Preparation and Evaluation of Rebamipide Colloidal Nanoparticles Obtained by Cogrinding in Ternary Ground Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of Samples
2.3. Physicochemical Properties of Samples
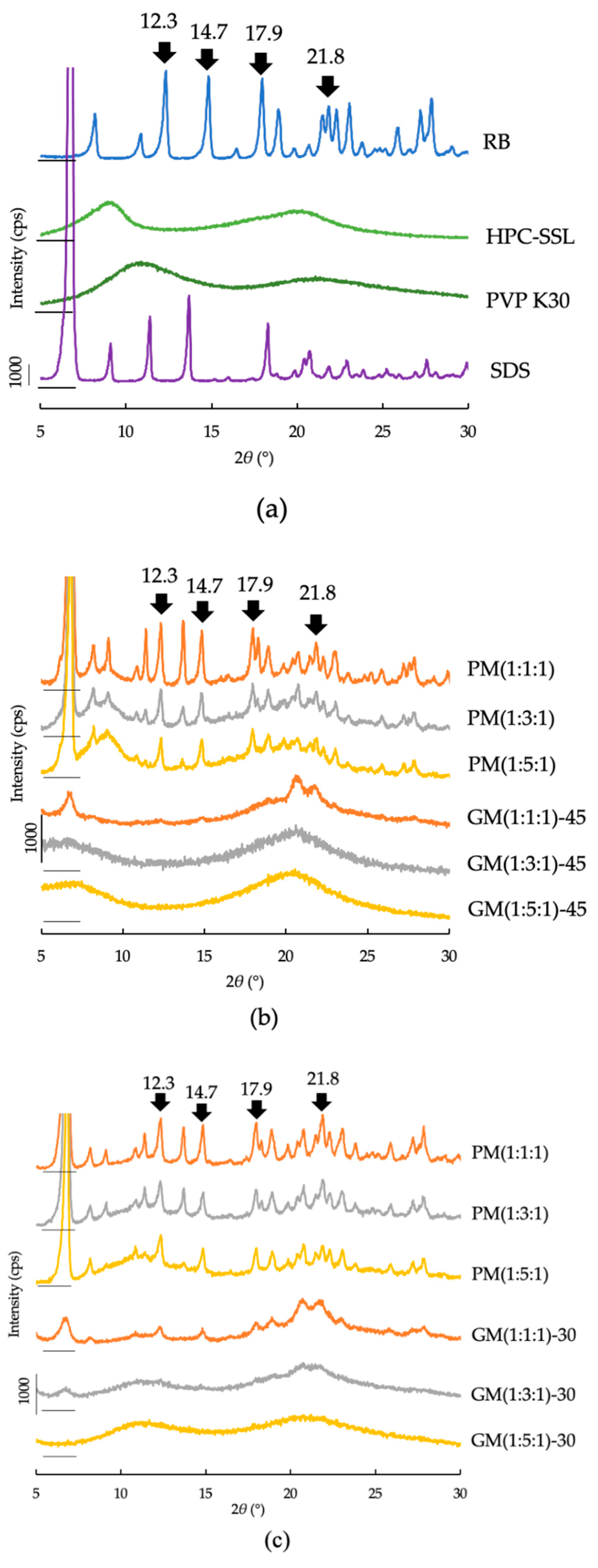
2.3.1. PXRD
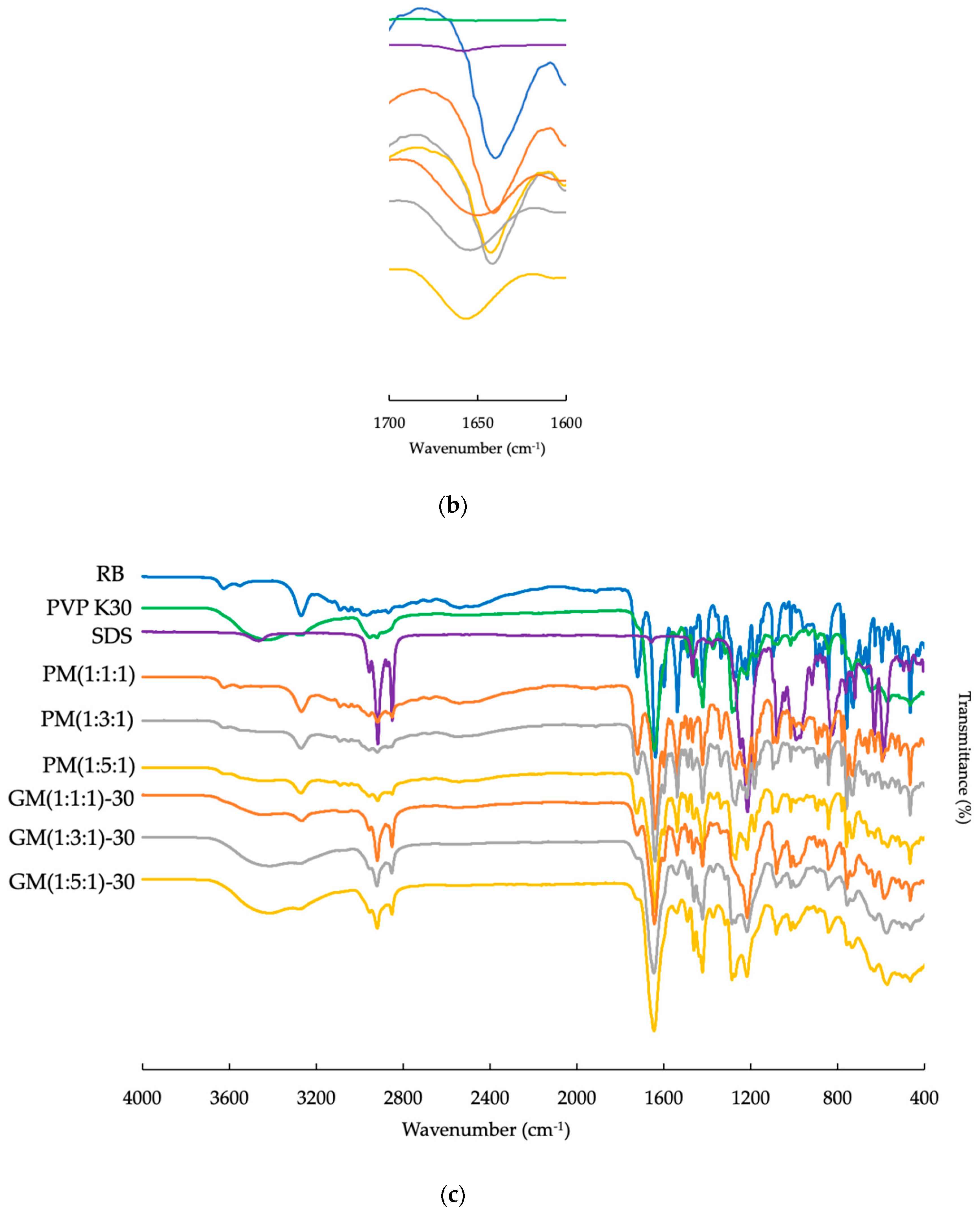
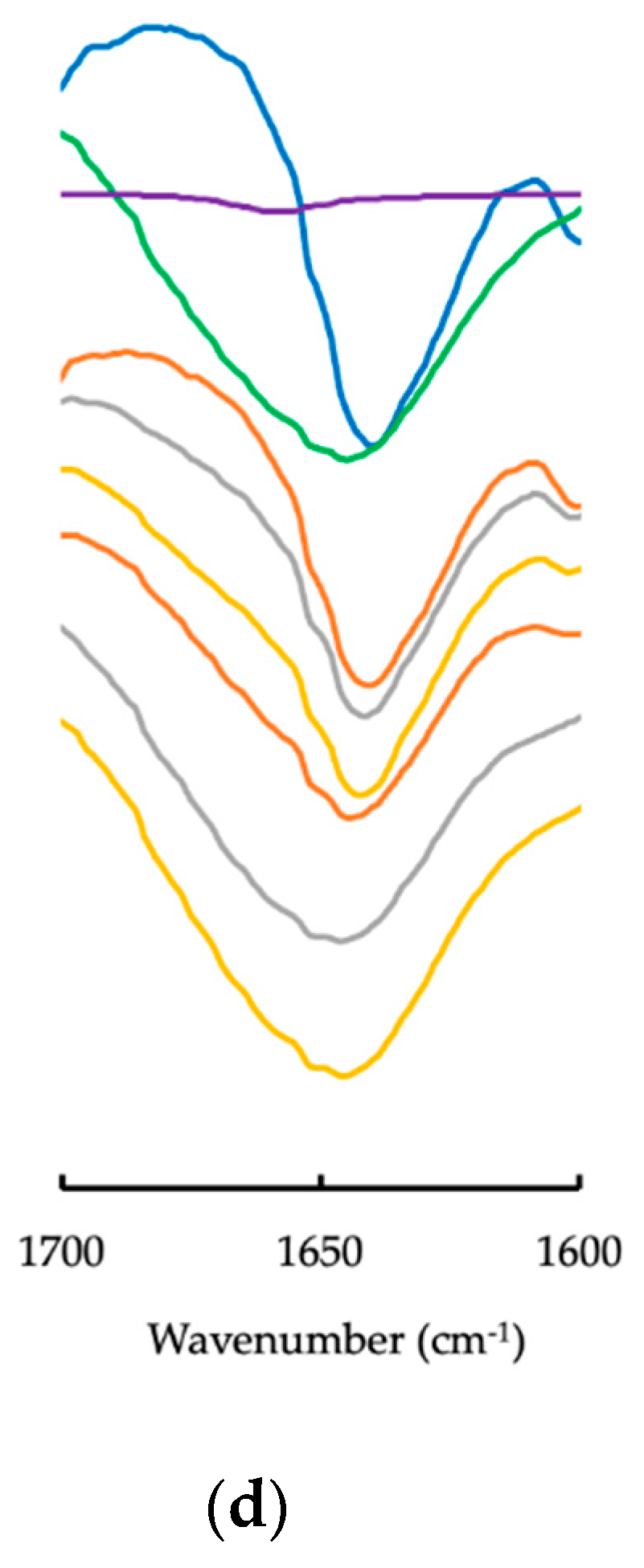
2.3.2. ATR-FTIR
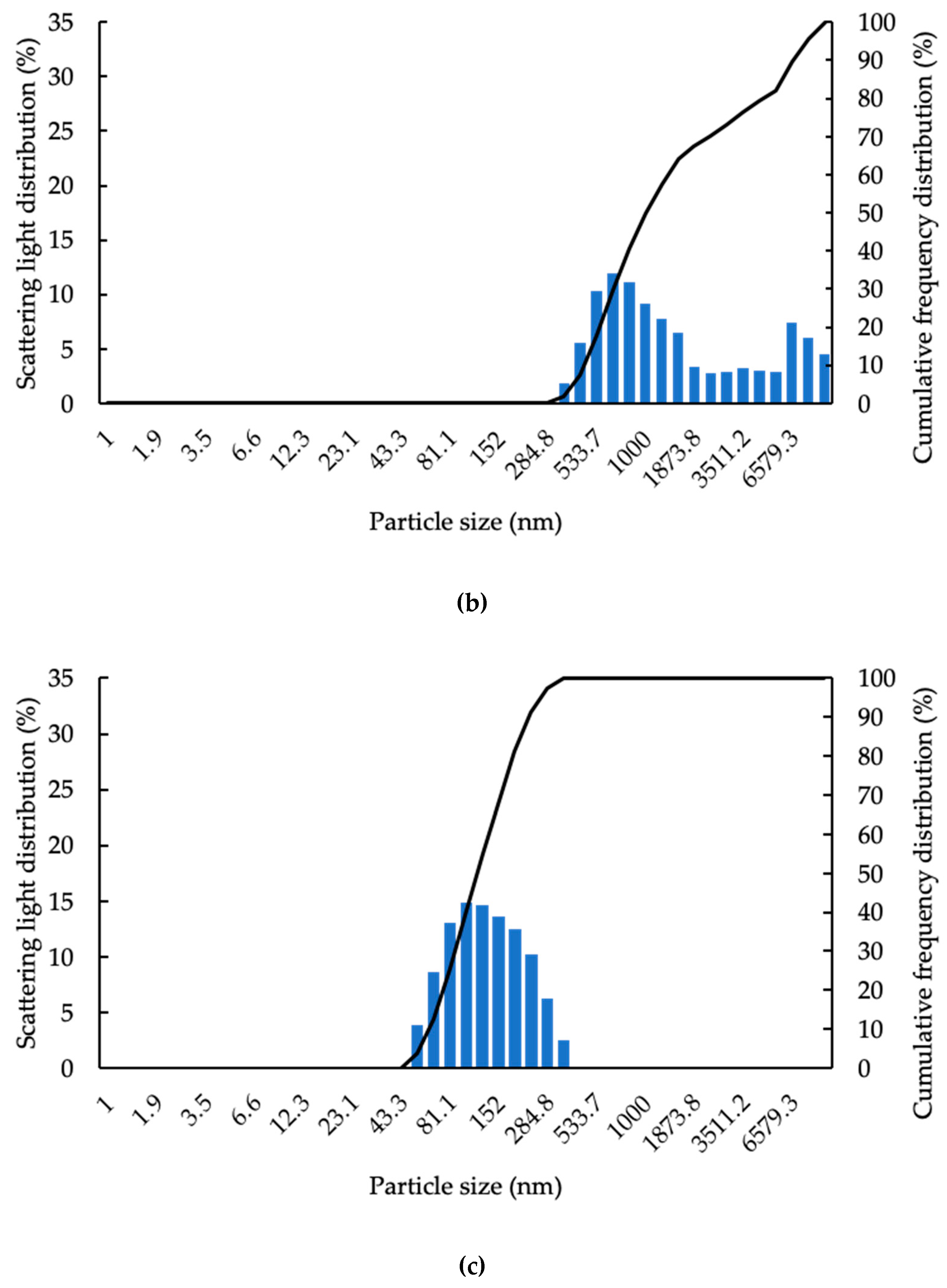
2.3.3. Particle Size and Zeta Potential in Water
2.3.4. Viscosity of Sample Solution
2.3.5. RB Solubility in Water
2.3.6. Evaluation of Dispersion Stability in Water
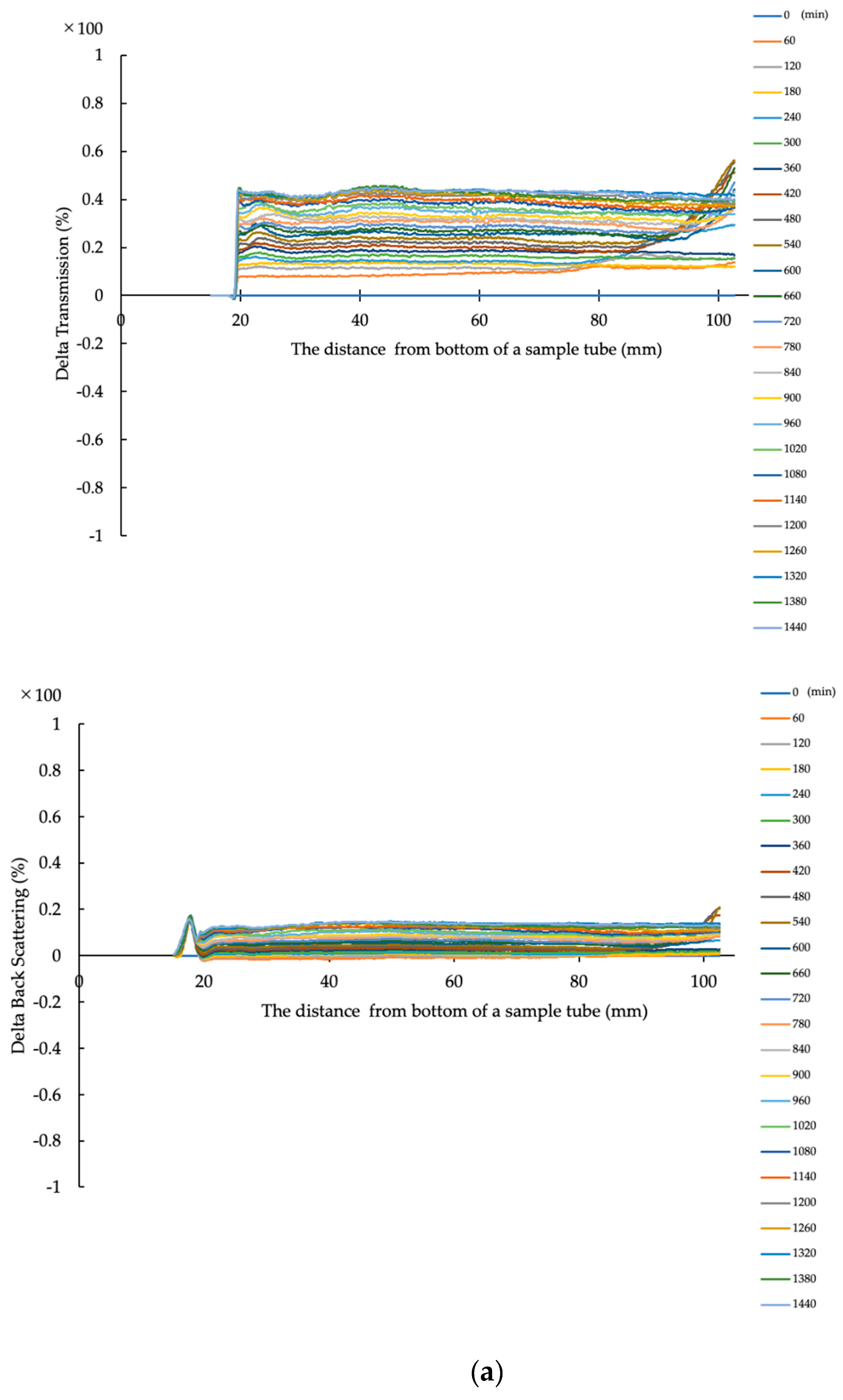
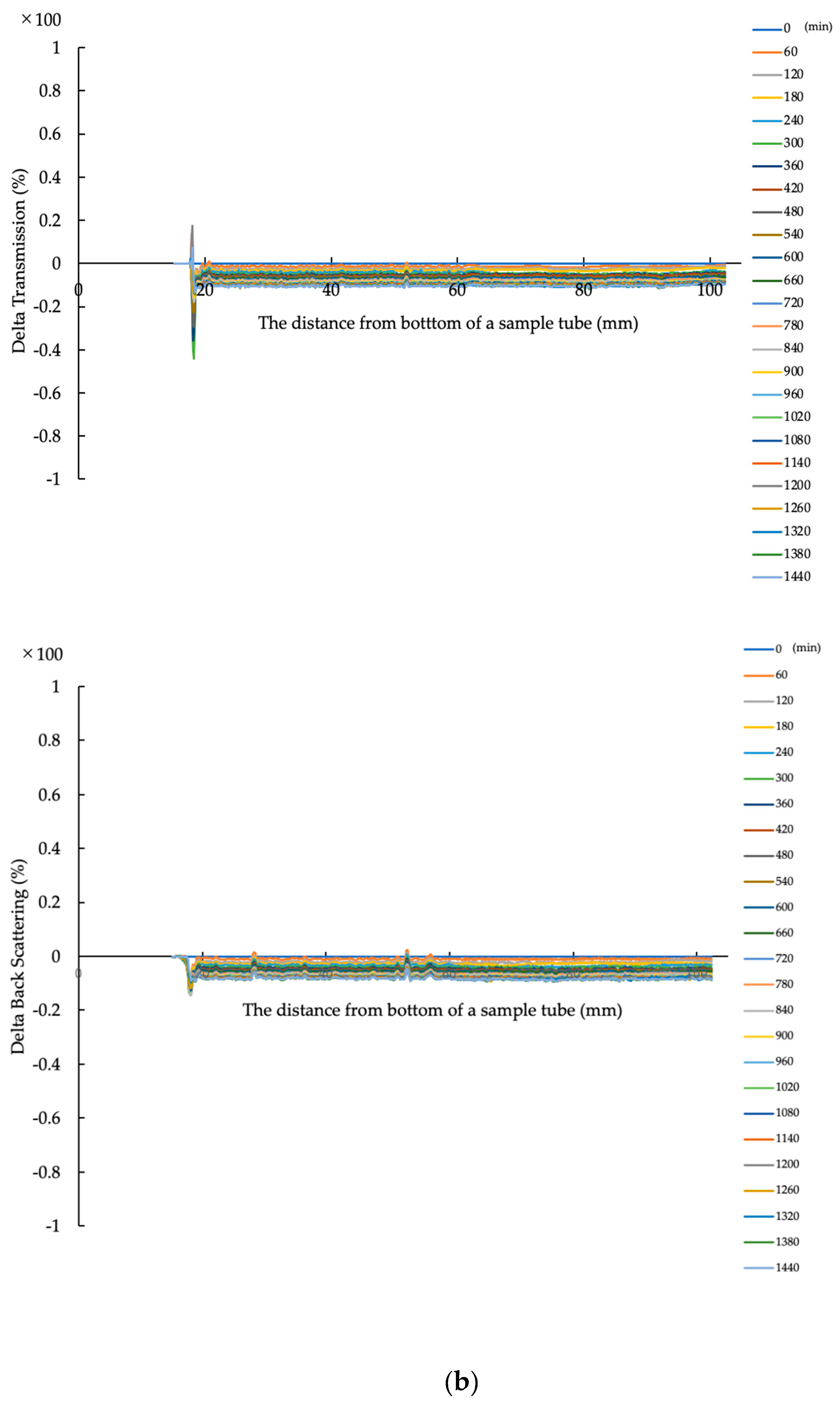
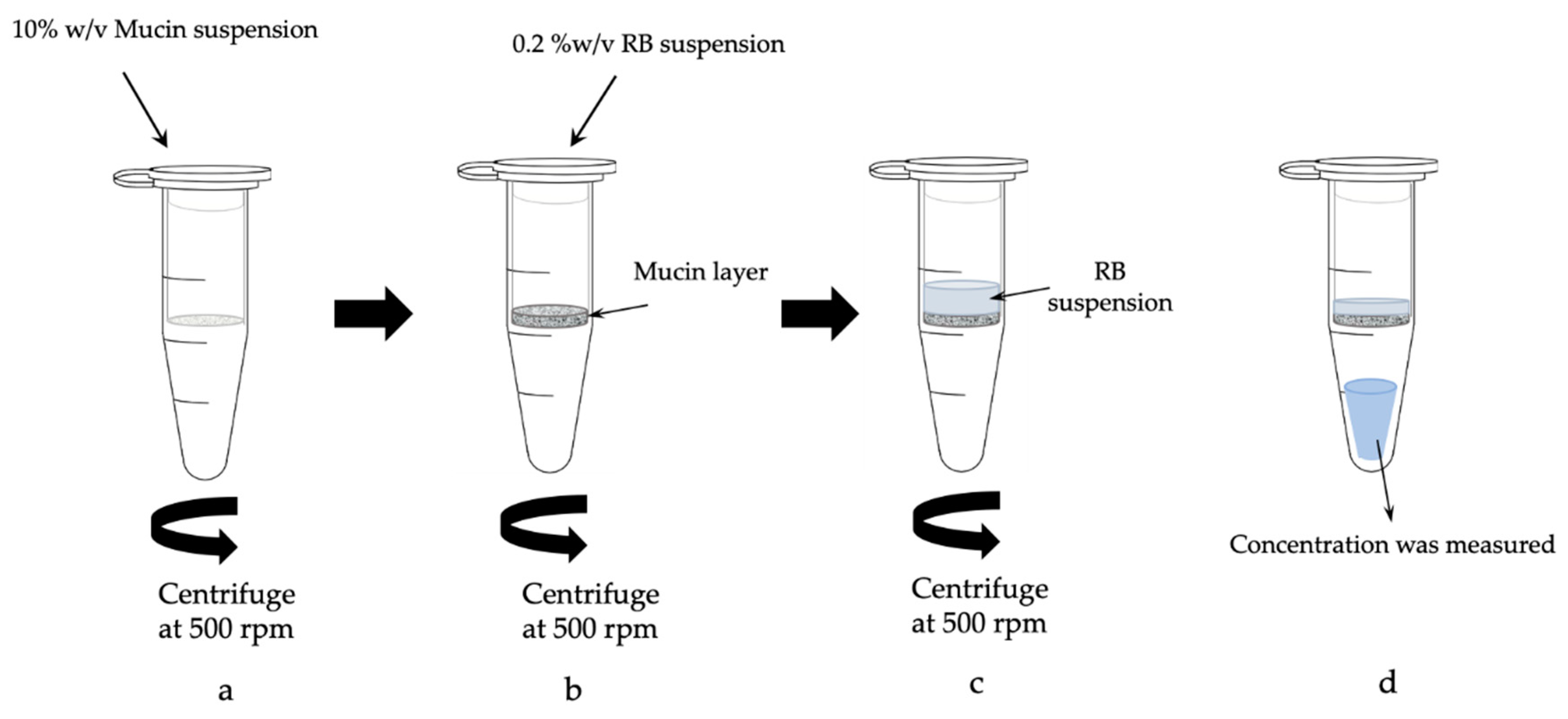
2.3.7. Evaluation of Mucus Permeation by RB Suspension
2.3.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 26 August 2020).
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer Nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Nie, S.; Wang, M.D. Nanotechnology applications in surgical oncology. Annu. Rev. Med. 2010, 61, 359–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carelle, N.; Piotto, E.; Bellanger, A.; Germanaud, J.; Thuillier, A.; Khayat, D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 2002, 95, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.; Abraham, S.; Kaye, S.B.; Sowerbutts, T.; Frewin, C.; Fox, R.M.; Tattersall, M.H. On the receiving end—Patient perception of the side-effects of cancer chemotherapy. Eur. J. Cancer Clin. Oncol. 1983, 19, 203–208. [Google Scholar] [CrossRef]
- Boer-Dennert, M.D.; Wit, R.D.; Schmitz, P.I.; Djontono, J.; Beurden, V.V.; Stoter, G.; Verweij, J. Patient perceptions of the side-effects of chemotherapy: The influence of 5ht3 antagonists. Br. J. Cancer 1997, 76, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef]
- Ishii, N.; Kawano, Y.; Sakai, H.; Hayashi, S.; Akizuki, N.; Komoda, M.; Hanawa, T. Effects of a rebamipide mouthwash on stomatitis caused by cancer chemotherapy-evaluation of the efficacy by patients themselves. Yakugaku Zasshi 2017, 137, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Tobin, P.; Clarke, S.J. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol. 2005, 6, 93–102. [Google Scholar] [CrossRef]
- Sonis, S.T. A Biological approach to mucositis. J. Support. Oncol. 2004, 2, 21–32, discussion 35. [Google Scholar]
- Akagi, S.; Fujiwara, T.; Nishida, M.; Okuda, A.; Nagao, Y.; Okuda, T.; Tokuda, H.; Takayanagi, K. The Effectiveness of rebamipide mouthwash therapy for radiotherapy and chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: A systematic review and meta-analysis. J. Pharm. Health Care Sci. 2019, 5, 16. [Google Scholar] [CrossRef]
- Kabeya, M.; Ina, K.; Yuasa, S.; Kikuchi, F.; Tajiri, C.; Kato, T.; Hibi, S.; Minagawa, Y.; Furuta, R.; Kikuchi, T.; et al. Effects of oral rinse using lemon-flavored water with or without rebamipide on fluoropyrimidine-induced stomatitis. J. Integr. Oncol. 2013, 2. [Google Scholar] [CrossRef]
- Kitagawa, J.; Nasu, M.; Okumura, H.; Shibata, A.; Makino, K.; Terada, H.; Matsumoto, S. Allopurinol gel mitigates radiation-induced mucositis and dermatitis. J. Radiat. Res. 2008, 49, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momo, K.; Nagaoka, H.; Kizawa, Y.; Bukawa, H.; Chiba, S.; Kohda, Y.; Homma, M. Assessment of indomethacin oral spray for the treatment of oropharyngeal mucositis-induced pain during anticancer therapy. Support. Care Cancer 2017, 25, 2997–3000. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Ogawa, T.; Takahashi, S.; Okami, K.; Fujii, T.; Tanaka, K.; Iwae, S.; Ota, I.; Ueda, T.; Monden, N.; et al. Efficacy and safety of rebamipide liquid for chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: A multicenter, randomized, double-blind, placebo-controlled, parallel-group phase ii study. BMC Cancer 2017, 17, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokomizo, H.; Yoshimatsu, K.; Hashimoto, M.; Ishibashi, K.; Umehara, A.; Yoshida, K.; Fujimoto, T.; Watanabe, K.; Ogawa, K. Prophylactic Efficacy of allopurinol ice ball for leucovorin/5-fluorouracil therapy-induced stomatitis. Anticancer Res. 2004, 24, 1131–1134. [Google Scholar]
- Shabanloei, R.; Ahmadi, F.; Vaez gharamaleki, J.; Hajizadeh, E.; Javadzadeh, Y. The effects of allopurinol mouthwash in the prevention of chemotherapy-induced stomatitis. Tehran Univ. Med. J. TUMS Publ. 2007, 65, 71–76. [Google Scholar]
- Panahi, Y.; Ala, S.; Saeedi, M.; Okhovatian, A.; Bazzaz, N.; Naghizadeh, M.M. Allopurinol mouth rinse for prophylaxis of fluorouracil-induced mucositis. Eur. J. Cancer Care 2010, 19, 308–312. [Google Scholar] [CrossRef]
- Montecucco, C.; Caporali, R.; Rossi, S.; Porta, C. Allopurinol mouthwashes in methotrexate-induced stomatitis. Arthritis Rheum. 1994, 37, 777–778. [Google Scholar] [CrossRef]
- Elzawawy, A. Treatment of 5-fluorouracil-induced stomatitis by allopurinol mouthwashes. Oncology 1991, 48, 282–284. [Google Scholar] [CrossRef]
- Fox, R.M.; Woods, R.L.; Tattersall, M.H.; Piper, A.A.; Sampson, D. Allopurinol modulation of fluorouracil toxicity. Cancer Chemother. Pharmacol. 1981, 5, 151–155. [Google Scholar] [CrossRef]
- Shibamori, M.; Sato, M.; Uematsu, N.; Nakashima, T.; Sato, A.; Yamamura, Y.; Sasabe, H.; Umehara, K.; Sakurai, K. Rebamipide does not interfere with the antitumor effect of radiotherapy or chemotherapy in human oral tumor-bearing nude mice. J. Pharmacol. Sci. 2015, 129, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsubaki, M.; Takeda, T.; Asano, R.T.; Matsuda, T.; Fujimoto, S.I.; Itoh, T.; Imano, M.; Satou, T.; Nishida, S. Rebamipide suppresses 5-fluorouracil-induced cell death via the activation of Akt/mTOR pathway and regulates the expression of Bcl-2 family proteins. Toxicol. Vitr. 2018, 46, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Bin Choy, Y.B.; Park, J.H.; Prausnitz, M.R. mucoadhesive microparticles engineered for ophthalmic drug delivery. J. Phys. Chem. Solids 2008, 69, 1533–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A. Physical Pharmacy, 4th ed.; BI Waverly Pvt Limited: New York, NY, USA, 1997. [Google Scholar]
- Ponchel, G.; Montisci, M.; Dembri, A.; Durrer, C.; Duchêne, D. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. Eur. J. Pharm. Biopharm. 1997, 44, 25–31. [Google Scholar] [CrossRef]
- Witten, J.; Samad, T.; Ribbeck, K. Selective permeability of mucus barriers. Curr. Opin. Biotechnol. 2018, 52, 124–133. [Google Scholar] [CrossRef]
- Takeuchi, H.; Yamamoto, H.; Kawashima, Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Adv. Drug Deliv. Rev. 2001, 47, 39–54. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Boisseau, P.; Loubaton, B. Nanomedicine, nanotechnology in medicine. C. R. Phys. 2011, 12, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Bhakay, A.; Merwade, M.; Bilgili, E.; Dave, R.N. Novel aspects of wet milling for the production of microsuspensions and nanosuspensions of water-soluble drugs. Drug Dev. Ind. Pharm. 2011, 37, 963–976. [Google Scholar] [CrossRef]
- Malamatari, M.; Taylor, K.M.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef]
- Laakson, T.; Liu, P.; Rahikkala, A.; Peltonen, L.; Kauppinen, E.I.; Hirvonen, J.; Järvinen, K.; Raula, J. Intact nanoparticulate indomethacin in fast-dissolving carrier particles by combined wet milling and aerosol flow reactor methods. Pharm. Res. 2011, 28, 2043. [Google Scholar] [CrossRef]
- Liu, K.; Rong, X.; Laru, J.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Laaksonen, T.; Peltonen, L. Nanosuspention of poorly soluble drugs: Preparation and development by wet milling. Int. J. Pharm. 2011, 411, 215–222. [Google Scholar] [CrossRef]
- Li, M.; Yaragudi, N.; Afolabi, A.; Dave, R.; Bilgili, E. Sub-100 nm drug particle suspensions prepared via wet milling with low bead contamination through novel process. Chem. Eng. Sci. 2015, 130, 207–220. [Google Scholar] [CrossRef]
- Loh, Z.H.; Samanta, A.K.; Heng, P.W.S. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Burbess, D.J. Wet milling induced physical and chemical instabilities of naproxen nano-crystalline suspensions. Int. J. Pharm. 2014, 466, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Upponi, J.R.; Jerajani, K.; Nagesha, D.K.; Kulkarni, P.; Sridhar, S.; Ferris, C.; Torchilin, V.P. Polymeric micelles: Theranostic co-delivery system for poorly water-soluble drugs and contrast agents. Biomaterials. 2018, 170, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ling, P.; Zhang, T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv. 2013. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanosized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, M.C.; Carbone, C.; Souto, E.B. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Kastner, E.; Verma, V.; Lowry, D.; Perrie, Y. Microfluidic-controlled manufacture of liposomes for the solubilization of poorly water soluble drug. Int. J. Pharm. 2015, 485, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Bolourchian, N.; Talamkhani, Z.; Nokhodchi, A. Preparation and physicochemical characterization of binary and ternary ground mixtures of carvedilol with PVP and SLS aimed to improve the drug dissolution. Pharm. Dev. Technol. 2019, 24, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Higashi, K.; Yamamoto, K.; Moribe, K. In situ molecular elucidation of drug supersaturation achieved by nano-sizing and amorphization of poorly water-soluble drug. Eur. J. Pharm. Sci. 2015, 77, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Penkina, A.; Semjonov, K.; Hakola, M.; Vuorinen, S.; Repo, T.; Yliruusi, J.; Aruväli, J.; Kogermann, K.; Veski, P.; Heinämäki, J. Towards improved solubility of poorly water-soluble drugs: Cryogenic co-grinding of piroxicam with carrier polymers. Drug Dev. Ind. Pharm. 2016, 42, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Liu, C.; Ren, T.; Wang, X.; Yang, Q.; Yang, Z.; Yang, Y.; Yang, S.; Gu, J.; Hu, C. Sodium salts and solvate of rebamipide: Synthesis, structure, and pharmacokinetic study. Cryst. Growth Des. 2016, 12, 3180–3189. [Google Scholar] [CrossRef]
- Shudo, J.; Pongpeeraoat, C.; Wanawongthai, C.; Moribe, K.; Yamamoto, K. In vivo assessment of oral administration of probucol nanoparticles in rats. Biol. Pharm. Bull. 2008, 31, 321–325. [Google Scholar] [CrossRef] [Green Version]
- TImpe, C. Drug Solubilization Strategies Applying nanoparticulate formulation and solid dispersion approaches in drug development. Am. Pharm. Rev. 2010, 13, 12–21. [Google Scholar]
- Mengual, O.; Meunier, G.; Cayre, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple Light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Rathbone, M.J.; Tucker, I.G. Mechanisms, barriers and pathways of oral mucosal drug permeation. Adv. Drug Deliv. Rev. 1993, 12, 41–60. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Friedl, H.; Dünnhaupt, S.; Hintzen, F.; Waldner, C.; Parikh, S.; Pearson, J.P.; Wilcox, M.D.; Bernkop-Schnürch, A. Development and evaluation of a novel mucus diffusion test system approved by self-nanoemulsifying drug delivery systems. J. Pharm. Sci. 2013, 102, 4406–4413. [Google Scholar] [CrossRef] [PubMed]





| Mucosta® Tablets 100 mg | 6 Tablets |
|---|---|
| Citric acid | 0.45 g |
| l-glutamine | 1.50 g |
| Simple syrup | 12.0 mL |
| Dextrin | 1.50 g |
| Preservation solution | 2.0 mL |
| Distilled water | Total of 300 mL |
| Sample | Mixing Weight Ratio | Grinding Time (min) | ||
|---|---|---|---|---|
| RB | HPC or PVP | SDS | ||
| PM (1:1:1) | 1 | 1 | 1 | |
| PM (1:3:1) | 3 | |||
| PM (1:5:1) | 5 | |||
| GM (1:1:1)-15 | 1 | 1 | 1 | 15 |
| GM (1:3:1)-15 | 3 | |||
| GM (1:5:1)-15 | 5 | |||
| GM (1:1:1)-30 | 1 | 1 | 1 | 30 |
| GM (1:3:1)-30 | 3 | |||
| GM (1:5:1)-30 | 5 | |||
| GM (1:1:1)-45 | 1 | 1 | 1 | 45 |
| GM (1:3:1)-45 | 3 | |||
| GM (1:5:1)-45 | 5 | |||
| Particle Size (nm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | HPC-SSL | HPC-SL | HPC-L | PVP K30 | PVP K90 | |||||
| Mean | PDI | Mean | PDI | Mean | PDI | Mean | PDI | Mean | PDI | |
| PM (1:1:1) | 1271.2 ± 194.2 | 0.485 ± 0.133 | 920.0 ± 238.9 | 0.339 ± 0.138 | 855.7 ± 101.7 | 0.612 ± 0.250 | 1520.3 ± 157.7 | 0.588 ± 0.133 | 1679.8 ± 614.5 | 0.600 ± 0.126 |
| PM (1:3:1) | 1679.7 ± 345.2 | 0.627 ± 0.193 | 904.6 ± 166.2 | 0.310 ± 0.105 | 996.2 ± 61.5 | 0.394 ± 0.110 | 1503.3 ± 225.4 | 0.641 ± 0.131 | 2016.5 ± 177.7 | 0.723 ± 0.194 |
| PM (1:5:1) | 796.5 ± 161.8 | 0.301 ± 0.064 | 925.2 ± 14.1 | 0.5132 ± 0.219 | 975.0 ± 79.4 | 0.539 ± 0.238 | 1869.0 ± 758.8 | 0.797 ± 0.450 | 1688.7 ± 778.6 | 0.593 ± 0.262 |
| GM (1:1:1)-15 | 243.9 ± 19.9 | 0.165 ± 0.005 | 318.2 ± 47.6 | 0.206 ± 0.045 | 364.5 ± 62.6 | 0.2378 ± 0.052 | 256.9 ± 36.1 | 0.169 ± 0.024 | 467.4 ± 99.1 | 0.296 ± 0.054 |
| GM (1:3:1)-15 | 220.0 ± 24.1 | 0.217 ± 0.030 | 212.3 ± 31.9 | 0.146 ± 0.025 | 264.6 ± 28.9 | 0.187 ± 0.017 | 217.7 ± 36.2 | 0.171 ± 0.016 | 1380.3 ± 658.5 | 0.587 ± 0.136 |
| GM (1:5:1)-15 | 229.3 ± 32.8 | 0.218 ± 0.035 | 209.7 ± 49.6 | 0.240 ± 0.079 | 274.8 ± 42.2 | 0.248 ± 0.055 | 186.4 ± 29.2 | 0.159 ± 0.013 | 1040.6. ± 171.6 | 0.591 ± 0.190 |
| GM (1:1:1)-30 | 221.4 ± 25.9 | 0.199 ± 0.060 | 239.4 ± 32.7 | 0.196 ± 0.071 | 309.2 ± 41.0 | 0.223 ± 0.038 | 222.9 ± 44.0 | 0.170 ± 0.016 | 378.3 ± 120.0 | 0.249 ± 0.040 |
| GM (1:3:1)-30 | 164.1 ± 16.0 | 0.226 ± 0.040 | 162.5 ± 4.2 | 0.168 ± 0.014 | 199.5 ± 3.7 | 0.202 ± 0.022 | 175.0 ± 32.5 | 0.177 ± 0.001 | 304.6 ± 56.5 | 0.271 ± 0.026 |
| GM (1:5:1)-30 | 166.8 ± 16.2 | 0.195 ± 0.072 | 171.4 ± 7.4 | 0.227 ± 0.025 | 175.2 ± 18.0 | 0.219 ± 0.031 | 145.0 ± 25.1 | 0.165 ± 0.016 | 451.6 ± 134.5 | 0.246 ± 0.036 |
| GM (1:1:1)-45 | 180.0 ± 49.1 | 0.217 ± 0.052 | 230.8 ± 46.0 | 0.246 ± 0.071 | 221.8 ± 34.6 | 0.206 ± 0.044 | 198.4 ± 50.07 | 0.181 ± 0.022 | 301.3 ± 68.6 | 0.284 ± 0.013 |
| GM (1:3:1)-45 | 112.4 ± 2.6 | 0.201 ± 0.033 | 130.5 ± 14.9 | 0.191 ± 0.071 | 173.5 ± 27.8 | 0.147 ± 0.011 | 140.0 ± 14.1 | 0.147 ± 0.011 | 509.9 ± 123.7 | 0.278 ± 0.016 |
| GM (1:5:1)-45 | 135.1 ± 40.8 | 0.206 ± 0.030 | 167.4 ± 11.5 | 0.191 ± 0.043 | 176.8 ± 13.0 | 0.232 ± 0.014 | 137.0 ± 22.6 | 0.128 ± 0.014 | 432.3 ± 8.0 | 0.236 ± 0.012 |
| Sample | Zeta Potential (mV) | Viscosity (mP•s) | Solubility (μg/mL) | |||
|---|---|---|---|---|---|---|
| HPC-SSL | PVP K30 | HPC-SSL | PVP K30 | HPC-SSL | PVP K30 | |
| PM (1:1:1) | −8.85 | −9.92 | 1.02 | 0.92 | 7.57 | 7.89 |
| PM (1:3:1) | −7.72 | −8.28 | 1.42 | 1.13 | 7.47 | 6.83 |
| PM (1:5:1) | −6.27 | −9.56 | 1.96 | 1.20 | 8.56 | 5.73 |
| GM (1:1:1)-15 | −9.19 | −13.93 | 1.01 | 1.03 | 11.40 | 12.33 |
| GM (1:3:1)-15 | −7.34 | −10.42 | 1.28 | 1.10 | 14.04 | 12.57 |
| GM (1:5:1)-15 | −7.18 | −8.41 | 1.78 | 1.16 | 16.89 | 15.09 |
| GM (1:1:1)-30 | −10.00 | −16.01 | 1.02 | 1.04 | 13.24 | 12.42 |
| GM (1:3:1)-30 | −6.60 | −11.78 | 1.20 | 1.08 | 17.64 | 14.04 |
| GM (1:5:1)-30 | −7.11 | −10.86 | 1.58 | 1.13 | 19.91 | 18.74 |
| GM (1:1:1)-45 | −8.82 | −13.51 | 0.99 | 1.00 | 14.78 | 17.07 |
| GM (1:3:1)-45 | −12.46 | −13.09 | 1.18 | 1.30 | 18.83 | 17.73 |
| GM (1:5:1)-45 | −11.02 | −11.656 | 1.48 | 1.20 | 21.09 | 24.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawano, Y.; Utsunomiya, Y.; Yokoyama, F.; Ishii, N.; Hanawa, T. Preparation and Evaluation of Rebamipide Colloidal Nanoparticles Obtained by Cogrinding in Ternary Ground Mixtures. Colloids Interfaces 2020, 4, 43. https://doi.org/10.3390/colloids4040043
Kawano Y, Utsunomiya Y, Yokoyama F, Ishii N, Hanawa T. Preparation and Evaluation of Rebamipide Colloidal Nanoparticles Obtained by Cogrinding in Ternary Ground Mixtures. Colloids and Interfaces. 2020; 4(4):43. https://doi.org/10.3390/colloids4040043
Chicago/Turabian StyleKawano, Yayoi, Yuiko Utsunomiya, Fumiya Yokoyama, Naoko Ishii, and Takehisa Hanawa. 2020. "Preparation and Evaluation of Rebamipide Colloidal Nanoparticles Obtained by Cogrinding in Ternary Ground Mixtures" Colloids and Interfaces 4, no. 4: 43. https://doi.org/10.3390/colloids4040043





