Electrical Conductivity and Viscosity in Binary Organic Liquid Mixtures: Participation of Molecular Interactions and Nanodomains
Abstract
:1. Introduction
1.1. Electrical Conduction in Non-Aqueous Liquids
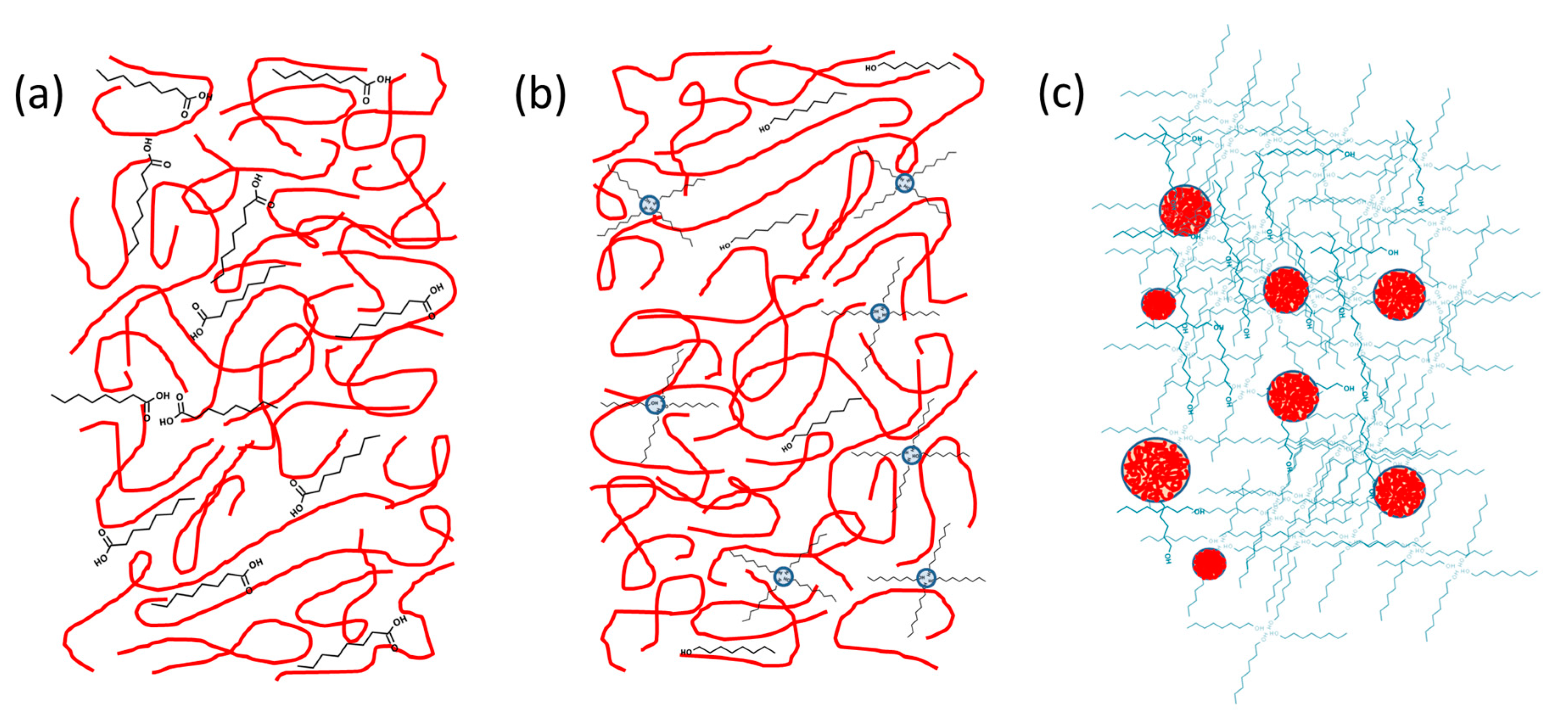
1.2. Evidence for Liquid Nanostructure
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
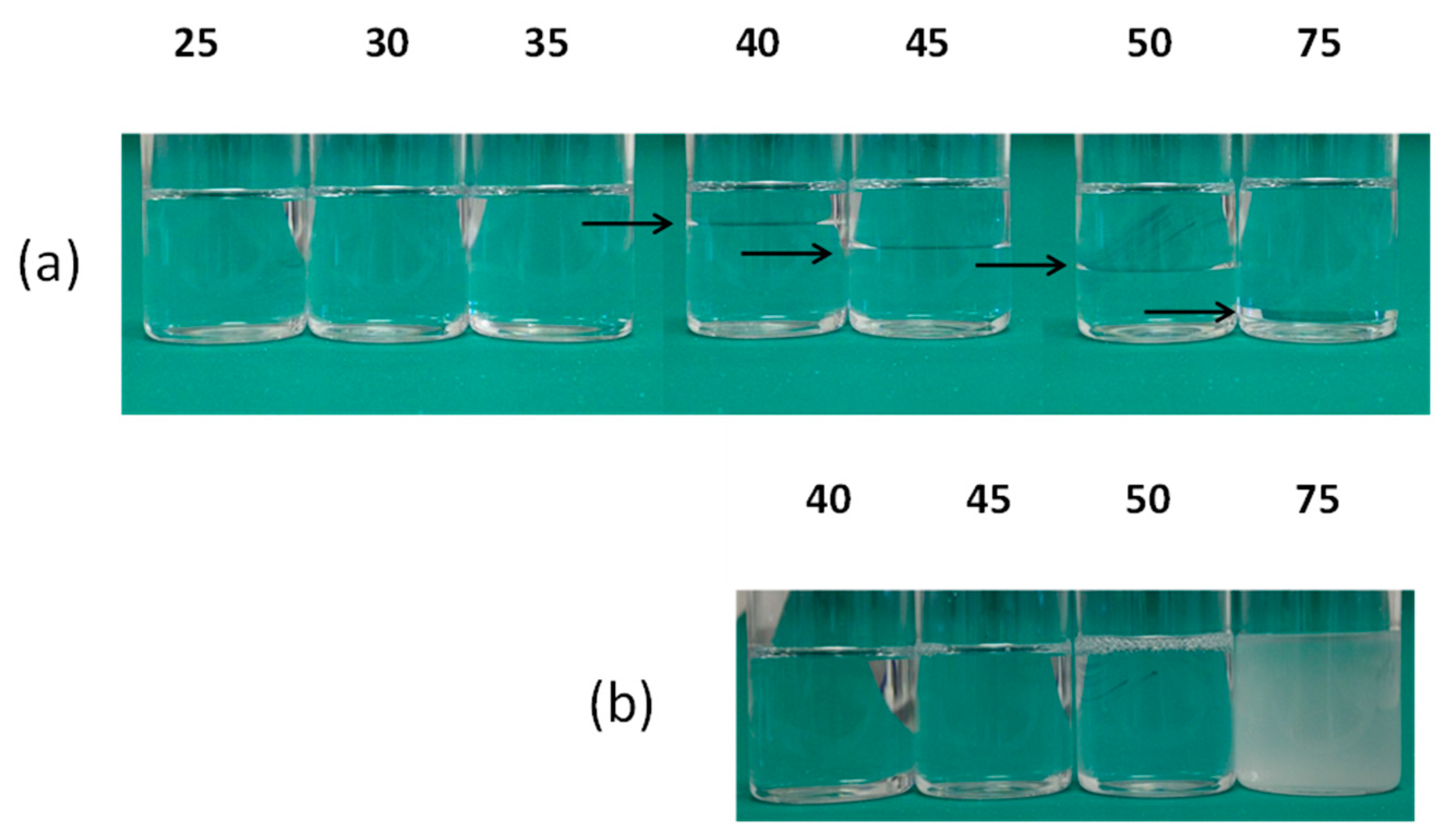
3.1. Properties of the Liquids
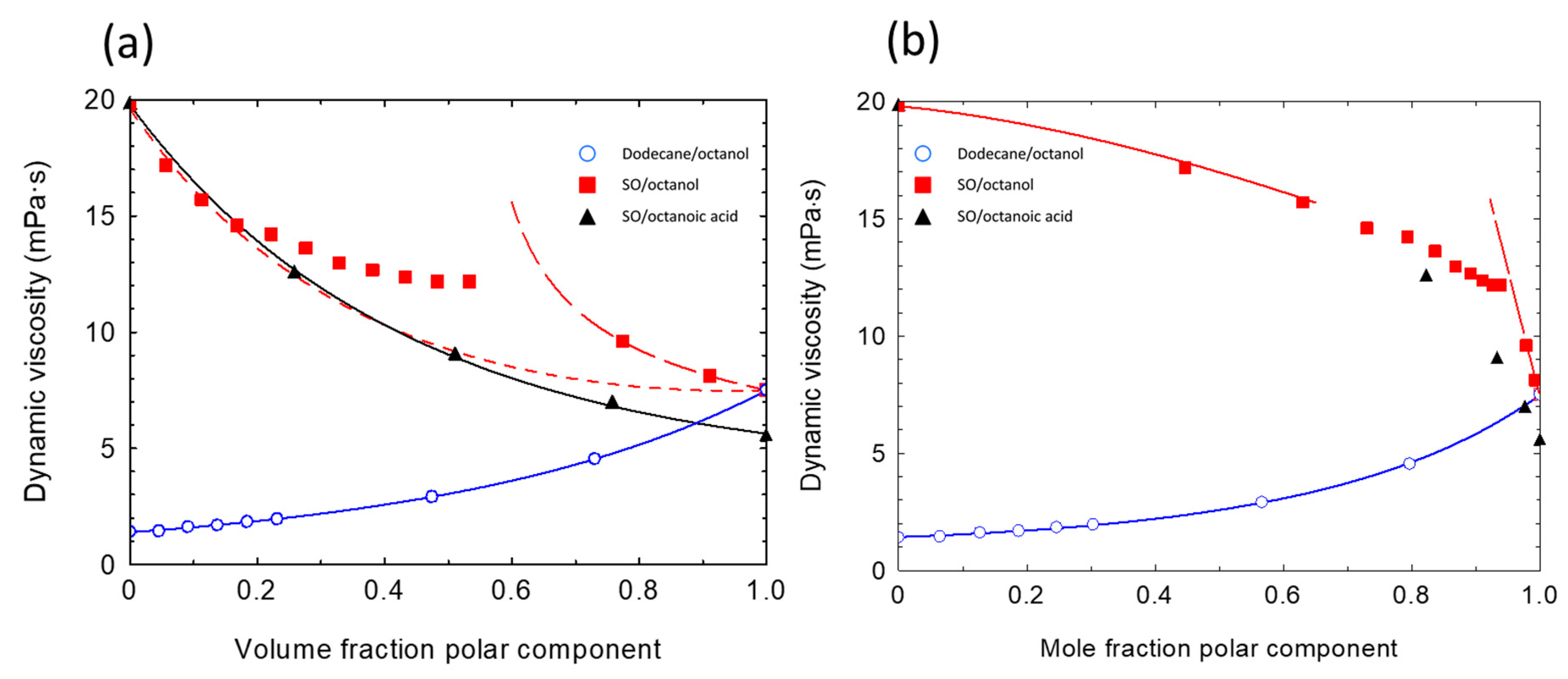
3.2. Dynamic Viscosity of Binary Mixtures
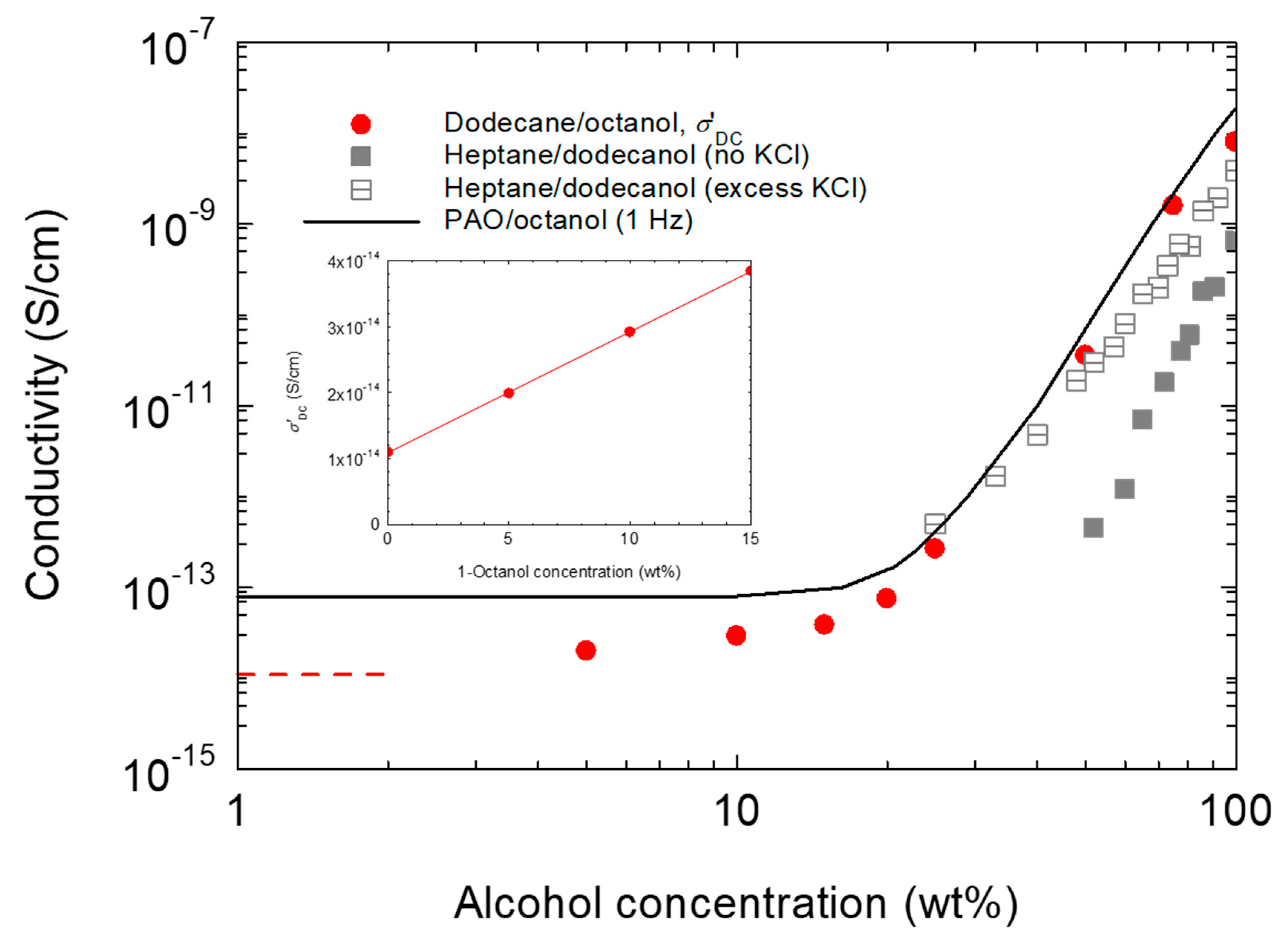
3.3. Dielectric and Electrical Conductivity Behavior of n-Dodecane/1-Octanol Mixtures
4. Discussion
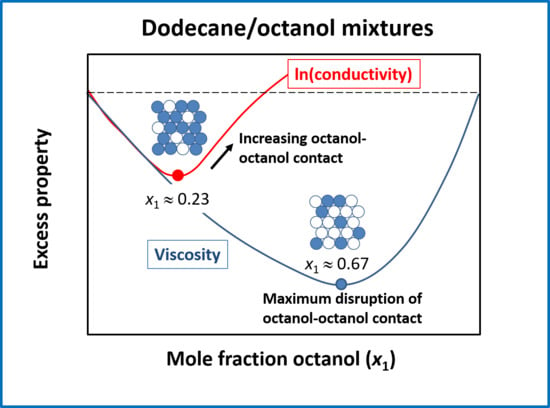
4.1. Evidence of Molecular Interactions from Mixture Viscosity
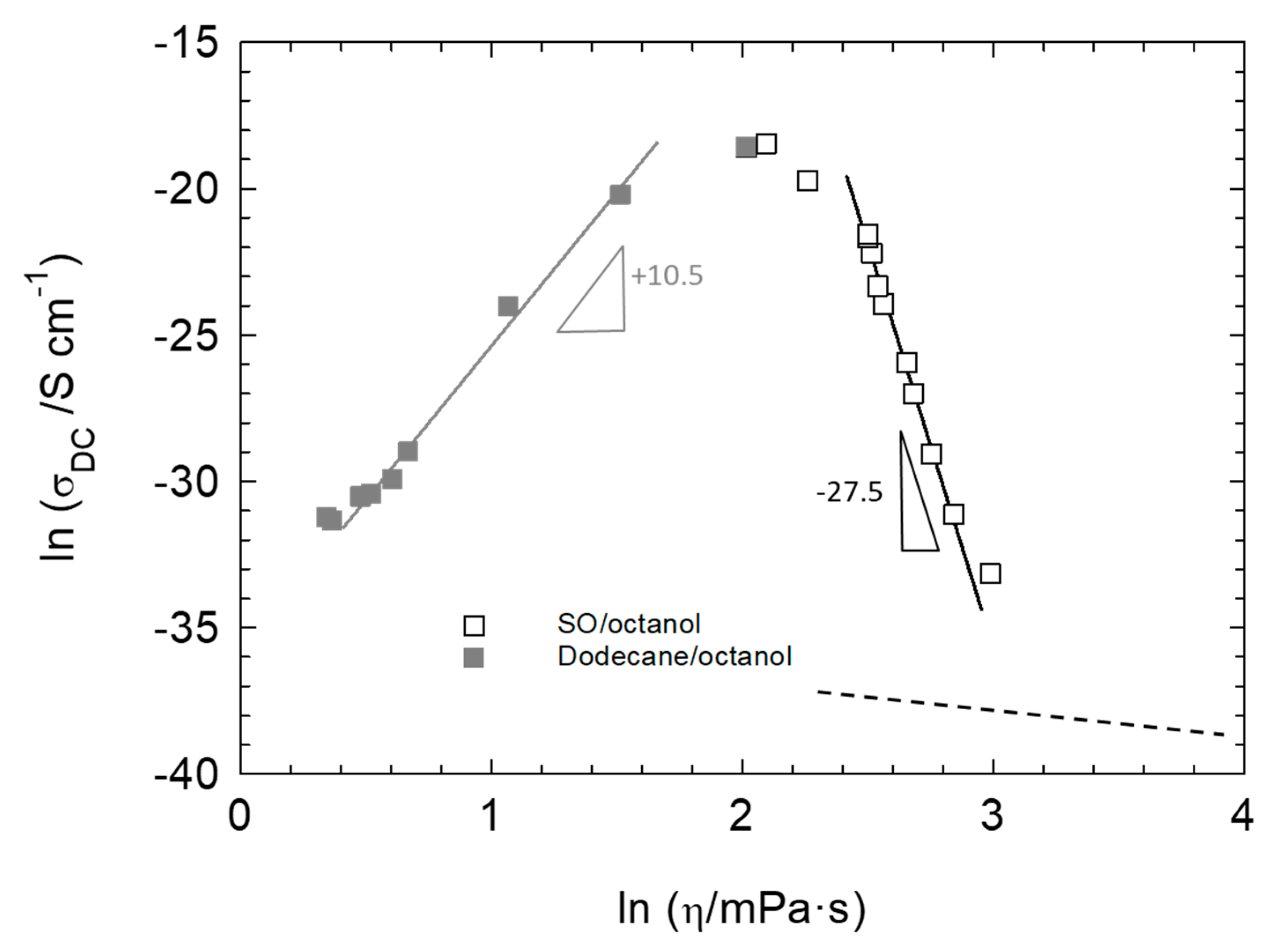
4.2. Electrical Conductivity Considerations
4.3. Relationship between Structure and Interactions, Viscosity, and Electrical Conductivity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berman, P.; Meiri, N.; Colnago, L.A.; Moraes, T.B.; Linder, C.; Levi, O.; Parmet, Y.; Saunders, M.; Wiesman, Z. Study of liquid-phase molecular packing interactions and morphology of fatty acid methyl esters (biodiesel). Biotechnol. Biofuels 2015, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, G.; Zong, H.; Yu, Q.; Lin, R. Thermal conductivity of alcohols with acetonitrile and N,N-dimethylformamide. J. Chem. Eng. Data 1993, 38, 332–335. [Google Scholar] [CrossRef]
- Tobitani, A.; Tanaka, T. Predicting thermal conductivity of binary liquid mixtures on basis of coordination number coordination model. Can. J. Chem. Eng. 1987, 65, 321–328. [Google Scholar] [CrossRef]
- Apelblat, A. The concept of associated solutions in historical development. Part 1. The 1884–1984 period. J. Mol. Liq. 2006, 128, 1–31. [Google Scholar] [CrossRef]
- Palombo, F.; Sassi, P.; Paolantoni, M.; Morresi, A.; Cataliotti, R.S. Comparison of hydrogen bonding in 1-octanol and 2-octanol as probed by spectroscopic techniques. J. Phys. Chem. B 2006, 110, 18017–18025. [Google Scholar] [CrossRef]
- Cevc, G.; Berts, I.; Fischer, S.F.; Rädler, J.O.; Nickel, B. Nanostructures in n-octanol equilibrated with additives and/or water. Langmuir 2018, 34, 6285–6295. [Google Scholar] [CrossRef]
- Wrzeszcz, W.; Tomza, P.; Kwasniewicz, M.; Mazurek, S.; Czarnecki, M.A. Microheterogeneity in binary mixtures of aliphatic alcohols and alkanes: ATR-IR/NIR spectroscopic and chemometric studies. RSC Adv. 2016, 6, 94294–94300. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Zhou, Y.; Shen, J.; Ji, Z.; Cheng, G. Microheterogeneous structure of 1-octanol in neat and water-saturated state. J. Phys. Chem. B 2007, 111, 10160–10165. [Google Scholar] [CrossRef]
- Henkel, S.; Misuraca, M.C.; Troselj, P.; Davidson, J.; Hunter, C.A. Polarisation effects on the solvation properties of alcohols. Chem. Sci. 2018, 9, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Hermens, J.L.M.; de Bruijn, J.H.M.; Brooke, D.N. The octanol-water partition coefficient: Strengths and limitations. Environ. Toxicol. Chem. 2013, 32, 732–733. [Google Scholar] [CrossRef]
- Dzida, M.; Marczak, W. Thermodynamic and acoustic properties of binary mixtures of alcohols and alkanes. II. Density and heat capacity of (ethanol + n-heptane) under elevated pressures. J. Chem. Thermodyn. 2005, 37, 826–836. [Google Scholar] [CrossRef]
- Dubey, G.P.; Sharma, M. Study of molecular interactions in binary liquid mixtures of 1-octanol with n-hexane, n-octane, and n-decane using volumetric, viscometric, and acoustic properties. J. Chem. Thermodyn. 2008, 40, 991–1000. [Google Scholar] [CrossRef]
- Estrada-Baltazar, A.; Iglesias-Silva, G.A.; Caballero-Cerón, C. Volumetric and transport properties of binary mixtures of n-octane + ethanol, +1-propanol, +1-butanol, and +1-pentanol from (293.15 to 323.15) K at atmospheric pressure. J. Chem. Eng. Data 2013, 58, 3351–3363. [Google Scholar] [CrossRef]
- Guzmán-López, A.; Iglesias-Silva, G.A.; Reyes-García, F.; Estrada-Baltazar, A. Densities and viscosities for binary liquid mixtures of n-undecane + 1-heptanol, 1-octanol, 1-nonanol, and 1-decanol from 283.15 to 363.15 K at 0.1 MPa. J. Chem. Eng. Data 2017, 62, 780–795. [Google Scholar] [CrossRef]
- Dannhauser, W. Dielectric relaxation in isomeric octyl alcohols. J. Chem. Phys. 1968, 48, 1918–1923. [Google Scholar] [CrossRef]
- Campbell, C.; Brink, G.; Glasser, L. Dielectric studies of molecular association. Concentration dependence of dipole moment of 1-octanol in solution. J. Phys. Chem. 1975, 79, 660–665. [Google Scholar] [CrossRef]
- Schwerdtfeger, S.; Köhler, F.; Pottel, R.; Kaatze, U. Dielectric relaxation of hydrogen bonded liquids: Mixtures of monohydric alcohols with n-alkanes. J. Chem. Phys. 2001, 115, 4186–4194. [Google Scholar] [CrossRef]
- Daruich, Y.; Magallanes, C.; Catenaccio, A. Dependence of permittivity of diluted octanol on temperature. Mol. Phys. 2001, 99, 1607–1611. [Google Scholar] [CrossRef]
- Kaatze, U.; Behrends, R.; Pottel, R. Hydrogen network fluctuations and dielectric spectrometry of liquids. J. Non Cryst. Solids 2002, 305, 19–28. [Google Scholar] [CrossRef]
- Wang, L.-M.; Shahriari, S.; Richert, R. Diluent effects on the Debye-type dielectric relaxation in viscous monohydroxy alcohols. J. Phys. Chem. B 2005, 109, 23255–23262. [Google Scholar] [CrossRef]
- Gainaru, C.; Meier, R.; Schildmann, S.; Lederle, C.; Hiller, W.; Rössler, E.A.; Böhmer, R. Nuclear-magnetic-resonance measurements reveal the origin of the Debye process in monohydroxy alcohols. Phys. Rev. Lett. 2010, 105, 258303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yomogida, Y.; Sato, Y.; Nozaki, R.; Mishina, T.; Nakahara, J. Comparative dielectric study of monohydric alcohols with terahertz time-domain spectroscopy. J. Mol. Struct. 2010, 981, 173–178. [Google Scholar] [CrossRef]
- Kaatze, U.; Behrends, R. Hydrogen bond fluctuations and dispersive interactions of alcohol/alkane mixtures. An ultrasonic relaxation study. Chem. Phys. Lett. 2011, 510, 67–72. [Google Scholar] [CrossRef]
- Power, G.; Nagaraj, M.; Vij, J.K.; Johari, G.P. Debye process and dielectric state of an alcohol in a nonpolar solvent. J. Chem. Phys. 2011, 134, 044525. [Google Scholar] [CrossRef] [PubMed]
- Jadżyn, J.; Świergiel, J. The viscous consequence of different trends in clustering of 1,2-diol and 1,n-diol molecules. Phys. Chem. Chem. Phys. 2018, 20, 21640–21646. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, L.; Lincoln, A.T. The dissociative power of solvents. J. Phys. Chem. 1899, 3, 12–35. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, A.T. The electrical conductivity of non-aqueous solutions. J. Phys. Chem. 1899, 3, 457–494. [Google Scholar] [CrossRef] [Green Version]
- Shaw, L.I. Studies on the electrical conductance of non-aqueous solutions. J. Phys. Chem. 1913, 17, 162–176. [Google Scholar] [CrossRef]
- Kraus, C.A.; Fuoss, R.M. Properties of electrolytic solutions. I. Conductance as influenced by the dielectric constant of the solvent medium. J. Am. Chem. Soc. 1933, 55, 21–36. [Google Scholar] [CrossRef]
- Freemantle, M. Introduction to Ionic Liquids; RSC Publishing: Cambridge, UK, 2010; p. 36. [Google Scholar]
- Harris, K.R. On the use of the Angell−Walden equation to determine the “ionicity” of molten salts and ionic liquids. J. Phys. Chem. B 2019, 123, 7014–7023. [Google Scholar] [CrossRef]
- Bombard, A.J.F.; Dukhin, A. Ionization of a nonpolar liquid with an alcohol. Langmuir 2014, 30, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- Dukhin, A.; Parlia, S. Ion-pair conductivity theory fitting measured data for various alcohol-toluene mixtures across entire concentration range. J. Electrochem. Soc. 2015, 162, H256–H263. [Google Scholar] [CrossRef]
- Gourdin-Bertin, S.; Chassagne, C. Application of classical thermodynamics to the conductivity in non-polar media. J. Chem. Phys. 2016, 144, 244501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourdin-Bertin, S.; Chassagne, C. Application of classical thermodynamics to conductivity in nonpolar media: Experimental confirmation. J. Phys. Chem. B 2018, 122, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Parlia, S.; Dukhin, A.; Somasundaram, P. Ion-pair conductivity theory: Mixtures of butanol with various non-polar liquids and water. J. Electrochem. Soc. 2016, 163, H570–H575. [Google Scholar] [CrossRef]
- Franks, N.P.; Abraham, M.H.; Lieb, W.R. Molecular organization of liquid n-octanol: An X-ray diffraction analysis. J. Pharm. Sci. 1993, 82, 466–470. [Google Scholar] [CrossRef]
- Tomšič, M.; Jamnik, A.; Fritz-Popovski, G.; Glatter, O.; Vlček, L. Structural properties of pure simple alcohols from ethanol, propanol, butanol, pentanol, to hexanol: Comparing Monte Carlo simulations with experimental SAXS data. J. Chem. Phys. B 2007, 111, 1738–1751. [Google Scholar] [CrossRef]
- MacCallum, J.L.; Tieleman, D.P. Structures of neat and hydrated 1-octanol from computer simulations. J. Am. Chem. Soc. 2002, 124, 15085–15093. [Google Scholar] [CrossRef]
- Tikhonov, A.M.; Pingali, S.V.; Schlossman, M.L. Molecular ordering and phase transitions in alkanol monolayers at the water–hexane interface. J. Chem. Phys. 2004, 120, 11822–11838. [Google Scholar] [CrossRef]
- Fletcher, A.N.; Heller, C.A. Self-association of alcohols in nonpolar solvents. J. Phys. Chem. 1967, 71, 3742–3756. [Google Scholar] [CrossRef]
- Aveyard, R.; Briscoe, B.J.; Chapman, J. Activity coefficients and association of n-alkanols in n-octane. J. Chem. Soc. Faraday Trans. I 1973, 69, 1772–1778. [Google Scholar] [CrossRef]
- Czarnecki, M.A. Effect of temperature and concentration on self-association of octan-1-ol studied by two-dimensional Fourier Transform near-infrared correlation spectroscopy. J. Phys. Chem. A 2000, 104, 6356–6361. [Google Scholar] [CrossRef]
- Ohta, A.; Murakami, R.; Urata, A.; Asakawa, T.; Miyagishi, S.; Aratono, M. Aggregation behavior of fluorooctanols in hydrocarbon solvents. J. Phys. Chem. B 2003, 107, 11502–11509. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.T.; Thomas, A.; Gibson, A.R.; Luebehusen, C.Y.; Donohue, M.D. Analysis of the self-association of aliphatic alcohols using Fourier Transform infrared (FT-IR) spectroscopy. Ind. Eng. Chem. Res. 2013, 52, 14456–14462. [Google Scholar] [CrossRef]
- Orzechowski, K.; Czarnecki, M.A. Association of 1-hexanol in mixtures with n-hexane: Dielectric, near-infrared and DFT studies. J. Mol. Liq. 2019, 279, 540–547. [Google Scholar] [CrossRef]
- Amenta, V.; Cook, J.L.; Hunter, C.A.; Low, C.M.R.; Sun, H.; Vinter, J.G. Interplay of self-association and solvation in polar liquids. J. Am. Chem. Soc. 2013, 135, 12091–12100. [Google Scholar] [CrossRef]
- DeBolt, S.E.; Kollman, P.A. Investigation of structure, dynamics, and solvation in 1-octanol and its water-saturated solution: Molecular Dynamics and free-energy perturbation studies. J. Am. Chem. Soc. 1995, 117, 5316–5340. [Google Scholar] [CrossRef]
- Golub, P.; Pogorelov, V.; Doroshenko, I. The structural peculiarities of liquid n-heptanol and n-octanol. J. Mol. Liq. 2012, 169, 80–86. [Google Scholar] [CrossRef]
- Czarnecki, M.A. Near-infrared spectroscopic study of self-association of octanoic acid. Chem. Phys. Lett. 2003, 368, 115–120. [Google Scholar] [CrossRef]
- Iwahashi, M.; Hayashi, Y.; Hachiya, N.; Matsuzawa, H.; Kobayashi, H. Self-association of octan-1-ol in the pure liquid state and in decane solutions as observed by viscosity, self-diffusion, nuclear magnetic resonance and near-infrared spectroscopy measurements. J. Chem. Soc. Faraday Trans. 1993, 89, 707–712. [Google Scholar] [CrossRef]
- Palombo, F.; Tassaing, T.; Danten, Y.; Besnard, M. Hydrogen bonding in liquid and supercritical 1-octanol and 2-octanol assessed by near and mid infrared spectroscopy. J. Chem. Phys. 2006, 125, 094503. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Graham, A.; Nemer, M.; Phinney, L.; Garcia, R.; Stirrup, E. Physical Properties of Low-Molecular Weight Polydimethylsiloxane Fluids; Sandia Report SAND2017-1242; Sandia National Laboratories: Albuquerque, NM, USA, 2017.
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005; Available online: http://www.hbcpnetbase.com (accessed on 22 August 2020).
- Nunes, R.J.; Saramago, B.; Marrucho, I.M. Surface tension of DL-menthol:octanoic acid eutectic mixtures. J. Chem. Eng. Data 2019, 64, 4915–4923. [Google Scholar] [CrossRef]
- Rondinini, S.; Longhi, P.; Mussini, P.R.; Mussini, T. Autoprotolysis constants in nonaqueous solvents and aqueous organic solvent. Pure Appl. Chem. 1987, 59, 1693–1702. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Singh, V.; Behrens, S.H. Electric charging in nonpolar liquids because of nonionizable surfactants. Langmuir 2009, 26, 3203–3207. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-S.; Yeh, K.-T.; Wu, C.-C.; Kao, D.-Y. IR study of monomer–dimer self-association of 2,2-dimethyl-3-ethyl-3-pentanol in n-octane: Determination of the molar absorptivities of monomer and dimer bands, and dimerization constants using novel equations. J. Solut. Chem. 2013, 42, 2269–2280. [Google Scholar] [CrossRef] [Green Version]
- Czarnecki, M.A.; Orzechowski, K. Effect of temperature and concentration on self-association of octan-3-ol studied by vibrational spectroscopy and dielectric measurements. J. Phys. Chem. A 2003, 107, 1119–1126. [Google Scholar] [CrossRef]
- Marczak, W.; Adamczyk, N.; Łęźniak, M. Viscosity of associated mixtures approximated by the Grunberg-Nissan model. Int. J. Thermophys. 2012, 33, 680–691. [Google Scholar] [CrossRef]
- El Hammami, N.; Bouanz, M.; Toumi, A. Thermodynamic properties of cyclohexane–methanol liquid from shear viscosity measurements. Fluid Phase Equilib. 2014, 384, 25–35. [Google Scholar] [CrossRef]
- Papir, Y.S.; Krieger, I.M. Rheological studies on dispersions of uniform colloidal spheres. II. Dispersions in nonaqueous media. J. Colloid Interface Sci. 1970, 34, 126–130. [Google Scholar] [CrossRef]
- Pal, R. Fundamental rheology of disperse systems based on single-particle mechanics. Fluids 2016, 1, 40. [Google Scholar] [CrossRef]
- Oswal, S.L.; Desai, H.S. Studies of viscosity and excess molar volume of binary mixtures. 1. Propylamine + 1-alkanol mixtures at 303.15 and 313.15 K. Fluid Phase Equilib. 1998, 149, 359–376. [Google Scholar] [CrossRef]
- Yang, C.; Xu, W.; Ma, P. Thermodynamic properties of binary mixtures of p-xylene with cyclohexane, heptane, octane, and N-methyl-2-pyrrolidone at several temperatures. J. Chem. Eng. Data 2004, 49, 1794–1801. [Google Scholar] [CrossRef]
- Choudhary, S.; Sengwa, R.J. Ionic conduction in binary mixtures of dipolar liquids. J. Mol. Liq. 2012, 175, 33–37. [Google Scholar] [CrossRef]
- Jasper, J.J.; Kring, E.V. The isobaric surface tensions and thermodynamic properties of the surfaces of a series of n-alkanes, C5 to C18, 1-alkenes, C6 to C16, and of n-decylcyclopentane, n-decylcyclohexane and n-decylbenzene. J. Phys. Chem. 1955, 59, 1019–1021. [Google Scholar] [CrossRef]
- Bell, W.; Daly, J.; North, A.M.; Pethrick, R.A.; Teik, P.B. Chain entanglement and normal mode motions in poly(dimethylsi1oxane) + toluene mixtures. J. Chem. Soc. Faraday Trans. II 1979, 75, 1452–1464. [Google Scholar] [CrossRef]
- Basta, M.; Picciarelli, V.; Stella, R. An introduction to percolation. Eur. J. Phys. 1994, 15, 97–101. [Google Scholar] [CrossRef]
- Ottavi, H.; Clerc, J.; Giraud, G.; Roussenq, J.; Guyon, E.; Mitescu, C.D. Electrical conductivity of a mixture of conducting and insulating spheres: An application of some percolation concepts. J. Phys. C Solid State Phys. 1978, 11, 1311–1328. [Google Scholar] [CrossRef]
- Ziff, R.M.; Torquato, S. Percolation of disordered jammed sphere packings. J. Phys. A Math. Theor. 2017, 50, 085001. [Google Scholar] [CrossRef] [Green Version]
- Domb, C.; Dalton, N.W. Crystal statistics with long-range forces. II. Asymptotic behavior of the equivalent neighbour model. Proc. Phys. Soc. 1966, 89, 859–871. [Google Scholar] [CrossRef]
- Walden, P. Über organische Lösungs- und Ionisierungsmittel. III. Teil: Innere Reibung und deren zusammenhang mit dem Leitvermögen. Z. Phys. Chem. 1906, 55, 207–246. [Google Scholar]
- Fuoss, R.M. Dependence of the Walden product on dielectric constant. Proc. Natl. Acad. Sci. USA 1959, 45, 807–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |






| Liquid | Electrical Conductivity (S/cm) a | Relative Permittivity | Dynamic Viscosity at 20 s−1 Shear Rate (mPa·s) | Surface Tension (mN/m) |
|---|---|---|---|---|
| n-Dodecane | 1.10 × 10−14 | 2.01 [54] | 1.41 | 25.4 [54] |
| Silicone oil (20 cSt) | 2.47 × 10−14 | 2.73 b | 19.8 | 19.0 [54] |
| 1-Octanol | 8.51 × 10−9 | 10.3 [54] | 7.50 | 27.1 [54] |
| 1-Octanoic acid | 6.33 × 10−13 | 2.85 [54] | 5.53 | 31 [55] |
| Binary System | Equation (1) | Equation (2) | ||||
|---|---|---|---|---|---|---|
| η1 (mPa·s) | η2 (mPa·s) | Φ12 | η1 (mPa·s) | η2 (mPa·s) | G12 | |
| Dodecane/ octanol | 7.50 | 1.38 | −0.236 | 7.49 | 1.42 | −0.940 |
| Silicone oil/ octanol | 7.50 a | 19.6 a | −1.08 a | 12.3 b,c | 19.8 b,c | 0.34 b,c |
| Silicone oil/ octanoic acid | 5.63 | 19.8 | −0.621 | b | b | b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, S.E.; Zeng, H. Electrical Conductivity and Viscosity in Binary Organic Liquid Mixtures: Participation of Molecular Interactions and Nanodomains. Colloids Interfaces 2020, 4, 44. https://doi.org/10.3390/colloids4040044
Taylor SE, Zeng H. Electrical Conductivity and Viscosity in Binary Organic Liquid Mixtures: Participation of Molecular Interactions and Nanodomains. Colloids and Interfaces. 2020; 4(4):44. https://doi.org/10.3390/colloids4040044
Chicago/Turabian StyleTaylor, Spencer E., and Huang Zeng. 2020. "Electrical Conductivity and Viscosity in Binary Organic Liquid Mixtures: Participation of Molecular Interactions and Nanodomains" Colloids and Interfaces 4, no. 4: 44. https://doi.org/10.3390/colloids4040044
APA StyleTaylor, S. E., & Zeng, H. (2020). Electrical Conductivity and Viscosity in Binary Organic Liquid Mixtures: Participation of Molecular Interactions and Nanodomains. Colloids and Interfaces, 4(4), 44. https://doi.org/10.3390/colloids4040044