Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties
Abstract
1. Introduction
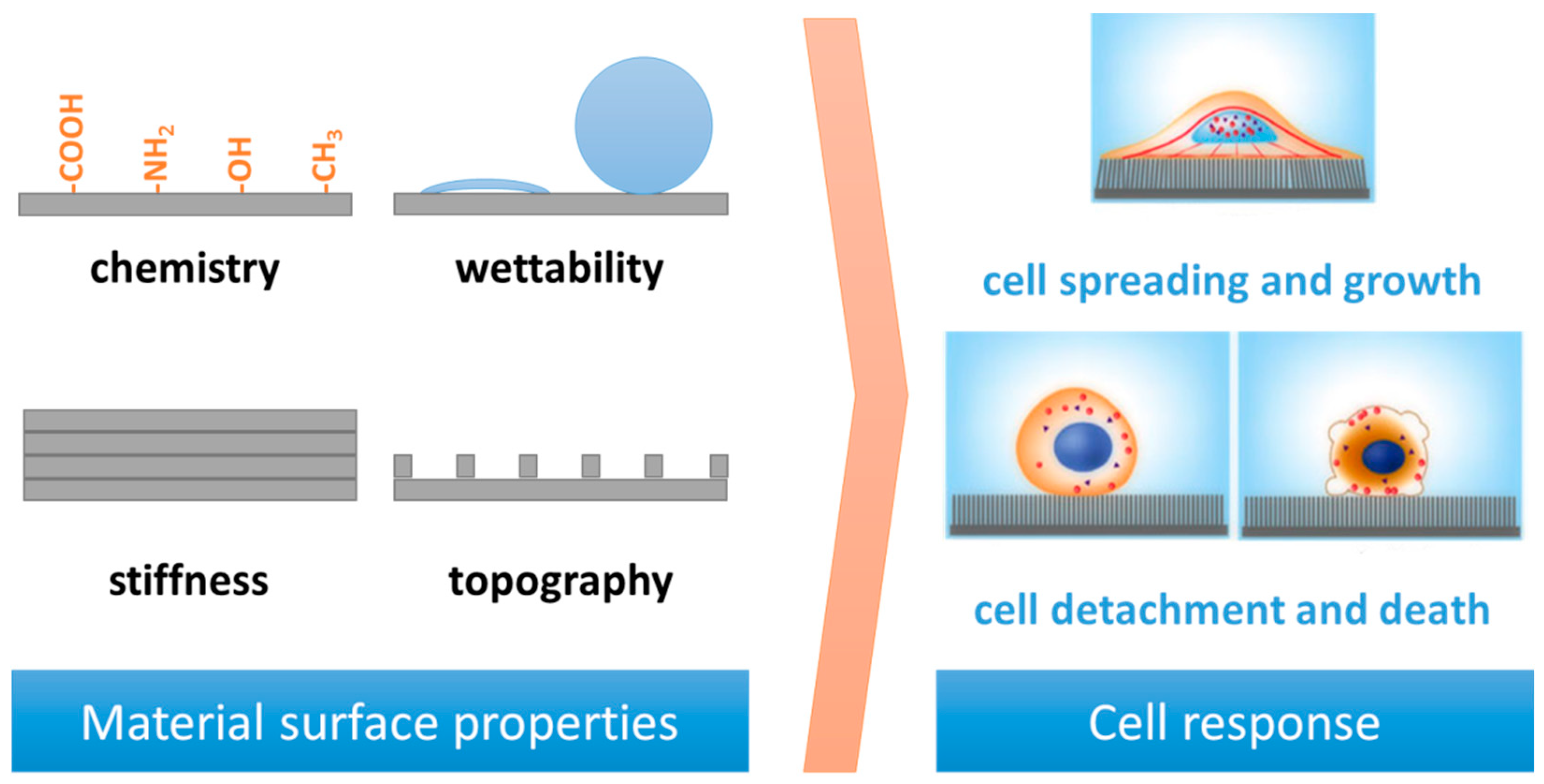
2. Surface Properties and Cell Response
2.1. Surface Stiffness
2.2. Surface Charge and Chemical Functionalities
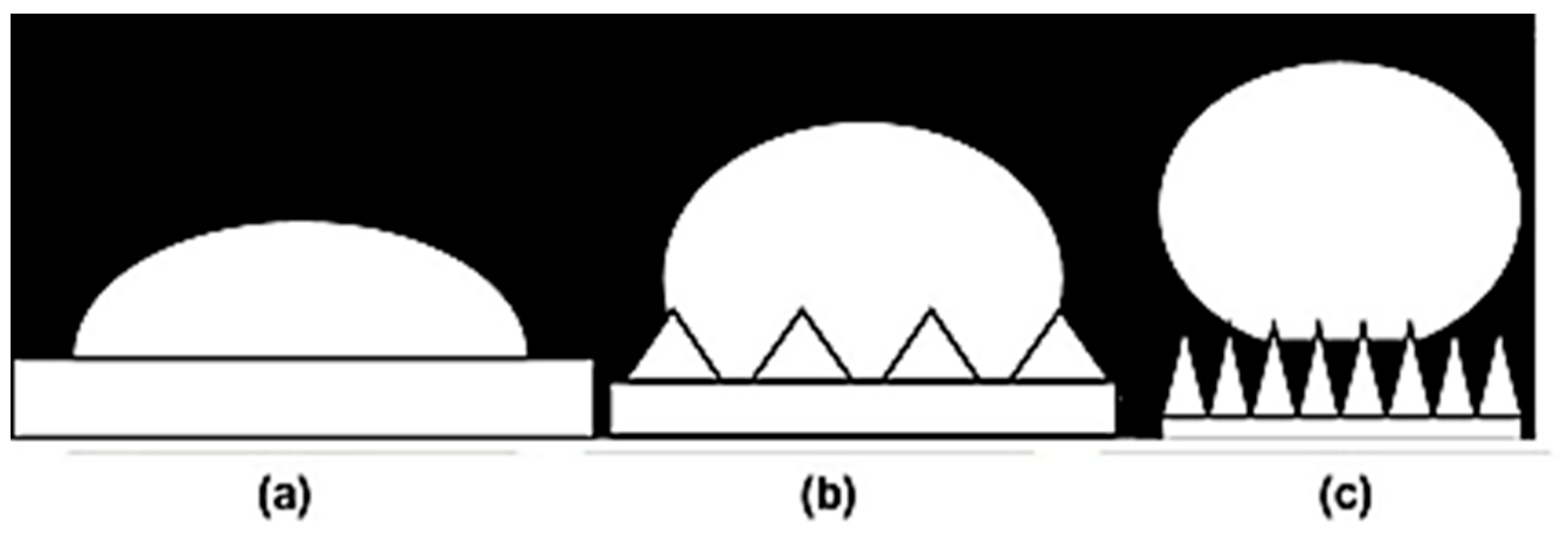
2.3. Surface Roughness
3. Dynamic Processes at the Interfaces
4. Case Studies
- The influence of the composition of poly(dimethylsiloxane) (PDMS) was investigated, studying the attachment and growth properties of several different types of mammalian cells: primary human umbilical artery endothelial cells (HUAECs), transformed 3T3 fibroblasts (3T3s), transformed osteoblast-like cells (MC3T3-E1), and transformed epithelial cells (HeLa). Cells’ growth has been studied on PDMS at different ratios of curing agent, that is, 10:1 v/v (normal PDMS, PDMSN), 10:3 v/v (PDMSCA), and 10:0.5 v/v (PDMSB), as well as on extracted PDMS (normal PDMS with reduced quantities of low molecular-weight oligomers, PDMSN, EX), normal PDMS extracted and then oxidized (PDMSN, EX, OX). Before the cell attachment step, all surfaces were exposed to a solution of fibronectin, being fibronectin-coated PDMS as suitable substrate for culturing mammalian cells [91]. The cell type appeared to be the most influencing factor of cells compatibility on some surfaces; 3T3 fibroblasts and MC3T3-E1 cells showed detachment from PDMSN, EX, OX, while HUAECs and HeLa cells detached from the PDMSCA surface. For most of the cell types on PDMSN, PDMSN, EX, and PDMSB, cell growth was comparable to standard tissue culture-treated polystyrene (TCPS). Despite Young’s moduli range, the growth rate was found to be similar for all cells on PDMS substrates and then independent on substrate stiffness.
- Bioinspired superhydrophobic surfaces were prepared based on intrinsically hydrophobic PDMS by roughening a structure using surface aggregates of nanoparticles [92]. The wettability data resulted in a direct dependence of WCA with the increasing of concentration of hydrophobic TiO2. A correlation between surface properties and cell–surface interactions supported the cell adhesion studies carried out in this work. Only the superhydrophobic sample showed a cell-repellent behavior, with a decreasing of cell viability up to 80% compared with the pure PDMS film. The surface energy was shown to play a key role in the cell-repellent behavior of the superhydrophobic sample, because of similarities in the roughness profiles of the two samples. This work underlines how surface wettability, roughness, and chemistry are parameters of optimization for developing biomaterial surfaces with controlled cell adhesion behavior.
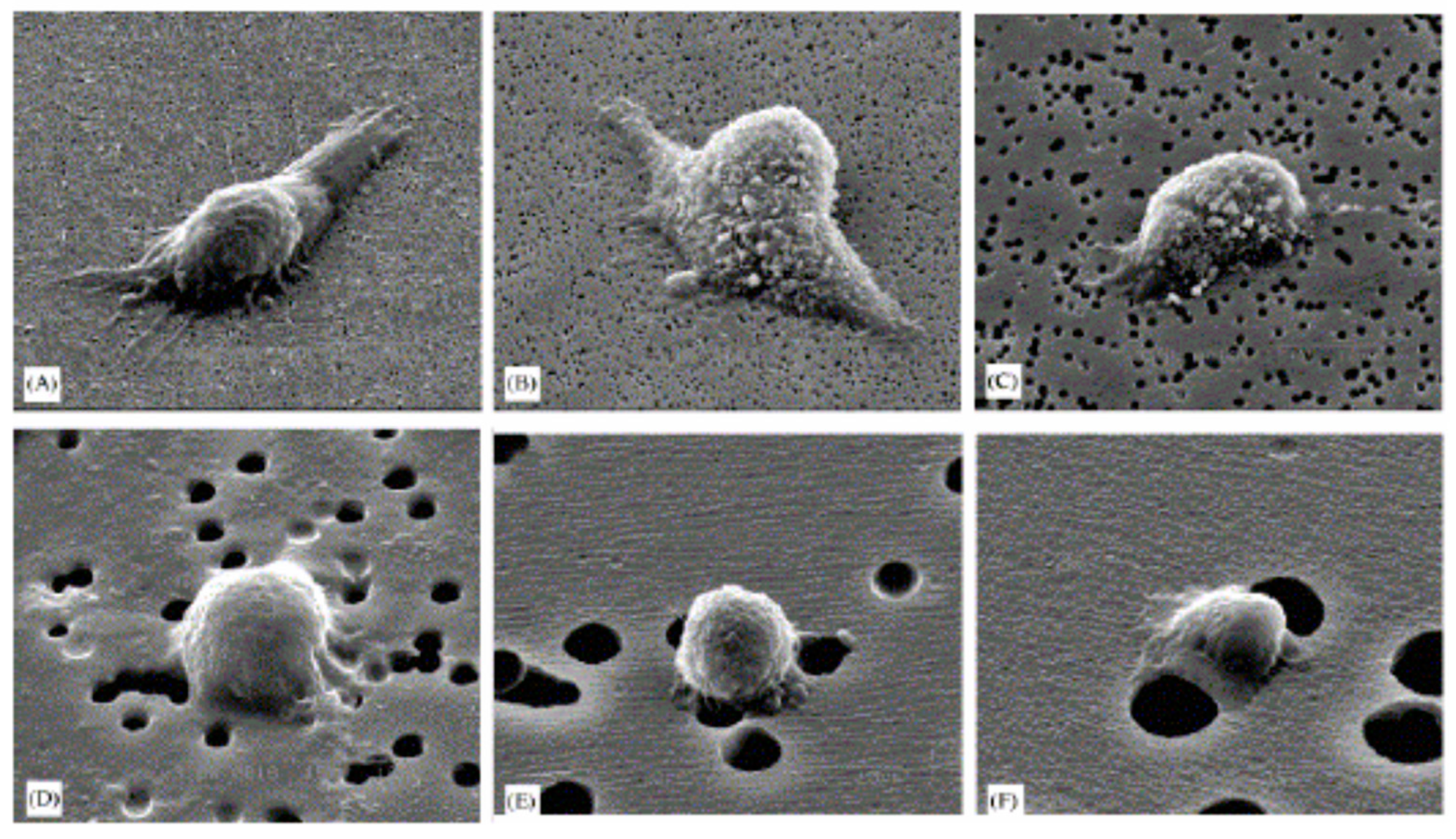
- The effect of enhancing the water repellence of the substrate was evaluated by determining the adhesion and spreading of human fibroblasts on untreated FEP–Teflon (hydrophobized), and was also compared with TCPS [93]. Ion etched Superhydrophobic FEP–Teflon was prepared and followed by oxygen glow-discharge, resulting in water contact angles of 140–150° (untreated FEP–Teflon: 109°). Compared with untreated FEP–Teflon (209 μm2 per cell), a significant decrease in the spreading of human skin fibroblasts was observed on superhydrophobic FEP–Teflon (158 μm2 per cell) (Figure 5). This work put in evidence that adhesion and spreading can be considered two different phenomena; in fact, while cell spreading on TCPS was significantly higher as compared with FEP–Teflon, the number of adhering cells on TCPS, however, was significantly higher than on the hydrophobic FEP–Teflon.
- Cell proliferation on a patterned ordered structure obtained by plasma CVD and VUV irradiation as a combination of both highly hydrophobic and hydrophilic areas was investigated in the work of [94]. The correlation between chemistry, physicochemical properties of the surface, and adhesive behaviour of cells was investigated using such a surface as a scaffold for cell culture. The cell selectivity for superhydrophilic areas was confirmed by comparison with the superhydrophobic part finding the first roughness structure intact. In facts, the cells distributed regularly as circular arrays along the surface pattern with a distance negative effect over a certain size (>400 μm) on the cell adhesive extension with the neighbours. Examining cell behaviour on superhydrophobic and superhydrophilic surfaces has demonstrated that cells adhered and proliferated on both surfaces; even on the superhydrophobic surface, they divide and proliferate in the presence of constant contact. In the study, the role of protein adsorption in the site selectivity in the final adhesion properties on different surfaces was underlined—far greater amounts of proteins adsorbed on the flat hydrophilic surface than on the flat hydrophobic surface.
- Cell attachment is governed by differences in surface energy—higher energy hydrophilic surfaces promote adhesion, while low surface energy substrates usually inhibit cell adhesion. With the aim of providing control of cell adhesion, a combination of superhydrophobic with a specific high energy component like polydopamine was investigated [95]. Superhydrophobic surfaces with their extremely low surface energy can reduce cell adhesion, but in the presence of polydopamine, coatings can become a suitable substrate for cell adhesion. In other words, a selective polydopamine coating on a cell-repellent superhydrophobic background can improve the precision in cell proliferation control systems.
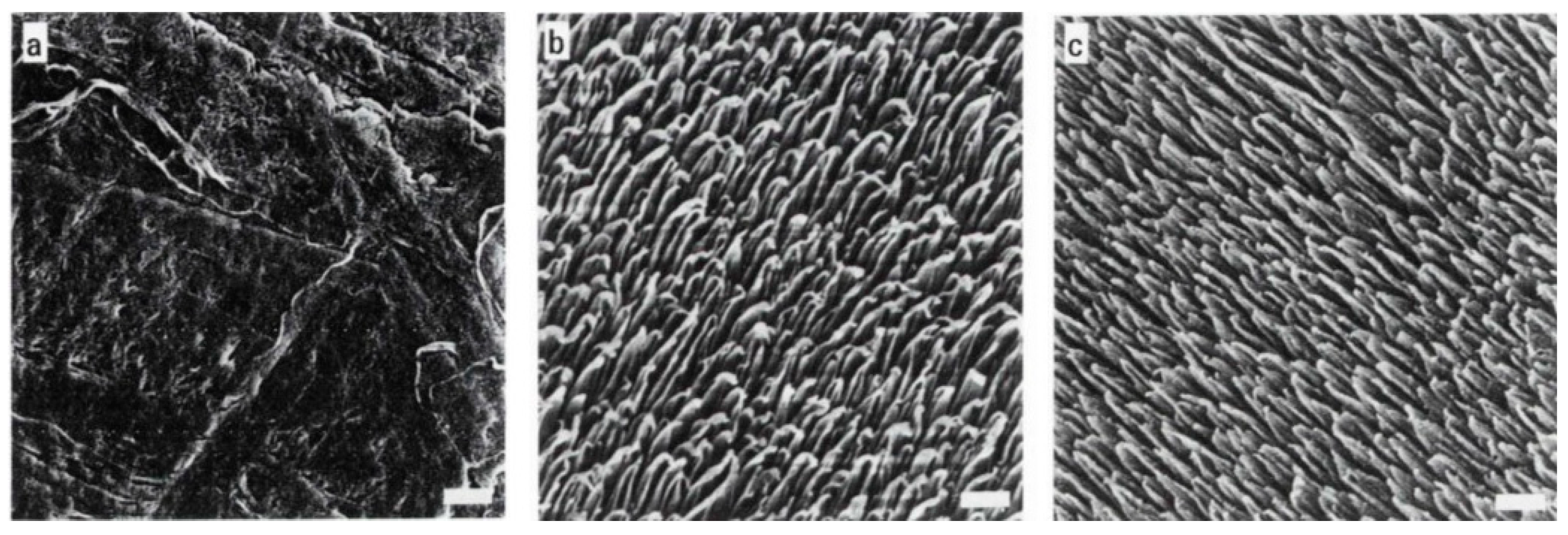
- Nonfouling superhydrophobic silicon nanowire (SiNW) substrate in a stable Cassie–Baxter state, with limited contact with the culture medium, was investigated by exploiting the interface between nanowires and living cells for applications in fields like biomedical implants, biosensors, or drug delivery [96]. Vertically aligned SiNW arrays prepared by the stain etching technique were chemically modified with octadecyltrichlorosilane (OTS), resulting in a superhydrophobic SiNW surface with a contact angle around 160°. Then, by standard optical lithography techniques, a micropatterned superhydrophilic/superhydrophobic SiNW surface was created for a K1 Chinese hamster ovary (CHO) cell culture investigation on patterned superhydrophilic/superhydrophobic silicon nanowire surfaces. As previously reported, superhydrophilic regions selectively discriminated cells’ adhesion from superhydrophobic areas, where cell adhesion was almost completely inhibited. The penetration of cell cytoplasmic projections into the hydrophilic silicon nanowires layer, leading to a strong adhesion through an intimate surface contact, was evidenced by transmission electron microscopy. In contrast, the cell cytoplasmic projections remained on the top of wires in superhydrophobic regions.
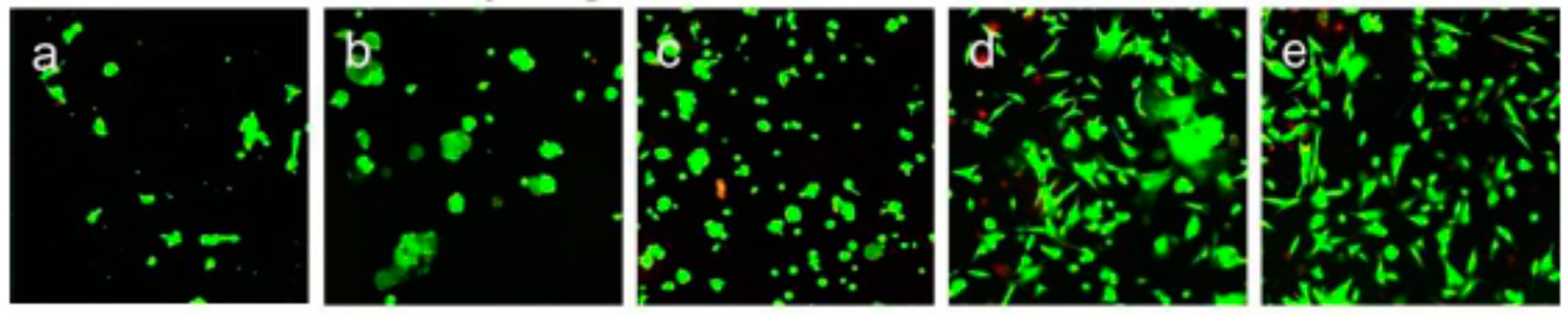
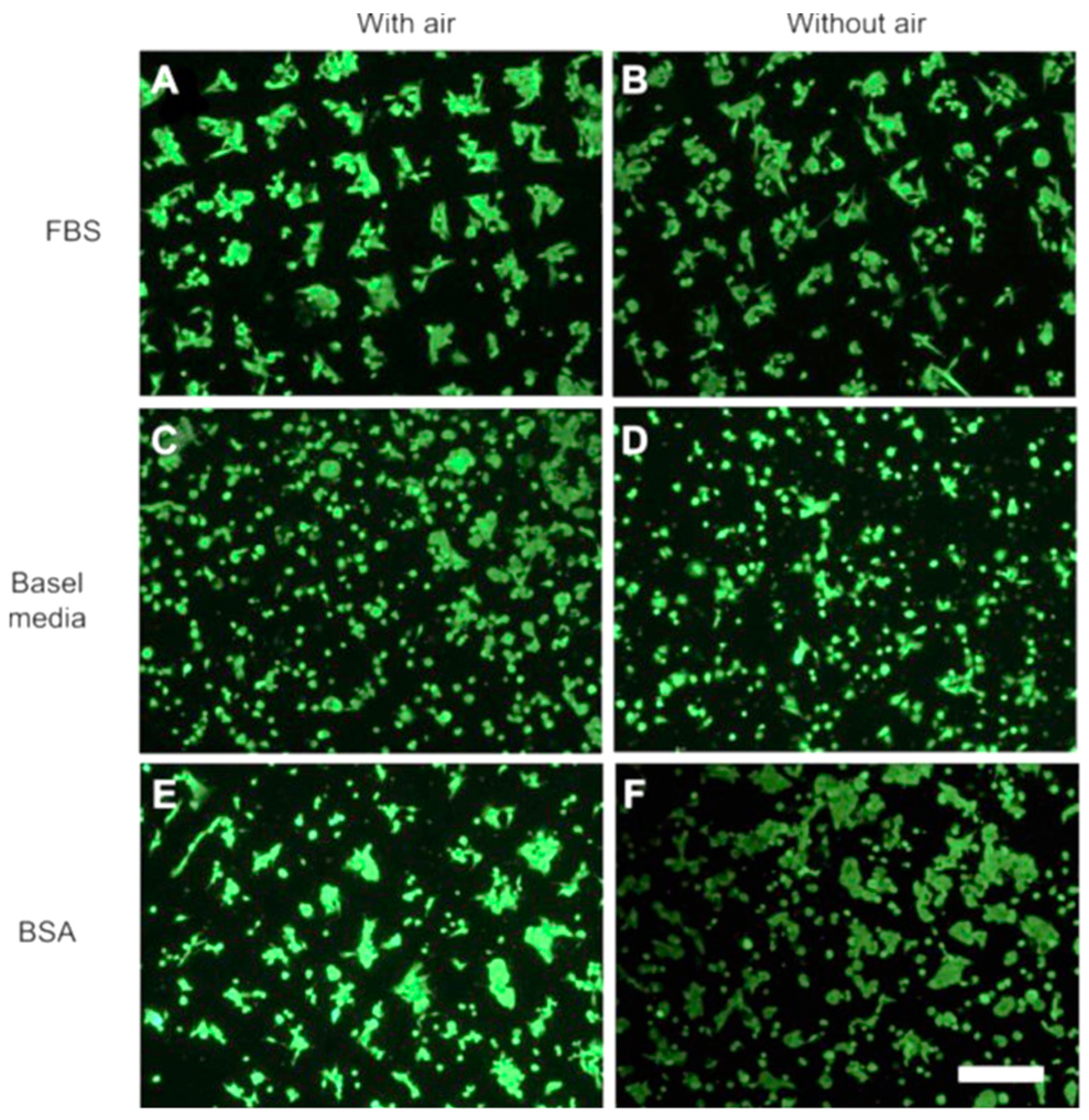
- The presence of trapped air in TiO2 nanotube microtemplated superhydrophobic/superhydrophilic surfaces plays a role in the formation of protein micropatterns, but not cells [97]. The superhydrophobic domains limit the adsorption of either bovine-serum albumin (BSA) or fetal-bovine serum (FBS) solutions, creating a strong contrast between superhydrophilic and superhydrophobic domains. It was observed that cell type and protein composition of the fluid phase influence micropatterns formation of cell (hFOB1.19, MG63, and HeLa), superhydrophilic domains are preferred by all cell types from each fluid phase (FBS, BSA, and basal media containing no protein). On the contrary, the attachment to superhydrophobic domains is not similar for all cell types: no attachment from FBS solutions, with-or-without trapped air, basal media suspensions promote cell attachment to superhydrophobic domains from, with-or-without trapped air, while mixed results are obtained from BSA-containing solutions (Figure 6). In fact, cell attachment seems to be controlled by interfacial tensions between cells, surfaces, and fluid phases. It was found that in the absence of trapped air, more proteins bind to superhydrophobic domains than to superhydrophilic ones [98]. In this case, the authors propose a system for creating patterns of multiple different cell types on one substrate. This method requires control of the spatial arrangement and geometry of different cell types, while keeping them separated and in close proximity for a long time in order to mimic and study a variety of biological processes in vitro. In comparison with existing patterning technologies limited to relatively simple geometry or, for the more complex, usually applicable to only one or two cell types, this approach can create pattern geometries of various complexity. Superhydrophobic borders built in a fine nanoporous polymer film confine the geometry of highly hydrophilic regions, allowing cell positioning in multiple cell-containing microreservoirs. As a case study, we showed the cross-talk between two cell populations via wingless-related integration site (Wnt) signaling molecules propagation during co-culture in a mutual culture medium.
- With the aim of improving the correlation of in vitro and in vivo cellular functions, the requirements of mimicking natural tissue properties (such as chemistry, three-dimensional structure, mechanical properties, etc) in comparison with traditional polystyrene treated flat tissue cell culture dishes for growing, subculturing, and studying cell behavior are widely assessed [99]. Interestingly, NIH 3T3 fibroblasts showed significantly greater adhesion and proliferation on XanoMatrix cell culture dishes; this substrate can be considered a versatile growth platform with the mimicked nanoscale geometry of natural tissue fibers with true, tortuous fiber beds.
- The physico-chemical characterization of an alternative platform surface affecting cells attachment and proliferation is proposed in a paper [100], in which wax-impregnated cotton fabrics were used as a microwell plate, easy to fabricate by a dipping and drying process. Microwell platforms are a widespread standard in cell-based assays and drug screening and, in this case, they represent a sustainable and environmentally friendly method. The influence of surface chemistry, hydrophobicity, and roughness was investigated on cultured human skin fibroblasts. The study underlines a potential use for future cell-based assay platforms, usually being made from non-biodegradable materials such as polystyrene or polyethylene or by the soft lithography and photolithography technique.
- In a recent work, a study on the influence of coating polyester fabric at different degrees of hydrophobicity on a few mammalian cell viability lines was reported [101]. The composition and structure of the mixed organic–inorganic coating with moderate to high water repellence can be finely modulated, resulting in controlling the hydrophobicity of the fabric on commercial, low cost fabric substrates, providing advanced performance. Cell viability on TCPS surfaces with this superhydrophobic coating has efficiently decreased, independent of the cell line type. Comparing the ratio values with those observed on uncoated surfaces and a less hydrophobic coating, the 3T3 or HaCaT cell line decreased their individual responses by 10 times the ratio values. In case of the HeLa line, the hydrophobic coating of polyester (PES) fabric was very efficient in minimizing viability in comparison with coating TCPS surfaces. From these results, tumor cell lines and non-tumor cell lines could be potentially discriminated based on their adhesion on PES fabrics.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pierres, A.; Benoliel, A.M.; Bongrand, P. Cell-cell interaction. In Physical Chemistry of Biological Interfaces; Baszkin, A., Norde, W., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 459–522. [Google Scholar]
- Brochard-Wyart, F.; de Gennes, P.G. Adhesion induced by mobile binders: Dynamics. Proc. Natl. Acad. Sci. USA 2002, 99, 7854–7859. [Google Scholar] [CrossRef] [PubMed]
- Lasky, L.A.; Singer, M.S.; Dowbenko, D.; Imai, Y.; Henzel, W.J.; Grimley, C.; Fennie, C.; Gillett, N.; Watson, S.R.; Rosent, S.D. An endothelial ligand for L-Selectin is a novel mucin-like molecule. Cell 1992, 69, 927–938. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Koch, A.E. Cell-cell interactions in synovitis: Endothelial cells and immune cell migration. Arthritis Res. 2000, 2, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ingber, D.E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1999, 1, E131. [Google Scholar] [CrossRef] [PubMed]
- Okegawa, T.; Pong, R.-C.; Li, Y.; Hsieh, J.-T. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim. Pol. 2004, 51, 445–457. [Google Scholar] [PubMed]
- Hirohashi, S.; Kanai, Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003, 94, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Perinpanayagam, H.; Zaharias, R.; Stanford, C.; Keller, J.; Schneider, G.; Brand, R. Early cell adhesion events differ between osteoporotic and non-osteoporotic osteoblasts. J. Orthop. Res. 2001, 19, 993–1000. [Google Scholar] [CrossRef]
- Cho, P.; Schneider, G.B.; Kellogg, B.; Zaharias, R.; Keller, J.C. Effect of glucocorticoid-induced osteoporotic-like conditions on osteoblast cell attachment to implant surface microtopographies. Implant. Dent. 2006, 15, 377–385. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Simon, S.; Green, C.E. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu. Rev. Biol. 2005, 7, 151–185. [Google Scholar] [CrossRef]
- Spangenberg, C.; Lausch, E.U.; Trost, T.M.; Prawitt, D.; May, A.; Keppler, R.; Fees, S.A.; Reutzel, D.; Bell, C.; Schmitt, S.; et al. ERBB2-mediated transcriptional up-regulation of the α5β1 integrin fibronectin receptor promotes tumor cell survival under adverse conditions. Cancer Res. 2006, 66, 3715–3725. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.X.; Liu, Y.; Pasquale, E.B.; Ruoslahti, E. Activated Src oncogene phosphorylates R-ras and suppresses integrin activity. J. Biol. Chem. 2002, 277, 1824–1827. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Cancer cells regulate biomechanical properties of human microvascular endothelial cells. J. Biol. Chem. 2011, 286, 40025–40037. [Google Scholar] [CrossRef] [PubMed]
- Schakenraad, J.M.; Busscher, H.J. Cell polymer interactions—the influence of protein adsorption. Colloids Surf. 1989, 42, 331–343. [Google Scholar] [CrossRef]
- Harnett, E.M.; Alderman, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf. B 2007, 55, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Kobayashi, J.; Okano, T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J. R Soc. Interf. 2009, 6, S293–S309. [Google Scholar] [CrossRef] [PubMed]
- Vagaska, B.; Bacakova, L.; Filova, E.; Balik, K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol. Res. 2010, 59, 309–322. [Google Scholar] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, S.; Wittemann, A.; Ballauff, M.; Breininger, E.; Bolze, J.; Dingenouts, N. Interaction of proteins with spherical polyelectrolyte brushes in solution as studied by small-angle X-ray scattering. Phys. Rev. E 2004, 70, 061403. [Google Scholar] [CrossRef] [PubMed]
- Wittemann, A.; Haupt, B.; Ballauff, M. Adsorption of proteins on spherical polyelectrolyte brushes in aqueous solution. Phys. Chem Chem Phys. 2003, 5, 1671–1677. [Google Scholar] [CrossRef]
- Worz, A.; Berchtold, B.; Moosmann, K.; Prucker, O.; Ruhe, J. Protein- resistant polymer surfaces. J. Mater. Chem. 2012, 22, 19547–19561. [Google Scholar] [CrossRef]
- Ko, Y.G.; Kim, Y.H.; Park, K.D.; Lee, H.J.; Lee, W.K.; Park, H.D.; Kim, S.H.; Lee, G.S.; Ahn, D.J. Immobilization of poly(ethylene glycol) or its sulfonate onto polymer surfaces by ozone oxidation. Biomaterials 2001, 22, 21152123. [Google Scholar] [CrossRef]
- Niepel, M.S.; Peschel, D.; Groth, T. Controlling fibroblast adhesion with pH modified polyelectrolyte multilayers. Int. J. Artif. Organs 2011, 34, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Biocompatibility of materials. In Biomaterials and Tissue Engineering. Biological and Medical Physics, Biomedical Engineering; Shi, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Ratner, B.D. The biocompatibility of implant materials. In Host Response to Biomaterials. The Impact of Host Response on Biomaterial Selection; BadylaK, S.F., Ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Kulinets, I. Biomaterials and their applications in medicine. In Regulatory Affairs for Biomaterials and Medical Devices. A Volume in Woodhead Publishing Series in Biomaterials; Amato, S.F., Ezzell, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Oliveira, S.M.; Alves, N.A.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2012, 28, 1–21. [Google Scholar] [CrossRef]
- Ramires, P.A.; Mirenghi, L.; Romano, A.R.; Palumbo, F.; Nicolardi, G. Plasma-treated PET surfaces improve the biocompatibility of human endothelial cells. J. Biomed. Mater. Res. 2000, 51, 535–539. [Google Scholar] [CrossRef]
- Pu, F.R.; Williams, R.L.; Markkula, T.K.; Hunt, J.A. Expression of leukocyte-endothelial cell adhesion molecules on monocyte adhesion to human endothelial cells on plasma treated PET and PTFE in vitro. Biomaterials 2002, 23, 4705–4718. [Google Scholar] [CrossRef]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Geckeler, K.E.; Wacker, R.; Martini, F.; Hack, A.; Aicher, W.K. Enhanced biocompatibility for SAOS-2 osteosarcoma cells by surface coating with hydrophobic epoxy resins. Cell. Physiol. Biochem. 2003, 13, 155–164. [Google Scholar] [CrossRef]
- Vasita, R.; Shanmugam, K.; Katti, D.S. Improved biomaterials for tissue engineering applications: Surface modification of polymers. Curr. Top. Med. Chem. 2008, 8, 341–353. [Google Scholar]
- Anselme, K.; Ploux, L.; Ponche, A. Cell/material interfaces: Influence of surface chemistry and surface topography on cell adhesion. J. Adhes. Sci. Technol. 2010, 24, 831–852. [Google Scholar] [CrossRef]
- Lourenço, B.N.; Marchioli, G.; Song, W.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; van Apeldoorn, A.; Mano, J.F. Wettability influences cell behavior on superhydrophobic surfaces with different topographies. Biointerphases 2012, 7, 46. [Google Scholar]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Khatiwala, C.B.; Peyton, S.R.; Metzke, M.; Putnam, D. The regulation of osteogenesis by ECM rigidity in MC3T3-E1 cells requires MAPK activation. J. Cell Physiol. 2007, 211, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Mason, B.N.; Califano, J.P.; Reinhart-King, C.A. Matrix stiffness: A regulator of cellular behavior and tissue formation. In Engineering Biomaterials for Regenerative Medicine; Bhatia, S., Ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Zemla, J.; Danilkiewicz, J.; Orzechowska, B.; Pabijan, J.; Seweryn, S.; Lekka, M. Atomic force microscopy as a tool for assessing the cellular elasticity and adhesiveness to identify cancer cells and tissues. Semin. Cell Dev. Biol. 2018, 73, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Szymon Prauzner-Bechcicki, S.; Joanna Raczkowska, J.; Madej, E.; Pabijan, J.; Lukes, J.; Sepitka, J.; Rysz, J.; Awsiuk, K.; Bernasik, A.; Budkowski, A.; et al. PDMS substrate stiffness affects the morphology and growth profiles of cancerous prostate and melanoma cells. J. Mech. Behav. Biomed. Mater. 2015, 41, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Raczkowska, J.; Prauzner-Bechcicki, S.; Lukes, J.; Sepitka, J.; Bernasik, A.; Awsiuk, K.; Paluszkiewicz, C.; Pabijan, J.; Lekka, M.; Budkowski, A. Physico-chemical properties of PDMS surfaces suitable as substrates for cell cultures. Appl. Surf. Sci. 2016, 389, 247–254. [Google Scholar] [CrossRef]
- Bartalena, G.; Loosli, Y.; Zambelli, T.; Snedeker, J.G. Biomaterial surface modifications can dominate cell–substrate mechanics: The impact of PDMS plasma treatment on a quantitative assay of cell stiffness. Soft Matter 2012, 8, 673–681. [Google Scholar] [CrossRef]
- Fairhurst, D.; Rowell, R.L.; Monahan, I.M.; Key, S.; Stieh, D.; McNeil-Watson, F.; Morfesis, A.; Mitchnick, M.; Shattock, R.A. Microbicides forHIV/AIDS. 2. Electrophoretic fingerprinting of CD4+ T-cell model systems. Langmuir 2007, 23, 2680–2687. [Google Scholar] [CrossRef]
- De Kerchove, A.J.; Elimelech, M. Impact of alginate conditioning film on deposition kinetics of motile and nonmotile Pseudomonas aeruginosa strains. Appl Environ. Microbiol. 2007, 73, 5227–5234. [Google Scholar] [CrossRef]
- Burello, E. Profiling the biological activity of oxide nanomaterials with mechanistic models. Comput. Sci. Discov. 2013, 6, 014009. [Google Scholar] [CrossRef][Green Version]
- Czeslik, C.; Jackler, G.; Hazlett, T.; Gratton, E.; Steitz, R.; Wittemann, A.; Bal-lauff, M. Salt-induced protein resistance of polyelectrolyte brushes studied using fluorescence correlation spectroscopy and neutron reflectometry. Phys. Chem Chem Phys. 2004, 6, 5557–5563. [Google Scholar] [CrossRef]
- Kim, S.E.E.A.; Kihm, K.D. Surface elasticity and charge concentration dependent endothelial cell attachment to copolymer polyelectrolyte hydrogel. Acta Biomater. 2009, 5, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Bet, M.R.; Goissis, G.; Vargas, S.; Selistre-de-Araujo, H.S. Cell adhesion and cytotoxicity studies over polyanionic collagen surfaces with variable negative charge and wettability. Biomaterials 2003, 24, 131–137. [Google Scholar] [CrossRef]
- Schneider, G.B.; English, A.; Abahram, M.; Zaharias, R.; Stanford, C.; Keller, J. The effect of hydrogel charge density on cell attachment. Biomaterials 2004, 25, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, M.; Pumberger, M.; Casper, M.E.; Shogren, K.; Giuliani, M.; Ruesink, T.; Hefferan, T.E.; Currier, B.L.; Yaszemski, M.J. The effects of fixed electrical charge on chondrocyte behavior. Acta Biomater. 2011, 7, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Makohliso, S.A.; Valentini, R.F.; Aebischer, P. Magnitude and polarity of a fluoroethylene propylene electret substrate charge influences neurite outgrowth in vitro. J. Biomed. Mater. Res. 1983, 7, 1075–1085. [Google Scholar]
- Lee, J.H.; Jung, H.W.; Kang, I.K.; Lee, H.B. Cell behaviour on polymer surfaces with different functional groups. Biomaterials 1994, 15, 705–711. [Google Scholar] [CrossRef]
- Thevenot, P.; Hu, W.J.; Tang, L.P. Surface chemistry influences implant biocompatibility. Curr. Top. Medic. Chem. 2008, 8, 270–280. [Google Scholar]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 5953–5957. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. A 2003, 66, 247–259. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Li, M.; Shi, L.; Ong, J.L.T.; Jańczewski, D.; Neoh, K.G. Parallel control over surface charge and wettability using polyelectrolyte architecture: Effect on protein adsorption and cell adhesion. ACS Appl. Mater. Interf. 2016, 8, 30552–30563. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Dourado, F.; Rodrigues, L.R. Overview on Cell-Biomaterial Interactions. In Advanced Polymers in Medicine; Puoci, F., Ed.; Springer: Cham, Switzerland, 2015; pp. 91–128. [Google Scholar]
- Donoso, M.G.; Méndez-Vilas, A.; Bruque, J.M.; González-Martin, M.L. On the relationship between common amplitude surface roughness parameters and surface area: Implications for the study of cell–material interactions. Int. Biodeter. Biodegr. 2007, 59, 245–251. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, J.S.; Park, K.S.; Khang, G.; Lee, Y.M.; Lee, H.B. Response of MG63 osteoblast-like cells onto polycarbonate membrane surfaces with different micropore sizes. Biomaterials 2004, 25, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- Bartolo, L.D.; Rende, M.; Morelli, G.G.; Salerno, S.; Piscioneri, A.; Gordano, A.; Di Vito, A.; Canonaco, M.; Drioli, E. Influence of membrane surface properties on the growth of neuronal cells isolated form hippocampus. J. Membr. Sci. 2008, 325, 139–149. [Google Scholar] [CrossRef]
- Chung, T.W.; Liu, D.Z.; Wang, S.Y.; Wang, S.S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Kim, M.H.; Kino-Oka, M.; Kawase, M.; Yagi, K.; Taya, M. Response of human epithelial cells to culture surfaces with varied roughnesses prepared by immobilizing dendrimers with/without D-glucose display. J. Biosci. Bioeng. 2007, 103, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J. Topographically induced direct cell mechanotransduction. Med. Eng. Phys. 2005, 27, 730–742. [Google Scholar] [CrossRef]
- Washburn, N.R.; Yamada, K.M.; Simon, C.G., Jr.; Kennedy, S.B.; Amis, E.J. High throughput investigation of osteoblast response to polymer crystallinity: Influence of nanometer-scale roughness on proliferation. Biomaterials 2004, 25, 1215–1224. [Google Scholar] [CrossRef]
- Ranella, A.; Barberoglou, M.; Bakogianni, S.; Fotakis, C.; Stratakis, E. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures. Acta Biomater. 2010, 6, 2711–2720. [Google Scholar] [CrossRef]
- Zheng, J.; Song, W.; Huang, H.; Chen, H. Protein adsorption and cell adhesion on polyurethane/Pluronic surface with lotus leaf-like topography. Colloids Surf. B 2010, 77, 234–239. [Google Scholar] [CrossRef]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, L.; Song, W.; Wu, Z.; Li, D. Biocompatible polymer materials: Role of protein-surface interactions. Prog. Polym. Sci. 2008, 33, 1059–1087. [Google Scholar] [CrossRef]
- Alves, N.M.; Pashkuleva, I.; Reis, R.L.; Mano, J.F. Controlling cell behavior through the design of polymer surfaces. Small 2010, 6, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Brown, X.Q.; Ookawa, K.; Wong, J.Y. Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: Interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials 2005, 26, 3123–3129. [Google Scholar] [CrossRef] [PubMed]
- Carré, A.; Mittal, K.L. Superhydrophobic Surfaces; VSP/Brill: Leiden, The Netherlands, 2009. [Google Scholar]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 0546–0550. [Google Scholar] [CrossRef]
- Bartolo, D.; Bouamrirene, F.; Verneuil, E.; Buguin, A.; Silberzan, P.; Moulinet, S. Bouncing or sticky droplets: Impalement transitions on superhydrophobic micropatterned surfaces. Europhys. Lett. 2006, 74, 299–305. [Google Scholar] [CrossRef]
- Reyssat, M.; Pépin, A.; Marty, F.; Chen, Y.; Quéré, D. Bouncing transitions on microtextured materials. Europhys. Lett. 2006, 74, 306–312. [Google Scholar] [CrossRef]
- Sbragaglia, M.; Peters, A.M.; Pirat, C.; Borkent, B.M.; Lammertink, R.G.H.; Wessling, M.; Lohse, D. Spontaneous breakdown of superhydrophobicity. Phys. Rev. Lett. 2007, 99, 156001. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhang, J. Air layer on superhydrophobic surface underwater. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 374–378. [Google Scholar] [CrossRef]
- Mohammadi, R.; Wassink, J.; Amirfazli, A. Effect of surfactants on wetting of super-hydrophobic surfaces. Langmuir 2004, 20, 9657–9662. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Ravera, F.; Rao, S.; Liggieri, L. Surfactant adsorption at superhydrophobic surfaces. Appl. Phys. Lett. 2006, 89, 053104. [Google Scholar] [CrossRef]
- Chang, F.M.; Sheng, Y.J.; Chen, H.; Tsao, H.K. From superhydrophobic to superhydrophilic surfaces tuned by surfactant solutions. Appl. Phys. Lett. 2007, 91, 094108. [Google Scholar] [CrossRef]
- Extrand, C.W. Designing for optimum liquid repellency. Langmuir 2006, 22, 1711–1714. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. A perfectly hydrophobic surface (θA/Θr = 180°/180°). J. Am. Chem. Soc. 2006, 128, 9052–9053. [Google Scholar] [CrossRef]
- Horbett, T.A. Protein adsorption on biomaterials. Biomater. Interf. Phenom. Appl. 1982, 199, 233–244. [Google Scholar]
- Young, B.R.; Pitt, W.G.; Cooper, S.L. Protein adsorption on polymeric biomaterials: II. Adsorption kinetics. J. Colloid Interf. Sci. 1988, 124, 28–43. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S. Nonfouling surfaces. Biomater. Sci. Introd. Mater. Med. 2004, 97–201. [Google Scholar]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Lih, E.; Oh, S.H.; Joung, Y.K.; Lee, J.H.; Han, D.K. Polymers for cell/tissue anti-adhesion. Prog. Polym. Sci. 2015, 44, 28–61. [Google Scholar] [CrossRef]
- Lee, J.N.; Jiang, X.; Ryan, D.; Whitesides, G.M. Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane). Langmuir 2004, 20, 11684–11691. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.Z.; Tabatabaei-Panah, P.; Seyfi, J. Emphasizing the role of surface chemistry on hydrophobicity and cell adhesion behavior of polydimethylsiloxane/TiO2nanocomposite films. Colloids Surf. B Biointerfaces 2018, 167, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Stokroos, I.; Golverdingen, J.G.; Schakenraad, J.M. Adhesion and spreading of human fibroblasts on superhydrophobic Fep.Teflon. Cells Mater. 1991, 1, 243–249. [Google Scholar]
- Ishizaki, T.; Saito, N.; Takai, O. Correlation of cell adhesive behaviors on superhydrophobic, superhydrophilic, and micropatterned superhydrophobic/superhydrophilic surfaces to their surface chemistry. Langmuir 2010, 26, 8147–8154. [Google Scholar] [CrossRef]
- Kang, S.M.; Choi, I.S. Control of Cell Adhesion on a superhydrophobic surface by polydopamine coating. Bull. Korean Chem. Soc. 2013, 34, 2525–2527. [Google Scholar] [CrossRef]
- Piret, G.; Galopin, E.; Coffinier, Y.; Boukherroub, R.; Legrand, D.; Slomianny, C. Culture of mammalian cells on patterned superhydrophilic/superhydrophobic silicon nanowire array. Soft Matter 2011, 7, 8642–8649. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, L.; Yang, Y.; Hu, R.; Vogler, E.A.; Lin, C. Role of trapped air in the formation of cell-and-protein micropatterns on superhydrophobic/superhydrophilic microtemplated surfaces. Biomaterials 2012, 33, 8213–8220. [Google Scholar] [CrossRef] [PubMed]
- Efremov, A.N.; Stanganello, E.; Welle, A.; Scholpp, S.; Levkin, P.A. Micropatterned superhydrophobic structures for the simultaneous culture of multiple cell types and the study of cell-cell communication. Biomaterials 2013, 34, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, G.; Webster, T.J. Increased NIH 3T3 fibroblast functions on cell culture dishes which mimic the nanometer fibers of natural tissues. Int. J. Nanomed. 2015, 10, 5293–5299. [Google Scholar]
- Wahab, N.M.; Jamil, S.A.; Riban, D.G.; Majid, F.A.A.; Kadir, M.R.A.; Wicaksono, D.H.B. Wax-Impregnated Cotton Fabrics as Cell Culture Platform. Adv. Mater. Res. 2015, 1112, 441–444. [Google Scholar] [CrossRef]
- Morán, M.C.; Ruano, G.; Cirisano, F.; Ferrari, M. Mammalian cell viability on hydrophobic and superhydrophobic fabrics. Mater. Sci. Eng. C 2019, 99, 241–247. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. https://doi.org/10.3390/colloids3020048
Ferrari M, Cirisano F, Morán MC. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids and Interfaces. 2019; 3(2):48. https://doi.org/10.3390/colloids3020048
Chicago/Turabian StyleFerrari, Michele, Francesca Cirisano, and M. Carmen Morán. 2019. "Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties" Colloids and Interfaces 3, no. 2: 48. https://doi.org/10.3390/colloids3020048
APA StyleFerrari, M., Cirisano, F., & Morán, M. C. (2019). Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids and Interfaces, 3(2), 48. https://doi.org/10.3390/colloids3020048