Polymeric Surfactant P84/Polyoxometalate α-PW12O403−—A Model System to Investigate the Interplay between Chaotropic and Hydrophobic Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cloud Point (CP) Measurements/Phase Diagrams
2.3. H-Nuclear Magnetic Resonance (1H-NMR)
2.4. Small Angle X-ray Scattering (SAXS)
2.5. Small Angle Neutron Scattering (SANS)
2.6. Light Scattering
3. Results and Discussion
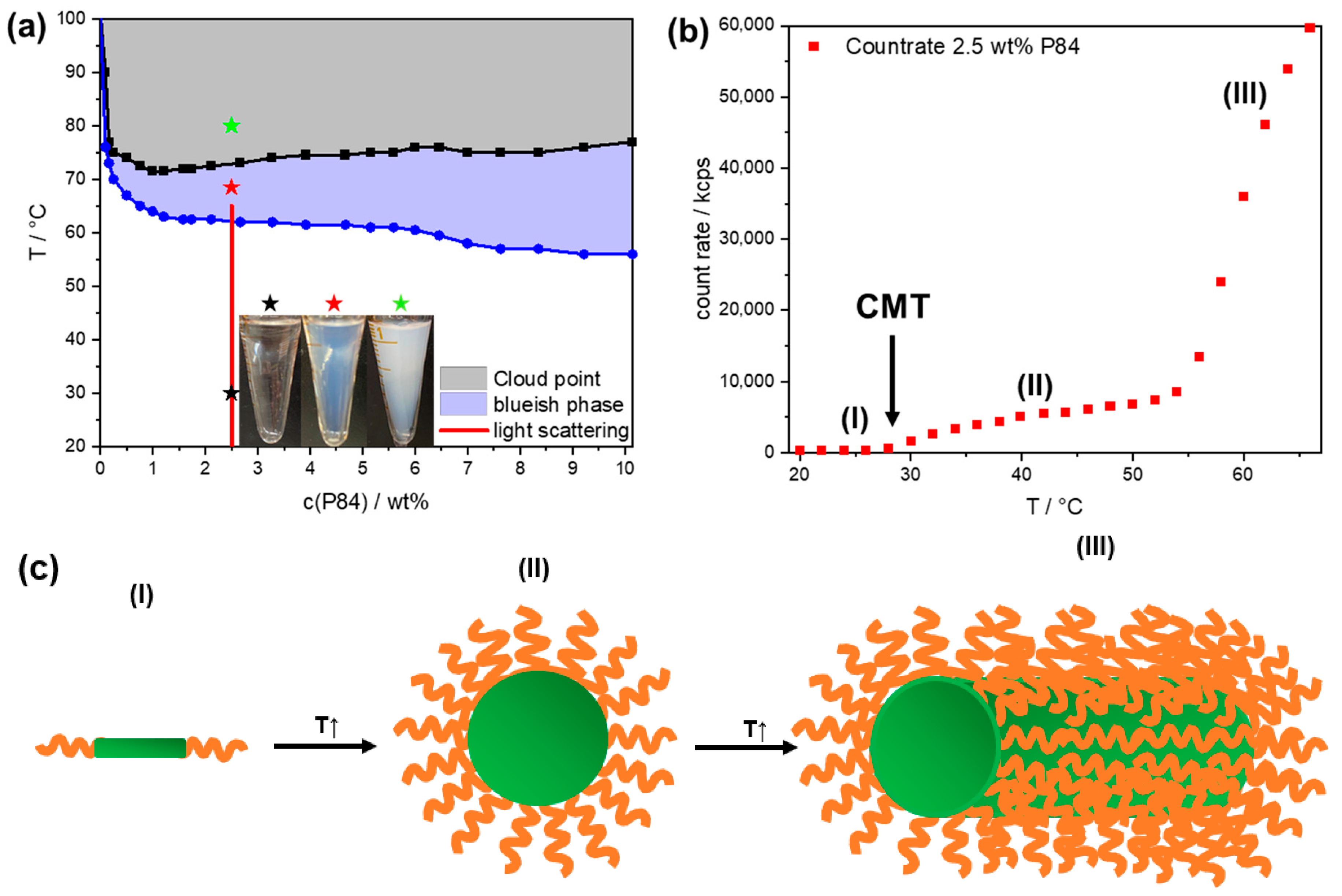
3.1. P84: Phase Diagram and Aggregation
3.2. P84: Cloud Point Evolution upon Addition of HPW
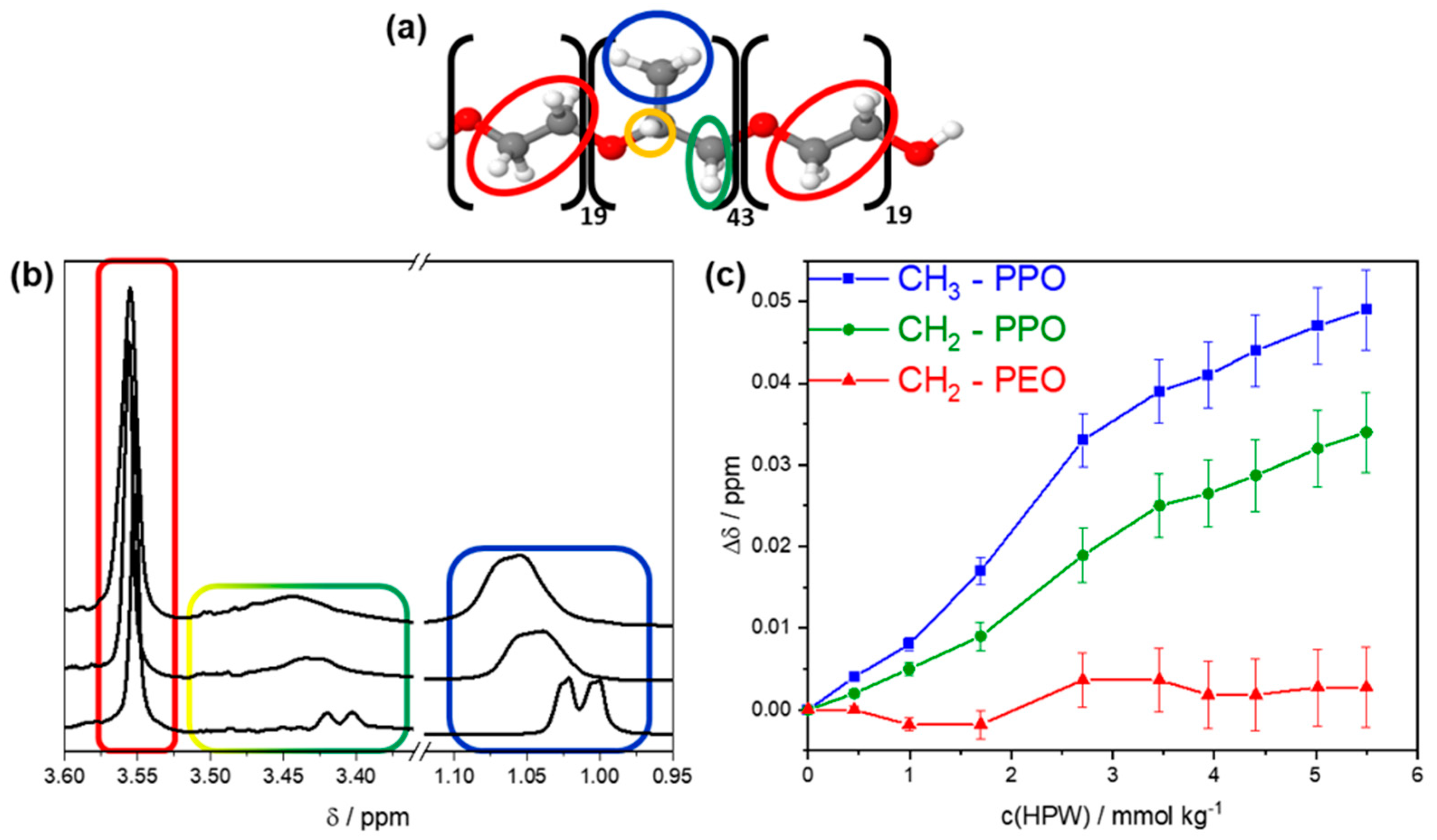
3.3. Association of HPW with P84 Unimers
- (i)
- The CH2 protons of PEO produced a sharp singlet at 3.55 ppm.
- (ii)
- The quaternary proton of PPO produced a multiplet at around 3.49 ppm as it coupled with all the neighboring CH2/CH3 protons in PPO via a 3J coupling.
- (iii)
- The CH2 protons of PPO were detected at around 3.41 ppm as a doublet that was overlayed by the multiplet of the PPO quaternary proton (ii).
- (iv)
- The CH3 protons of PPO produced a doublet at 1.01 ppm.
3.4. Association of HPW with P84 Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernardini, G.; Wedd, A.G.; Zhao, C.; Bond, A.M. Photochemical oxidation of water and reduction of polyoxometalate anions at interfaces of water with ionic liquids or diethylether. Proc. Natl. Acad. Sci. USA 2012, 109, 11552–11557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Krickl, S.; Buchecker, T.; Meyer, A.U.; Grillo, I.; Touraud, D.; Bauduin, P.; König, B.; Pfitzner, A.; Kunz, W. A systematic study of the influence of mesoscale structuring on the kinetics of a chemical reaction. Phys. Chem. Chem. Phys. 2017, 19, 23773–23780. [Google Scholar] [CrossRef] [PubMed]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327–357. [Google Scholar] [CrossRef]
- Fukuma, M.; Seto, Y.; Yamase, T. In vitro antiviral activity of polyoxotungstate (PM-19) and other polyoxometalates against herpes simplex virus. Antivir. Res. 1991, 16, 327–339. [Google Scholar] [CrossRef]
- Stephan, H.; Kubeil, M.; Emmerling, F.; Müller, C.E. Polyoxometalates as versatile enzyme inhibitors. Eur. J. Inorg. Chem. 2013, 2013, 1585–1594. [Google Scholar] [CrossRef]
- Yamase, T. Photo-and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–326. [Google Scholar] [CrossRef]
- Bijelic, A.; Rompel, A. The use of polyoxometalates in protein crystallography-An attempt to widen a well-known bottleneck. Coord. Chem. Rev. 2015, 299, 22–38. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.; Kozma, K.; Streb, C.; Nyman, M. Beyond charge balance: Counter-cations in polyoxometalate chemistry. Angew. Chem. Int. Ed. 2019, 59, 596–612. [Google Scholar] [CrossRef] [Green Version]
- Bera, M.K.; Qiao, B.; Seifert, S.; Burton-Pye, B.P.; Olvera De La Cruz, M.; Antonio, M.R. Aggregation of heteropolyanions in aqueous solutions exhibiting short-range attractions and long-range repulsions. J. Phys. Chem. C 2016, 120, 1317–1327. [Google Scholar] [CrossRef]
- Naskar, B.; Diat, O.; Nardello-Rataj, V.; Bauduin, P. Nanometer-size polyoxometalate anions adsorb strongly on neutral soft surfaces. J. Phys. Chem. C 2015, 119, 20985–20992. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888, 25, 1–30. [Google Scholar] [CrossRef]
- Kunz, W.; Henle, J.; Ninham, B.W. “Zur Lehre von der Wirkung der Salze” (about the science of the effect of salts): Franz Hofmeister’s historical papers. Curr. Opin. Colloid Interface Sci. 2004, 9, 19–37. [Google Scholar] [CrossRef]
- Assaf, K.I.; Ural, M.S.; Pan, F.; Georgiev, T.; Simova, S.; Rissanen, K.; Gabel, D.; Nau, W.M. Water structure recovery in chaotropic anion recognition: High-affinity binding of dodecaborate clusters to γ-cyclodextrin. Angew. Chem. Int. Ed. 2015, 54, 6852–6856. [Google Scholar] [CrossRef] [Green Version]
- Buchecker, T.; Schmid, P.; Renaudineau, S.; Diat, O.; Proust, A.; Pfitzner, A.; Bauduin, P. Polyoxometalates in the Hofmeister series. Chem. Commun. 2018, 54, 1833–1836. [Google Scholar] [CrossRef] [PubMed]
- Assaf, K.I.; Nau, W.M. The chaotropic effect as an assembly motif in chemistry. Angew. Chem. Int. Ed. 2018, 57, 13968–13981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, B.; An, S.; Luo, J.; Liu, T.; Song, Y.-F. Enhanced macroanion recognition of superchaotropic keggin clusters achieved by synergy of anion–π and anion–cation interactions. Chem. A Eur. J. 2020, 26, 16802–16810. [Google Scholar] [CrossRef] [PubMed]
- Merhi, A.T.; Jonchère, A.; Girard, L.; Diat, O.; Nuez, M.; Viñas, C.; Bauduin, P. Highlights on the Binding of Cobalta-Bis-(Dicarbollide) with Glucose Units. Chem. A Eur. J. 2020, 26, 13935–13947. [Google Scholar] [CrossRef]
- Hohenschutz, M.; Grillo, I.; Diat, O.; Bauduin, P. How nano-ions act like ionic surfactants. Angew. Chem. Int. Ed. 2020, 59, 8084–8088. [Google Scholar] [CrossRef]
- Buchecker, T.; LeGoff, X.; Naskar, B.; Pfitzner, A.; Diat, O.; Bauduin, P. Polyoxometalate/polyethylene glycol interactions in water: From nano-assemblies in water to crystal formation by electrostatic screening. Chem. A Eur. J. 2017, 23, 8434–8442. [Google Scholar] [CrossRef]
- Buchecker, T.; Schmid, P.; Grillo, I.; Prévost, S.; Drechsler, M.; Diat, O.; Pfitzner, A.; Bauduin, P. Self-assembly of short chain Poly-N-isopropylacrylamid (PNIPAM) induced by superchaotropic Keggin Polyoxometalates: From globules to sheets. J. Am. Chem. Soc. 2019, 141, 6890–6899. [Google Scholar] [CrossRef]
- Falaise, C.; Moussawi, M.A.; Floquet, S.; Abramov, P.A.; Sokolov, M.N.; Haouas, M.; Cadot, E. Probing dynamic library of metal-oxo building blocks with γ-cyclodextrin. J. Am. Chem. Soc. 2018, 140, 11198–11201. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Falaise, C.; Ivanov, A.A.; Leclerc, N.; Hohenschutz, M.; Haouas, M.; Landy, D.; Shestopalov, M.A.; Bauduin, P.; Cadot, E. Hofmeister effect in the Keggin-type polyoxotungstate series. Inorg. Chem. Front. 2021, 8, 12–25. [Google Scholar] [CrossRef]
- Assaf, K.I.; Begaj, B.; Frank, A.; Nilam, M.; Mougharbel, A.S.; Kortz, U.; Nekvinda, J.; Grüner, B.; Gabel, D.; Nau, W.M. High-affinity binding of metallacarborane cobalt bis(dicarbollide) anions to cyclodextrins and application to membrane translocation. J. Org. Chem. 2019, 84, 11790–11798. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Buchecker, T.; Khoshsima, A.; Touraud, D.; Diat, O.; Kunz, W.; Pfitzner, A.; Bauduin, P. Self-assembly of a short amphiphile in water controlled by superchaotropic Polyoxometalates: H4SiW12O40 vs. H3PW12O40. J. Coll. Interface Sci. 2021, 587, 347–357. [Google Scholar] [CrossRef]
- Grillo, I.; Morfin, I.; Prévost, S. Structural characterization of pluronic micelles swollen with perfume molecules. Langmuir 2018, 34, 13395–13408. [Google Scholar] [CrossRef]
- Šturcová, A.; Schmidt, P.; Dybal, J. Role of hydration and water coordination in micellization of Pluronic block copolymers. J. Colloid Interface Sci. 2010, 352, 415–423. [Google Scholar] [CrossRef]
- Álvarez-Ramírez, J.G.; Fernández, V.V.A.; Macías, E.R.; Rharbi, Y.; Taboada, P.; Gámez-Corrales, R.; Puig, J.E.; Soltero, J.F.A. Phase behavior of the Pluronic P103/water system in the dilute and semi-dilute regimes. J. Coll. Interface Sci. 2009, 333, 655–662. [Google Scholar] [CrossRef]
- Jain, N.J.; Aswal, V.K.; Goyal, P.S.; Bahadur, P. Salt induced micellization and micelle structures of PEO/PPO/PEO block copolymers in aqueous solution. Colloids Surf. A 2000, 173, 85–94. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Prévost, S.; Gradzielski, M. Solubilisation of oils of different polarity in aqueous solutions of pluronic triblock copolymers. Z. Phys. Chem. 2012, 226, 675–694. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Yue, M.B.; Chun, Y.; Cao, Y.; Dong, X.; Zhu, J.H. CO2 capture by as-prepared SBA-15 with an occluded organic template. Adv. Funct. Mater. 2006, 16, 1717–1722. [Google Scholar] [CrossRef]
- Bronich, T.K.; Bontha, S.; Shlyakhtenko, L.S.; Bromberg, L.E.; Hatton, V.; Kabanov, T.A.A.V. Template-assisted synthesis of nanogels from Pluronic-modified poly (acrylic acid). J. Drug Target 2006, 14, 357–366. [Google Scholar] [CrossRef]
- Dewhurst, C.D.; Grillo, I.; Honecker, D.; Bonnaud, M.; Jacques, M.; Amrouni, C.; Perillo-Marcone, A.; Manzin, G.; Cubitt, R. The small-angle neutron scattering instrument D33 at the Institut Laue–Langevin. J. Appl. Crystallogr. 2016, 49, 1–14. [Google Scholar] [CrossRef]
- Patel, T.; Bahadur, P.; Mata, J. The clouding behaviour of PEO-PPO based triblock copolymers in aqueous ionic surfactant solutions: A new approach for cloud point measurements. J. Colloid Interface Sci. 2010, 345, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymers association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Khimani, M.; Rao, U.; Bahadur, P.; Bahadur, P. Calorimetric and scattering studies on micellization of pluronics in aqueous solutions: Effect of the size of hydrophilic PEO end blocks, temperature, and added salt. J. Dispers. Sci. Technol. 2014, 35, 1599–1610. [Google Scholar] [CrossRef]
- Mohan, J. Organic Spectroscopy: Principles and Applications, 2nd ed.; Alpha Science: Paris, France, 2004. [Google Scholar]
- Rogers, B.A.; Okur, H.I.; Yan, C.; Yang, T.; Heyda, J.; Cremer, P.S. Weakly hydrated anions bind to polymers but not monomers in aqueous solutions. Nat. Chem. 2022, 14, 40–45. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Huang, J.S. Small-Angle Neutron Scattering Analysis of the Structure and Interaction of Triblock Copolymer Micelles in Aqueous Solution. Macromolecules 1998, 9297, 2236–2244. [Google Scholar] [CrossRef]
- Hayter, J.B.; Penfold, J. An analytic structure factor for macroion solutions. Mol. Phys. 1981, 42, 109–118. [Google Scholar] [CrossRef]
- Hansen, J.; Hayter, J.B. A rescaled MSA structure factor for dilute charged colloidal dispersions. Mol. Phys. 1982, 46, 651–656. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, P.; Graß, X.; Bahadur, P.; Grillo, I.; Diat, O.; Pfitzner, A.; Bauduin, P. Polymeric Surfactant P84/Polyoxometalate α-PW12O403−—A Model System to Investigate the Interplay between Chaotropic and Hydrophobic Effects. Colloids Interfaces 2022, 6, 16. https://doi.org/10.3390/colloids6010016
Schmid P, Graß X, Bahadur P, Grillo I, Diat O, Pfitzner A, Bauduin P. Polymeric Surfactant P84/Polyoxometalate α-PW12O403−—A Model System to Investigate the Interplay between Chaotropic and Hydrophobic Effects. Colloids and Interfaces. 2022; 6(1):16. https://doi.org/10.3390/colloids6010016
Chicago/Turabian StyleSchmid, Philipp, Xaver Graß, Pratap Bahadur, Isabelle Grillo, Olivier Diat, Arno Pfitzner, and Pierre Bauduin. 2022. "Polymeric Surfactant P84/Polyoxometalate α-PW12O403−—A Model System to Investigate the Interplay between Chaotropic and Hydrophobic Effects" Colloids and Interfaces 6, no. 1: 16. https://doi.org/10.3390/colloids6010016
APA StyleSchmid, P., Graß, X., Bahadur, P., Grillo, I., Diat, O., Pfitzner, A., & Bauduin, P. (2022). Polymeric Surfactant P84/Polyoxometalate α-PW12O403−—A Model System to Investigate the Interplay between Chaotropic and Hydrophobic Effects. Colloids and Interfaces, 6(1), 16. https://doi.org/10.3390/colloids6010016