Abstract
The growing interest in probiotic bacteria within the food industry is driven by their recognized health benefits for consumers. However, preserving their therapeutic viability and stability during gastrointestinal transit remains a formidable challenge. Hence, this research aimed to enhance the viability of Lactobacillus reuteri through microencapsulation using a binary polysaccharide mixture composed of low acyl gellan gum (LAG), high acyl gellan gum (HAG), and calcium for the microencapsulation of L. reuteri. To achieve this, the Box–Behnken design was applied, targeting the optimization of L. reuteri microencapsulated to withstand simulated gastrointestinal conditions. The microcapsules were crafted using the internal ionic gelation method, and optimization was performed using response surface methodology (RSM) based on the Box–Behnken design. The model demonstrated robust predictive power, with R2 values exceeding 95% and a lack of fit greater than p > 0.05. Under optimized conditions—0.88% (w/v) LAG, 0.43% (w/v) HAG, and 24.44 mM Ca—L. reuteri reached a viability of 97.43% following the encapsulation process. After 4 h of exposure to simulated gastric fluid (SGF) and intestinal fluid (SIF), the encapsulated cells maintained a viable count of 8.02 log CFU/mL. These promising results underscore the potential of biopolymer-based microcapsules, such as those containing LAG and HAG, as an innovative approach for safeguarding probiotics during gastrointestinal passage, paving the way for new probiotic-enriched food products.
1. Introduction
Probiotics are defined as live microorganisms that, when administered in adequate quantities, confer health benefits to the host by enhancing the microbial balance of the intestinal tract [1]. These benefits include the prevention of gastrointestinal infections and potential protective effects against cancer, cardiovascular diseases, and certain allergic responses. Additionally, probiotics have been reported to exhibit antagonistic activity against both Gram-positive and Gram-negative bacteria, including pathogenic strains [2]. Among probiotic microorganisms, lactic acid bacteria are particularly significant [3]. Lactobacillus reuteri, a species naturally found in the human gastrointestinal tract, exhibits strong probiotic characteristics and colonization ability [4]. This bacterium is known to synthesize reuterin (β-hydroxypropionaldehyde), a broad-spectrum antimicrobial compound effective against bacteria, yeasts, fungi, and protozoa [5].
The gastrointestinal tract presents multiple physiological barriers that significantly challenge the survival of orally administered probiotic bacteria. The stomach, with its highly acidic environment and the proteolytic activity of pepsin during an average transit time of 90–120 min, acts as a primary defence against microbial passage to the intestine [6]. Following gastric exposure, probiotics must also withstand the harsh conditions of the small intestine, where they encounter bile salts, pancreatic enzymes, and a typically alkaline pH of approximately 8.0. As a result, considerable research has focused on developing effective strategies to enhance probiotic survival under these adverse gastrointestinal conditions.
Given that probiotics are typically delivered via oral administration, they must be protected against the destructive effects of gastric acid, bile salts, and digestive enzymes. To address this, various techniques have been explored to improve both the viability and stability of probiotic formulations, ensuring that enough live cells reach their intended site of action in the intestine. The viability of probiotic organisms is a critical requirement for conferring health benefits, as only live cells can exert the desired physiological effects [7]. However, passage through the gastroduodenal tract often results in a reduction of viable cell counts, making tolerance to gastric acidity and bile salts a key attribute of effective probiotic strains [8]. The survival rate of probiotics is strain-dependent, but several studies suggest that to exert a beneficial effect, a probiotic product should deliver at least 106 to 107 colony-forming units (CFU) per gram at the time of consumption [9,10]. Furthermore, for products claiming probiotic health benefits, a minimum of 106 CFU/mL must be maintained until the end of their shelf life, as the recommended daily intake for a therapeutic effect range from 108 to 109 viable cells [11].
Recent studies have highlighted microencapsulation as one of the most promising and effective strategies for enhancing the survival of probiotic cells. This technique improves the delivery of viable probiotics in food products by providing a physical barrier through the encapsulating matrix, which helps protect the cells from environmental stressors [12]. Microencapsulation is widely recognized as a practical method to preserve probiotic viability, maintain their functional properties, and enhance overall stability [13]. The process involves enclosing active probiotic cells within a protective matrix that shields them from adverse conditions such as acidity, bile salts, oxygen, and temperature fluctuations. Various microencapsulation techniques have been developed for the oral delivery of live probiotics, including extrusion, emulsion, fluidized bed coating, spray drying, and freeze drying [14].
Among these, internal ionic gelation has emerged as a widely used method due to its simplicity and the mild processing conditions it requires—namely, the absence of high temperatures or organic solvents—making it particularly suitable for sensitive probiotic cultures [15]. Regarding wall materials, gellan gum is one of the most employed biopolymers in probiotic microencapsulation. Its ability to form stable gels through interactions with divalent cations, along with its biocompatibility and safety for both microorganisms and consumers, makes it an attractive encapsulating agent. Gellan gum is an anionic extracellular heteropolysaccharide synthesized by the bacterium Sphingomonas elodea. Its structure is composed of repeating tetra saccharide units consisting of 1,3-β-D-glucose, 1,4-β-D-glucuronic acid, 1,4-β-D-glucose, and 1,4-α-L-rhamnose. Gellan gum is commercially available in two main forms: high-acyl (HAG) and low-acyl (LAG). The LAG variant is produced by subjecting native gellan gum to alkaline hydrolysis at elevated temperatures, which reduces the content of acyl groups. LAG is known for forming firm, thermally stable gels that are resistant to low pH and enzymatic degradation [16]. Both HAG and LAG have been independently employed in the microencapsulation of probiotic bacteria [16,17]. LAG has been used as a wall material in combination with other compounds such as chitosan and alginate to protect the viability of Lactobacillus casei under simulated gastric fluids [18]. Similarly, Rosas-Flores et al. [19] used a combination of LAG and alginate to microencapsulate Lactobacillus helveticus and Lactobacillus delbrueckii through internal ionic gelation, achieving high microencapsulation efficiencies exceeding 92.83%. LAG has also been employed in combination with chitosan to microencapsulate proteins. Xie et al. [20] microencapsulated Lactobacillus plantarum in mixtures of gelatin and LAG, evaluating their stability under various adverse conditions. Their results demonstrated that this type of microcapsule effectively protects probiotics under diverse stresses, showing excellent potential for functional food applications. However, there are few studies on the use of LAG and HAG mixtures as wall materials for the microencapsulation of probiotic bacteria.
Once the microencapsulation method is selected, the next critical step involves choosing suitable materials and optimizing their concentrations to maximize key performance indicators such as encapsulation efficiency and probiotic cell viability [16]. For instance, Rojas-Espina [21] employed calcium chloride, inulin, and chitosan as independent variables in optimizing the viability of Lactiplantibacillus plantarum using response surface methodology for targeted probiotic delivery. Accordingly, the primary objective of the present study was to optimize the microencapsulation of Lactobacillus reuteri using gellan gum-based microcapsules. This was achieved through a Box–Behnken Design (BBD) to identify the optimal concentrations of key components that yield the highest encapsulation efficiency and bacterial viability. In a subsequent phase, the study evaluated the survival of microencapsulated L. reuteri under simulated gastric conditions. The results are expected to offer valuable insights for the development of robust probiotic microcapsules suitable for incorporation into functional food products.
2. Materials and Methods
2.1. Preparation of the Probiotic Cells
Lactobacillus reuteri (provided by the Microbiology Laboratory of the University of Cartagena, Colombia) was initially propagated in Man Rogosa Sharpe (MRS) broth at 37 °C under constant agitation (100 rpm) for 24 h. To achieve bacterial activation, a second incubation was performed in nutrient broth under microaerophilic conditions for an additional 24 h. After incubation, the bacterial cells were harvested by centrifugation at 2500 rpm for 10 min at 4 °C and subsequently washed twice with sterile distilled water. The resulting pellet was resuspended in a 0.1% peptone solution to obtain a final concentration of at least 10 log CFU/mL, which was then used for the microencapsulation procedure.
2.2. Microencapsulation of Lactobacillus reuteri
2.2.1. Dispersion Preparation
Polymeric solutions were formulated by individually dissolving low-acyl gellan gum (LAG) and high-acyl gellan gum (HAG) (Modernist Pantry, Eliot, Maine, USA) in deionized water at varying concentrations, as specified in Table 1. Calcium carbonate (8–40 mM) was incorporated as a source of calcium ions to facilitate the crosslinking of gellan gum helices. The resulting dispersions were heated and continuously stirred at 90 °C for 10 min using a hot plate stirrer.

Table 1.
Factors for the Box–Behnken design and their levels.
2.2.2. Emulsion Preparation
The polymer solution containing LAG, HAG, and calcium was allowed to cool to room temperature before incorporating L. reuteri, previously suspended at a concentration of 10.00 log CFU/mL. The microbial concentration was determined using plate counts on MRS agar. Emulsions were prepared by adding 0.20% v/v Span 80 (sorbitan monooleate) (Sigma-Aldrich, St. Louis, Mo, USA) to vegetable oil under continuous stirring at 700 rpm with a hot plate stirrer. Gelation was initiated by adding α-gluconolactone (Sigma-Aldrich, St. Louis, Mo, USA) to the polymer solution until the pH reached 4.50. The polymer mixture was then quickly transferred into the oil phase under constant agitation for 10 min, followed by a settling period. The oil phase was subsequently removed through adsorption, and the microcapsules formed in the aqueous phase were recovered by two centrifugation steps at 5000 rpm for 10 min using a saline solution. The obtained microcapsules were stored at 4 °C until further analysis.
2.3. Microencapsulation Efficiency of Microencapsulated Lactobacillus reuteri
To release the entrapped bacteria, 1 mL of the microcapsule suspension was homogenized in 9 mL of sodium citrate solution (0.1 g/100 g) for 10 min, followed by gentle stirring, serial dilution, and plating on MRS agar. The plates were incubated at 37 °C for 48 h, and the number of released bacteria was expressed as colony-forming units per millilitre (CFU/mL). Encapsulation efficiency (%EE), which reflects both the entrapment capability and the viability of cells following the microencapsulation process, was calculated using the following equation:
where A is the initial bacterial concentration in the suspension and B is the concentration of non-encapsulated bacteria present in the supernatant. Microcapsule morphology and size were examined using a light microscope (DM500, Leica, Wetzlar, Hesse, Germany), and image analysis was performed with Image Pro-Plus software (version 5.1).
2.4. Sizing of Microcapsules by Light Microscopy
The size of the microcapsules was determined using a light microscope (DM500, Leica, Wetzlar, Hesse, Germany) equipped with a digital imaging system. For analysis, a 20 µL aliquot of the microcapsule suspension was diluted in sterile saline solution. The diameters of approximately 50 microcapsules per experimental condition were measured using Image-Pro Plus software (version 5.1).
2.5. Optimization of Microcapsules’ Wall Material Through Box–Behnken Experimental Design
To optimize the microencapsulation process in terms of encapsulation efficiency and bacterial viability, three independent variables were selected: low-acyl gellan gum (LAG, 0.18–1.0% w/v), high-acyl gellan gum (HAG, 0.18–1.0% w/v), and calcium concentration (5.00–40 mM). The selection of variable ranges was based on the research group’s prior experience and expertise in working with these materials. The optimization was carried out using a Box–Behnken design. The experimental matrix included 15 runs, with three replicates at the centre point to estimate experimental error. A second-order polynomial model (Equation (2)) was used to describe the relationship between the response variables and the independent variables (X1, X2, and X3) (see Table 1). In this model, Y denotes the predicted response, β0 is the intercept, and X1, X2, and X3 represent the independent factors. The coefficients β1, β2, and β3 correspond to the linear effects, β11, β22, and β33 to the quadratic effects, and β12, β13, and β23 to the interaction effects between variables. Analysis of variance (ANOVA) was performed to determine the significance of each model term. The model’s predictive accuracy and reliability were evaluated based on the coefficient of determination (R2), adjusted R2, predicted R2, and the lack-of-fit test, thereby confirming the validity of the polynomial model:
2.6. Statistical Analysis
All experiments were conducted in triplicate, and the data are reported as mean values ± standard deviation. Statistical analysis was performed using analysis of variance (ANOVA) to evaluate the significance of the model terms. Differences were considered statistically significant at a confidence level of p < 0.05.
2.7. Simulated Gastrointestinal Digestion In Vitro
Simulated gastric fluid (SGF) was prepared by dissolving 1 g of pepsin in 50 mL of distilled water, adjusting the final volume to 100 mL, and setting the pH to 2.0 throughout the analysis using 1 mol/L HCl [19]. Simulated intestinal fluid (SIF) was prepared by mixing Solution A and Solution B in a 2:1 (v/v) ratio and adjusting the pH to 8.0. Solution A consisted of 0.1 g pancreatin, (Sigma-Aldrich, St. Louis, Mo, USA) 1.1 g sodium bicarbonate (NaHCO3), and 0.2 g sodium chloride (NaCl) dissolved in 50 mL of distilled water, with the final volume adjusted to 100 mL and pH set to 8.0. Solution B was prepared by dissolving 0.9 g of bile salts (Sigma-Aldrich, St. Louis, Mo, USA) in 50 mL of distilled water and adjusting the volume to 100 mL [22]. Probiotic microcapsules containing L. reuteri (9.6 log CFU/mL) were exposed to 1 mL of SGF and incubated at 37 °C in a shaking incubator at 100 rpm. Viable cell counts were performed every 30 min for a total of 2 h. Following this, an equal volume of SIF was added to the mixture, and incubation continued under the same conditions for an additional 2 h. Non-encapsulated L. reuteri was used as the control. Survivability was assessed by plating samples on MRS agar and incubating at 37 °C for 48 h. It is important to mention that the SGF and SIF solutions were prepared on the same day of the analysis, and their pH was monitored throughout the entire experiment in order to prevent any changes in their values.
3. Results and Discussion
3.1. Microcapsules’ Size
During the microencapsulation process of living cells, several critical factors must be considered to ensure the success of the microcapsules in their final application. Among these factors, the most relevant are the microcapsule size and the microencapsulation efficiency. The former was assessed through microscopic analysis, while the latter was determined by counting viable cells before and after the microencapsulation process. Microcapsule size plays a fundamental role in food applications. Larger capsules may lead to poor distribution within food matrices and can negatively affect sensory attributes. Conversely, excessively small capsules may provide insufficient protection of the active compound against deleterious environmental conditions [23,24]. For instance, Kailasapathy et al. [25] reported that microcapsules approximately 300 µm in diameter containing probiotic bacteria significantly increased yogurt smoothness compared to yogurt containing free cells. Similarly, Truelstrup-Hansen et al. [26] suggested that microcapsules smaller than 100 µm may help prevent undesirable sensory impacts in food products.
Figure 1 presents the diameters obtained by varying the concentrations of LAG, HAG and calcium (Ca). The results show that microcapsule diameters ranged from 20 to 120 µm, indicating that the size was not significantly affected by the proportions of LAG, HAG, and Ca used. Notably, the microcapsule sizes achieved in this study are considered optimal for food applications, including both solid and liquid matrices—particularly in dairy products [27]. Previous studies have indicated that microcapsule diameter in ionic gelation processes is primarily influenced by the stirring speed (rpm) used during emulsification and the concentration of surfactant employed [17]. Based on this, it is reasonable to obtain similar diameters when varying the concentrations of gellan gum and calcium, provided that stirring speed and surfactant concentration are kept constant across all treatments.
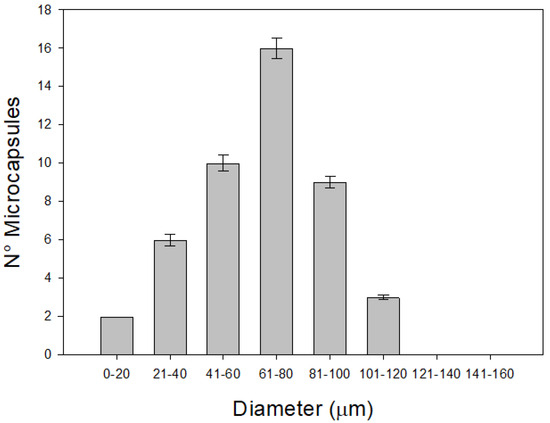
Figure 1.
Average diameter distribution of the microcapsules obtained with LAG, HAG, and calcium.
Similar findings were reported by Gonzalez et al. [16], who developed microcapsules using varying concentrations of gellan gum and bacterial cellulose to enhance the viability of Lactobacillus plantarum exposed to simulated gastric juice. The resulting diameters ranged from 15 to 120 µm, regardless of the proportions of gellan gum and bacterial cellulose. Taken together, these findings support the use of LAG, HAG, and Ca at the concentrations reported herein as suitable for producing microcapsules with sizes appropriate for food applications.
3.2. Optimization of the Lactobacillus reuteri Microencapsulation
In the Box–Behnken design applied to model the internal ionic gelation process for the microencapsulation of Lactobacillus reuteri, three critical variables were selected due to their influence on microencapsulation efficiency and bacterial viability: low-acyl gellan gum concentration (LAG, X1), high-acyl gellan gum concentration (HAG, X2), and calcium concentration (Ca, X3). A total of 15 experimental runs were conducted, each in triplicate, to develop the predictive models. Table 2 presents the full experimental design along with the corresponding values of the response variables obtained for each treatment. It is important to note that the initial cell counts of L. reuteri were adjusted to meet the minimum recommended levels for incorporation into food matrices. Several studies [28,29] have suggested that a probiotic intake of approximately 8 to 9 log CFU/g is required to confer beneficial health effects to consumers.

Table 2.
Comparison between the experimental response and RSM predicted response.
The experimental encapsulation efficiency (%EE) values ranged from 74.4% to 95.5%. These results are higher than those reported by Gonzalez et al. [16], who obtained values ranging from 53% to 86% for Lactobacillus plantarum microencapsulated in gellan gum and bacterial cellulose mixtures. Similarly, Graff et al. [30] reported microencapsulation efficiencies of approximately 53% for Saccharomyces boulardii encapsulated in chitosan–alginate using vibrating technology. Vodnar and Socaciu [31] observed even lower efficiencies, around 37.5%, when co-encapsulating Bifidobacterium sp. with green tea extract in an alginate–chitosan matrix using the extrusion method. In the case of Lactobacillus reuteri, Corbo et al. [32] reported an encapsulation efficiency of approximately 54.8% using alginate as the wall material. These findings underscore the significant impact of the encapsulation technique on the efficiency of probiotic microorganism entrapment, with internal ionic gelation emerging as a particularly probiotic-friendly method.
Regarding the viability values obtained after the microencapsulation process, the highest percentage was achieved with a high concentration of LAG (1.0% w/v), a low concentration of HAG (0.18% w/v), and an intermediate calcium concentration (22.5 mM). Conversely, the lowest viability percentage (81.50 ± 2.77) was obtained using a low concentration of LAG (0.18% w/v), an intermediate concentration of HAG (0.59% w/v), and a low calcium concentration (5.0 mM). This behaviour may be explained by the different gelation mechanisms of high- and low-acyl gellan gums. While LAG requires calcium ions to form ionic crosslinks and establish the three-dimensional structure of the microcapsule, HAG gels through the formation of hydrogen bonds, resulting in more elastic gels. Therefore, LAG is more efficient in preserving bacterial viability.
Moreover, these results indicate a higher viability compared to those reported by Rojas-Espina et al. [21], who microencapsulated Lactiplantibacillus plantarum in a chitosan–inulin mixture using the freeze-drying method, obtaining viability values between 49% and 70%, with the highest survival rate observed at 2% CaCl2, 1% chitosan, and 1.25% inulin. Similarly, Schell and Beermann [4] reported a viability of approximately 42.85 ± 4.62 for L. reuteri microencapsulated with sweet whey and shellac using the fluidized bed technique. These results confirm that internal ionic gelation is a more suitable microencapsulation technique for preserving the viability of probiotic bacteria.
The data summarized in Table 2 were employed to construct two second-order polynomial models, as defined by Equation (1). The ANOVA outcomes for the response variables—microencapsulation efficiency (%EE) and bacterial viability—are presented in Table 3. A p-value below 0.05 was considered statistically significant [33], indicating that at least one term in the regression model has a meaningful association with the corresponding response variable (%EE or viability). Nevertheless, it is important to consider the intrinsic variability of microbiological systems, which may contribute to deviations in the experimental responses.

Table 3.
ANOVA and regression coefficient δ of the predicted second-order polynomials model for viability and EE.
The final model equation describing the correlation between microencapsulation efficiency (%EE) of Lactobacillus reuteri and the independent variables X1, X2, and X3 was established (Equation (3)). The polynomial equation developed to describe the efficiency response was evaluated using key statistical indices: R2, adjusted R2, and predicted R2. R2 quantifies the proportion of variability in the response variable explained by the model, while the adjusted R2 accounts for the number of model terms, making it useful for evaluating model complexity. Predicted R2 assesses the model’s accuracy in predicting new observations [34]. According to the ANOVA results, only the interaction term X2·X3 exhibited a p-value greater than 0.05, indicating a lack of statistical significance between HAG and calcium. This supports the understanding that HAG does not require calcium ions to undergo gelation. The R2 value for %EE was 99.55%, reflecting a strong agreement between the predicted and experimental data [35] and suggesting that the model accounts for most of the variability in the response. Furthermore, the predicted R2 (95.93%) closely matched the adjusted R2 (98.73%), with a difference of less than 0.2, further confirming the model’s robustness and predictive capability. In addition, the lack-of-fit test resulted in a p-value of 0.656 (>0.05), indicating that the lack of fit was not statistically significant. This outcome confirms the suitability of the proposed regression model for predicting the %EE of Lactobacillus reuteri microencapsulated within a multicomponent matrix:
In practical terms, the previously described mathematical model can be interpreted by examining the magnitude of its coefficients. Positive coefficients for X1 and X3 suggest that increases in these variables have a favourable effect on the response within the defined experimental range. Among the studied factors, X1 (concentration of LAG) and X3 (calcium concentration) had the most significant impact, with coefficients of 3.88 and 1.07, respectively. Notably, these two variables exhibited a synergistic interaction, as indicated by the positive interaction term (0.605 for X1·X3) in the model. Positive coefficients in the polynomial model represent synergistic effects between factors that enhance the response, whereas negative coefficients indicate antagonistic effects that suppress it [36].
Regarding the ANOVA results for viability values, the factors X1, X2, and X3, along with their interactions (X1·X2, X1·X3, and X2·X3) and quadratic terms (, , and ), exhibited statistically significant effects, with p-values lower than 0.05. Consequently, all these terms were included in the development of the polynomial model used to describe the viability of Lactobacillus reuteri subjected to a microencapsulation process. Statistical indicators such as the coefficient of determination (R2), adjusted R2, and predicted R2 were again employed to assess the adequacy of the model. As shown in Table 3, the R2 value was 99.80%, indicating that only 0.2% of the total variability remained unexplained. The high predicted R2 (97.11%) and adjusted R2 (99.44%) values further supported the model’s robustness in capturing the relationship between the process variables and viability [37]. Moreover, the lack-of-fit test yielded a p-value of 0.537, confirming the model’s suitability for predicting the viability of L. reuteri microencapsulated via internal ionic gelation:
When comparing the magnitudes of the coefficients in the developed model (Equation (4)), positive values were observed for X1 (1.562) and X3 (2.250), with calcium (X3) exhibiting the strongest effect. This indicates a significant influence of calcium on probiotic viability, which can be attributed to its role in the gelation mechanism of alginate, as previously reported by González et al. [38]. These results suggest that both models reliably describe the design space, effectively capturing the relationship between the independent variables (X1, X2, and X3) and the response variables (encapsulation efficiency and probiotic viability) [39]. The close agreement between the experimental and predicted values further supports the accuracy and predictive capability of the polynomial models within the studied system. Finally, both equations (3 and 4) were used to generate response surface plots, which illustrate the interactive effects of the significant variables on probiotic viability.
3.3. Response Surface Plots
Three-dimensional response surface plots were constructed based on the polynomial models described in Equations (3) and (4), enabling visualization of the effects of variables X1, X2, and X3 on both encapsulation efficiency (%EE) and the viability of Lactobacillus reuteri microencapsulated via internal ionic gelation. These graphical representations offer a detailed statistical and visual overview, supporting the identification of optimal process conditions [39]. Such insights are crucial for improving encapsulation performance and maximizing probiotic survival, thereby enhancing the overall protective effect of the encapsulation system.
Figure 2a presents the relationship between the statistically significant factors LAG and HAG in influencing the %EE of L. reuteri microcapsules. The response surface indicates that increasing HAG concentration up to 0.4% leads to an encapsulation efficiency above 95%. In contrast, achieving similar efficiency levels with LAG requires concentrations close to 0.8%. This difference may be attributed to the high viscosity exhibited by HAG at elevated concentrations, which can hinder the proper formation of microcapsules.
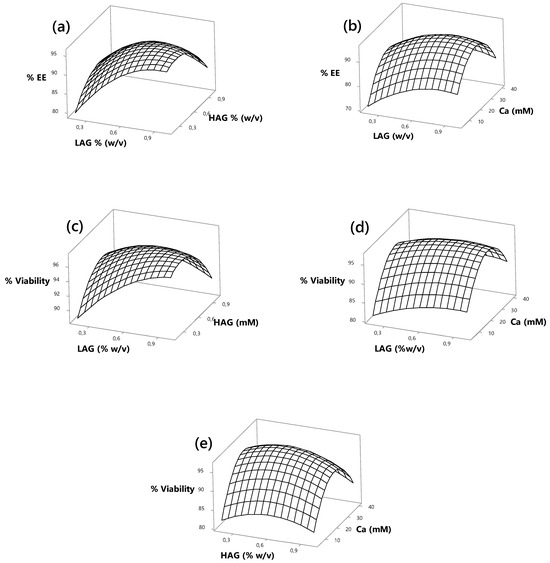
Figure 2.
Three-dimensional response surfaces for the effects of the LAG, HAG, and Ca concentrations on the encapsulation efficiency of L. reuteri.
As shown in Figure 2b, the interaction between LAG and calcium exhibits a parabolic trend, where both low and high calcium concentrations result in reduced encapsulation efficiency. In contrast, intermediate calcium levels yield optimal %EE, exceeding 95%. Previous studies have reported that insufficient calcium concentrations may not provide enough crosslinking to form a compact three-dimensional gel network through electrostatic interactions [40], leading to poor encapsulation efficiency due to incomplete coverage of probiotic cells. Similarly, Anani et al. [41] observed that higher calcium concentrations resulted in smaller, more rigid microcapsules. However, excessive calcium ion levels may disrupt electrostatic interactions between gellan gum helices and the ionic balance of probiotic cells, thereby reducing %EE [18]. In addition, oversaturation of calcium-binding sites on gellan gum helices may induce osmotic stress, further compromising encapsulation performance [42].
The effects of factors X1, X2, and X3 are illustrated in Figure 2c,d, where a parabolic response pattern—like that observed for %EE—was identified. The highest viability percentages of L. reuteri were achieved at intermediate calcium concentrations, both in interaction with LAG and HAG. This highlights a protective effect of calcium on probiotic viability up to an optimal level. Beyond this point, a decline in viability was observed. Yuan et al. [43] reported that increasing calcium concentrations led to decreased viability of L. rhamnosus cells. This phenomenon can be attributed to the interaction between the anionic groups of the polysaccharide and calcium ions during gel formation. As noted by Łetocha et al. [44], an excess of divalent cations may increase the rigidity of the microcapsule wall, potentially hinder the controlled release of the encapsulated content, and negatively affect cell viability.
The critical concentrations of X1, X2, and X3 required to optimize the viability of L. reuteri microencapsulated via internal ionic gelation were determined using the “Response Optimiser” function in Minitab® statistical software (version 17.00) (see Supplementary Materials). According to the response surface model, the optimal concentrations were 0.8850% w/v for X1, 0.4368% w/v for X2, and 24.444 mM for X3, yielding a predicted viability of 97.43%. Microcapsules were subsequently produced under these optimal conditions, and their performance was evaluated under simulated gastric conditions to assess the resistance of the encapsulated L. reuteri. These results provide insight into the potential application of this microencapsulation system in the development of functional food matrices enriched with probiotics.
3.4. Viability of Lactobacillus reuteri in Simulated Gastric Juice
L. reuteri microencapsulated under optimal conditions was subjected to simulated gastric conditions for a period of 2 h. A known concentration of encapsulated L. reuteri was used for this experiment. As shown in Figure 3, the viability of microencapsulated cells decreased from 9.50 to 8.46 log CFU/mL. In contrast, free cells exhibited a significantly greater reduction, declining from 9.50 to 2.90 log CFU/mL over the same incubation period, likely due to the adverse effects of the acidic environment. These findings demonstrate that microencapsulation using binary blends of gellan gum improves the survival of L. reuteri, possibly due to the delayed dissolution of the capsule wall structure, which reduces direct exposure of probiotic cells to the acidic medium, thereby enhancing the protective effect of the wall material.
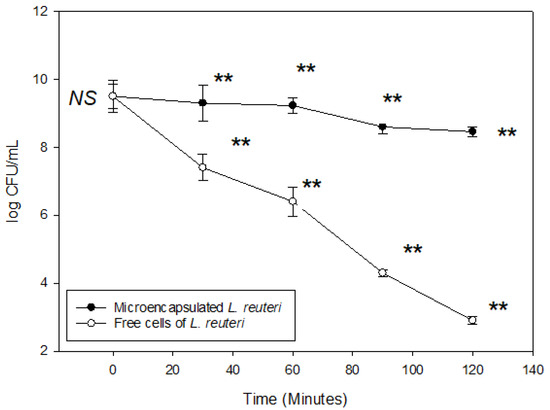
Figure 3.
Viability of Lactobacillus reuteri microencapsulated under optimized conditions and subjected to simulated gastric juices for 2 h. (** significant difference at the same incubation time in p < 0.05 according to Tukey test; NS: there was no significant difference in p < 0.05). Vertical bars indicate standard error of the means.
Similar results were reported by Trabelsi et al. [45], who found that microcapsules made from alginate and chitosan enhanced the viability of L. plantarum after exposure to simulated gastric juice at pH 2, whereas free cells completely lost viability under the same conditions. Likewise, Kim et al. [46] showed that non-encapsulated L. acidophilus ATCC 43121 was fully inactivated after exposure to simulated gastric juice at pH 1.2 and 1.5, while encapsulated samples showed only a 3-log reduction in viable cell count. This protective effect has also been reported by Gómez-Mascaraque et al. [7], who encapsulated L. plantarum in a whey protein concentrate matrix using electrospinning, demonstrating that this approach provided effective protection under simulated gastric conditions. However, it is important to note that the acid tolerance of Lactobacillus species can vary significantly due to physiological differences between strains [25,26].
Although previous studies have indicated that L. reuteri is particularly sensitive to simulated gastric environments [47,48], the results of the present study agree with findings by Iyer and Kailasapathy [49], who reported that microencapsulated L. reuteri retained its viability in simulated gastric media. Similarly, Wang et al. [50] observed no significant differences in the viability of L. plantarum encapsulated with trehalose and a whey protein concentrate/pullulan matrix after 4 h of incubation in simulated gastric juice. In another study, Li et al. [40] evaluated the impact of a carboxymethyl dextran–whey protein conjugate on the viability of L. plantarum exposed to simulated gastric conditions. They observed a reduction in viable counts from 10.07 to 9.53 and 9.24 log CFU/mg after 1 and 2 h of incubation, respectively. When the same microcapsules were subsequently subjected to simulated intestinal fluid for an additional 2 h, a further decrease from 9.24 to 8.89 log CFU/mg was recorded. These findings suggest that the microcapsules provide substantial protection under acidic conditions, allowing enough viable probiotic cells to survive and potentially reach the intestinal tract [51].
3.5. Viability of Lactobacillus reuteri in Simulated Intestinal Juice
After withstanding gastric conditions, probiotic bacteria must also survive in the environment of the small intestine, which is characterized by the presence of bile salts. In this context, the viability of microencapsulated L. reuteri was assessed under simulated intestinal conditions for a period of 2 h. It is important to note that prior to this evaluation, the microorganism had already been exposed to simulated gastric juice to more realistically replicate the gastrointestinal transit experienced in the human body. As shown in Figure 4, the viability trend of microencapsulated L. reuteri was consistent with the results obtained under simulated gastric conditions—namely, significantly higher viable cell counts were observed in the encapsulated samples compared to free cells. After 2 h of exposure to simulated intestinal fluid, the viability of microencapsulated L. reuteri decreased from 8.46 to 8.02 log CFU/mL, while the free cells showed a more pronounced decline, from 3.00 log CFU/mL to until no longer detectable. Supporting these findings, Jantzen et al. [52] reported an overall survival rate of 54% for L. reuteri DSM 20016 encapsulated using spray-dried whey protein, under simulated gastrointestinal conditions. This represented a 32% higher survival rate compared to unencapsulated cells.
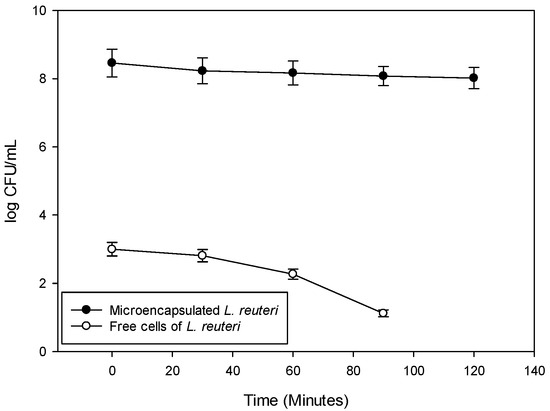
Figure 4.
Viability of Lactobacillus reuteri microencapsulated under optimized conditions and subjected to simulated intestinal juices for 2 h.
These findings are consistent with those reported by Trabelsi et al. [45], who observed that L. plantarum microencapsulated in alginate exhibited a reduction in viability from 5.3 to 4.5 log CFU/mL under simulated intestinal conditions, whereas free cells showed a more pronounced decrease, from 5.3 to 4.2 log CFU/mL. However, the results of the present study suggest that microencapsulation using gellan gum provides superior protection against bile salt-induced stress compared to alginate. This enhanced protective effect may be attributed to the fact that alginate capsules are prone to degradation in alkaline environments, while in acidic conditions, alginate converts into insoluble alginic acid, which acts as a barrier limiting the diffusion of undesirable compounds into the encapsulating matrix [53].
4. Conclusions
This study demonstrated that microencapsulation of Lactobacillus reuteri via internal ionic gelation using binary blends of gellan gum (LAG and HAG) and calcium is an effective strategy to enhance both encapsulation efficiency and probiotic viability. Response surface modelling along with the Box–Behnken design enabled the identification of optimal conditions—0.88% (w/v) LAG, 0.43% (w/v) HAG, and 24.44 mM Ca— that maximized encapsulation efficiency (>95%) and cell viability (97.43%), highlighting the relevance of intermediate calcium concentrations and appropriate gum ratios in forming a dense, protective gel matrix. Simulated gastrointestinal condition tests revealed that the developed microcapsules provided significant protection against acid stress and bile salt exposure compared to free cells, which is attributed to the matrix’s ability to limit the direct exposure of bacterial cells to the external environment. These findings support the use of gellan gum-based encapsulation systems as a promising technological tool for the development of functional foods with controlled release of viable probiotics during gastrointestinal transit.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcs9080419/s1, Figure S1: Residual plot for Microencapsulation of Lactobacillus reuteri; Figure S2: Residual plot for Viability of Lactobacillus reuteri microencapsulated; Figure S3: Viability optimization of microencapsulated Lactobacillus reuteri microencapsulated.
Author Contributions
Conceptualization, R.G.-C. and R.O.-T.; data curation, J.H.-F.; investigation, R.G.-C.; methodology, R.G.-C.; resources, R.G.-C., J.H.-F. and R.O.-T.; software, R.G.-C. and J.H.-F.; supervision, R.G.-C.; validation, R.G.-C., J.H.-F. and R.O.-T.; visualization, R.O.-T.; writing—original draft, R.G.-C.; writing—review and editing, J.H.-F. and R.O.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors thank the Universidad de Cartagena for providing the equipment and reagents to conduct this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations/World Health Organization: London, UK, 2002. [Google Scholar]
- Goderska, K.; Agudo Pena, S. An in vitro gastrointestinal model to evaluate the tolerance of encapsulated Lactobacillus and Lactococcus strains with synbiotic containing lactobionic acid via lyophilization technique to harsh gastric conditions during storage time. Eur. J. Pharm. Biopharm. 2024, 197, 114147. [Google Scholar] [CrossRef]
- Axelsson, L.T. Lactic acid bacteria: Classification and physiology. In Lactic Acid Bacteria; Salminen, S., von Wright, A., Eds.; Marcel Dekker Inc.: New York, NY, USA; Basel, Switzerland, 1993; pp. 1–64. [Google Scholar]
- Schell, D.; Beermann, C. Fluidized Bed Microencapsulation of Lactobacillus reuteri with Sweet Whey and Shellac for Improved Acid Resistance and In Vitro Gastrointestinal Survival. Food Res. Int. 2014, 62, 308–314. [Google Scholar] [CrossRef]
- Ortiz-Rivera, Y.; Sánchez-Vega, R.; Gutiérrez-Méndez, N.; León-Félix, J.; Acosta-Muñiz, C.; Sepulveda, D. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef]
- Zhou, X.X.; Pan, Y.J.; Wang, Y.B.; Li, W.F. In vitro assessment of gastrointestinal viability of two photosynthetic bacteria, Rhodopseudomonas palustris and Rhodobacter sphaeroides. J. Zhejiang Univ.-Sci. B 2007, 8, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mascaraque, L.G.; Cruz Morfin, R.; Pérez-Masiá, R.; Sanchez, G.; Lopez-Rubio, A. Optimization of Electrospraying Conditions for the Microencapsulation of Probiotics and Evaluation of Their Resistance During Storage and In-Vitro Digestion. LWT—Food Sci. Technol. 2016, 69, 438–446. [Google Scholar] [CrossRef]
- Lotfipour, F.; Mirzaeei, S.; Maghsoodi, M. Preparation and characterization of alginate and psyllium beads containing Lactobacillus acidophilus. Sci. World J. 2012, 2012, 680108. [Google Scholar] [CrossRef] [PubMed]
- Del Piano, M.; Carmagnola, S.; Andorno, S.; Pagliarulo, M.; Tari, R.; Mogna, L.; Strozzi, G.P.; Sforza, F.; Capurso, L. Evaluation of the intestinal colonization by microencapsulated probiotic bacteria in comparison with the same uncoated strains. J. Clin. Gastroenterol. 2010, 44, S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Piątek, J.; Gibas-Dorna, M.; Olejnik, A.; Krauss, A.; Wierzbicki, K.; Żukiewicz-Sobczak, W.; Głowacki, M. The viability and intestinal epithelial cell adhesion of probiotic strain combination: In vitro study. Ann. Agric. Environ. Med. 2012, 19, 99–102. [Google Scholar] [PubMed]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- González Cuello, R.E.; Perez Mendoza, J.; Moron Alcazar, L.B. Efecto de la Microencapsulación sobre la Viabilidad de Lactobacillus delbrueckii sometido a Jugos Gástricos Simulados. Inf. Tecnol. 2015, 26, 11–16. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Nagarajan, M.; Kumar, P.K.; Singh, S.S.; Manvi, D.; Gowda, N.N. A comprehensive review on microencapsulation of probiotics: Technology, carriers and current trends. Appl. Food Res. 2023, 3, 100248. [Google Scholar] [CrossRef]
- Canga, E.M.; Dudak, F.C. Improved digestive stability of probiotics encapsulated within poly (vinyl alcohol)/cellulose acetate hybrid fibers. Carbohydr. Polym. 2021, 264, 117990. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalinejhad, S.; Almonaitytė, A.; Jensen, I.-J.; Kurek, M.; Lerfall, J. Alginate microbeads incorporated with anthocyanins from purple corn (Zea mays L.) using electrostatic extrusion: Microencapsulation optimization, characterization, and stability studies. Int. J. Biol. Macromol. 2023, 246, 125684. [Google Scholar] [CrossRef] [PubMed]
- González-Cuello, R.; Hernández-Fernández, J.; Ortega-Toro, R. Response surface methodology-based optimization for enhancing the viability of microencapsulated Lactobacillus plantarum in composite materials. J. Compos. Sci. 2025, 9, 189. [Google Scholar] [CrossRef]
- González, R.E.; Salazar, J.A.; Pérez, J.A. Obtaining Size-Controlled Microcapsules by Ionic Gelation with High and Low Acyl Gellans Containing Lactococcus lactis. Rev. Colomb. Biotecnol. 2013, 15, 70. [Google Scholar] [CrossRef]
- Thinkohkaew, K.; Jonjaroen, V.; Niamsiri, N.; Panya, A.; Suppavorasatit, I.; Potiyaraj, P. Microencapsulation of Probiotics in Chitosan-Coated Alginate/Gellan Gum: Optimization for Viability and Stability Enhancement. Food Hydrocoll. 2024, 151, 109788. [Google Scholar] [CrossRef]
- Rosas-Flores, W.; Ramos-Ramírez, E.G.; Salazar-Montoya, J.A. Microencapsulation of Lactobacillus helveticus and Lactobacillus delbrueckii Using Alginate and Gellan Gum. Carbohydr. Polym. 2013, 98, 1011–1017. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, K.; Ma, X.; Zhang, Y.; Wu, X.; Ma, L.; Zou, L.; Liu, W. Lactobacillus plantarum P9 Encapsulated in Gelatin/Gellan Gum Microcapsules with Lipid Filling and Ca2⁺ Crosslinking: Enhancing Digestive Vitality and Storage Stability. Food Biosci. 2025, 106671, in press. [Google Scholar] [CrossRef]
- Rojas-Espina, D.; Urriola-Urriola, N.; Cañas-Sarazúa, R.; Briones-Labarca, V. Optimizing the viability of microencapsulated Lactiplantibacillus plantarum using response surface methodology for dietary probiotic delivery. Future Foods 2024, 9, 100329. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Y.; Li, S.; Yun, L.; Wu, T.; Zhang, M. Improving the Physical Stability of Lactobacillus plantarum LP90 During Storage by Mixing Carboxymethylated Dextran-Whey Protein Conjugates and Small-Molecule Sugars. Food Res. Int. 2025, 203, 115834. [Google Scholar] [CrossRef]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Coorey, R. A Review on Probiotic Microencapsulation and Recent Advances of Their Application in Bakery Products. Food Bioproc. Technol. 2022, 15, 1677–1699. [Google Scholar] [CrossRef]
- Zarali, M.; Sadeghi, A.; Jafari, S.M.; Ebrahimi, M.; Mahoonak, A.S. Enhanced Viability and Improved In Situ Antibacterial Activity of the Probiotic LAB Microencapsulated Layer-by-Layer in Alginate Beads Coated with Nisin. Food Biosci. 2023, 53, 102593. [Google Scholar] [CrossRef]
- Kailasapathy, K. Survival of Free and Encapsulated Probiotic Bacteria and Effect on the Sensory Properties of Yoghurt. LWT—Food Sci. Technol. 2006, 39, 1221–1227. [Google Scholar] [CrossRef]
- Truelstrup Hansen, L.; Jin, Y.L.; Allan-Wojtas, P.M.; Paulson, A.T. Survival of Ca-Alginate Microencapsulated Bifidobacterium spp. in Milk and Simulated Gastrointestinal Conditions. Food Microbiol. 2002, 19, 35–45. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of Probiotic Living Cells: From Laboratory Scale to Industrial Applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Aureli, P.; Capurso, L.; Castellazzi, A.; Clerici, M.; Giovannini, M.; Morelli, L.; Poli, A.; Pregliasco, F.; Salvini, F.; Zuccotti, G. Probiotics and Health: An Evidence-Based Review. Pharmacol. Res. 2011, 63, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Kenifel, W.; Ouwehand, A. Probiotics, Applications in Dairy Products. In Encyclopedia of Dairy Sciences; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: San Diego, CA, USA, 2011. [Google Scholar]
- Graff, S.; Hussain, S.; Chaumeil, J.C.; Charrueau, C. Increased Intestinal Delivery of Viable Saccharomyces boulardii by Encapsulation in Microspheres. Pharm. Res. 2007, 25, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C.; Socaciu, C. Selenium Enriched Green Tea Increases Stability of Lactobacillus casei and Lactobacillus plantarum in Chitosan-Coated Alginate Microcapsules during Exposure to Simulated Gastrointestinal and Refrigerated Conditions. LWT—Food Sci. Technol. 2014, 57, 406–411. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Sinigaglia, M. Shelf Life of Alginate Beads Containing Lactobacilli and Bifidobacteria: Characterisation of Microspheres Containing Lactobacillus delbruekii subsp. bulgaricus. Int. J. Food Sci. Technol. 2011, 46, 2212–2217. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, B.; Mishra, I.M. Process Parametric Study for Ethene Carboxylic Acid Removal onto Powder Activated Carbon Using Box–Behnken Design. Chem. Eng. Technol. 2007, 30, 932–937. [Google Scholar] [CrossRef]
- Montgomery, D.C. Introduction to Statistical Quality Control, 6th ed.; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Körbahti, B.K. Response Surface Optimization of Electrochemical Treatment of Textile Dye Wastewater. J. Hazard. Mater. 2007, 145, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Rifa’i Muhaimin, M.F.; Atho’illah, S.N.; Arifah, A.R.; Suharto, A.R.; Fadhilla, A.N.; Sa’adah, N.A.M.; Ardiansyah, E.; Izati, R.; Al Faizah, B.N.; Fadlilah, D.N.; et al. Physicochemical and Functional Optimization of Probiotic Yogurt with Encapsulated Lacticaseibacillus paracasei E1 Enriched with Green Tea Using Box–Behnken Design. Appl. Food Res. 2025, 5, 100690. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Surya, S.M.; Naganyashree, S.; Shivamathi, C.S. Response Surface Optimization of Ultrasound Assisted Extraction of Pectin from Pomegranate Peel. Int. J. Biol. Macromol. 2015, 72, 1323–1328. [Google Scholar] [CrossRef]
- González, R.; Ramos, G.; Cruz, A.; Salazar, A. Rheological Characterization and Activation Energy Values of Binary Mixtures of Gellan. Eur. Food Res. Technol. 2012, 234, 305–313. [Google Scholar] [CrossRef]
- Henseler, J.; Sarstedt, M. Goodness-of-Fit Indices for Partial Least Squares Path Modeling. Comput. Stat. 2013, 28, 565–580. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; He, J.; Huang, Y. A New Insight to the Effect of Calcium Concentration on Gelation Process and Physical Properties of Alginate Films. J. Mater. Sci. 2016, 51, 5791–5801. [Google Scholar] [CrossRef]
- Anani, J.; Noby, H.; Zkria, A.; Yoshitake, T.; ElKady, M. Monothetic Analysis and Response Surface Methodology Optimization of Calcium Alginate Microcapsules Characteristics. Polymers 2022, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.Y.; How, Y.H.; Pui, L.P. Microencapsulation of Bifidobacterium lactis Bi-07 with Galactooligosaccharides Using Co-Extrusion Technique. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e2416. [Google Scholar] [CrossRef]
- Yuan, Y.; Yin, M.; Chen, L.; Liu, F.; Chen, M.; Zhong, F. Effect of Calcium Ions on the Freeze-Drying Survival of Probiotic Encapsulated in Sodium Alginate. Food Hydrocoll. 2022, 130, 107668. [Google Scholar] [CrossRef]
- Łetocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, I.; Bejar, W.; Ayadi, D.; Chouayekh, H.; Kammoun, R.; Bejar, S.; Ben Salah, R. Encapsulation in Alginate and Alginate Coated-Chitosan Improved the Survival of Newly Probiotic in Oxgall and Gastric Juice. Int. J. Biol. Macromol. 2013, 61, 36–42. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cho, S.Y.; Kim, S.H.; Song, O.J.; Shin, I.S.; Cha, D.S.; Park, H.J. Effect of Microencapsulation on Viability and Other Characteristics in Lactobacillus acidophilus ATCC 43121. LWT—Food Sci. Technol. 2008, 41, 493–500. [Google Scholar] [CrossRef]
- De Prisco, A.; Maresca, D.; Ongeng, D.; Mauriello, G. Microencapsulation by Vibrating Technology of the Probiotic Strain Lactobacillus reuteri DSM 17938 to Enhance Its Survival in Foods and in Gastrointestinal Environment. LWT—Food Sci. Technol. 2015, 61, 452–462. [Google Scholar] [CrossRef]
- Malmo, C.; La Storia, A.; Mauriello, G. Microencapsulation of Lactobacillus reuteri DSM 17938 Cells Coated in Alginate Beads with Chitosan by Spray Drying to Use as a Probiotic Cell in a Chocolate Souffle. Food Bioprocess Technol. 2013, 6, 795–805. [Google Scholar] [CrossRef]
- Iyer, C.; Kailasapathy, K. Effect of Co-Encapsulation of Probiotics with Prebiotics on Increasing the Viability of Encapsulated Bacteria in Simulated Gastrointestinal Conditions and in Yoghurt. J. Food Sci. 2005, 70, 18–23. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Wu, J.; Wang, Y.; Ai, Z.; Wang, X.; Nan, B.; Cao, Y.; Li, X.; Liu, J.; et al. Improved Viability of Trehalose on Lactobacillus plantarum Embedded with Whey Protein Concentrate/Pullulan in Simulated Gastrointestinal Conditions and Its Application in Acid Juice. Food Sci. Hum. Wellness 2024, 13, 3614–36231. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending Viability of Bifidobacterium longum in Chitosan-Coated Alginate Microcapsules Using Emulsification and Internal Gelation Encapsulation Technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef]
- Jantzen, M.; Göpel, A.; Beermann, C. Direct Spray Drying and Microencapsulation of Probiotic Lactobacillus reuteri from Slurry Fermentation with Whey. J. Appl. Microbiol. 2013, 115, 1029–1036. [Google Scholar] [CrossRef]
- Motalebi Moghanjougi, Z.; Rezazadeh Bari, M.; Khaledabad, M.A.; Almasi, H.; Amiri, S. Bio-Preservation of White Brined Cheese (Feta) by Using Probiotic Bacteria Immobilized in Bacterial Cellulose: Optimization by Response Surface Method and Characterization. LWT—Food Sci. Technol. 2020, 117, 108603. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).