2.1. Simple Macroscopic Model
Let us consider a narrow circular slit with radius
R and thickness
H embedded in a soft solid. The slit is assumed to be completely filled with condensate and connected to the ambient vapor outside the soft solid. This connection (which is not shown in
Figure 1) could be a channel between the micropore and an adjacent mesopore, which itself is part of a mesopore system connected to the ambient vapor outside the porous solid. It is useful to introduce the relative vapor pressure
where
is the vapor pressure of the ambient vapor and
the saturation pressure of the bulk fluid.
m may vary between a value above the threshold for capillary condensation and one. In this case, a slit pore is either filled with condensate or completely compressed. According to the classical theory of capillarity, the condensate exerts a compressive stress
on pore walls, where
is the particle number density of the condensate,
T the temperature and
the Boltzmann constant. As a surprising result, Israelachvili [
14] pointed out that this formula for
leads to formally correct results for adhesion forces between solid surfaces even at rather low relative vapor pressure (
), when the corresponding meniscus radius turns out to be equal to a molecular diameter. In a previous publication [
16], we have used the capillary pressure
(Kelvin’s equation) to derive the compression of slits in soft materials. In very soft solids, complete slit compression can also occur if its thickness
H is relatively large. Hardened cement pastes are not very soft materials. For these porous pastes, length change measurements [
17] support the assumption that the thickness of very narrow water-filled slit-like spaces (called interlayer space in the cement literature) and the number of water molecules absorbed in these slits
N are directly proportional (
). Based on this proportionality, a more general approach leads to a solvation pressure, which is the sum of a humidity-dependent and a humidity-independent term (
Appendix A):
The humidity-dependent term:
is proportional to a dimensionless constant factor
q, which should not deviate much from one. Here, we always assume that
, which leads to the formal coincidence
.
If the solid is soft and Young’s modulus
E is low, tension
is capable of compressing narrow slits. We define
as the value of the slit width
H at saturation pressure (
), which is accompanied by vanishing condensate tension (
). When the relative vapor pressure is reduced from
to a lower value, the tension can change the pore thickness from
to
. Reduction of the pore thickness produces elastic deformations in the solid skeleton of the soft solid. The elastic free energy of the deformations resulting from a compressed slit can be approximately evaluated by using the dislocation theory of solids [
18,
19].
A closed dislocation loop is formally produced by a Volterra process [
18,
20,
21], which is used in the theory of edge dislocations. In the monograph [
18], a circular dislocation loop is considered. The production of a circular dislocation loop by a Volterra process is comprised of two steps, where the first step produces the open pore. After the second step, the circular dislocation loop appears at the edge of the former circular slit pore:
Step 1: An open circular slit is produced by removing all atoms of the solid in a disk-like region. The resulting structure appears as a circular slit with a thickness equal to the length b of Burgers vector, which corresponds to an open slit pore with thickness b.
Step 2: The opposite slit walls are pressed together, and the touching slit interfaces are glued. In the physics of solids, chemical bonds bridge both sides of the former slit-like cavity, but we assume that a compressive adhesion force hampers reopening of the pore.
This procedure can also be applied to planar circular slits in amorphous materials, where the slit thickness cannot be identified with the length of a Burgers vector (vector of a crystal lattice) because the elasticity theory is a macroscopic description of matter.
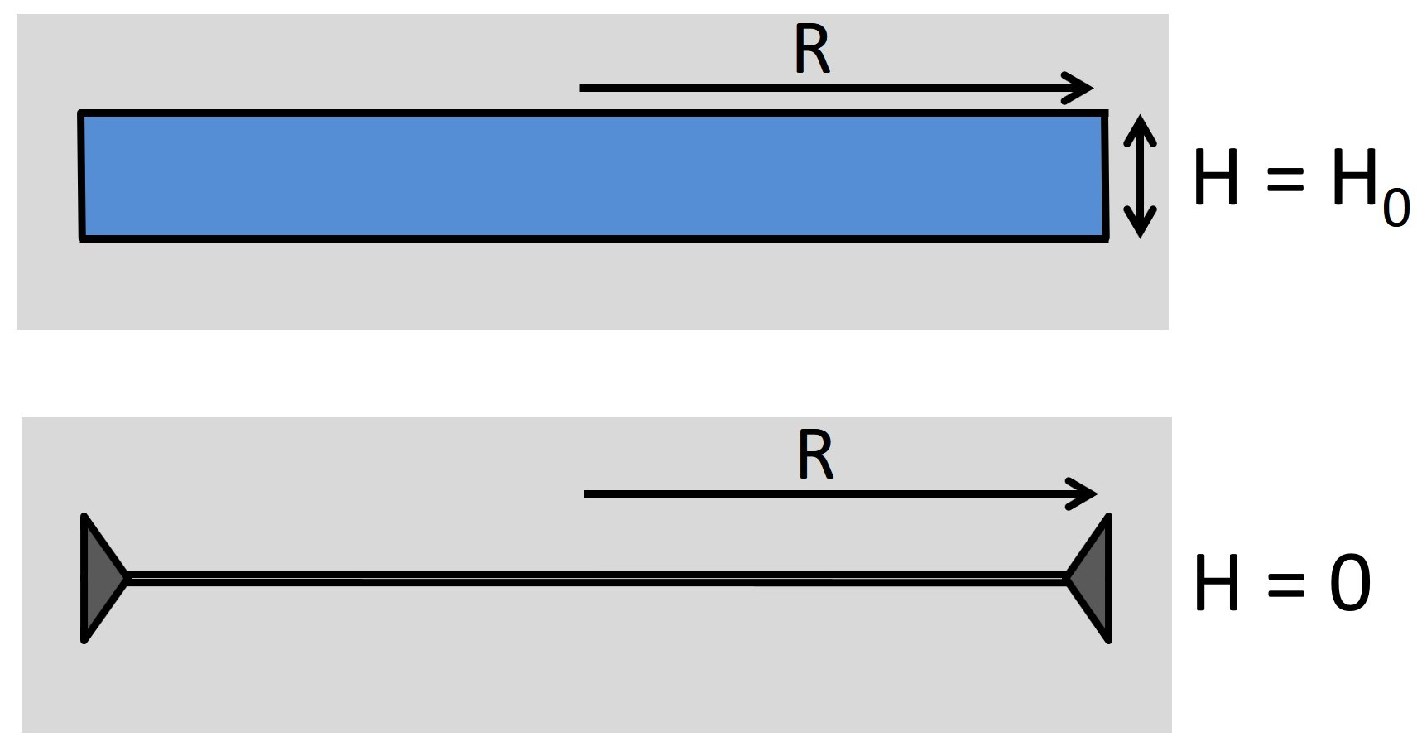
Analogously to the first step of the Volterra process, we can produce the open slit that is shown in the upper part of
Figure 1. The slit with thickness
and radius
R is created by removing all molecules of the disk-shaped region. Then, the slit is filled with water at humidity
. The contribution
to the solvation force (Equation (
1)) is zero for
because at saturation pressure (
), adhesion appears only in the borderline case
(touching surfaces), as we only consider the simplest macroscopic description in this section (a force
with a finite range
will be considered in
Section 2.2). Hence,
(Equation (
2)) defines the force (per unit area) between separated walls. As
, the water-filled cavity retains its thickness
, and there are no deformations of the solid skeleton caused by slit compression. For this state (
), the elastic free energy
should be equal to zero, and the water-filled slit is in mechanical equilibrium (
). If humidity
m is reduced, the tension
compresses the slit. Assuming a Hookean behavior, the elastic free energy (divided by the area
) for the deformed solid is expected to be a quadratic function of the slit wall displacement
. The ansatz:
becomes quite plausible if
is approximately evaluated by using a Taylor series (
Appendix B, Equation (
A11)). An approximate expression for
can be obtained by using the free energy per slit area
resulting from inserting
into Equation (
3). This completely compressed slit configuration (
) should appear when the compressive tension
is strong enough. On the other hand, the same closed slit configuration appears after the second step of the Volterra process to create a circular edge dislocation loop with
for the magnitude of the Burgers vectors (
Figure 1). The second step of the Volterra process is equivalent to slit closing. Hence, the elastic free energy for a circular edge dislocation loop
is equal to the elastic free energy
of a completely compressed slit. Equation
for
allows us to evaluate an approximate expression for the coefficient
in Equation (
3). As is demonstrated in the
Appendix B, coefficient
turns out to be independent of
. Thus, we use the notation
instead of
in the subsequent equations of this paper. Then, the elastic free energy divided by the slit area
can be written as:
where (
Appendix B, Equation (
A15)):
E is Young’s modulus,
Poisson’s ratio and radius
is comparable to the core radius of the dislocation. For simplicity, we assume that
. If the condensate tension is strong enough to completely compress the slit (
), we obtain the free energy
, which is equal to the elastic free energy of a circular dislocation loop (
Figure 1). The closed slit configuration
is stabilized by adhesion of the touching slit walls. In previous papers [
11,
12,
13], we have considered the simple macroscopic model for the adhesion potential
where
w is equal to the work per unit area to separate two touching planar surfaces at saturation pressure (
). The difference of the grand potentials (divided by the slit area
A) for the open and the closed slit configuration
or
can be written as [
11]:
The last term
is equal to the elastic free energy
for
. The condition for mechanical stability
for open pores (
) yields:
The potential difference is equal to zero if there is an equilibrium between the open () and the closed pore configuration (). If , the open slit is absolutely stable, and if , the closed configuration is the absolutely stable state.
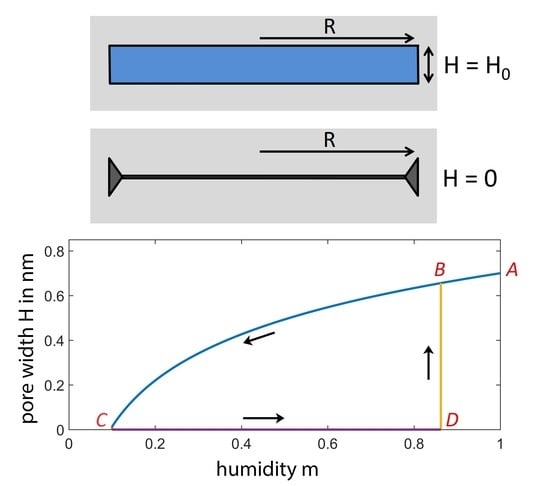
Let us first briefly discuss the mechanism that leads to sorption hysteresis.
Figure 2 illustrates the humidity-dependent width
H of a narrow circular slit pore with radius
nm. At saturation pressure (
), the value of the pore width
is chosen to be
nm. The slit is embedded in a soft solid with Young’s modulus
GPa, Poisson’s ratio
and adhesion energy per unit area
N/m. The number density of water is
nm
at the chosen temperature
K. The narrow slit is supposed to be always filled with condensate if
. However, instead of evaporation, the amount of condensate in the pore can decrease by compression of the pore space. Condensate can be squeezed out of the slit pore, when the compression force (Equation (
1)) increases with decreasing humidity
m. This process occurs along the desorption branch in
Figure 2 (Curve
). According to Equation (
8), the slit thickness
H is equal to
at saturation pressure (
), which refers to Point A in
Figure 2.
After decreasing
m to
, the slit is completely closed (Point
C), and the onset of the short-range adhesion stabilizes the closed slit configuration (
). This configuration remains closed along the line
as long as the difference of grand potentials
(Equation (
7)) is positive. When the humidity
m is increased to values exceeding
, the potential difference
is negative, and the closed slit pore can reopen. We evaluate the threshold for pore reopening from the condition
, which refers to the thermodynamic equilibrium between the open and the closed configuration. Thus, the adsorption branch starts at Point
C, moves to Point
D, jumps to Point
B and finally arrives at Point
A when
m is further increased. The desorption-adsorption cycle (
) can be repeated many times without serious material damage, since mainly elastic deformations occur. In a region close to the dislocation line, however, the large strain may cause some plastic deformations.
For hardened cement pastes, experimental data for the average distance between adjacent C-S-H sheets (basal spacing) are available, and thus, the relation between
m and
H can be tested. Let us denote changes of the basal spacing by
. Humidity-dependent changes of the slit width H are equal to
. According to Equation (
8), the change of the basal spacing
by varying humidity
m should be proportional to
. The lower diagram in
Figure 2 shows a semi-log plot for illustrating this logarithmic humidity dependence of
H. In the plots for experimental data, the absolute vapor pressure
is often chosen instead of the relative humidity
. Because
, this different choice is not important. Using
instead of
m only causes a uniform shift of data points parallel to the humidity axis in semi-log plots. In
Figure 2, the desorption branch
A—
C appears as a sloped straight line. Furthermore, the absorption branch consists of a straight line
C—
D parallel to the humidity axis and a perpendicular line
D—
B describing a discontinuity appearing when the closed slit reopens. This plot can be compared with analogous semi-log plots for experimental data obtained from X-ray measurements. For the branches
A—
C and
C—
D, Smith [
7] found straight lines as depicted in
Figure 2. Some deviations from the linear behavior at high relative humidity along the desorption branch can readily be explained by water-filled mesopores above the threshold of capillary condensation, because the condensed water in mesopores exerts a compression force on the solid skeleton [
1,
16]. Finally, the experimentally-observed data reveal a rapid change of the basal spacing after pore reopening. The experimental curves are relatively steep, but not perpendicular to the humidity axis, while in
Figure 2, the depicted straight line
D—
B is perpendicular to the humidity axis. This deviation could be explained by a slit size distribution, as illustrated in a previous paper [
11].
It should be noted, however, that some publications report on the appearance of jumps along the desorption branch in semi-log plots of the basal spacing versus humidity [
15,
22]. The macroscopic model that uses the discontinuous adhesion potential (Equation (
6)) is not capable of describing a discontinuity of the basal spacing for the desorption branch. In this paper, a more realistic adhesion potential that has a finite interaction range is used. We want to check if this extension is capable of describing a discontinuous dependence of
H on
m.
2.2. Extended Theoretical Model
The considered model for the adhesion potential (Equation (
6)) is only a proper approximation in cases where the interaction force
per unit area decays rapidly with increasing slit wall distance
H. For elucidating the impact of a finite interaction range on sorption diagrams, we can use the force:
where the length
is a measure of the interaction distance and
A is the magnitude of
. The choice
leads to a compression force accompanied by a cohesion of slit walls. The constants
A and
in Equation (
9) could be fitted to results of Monte Carlo simulations. In hardened cement paste, the cohesion force between C-S-H sheets is supposed to originate from calcium ions in the interlayer water [
23]. Equation (
7) is replaced by (
Appendix A, Equation (
A10)):
where
is a reference width of the slit at vanishing solvation pressure
introduced in the
Appendix A. It is expedient to define the reference width as the value of
H at vapor saturation (
). This width would be equal to
if condition
were satisfied. Usually, condition
is not satisfied because the attraction force between walls is not equal to zero, even at vapor saturation (
). Actually, the condition for mechanical stability of an open slit
leads to:
At saturation pressure of the vapor, we have
, and according to Equation (
11), the equilibrium slit thickness deviates from
if
. It is expedient to introduce a slightly shifted slit thickness
by the equation:
It should be noted, however, that the difference between
and
is rather small, as the force magnitude
is a strongly decreasing function of the wall distance
H. Thus, the condition
holds. Replacing
in Equation (
10) by using Equation (
12),
is transformed into:
where an adhesion potential:
is introduced, and
is a constant, independent of
m. Now, the condition for mechanical stability
leads to:
This equation allows us to evaluate how the width
H of an open slit depends on the relative vapor pressure
m. Obviously, Equation (
15) satisfies the condition
, and thus, the value of the wall width
H at saturation pressure (
) is equal to
. In a similar way, as has been done previously, we define a work per unit area
w necessary to separate two touching planar surfaces and to restore the original slit width
at saturation pressure (
). According to Equation (
14), this work can be evaluated by
or:
Combining Equations (
9) and (
16) yields:
where:
is a constant. Finally, inserting
(Equation (
17)) into Equation (
14), we obtain the adhesion potential:
Figure 3 illustrates the adhesion potential
for three different interaction distances
. If
, the force
deviates from zero only in the case of touching wall surfaces (
), as in the simplest macroscopic adhesion model (Equation (
6)).
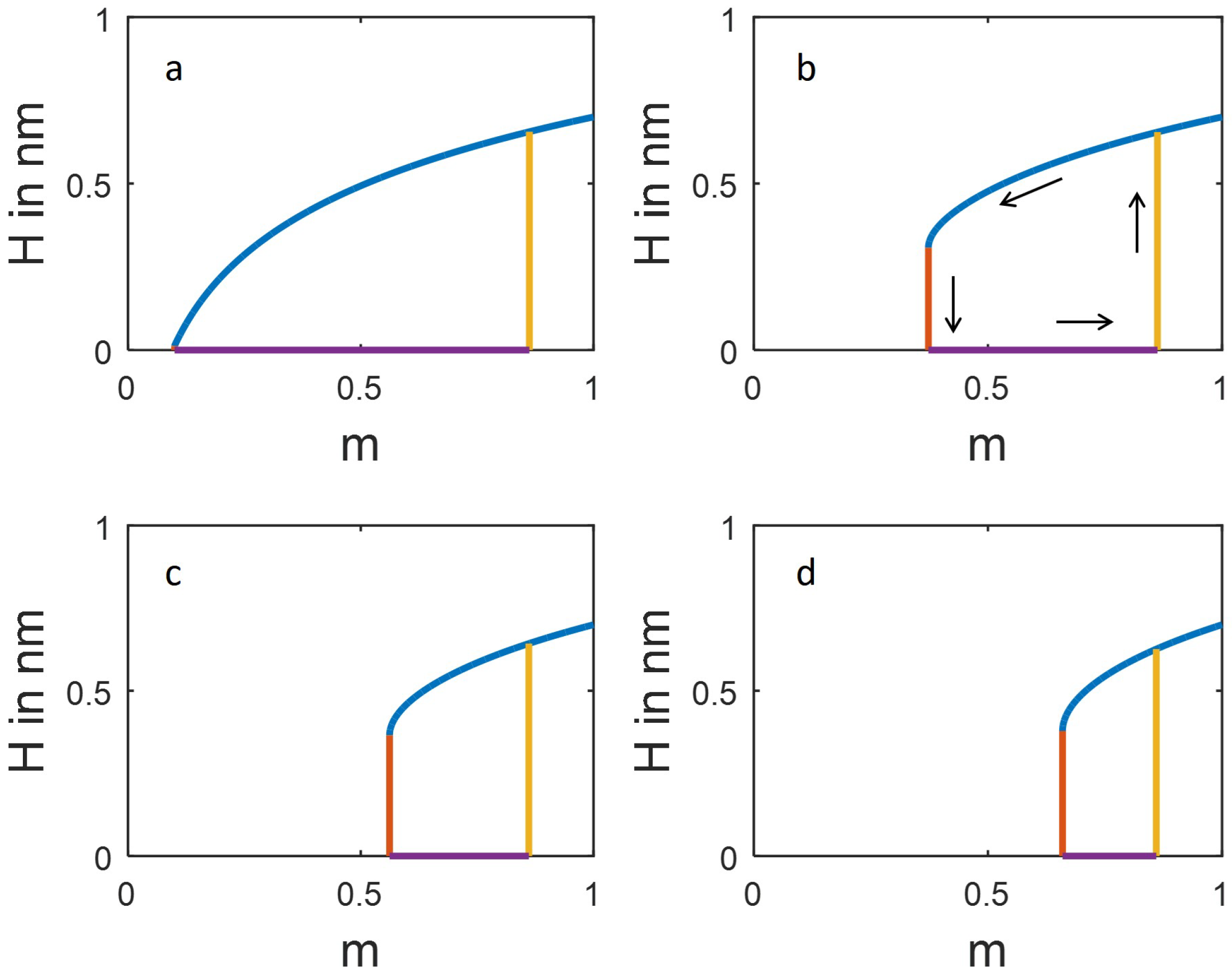
2.3. Mechanical Stability of the Open and the Closed Slit Configuration
In the previous section, we have introduced the difference
of grand potentials for deciding whether an open (
) or closed slit configuration (
) has a lower grand potential. Checking whether or not
has a negative value provides us the preferred state of the slit, but mechanical stability is not guaranteed. Let us first check the stability of the open slit configuration (
). Mechanical stability requires that the grand potential
as a function of
H has a local minimum. Apart from the equilibrium condition
, the condition for a local minimum of the grand potential
must be satisfied. Hence, taking into account that the grand potential (divided by the constant slit area
) can be written as:
we obtain the stability condition for an open slit
where the width
is evaluated by Equation (
15). The point on the desorption branch where the open slit configuration loses its stability is the critical point of a fold in the framework of catastrophe theory [
24]. At this point, the open pore collapses on the desorption branch, and the closed slit configuration (
) is the only stable state at low humidity (
Figure 4). Unstable branches of the fold catastrophe are not depicted in the diagrams shown in
Figure 4. In the borderline case
, the extended model for sorption hysteresis based on a continuous function
(Equation (
18)) reduces to the simpler model with a discontinuous adhesion potential (Equation (
6)). Taking into account that
and:
for
and arbitrary values of
n, the stability condition for open pores (Equation (
20) turns out to be always satisfied in the case of the discontinuous adhesion model (Equation (
6)).
For confirming the consistency of the considered model, we also check the mechanical stability of the closed slit configuration (
). Stability considerations for the closed slit state are more subtle because the width
H must be always positive (
). This constraint for
H has the consequence that the condition
is not necessary for the closed slit (
) [
24]. Instead, the closed slit configuration is stable if condition
holds. From a physical point of view, this condition allows for a compressive stress
in the plane bounded by the circular dislocation loop (
Figure 1). Using Equation (
19), condition
yields:
Obviously, for sufficiently small values of the interaction length
, the condition for local stability (Equation (
22)) of closed slits is satisfied in the whole range of relative vapor pressure (or relative humidity)
. Using the stability conditions (Equations (
20) and (
22)), the mechanical stability of all states defined by the isotherms illustrated in this paper can also be checked.