Lysozyme Influence on Monolayers of Individual and Mixed Lipids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Techniques
3. Results
3.1. Surface Lysozyme Behaviour
3.2. Influence of Lysozyme over Lipid Monolayers
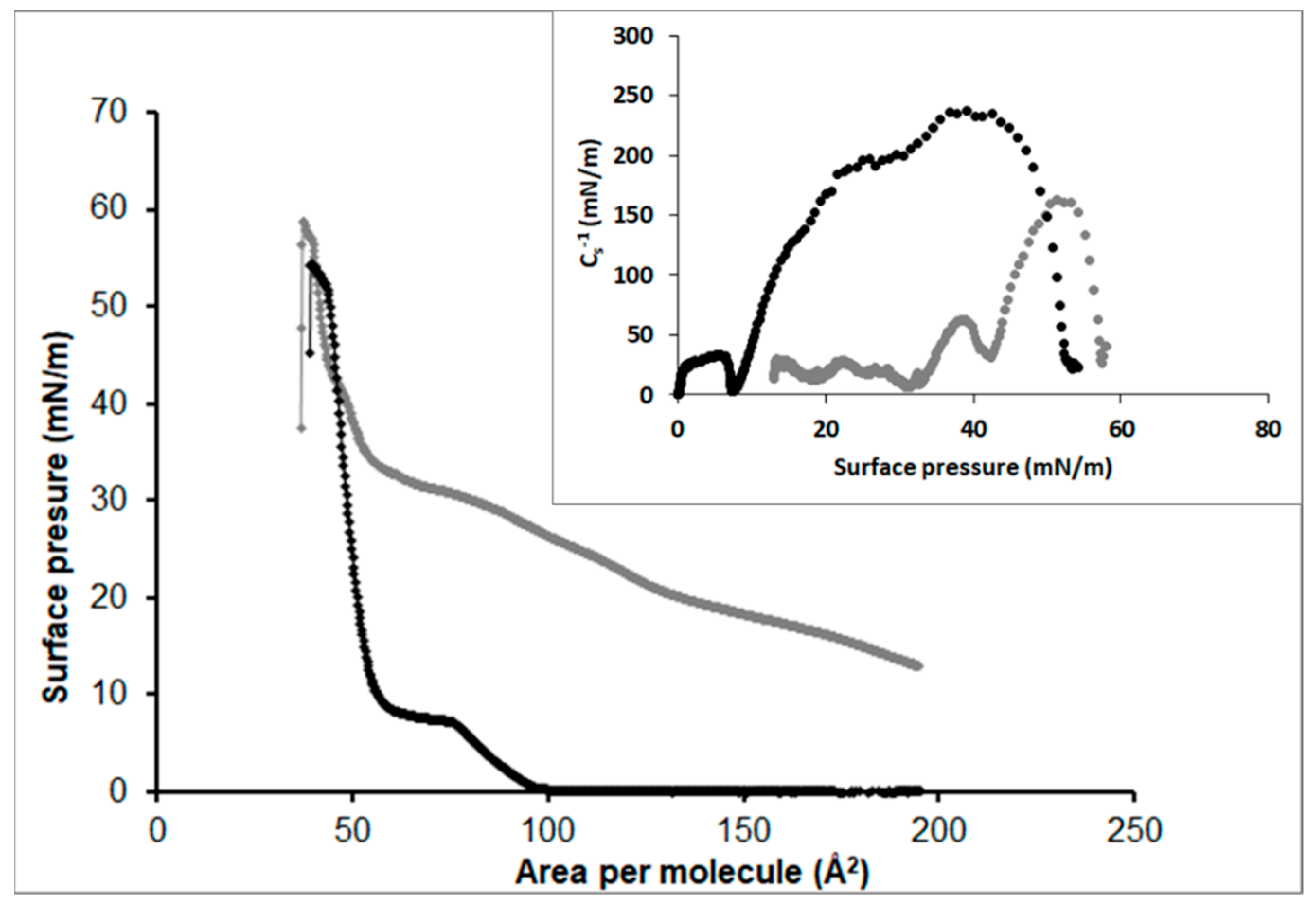
3.2.1. Stearic Acid
3.2.2. Oleic Acid
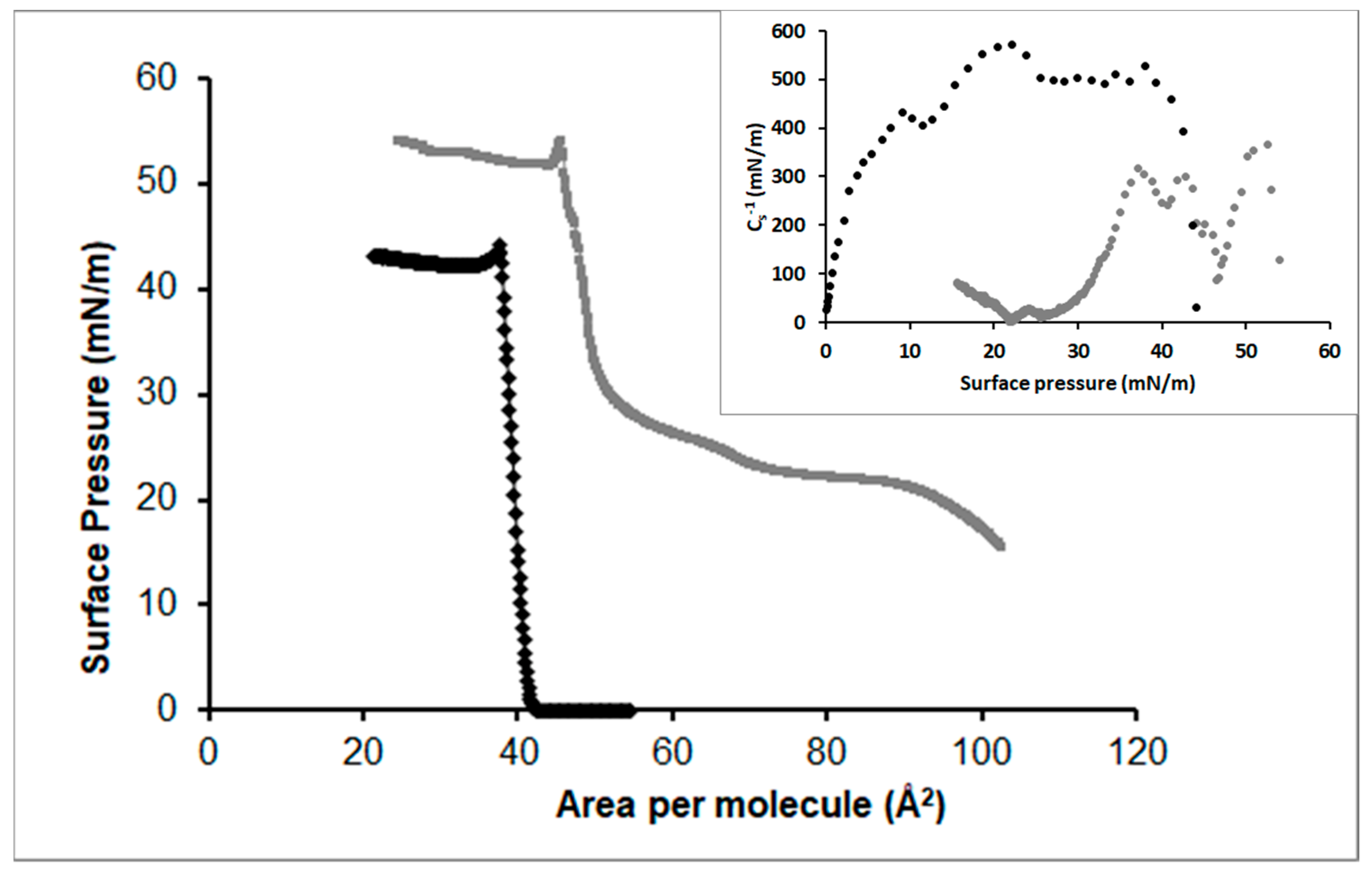
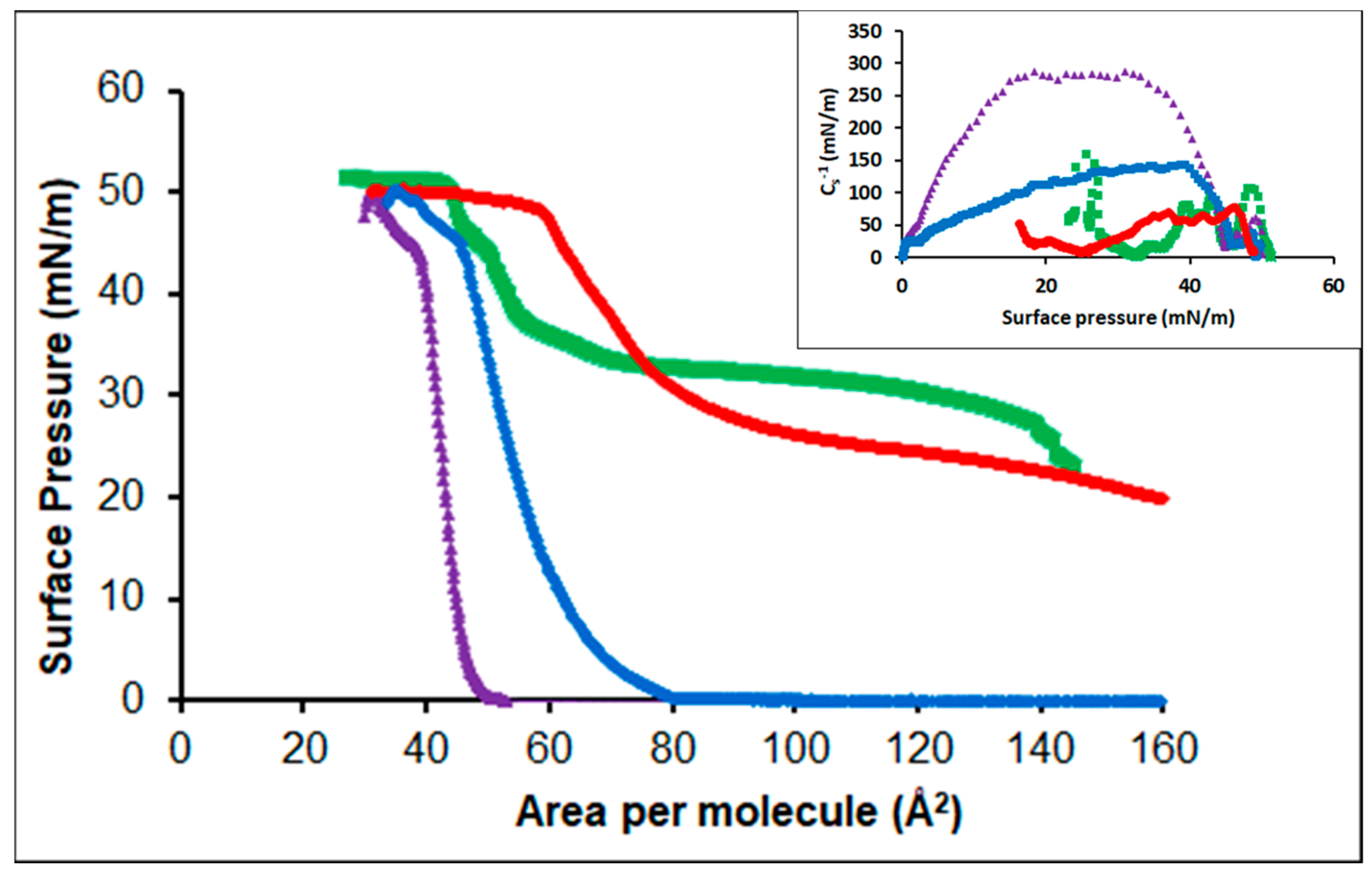
3.2.3. DPPC
3.2.4. POPC
3.2.5. Cholesterol
3.3. Influence of Lysozyme over Mixed Lipid Monolayers
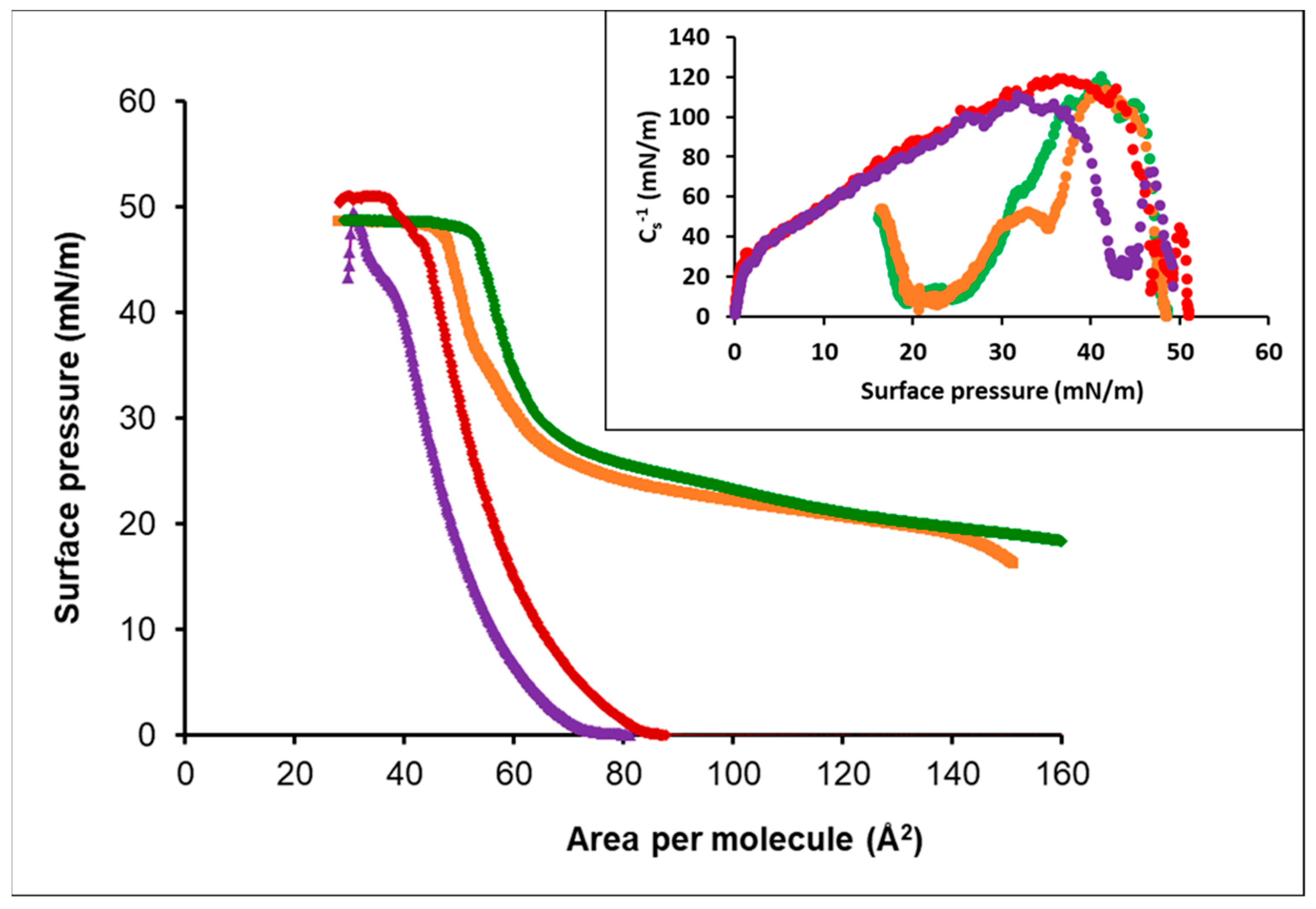
3.3.1. Oleic Acid-DPPC
3.3.2. Oleic Acid-POPC
3.3.3. Cholesterol-POPC
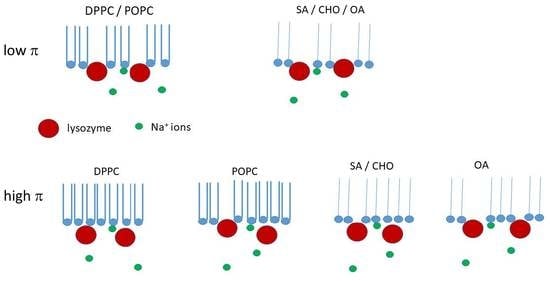
4. Discussion
4.1. Individual Lipids
4.2. Mixed Lipids
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Ulman, A. An Introduction to Ultrathin Organic Films; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Petty, M.C. Langmuir-Blodgett Films: An Introduction; Cambridge University Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Richardson, T.H. (Ed.) Functional Organic and Polymeric Materials: Molecular Functionality-Macroscopic Reality; Wiley: Chichester, UK, 2000. [Google Scholar]
- Torrent-Burgués, J. Thermodynamic behaviour of mixed films of an unsaturated and a saturated polar lipid. (Oleic acid-stearic acid and POPC-DPPC). Colloids Interfaces 2018, 2, 17. [Google Scholar] [CrossRef]
- Eftaiha, A.F.; Brunet, S.M.K.; Paige, M.F. Thermodynamic and structural characterization of a mixed perfluorocarbon–phospholipid ternary monolayer surfactant system. J. Colloid Interface Sci. 2012, 368, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Gzyl-Malcher, B.; Paluch, M. Studies of lipid interactions in mixed Langmuir monolayers. Thin Solid Films 2008, 516, 8865–8872. [Google Scholar] [CrossRef]
- Hac-Wydro, K.; Wydro, P. The influence of fatty acids on model cholesterol-phospholipd membranes. Chem. Phys. Lipids 2007, 150, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Hac-Wydro, K.; Jedrzejek, K.; Dynarowicz-Łatka, P. Effect of saturation degree on the interactions between fatty acids and phosphatidylcholines in binary and ternary Langmuir monolayers. Colloids Surf. B Biointerfaces 2009, 72, 101–111. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Sun, R.; Hao, C.; Yang, J.; Wang, M.; Zhang, L. Thermodynamic analysis and AFM study of the interaction of palmitic acid with DPPE in Langmuir monolayers. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 184–194. [Google Scholar] [CrossRef]
- Hao, C.; Sun, R.; Zhang, J. Mixed onolayers of DOPC and palmitic acid at the liquid–air interface. Colloids Surf. B Biointerfaces 2013, 112, 441–445. [Google Scholar] [CrossRef]
- Hoda, K.; Nakahara, H.; Nakamura, S.; Nagadome, S.; Sugihara, G.; Yoshino, N.; Shibata, O. Langmuir monolayer properties of the fluorinated-hydrogenated hybrid amphiphiles with dipalmitoylphosphatidylcholine (DPPC). Colloids Surf. B Biointerfaces 2006, 47, 165–175. [Google Scholar] [CrossRef]
- Hoda, K.; Kawasaki, H.; Yoshino, N.; Chang, C.-H.; Morikawa, Y.; Sugihara, G.; Shibata, O. Mode of interaction of two fluorinated-hydrogenated hybrid amphiphiles with dipalmitoylphosphatidylcholine (DPPC) at the air-water interface. Colloids Surf. B Biointerfaces 2006, 53, 37–50. [Google Scholar] [CrossRef]
- Nakahara, H.; Nakamura, S.; Kawasaki, H.; Shibata, O. Properties of two-component Langmuir monolayer of single chain perfluorinated carboxylic acids with dipalmitoylphosphatidylcholine (DPPC). Colloids Surf. B Biointerfaces 2005, 41, 285–298. [Google Scholar] [CrossRef]
- Petelska, A.D.; Figaszewski, Z.A. The equilibria of phosphatidylcholine–fatty acid and phosphatidylcholine–amine in monolayers at the air/water interface. Colloids Surf. B Biointerfaces 2011, 82, 340–344. [Google Scholar] [CrossRef]
- Torrent-Burgués, J. Oleamide and oleamide-lipid mixed monolayers. Bionanoscience 2011, 1, 202–209. [Google Scholar] [CrossRef]
- Miñones, J., Jr.; Pais, S.; Miñones, J.; Conde, O.; Dynarowicz-Łątka, P. Interactions between membrane sterols and phospholipids in model mammalian and fungi cellular membranes—A Langmuir monolayer study. Biophys. Chem. 2009, 140, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-H.; Chang, C.-H. Thermodynamic behavior and relaxation processes of mixed DPPC/cholesterol monolayers at the air/water interface. Colloids Surf. B 2000, 17, 71–79. [Google Scholar] [CrossRef]
- Drolle, E.; Kucerka, N.; Hoopes, M.I.; Choi, Y.; Katsaras, J.; Karttunen, M.; Leonenko, Z. Effect of melatonin and cholesterol on the structure of DOPC and DPPC membranes. Biochim. Biophys. Acta 2013, 1828, 2247–2254. [Google Scholar] [CrossRef]
- Dynarowicz-Łatka, P.; Haç-Wydro, K. Interactions between phosphatidylcholines and colesterol in monolayers at the air/water interface. Colloids Surf. B 2004, 37, 21–25. [Google Scholar] [CrossRef]
- Haç-Wydro, K.; Wydro, P.; Dynarowicz-Łatka, P.; Paluch, M. Cholesterol and phytosterols effect on sphingomyelin/phosphatidylcholine model Membranes-Thermodynamic analysis of the interactions in ternary monolayers. J. Colloid Interface Sci. 2009, 329, 265–272. [Google Scholar] [CrossRef]
- Korchowiec, B.; Paluch, M.; Corvis, Y.; Rogalska, E. A Langmuir film approach to elucidating interactions in lipid membranes: 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine/cholesterol/metal cation Systems. Chem. Phys. Lipids 2006, 144, 127–136. [Google Scholar] [CrossRef]
- Wydro, P. The influence of cholesterol on multicomponent Langmuir monolayers imitating outer and inner leaflet of human erythrocyte membrane. Colloids Surf. B 2013, 103, 67–74. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Retzlaff, I.; Won, J.Y.; Gunes, D.Z.; Gochev, G.; Gehin-Delval, C.; Leser, M.; Noskov, B.A.; Miller, R. β-Lactoglobulin adsorption layers at the water/air surface: 2. Dilational rheology: Effect of pH and ionic strength. Colloids Surf. A 2017, 521, 167–176. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Gaillard, C.; Boué, F.; Axelos, M.A.V.; Riaublanc, A. Self-similar assemblies of globular whey proteins at the air–water interface: Effect of the structure. J. Colloid Interface Sci. 2010, 345, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Miñones-Conde, M.; Conde, O.; Trillo, J.M.; Miñones, J., Jr. How to obtain a well-spread monolayer of lysozyme at the air-water interfaces. J. Colloid Interface Sci. 2011, 361, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.K.; Kundu, S. Modification of hysteresis behaviors of protein monolayer and the corresponding structures with the variation of the protein surface charges. Colloids Surf. B 2017, 159, 696–704. [Google Scholar] [CrossRef]
- Tihonov, M.M.; Milyaeva, O.Y.; Noskov, B.A. Dynamic surface properties of lysozyme solutions. Impact of urea and guanidine hydrochloride. Colloids Surf. B 2015, 129, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Chernysheva, M.G.; Badun, G.A.; Shnitko, A.V.; Petrova, V.I.; Ksenofontov, A.L. Lysozyme-surfactant adsorption at the aqueous-air and aqueous-organic liquid interfaces as studied by tritium probe. Colloids Surf. A 2018, 537, 351–360. [Google Scholar] [CrossRef]
- Girard-Egrot, A.P.; Godoy, S.; Blum, L.J. Enzyme association with lipidic Langmuir–Blodgett films: Interests and applications in nanobioscience. Adv. Colloid Interface Sci. 2005, 116, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Kamylya, T.; Pal, P.; Talapatra, G.B. Incorporation of ovalbumin within cationic octadecylamine monolayer and a comparative study with zwitterionic DPPC and anionic stearic acid monolayer. J. Colloid Interface Sci. 2007, 315, 464–474. [Google Scholar] [CrossRef]
- Lai, L.; Wei, X.-Q.; Huang, W.-H.; Mei, P.; Ren, Z.-H.; Liu, Y. Impact of carbon quantum dots on dynamic properties of BSA and BSA/DPPC adsorption layers. J. Colloid Interface Sci. 2017, 506, 245–254. [Google Scholar] [CrossRef]
- Nishimura, S.Y.; Magana, G.M.; Ketelson, H.A.; Fuller, G.G. Effect of Lysozyme Adsorption on the Interfacial Rheology of DPPC and Cholesteryl Myristate Films. Langmuir 2008, 24, 11728–11733. [Google Scholar] [CrossRef]
- Ohno, M.; Toyota, T.; Nomoto, T.; Fujinami, M. Interfacial tension in adsorption of lysozyme onto a lipid monolayer formed at a water/choloform interface. Colloids Surf. A 2015, 480, 85–90. [Google Scholar] [CrossRef]
- Vázquez, R.F.; Daza Millone, M.A.; Pavinatto, F.J.; Herlax, V.S.; Bakás, L.S.; Oliveira, O.N., Jr.; Vela, M.E.; Maté, S.M. Interaction of acylated and unacylated forms of E. coli alpha-hemolysin with lipid monolayers: A PM-IRRAS study. Colloids Surf. B 2017, 158, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Derde, M.; Nau, F.; Lechevalier, V.; Guérin-Dubiard, C.; Paboeuf, G.; Jan, S.; Baron, F.; Gautier, M.; Vié, V. Native lysozyme and dry-heated lysozyme interactions with membrane lipid monolayers: Lateral reorganization of LPS monolayer, model of the Escherichia coli outer membrane. Biochim. Biophys. Acta 2015, 1848, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Derde, M.; Nau, F.; Guérin-Dubiard, C.; Lechevalier, V.; Paboeuf, G.; Jan, S.; Baron, F.; Gautier, M.; Vié, V. Native and dry-heated lysozyme interactions with membrane lípid monolayers: Lipid packing modifications of a phospholipid mixture, model of the Escherichia coli cytoplasmic membrane. Biochim. Biophys. Acta 2015, 1848, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Gorbenko, G.P.; Ioffe, V.M.; Kinnunen, P.K.J. Binding of Lysozyme to Phospholipid Bilayers: Evidence for Protein Aggregation upon Membrane Association. Biophys. J. 2007, 93, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, C.; Pezennec, S.; Bouchoux, A.; Cabane, B.; Lechevalier, V.; Le Floch-Fouéré, C.; Paboeuf, G.; Pasco, M.; Dollet, B.; Lee, L.-T.; et al. Protein Transport upon Advection at the Air/Water Interface: When Charge Matters. Langmuir 2021, 37, 12278–12289. [Google Scholar] [CrossRef]
- Torrent-Burgués, J.; Raju, R. Effect of lysozyme subphase and insertion on several lipid films. Adv. Mater. Sci. 2019, 4, 1–7. [Google Scholar]
- Nobre, T.M.; Pavinatto, F.J.; Caselli, L.; Barros-Timmons, A.; Dynarowicz-Latka, P.; Oliveira, O.N., Jr. Interactions of bioactive molecules & nanomaterials with Langmuir monolayers as cell membrane models. Thin Solid Films 2015, 593, 158–188. [Google Scholar]
- Torrent-Burgués, J.; Hoyo, J.; Tzanov, T. Lipid artificial tears at a mimetic ocular interface. Chem. Phys. Lipids 2021, 238, 105087. [Google Scholar] [CrossRef]
- Crawford, N.F.; Micic, M.; Orbulescu, J.; Weissbart, D.; Leblanc, R.M. Surface chemistry and spectroscopy of the β-galactosidase Langmuir monolayer. J. Colloid Interface Sci. 2015, 453, 202–208. [Google Scholar] [CrossRef]
- Krajewska, B.; Wydro, P.; Janczyk, A. Probing the Modes of Antibacterial Activity of Chitosan. Effects of pH and Molecular Weight on Chitosan Interactions with Membrane Lipids in Langmuir Films. Biomacromolecules 2011, 12, 4144–4152. [Google Scholar] [CrossRef]
- Vitovič, P.; Nikolelis, D.P.; Hianik, T. Study of calix [4] resorcinarene–dopamine com-plexation in mixed phospholipid monolayers formed at the air|water interface. Biochim. Biophys. Acta 2006, 1758, 1852–1861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davies, J.T.; Rideal, E.K. Interfacial Phenomena; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Caseli, L.; Crespilho, F.N.; Nobre, T.M.; Zaniquelli, M.D.; Zucolotto, V.; Oliveira, O.N., Jr. Using phospholipid Langmuir and Langmuir–Blodgett films as matrix for urease immobilization. J. Colloid Interface Sci. 2008, 319, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D. Lateral pressure in membranes. Biochim. Biophys. Acta 1996, 1286, 183–223. [Google Scholar] [CrossRef]
- Tomoaia-Cotisol, M.; Zsako, J.; Mocanu, A.; Lupea, M.; Chifu, E. Insoluble mixed monolayers: III. The ionization characteristics of some fatty acids at the air/water interface. J. Colloid Interface Sci. 1987, 117, 464–476. [Google Scholar] [CrossRef]





| Π (mN·m−1) | OA-DPPC 1 | OA-DPPC 2 | OA-POPC 3 | OA-POPC 4 | CHO-POPC 5 | CHO-POPC 6 |
|---|---|---|---|---|---|---|
| 5 | −8.2 | −6.8 | −7.1 | −5.0 | −14.9 | −11.3 |
| 15 | −0.9 | −0.7 | −5.1 | −3.3 | −10.1 | −8.2 |
| 25 | −1.4 | −4.2 | −4.2 | −2.3 | −7.4 | −5.9 |
| 35 | −5.7 | −4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrent-Burgués, J. Lysozyme Influence on Monolayers of Individual and Mixed Lipids. Colloids Interfaces 2022, 6, 15. https://doi.org/10.3390/colloids6010015
Torrent-Burgués J. Lysozyme Influence on Monolayers of Individual and Mixed Lipids. Colloids and Interfaces. 2022; 6(1):15. https://doi.org/10.3390/colloids6010015
Chicago/Turabian StyleTorrent-Burgués, Juan. 2022. "Lysozyme Influence on Monolayers of Individual and Mixed Lipids" Colloids and Interfaces 6, no. 1: 15. https://doi.org/10.3390/colloids6010015
APA StyleTorrent-Burgués, J. (2022). Lysozyme Influence on Monolayers of Individual and Mixed Lipids. Colloids and Interfaces, 6(1), 15. https://doi.org/10.3390/colloids6010015