Abstract
In this work, we investigated the potential of Bacillus subtilis UCP 0146 in the bioconversion of a medium containing 100% cassava flour wastewater to obtain a bioemulsifier. The evaluation of the production was carried out by the emulsification index (IE24) and the surface tension (ST). The ionic charge, stability (temperature, salinity, and pH measured by IE24 and viscosity), and ability to remove and disperse oil and textile dye were investigated. B. subtilis produced an anionic bioemulsifier in the medium containing 100% cassava wastewater under Condition 4 of the factorial design (inoculum 9% at a temperature of 35 °C and shaken at 100 rpm), and showed a surface tension of 39 mN/m, an IE24 of 95.2%, and a yield of 2.69 g·L−1. The bioemulsifier showed stability at different pH (2–8), temperatures (0–120 °C), and NaCl concentrations, a dispersion oil displacement area (ODA) test of 55.83 cm2, and a reduction of the viscosity of the burned engine oil (90.5 Cp). The bioemulsifier was able to remove petroleum (94.4%) and methylene blue azo dye (62.2%). The bioemulsifier and its synthesis from bacteria also emphasizes the role of surfactants in oil remediation.
1. Introduction
Bioemulsifiers are amphiphilic compounds with a high molecular weight that have a high response to surface tension. They have a hydrophobic (amino acids, mono/disaccharide peptides, polysaccharides) and hydrophilic (saturated or unsaturated fatty acids) portion, allowing interaction with fluids of different polarities (oil/water and water/oil), the forming of microemulsions, and provide detergency, solubilizing, and emulsifying actions [1,2,3].
A variety of microorganisms are producers of biomulsifiers; some have a higher production capacity, such as bacteria and yeasts, and to a lesser extent filamentous fungi. Regarding bacteria, there is the Bacillus species, in particular Bacillus subtilis, which has the ability to produce lipopeptides, degrade long-chain alkanes, and reduce the viscosity of hydrocarbons [4,5,6,7,8]. In recent years, bioemulsifiers have been cited as leading biomolecules in the therapeutic and biomedical sector as well as in agriculture, pharmacological products, dermatology, the food and cosmetic industries, and bioremediation [9,10,11].
Their use in the petrochemical industry has occurred as a result of bioemulsifiers being considered better than synthetic chemicals due to the characteristics of biodegradability, low toxicity, and production from renewable sources [12,13,14,15,16,17,18].
The use of agroindustrial residues as a low-cost raw material for the microbial production of bioemulsifiers is a well-explored strategy in the field of biotechnology to reduce production costs and put into practice current environmental policies [19,20,21,22,23,24].
Cassava wastewater is the main effluent from the cassava press due to the industrial production of flour. The chemical composition basically constitutes carbohydrates, nitrogen, and several mineral salts, characterizing itself as a supplement with significant potential [25,26,27,28,29,30,31,32,33].
The main objective of this work was to use Bacillus subtilis UCP 0146 to produce a bioemulsifier by applying an ecofriendly methodology that uses cassava wastewater as a substrate in order to improve our understanding of the stability of the emulsion properties and the applicability of this biomolecule.
2. Materials and Methods
2.1. The Microorganism and the Culture Conditions
The microorganism used was Bacillus subtilis UCP 0146 isolated from mangrove sediments (Rio Formoso-PE). It is maintained by the Culture Collection UCP (Universidade Católica de Pernambuco), Catholic University of Pernambuco, Recife-PE, Brazil, which is registered with the World Federation for Culture Collection (WFCC). The bacterium was maintained in nutrient agar (peptone 5.0 g·L−1; meat extract 3.0 g·L−1; and agar 18 g·L−1) at 5 °C, and transferred to a nutrient broth (peptone 5.0 g·L−1 and meat extract 3.0 g·L−1).
2.2. Substrates
Cassava wastewater, a by-product of cassava (Manihot esculenta) processing for flour production, was kindly supplied by the flour house of the municipality of Pombos-PE, Brazil. Methylene blue dye (molecular mass −333.6 g/mol) was obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.3. Production of the Bioemulsifier
The inoculum was prepared by transferring B. subtillis cells from the solid medium to the nutrient broth, which was maintained at 30 °C for 12 h at 150 rpm. Then, 5% of the inoculum containing 108 colony-forming units (CFU)/mL were transferred to Erlenmeyer flasks with a 250 mL capacity with the bioemulsifier production medium, which contained 100% cassava wastewater, as described by Nitschke and Pastore [33]. The flasks were kept under orbital shaking at 150 rpm for 96 h at 30 °C. After this time, the samples were centrifuged at 4000 rpm and filtered for separation of the cell-free supernatant. The experiments were performed in triplicate.
2.4. The Full Factorial Design
The effects and interactions between the inoculum size, the temperature, and the agitation speed for the bioemulsifier’s production were investigated using a 23 factorial design, as shown in Table 1. STATISTICA software version 6.0 of StatSoft® was used for the statistical analysis of the results.

Table 1.
The levels of the variables that were studied in the 23 full factorial design for bioemulsifier production by Bacillus subtilis UCP 0146.
2.5. Determination of the pH
The pH of the bioemulsifier production medium was determined in the metabolic liquid by an Orion potentiometer (Model 310) (Orion Research Inc., Cambridge, MA, USA).
2.6. Determination of the Surface Tension (ST)
The surface tension was determined through the on cell-free supernatant using a tensiometer, model Sigma 70 (KSV Instruments Ltd., Helsinki, Finland), by the Du Nouyring method at room temperature (±28 °C) as reported by Kuyukina et al. [34].
2.7. Determination of the Emulsification Index (EI24)
The emulsification index of the cell-free supernatant was analyzed according to Cooper and Goldenberg [35]. The hydrophobic substrates used were vegetable oils (soybean, corn, canola, olive, and waste soybean oil) and petroleum derivatives (diesel and burned engine oil). After 24 h, the emulsification index (EI24) was determined and is expressed as a percentage. In addition, the shelf life was evaluated by the emulsification index and viscosity for 150 days.
2.8. Optical Microscopic Analysis of Emulsions
The analysis of emulsions was carried out after adding 2 mL of hydrophobic substrates and 1 mL of cell-free supernatant collected at 24, 48, 72, and 96 h in test tubes, and then they were vortexed at high speed for 1 min. The emulsions were observed through an optical microscope with a magnification of 40×, and a digital camera was used to capture the images.
2.9. Determination of Viscosity
The effect of the bioemulsifier on the viscosity of different oils (soybean, corn, waste soybean, and burned motor oil) was investigated in test tubes containing 6 mL of the respective oils and 2 mL of 1% bioemulsifier solution (w/v). Then, the tubes were vortexed for 1 min and the viscosity was measured at 25 °C in an automatic viscometer (Brookfield Middleboro, Middleborough, MA, USA; TC 500). Anionic surfactant sodium dodecyl sulfate (SDS) and commercial detergent were used as controls. The viscosity results are expressed in centipoise (Cp) and as a percentage (%).
2.10. Determination of the Stability
The stability of the bioemulsifier was evaluated in the cell-free supernatant submitted to different pH (2, 4, 6, 8, 10, 12, and 14), NaCl concentrations (0%, 2%, 4%, 6%, 8%, 10%, 12%, and 14%), and temperatures (0, 5, 70, 100, and 120 °C) for 10 min.
2.11. Extraction and Yield of the Bioemulsifier
The bioemulsifier of B. subtilis was extracted from the cell-free supernatant using the ethanol precipitation method according to Nahato et al. [36]. After extraction, the crude bioemulsifier was washed twice with distilled water and the yield expressed in g·L−1.
2.12. Determination of Ionic Charge
The ionic charge of the bioemulsifier was investigated using 100 mg of the solubilized biomolecule in 5 mL of distilled water. The ionic character was determined with a Zeta potentiometer ZM3-D-G, Zeta Meter System 3.0+, and the direct images were recorded in a Zeta Meter video, San Francisco, CA, USA.
2.13. Application of the Bioemulsifier in an Oil Spreading Test and Dye Removal in Water
The ability of the bioemulsifier to disperse burned motor oil was investigated using 50 mL of distilled water added to a large Petri dish (15 cm in diameter) followed by adding 20 µL of burned motor oil to the water’s surface and 10 µL of the cell-free supernatant. A clear halo was visible under light. The area of this circle was measured and calculated to determine the oil displacement area (ODA) using the following equation. The appearance of a clear zone after the addition of the cell-free supernatant containing the bioemulsifier indicated the dispersing capacity of the bioemulsifier. The clear zone’s diameter was measured as the oil displacement area (ODA), and the results are expressed in cm−1 as per Techaoei et al. [37].
The bioemulsifier produced by B. subtillis was tested for its potential to remove methylene blue dye (molecular mass −333.6 g/mol) according to the methodology proposed by Asku et al. [38], modified by the use of the bioemulsifier to replace the chemical surfactant sodium dodecyl sulfate (SDS). The experiments were carried out in 250 mL Erlenmeyer flasks containing a methylene blue solution prepared from a standard solution (1 g·L−1) and the crude bioemulsifier (1 g·L−1), suspended in distilled water. The dye solution without a bioemulsifier was used as a negative control. The experiments were performed in triplicate. The flasks containing methylene blue solution and bioemulsifier were maintained under orbital shaking at 150 rpm for 48 h with the removal of 5 mL aliquots to determine the biosorption kinetics of the dye by the bioemulsifier. All samples were centrifuged (4000 rpm) for 5 min, and the supernatant was analyzed in a spectrophotometer at a wavelength 663 nm. The biosorption efficiency was calculated according to Equation (1):
where A is the initial absorbance and B is the absorbance of the decolorized solution.
2.14. Application in the Removal of Oil in Marine Soil
The ability of the bioemulsifier to remove oil in marine soil was investigated using 20 g of marine soil that was collected from the beach (Recife-PE), impregnated with 5 mL of burned motor oil, and transferred to a 250 mL Erlenmeyer flask. Then, 30 mL of the cell-free supernatant containing the bioemulsifier was added and the flasks were kept under orbital shaking at 150 rpm and 28 °C for 48 h, according to the methodology of Nitschke and Pastore [4]. The amount of oil that was removed was determined by washing the marine soil with hexane, and the results are expressed as a percentage.
3. Results
3.1. Production of a Bioemulsifier by Bacillus subtilis UCP 0146 in a Medium Containing Cassava Wastewater
The production of a bioemulsifier by Bacillus subtilis in a medium that was formulated with cassava flour wastewater (100%) reduced the surface tension from 72 to 39 mN/m and resulted in 95% emulsification using motor-burned oil (Table 2).

Table 2.
The surface tension and the emulsification index (EI24) for different hydrophobic substrates that were obtained by the bioemulsifier produced from Bacillus subtilis UCP 0146 in a medium containing cassava wastewater.
From the identification of the occurrence of the production of the bioemulsifier by Bacillus subtilis, a complete factorial design was performed (Table 1) to evaluate the effect of temperature, agitation, and inoculum volume on the production of the bioemulsifier. The results showed that the bioemulsifier that was produced under Condition 4 of the factorial design (temperature 35 °C, 100 rpm, and inoculum of 9%) maintained a high emulsifying capacity (95.2%) after 150 days (IE150) using motor-burned oil as a hydrophobic substrate (Table 3). The bioemulsifier that was produced under Condition 6 of the factorial design resulted in IE24 values of 95%, similar to that of Condition 4 (95.2%). However, it did not maintain stability for 150 days (IE150) with the motor-burned oil; thus, Condition 4 of the factorial design was chosen.

Table 3.
The results of a factorial design of 23 to evaluate the influence of temperature, agitation, and inoculum volume on the production of a bioemulsifier by Bacillus subtilis UCP 0146, evaluated during 150 days by the emulsification index EI24 (%) and EI150 (%).
Under Condition 4 of the factorial design (temperature 35 °C, 100 rpm, and 9% inoculum), the crude bioemulsifier yield was 2.69 g·L−1. The cell-free supernatant containing the bioemulsifier was able to form a mixture with the burned motor oil and reduced the viscosity of this oil from 170 to 90.5 Cp after 24 h. This assay was followed for 150 days to identify the ability of the bioemulsifier to keep the viscosity of the formed emulsions stable. The results showed that there were few variations in viscosity during the 150 h, resulting in values ranging from 68.8 Cp to 90.5 Cp. However, it was confirmed that the metabolic liquid cultured at 60 h was the best at maintaining the viscosity and stability of the emulsions, with values of 88.9 Cp after 150 h.
3.2. Determination of the Influence of Inoculum Size, Temperature, and Agitation on Bioemulsifier Production by Bacillus subtilis
The effect of the independent variables volume of the inoculum, temperature, and speed on the emulsification index (IE24), using burned engine oil as the hydrophobic substrate, is represented in the Pareto Diagram (Figure 1) with a confidence level of 95%. According to this diagram, the increase in inoculum volume exercised a statistically positive effect in the increase of IE24. In addition, it was also observed that the interaction of the inoculum volume variable and the agitation variable exercised a statistically negative effect on the increase of IE24.
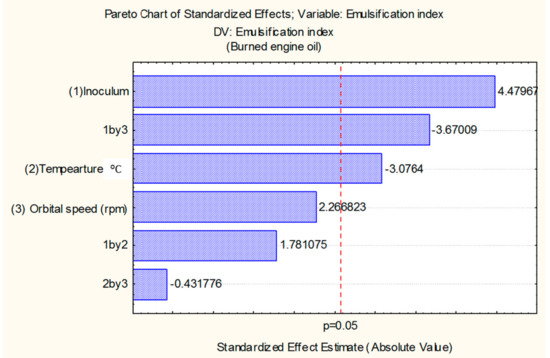
Figure 1.
The Pareto chart for determining the influence of inoculum size, temperature, and agitation on bioemulsifier production by Bacillus subtilis.
3.3. Characterization of Emulsion Droplets Using Burned Engine Oil
The emulsions that were formed by the bioemulsifier using burned engine oil were measured by optical microscopy (Figure 2), and characterized according to the main properties, emulsification index, and viscosity shown in Table 4. The results showed the formation of water-in-oil-type emulsions under Condition 4 of the factorial design (9% inoculum, cultivation at 35 °C, and agitation at 100 rpm) in medium containing 100% cassava wastewater. The cell-free supernatant cultured at 24 h showed the presence of large and thermodynamic unstable droplets with a globose and heterogeneous appearance and an EI24 of 80% (Figure 2A).
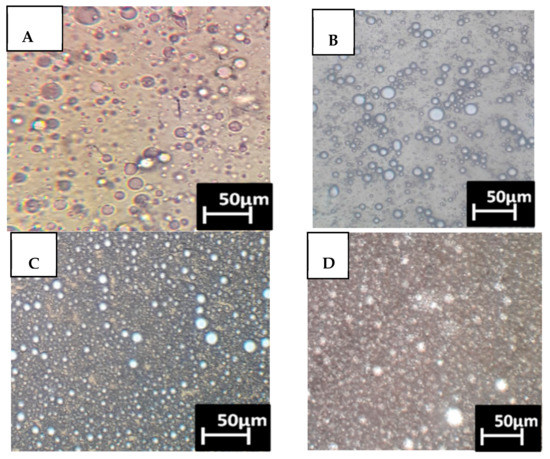
Figure 2.
Microscopic observations of the droplets that were formed from the bioemulsifier: (A) Metabolic liquid at 24 h; (B) Metabolic liquid at 48 h; (C) Metabolic liquid at 72 h; (D) Metabolic liquid at 96 h.

Table 4.
Evaluation of the stability of the bioemulsifier after exposure to different pH, concentrations of NaCl, and temperatures by viscosity determinations.
Figure 2B shows the metabolic liquid cultured at 48 h where no significant changes occurred, but flocculation of the droplets and greater free space were observed. Figure 2C shows the metabolic liquid cultured at 72 h with the formation of smaller, larger, homogeneous, and stable droplets with an EI24 of 90%. However, the formation of globose, homogeneous, and stable droplets with a smaller diameter (0.3 μm) and a high emulsification index (90%) occurred in the metabolic liquid cultured at 96 h (Figure 2D). Similar characteristics of stability, size, and type of emulsion obtained using the chemical surfactant sodium dodecyl sulfate (SDS) were mentioned by Souza et al. (2016) and are compatible with those observed in this study using the metabolic liquid of B. subtilis after 96 h of cultivation in cassava wastewater (Figure 2D).
3.4. Stability of the Bioemulsifier by Determination of the Viscosity and the Emulsification Index
The B. subtilis bioemulsifier was able to maintain a high emulsifying capacity, with IE24 values above 80% after abrupt pH variations (with the exception of pH 10 and 14) (Figure 3A). In relation to high concentrations of NaCl (Figure 3B), it was shown that the emulsions with motor oil remained stable in all tested concentrations of NaCl, with values around 90%. The thermal stability of the bioemulsifier (Figure 3C) was tested between 0 and 120 °C, revealing that the emulsions with burned motor oil had a decrease of 10% at 120 °C.
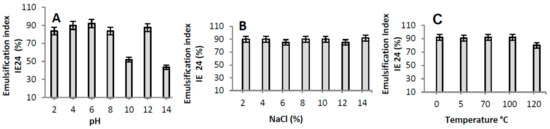
Figure 3.
The stability of the bioemulsifier that was produced by Bacillus subtilis UCP 0146 according to the emulsification index (EI24): pH (A); NaCl (B); and temperature (C).
The stability of the bioemulsifier according to the viscosity was also investigated, and it was demonstrated that the maximum reduction of the viscosity of the emulsions (10–20 Cp) occurred after the change in the pH of the cell-free supernatant, except at pH 4 (Table 5).

Table 5.
The characteristics and properties of the bioemulsifier produced by Bacillus subtilis UCP 0146.
Significant emulsifying activity, stability over a wide range of pH, extreme temperatures, and high salt concentrations, in addition to reducing oil viscosity, indicates that the bioemulsifier is suitable for use in the petroleum industry and other environmental activities, such as the recovery of oil, the cleaning of reservoirs, and the transportation of crude oil.
3.5. Efficiency of the Bioemulsifier in Petroderivative Dispersal and Pollutants Removal
The anionic bioemulsifier that was obtained by B. subtilis in medium containing 100% cassava wastewater, under Condition 4 of the full factorial design (FFD), was investigated as to the biosorption potential of the cationic dye methylene blue present in water. The biosorption results was promising to the isolate biomulsifier produced by Bacillus subtilis was able to removal 62.2% of the dye after 12 h and 55% at 1 h, respectively. The control used SDS solution (1%) showed allow efficiency of removal of the methylene blue dye at 1 h was 37.2%. In addition, the contact of 12 h of the synthetic emulsifier with dye reduced the removal value to 15.3% (Figure 4).
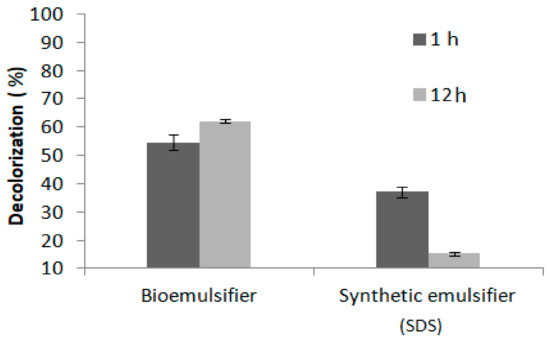
Figure 4.
Methylene blue dye removal by the bioemulsifier produced from Bacillus subtilis during 1 h and 12 h.
The bioemulsifier that was produced by B. subtilis in the best condition of the full factorial design (Assay 4) was applied in the dispersion and removal of burned engine oil present in water and marine soil, respectively. The dispersion efficiency—measured as the oil displacement area (ODA)—demonstrated that the bioemulsifier exhibited an excellent capacity for dispersion (55.38 cm2 ODA) after being compared with the dispersion by the commercial detergent as shown in Figure 5.
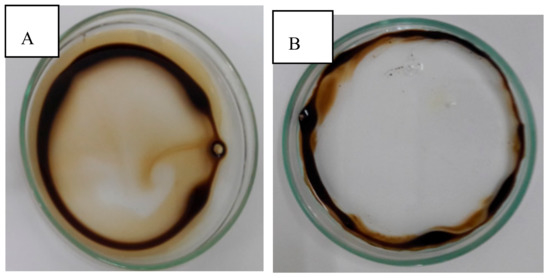
Figure 5.
Dispersion of burned engine oil in water. (A) Commercial detergent as the control, and (B) The bioemulsifier produced by Bacillus subtilis.
On the other hand, the bioemulsifier of B. subtilis was also able to demonstrate a significant capacity to act to remove petroderivatives, as we detected the removal of 94.4% of the burned engine oil that was impregnated in the marine soil.
4. Discussion
The results obtained in this work (a reduction in surface tension to 39 mN/m and an emulsification of 95%) using motor-burned oil suggest that the biomolecules produced by B. subtilis have the characteristics of a bioemulsifier. These results are in accordance with Rahman et al. [39], which affirms that bioemulsifiers do not cause significant changes in the reduction of surface tension between liquids and have high emulsifying activity.
According to the Pareto diagram, the increase in inoculum volume exercised a statistically positive effect on the increase of IE24. In addition, it was also observed that the interaction of the inoculum volume variable and the agitation variable exercised a statistically negative effect on the increase of IE24.
In this study, B. subtilis produced a bioemulsifier with great potential to maintain stability with IE24 values above 80% after abrupt pH, temperature, and NaCl variations. These results are very significant considering that Willumsen, Chiistian, and Ruzicka [40] affirm that the main characteristic of emulsifying agents is their ability to maintain the stability of substances with different degrees of polarity, which are represented by emulsification values (EI24) above 50% after 24 h. Presenting similar results [40,41,42], we obtained significant values throughout the pH range, except for when the biosurfactant was exposed to pH 12. Sarrubbo et al. [41] reported that pH extremes denatured protein components by increasing ionization.
The bioemulsifier produced by B. subtilis in this study was more effective for emulsification stability than the biosurfactant produced by C. lipolytica, which is grown in corn steep liquor, and waste soybean oil also obtained emulsion activity with the burned motor oil, which was unchanged at up to 10% NaCl and lost activity at a 12% NaCl concentration [40,41,42,43,44,45]. The bioemulsifier produced by C. lipolytica in a medium that was supplemented with corn steep liquor and waste soybean oil did not obtain emulsifying activity at values higher than 5% NaCl [3].
Similar results of emulsification stability were obtained by Santos et al. [40] and showed stability at low and high temperatures, exhibiting a 20% (73%) reduction in the emulsification index at 120 °C. Studies conducted by Regina and Silva [14] and Souza et al. [3] maintained emulsions that were formed in 65% and 80% motor oil, respectively, when exposing the bioemulsifier to a temperature of 100 °C.
According to Wei et al. [44], biosurfactants capable of reducing the viscosity of oils are suitable for use in the petroleum industry, since their low viscosity facilitates the removal of a considerable amount of oil. The bioemulsifier obtained in this work was able to reduce the viscosity of the burned motor oil from 170 to 90.5 Cp. There are few reports in the literature indicating bioemulsifiers’ potential to reduce the viscosity of oils. On the other hand, the study by Regina and Silva [14] added a bioemulsifier to motor oil and obtained a viscosity increase from 148.9 Cp to 210.7 Cp.
The removal of cationic dyes by anionic agents can be explained by the fact that they are compounds of opposite charges that enter into equilibrium in aqueous solutions and form pairs of hydrophobic ions, or more complexes associated with, or even aggregates between, the cationic dye and the anionic bioemulsifier in the solution [40,41,42,43,44,45]. In this context, was investigated in this work the efficiency of the bioemulsifier of Bacillus subtilis in biosorption of the cationic dye methylene blue, and the results showed biosorption efficiency after 12 h. Thus, these results indicate the possible use of the bioemulsifier of Bacillus subtilis in the treatment and removal dye pigments from industrial effluents. The application of a bioemulsifier in biosorption of methylene blue dye is pioneer in this work.
The bioemulsifier of Bacillus subtilis showed an excellent capacity to disperse burned motor oil (55.38 cm2 ODA). The smallest capacity to disperse oil was obtained by Andrade et al. [14], using a bioemulsifier isolated from the fermentation of Cunninghamella echinulata in waste soybean oil and corn steep liquor, with an oil dispersion area of 37.36 cm2. Similar results on the dispersion capacity of burned motor oil were obtained by Souza et al. [3] after application of a bioemulsifier from Candida lipolytica, with a dispersibility of 45.34 cm2.
On the other hand, the biosurfactant produced by C. lipolytica UCP 0988 that was investigated by Santos et al. [40] removed 92.3% of the burned motor oil, and, in the study by Andrade et al. [38], the biosurfactant produced by Candida glabrata removed 95.7% of the burned engine oil through the washing method.
5. Conclusions
The bioemulsifier produced in this work by Bacillus subtilis UCP 0146 in a medium containing exclusively cassava wastewater showed important properties that are useful for application in a bioremediation process, in particular the capacity to disperse burnt motor oil in water and the capacity to remove effluents from the textile industry. In addition, the bioemulsifier was able to form stable emulsions, suggesting that it has potential application in the pharmaceutical industry.
Author Contributions
Conceptualization, G.M.C.T. and R.F.S.A.; Methodology, P.C.V.S.M.; Software, M.A.C.L., T.A.L.S., and V.P.S.; Validation, P.C.V.S.M., M.A.C.L., and V.P.S.; Formal Analysis, G.M.C.T., M.A.C.L., and A.S.F.; Investigation, P.C.V.S.M.; Resources, P.C.V.S.M. and T.A.L.S.; Data Curation, G.M.C.T., P.C.V.S.M., A.S.F., and M.A.C.L.; Writing (Original Draft Preparation), P.C.V.S.M.; Writing (Review & Editing), R.F.S.A.; Visualization, P.C.V.S.M., R.F.S.A., and G.M.C.T.; Supervision, G.M.C.T.; Project Administration, G.M.C.T.; Funding Acquisition, G.M.C.T.
Funding
CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) under Process Nr. 311373/2014-3, FACEPE (Fundação de Amparo à Ciência e Tecnologia de Pernambuco) Process Nr. APQ-0291-2.12/15, and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior): PNPD fellowship to R.F.S.A and T.A.L.S, and support to P.C.V.S.M.
Acknowledgments
The authors are also grateful to the NPCIAMB (Nucleus of Research in Environmental Sciences and Biotechnology), Catholic University of Pernambuco (Recife-PE, Brazil), for the use of its laboratories.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J.; Rahman, P.K.S.M. Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 2015, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Bardone, E.; Bravi, M.; Keshavarz, T.; Secato, J.F.F.; Coelho, D.F.; Rosa, N.G.J.; Costa, L.D.L.; Tambourgi, E.B. Biosurfactant Production Using Bacillus subtilis and Industrial Waste as Substrate. Chem. Eng. Trans. 2016, 49, 103. [Google Scholar] [CrossRef]
- Souza, A.F.; Rodriguez, D.M.; Ribeaux, D.R.; Luna, M.A.C.; Lima, E.; Silva, T.A.; Andrade, R.F.S.; Gusmão, N.B.; Campos-Takaki, G.M. Waste Soybean Oil and Corn Steep Liquor as Economic Substrates for Bioemulsifier and Biodiesel Production by Candida lipolytica UCP 0998. Int. J. Mol. Sci. 2016, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Pastore, G.M. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour. Technol. 2006, 97, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Sakthipriya, N.; Doble, M.; Sangwai, J.S. Action of biosurfactant producing thermophilic Bacillus subtilis on waxy crude oil and long chain paraffins. Int. Biodeterior. Biodegrad. 2015, 105, 168–177. [Google Scholar] [CrossRef]
- Parthipan, P.; Preetham, E.; Machuca, L.L.; Rahman, P.K.S.M.; Murugan, K.; Rajasekar, A. Biosurfactant and degradative enzymes mediated crude oil degradation by bacterium Bacillus subtilis A1. Front. Microbiol. 2017, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Falode, O.A.M.A.A. Evaluation of Indigenous Biosurfactant-producing Bacteria for De-emulsification of Crude Oil. Microbiol. Res. J. Int. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.F.S.; Antunes, A.A.; Lima, R.A.; Araújo, H.W.C.; Resende-Stoianoff, M.A.; Franco, L.O.; Campos-Takaki, G.M. Enhanced Production of an Glycolipid Biosurfactant Produced by Candida glabrata UCP/WFCC1556 for Application in Dispersion and Removal of Petroderivatives. Int. J. Curr. Microbiol. 2015, 4, 563–576. [Google Scholar]
- Saha, P.; Rao, K.V. Biosurfactants—A Current Perspective on Production and Applications. Nat. Environ. Pollut. Technol. 2017, 16, 181–188. [Google Scholar]
- Satpute, S.K.; Płaza, G.A.; Banpurkar, A.G. Management Systems in Production Engineering biosurfactants production from renewable natural resources: Example of innovative and smart technology in circular bioeconomy. Manag. Syst. Product. Eng. 2017, 25, 46–54. [Google Scholar] [CrossRef]
- Bouassida, M.; Fourati, N.; Ghazala, I.; Ellouze-Chaabouni, S.; Ghribi, D. Potential application of Bacillus subtilis SPB1 biosurfactants in laundry detergent formulations: Compatibility study with detergent ingredients and washing performance. Eng. Life Sci. 2018, 18, 70–77. [Google Scholar] [CrossRef]
- Andrade Silva, N.R.; Luna, M.A.C.; Santiago, A.L.C.M.A.; Franco, L.O.; Silva, G.K.B.; de Souza, P.M.; Okada, K.; Albuquerque, C.D.C.; da Silva, C.A.A.; Campos-Takaki, G.M. Biosurfactant-and-Bioemulsifier Produced by a Promising Cunninghamella echinulata Isolated from Caatinga Soil in the Northeast of Brazil. Int. J. Mol. Sci. 2014, 15, 15377–15395. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, D.G.; Soares Da Silva, R.D.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J.; Upasani, V.N. Critical Review on Biosurfactant Analysis, Purification and Characterization Using Rhamnolipid as a Model Biosurfactant. Bioresour. Technol. 2017, 232, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Nayarisseri, A.; Singh, P.; Singh, S.K. Screening, isolation and characterization of biosurfactant producing Bacillus subtilis strain ANSKLAB03. Bioinformation 2018, 14, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Glick, B.R.; Rathore, D. Biosurfactants as a Biological Tool to Increase Micronutrient Availability in Soil: A Review. Pedosphere 2018, 28, 170–189. [Google Scholar] [CrossRef]
- Jain, R.M.; Mody, K.; Joshi, N.; Mishra, A.; Jha, B. Effect of unconventional carbon sources on biosurfactant production and its application in bioremediation. Int. J. Biol. Macromol. 2013, 62, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bouassida, M.; Ghazala, I.; Ellouze-Chaabouni, S.; Ghribi, D. Improved biosurfactant production by Bacillus subtilis SPB1 mutant obtained by random mutagenesis and its application in enhanced oil recovery in a sand system. J. Microbiol. Biotechnol. 2018, 28, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant Production: Emerging Trends and Promising Strategies. J. Appl. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Das, M.D. Application of biosurfactant produced by an adaptive strain of C. tropicalis MTCC230 in microbial enhanced oil recovery (MEOR) and removal of motor oil from contaminated marine soil and water. J. Pet. Sci. Eng. 2018, 170, 40–48. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Manuel Cruz, J.; Moldes, A.B. Biological Surfactants vs. Polysorbates: Comparison of Their Emulsifier and Surfactant Properties. Tens. Surf. Deterg. 2018, 55, 273–280. [Google Scholar] [CrossRef]
- Selvam, K.; Selvankumar, T.; Rajiniganth, R.; Srinivasan, P.; Sudhakar, C.; Senthilkumar, B.; Govarthanan, M. Enhanced production of amylase from Bacillus sp. using groundnut shell and cassava waste as a substrate under process optimization: Waste to wealth approach. Biocatal. Agric. Biotechnol. 2016, 7, 250–256. [Google Scholar] [CrossRef]
- Saha, P.; Nath, D.; Choudhury, M.D.; Talukdar, A.D. Probiotic biosurfactants: A potential therapeutic exercises in biomedical sciences. In Microbial Biotechnology; Patra, J.K., Das, G., Shin, H., Eds.; Springer: Singapore, 2018; pp. 499–514. [Google Scholar]
- Perfumo, A.; Rudden, M.; Marchant, R.; Banat, I.M. Biodiversity of biosurfactants and roles in enhancing the (bio)availability of hydrophobic substrates. Cell. Ecophysiol. Microbe 2018. [Google Scholar] [CrossRef]
- Marine Soilri, D.; Kholiq, M.A. Biosurfactant producing bacteria from oil contaminated soil: Screening, identification, and process optimization. Asian J. Environ. Biotechnol. 2018, 1, 49–56. [Google Scholar]
- George, S.; Jayachandran, K. Biosurfactants from Processed Wastes. In Waste to Wealth; Springer: Singapore, 2018; pp. 45–58. [Google Scholar]
- De Oliveira, D.W.; Cara, A.B.; Lechuga-Villena, M.; García-Román, M.; Melo, V.M.; Gonçalves, L.R.; Vaz, D.A. Aquatic toxicity and biodegradability of a surfactant produced by Bacillus subtilis ICA56. J. Environ. Sci. Health Part A 2017, 52, 174–181. [Google Scholar] [CrossRef] [PubMed]
- John, U.S.; John, M.C. Production and Application of Microbial Surfactant from Cassava Wastewater. Am. J. Eng. Technol. Soc. 2015, 2, 85–89. [Google Scholar] [CrossRef]
- Elijah, A.; Asamudo, N. Molecular Characterization and Potential of Fungal Species Associated with Cassava Waste. Br. Biotechnol. J. 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Larissa, K.; Nat Aacutessia, J.C.; Glaucia, M.P.; Ana, P.R.S.; Vander, F.M.; Simone, D.G. Adsorption of copper, zinc and lead on biosurfactant produced from cassava wastewater. Afr. J. Biotechnol. 2016, 15, 110–117. [Google Scholar] [CrossRef]
- Nitschke, M.; Pastore, G.M. Biossurfactantes: Propriedades e aplicações. Quim. Nova 2002, 25, 772–776. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B.; Makarov, S.O.; Litvinenko, L.V.; Cunningham, C.J.; Philp, J.C. Effect of biosurfactants on crude oil desorption and mobilization in a soil system. Environ. Int. 2005, 31, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [PubMed]
- Techaoei, S.; Leelapornpisid, P.; Santiarwarn, D.; Lumyong, S.; Mai, C. Preliminary Screening of Biosurfactant-Producing Microorganisms Isolated from Hot Spring and Garages in Northern Thailand. Curr. Appl. Sci. Technol. 2007, 7, 38–43. [Google Scholar]
- Aksu, Z.; Kutsal, T.; Gün, S.; Haciosmanoglu, N.; Gholaminejad, M. Investigation of biosorption of Cu (II), Ni(II) and Cr(VI) ions to activated sludge bacteria. Environ. Technol. 1991, 12, 915–921. [Google Scholar] [CrossRef]
- Rahman, P.K.S.M.; Sekhon Randhawa, K.K. Editorial: Microbiotechnology based surfactants and their applications. Front. Microbiol. 2015, 6, 1344. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, B.; Christian, G.D.; Ruzicka, J. Flow injection renewable surface immunoassay for real time monitoring of biospecific interactions. Anal. Chem. 1997, 69, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Salgueiro, A.A.; Sarubbo, L.A. Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J. Pet. Sci. Eng. 2013, 105, 43–50. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Farias, C.B.B.; Campos-Takaki, G.M. Co-utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Curr. Microbiol. 2007, 54, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.B.; Gonzales-Limache, E.E.; Sousa, S.T.P.; Dellagnezze, B.M.; Sartoratto, A.; Silva, L.C.F.; Sousa, M.P. Exploring the potential of halophilic bacteria from oil terminal environments for biosurfactant production and hydrocarbon degradation under high-salinity conditions. Int. Biodeterior. Biodegrad. 2018, 126, 231–242. [Google Scholar] [CrossRef]
- De Souza, C.G. Simultaneous quantification of lipopeptide isoforms by UPLC-MS in the fermentation broth from Bacillus subtilis CNPMS22. Anal. Bioanal. Chem. 2018, 410, 6827–6836. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.F.; Mather, R.R.; Fotheringham, A.F. Oil removal from used sorbents using a biosurfactant. Bioresour. Technol. 2005, 96, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Prévost, S.; Wattebled, L.; Laschewsky, A.; Gradzielski, M. Formation of monodisperse charged vesicles in mixtures of cationic gemini surfactants and anionic SDS. Langmuir 2011, 27, 582–591. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).