Adsorption of Hyperbranched Arabinogalactan-Proteins from Plant Exudate at the Solid–Liquid Interface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Surface Preparation
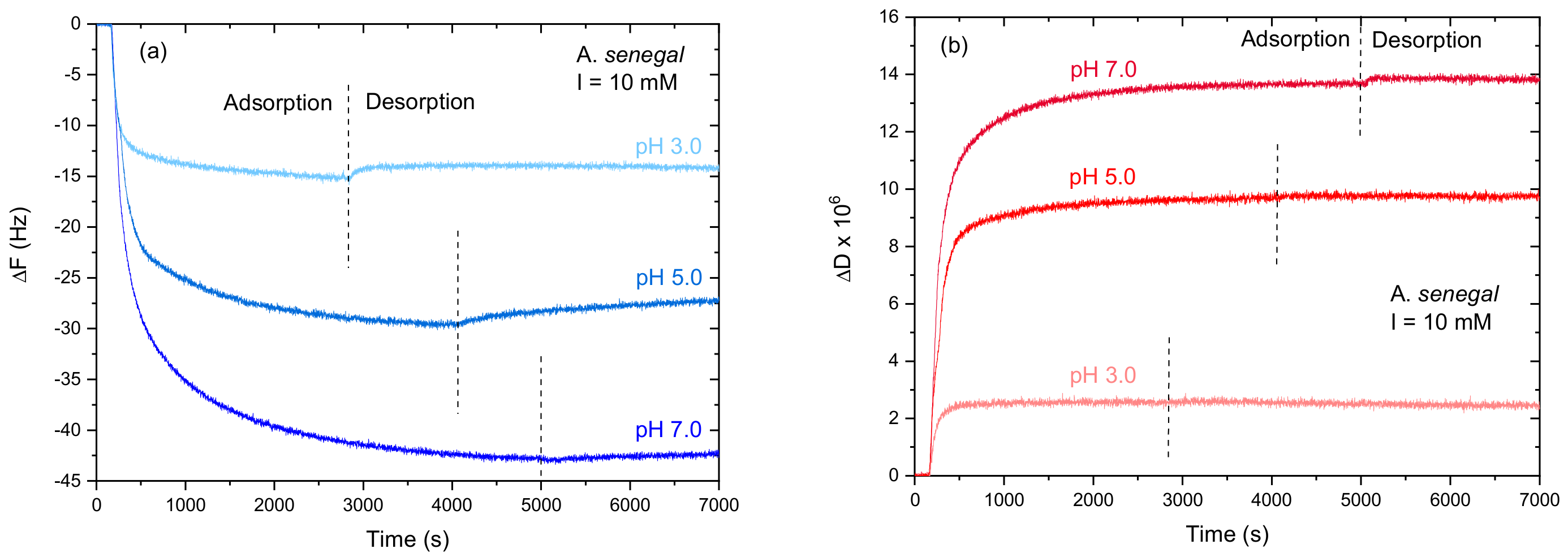
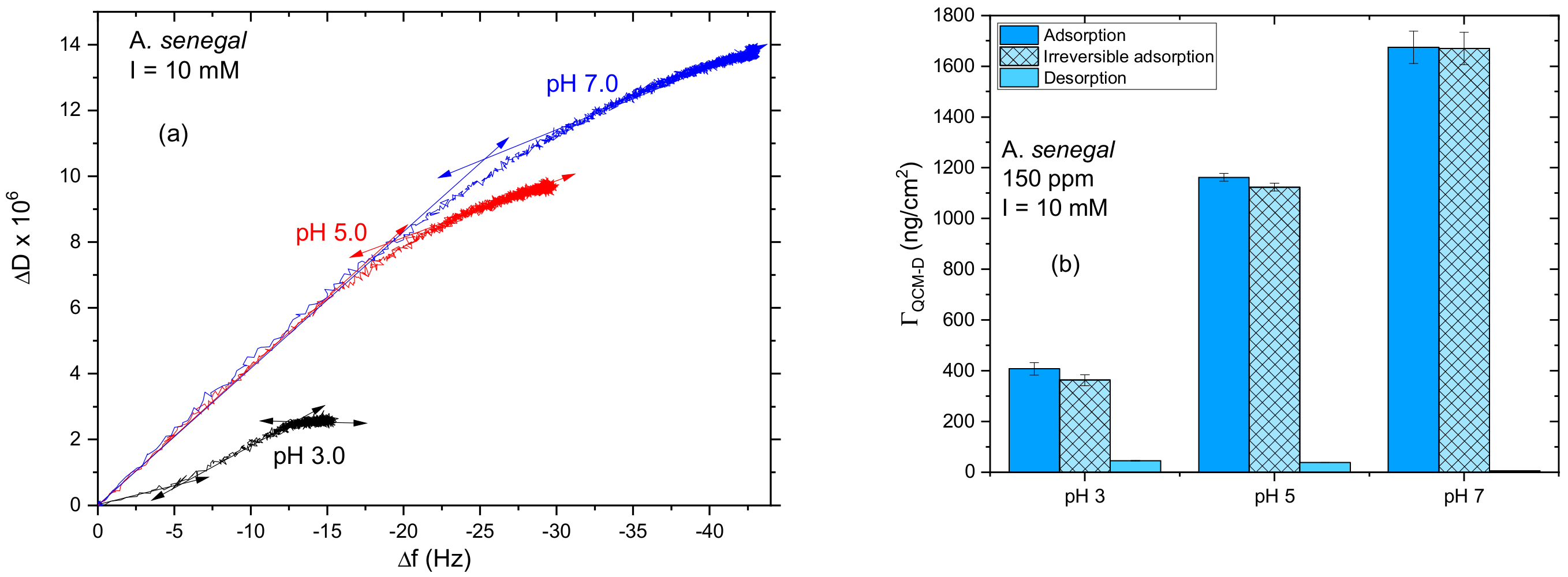
2.2.2. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
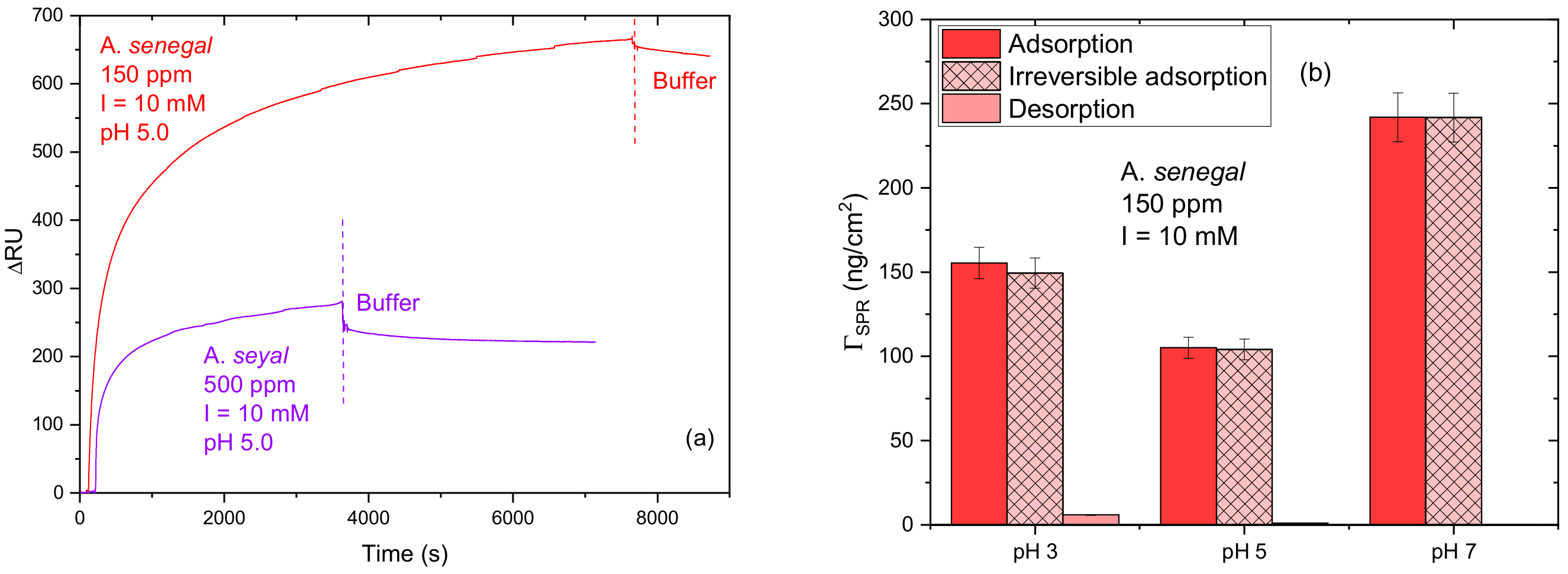
2.2.3. Surface Plasmon Resonance (SPR)
2.2.4. Hydration State of the Adsorbed Film
2.2.5. Electrophoretic Mobility
2.2.6. Atomic Force Microscopy (AFM)
3. Results
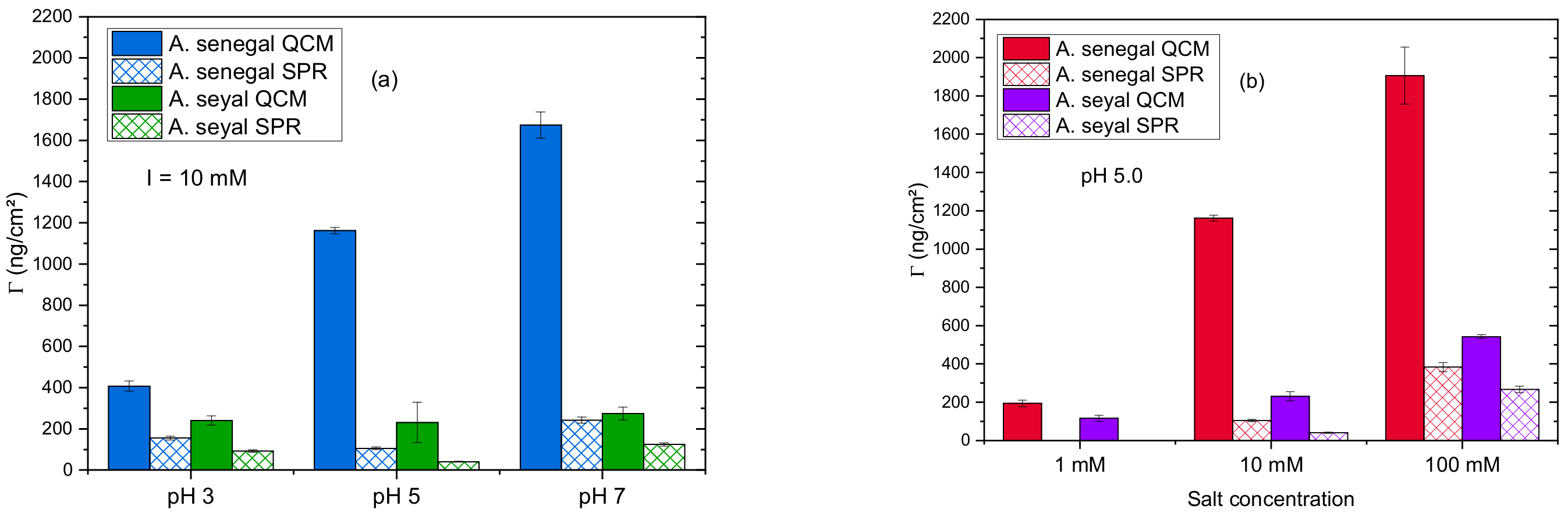
3.1. A. senegal Gum Adsorption Behavior
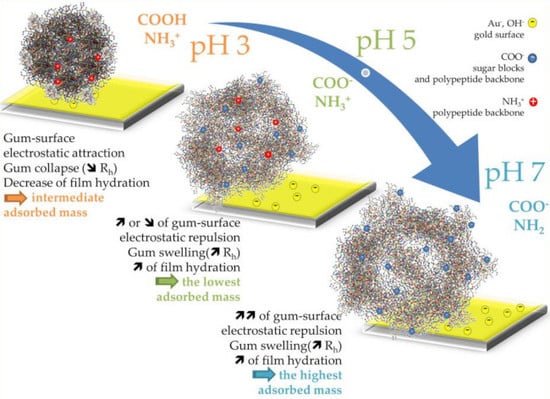
3.1.1. Influence of pH
3.1.2. Influence of Salt Concentration
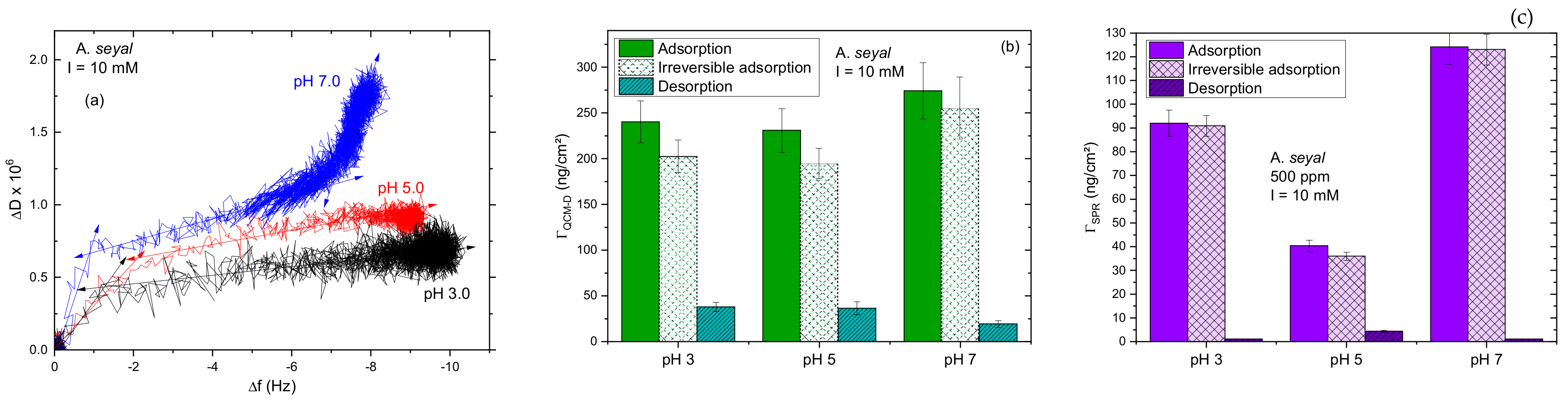
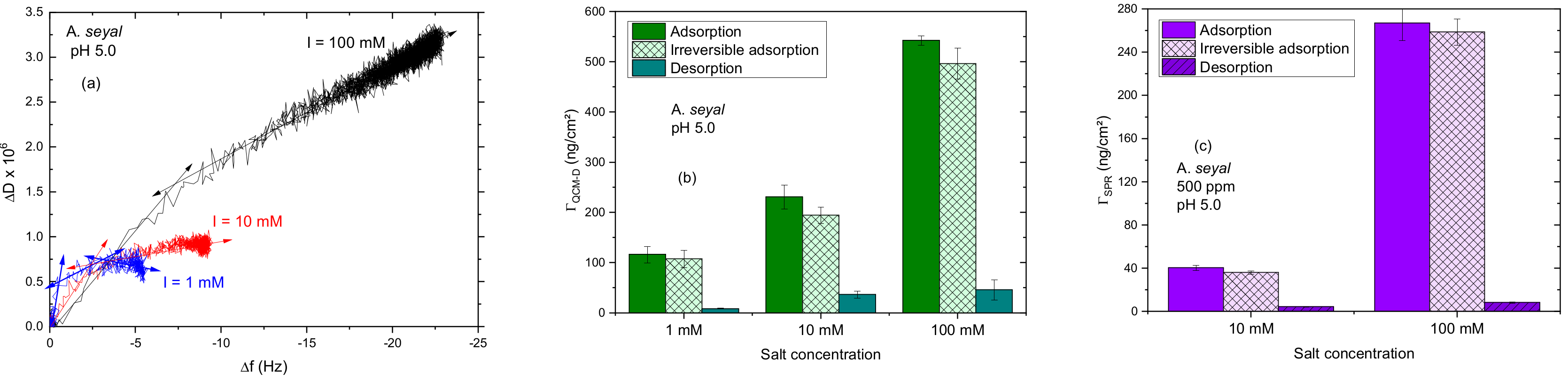
3.2. A. seyal Gum Adsorption Behavior
3.2.1. Influence of pH
3.2.2. Impact of the Salt Concentration
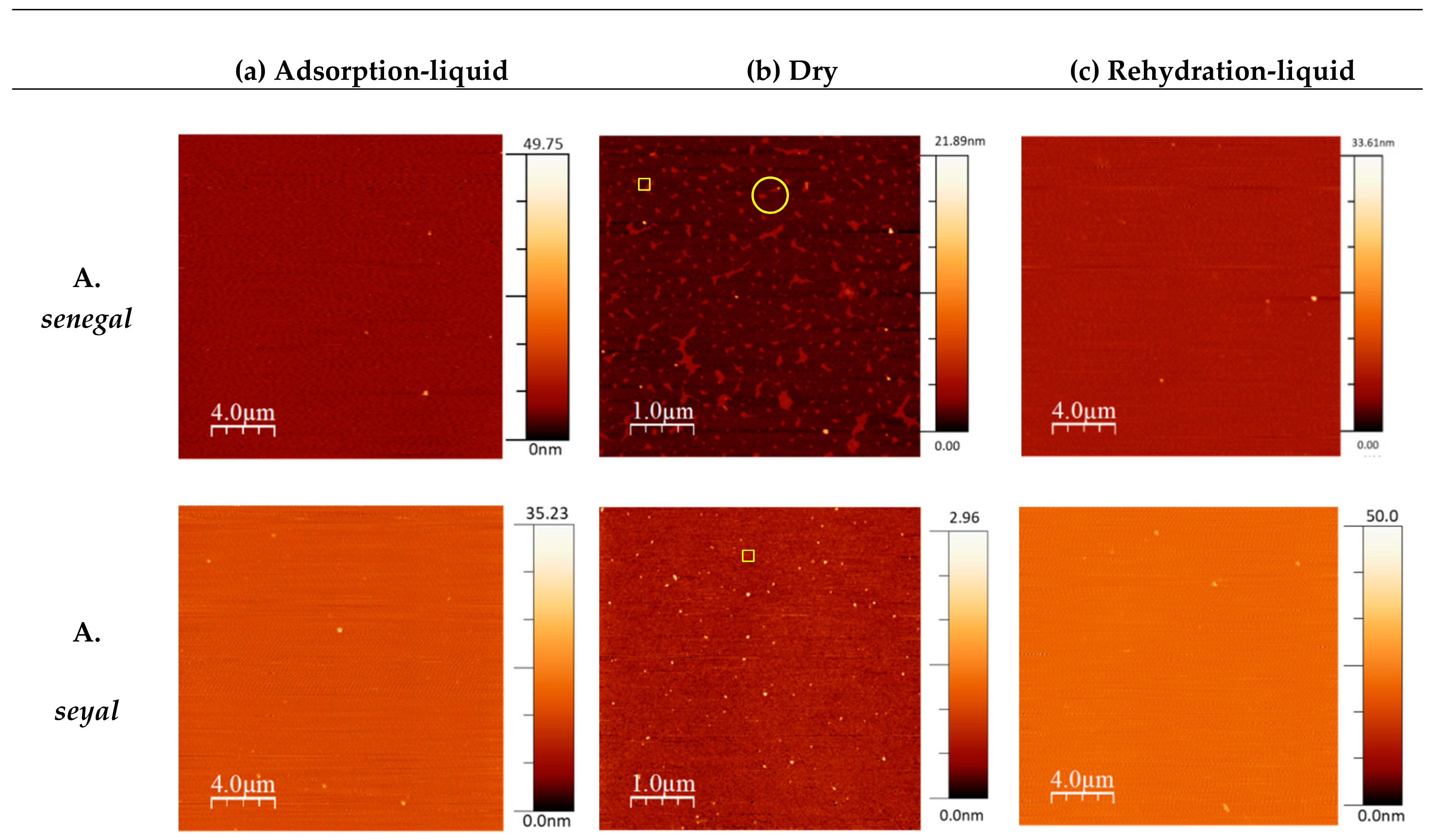
3.3. Morphological Characterization of Gum Films
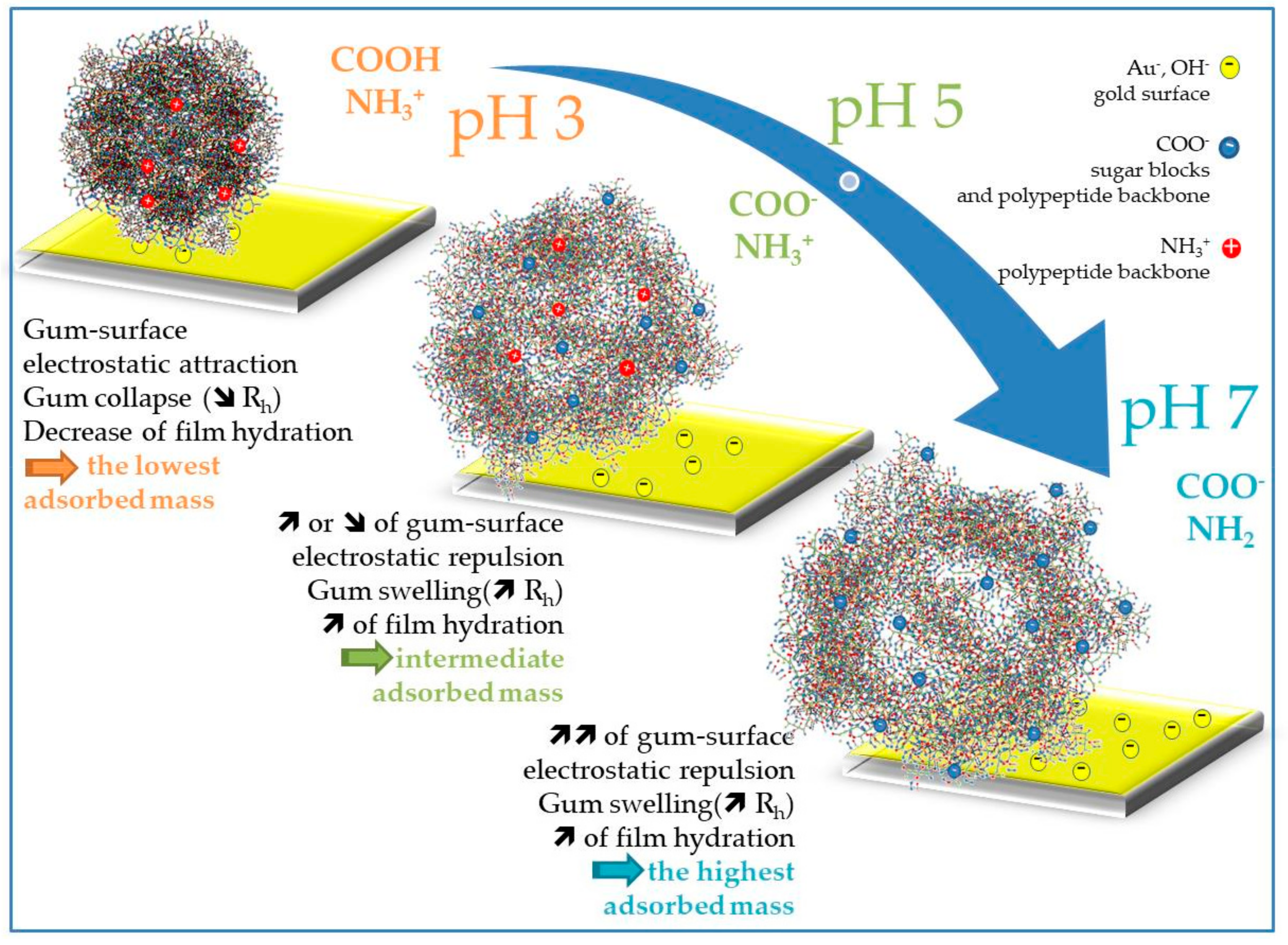
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchez, C.; Nigen, M.; Mejia Tamayo, V.; Doco, T.; Williams, P.; Amine, C.; Renard, D. Acacia gum: History of the future. Food Hydrocoll. 2018, 78, 140–160. [Google Scholar] [CrossRef]
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate gums: Occurrence, production, and applications. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar] [CrossRef]
- Nussinovitch, A. Plant Gum Exudates of the World: Sources, Distribution, Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978−1-4200-5224-4. [Google Scholar]
- Randall, R.C.; Phillips, G.O.; Williams, P.A. Fractionation and characterization of gum from Acacia senegal. Food Hydrocoll. 1989, 3, 65–75. [Google Scholar] [CrossRef]
- Renard, D.; Lavenant-Gourgeon, L.; Ralet, M.-C.; Sanchez, C. Acacia senegal Gum: Continuum of Molecular Species Differing by Their Protein to Sugar Ratio, Molecular Weight, and Charges. Biomacromolecules 2006, 7, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Torrez, L.; Nigen, M.; Williams, P.; Doco, T.; Sanchez, C. Acacia senegal vs. Acacia seyal gums—Part 1: Composition and structure of hyperbranched plant exudates. Food Hydrocoll. 2015, 51, 41–53. [Google Scholar] [CrossRef]
- Mejia Tamayo, V.; Nigen, M.; Apolinar-Valiente, R.; Doco, T.; Williams, P.; Renard, D.; Sanchez, C. Flexibility and Hydration of Amphiphilic Hyperbranched Arabinogalactan-Protein from Plant Exudate: A Volumetric Perspective. Colloids Interfaces 2018, 2, 11. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Andres-Brull, M.; Cirre, J.; Phillips, G.O. Structural Changes Following Industrial Processing of Acacia Gums. In Gum Arabic; RSC Publishing: Cambridge, UK, 2011; pp. 153–168. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley-Blackwell: New York, NY, USA, 1997; ISBN 978-0-471−14873-9. [Google Scholar]
- Redgwell, R.J.; Schmitt, C.; Beaulieu, M.; Curti, D. Hydrocolloids from coffee: Physicochemical and functional properties of an arabinogalactan-protein fraction from green beans. Food Hydrocoll. 2005, 19, 1005–1015. [Google Scholar] [CrossRef]
- Amiri, A.; Shanbedi, M.; Eshghi, H.; Heris, S.Z.; Baniadam, M. Highly Dispersed Multiwalled Carbon Nanotubes Decorated with Ag Nanoparticles in Water and Experimental Investigation of the Thermophysical Properties. J. Phys. Chem. C 2012, 116, 3369–2275. [Google Scholar] [CrossRef]
- Bandyopadhyaya, R.; Nativ-Roth, E.; Regev, O.; Yerushalmi-Rozen, R. Stabilization of Individual Carbon Nanotubes in Aqueous Solutions. Nano Lett. 2002, 2, 25–28. [Google Scholar] [CrossRef]
- Dror, Y.; Pyckhout-Hintzen, W.; Cohen, Y. Conformation of Polymers Dispersing Single-Walled Carbon Nanotubes in Water: A Small-Angle Neutron Scattering Study. Macromolecules 2005, 38, 7828–7836. [Google Scholar] [CrossRef]
- Edri, E.; Regev, O. Cryo-staining techniques in cryo-TEM studies of dispersed nanotubes. Ultramicroscopy 2010, 110, 751–757. [Google Scholar] [CrossRef]
- Kim, M.T.; Park, H.S.; Hui, D.; Rhee, K.Y. Carbon Nanotube Modification Using Gum Arabic and Its Effect on the Dispersion and Tensile Properties of Carbon Nanotubes/Epoxy Nanocomposites. J. Nanosci. Nanotechnol. 2011, 11, 7369–7373. [Google Scholar] [CrossRef]
- Krishna Kumar, M.; Leela Mohana Reddy, A.; Ramaprabhu, S. Exfoliated single-walled carbon nanotube-based hydrogen sensor. Sens. Actuators B Chem. 2008, 130, 653–660. [Google Scholar] [CrossRef]
- Najeeb, C.K.; Lee, J.-H.; Chang, J.; Kim, J.-H. The effect of surface modifications of carbon nanotubes on the electrical properties of inkjet-printed SWNT/PEDOT–PSS composite line patterns. Nanotechnology 2010, 21, 385302. [Google Scholar] [CrossRef] [PubMed]
- Batalha, I.L.; Hussain, A.; Roque, A.C.A. Gum Arabic coated magnetic nanoparticles with affinity ligands specific for antibodies. J. Mol. Recognit. 2010, 23, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Gils, P.S.; Ray, D.; Sahoo, P.K. Designing of silver nanoparticles in gum arabic based semi-IPN hydrogel. Int. J. Biol. Macromol. 2010, 46, 237–244. [Google Scholar] [CrossRef]
- Kannan, R.; Zambre, A.; Chanda, N.; Kulkarni, R.; Shukla, R.; Katti, K.; Upendran, A.; Cutler, C.; Boote, E.; Katti, K.V. Functionalized radioactive gold nanoparticles in tumor therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 42–51. [Google Scholar] [CrossRef]
- Kattumuri, V.; Katti, K.; Bhaskaran, S.; Boote, E.J.; Casteel, S.W.; Fent, G.M.; Robertson, D.J.; Chandrasekhar, M.; Kannan, R.; Katti, K.V. Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: In vivo pharmacokinetics and X-ray-contrast-imaging studies. Small Weinh. Bergstr. Ger. 2007, 3, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Levard, C.; Marinakos, S.M.; Cheng, Y.; Liu, J.; Michel, F.M.; Brown, G.E.; Lowry, G.V. Size-controlled dissolution of organic-coated silver nanoparticles. Environ. Sci. Technol. 2012, 46, 752–759. [Google Scholar] [CrossRef]
- Roque, A.C.A.; Wilson, O.C. Adsorption of gum Arabic on bioceramic nanoparticles. Mater. Sci. Eng. C 2008, 28, 443–447. [Google Scholar] [CrossRef]
- Song, J.E.; Phenrat, T.; Marinakos, S.; Xiao, Y.; Liu, J.; Wiesner, M.R.; Tilton, R.D.; Lowry, G.V. Hydrophobic interactions increase attachment of gum Arabic- and PVP-coated Ag nanoparticles to hydrophobic surfaces. Environ. Sci. Technol. 2011, 45, 5988–5995. [Google Scholar] [CrossRef]
- Balantrapu, K.; Goia, D.V. Silver nanoparticles for printable electronics and biological applications. J. Mater. Res. 2009, 24, 2828–2836. [Google Scholar] [CrossRef]
- Song, J.K.; Choi, H.J.; Chin, I. Preparation and properties of electrophoretic microcapsules for electronic paper. J. Microencapsul. 2007, 24, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Zhao, X.P. Microencapsulated electric ink using gelatin/gum arabic. J. Microencapsul. 2009, 26, 37–45. [Google Scholar] [CrossRef]
- Gashua, I.B.; Williams, P.A.; Baldwin, T.C. Molecular characteristics, association and interfacial properties of gum Arabic harvested from both Acacia senegal and Acacia seyal. Food Hydrocoll. 2016, 61, 514–522. [Google Scholar] [CrossRef]
- Snowden, M.J.; Phillips, G.O.; Williams, P.A. Functional characteristics of gum arabic. Food Hydrocoll. 1987, 1, 291–300. [Google Scholar] [CrossRef]
- Nishino, M.; Katayama, T.; Sakata, M.; Al-Assaf, S.; Phillips, G.O. Effect of AGP on Emulsifying Stability of Gum Arabic. In Gum Arabic; RSC Publishing: Cambridge, UK, 2011; pp. 269–274. [Google Scholar]
- Al-Assaf, S.; Phillips, G.O.; Aoki, H.; Sasaki, Y. Characterization and properties of Acacia senegal (L.) Willd. var. senegal with enhanced properties (Acacia (sen) SUPER GUMTM): Part 1—Controlled maturation of Acacia senegal var. senegal to increase viscoelasticity, produce a hydrogel form and convert a poor into a good emulsifier. Food Hydrocoll. 2007, 21, 319–328. [Google Scholar]
- Aoki, H.; Al-Assaf, S.; Katayama, T.; Phillips, G.O. Characterization and properties of Acacia senegal (L.) Willd. var. senegal with enhanced properties (Acacia (sen) SUPER GUMTM): Part 2—Mechanism of the maturation process. Food Hydrocoll. 2007, 21, 329–337. [Google Scholar] [CrossRef]
- Aoki, H.; Katayama, T.; Ogasawara, T.; Sasaki, Y.; Al-Assaf, S.; Phillips, G.O. Characterization and properties of Acacia senegal (L.) Willd. var. Senegal with enhanced properties (Acacia (sen) SUPER GUMTM): Part 5. Factors affecting the emulsification of Acacia senegal and Acacia (sen) SUPER GUMTM. Food Hydrocoll. 2007, 21, 353–358. [Google Scholar] [CrossRef]
- Jachimska, B.; Świątek, S.; Loch, J.I.; Lewiński, K.; Luxbacher, T. Adsorption effectiveness of β-lactoglobulin onto gold surface determined by quartz crystal microbalance. Bioelectrochemistry 2018, 121, 95–104. [Google Scholar] [CrossRef]
- Jachimska, B.; Łapczyńska, M.; Zapotoczny, S. Reversible Swelling Process of Sixth-Generation Poly(amido amine) Dendrimers Molecule As Determined by Quartz Crystal Microbalance Technique. J. Phys. Chem. C 2013, 117, 1136–1145. [Google Scholar] [CrossRef]
- Jachimska, B.; Tokarczyk, K. Combining Surface Plasmon Resonance and Quartz Crystal Microbalance to Determine Hydration of Dendrimer Monolayers. J. Phys. Chem. C 2016, 120, 19678–19685. [Google Scholar] [CrossRef]
- Orelma, H.; Filpponen, I.; Johansson, L.-S.; Laine, J.; Rojas, O.J. Modification of Cellulose Films by Adsorption of CMC and Chitosan for Controlled Attachment of Biomolecules. Biomacromolecules 2011, 12, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Kim, S.-O.; Linardy, E.; Dreaden, E.C.; Zhdanov, V.P.; Hammond, P.T.; Cho, N.-J. Adsorption of hyaluronic acid on solid supports: Role of pH and surface chemistry in thin film self-assembly. J. Colloid Interface Sci. 2015, 448, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Sedeva, I.G.; Fornasiero, D.; Ralston, J.; Beattie, D.A. Reduction of Surface Hydrophobicity Using a Stimulus-Responsive Polysaccharide. Langmuir 2010, 26, 15865–15874. [Google Scholar] [CrossRef] [PubMed]
- Tokarczyk, K.; Jachimska, B. Quantitative interpretation of PAMAM dendrimers adsorption on silica surface. J. Colloid Interface Sci. 2017, 503, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.R.; Macakova, L.; Chojnicka-Paszun, A.; de Kruif, C.G.; de Jongh, H.H.J. Lubrication, Adsorption, and Rheology of Aqueous Polysaccharide Solutions. Langmuir 2011, 27, 3474–3484. [Google Scholar] [CrossRef] [PubMed]
- Malmström, J.; Agheli, H.; Kingshott, P.; Sutherland, D.S. Viscoelastic Modeling of Highly Hydrated Laminin Layers at Homogeneous and Nanostructured Surfaces: Quantification of Protein Layer Properties Using QCM-D and SPR. Langmuir 2007, 23, 9760–9768. [Google Scholar] [CrossRef] [PubMed]
- Hoypierres, J.; Dulong, V.; Rihouey, C.; Alexandre, S.; Picton, L.; Thébault, P. Two Methods for One-Point Anchoring of a Linear Polysaccharide on a Gold Surface. Langmuir 2015, 31, 254–261. [Google Scholar] [CrossRef]
- Mohan, T.; Rathner, R.; Reishofer, D.; Koller, M.; Elschner, T.; Spirk, S.; Heinze, T.; Stana-Kleinschek, K.; Kargl, R. Designing Hydrophobically Modified Polysaccharide Derivatives for Highly Efficient Enzyme Immobilization. Biomacromolecules 2015, 16, 2403–2411. [Google Scholar] [CrossRef]
- Villares, A.; Moreau, C.; Dammak, A.; Capron, I.; Cathala, B. Kinetic aspects of the adsorption of xyloglucan onto cellulose nanocrystals. Soft Matter. 2015, 11, 6472–6481. [Google Scholar] [CrossRef] [PubMed]
- Bano, F.; Carril, M.; Di Gianvincenzo, P.; Richter, R.P. Interaction of Hyaluronan with Cationic Nanoparticles. Langmuir 2015, 31, 8411–8420. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Williams, P.; Nigen, M.; Tamayo, V.M.; Doco, T.; Sanchez, C. Recovery, structure and physicochemical properties of an aggregate-rich fraction from Acacia senegal gum. Food Hydrocoll. 2018, 89, 864–873. [Google Scholar] [CrossRef]
- Höök, F.; Rodahl, M.; Brzezinski, P.; Kasemo, B. Energy Dissipation Kinetics for Protein and Antibody−Antigen Adsorption under Shear Oscillation on a Quartz Crystal Microbalance. Langmuir 1998, 14, 729–734. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Krozer, A.; Brzezinski, P.; Kasemo, B. Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum. 1995, 66, 3924–3930. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Saftics, A.; Prósz, G.A.; Türk, B.; Peter, B.; Kurunczi, S.; Horvath, R. In situ viscoelastic properties and chain conformations of heavily hydrated carboxymethyl dextran layers: A comparative study using OWLS and QCM-I chips coated with waveguide material. Sci. Rep. 2018, 8, 11840. [Google Scholar] [CrossRef]
- Voinova, M.V.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic Acoustic Response of Layered Polymer Films at Fluid-Solid Interfaces: Continuum Mechanics Approach. Phys. Scr. 1999, 59, 391. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B.; Nylander, T.; Fant, C.; Sott, K.; Elwing, H. Variations in Coupled Water, Viscoelastic Properties, and Film Thickness of a Mefp−1 Protein Film during Adsorption and Cross-Linking: A Quartz Crystal Microbalance with Dissipation Monitoring, Ellipsometry, and Surface Plasmon Resonance Study. Anal. Chem. 2001, 73, 5796–5804. [Google Scholar] [CrossRef]
- Reviakine, I.; Johannsmann, D.; Richter, R.P. Hearing What You Cannot See and Visualizing What You Hear: Interpreting Quartz Crystal Microbalance Data from Solvated Interfaces. Anal. Chem. 2011, 83, 8838–8848. [Google Scholar] [CrossRef]
- Jung, L.S.; Campbell, C.T.; Chinowsky, T.M.; Mar, M.N.; Yee, S.S. Quantitative interpretation of the response of surface plasmon resonance sensors to adsorbed films. Langmuir 1998, 14, 5636–5648. [Google Scholar] [CrossRef]
- Stenberg, E.; Persson, B.; Roos, H.; Urbaniczky, C. Quantitative Determination of Surface Concentration of Protein with Surface Plasmon Resonance Using Radiolabeled Proteins. J. Colloid Interface Sci. 1991, 143, 513–526. [Google Scholar] [CrossRef]
- Lopez Torrez, D.L. Characterisation of Acacia Gums (A. senegal and A. seyal) and Development of Heat-Induced Acacia Gum/Potato Protein Microparticles; Montpellier SupAgro: Montpellier, France, 2017. [Google Scholar]
- Jayme, M.L.; Dunstan, D.E.; Gee, M.L. Zeta potentials of gum arabic stabilised oil in water emulsions. Food Hydrocoll. 1999, 13, 459–465. [Google Scholar] [CrossRef]
- Schrems, A.; Kibrom, A.; Küpcü, S.; Kiene, E.; Sleytr, U.B.; Schuster, B. Bilayer Lipid Membrane Formation on a Chemically Modified S-Layer Lattice. Langmuir 2011, 27, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- Grein-Iankovski, A.; Ferreira, J.G.L.; Orth, E.S.; Sierakowski, M.-R.; Cardoso, M.B.; Simas, F.F.; Riegel-Vidotti, I.C. A comprehensive study of the relation between structural and physical chemical properties of acacia gums. Food Hydrocoll. 2018, 85, 167–175. [Google Scholar] [CrossRef]
- Ma, F.; Bell, A.E.; Davis, F.J. Effects of high-hydrostatic pressure and pH treatments on the emulsification properties of gum arabic. Food Chem. 2015, 184, 114–121. [Google Scholar] [CrossRef]


| Ibuffer (M) 1 | 1.75 × 10−4 | 6.4 × 10−3 | 9.94 × 10−3 |
|---|---|---|---|
| pH 3 | pH 5 | pH 7 | |
| κ (nm) 2 | 23.0 | 3.8 | 3.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davantès, A.; Nigen, M.; Sanchez, C.; d’Orlando, A.; Renard, D. Adsorption of Hyperbranched Arabinogalactan-Proteins from Plant Exudate at the Solid–Liquid Interface. Colloids Interfaces 2019, 3, 49. https://doi.org/10.3390/colloids3020049
Davantès A, Nigen M, Sanchez C, d’Orlando A, Renard D. Adsorption of Hyperbranched Arabinogalactan-Proteins from Plant Exudate at the Solid–Liquid Interface. Colloids and Interfaces. 2019; 3(2):49. https://doi.org/10.3390/colloids3020049
Chicago/Turabian StyleDavantès, Athénaïs, Michaël Nigen, Christian Sanchez, Angelina d’Orlando, and Denis Renard. 2019. "Adsorption of Hyperbranched Arabinogalactan-Proteins from Plant Exudate at the Solid–Liquid Interface" Colloids and Interfaces 3, no. 2: 49. https://doi.org/10.3390/colloids3020049
APA StyleDavantès, A., Nigen, M., Sanchez, C., d’Orlando, A., & Renard, D. (2019). Adsorption of Hyperbranched Arabinogalactan-Proteins from Plant Exudate at the Solid–Liquid Interface. Colloids and Interfaces, 3(2), 49. https://doi.org/10.3390/colloids3020049