Abstract
This study aimed to evaluate the production of a surfactant by Cunninghamella echinulata, using agro-industrial residues, corn steep liquor (CSL), and soybean oil waste (SOW). The study had a factorial design, using as a variable response to the reduction of surface tension. C. echinulata was able to produce biosurfactant in assay, CSL (8.82%) and SOW (2%). The results showed that the biosurfactant was successfully produced by C. echinulata and had attractive properties, such as a low surface tension (31.7 mN/m), a yield of 5.18 g/L at 120 h of cultivation, and an anionic profile. It also achieved a reduction in surface tension stability in a wide range of pH values, temperatures, and salinity values. The biosurfactant produced by C. echinulata showed an absence of toxicity to Artemia salina. The influence of the biosurfactant on the viscosity of engine oil, burnt engine oil, diesel, soybean oil post-frying, canola oil, and water was investigated. The results reveal a mechanism for the decrease of the viscosity using hydrophobic substrates and the new biosurfactant solution at 1.5% of the (CMC). This enables the formulation of a low-cost culture medium alternative, based on corn steep liquor and the reuse of soybean oil after frying to produce a biosurfactant. Additionally, performance of the biosurfactant isolated from C. echinulata showed an excellent ability to remove spilled oil, such as diesel (98.7%) and kerosene (92.3%) from marine sand.
1. Introduction
Microbial components that exhibit high surface activity and emulsifying activity are classified as biosurfactants. These are metabolic products made by different microorganisms such as bacteria, yeasts and filamentous fungi, using various low-cost substrates including sugars, oils, alkanes, and industrial and agricultural wastes. Oil industry waste in the form of by-products generated during the manufacture of vegetable oil is very accessible. Therefore, our main purpose is to consider various oil industry wastes for the production of biosurfactants, as efficient and economical raw materials in order to lower the cost of biosurfactant production [1,2,3]. Although soybean oil has been shown to have a good ability to stimulate biosurfactant production by Pseudomonas aeruginosa MR01, production yield increases by more than 1.6 times when using soybean oil wastes. This fact was paralleled by the increase in biodegradation yields in cultures containing waste. However, further studies are necessary to explore this phenomenon at the molecular and structural level. In conclusion, using soybean oil wastes not only achieves highly efficient biosurfactant production when compared to the results of other researchers [4,5], but it can also be regarded as contributing to ecological preservation [6].
Biosurfactants have many applications ranging from environmental, food and biomedical applications, through to applications in the cosmetic and pharmaceutical industries. The biosurfactants isolated from Candida lipolytica, Candida antarctica, Candida bombicola, Torulopsis bombicola and Aspergillus ustus have been found to be the best choices in microbially enhanced oil recovery [7,8,9].
Biosurfactants also have a wide range of biotechnological applications in the dairy, food, beverage, cosmetics, detergent, textile, paint, mining, petroleum, paper pulp, and pharmaceutical industries [10,11]. Extreme environments are valuable sources of microorganisms that may secrete novel biosurfactants with a wider adaptability and stability toward adverse environmental conditions [12,13]. These properties make them suitable for applications in the bioremediation of oil, hydrocarbon, and toxic metals from contaminated soil and wastewaters [14,15,16].
There is a worldwide concern about the release of hydrocarbons into the environment from industrial activities and from accidental oil spills and their related components [17]. The amphipathic structure of biosurfactants acts on hydrocarbons, emulsifying them through the formation of micelles which accumulate at the interface between liquids of different polarities. This produces hydrophobic soil pollutants, such as polycyclic aromatic hydrocarbons (PAHs), and confers the ability to carry out the detergency, emulsification, lubrication, solubilization, and dispersion phases [18,19,20].
The present study aimed to evaluate the production and stability of the surfactant produced by the filamentous fungus Cunninghamella echinulata, using low-cost corn steep liquor (CSL) and soybean oil waste (SOW). This study also investigated the capacity of this biosurfactant to solubilize and facilitate the recovery of spilled oil, and to reduce surface tension under different environmental conditions.
2. Materials and Methods
2.1. Microorganism
Cunninghamella echinulata UCP 1297 was isolated from Caatinga soil of Pernambuco, Brazil, and deposited in the Culture Collection of the Catholic University of Pernambuco. It was registered in the World Federation of Culture Collection (WFCC). The fungus was maintained at 5 °C on Sabouraud dextrose agar, and was transferred at four months to the same medium.
2.2. Detection of Biosurfactant Production by Hemolysis
A 0.5 cm disc of C. echinulata was placed in the plate center (blood agar) and incubated for 48 h at 37 °C. The plates were visually inspected for zones of clearing around the colonies, indicative of biosurfactant production. The diameter of the clear zones depends on the concentration of the biosurfactant [21]. The zones of clearing were scored as follows: No hemolysis (−); incomplete hemolysis (+); complete hemolysis with a diameter of lysis <1 cm (++); complete hemolysis with a diameter of lysis >1 cm but <3 cm (+++); and complete hemolysis with a diameter of lysis >3 cm and green colonies (++++). Two plates for each strain were inoculated and clear zones in several different areas of each plate were analyzed.
2.3. Biosurfactant Production
Erlenmeyer flasks of 250 mL capacity were used. They contained 100 mL of medium with corn steep liquor (CSL), which is the waste obtained from the processing of corn obtained from Corn Products Brazil, and soybean oil waste (SOW) with a pH of 5.5. The experiments were monitored by a 22) with a variable response reduction in surface tension (Table 1). The culture media were autoclaved at 121 °C for 15 min. An aliquot (1 mL) of spore suspension containing 107 sporangiospores/mL from Cunninghamella echinulate UCP1297 was inoculated in the Erlenmeyer flasks. The samples were agitated (150 rpm) for 120 h at 28 °C.

Table 1.
Levels of Central Composition Rotational Design (CCRD 22) to biosurfactant production by Cunninghamella echinulata.
2.4. Determination of the Surface Tension, Interfacial Tension and pH
The surface tension of the cell-free metabolic liquid cells containing the biosurfactant after 120 h of cultivation, was measured in a semi-automatic tensiometer using a DU NUOY ring [22]. The Interfacial tension was measured against the n-hexadecane in the cell-free broth following cell removal with a Millipore (0.45 µm). Changes in the pH of the culture media were monitored throughout the growth using a potentiometer. The pH range at each point was the mean of three measurements.
2.5. Isolation of Biosurfactant
The samples were centrifuged at 4000 rpm and 28 °C for 20 min and the cell-free metabolic liquid was analyzed according to the method of Shavandi et al. [4]. The liquid containing the active tension had ethanol added (70%) 1:2, and was kept under refrigeration for 24 h until complete precipitation. It was then centrifuged at 4000 rpm for 15 min. The crude product was dialyzed for 48 h at 4 °C (12,000 Da cut off dialysis tubing, Sigma) and lyophilized.
2.6. Determination of Critical Micelle Concentration (CMC)
The experimental method to self-consistently determine the line interfacial tension between the oil/water and air/water surfaces. These effects are proportional to the concentration of active tension, dissolving until it reaches the critical micelle concentration (CMC). The CMC was determined by measuring the surface tension dilutions of purified biosurfactant in distilled water against a constant value of surface tension. The value of the CMC was determined as g/L biosurfactant.
2.7. Determination of the Stability of the Biosurfactant
The stability of the biosurfactant was evaluated from the condition selected using the cell-free metabolic liquid by surface tension. The evaluation was done using different pH levels (2, 4, 6, 8, 10, and 12), different concentrations of NaCl (2.5, 5, 10, 15, and 20%), and temperatures of 100 °C for 15, 20, 40, 60, 100, and 140 min, and 121 °C for 15, 20, 40, and 60 min [23].
2.8. Determination of the Biochemical Composition of the Biosurfactant
The total protein of the biosurfactant was determined by kit for total protein analysis from Labtest Diagnostica SA Brazil. The total sugars were determined according to the methodology described by Dubois et al. [24]. The amounts of lipids present in the biosurfactant were determined using chloroform and methanol in different ratios (1:1 to 1:2 v/v). The organic extract was evaporated, and the lipid content was then determined by gravimetry [24,25].
2.9. Determination of the Ionic Charge of Biosurfactant and Oil Viscosity Determination
The ionic charge of the biosurfactant was determined by measuring the zeta potential, 100 volts, ¼ scale, at a temperature of 22 °C. In order to investigate the effects of the biosurfactant produced on the viscosity, tests were carried out in which the test was fixed to a volume of 6 mL. These viscosities were measured using a standard viscometer (Brookfield-TC 500) at 25 °C. The hydrophobic substrates of engine oil, burnt engine oil, diesel, soybean post-frying oil, canola oil, and water with biosurfactants and water as a control, were tested. In the same sequence as the hydrophobic substrates, we added 2 mL of solution of biosurfactant at 1.5% (w/v), subjected it to vortex for 1 min, and then the viscosity was measured again. The viscosity results were expressed in cP and %.
2.10. Toxicity Assay
The toxicity assay was performed with the isolated biosurfactant using the microcrustacean Artemia salina as the toxicity indicator. Brine shrimp eggs were obtained from a local store. Larvae were used within 1 day of hatching. Following dilution of a biosurfactant solution (3.75, 7.5, 11.3, and 15 mg/mL), the assays were conducted in penicillin tubes of 10 mL capacity containing 10 brine shrimp larvae in 5 mL of saline water per tube (33.3 g/L), incubated for 24 h. A cell-free broth containing the biosurfactant was also tested. The brine shrimp larvae in each tube were tested using a 1.5 mL per concentration level of biosurfactant solution. They were observed for 24 h to calculate mortality [26]. The toxicity threshold concentration, expressed as biosurfactant concentration per 100 ml of saline water, was defined as the lowest concentration that killed all tested brine shrimp within 24 h. Each test was run in duplicate and saline water was used as the control. Then the count of surviving organisms was conducted, thereby determining a threshold dose (LC50) of biosurfactant. The calculations were undertaken by the StatPlus 2009 program using the Probit Analysis Method.
2.11. Application of Biosurfactant to Remove Spilled Oils from Polluted Marine Sand
The suitability of the biosurfactant for oil in sand separation by gravity was determined using 10 g of marine sand impregnated with 1.0 mL of burnt engine oil, kerosene, diesel, and soybean post-frying oil, which was transferred to 125 mL Erlenmeyer flasks followed by the addition of 30 mL of the biosurfactant produced by the Cunninghamella echuinulata cultivated in the optimized medium (CSL 8.82% added SOW 2.0%) or 30 mL of distilled water (control). The samples were incubated on a rotatory shaker (150 rpm) for 24 h at 28 °C and centrifuged at 5000 g for 10 min to separate the laundering solution and marine sand. The amount of oil in the marine sand after contact with the biosurfactant was gravimetrically determined as the amount of material extracted by solubilization using hexane [27].
3. Results and Discussion
3.1. Biosurfactant Production through Hemolysis Formation by C. echinulate
As mentioned above, the lysis of sheep red blood cells has been recommended as a simple and easy method to test for biosurfactant activity [28,29]. Mulligan et al. [20] recommended the blood agar lysis method as a preliminary screening method for the detection of biosurfactant production.
The diameter of the clear zones depends on the concentration of the biosurfactant. The zones of clearing were scored as follows: ‘−’ (no hemolysis); ‘+’ (incomplete hemolysis); ‘++’ (complete hemolysis with a diameter of lysis <1 cm); ‘+++’ (complete hemolysis with a diameter of lysis >1 cm but <3 cm); and ‘++++’ (complete hemolysis with a diameter of lysis >3 cm and green colonies). However, not all biosurfactants have hemolytic activity and compounds other than biosurfactants may cause hemolysis [21].
The results show complete hemolysis (2.0 cm) represented by (+++) with a diameter of lysis >1 cm but <3 cm (Figure 1).
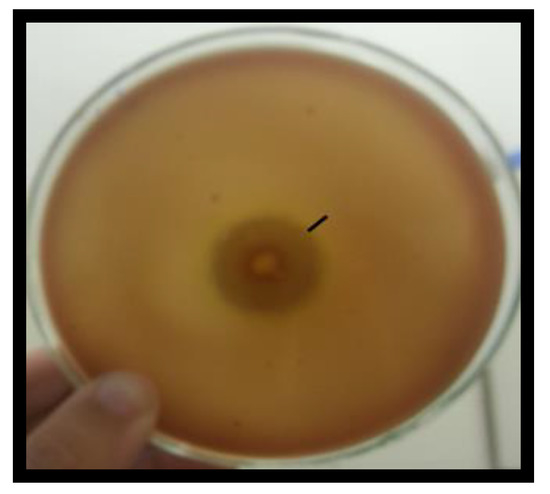
Figure 1.
Blood agar method: Halo produced by Cunninghamella echinulata in the culture medium of blood agar, incubated at 37 °C for 48 h.
3.2. Determination of the Surface Tension, Interfacial Tension and pH
Haba et al. [30] consider those microorganisms which reduce water surface activity from 72 mN/m to 40 mN/m to be good producers of biosurfactants. Zhou and Kosaric [31], using canola oil and lactose present in whey cheese processing for the production of biotenso-actives with Candida bombicola as their carbon source, obtained a reduction of the surface tension (air/water interface) from 72 mN/m to 33 mN/m.
The results obtained revealed that C. echinulata was able to produce a biosurfactant during growth in a low-cost medium, containing corn steep liquor (8.82%) and soybean oil after frying (2%). The compound decreased the water surface tension from 72.0 mN/m to 31.7 mN/m, the interfacial tension (12 mN/m), and the final pH (6.5) after 120 h of cultivation. Table 2 presents the surface tension and pH of the cell-free metabolic liquid produced by C. echinulata, according to the factorial design, DCCR 22, presented in Table 1.

Table 2.
Surface tension according to the factorial design, CCRD 22, using Cunninghamella echinulata after 120 h of culture under agitation.
The analysis of the Pareto Diagram (Figure 2) suggests that under the conditions being evaluated, concentrations of soybean oil post-frying and corn steep liquor significantly facilitated the reduction of the surface tension. It was also observed that the interaction effect between corn steep liquor and soybean oil post-frying showed no statistically significant effect in reducing the surface tension.
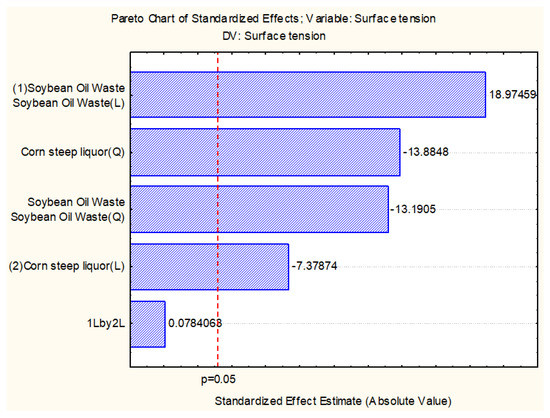
Figure 2.
Diagram of Pareto Factorial Design I CCRD 22, using Cunninghamella echinulata after 120 h under agitation.
These results demonstrate that the biosurfactant produced by Cunninghamella echinulata has a greater capacity to reduce surface tension in comparison to biosurfactants from Candida lipolytica (32 mN/m) [32], C. glabrata (31 mN/m) [33], C. antarctica (35 mN/m) [34], and Y. lipolytica (50 mN/m) [35].
Corn steep liquor is also being used to produce biosurfactants. The composition of corn steep liquor is 21–45% protein, 20–26% lactic acid, about 8% ash (containing Ca2+, Mg2+, K2+, and more), about 3% carbohydrates, and low levels of fat (0.9–1.2%), according to the literature [36,37].
3.3. Stability Studies
The biosurfactant produced by Cunninghamella echinulata showed low surface tension stability over a wide range of pH values, temperatures and exposure to high salinity. Regarding the influence of the temperature on the surface tension of the cell-free medium containing the biosurfactant (Figure 3A,B), it was observed that the same stable front remained regardless of the studied temperatures.
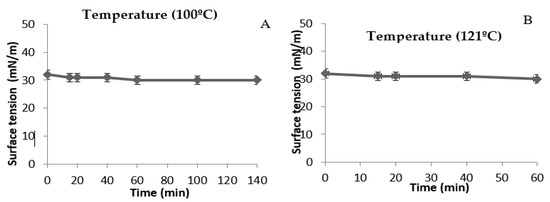
Figure 3.
Influence of temperatures of 100 °C (A) and 121 °C (B) on the surface tension of the metabolic cell-free produced by Cunninghamella echinulata in the culture medium: Corn steep liquor (8.82%) added to post-frying soybean oil (2%).
The surface tension of the cell-free broth containing the biosurfactant showed stability independent of the concentration of added salt and the pH (Figure 4A,B), respectively.
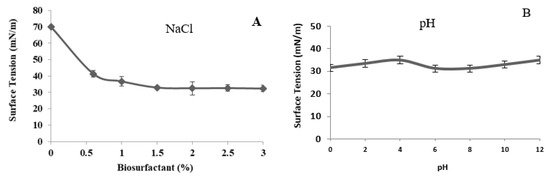
Figure 4.
The influence of different concentrations of sodium chloride (A) and different pH values (B) on the surface tension of the metabolic cell-free produced by Cunninghamella echinulata in the culture medium: CSL (8.82%) with added SOW (2%).
These results agreed with the surface tension values found for the biosurfactant of Nocardia sp. L-417, which remained stable at all the pH values tested (from 2 to 12), indicating that the variation of the pH did not have a significant effect on the superficial tension [38]. The surface tension of the biosurfactant produced by Bacillus subtillis was also stable under different pH values [39], although the effectiveness of the liposan of C. lipolytica as an emulsifier was limited to the acidic to neutral pH values [40].
3.4. Surface Tension Reduction and Critical Micelle Concentration (CMC) of the Biosurfactant
The ability to reduce surface tension depends on the specific concentration of the surface-active compound—CMC, which is defined as the minimum concentration of biosurfactant required to yield the maximum surface tension reduction in water and initiate micelle formation (Figure 5). Efficient surfactants are required to reduce surface tension [41]. The CMC of the biosurfactant produced by Cunninghamella echinulata was approximately 1.5% and the surface tension at that point was 31.7 mN/m.
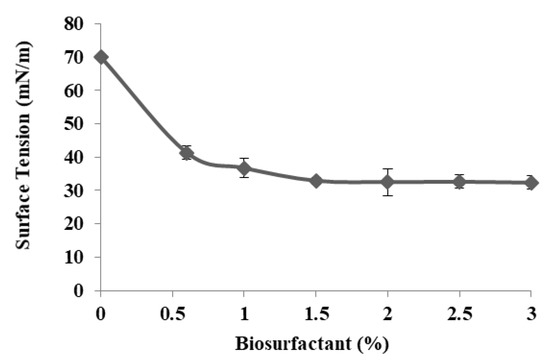
Figure 5.
Surface tension related to the concentration of the biosurfactant produced by the fungus Cunninghamella echinulata in CSL (8.82%) and SOW (2%).
3.5. Yield, Ionic Charge and Preliminary Chemical Composition of the Biosurfactant
The biosurfactant showed a CMC of 1.5% and an anionic profile using a zeta meter with −100 V, 2.340 µS/cm at 22 °C, ¼ scale. The zeta potential determines the function of the surface charge of the particles that serve to predict and control the stability of colloidal suspensions and emulsions. It confirmed that the higher values obtained (square) indicate a good stability due to the repulsion between hydrophilic particles, as per the literature [42,43,44].
The chemical characterization of the isolate molecule of the biosurfactant revealed the presence of lipids (79.5%), carbohydrates (4.5%), and proteins (12%).
3.6. Biosurfactant Toxicity
The lethal dose (LD50) is the dose required for a given substance or type of radiation to kill 50% of a population under testing (Figure 6). The LD50 is often used as an indicator of the acute toxicity of a substance, where a higher lethal dose is considered less toxic. The biosurfactant from Cunninghamella echinulata was first tested with regards to toxicity in short-term bioassays using brine shrimp. Counting the surviving organisms determined the dose limit concentration (LD50: 18.11 mg/mL) of biosurfactant (Figure 6), indicating the absence of toxicity of the biosurfactant.
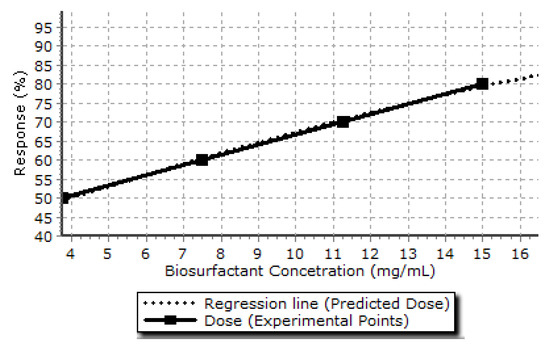
Figure 6.
Determination of the dose limit concentration of biosurfactant produced by Cunninghamella echinulate.
3.7. Effect of the Biosurfactant on Viscosity
We determined the viscosity in engine oil, engine burning oil, diesel, canola oil, soybean waste oil, and water (control) isolate, before and after the addition of the biosurfactant isolate from C. echinulate (Table 3). The determination was made with regard to the centipoise (cP) and percentage (%) of the hydrophobic substrates before and post solubilization by the use of the biosurfactant at 1.5% solution over 30 min. The best results were achieved with the use of the biosurfactant. The viscosity determination was decreased for engine oil from 736.6 cP to 46.9 cP; engine burning oil from 148.9 cP to 46.9 cP; diesel from 154.1 cP to 2.09 cP; canola oil from 374.0 cP to 35.4 cP, and soybean waste oil from 380.1 cP to 21.6 cP. The viscosity determination was increased after the biosurfactant addition for water (control) from 0.96 cP to 37.2 cP.

Table 3.
Proprieties of reduction and increase of the viscosity of substrates by the biosurfactant produced by Cunninghamella chinulata.
The results reveal a mechanism for the decrease of the viscosity using hydrophobic substrates, with the use of the new biosurfactant solution at 1.5%. The viscosity results suggest the new biosurfactant is a candidate for mediated enhanced oil recovery [10,45].
3.8. Application of Biosurfactant to the Removal of a Hydrophobic Contaminant Adsorbed in a Sand Beach
Biosurfactants emulsify hydrocarbons by enhancing their water solubility, decreasing surface tension and increasing the displacement of the oil substances from soil particles. Results on the removal of soybean oil, diesel, kerosene, and engine oil adsorbed in the samples of sand beach tested by the cell-free culture medium from C. echinulata were obtained after 120 h and compared with distilled water (control) (Table 4).

Table 4.
Removal of oils from a sand beach by the biosurfactant produced by Cunninghamella echinulate.
The performance of biosurfactants from C. echinulata was observed to be excellent, as it alone removed 98.7% of the diesel from the marine sand from beach compared to a 12.5% recovery by the control (distilled water). A total of 92.3% of the kerosene was recovered in the presence of the biosurfactant, in comparison to 27% by the control (distilled water).
4. Conclusions
The results suggest the successful formulation of a low-cost culture medium alternative based on corn steep liquor, as well as the reuse of soybean oil after frying, to produce biosurfactants. This work has shown that the biosurfactant produced by Cunninghamella echinulata has attractive properties such as a low surface tension. Stability over a wide range of pH values, temperatures, and exposure to high salinity was achieved, but it cannot act as an emulsifier. The biosurfactant from Cunninghamella echinulata showed an absence of toxicity in the tenso-active, and the viscosity results show that the biosurfactant is a candidate for mediated enhanced oil recovery. The performance of biosurfactants from C. echinulata is excellent for the removal of diesel and kerosene from marine sand. These characteristics indicate the potential use of the biosurfactant in bioremediation and the oil industry.
Author Contributions
P.M.d.S., C.D.C.A. and G.M.C.-T., methodology; P.M.d.S., N.R.A.S., D.G.S. and T.A.L.e.S., validation; M.C.F.-S., R.F.S.A. and G.K.B.S., formal analysis; P.M.d.S. and M.C.F.-S., investigation, resources; P.M.d.S., N.R.A.S., D.G.S. and M.C.F.-S., data curation; P.M.d.S. and G.M.C.-T., writing—original draft preparation; P.M.d.S., R.F.S.A., A.S.M. and G.M.C.-T., writing—review and editing; A.S.M., C.D.C.A. and G.M.C.-T., visualization.
Funding
CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) under Process No. 311373/2014-3 FACEPE (Fundação de Amparo à Ciência e Tecnologia de Pernambuco) Process No. APQ-0291-2.12/15 CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior)–PNPD.
Acknowledgments
The authors are also grateful to the NPCIAMB (Nucleus of Research in Environmental Sciences and Biotechnology), Catholic University of Pernambuco (Recife-PE, Brazil) for the use of its laboratories.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lima, R.A.; Andrade, R.F.S.; Rodriguez, D.M.; Araujo, H.W.C.; Santos, V.P.; Campos-Takaki, G.M. Production and characterization of biosurfactant isolated from Candida glabrata using renewable substrates. Afr. J. Microbiol. Res. 2017, 11, 237–244. [Google Scholar]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ribeaux, D.; Andrade, R.F.S.; Silva, G.S.; Holanda, R.A.; Pele, M.A.; Nunes, P.; Junior, J.C.V.; Campos-Takaki, G.M. Promising biosurfactant produced by a new Candida tropicalis UCP 1613 strain using substrates from renewable-resources. Afr. J. Microbiol. Res. 2017, 11, 981–991. [Google Scholar]
- Shavandi, M.; Mohebali, G.; Haddadi, A.; Shakarami, H.; Nuhi, A. Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloids Surf. B 2011, 82, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, N.; Li, D.; Long, S.; Tang, X.; Xiao, G.; Wang, L. Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ. Sci. Pollut. Res. 2018, 25, 14934–14943. [Google Scholar] [CrossRef] [PubMed]
- Partovi, M.; Lotfabad, T.B.; Roostaazad, R.; Bahmaei, M.; Tayyebi, S. Management of soybean oil refinery wastes through recycling them for producing biosurfactant using Pseudomonas aeruginosa MR01. World J. Microbiol. Biotechnol. 2013, 29, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Aulwar, U.; Awasthi, R.S. Production of Biosurfactant and their Role in Bioremediation. J. Ecosys Ecograph 2016, 6, 1–5. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Resende, A.H.M.; Almeida, R.C.; Soares da Silva, F.; Rufino, R.D.; Luna, J.M.; Banat, I.M.; Sarubbo, L.A. Candida lipolytica UCP0988 biosurfactant: Potential as a bioremediation agent and in formulating a commercial related product. Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Perfumo, A.; Banat, I.M.; Marchant, R. Going green and cold: Biosurfactants from low-temperature environments to biotechnology applications. Trends Biotechnol. 2018, 36, 227–289. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.F.S.; Antunes, A.A.; Lima, R.A.; Araújo, H.W.; Resende-Stoianoff, M.A.; Franco, L.O.; Campos-Takaki, G.M. Enhanced Production of an Glycolipid Biosurfactant Produced by Candida glabrata UCP/WFCC1556 for Application in Dispersion and Removal of Petroderivatives. Int. J. Cur. Microbiol. Appl. Sci. 2015, 4, 563–576. [Google Scholar]
- Cameotra, S.S.; Makkar, R.S. Biosurfactant-enhanced bioremediation of hydrophobic pollutants. Pure Appl. Chem. 2010, 82, 97–116. [Google Scholar] [CrossRef]
- Grbavčić, S.; Marković, D.; Rajilić-Stojanović, M.; Antov, M.; Šćiban, M.; Karadžić, I.; Knežević-Jugović, Z. Development of an environmentally acceptable detergent formulation for fatty soils based on the lipase from the indigenous extremophile Pseudomonas aeruginosa strain. J. Surfact. Deterg. 2015, 18, 383–395. [Google Scholar]
- Almeida, D.G.; Soares da Silva, R.C.F.; Brasileiro, P.P.F.; Luna, J.M.; Silva, M.G.C.; Rufino, R.D.; Costa, A.F.S.; Santos, V.A.; Sarubbo, L.A. Application of a Biosurfactant from Candida tropicalis UCP 0996 Produced in Low-Cost Substrates for Hydrophobic Contaminants Removal. Chem. Eng. Trans. 2018, 64, 1–6. [Google Scholar]
- Pele, M.A.; Montero-Rodriguez, D.; Rubio-Ribeaux, D.; Souza, A.F.; Luna, M.A.C.; Santiago, M.F.; Andrade, R.F.S.; Silva, T.A.L.; Santiago, A.L.C.M.A.; Campos-Takaki, G.M. Development and improved selected markers to biosurfactant and bioemulsifier production by Rhizopus strains isolated from Caatinga soil. Afr. J. Biotechnol. 2018, 17, 150–157. [Google Scholar]
- Silva, V.; Correa, P.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Recovery of contaminated marine environments by biosurfactant-enhanced bioremediation. Colloids Surf. B 2018, 72, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Mahanti, P.; Kumar, S.; Patra, J.K. Biosurfactants: An Agent to Keep Environment Clean. In Microbial Biotechnology; Das, G., Ed.; Springer: Singapore, 2017; pp. 413–428. [Google Scholar]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; Dirisala, V.R.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 2018, 3, 241–249. [Google Scholar] [CrossRef]
- Oje, A.O.; Okpashi, V.E.; Uzor, J.C.; Uma, U.O.; Irogbolu, A.O.; Onwurah, I.N.E. Effect of Acid and Alkaline Pretreatment on the Production of Biosurfactant from Rice Husk Using Mucor indicus. Res. J. Environ. Toxicol. 2016, 10, 60–67. [Google Scholar] [CrossRef][Green Version]
- Mulligan, C.N.; Cooper, D.G.; Neufeld, R.J. Selection of microbes producing biosurfactants in media without hydrocarbons. J. Ferm. Technol. 1984, 62, 311–314. [Google Scholar]
- Derguine-Mecheri, L.; Kebbouche-Gana, S.; Khemili-Talbi, S.; Djenane, D. Screening and biosurfactant/bioemulsifier production from a high-salt-tolerant halophilic Cryptococcus strain YLF isolated from crude oil. J. Petroleum Sci. Engin. 2018, 161, 712–724. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B.; Philp, J.C.; Christofi, N.; Dunbar Ritchkov, S.A.; Ritchkov, M.I. Recovery of Rhodococcus biosurfactants using methyl tertiary-butyl ether extraction. J. Microbiol. Methods 2001, 46, 109–120. [Google Scholar] [CrossRef]
- Barros, F.F.C.; Quadros, C.P.; Pastore, G.M. Studies of emulsifying properties and stability of the biosurfactant produced by Bacillus subtilis in cassava wastewater. Food Sci. Technol. 2008, 28, 979–985. [Google Scholar] [CrossRef]
- Manocha, M.S.; San-Blas, G.; Centeno, S. Lipid composition of Paracciodioids brasilienses: Possible correlation winter virulence of different strains. Microbiolgy 1980, 117, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 29, 350–356. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, J.B.; Nichols, D.E.; Mclaughlin, J.L. Brine shrimp; a convenient general bioassay for activeplant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Pastore, G.M. Biosurfactantes: Propriedades e aplicações. Quim. Nova 2002, 25, 772–776. [Google Scholar] [CrossRef]
- Banat, I.M. The isolation of a thermophilic biosurfactant-producing Bacillus species. Biotechnol. Lett. 1993, 6, 591–594. [Google Scholar] [CrossRef]
- Yonebayashi, H.; Yoshida, S.; Ono, K.; Enomoto, H. Screening of microorganisms for microbial enhanced oil recovery process. Sekiyu Gakkaishi 2000, 43, 59–69. [Google Scholar] [CrossRef]
- Haba, E.; Espuny, M.J.; Busquets, M.; Manresa, A. Screening and Production of Rhamnolipids by Pseudomonas aeruginosa 47T2 NCIB 40044 from Waste Frying Oils. J. Appl. Microbiol. 2000, 88, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.H.; Kosaric, N. Utilization of canola oil and lactose to produce biosurfactant with Candida bombicola. J. Am. Oil Chem. Soc. 1995, 72, 67–71. [Google Scholar] [CrossRef]
- Rufino, R.D.; Sarubbo, L.A.; Neto, B.B.; Campos-Takaki, G.M. Experimental design for the production of tensio-active agent by Candida lipolytica. J. Ind. Microbiol. Biotechnol. 2008, 35, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, L.A.; Luna, J.M.; Campos-Takaki, G.M. Production and stability studies of the bioemulsifier obtained from a new strain of Candida glabrata UCP 1002. Electron. J. Biotechnol. 2006, 9, 400–406. [Google Scholar]
- Shepherd, R.; Rockey, J.; Shutherland, I.W.; Roller, S. Novel bioemulsifier from microorganisms for use in food. J. Biotechnol. 1995, 40, 207–217. [Google Scholar] [CrossRef]
- Gallert, C.; Winter, J. Solid and liquid residues as raw materials for biotechnology. Naturwissenschaften 2002, 89, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, E.V.; Hedrick, L.R. Microbiological assay of corn steep liquor for amino acid content. J. Biol. Chem. 1948, 172, 609–612. [Google Scholar] [PubMed]
- Akhtar, M.; Lentz, M.J.; Blanchette, R.A.; Kirk, T.K. Corn steep liquor lowers the amount of inoculum for biopulping. Tappi J. 1997, 80, 161–164. [Google Scholar]
- Kim, S.H.; Lim, E.J.; Lee, S.O.; Lee, J.D.; Lee, T.H. Purification and characterization of biosurfactants from Nocardia sp. L-417. Biotechnol. Appl. Biochem. 2000, 31, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.S.; Cameotra, S.S. Production of biosurfactant at mesophilic and thermophilic conditions by a strain of Bacillus subtillis. J. Microbiol. Biotechnol. 1998, 20, 48–52. [Google Scholar] [CrossRef]
- Cirigliano, M.C.; Carman, G.M. Isolation of a bioemulsifier from Candida lipolytica. Appl. Environ. Microbiol. 1984, 48, 747–750. [Google Scholar] [PubMed]
- Ramnani, P.; Kumar, S.S.; Gupta, R. Concomitant production and downstream processing of alkaline protease and biosurfactant from Bacillus licheniformis RG1: Bioformulation as detergent additive. Process Biochem. 2005, 40, 3352–3359. [Google Scholar] [CrossRef]
- Patil, J.R.; Chopade, B.A. Studies on bioemulsifier production by Acinetobacter strains isolated from healthy human skin. J. Appl. Microbiol. 2001, 91, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Pornsunthorntawee, O.; Wongpanit, P.; Chavadej, S.; Abe, M.; Rujiravanit, R. Structural and physicochemical characterization of crude biosurfactant produced by Pseudomonas aeruginosa SP4 isolated from petroleum-contaminated soil. Bioresour. Technol. 2008, 99, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.M.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Sen, R. Biotechnology in petroleum recovery: The microbial EOR. Prog. Energ. Comb. Sci. 2008, 34, 714–724. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).