Abstract
Traditional approaches to achieve sustained delivery of pharmaceutical peptides traditionally use co-excipients (e.g., microspheres and hydrogels). Here, we investigate the release of an amyloidogenic glucagon analogue (3474) from an aggregated state and the influence of surfactants on this process. The formulation of peptide 3474 in dodecyl maltoside (DDM), rhamnolipid (RL), and sophorolipid (SL) led to faster fibrillation. When the aggregates were subjected to multiple cycles of release by repeated resuspension in fresh buffer, the kinetics of the release of soluble peptide 3474 from different surfactant aggregates all followed a simple exponential decay fit, with half-lives of 5–18 min and relatively constant levels of release in each cycle. However, different amounts of peptide are released from different aggregates, ranging from 0.015 mg/mL (3475-buffer) up to 0.03 mg/mL (3474-DDM), with 3474-buffer and 3474-RL in between. In addition to higher release levels, 3474-DDM aggregates showed a different amyloid FTIR structure, compared to 3474-RL and 3474-SL aggregates and a faster rate of degradation by proteinase K. This demonstrates that the stability of organized peptide aggregates can be modulated to achieve differences in release of soluble peptides, thus coupling aggregate polymorphism to differential release profiles. We achieved aggregate polymorphism by the addition of different surfactants, but polymorphism may also be reached through other approaches, including different excipients as well as changes in pH and salinity, providing a versatile handle to control release profiles.
1. Introduction
The controlled release of pharmaceutical drugs is subject to intense research efforts. Many strategies involve different forms of immobilization of the drug in microspheres or hydrogels [1,2,3,4,5,6], e.g., through cross-linking of various molecules to generate biodegradable microsphere polymers, which trap the active drug compound. There is a large range of polymers with different biodegradation and drug release profiles [7,8], including both micelles [9], peptide nanorods [10] and composites of polysaccharides and polylactides [11]. Delivery of self-assembled peptides may offer another strategy to generate an injectable drug with extended release. A good example is provided by insulin degludec, in which the natural propensity of insulin to form fibers consisting of concatemers of hexameric subunits with zinc is exploited for sustained release of insulin over 42 h [12]. Another way to assemble proteins is via amyloid aggregates, i.e., cross β-sheet fiber aggregates formed by proteins and peptides [13]. Amyloids in the form of misfolded proteins are associated with at least 50 human diseases [14], but also find use in nature in a wide range of applications, including cell-cell contacts, biofilm strengthening, and cellular structures [15,16,17]. Amyloid formation occurs above the equilibrium solubility of the monomeric species and is usually detected with the dye thioflavin T, which undergoes a marked increase in fluorescence upon binding fibrils [18]. Thus, aggregates of the amyloid β peptide (1-40) are in a dynamic equilibrium with the soluble peptide population and aggregates diluted into phosphate buffered saline (PBS) release peptides until the critical concentration (equilibrium solubility) is reached over a timespan of ~24 h [19,20]. It has been suggested that most, if not all, proteins and peptides can form amyloid structures [21,22,23]. Amyloids may offer a general approach to depot formation. Their high resistance to proteolysis, pH changes, physical stress, and chemical stability [24,25,26,27] offer the prospect of a common strategy for the release of peptides and unstructured proteins. Indeed, Riek and coworkers [28] have shown that long-acting gonadotropin-releasing hormone analogues act by forming amyloids with long-lasting peptide release (half-life times ranging from 15–30 days). In contrast, most short-acting variants did not form amyloids.
Aggregation of proteins and peptides is strongly influenced by surfactants [29], ranging from inhibition to promotion of aggregation. The promotion of aggregation can be driven by destabilization of the native protein structure by surfactants [30,31], assembly of proteins through the formation of shared micelles [32], or micellar interactions similar to membrane interactions [33,34,35]. In addition, surfactants may also lead to off-pathway aggregation by electrostatic interactions stabilizing oligomeric interactions [36] or inhibition of aggregation, e.g., observed in insulin by modulating the effective polarity of water [37] and by hydrophobic interactions with recombinant human growth hormone (rHGH) that prevent aggregation from the molten globule state [38,39].
Here we investigate the potential use of amyloid aggregates as injectable particles for subcutaneous depot formation and its manipulation through different ways of forming aggregates. As a model system, we use an inactive analogue of the peptide hormone glucagon (peptide 3474) which fibrillates in the presence of micelles of different surfactants, namely the nonionic surfactant dodecyl maltoside (DDM) and the two glycolipid biosurfactants rhamnolipid (RL) and sophorolipid (SL), both of which have a carboxyl group that is ionized above pH 5–6. Peptide 3474 is a variant of the native glucagon sequence, in which five amino acids have been changed, conferring a global charge of −2 at neutral pH. These changes lead to improved solubility at neutral pH, compared to native glucagon, that has a global charge of 0 at neutral pH. We find that the DDM-induced aggregates lead to higher levels of release of 3474, compared to the aggregates formed in RL and SL, and the difference in release can be correlated to changes in aggregate structure.
2. Materials and Methods
Chemicals: Rhamnolipid (RL), 90% pure, was from Agae Technologies (Corvallis, OR), 87% pure sophorolipid was from Ecover (Malle, Belgium), dodecyl maltoside (DDM) came from Avanti Lipids (Alabaster, AL), proteinase K was from MP Biomedicals (Santa Ana, CA), and all other chemicals were from Sigma (St. Louis, MO). Peptide 3474 (sequence HSQGTFTSDYSKYLDSARAEDFVAWLERT) was synthesized and purified to 98% as a trifluoroacetate-lyophilisate purity at Zealand Pharma A/S (Glostrup, Denmark).
Thioflavin T (ThT) fibrillation assay to prepare peptide aggregates: Fibrillation of 3474 was performed on a Tecan Genios Pro Plate Reader (Tecan, Switzerland) at 40 °C with constant agitation (300 rpm orbital shaking). ThT was prepared in milliQ water stocks at 1 mM and diluted to a final concentration of 20 µM. The 3474 was dissolved in milliQ water-HCl pH 2.5 at a concentration of 5 mg/mL and centrifuged for 10 min at 21.130 g on an Eppendorf microcentrifuge 5424 R (Eppendorf, Germany) before dilution to a final concentration of 1 mg/mL in 50 mM phosphate pH 7.0 buffer. ThT fluorescence was monitored with excitation at 448 nm and emission at 485 nm. Data was collected every 10 min over 250 h.
ATR-FTIR: Prior to measurement, aggregated samples of 3474 were washed by 3 cycles of resuspension in water followed by centrifugation at 21.130 g for 10 min on an Eppendorf microcentrifuge 5424 R (Eppendorf, Germany). Spectra were collected on a Tensor 27 FTIR spectrometer (Bruker Optics, Billerica, MA). To record spectra, 2 µL of peptide samples were dried under nitrogen flow. Data were collected as an average of 68 scans and a resolution of 2 cm−1. Data processing and peak identification were carried out as described [40].
Circular dichroism: Spectra were recorded on a JASCO J-810 spectropolarimeter (Jasco Spectroscopic Co. Ltd., Japan). The samples contained 1 mg/mL 3474 and were measured in a 0.2 mm cuvette over 260–190 nm with 5 repetitions at a scanning speed of 100 nm/min. Spectra of 3474 in SL, RL, and sodium dodecyl sulphate (SDS) were reconstructed as weighted averages of the spectra of 3474 in SDS (fraction fSDS) and in buffer (1-fSDS) in Excel Solver.
Release Assay for 3474 Aggregates: Peptide aggregates were washed in 11.8 mM NaP with 137 mM NaCl and 2.7 mM KCl (PBS) with a triple cycle of centrifugation and resuspension in buffer. The centrifugation was done in an Eppendorf microcentrifuge 5424 R (Eppendorf, Germany) at 21.130 g. The washed aggregates were resuspended in PBS at 1.0 mg/mL, after which they were left to incubate at 37 °C in darkness. Samples were extracted at different time intervals (cycles 1, 2 or 3—0, 0.25, 0.5, 1, 2, and 4 h, cycle 4—0, 0.25, and 12 h). For each time-point, samples were centrifuged at 21.130 g for 5 min and supernatant was collected. Between each cycle, the entire supernatant was removed after the centrifugation step and fresh PBS was added to the original sample volume (1 mg/mL). The concentration of peptide in the supernatant samples was measured on a TSKgel G2000SW column (Tosoh Bioscience, Japan) using an Ultimate 3000 HPLC (Thermo Fisher Scientific, Massachusetts, USA) with a fluorescence or UV detector. The UV was collected at 280 nm on an Ultimate 3000 RS Diode array detector and fluorescence with excitation at 280 nm and emission at 350 nm on an Ultimate 3000 RS Fluorescence detector. The elution buffer consisted of 45% (V/V) acetonitrile and 0.1% trifluoroacetic acid in MilliQ water. Chromeleon 7 software was used to determine the peak area of the samples, which was converted into concentration using a 3474-standard diluted from a known stock solution.
Transmission Electron Microscopy: Samples of aggregated 3474 were transferred to a carbon-coated glow-discharged 400 mesh nickel grid, stained with 1% phosphotungstic acid and blotted dry. A JEOL 1010 electron microscope was used at 60 kW with an Olympus KeenView camera.
Proteinase K amyloid digestion assay: The 3474 aggregates were washed twice in MQ water and twice in PBS by centrifugation at 21.130 g for 10 min on an Eppendorf microcentrifuge 5424 R (Eppendorf, Germany) by removing the supernatant. Proteinase K was added to the aggregates at molar protease–peptide ratios of 1:1, 1:5, 1:10, 1:50, 1:100, 1:500, 1:1000, and 0:1. ThT was added to 40 µM and the decline in ThT fluorescence was used to estimate proteolysis of the 3474 aggregates. ThT fluorescence was measured over 72 h with excitation at 448 nm, emission at 485 nm and a gain of 75, and slit widths of 9.0/9.0 on a Synergy H4 Hybrid Reader (BioTek, Vermont USA). The experiment was run at 37 °C with 300 rpm shaking for 15 s between each time point (equal to shaking 10% of the time).
3. Results
3.1. The Peptide 3474 Aggregates in Buffer and in the Presence of Different Surfactants
In this study we investigate release of an inactive fibrillating glucagon analogue (3474) in a simple dissolution model. Although 3474 is more soluble than native glucagon at neutral pH, the peptide still forms ThT-positive aggregates in an accelerated stress assay (constant shaking at 40 °C). There is an abrupt increase in ThT signal after 130–200 h, leading to a large overshoot and then a decline, over a ~30 h period, to a plateau level well above the start value (Figure 1A with sample in buffer). This overshoot is reproducible and may reflect a very efficient cycle of fragmentation and growth, which sets in once a sufficient number of fibrils have formed. Over longer time scales, these fibrils may rearrange and bundle together to reduce the number of ThT binding sites, leading to a reduction in ThT fluorescence, as reported in numerous other fibrillation studies. FTIR confirmed that these aggregates contained amyloid with a peak around 1627 cm−1 in a second derivative spectrum (Figure 1B), which is characteristic for cross-β structures [41].
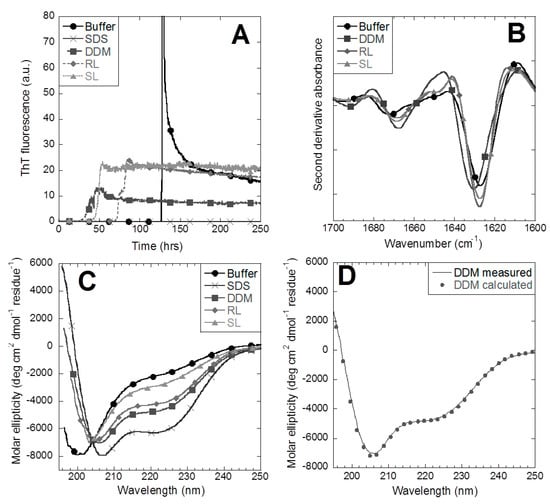
Figure 1.
Amyloidogenic propensity of 3474 in buffer and surfactants. (A) Incubation of 3474 at 40 °C under constant agitation and monitoring amyloid formation, using the amyloid dye ThT, shows that fibrillation of 3474 is accelerated by the addition of dodecyl maltoside (DDM), rhamnolipid (RL), and sophorolipid (SL), while sodium dodecyl sulphate (SDS) inhibits aggregation completely. (B) FTIR confirms the amyloid peak of 3474. (C) The far-UV circular dichroism signal of 3474 before aggregation shows increasing α-helicity with surfactants. (D) Comparison of measured circular dichroism (CD) structure and the calculated signal of 3474-DDM based on linear combinations of its structure in buffer and SDS.
To form additional types of aggregates for slow-release studies, we turned to surfactants which are known to induce protein aggregation under some circumstances [29]. At 10 mM surfactant (which is well above the critical micelle concentration (cmc) for all tested surfactants), we find that SDS completely suppresses aggregation, whereas DDM, RL, and SL shorten the lag-time of 3474 fibrillation and suppress the overshoot phase, leading to a simpler sigmoidal ThT time profile (Figure 1A). To elucidate the basis for these effects, we investigated changes to the secondary structure of 3474 by far-UV circular dichroism (CD) in the presence of these surfactants (Figure 1C). The 3474 is mostly a random coil in buffer solution, while SDS induces an α-helix structure as expected. DDM, RL, and SL influence secondary structure to a lesser degree than SDS, with DDM resembling the SDS-induced α-helix spectrum the most and SL the least (Figure 1C). All the spectra intercept in an isodichroic point around 204 nm, suggesting that they are linear combinations of only two different spectra. In fact, the spectra for 3474 in SL, RL, and DDM can be deconvoluted as weighted averages of the spectra of 3474 in buffer and in SDS (a representative sample for DDM is shown in Figure 1D). The SDS component contributes 17%, 51% and 63% to the spectra of SL, RL, and DDM, respectively, while the remaining percentages come from 3474 in the random coil conformation.
3.2. 3474 Aggregates Show Burst Release of Soluble Peptides, Particularly Those Formed in DDM
We considered several ways to mimic a drug release profile. As an initial attempt to follow the release of soluble peptides from 3474 aggregates, we placed the aggregates inside a dialysis membrane with a 10 kDa cut-off. However, background binding of 3474 to the cellulose ester membrane made this approach unfeasible. We had more success with a simple setup where 3474 aggregates were kept suspended in solution for an extended period. At different time points, the aggregates were spun down and samples were withdrawn to measure the concentration of peptide in the solution, using absorbance and fluorescence from an Ultimate 3000 chromatography system. Before the start of this incubation period, residual soluble 3474 (<5%) was removed in several centrifugation steps.
We focused on the release profile from the aggregates generated in the buffer, DDM, RL, and SL over a period of 24 h, divided into 4 cycles. In each cycle, the aggregate was resuspended into the PBS buffer and samples were withdrawn at regular intervals. The amount of released peptide was determined by spinning down the sample suspension and measuring the concentration of monomeric peptide by HPLC. After each cycle, the original suspension was centrifuged, the supernatant was removed, and the pellet was resuspended in fresh PBS solution. In this way we attempted to model a situation in which the released peptide was transported away at regular intervals, enabling a new release equilibrium to establish itself. In all cases, there was a rapid release of peptide that reached a plateau within 0–2 h, with 50–100% total release around the first sample point (15 min) (Figure 2). DDM aggregates were released so rapidly that we could only estimate a lower limit for the half-life of release of ≤5 min. In the remaining three cases, the release profile can be fitted to a simple exponential decay with half-lives of 18 ± 4, 14 ± 3, and 15 ± 3 min in the buffer, RL, and SL, respectively. The 3474-DDM aggregates reached the highest plateau at 0.031 ± 0.001 mg/mL, which was sustained within the duration of the experiment (12 h in the present study and up to 28 days in other studies (data not shown)), indicating that the equilibrium between aggregated and soluble 3474 is reached quickly. The 3474 aggregates formed in the buffer, RL, and SL reached lower plateau concentrations (0.0154 ± 0.001, 0.021 ± 0.001 and 0.019 ± 0.002 mg/mL, respectively, based on averages from 4 cycles). Thus, each cycle only released 1.5–3% of the total protein, leading cumulatively to ca. 13% for DDM over 4 cycles and correspondingly less for the other aggregates.
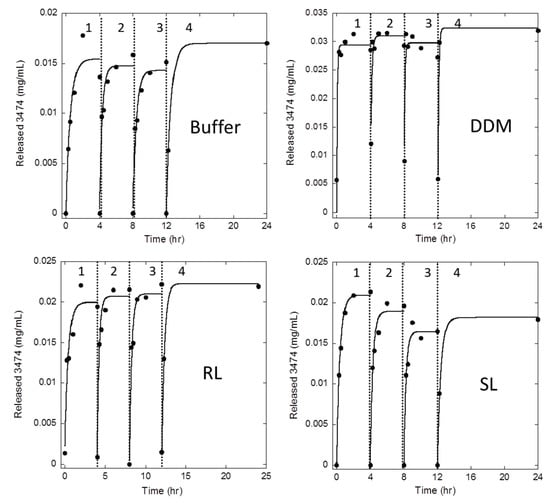
Figure 2.
Soluble 3474 is released at the same final level in consecutive release cycles from each type of aggregate. The 3474 aggregates were prepared in the buffer, 10 mM DDM, 10 mM RL, or 10 mM SL and spun down. In each cycle, the aggregate was washed and resuspended in a PBS solution to 1 mg/mL and samples were withdrawn at different time points and centrifuged separately to measure the release of soluble 3474 over time. At the end of each cycle, the aggregate was spun down, and subsequently resuspended in a new cycle. The release of 3474 from different aggregates could all be fitted to a single exponential decay with half-lives of 14–18 min, except for DDM aggregates which reached equilibrium within the first measurement point (see main text). Release figures show release levels in mg/mL from a suspension of originally 1 mg/mL aggregate. Thus, the release figures in mg/mL also correspond to the fraction of release (disregarding the small reduction in total protein after each cycle).
3.3. Fibril Structure Differences is Reflected by Significant Differences in Proteolysis Susceptibility
To rationalize these differences in release, we turned to a closer inspection of the different aggregates. Transmission electron micrographs showed short unbranched fibrils of 3474 in the buffer (Figure 3A), with no obvious changes to fiber structure or morphology compared to fibers formed in RL or SL (Figure 3B,C), while fibers formed in DDM had weaker contours and showed signs of partial dissolution (Figure 3D). FTIR spectra of the washed aggregates revealed an important difference (Figure 1B). While the pronounced peak in the 1625–1630 cm−1 region confirms the amyloid cross-β structure of all four types of aggregates, the position of the amyloid peak is almost identical in the buffer (1627.3 cm−1), SL (1627.5 cm−1), and RL (1627.8 cm−1) but shifts by 3 nm in DDM (1630.3 cm−1).
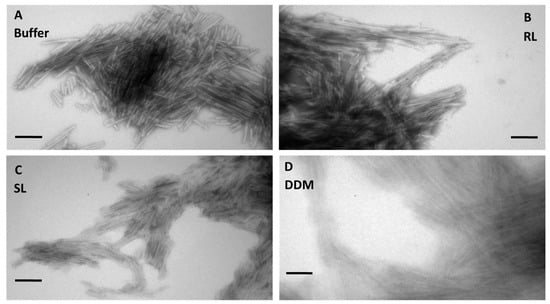
Figure 3.
The 3474 aggregates formed in buffer (A), RL (B) and SL (C) are morphologically indistinguishable. Electron microscopy shows the aggregates formed in DDM (D) are morphologically different compared to those formed in pure buffer and RL/SL. The scale bar indicates 100 nm.
Finally, we investigated peptide aggregate stability by a simple protease assay, in which the broad-specificity proteinase K (protK) was incubated with peptide aggregates at protK–peptide molar ratios ranging from 1:1 to 1:1000, while following the change in ThT fluorescence over time (data for DDM shown in Figure 4A). The 3474 aggregated in the buffer, DDM, and RL displayed rapid degradation as a decay in the ThT fluorescence, reaching zero fluorescence after 2–20 h. The decay in ThT fit an exponential decay function satisfactorily, yielding a half-life t½.
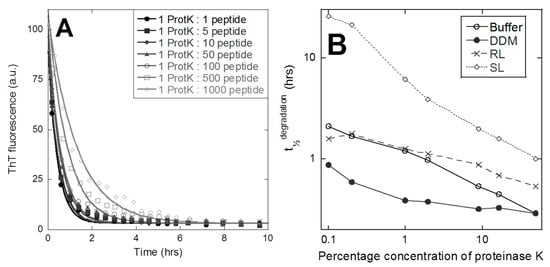
Figure 4.
Proteolysis of 3474 aggregates correlates with levels of release. Degradation of 3474 aggregates by proteinase K. (A) ThT fluorescence over time of 3474-DDM aggregates incubated with different molar ratios (proteinase K-peptide) of proteinase. (B) The t½ values from ThT-fluorescence decays of the four different types of 3474 aggregates follow an approximately linear relationship in a log–log plot, when plotted versus the fraction of proteinase K.
In the case of 3474-SL, the ThT fluorescence initially increases before a rapid decline towards zero (data not shown). In this case, we estimated the apparent half time visually as the time needed to reduce the ThT fluorescence signal to 50% of the value at time t = 0. As summarized in Figure 4B, all concentrations of protK degrade 3474 and there is a roughly linear decline in t½ versus protK concentration for all 4 aggregates in a log–log plot. It is evident that 3474-DDM is degraded the fastest at low protK ratios, while 3474 in the buffer and RL remain very similar. The 3474-SL stands out with its non-trivial degradation behavior and much higher t½ at low protK ratios. Aggregate degradation by protK correlates well with the extent of release (Figure 2), 3474-DDM degrades the quickest, and 3474-SL the slowest.
4. Discussion and Conclusions
We have studied the aggregation and subsequent release of soluble peptides from 3474 fibrils. The lag-time of fibrillation in the buffer was very long, but could be shortened by inclusion of mild surfactants (DDM, RL, and SL) and completely prevented by a strongly binding anionic surfactant (SDS). The inhibitory effect of SDS on aggregation is expected, as surfactants are known to influence aggregation of proteins and peptides. While low concentrations of SDS can promote aggregation, due to formation of e.g., shared micelles [29,30,36], concentrations above the cmc invariably inhibit aggregation [30,36,42] due to the solubilizing and dispersing power of SDS micelles. Despite being anionic, biosurfactants RL and SL only weakly interact with proteins, probably because the negative charge is more delocalized on the carboxylate group, compared to SDS’ sulfate group [43,44,45].
We found that 3474 release was influenced by the conditions of aggregation. The peptide release had a short t½ that fit a simple exponential decay. The 3474-DDM aggregates released more 3474 into the solution than 3474 in the buffer, RL, and SL aggregates. The release is in agreement with the release behavior reported for gonadotropin-releasing hormone aggregates [28], albeit with much faster kinetics (minutes rather than days). The in vivo release profile may be altered by various serum components known to interact with proteins and protein aggregates, such as glycosaminoglycans [46] and amyloid P protein [47]. We did not address potential immunogenicity of 3474 aggregates, but note that there is large variance between different aggregates [48,49]. For example, soluble aggregates of human gamma globulin elicit a higher immunogenic response compared to non-aggregated globulin [50], while aggregates of recombinant human interferon beta are only immunogenic if oxidized [51]. In all cases, we observed that multiple cycles of aggregate resuspension followed by centrifugation and replacement of the supernatant with fresh buffer solution led to the same overall levels of release in each cycle. This is consistent with the rapid formation of an equilibrium between the solution and aggregate, in which the existence of a separate insoluble phase (rather than the total amount of insoluble material) determines the solubility of the peptide.
The underlying mechanism behind the different release from 3474-DDM compared to 3474-RL/SL probably relates to different aggregate structures at several different levels. Firstly, DDM-induced aggregation gives a lower ThT fluorescence than aggregation in the buffer, RL, and SL. Secondly, FTIR spectroscopy confirms that 3474-DDM aggregates differ from the other 3474 aggregates in secondary structure (FTIR spectra), and thirdly 3474-DDM aggregates are more susceptible to proteolysis by proteinase K. In a previous study on the closely related glucagon analogue ZP-GA-1 [45], we have demonstrated that DDM interacts more strongly with the peptide than the biosurfactants RL and SL 3474 (and also more than the other surfactants surfactin and Tween 20), though much more weakly than SDS does. Thus, the different types of aggregates formed clearly reflect different levels of binding affinity. We speculate that the DDM-peptide interactions are weak enough to allow the peptide to form stabilizing intermolecular interactions favoring aggregation, but strong enough to destabilize the aggregates sufficiently for release of most of the peptide. In this model, the weaker interactions formed by RL and SL do not “prime” the aggregate to the same extent. It should be noted that the level of release in all cases is relatively low (cumulatively up to ca. 13% for DDM), though additional release will likely occur upon further dilution. Nevertheless, the difference in release between the different aggregates provides a useful way to modulate the biological accessibility of the peptide in vivo.
Polymorphic control over release is also observed in chemical drugs, e.g., chloramphenicol-palmitate displays vastly different dissolution kinetics between two polymorphic structures [52]. Similarly, native glucagon also displays polymorphic variance of aggregates that expresses itself in different morphologies of aggregates (e.g., twist and no twist along the fibrillar axis, typically visualized with EM) and differences in stability (determined by urea and thermal stability) [53]. In the case of glucagon, different polymorphic states can be induced by changing the concentration of glucagon, including shear stress and inclusion of sulfate [53,54,55]. It is generally accepted that most proteins and peptides display some degree of polymorphism in their aggregation [56]. By exploiting this polymorphism, we can access different types of aggregates with different release profiles, highlighting that amyloid aggregates can be versatile tools to generate long-acting drugs. In the present work we have achieved this by incubating 3474 with surfactants during aggregation, but there may be many other means to influence peptide aggregation and generate different polymorphs. Native glucagon forms aggregates of differing stability when incubated under different conditions, such as variations in temperature, salinity, and glucagon concentration [53,57]. Mutating the amino acid sequence may also be utilized to control equilibrium solubility, which is illustrated by the 8-fold higher kinetic solubility of amyloid β(26-40) compared to amyloid β(26-43) [20].
Author Contributions
Conceptualization, D.E.O., J.K.M. and L.G. Methodology: J.K.M. and G.C. Formal analysis: J.K.M., G.C. and D.E.O. Writing-Original Draft Preparation, J.K.M.; Writing-Review & Editing, D.E.O.; Visualization, J.K.M.; Supervision, D.E.O. and L.G.; Project Administration, D.E.O. and L.G.; Funding Acquisition, D.E.O. and L.G.
Funding
This research was co-funded by The Danish Innovation Foundation (grant number 1355-00136) and Zealand Pharma (salaries for J.K.M. and L.G.).
Acknowledgements
We thank Lene Jørgensen at Copenhagen University for assisting with expertise on drug release design.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| SDS | sodium dodecyl sulphate |
| DDM | dodecyl maltoside |
| RL | rhamnolipid |
| SL | sophorolipid |
| NaP | sodium phosphate |
| PBS | phosphate buffered saline |
| 3474 | glucagon analogue |
References
- Goosen, M.F.; Leung, Y.F.; Chou, S.; Sun, A.M. Insulin-albumin microbeads: An implantable, biodegradable system. Biomater. Med. Devices Artif. Organs 1982, 10, 205–218. [Google Scholar] [CrossRef]
- Garbayo, E.; Ansorena, E.; Lanciego, J.L.; Aymerich, M.S.; Blanco-Prieto, M.J. Sustained release of bioactive glycosylated glial cell-line derived neurotrophic factor from biodegradable polymeric microspheres. Eur. J. Pharm. Biopharm. 2008, 69, 844–851. [Google Scholar] [CrossRef]
- Liu, B.; Dong, Q.; Wang, M.; Shi, L.; Wu, Y.; Yu, X.; Shi, Y.; Shan, Y.; Jiang, C.; Zhang, X.; et al. Preparation, characterization, and pharmacodynamics of exenatide-loaded poly(DL-lactic-co-glycolic acid) microspheres. Chem. Pharm. Bull. (Tokyo) 2010, 58, 1474–1479. [Google Scholar] [CrossRef]
- Kamath, K.R.; Park, K. Biodegradable hydrogels in drug delivery. Adv. Drug Deliv. Rev. 1993, 11, 59–84. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained release intraocular drug delivery devices for treatment of uveitis. J. Ophthalmic. Vis. Res. 2011, 6, 317–329. [Google Scholar] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; He, W.; Zhao, D.; Song, A.; Luan, Y. Disulfide-Linked Amphiphilic Polymer-Docetaxel Conjugates Assembled Redox-Sensitive Micelles for Efficient Antitumor Drug Delivery. Biomacromolecules 2016, 17, 1621–1632. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Zhao, Q.; Zhang, Y.; Cao, L.; Luan, Y. Doxorubicin-loaded polypeptide nanorods based on electrostatic interactions for cancer therapy. J. Colloid Interface Sci. 2016, 464, 126–136. [Google Scholar] [CrossRef]
- Rogovina, S.Z. Biodegradable polymer composites based on synthetic and natural polymers of various classes. Prog. Polym. Sci. 2016, 58, 1–26. [Google Scholar] [CrossRef]
- Vora, J.; Cariou, B.; Evans, M.; Gross, J.L.; Harris, S.; Landstedt-Hallin, L.; Mithal, A.; Rodriguez, M.R.; Meneghini, L. Clinical use of insulin degludec. Diabetes Res. Clin. Pract. 2015, 109, 19–31. [Google Scholar] [CrossRef]
- Sunde, M.; Serpell, L.C.; Bartlam, M.; Fraser, P.E.; Pepys, M.B.; Blake, C.C. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 1997, 273, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Seuring, C.; Nespovitaya, N.; Rutishauser, J.; Spies, M.; Riek, R. Hormone amyloids in sickness and in health. In Amyloid Fibrils and Prefibrillar Aggregates; Otzen, D.E., Ed.; WileyVCH Verlag & Co. KGaA: Berlin/Heidelberg, Germany, 2013; pp. 395–410. [Google Scholar]
- Otzen, D.E.; Nielsen, P.H. We find them here, we find them there: Functional bacterial amyloid. Cell. Mol. Life Sci. 2008, 65, 910–927. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.F.B.; Schafer, N.; Wolf-Perez, A.; Madsen, D.J.; Otzen, D.E. Bacterial amyloids: Biogenesis and biomaterials. In Biological and Bio-Inspired Nanomaterials: Assembly Mechanisms and Properties; Perrett, S., Buell, A.K., Knowles, T.J., Eds.; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- LeVine, H., 3rd. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999, 309, 274–284. [Google Scholar]
- O’Nuallain, B.; Shivaprasad, S.; Kheterpal, I.; Wetzel, R. Thermodynamics of Aβ(1−40) Amyloid Fibril Elongation. Biochemistry 2005, 44, 12709–12718. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, J.T.; Costa, P.R.; Griffin, R.G.; Lansbury, P.T. Models of the beta Protein C-Terminus: Differences in Amyloid Structure May Lead to Segregation of “Long” and “Short” Fibrils. J. Am. Chem. Soc. 1994, 116, 9741–9742. [Google Scholar] [CrossRef]
- Baldwin, A.J.; Knowles, T.P.; Tartaglia, G.G.; Fitzpatrick, A.W.; Devlin, G.L.; Shammas, S.L.; Waudby, C.A.; Mossuto, M.F.; Meehan, S.; Gras, S.L.; et al. Metastability of native proteins and the phenomenon of amyloid formation. J. Am. Chem. Soc. 2011, 133, 14160–14163. [Google Scholar] [CrossRef]
- Gazit, E. The “Correctly Folded” state of proteins: Is it a metastable state? Angew. Chem. Int. Ed. Engl. 2002, 41, 257–259. [Google Scholar] [CrossRef]
- Dobson, C.M. The structural basis of protein folding and its links with human disease. Philos. Trans. R. Soc. Lond B Biol. Sci. 2001, 356, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Morel, B.; Varela, L.; Conejero-Lara, F. The thermodynamic stability of amyloid fibrils studied by differential scanning calorimetry. J. Phys. Chem. B 2010, 114, 4010–4019. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Reches, M.; Sedman, V.L.; Allen, S.; Tendler, S.J.; Gazit, E. Thermal and chemical stability of diphenylalanine peptide nanotubes: Implications for nanotechnological applications. Langmuir 2006, 22, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; Fitzpatrick, A.W.; Meehan, S.; Mott, H.R.; Vendruscolo, M.; Dobson, C.M.; Welland, M.E. Role of intermolecular forces in defining material properties of protein nanofibrils. Science 2007, 318, 1900–1903. [Google Scholar] [CrossRef] [PubMed]
- Nordstedt, C.; Naslund, J.; Tjernberg, L.O.; Karlstrom, A.R.; Thyberg, J.; Terenius, L. The Alzheimer A beta peptide develops protease resistance in association with its polymerization into fibrils. J. Biol. Chem. 1994, 269, 30773–30776. [Google Scholar] [PubMed]
- Maji, S.K.; Schubert, D.; Rivier, C.; Lee, S.; Rivier, J.E.; Riek, R. Amyloid as a depot for the formulation of long-acting drugs. PLoS Biol. 2008, 6, e17. [Google Scholar] [CrossRef]
- Otzen, D.E. Amyloid formation in surfactants and alcohols: Membrane mimetics or structural switchers? Curr. Protein Pept. Sci. 2010, 11, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Giehm, L.; Oliveira, C.L.; Christiansen, G.; Pedersen, J.S.; Otzen, D.E. SDS-induced fibrillation of alpha-synuclein: An alternative fibrillation pathway. J. Mol. Biol. 2010, 401, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Leffers, K.W.; Schell, J.; Jansen, K.; Lucassen, R.; Kaimann, T.; Nagel-Steger, L.; Tatzelt, J.; Riesner, D. The Structural Transition of the Prion Protein into its Pathogenic Conformation is Induced by Unmasking Hydrophobic Sites. J. Mol. Biol. 2004, 344, 839–853. [Google Scholar] [CrossRef]
- Andersen, K.K.; Oliveira, C.L.P.; Larsen, K.L.; Poulsen, F.M.; Callisen, T.H.; Westh, P.; Pedersen, J.S.; Otzen, D.E. The role of decorated SDS micelles in sub-cmc protein denaturation and association. J. Mol. Biol. 2009, 391, 207–226. [Google Scholar] [CrossRef]
- Patil, S.M.; Xu, S.; Sheftic, S.R.; Alexandrescu, A.T. Dynamic alpha-helix structure of micelle-bound human amylin. J. Biol. Chem. 2009, 284, 11982–11991. [Google Scholar] [CrossRef]
- Knight, J.D.; Miranker, A.D. Phospholipid catalysis of diabetic amyloid assembly. J. Mol. Biol. 2004, 341, 1175–1187. [Google Scholar] [CrossRef]
- Chirita, C.N.; Necula, M.; Kuret, J. Anionic micelles and vesicles induce tau fibrillization in vitro. J. Biol. Chem. 2003, 278, 25644–25650. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Rocha, S.; Mdo, C.P. Charged surfactants induce a non-fibrillar aggregation pathway of amyloid-beta peptide. J. Pept. Sci. 2013, 19, 581–587. [Google Scholar] [CrossRef]
- Lougheed, W.D.; Albisser, A.M.; Martindale, H.M.; Chow, J.C.; Clement, J.R. Physical stability of insulin formulations. Diabetes 1983, 32, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Bam, N.B.; Cleland, J.L.; Yang, J.; Manning, M.C.; Carpenter, J.F.; Kelley, R.F.; Randolph, T.W. Tween protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions. J. Pharm. Sci. 1998, 87, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Bam, N.B.; Cleland, J.L.; Randolph, T.W. Molten globule intermediate of recombinant human growth hormone: Stabilization with surfactants. Biotechnol. Prog. 1996, 12, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, N.; Nielsen, S.B.; Buell, A.K.; Kaspersen, J.D.; Arosio, P.; Vad, B.S.; Paslawski, W.; Christiansen, G.; Valnickova-Hansen, Z.; Andreasen, M.; et al. The role of stable α-synuclein oligomers in the molecular events underlying amyloid formation. J. Am. Chem. Soc. 2014, 136, 3859–3868. [Google Scholar] [CrossRef]
- Zandomeneghi, G.; Krebs, M.R.; McCammon, M.G.; Fandrich, M. FTIR reveals structural differences between native beta-sheet proteins and amyloid fibrils. Protein Sci. 2004, 13, 3314–3321. [Google Scholar] [CrossRef]
- Steiner, S.S.; Li, M.; Hauser, R.; Pohl, R. Stabilized glucagon formulation for bihormonal pump use. J. Diabetes Sci. Technol. 2010, 4, 1332–1337. [Google Scholar] [CrossRef]
- Madsen, J.K.; Pihl, R.; Møller, A.L.B.; Madsen, A.T.; Otzen, D.E.; Andersen, K.K. The anionic biosurfactant rhamnolipid does not denature industrial enzymes. Front. Microbiol. 2015, 6, 292. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.K.; Otzen, D.E. Denaturation of a-lactalbumin and myoglobin by the anionic biosurfactant Rhamnolipid. Biochim. Biophys. Acta 2014, 1844, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.K.; Giehm, L.; Otzen, D.E. The use of surfactants to solubilise a glucagon analogue. Pharm. Res. 2018, 35, 235. [Google Scholar] [CrossRef]
- Malmos, K.G.; Bjerring, M.; Jessen, C.M.; Nielsen, E.H.; Poulsen, E.T.; Christiansen, G.; Vosegaard, T.; Skrydstrup, T.; Enghild, J.J.; Pedersen, J.S.; et al. How Glycosaminoglycans Promote Fibrillation of Salmon Calcitonin. J. Biol. Chem. 2016, 291, 16849–16862. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.; de Frutos, P.G.; Carlemalm, E.; Dahlback, B.; Eriksson, S. Inhibition of Alzheimer beta-peptide fibril formation by serum amyloid P component. J. Biol. Chem. 1995, 270, 26041–26044. [Google Scholar] [CrossRef] [PubMed]
- Fradkin, A.H.; Carpenter, J.F.; Randolph, T.W. Immunogenicity of aggregates of recombinant human growth hormone in mouse models. J. Pharm. Sci. 2009, 98, 3247–3264. [Google Scholar] [CrossRef]
- Weltzien, R.B.; Pachter, J.S. Visualization of beta-amyloid peptide (Abeta) phagocytosis by human mononuclear phagocytes: Dependency on Abeta aggregate size. J. Neurosci. Res. 2000, 59, 522–527. [Google Scholar] [CrossRef]
- Gamble, C.N. The role of soluble aggregates in the primary immune response of mice to human gamma globulin. Int. Arch. Allergy Appl. Immunol. 1966, 30, 446–455. [Google Scholar] [CrossRef]
- van Beers, M.M.; Sauerborn, M.; Gilli, F.; Brinks, V.; Schellekens, H.; Jiskoot, W. Oxidized and aggregated recombinant human interferon beta is immunogenic in human interferon beta transgenic mice. Pharm. Res. 2011, 28, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.J.; Krc, J., Jr.; Kinkel, A.W.; Samyn, J.C. Effect of polymorphism on the absorption of chloramphenicol from chloramphenicol palmitate. J. Pharm. Sci. 1967, 56, 847–853. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Dikov, D.; Flink, J.L.; Hjuler, H.A.; Christiansen, G.; Otzen, D.E. The changing face of glucagon fibrillation: Structural polymorphism and conformational imprinting. J. Mol. Biol. 2006, 355, 501–523. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Andersen, C.B.; Otzen, D.E. Amyloid structure—One but not the same: The many levels of fibrillar polymorphism. FEBS J. 2010, 277, 4591–4601. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Otzen, D.E. Amyloid—A state in many guises: Survival of the fittest fibril fold. Protein Sci. 2008, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tycko, R. Amyloid Polymorphism: Structural Basis and Neurobiological Relevance. Neuron 2015, 86, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.S. The nature of amyloid-like glucagon fibrils. J. Diabetes Sci. Technol. 2010, 4, 1357–1367. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).