food Chem. X 2021, 12, 100170. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Zhang, H.; Wang, L.; Zhang, H.; Qian, H.; Qi, X. Isolation, Purification and Identification of Two Antioxidant Peptides from Water Hyacinth Leaf Protein Hydrolysates (WHLPH). Eur. Food Res. Technol. 2018, 244, 83–96. [Google Scholar] [CrossRef]
- Yan, X.; Liang, S.; Peng, T.; Zhang, G.; Zeng, Z.; Yu, P.; Gong, D.; Deng, S. Influence of Phenolic Compounds on Physicochemical and Functional Properties of Protein Isolate from Cinnamomum camphora Seed Kernel. Food Hydrocoll. 2020, 102, 105612. [Google Scholar] [CrossRef]
- Böcker, U.; Wubshet, S.G.; Lindberg, D.; Afseth, N.K. Fourier-Transform Infrared Spectroscopy for Characterization of Protein Chain Reductions in Enzymatic Reactions. Analyst 2017, 142, 2812–2818. [Google Scholar] [CrossRef]
- del Mar Contreras, M.; Lama-Muñoz, A.; Manuel Gutiérrez-Pérez, J.; Espínola, F.; Moya, M.; Castro, E. Protein Extraction from Agri-Food Residues for Integration in Biorefinery: Potential Techniques and Current Status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef]
- Famuwagun, A.A.; Alashi, A.M.; Gbadamosi, S.O.; Taiwo, K.A.; Oyedele, D.J.; Adebooye, O.C.; Aluko, R.E. Comparative Study of the Structural and Functional Properties of Protein Isolates Prepared from Edible Vegetable Leaves. Int. J. Food Prop. 2020, 23, 955–970. [Google Scholar] [CrossRef]
- Akbari, N.; Mohammadzadeh Milani, J.; Biparva, P. Functional and Conformational Properties of Proteolytic Enzyme-modified Potato Protein Isolate. J. Sci. Food Agric. 2020, 100, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Miss-Zacarías, D.M.; Iñiguez-Moreno, M.; Calderón-Santoyo, M.; Ragazzo-Sánchez, J.A. Optimization of Ultrasound-Assisted Microemulsions of Citral Using Biopolymers: Characterization and Antifungal Activity. J. Dispers. Sci. Technol. 2022, 43, 1373–1382. [Google Scholar] [CrossRef]
- Ricci, L.; Umiltà, E.; Righetti, M.C.; Messina, T.; Zurlini, C.; Montanari, A.; Bronco, S.; Bertoldo, M. On the Thermal Behavior of Protein Isolated from Different Legumes Investigated by DSC and TGA. J. Sci. Food Agric. 2018, 98, 5368–5377. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Thompson, E.O.P. Halogenation of Tyrosine during Acid Hydrolysis. Biochim. Biophys. Acta 1963, 71, 468–471. [Google Scholar] [CrossRef]
- Famuwagun, A.A.; Alashi, A.M.; Gbadamosi, O.S.; Taiwo, K.A.; Oyedele, D.; Adebooye, O.C.; Aluko, R.E. Antioxidant and Enzymes Inhibitory Properties of Amaranth Leaf Protein Hydrolyzates and Ultrafiltration Peptide Fractions. J. Food Biochem. 2021, 45, e13396. [Google Scholar] [CrossRef]
- Tenorio, A.T.; Kyriakopoulou, K.E.; Suarez-Garcia, E.; van den Berg, C.; van der Goot, A.J. Understanding Differences in Protein Fractionation from Conventional Crops, and Herbaceous and Aquatic Biomass—Consequences for Industrial Use. Trends Food Sci. Technol. 2018, 71, 235–245. [Google Scholar] [CrossRef]
- WHO/FAO. Report of a Joint WHO/FAO/UNU Expert Consultation 2007; World Health Organization/Food and Agricultural Organization: Geneva, Switzerland, 2007.
- Kchaou, H.; Jridi, M.; Benbettaieb, N.; Debeaufort, F.; Nasri, M. Bioactive Films Based on Cuttlefish (Sepia Officinalis) Skin Gelatin Incorporated with Cuttlefish Protein Hydrolysates: Physicochemical Characterization and Antioxidant Properties. Food Packag. Shelf Life 2020, 24, 100477. [Google Scholar] [CrossRef]
- Trigui, I.; Yaich, H.; Zouari, A.; Cheikh-Rouhou, S.; Blecker, C.; Attia, H.; Ayadi, M.A. Structure-Function Relationship of Black Cumin Seeds Protein Isolates: Amino-Acid Profiling, Surface Characteristics, and Thermal Properties. Food Struct. 2021, 29, 100203. [Google Scholar] [CrossRef]
- Saha, J.; Deka, S.C. Functional Properties of Sonicated and Non-Sonicated Extracted Leaf Protein Concentrate from Diplazium esculentum. Int. J. Food Prop. 2017, 20, 1051–1061. [Google Scholar] [CrossRef]
- Gómez, A.; Gay, C.; Tironi, V.; Avanza, M.V. Structural and Antioxidant Properties of Cowpea Protein Hydrolysates. Food Biosci. 2021, 41, 101074. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Liu, C.; Luo, L.; Chen, J.; Luo, S.; McClements, D.J.; Wu, L. Effect of Limited Enzymatic Hydrolysis on Structure and Emulsifying Properties of Rice Glutelin. Food Hydrocoll. 2016, 61, 251–260. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, G.; Zhao, J.; Ma, M.; Bao, X.; Zeng, Z.; Gong, X.; Yu, P.; Wen, X.; Gong, D. Influence of Phenolic Compounds on the Structural Characteristics, Functional Properties and Antioxidant Activities of Alcalase-Hydrolyzed Protein Isolate from Cinnamomum camphora Seed Kernel. LWT 2021, 148, 111799. [Google Scholar] [CrossRef]
- Marcelino, A.M.C.; Gierasch, L.M. Roles of β-Turns in Protein Folding: From Peptide Models to Protein Engineering. Biopolymers 2008, 89, 380–391. [Google Scholar] [CrossRef] [PubMed]
- López, D.N.; Ingrassia, R.; Busti, P.; Bonino, J.; Delgado, J.F.; Wagner, J.; Boeris, V.; Spelzini, D. Structural Characterization of Protein Isolates Obtained from Chia (Salvia hispanica L.) Seeds. LWT 2018, 90, 396–402. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Yao, L.; Qin, F.; Hou, G.; Chen, B.; Jin, L.; Deng, J.; Shen, Y. Characterisation, Physicochemical and Functional Properties of Protein Isolates from Amygdalus pedunculata Pall Seeds. Food Chem. 2020, 311, 125888. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E. Physicochemical and Functional Properties of Membrane-Fractionated Heat-Induced Pea Protein Aggregates. Front. Nutr. 2022, 9, 852225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, Y.; Zhao, M.; Ren, J.; Yang, B. Enzymatic Hydrolysis and Their Effects on Conformational and Functional Properties of Peanut Protein Isolate. Food Chem. 2011, 127, 1438–1443. [Google Scholar] [CrossRef]
- Calderón-Chiu, C. (Instituto Tecnológico de Tepic, Tepic, Mexico); Ragazzo-Sánchez, J.A. (Instituto Tecnológico de Tepic, Tepic, Mexico); Calderón-santoyo, M. (Instituto Tecnológico de Tepic, Tepic, Mexico). Personal Communication, Non-Published Material. 2022.
- Tang, C.-H.; Ma, C.-Y. Heat-Induced Modifications in the Functional and Structural Properties of Vicilin-Rich Protein Isolate from Kidney (Phaseolus vulgaris L.) Bean. Food Chem. 2009, 115, 859–866. [Google Scholar] [CrossRef]
- Fu, H.; Grimsley, G.R.; Razvi, A.; Scholtz, J.M.; Pace, C.N. Increasing Protein Stability by Improving Beta-Turns. Proteins Struct. Funct. Bioinforma 2009, 77, 491–498. [Google Scholar] [CrossRef]
- Damodaran, S. Amino Acids, Peptides, and Protein. In Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2017; pp. 235–356. ISBN 9781315372914. [Google Scholar]
- Zheng, X.; Wang, J.; Liu, X.; Sun, Y.; Zheng, Y.; Wang, X.; Liu, Y. Effect of Hydrolysis Time on the Physicochemical and Functional Properties of Corn Glutelin by Protamex Hydrolysis. Food Chem. 2015, 172, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Huang, X.; Jin, Y.; Zhang, S.; Ma, M. Characterization of Enzymatically Modified Liquid Egg Yolk: Structural, Interfacial and Emulsifying Properties. Food Hydrocoll. 2020, 105, 105763. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Liu, Y. Effects of Partial Hydrolysis on the Structural, Functional and Antioxidant Properties of Oat Protein Isolate. Food Funct. 2020, 11, 3144–3155. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chi, Y.-J.; Zhao, M.-Y.; Xu, W. Influence of Degree of Hydrolysis on Functional Properties, Antioxidant and ACE Inhibitory Activities of Egg White Protein Hydrolysate. Food Sci. Biotechnol. 2012, 21, 27–34. [Google Scholar] [CrossRef]
- Chang, C.; Tu, S.; Ghosh, S.; Nickerson, M.T. Effect of PH on the Inter-Relationships between the Physicochemical, Interfacial and Emulsifying Properties for Pea, Soy, Lentil and Canola Protein Isolates. Food Res. Int. 2015, 77, 360–367. [Google Scholar] [CrossRef]
- Ruiz-Álvarez, J.M.; del Castillo-Santaella, T.; Maldonado-Valderrama, J.; Guadix, A.; Guadix, E.M.; García-Moreno, P.J. PH Influences the Interfacial Properties of Blue Whiting (M. Poutassou) and Whey Protein Hydrolysates Determining the Physical Stability of Fish Oil-in-Water Emulsions. Food Hydrocoll. 2022, 122, 107075. [Google Scholar] [CrossRef]
| H–Pep at Different Hydrolysis Times (min) | |||||||
|---|---|---|---|---|---|---|---|
| Amino Acid | Leaf Flour | LPC | 30 | 60 | 120 | 180 | 240 |
| Alanine | 1.68 ± 0.17 b | 6.55 ± 0.19 e | 3.1 ± 0.12 a | 2.57 ± 0.1 ac | 2.29 ± 0.68 bc | 3.19 ± 0.06 a | 4.22 ± 0.42 d |
| Glycine | 0.32 ± 0.15 c | 0.97 ± 0.05 d | 0 ± 0 a | 0.13 ± 0.01 ab | 0.13 ± 0.04 ab | 0.17 ± 0 bc | 0 ± 0 a |
| Valine | 1.77 ± 0.2 d | 8.62 ± 0.42 e | 5.58 ± 0.8 c | 3.77 ± 0.11 a | 3.53 ± 0.72 a | 4.25 ± 0.27 ab | 5.17 ± 0.84 bc |
| Leucine | 2.52 ± 0.14 c | 11.26 ± 0.13 e | 6.9 ± 0.65 d | 4.44 ± 0.05 ab | 3.87 ± 0.29 ac | 4.8 ± 0.15 ab | 5.77 ± 1.33 bd |
| Isoleucine | 1.36 ± 0.12 d | 6.02 ± 0.01 e | 3.91 ± 0.3 c | 2.56 ± 0.08 a | 2.38 ± 0.41 a | 2.83 ± 0.22 ab | 3.67 ± 0.93 bc |
| Proline | 1.46 ± 0.08 a | 2.68 ± 0.06 b | 1.11 ± 0.15 a | 1.28 ± 0.12 a | 1.04 ± 0.79 a | 1.01 ± 0.01 a | 0.89 ± 0.03 a |
| Serine | 0.28 ± 0.03 e | 2.87 ± 0.11 d | 1.49 ± 0.01b c | 1.27 ± 0.05 bc | 1.06 ± 0.05 a | 1.73 ± 0.05 c | 2.72 ± 0.23 d |
| Threonine | 0.76 ± 0.05 a | 5.02 ± 0.05 b | 2.66 ± 0.11 c | 2.14 ± 0.23 d | 1.7 ± 0.42 e | 3.22 ± 0.01 f | 4.05 ± 0.04 g |
| Phenylalanine | 1.27 ± 0.06 c | 5.27 ± 0.11 d | 3.43 ± 0.03 a | 2.62 ± 0.91 ab | 1.95 ± 0.13 bc | 2.77 ± 0.01 ab | 2.89 ± 0.07 a |
| Aspartic acid | 1.65 ± 0.11 a | 5.87 ± 0.08 f | 2.85 ± 0.15 c | 2.55 ± 0.01 bc | 2.05 ± 0.45 ab | 3.81 ± 0.27 d | 4.68 ± 0.08 e |
| Glutamic acid | 1.06 ± 0.12 a | 5.66 ± 0.07 d | 1.94 ± 0.12 ac | 2.71 ± 0.04 bc | 1.43 ± 1.27 a | 3.38 ± 0.02 b | 3.4 ± 0.06 b |
| Tyrosine | 3.34 ± 0.36 a | 3.41 ± 0.08 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| HAA | 13.41 ± 0.43 a | 43.81 ± 0.16 b | 24.02 ± 0.13 c,A | 17.24 ± 0.5 d,A | 15.07 ± 0.33 e,A | 18.84 ± 0.11 f,A | 22.62 ± 0.28 g,A |
| AAA | 4.62 ± 0.86 c | 8.68 ± 0.23 d | 3.43 ± 0.15 b,A | 2.62 ± 0.31 ab | 1.95 ± 0.45 a,A | 2.77 ± 0.27 ab,A | 2.89 ± 0.13 ab,A |
| EAA | 7.69 ± 0.11 b | 36.19 ± 0.17 f | 22.47 ± 0.2 a,A | 15.54 ± 0.23 d,A | 13.44 ± 1.8 c,A | 17.87 ± 0.41 e,A | 21.56 ± 1.27 a,A |
| TAA | 17.48 ±1.03 b | 64.19± 0.1 f | 32.96 ± 0.23 a,A | 26.04 ± 0.82 d,A | 21.45 ± 1.41 c,A | 31.15 ± 0.44 a,A | 37.47 ± 1.24 e,A |
| Alanine | 1.68 ± 0.17 a | 6.55 ± 0.19 e | 2.91 ± 0.26 bc | 1.92 ± 0.17 a | 3.04 ± 0.06 c | 3.61 ± 0.19 d | 2.54 ± 0.12 b |
| Glycine | 0.32 ± 0.15 b | 0.97 ± 0.05 c | 3.13 ± 0.27 d | 0.79 ± 0.15 ac | 0.65 ± 0.03 ac | 0.59 ± 0.05 ab | 0.48 ± 0.08 ab |
| Valine | 1.77 ± 0.2 c | 8.62 ± 0.42 e | 3.07 ± 0.3 a | 3.07 ± 0.2 a | 4.75 ± 0.65 b | 4.99 ± 0.71 b | 6.73 ± 0.8 d |
| Leucine | 2.52 ± 0.14 c | 11.26 ± 0.13 e | 3.84 ± 0.33 a | 3.59 ± 0.14 a | 5.89 ± 0.86 b | 5.86 ± 0.13 b | 7.18 ± 0.65 d |
| Isoleucine | 1.36 ± 0.12 c | 6.02 ± 0.01 e | 2.09 ± 0.18 a | 2.19 ± 0.12 a | 3.41 ± 0.47 b | 3.61 ± 0.01 b | 4.31 ± 0.3 d |
| Proline | 1.46 ± 0.08 b | 2.68 ± 0.06 c | 4.62 ± 0.24 d | 3.29 ± 0.08 a | 3.26 ± 0.01 a | 3.31 ± 0.06 a | 7.57 ± 0.15 e |
| Serine | 0.28 ± 0.03 b | 2.87 ± 0.11 f | 2.23 ± 0.08 e | 0.62 ± 0.03 c | 1.04 ± 0.11 a | 1.62 ± 0.11 d | 1.03 ± 0 a |
| Threonine | 0.76 ± 0.05 b | 5.02 ± 0.05 f | 2.65 ± 0.12 a | 1.39 ± 0.05 c | 2.49 ± 0.03 a | 2.95 ± 0.2 d | 3.3 ± 0.11 e |
| Phenylalanine | 1.27 ± 0.06 b | 5.27 ± 0.11 d | 1.97 ± 1.48 ab | 1.67 ± 0.06 ab | 3.15 ± 0.37 ac | 2.9 ± 0.74 ac | 4.21 ± 0.03 cd |
| Aspartic acid | 1.65 ± 0.11 c | 5.87 ± 0.08 e | 3.73 ± 0.45 a | 0.71 ± 0.11 b | 2.54 ± 0.57 d | 3.65 ± 0.4 a | 3.64 ± 0.15 a |
| Glutamic acid | 1.06 ± 0.12 b | 5.66 ± 0.07 c | 3.28 ± 0.71 a | 0.64 ± 0.12 b | 2.83 ± 1.19 a | 3.99 ± 0.08 a | 2.8 ± 0.69 a |
| Tyrosine | 3.34 ± 0.36 a | 3.41 ± 0.08 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0.73 b |
| HAA | 13.41 ±0.43 b | 43.81 ± 0.16 f | 18.51 ± 0.1 d,B | 15.73 ± 0.43 c,B | 23.5 ± 0.43 a,B | 24.28 ± 0.25 a,B | 32.54 ± 1.34 e,B |
| AAA | 4.62 ± 0.86 b | 8.68 ± 0.23 a | 1.97 ± 0.08 b,B | 1.67 ± 0.06 b,B | 3.15 ± 0.09 c,B | 2.9 ± 0.02 c,A | 4.21 ± 0.15 b,B |
| EAA | 7.69 ± 0.11 b | 36.19 ± 0.17 f | 13.63 ± 0.27 d,B | 11.9 ± 0.11 c,B | 19.69 ± 0.57 a,B | 20.31 ± 0.01 a,B | 25.73 ± 1.61 e,B |
| TAA | 17.48 ±1.03 b | 64.19 ± 0.1 f | 33.53 ± 0.33 a,A | 19.88 ± 1.03 c,B | 33.04 ± 0.1 a,B | 37.08 ± 0.23 d,B | 43.8 ± 0.17 e,B |
| IR Spectral Bands (cm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Hydrolysis Time (min) | Amide A | Amide B | Amide I | Amide II | CH3 Bending | Amide III | C-O |
| LPC | 0 | 3280.3 | 2923.0 | 1605.0 | 1517.2 | nd | 1235.2 | 1062.1 |
| H–Pep | 30 | 3305.9 | 2918.0 | 1636.0 | 1594.8 | 1398.6 | 1257.4 | 1065.0 |
| 60 | 3289.0 | 2919.0 | 1635.0 | 1598.7 | 1395.3 | 1258.8 | 1065.0 | |
| 120 | nd | nd | 1636.0 | 1596.3 | 1399.1 | 1253.5 | 1064.0 | |
| 180 | 3283.2 | 2917.0 | 1636.0 | 1596.3 | 1395.3 | 1255.9 | 1062.1 | |
| 240 | 3289.0 | 2961.4 | 1636.8 | 1540.8 | 1399.6 | 1260.2 | 1078.0 | |
| H–Pan | 30 | 3247.1 | 2936.0 | 1635.0 | 1599.2 | 1387.1 | 1242.4 | 1069.3 |
| 60 | 3288.0 | 2925.0 | 1636.3 | 1599.7 | 1388.5 | 1235.2 | 1069.3 | |
| 120 | 3323.2 | 2925.0 | 1636.0 | 1598.7 | 1389.9 | 1242.9 | 1068.4 | |
| 180 | 3239.0 | nd | 1636.0 | 1596.3 | 1388.1 | 1238.1 | 1068.9 | |
| 240 | 3262.0 | nd | 1636.0 | 1598.2 | 1388.0 | 1243.9 | 1067.4 | |
| Sample | Hydrolysis Time (min) | Stages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | Final Residue (%) | ||||||
| Temperature Range (°C) | Weight Loss (%) | Temperature Range (°C) | Weight Loss (%) | Temperature Range (°C) | Weight Loss (%) | Temperature Range (°C) | Weight Loss (%) | |||
| LPC | 0 | 28.3–39.2 ± 0.23 a | 3.93 ± 0.03 a | 252.4–370.4 ± 0.18 a | 61.65 ± 0.80 a | *** | *** | *** | *** | 34.42 ± 0.12 a |
| H–Pep | 30 | 34.5–47.0 ± 0.13 b,A | 1.97 ± 0.02 b,A | 230.7–357.2 ± 0.07 b,A | 23.60 ± 0.03 b,A | 736.5–774.6 ± 0.26 a,A | 67.58 ± 0.21 a,A | *** | *** | 6.85 ± 0.07 b,A |
| 60 | 35.3–36.9 ± 0.22 c,A | 1.93 ± 0.13 b,A | 228.2–334.8 ± 0.43 c,A | 28.92 ± 0.12 c,A | 736.6–764.8 ± 0.15 b,A | 57.33 ± 0.41 b,A | *** | *** | 11.82 ± 0.18 c,A | |
| 120 | 30.2–47.6 ± 0.16 d,A | 4.33 ± 0.02 c,A | 192.2–295.8 ± 0.32 d,A | 27.50 ± 0.14 d,A | 725.2–767.7 ± 0.08 c,A | 64.27 ± 0.26 c,A | *** | *** | 3.90 ± 0.23 d,A | |
| 180 | 30.8–42.4 ± 0.25 e,A | 5.11 ± 0.10 d,A | 202.0–318.4 ± 0.13 e,A | 24.61 ± 0.22 e,A | 426.4–515.5 ± 0.23 d,A | 21.79 ± 0.05 d,A | 719.7–762.6 ± 0.33 a | 42.32 ± 0.21 a | 6.18 ± 0.17 e,A | |
| 240 | 30.3–37.1 ± 0.06 c,A | 4.94 ± 0.07 e,A | 227.2–328.6 ± 0.26 f,A | 25.54 ± 0.32 f,A | 439.1–524.1 ± 0.29 e,A | 21.36 ± 0.39 d,A | 727.4–761.6 ± 0.27 a | 47.60 ± 0.32 b | 0.56 ± 0.05 f,A | |
| H–Pan | 30 | 35.2–45.2 ± 0.02 b,B | 5.13 ± 0.12 b,B | 218.5–353.8 ± 0.11 b,B | 44.27 ± 0.07 b,B | 746.6–785.3 ± 0.39 a,B | 18.61 ± 0.12 a,B | *** | *** | 31.99 ± 0.12 b,B |
| 60 | 35.0–45.8 ± 0.17 b,B | 5.55 ± 0.15 b,B | 210.4–334.1 ± 0.34 c,A | 44.13 ± 0.14 b,B | 763.5–784.9 ± 0.23 a,B | 23.82 ± 0.19 b,B | *** | *** | 26.51 ± 0.18 c,B | |
| 120 | 31.6–47.1 ± 0.09 c,B | 7.94 ± 0.13 c,B | 211.3–322.9 ± 0.18 d,B | 34.74 ± 0.21 c,B | 461.8–605.0 ± 0.36 c,B | 37.50 ± 0.07 c,B | *** | *** | 19.82 ± 0.11 d,B | |
| 180 | 32.0–46.8 ± 0.18 d,B | 7.32 ± 0.28 d,B | 220.8–327.0 ± 0.25 e,B | 38.94 ± 0.56 d,B | 456.1–595.4 ± 0.19 d,B | 41.92 ± 0.11 d,B | *** | *** | 11.83 ± 0.15 e,B | |
| 240 | 32.8–41.9 ± 0.21 e,B | 6.54 ± 0.02 e,B | 213.2–321.3 ± 0.71 f,B | 37.73 ± 0.97 e,B | 429.4–518.8 ± 0.22 e,B | 44.27 ± 0.16 e,B | *** | *** | 11.46 ± 0.45 e,B | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Chiu, C.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; Díaz, J.A.; Ragazzo-Sánchez, J.A. Structural Modification of Jackfruit Leaf Protein Concentrate by Enzymatic Hydrolysis and Their Effect on the Emulsifier Properties. Colloids Interfaces 2022, 6, 52. https://doi.org/10.3390/colloids6040052
Calderón-Chiu C, Calderón-Santoyo M, Barros-Castillo JC, Díaz JA, Ragazzo-Sánchez JA. Structural Modification of Jackfruit Leaf Protein Concentrate by Enzymatic Hydrolysis and Their Effect on the Emulsifier Properties. Colloids and Interfaces. 2022; 6(4):52. https://doi.org/10.3390/colloids6040052
Chicago/Turabian StyleCalderón-Chiu, Carolina, Montserrat Calderón-Santoyo, Julio César Barros-Castillo, José Alfredo Díaz, and Juan Arturo Ragazzo-Sánchez. 2022. "Structural Modification of Jackfruit Leaf Protein Concentrate by Enzymatic Hydrolysis and Their Effect on the Emulsifier Properties" Colloids and Interfaces 6, no. 4: 52. https://doi.org/10.3390/colloids6040052
APA StyleCalderón-Chiu, C., Calderón-Santoyo, M., Barros-Castillo, J. C., Díaz, J. A., & Ragazzo-Sánchez, J. A. (2022). Structural Modification of Jackfruit Leaf Protein Concentrate by Enzymatic Hydrolysis and Their Effect on the Emulsifier Properties. Colloids and Interfaces, 6(4), 52. https://doi.org/10.3390/colloids6040052