Cellulose Acetate–PHB Biocomposite from Saccharum officinarum for Ni (II) Adsorption: Equilibrium and Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
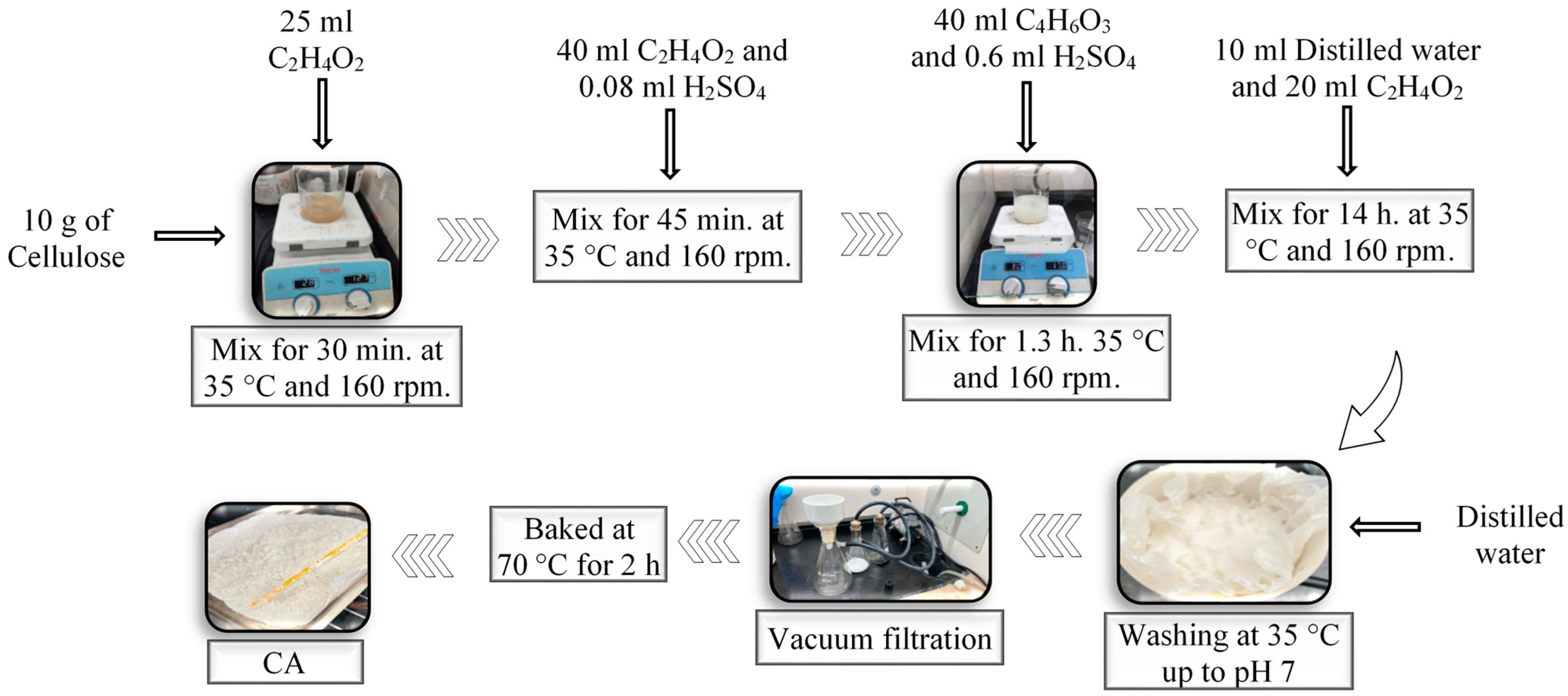
2.2. Obtaining Cellulose Acetate from Sugarcane Bagasse Cellulose
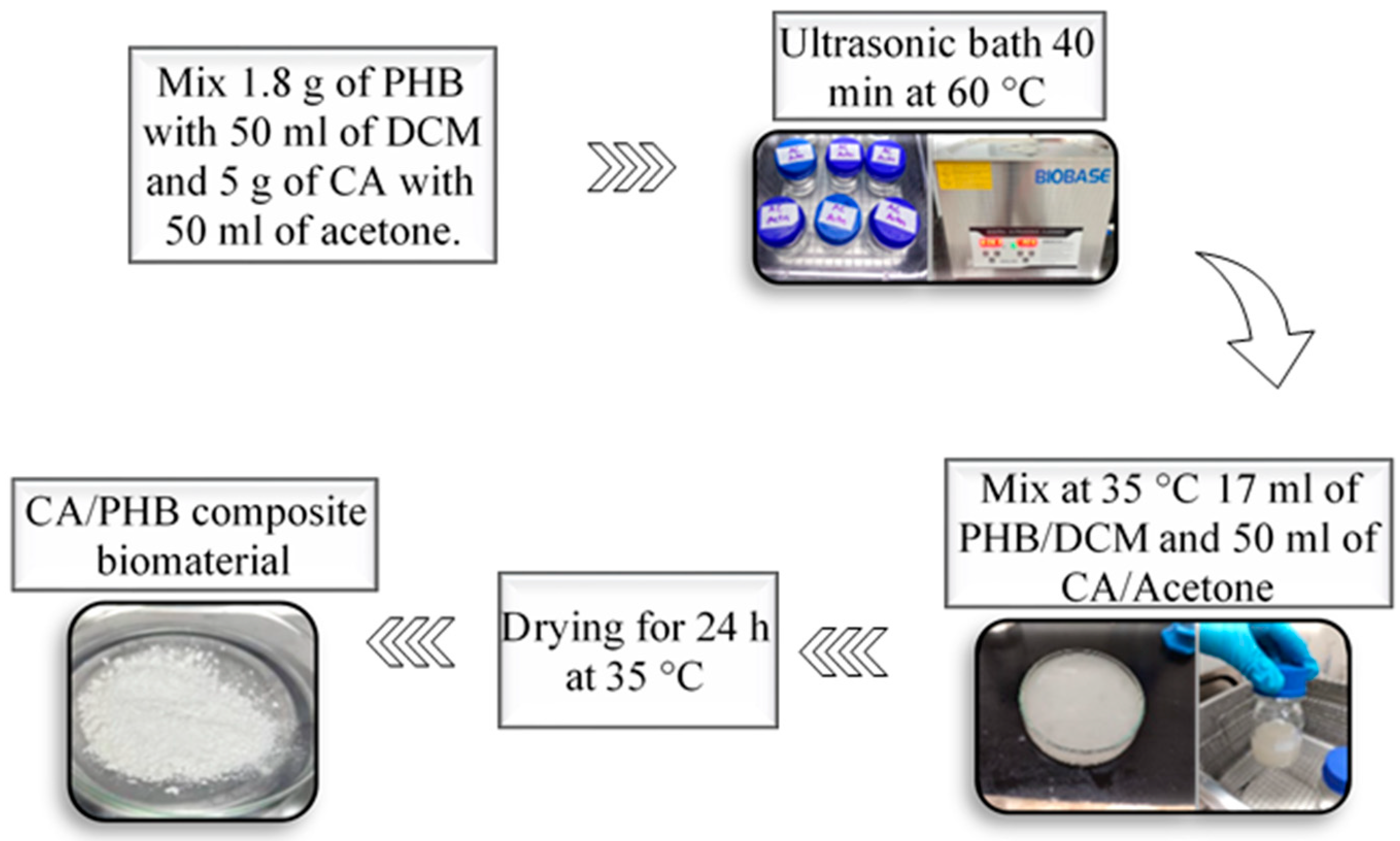
2.3. Synthesis of the Composite Biomaterial (CA/PHB)
2.4. Determination of the pH at the Point of Zero Charge (pHpzc)
2.5. Characterisation of the Biocomposite CA/PHB
2.6. Evaluation of the Adsorption Capacity of the CA/PHB Biocomposite
2.7. Identification of Adsorption Mechanisms
2.8. Equilibrium in the Removal of Ni (II)
3. Results and Discussion
3.1. Synthesis of the Biocomposite from Cellulose Acetate and Polyhydroxybutyrate (CA/PHB)
3.2. Determination of the pH at the Zero Load Point (pHcc) for CA/PHB
3.3. Evaluation of Adsorption Capacity
3.4. FTIR Analysis of the Biomaterial Before and After the Ni (II) Adsorption Process
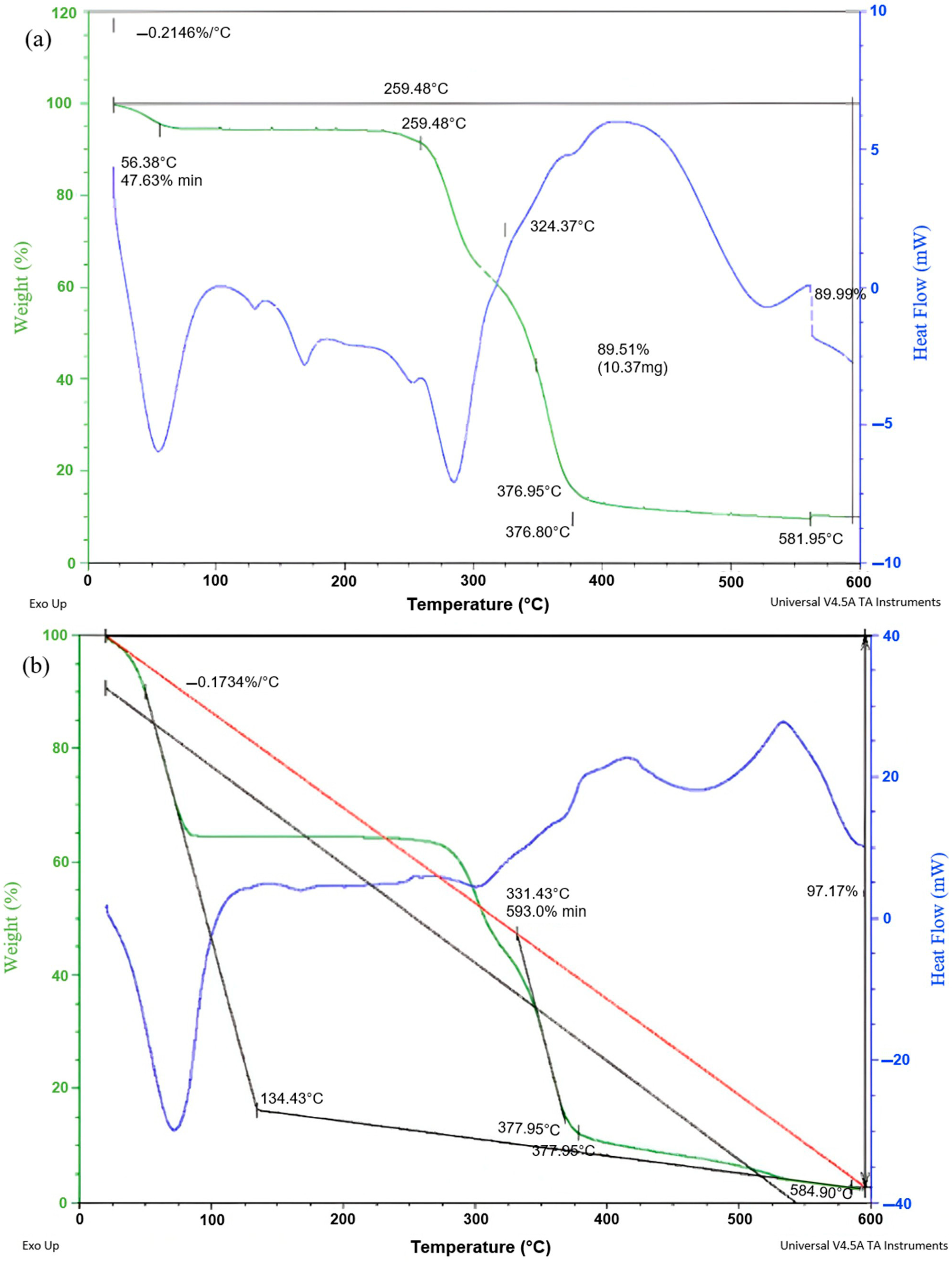
3.5. TGA Analysis of CA/PHB Before and After Ni (II) Adsorption Process
3.6. SEM Analysis of the Biocomposite Before and After the Ni (II) Adsorption Process
3.7. Identification of the Adsorption Mechanisms Using the CA/PHB Biocomposite
4. Conclusions
- The incorporation of the results showed that the synthesis efficiency of cellulose acetate (CA) from commercial cellulose using the acetylation method is high, with a yield percentage of 96.32%. This demonstrates the enormous potential of physicochemical modifications of cellulose in terms of cost and performance.
- It was found that the adsorption capacity qe of the CA/PHB composite biomaterial was excellent, demonstrating a result of 5.042 mg/g at a concentration of 35 mg/L of Ni (II), resulting in a removal rate of 86.44%. This indicates that the composite biomaterial presents suitable and promising properties for its application in heavy metal adsorption processes.
- The pseudo-second-order adsorption kinetic model was found to provide the best fit to the experimental data, with an R2 = 0.99997, suggesting an adsorption mechanism dominated by chemical interactions. In the adsorption equilibrium study, the Freundlich isotherm model was found to provide the best fit to the data.
- The efficiency of the CA/PHB biocomposite is notable, since considering the results of the adsorption kinetics analysis, it can be observed that, from 5 min in terms of contact time with the contaminant, the biocomposite presents adsorption capacities similar to those of the same sample with a contact time of 24 h.
- The characterisations revealed information on the presence of functional groups C–H, C=O, O–H, and C–N, which, considering the FTIR analysis, indicate their involvement in the adsorption process of Ni (II) ions. TGA analysis revealed three stages of degradation, showing a decrease in the thermal stability of the biocomposite after adsorption, suggesting an effective interaction between the material and the metal ions. Meanwhile, SEM revealed a porous surface before adsorption and morphological changes after the process, supporting the retention of Ni (II) on the adsorbent surface.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, Y.; Yan, Q.; Gao, S.; Xiong, B.; Tang, X.; Liu, Z.; Li, P.; Huang, M.; Wang, Z.; Le, X.; et al. Adsorption of Ni2+ in aqueous solution by KMnO4 modified biomass: Investigation on adsorption kinetics and modification mechanism. Environ. Technol. 2022, 43, 2855–2866. [Google Scholar] [CrossRef] [PubMed]
- Balram; Singh Kaith, B. Removal of hazardous metal ions from polluted water using biomaterial-based ion- exchangers: A review. Mater. Today Proc. 2022, 53, 174–178. [Google Scholar] [CrossRef]
- El Mansouri, F.; El Farissi, H.; Cacciola, F.; Talhaoui, A.; El Bachiri, A.; Tahani, A.; Esteves da Silva, J.C.G.; Brigui, J. Rapid elimination of copper (II), nickel (II) and chromium (VI) ions from aqueous solutions by charcoal modified with phosphoric acid used as a green biosorbent. Polym. Adv. Technol. 2022, 33, 2254–2264. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Bui, N.T.; Nguyen, V.H.; Le, D.T.; Van Tran, T.T.; Bui, T.H. Superparamagnetic cobalt ferric nanoparticles incorporated biopolymers extracted from dragon fruit (hylocereus undatus) peels for nickel(II) removal. Environ. Technol. Innov. 2021, 23, 101773. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, Á.; Ortega-Toro, R. Removal of metals and dyes in water using low-cost agro-industrial waste materials. Appl. Sci. 2023, 13, 8481. [Google Scholar] [CrossRef]
- Sánchez-Ponce, L.; Díaz-de-Alba, M.; Casanueva-Marenco, M.J.; Gestoso-Rojas, J.; Ortega-Iguña, M.; Galindo-Riaño, M.D.; Granado-Castro, M.D. Potential Use of Low-Cost Agri-Food Waste as Biosorbents for the Removal of Cd(II), Co(II), Ni(II) and Pb(II) from Aqueous Solutions. Separations 2022, 9, 309. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Z.; Liu, W.; Lin, J. Centrifugally spun lignin fibers with high Cr(VI) adsorption capacity. Ind. Crops Prod. 2022, 189, 115833. [Google Scholar] [CrossRef]
- Harripersadth, C.; Musonge, P.; Makarfi Isa, Y.; Morales, M.G.; Sayago, A. The application of eggshells and sugarcane bagasse as potential biomaterials in the removal of heavy metals from aqueous solutions. S. Afr. J. Chem. Eng. 2020, 34, 142–150. [Google Scholar] [CrossRef]
- Ding, R.; Hu, S.; Xu, M.; Hu, Q.; Jiang, S.; Xu, K.; Tremblay, P.L.; Zhang, T. The facile and controllable synthesis of a bacterial cellulose/polyhydroxybutyrate composite by co-culturing Gluconacetobacter xylinus and Ralstonia eutropha. Carbohydr. Polym. 2021, 252, 117137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wei, Z.; Sun, L.; Wang, Y.; Wu, X.; Wang, T.; Wang, Z.; Fu, Y. A Novel Cellulose-Based Composite Hydrogel Microsphere Material: For Efficient Adsorption of Co(II) and Ni(II) Ions in Water. J. Inorg. Organomet. Polym. Mater. 2024, 35, 898–918. [Google Scholar] [CrossRef]
- Matsedisho, B.; Otieno, B.; Kabuba, J.; Leswifi, T.; Ochieng, A. Removal of Ni(II) from aqueous solution using chemically modified cellulose nanofibers derived from orange peels. Int. J. Environ. Sci. Technol. 2024, 22, 2905–2916. [Google Scholar] [CrossRef]
- Candido, R.G.; Godoy, G.G.; Gonçalves, A. Characterization and application of cellulose acetate synthesized from sugarcane bagasse. Carbohydr. Polym. 2017, 167, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.V.; Joseph, K.; Thomas, S.; Pillai, C.K.S.; Prasad, V.S.; Groeninckx, G.; Sarkissova, M. The thermal and crystallisation studies of short sisal fibre reinforced polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2003, 34, 253–266. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, H.; Liu, R.; Wu, C. Preparation of long sisal fiber-reinforced polylactic acid biocomposites with highly improved mechanical performance. Polymers 2021, 13, 1124. [Google Scholar] [CrossRef] [PubMed]
- Sutthasupa, S.; Koo-amornpattana, W.; Worasuwannarak, N.; Prachakittikul, P.; Teachawachirasiri, P.; Wanthong, W.; Thungthong, T.; Inthapat, P.; Chanamarn, W.; Thawonbundit, C.; et al. Sugarcane bagasse-derived granular activated carbon hybridized with ash in bio-based alginate/gelatin polymer matrix for methylene blue adsorption. Int. J. Biol. Macromol. 2023, 253, 127464. [Google Scholar] [CrossRef] [PubMed]
- Voronova, M.; Rubleva, N.; Kochkina, N.; Afineevskii, A.; Zakharov, A.; Surov, O. Preparation and characterization of polyvinylpyrrolidone/cellulose nanocrystals composites. Nanomaterials 2018, 8, 1011. [Google Scholar] [CrossRef] [PubMed]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef] [PubMed]
- Farch, S.; Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Lefnaoui, S.; Kebir, M.; Zamouche, M.; Amrane, A.; Zhang, J.; Hadadi, A.; et al. Application of Walnut Shell Biowaste as an Inexpensive Adsorbent for Methylene Blue Dye: Isotherms, Kinetics, Thermodynamics, and Modeling. Separations 2023, 10, 60. [Google Scholar] [CrossRef]
- Debnath, S.; Das, R. Strong adsorption of CV dye by Ni ferrite nanoparticles for waste water purification: Fits well the pseudo second order kinetic and Freundlich isotherm model. Ceram. Int. 2023, 49, 16199–16215. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Kamińska, A.; Niedoba, O.; Michalkiewicz, B. CO2 Adsorption on Activated Carbons Prepared from Molasses: A Comparison of Two and Three Parametric Models. Mater 2021, 14, 7458. [Google Scholar] [CrossRef] [PubMed]
- Villabona-Ortíz, Á.; Ortega-Toro, R.; Pedroza-Hernández, J. Biocomposite based on polyhydroxybutyrate and cellulose acetate for the adsorption of methylene blue. J. Compos. Sci. 2024, 8, 234. [Google Scholar] [CrossRef]
- Guerrero, Y. Extracción de la Celulosa a Partir de Los Residuos de Pasto Común (Festuca Arundinacea) Para la Elaboración de Acetato de Celulosa. Bachelor’s Thesis, University Politécnica Salesiana, Sede Cuenca, Cuenca, 2021. [Google Scholar]
- Sinyeue, C.; Garioud, T.; Lemestre, M.; Meyer, M.; Brégier, F.; Chaleix, V.; Sol, V.; Lebouvier, N. Biosorption of nickel ions Ni2+ by natural and modified Pinus caribaea Morelet sawdust. Heliyon 2022, 8, e08842. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Tovar, C.; Villabona-Ortiz, Á.; Ortega-Toro, R. Kinetic, Isothermal and Thermodynamic Study on the Removal of Hexavalent Chromium with Biocomposites (Cellulose–PLA). J. Compos. Sci. 2025, 9, 36. [Google Scholar] [CrossRef]
- Mohamed Noor, N.; Chan Abdullah, E.; Othman, R.; Mujawar Nasibab, M. Removal of cadmium ions via the magnetic biochar synthesised from sugarcane bagasse: Factors affecting yield and adsorption capability. Penerbit UMT J. Sustain. Sci. Manag. 2023, 18, 70–85. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.Z.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.S. Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef] [PubMed]
- Din, M.I.; Mujahid, A.; Bock, U.; Khalid, R.; Hussain, Z. A kinetic and thermodynamic investigation for adsorption of cadmium (ii) ions on the microwave modified sugar cane bagasse. Desalin. Water Treat. 2024, 317, 100194. [Google Scholar] [CrossRef]
- Lang’at, E.K.; Omolo, J.O.; Ongoma, P.O. Sugarcane Bagasse Based Adsorbents and their Adsorption Efficacy on Removal of Heavy Metals from Nakuru Industrial Wastewater: Optimization, Kinetic and Thermodynamic Aspects. Asian J. Appl. Chem. Res. 2024, 15, 276–293. [Google Scholar] [CrossRef]
- Ferreira Piazzi Fuhr, A.C.; Raupp, I.N.; Oliveira Silva, L.F.; Crissien, T.J.; Philippi, V.P.; Alomar, S.Y.; Dotto, G.L. Alternative interpretations for the nickel adsorption using different agricultural wastes and activated carbon: A statistical physics approach to evaluate the characteristics that influence the effectiveness of adsorbents. J. Mol. Liq. 2025, 421, 126913. [Google Scholar] [CrossRef]
- Pagala, B. Biosorption of Chromium and Lead from Electroplating Industry Effluent Using Modified Cane Bagasse. J. Inst. Eng. Ser. D 2023, 105, 1655–1666. [Google Scholar] [CrossRef]
- Omer, A.M.; Abd El-Monaem, E.M.; Eltaweil, A.S. Novel reusable amine-functionalized cellulose acetate beads impregnated aminated graphene oxide for adsorptive removal of hexavalent chromium ions. Int. J. Biol. Macromol. 2022, 208, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Hsini, A.; Essekri, A.; Aarab, N.; Laabd, M.; Ait Addi, A.; Lakhmiri, R.; Albourine, A. Elaboration of novel polyaniline@Almond shell biocomposite for effective removal of hexavalent chromium ions and Orange G dye from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 15245–15258. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Shaikh, A.A.; Yeasmin, S.; Gafur, A.; Hossain, I.; Alam, A.; Khan, S.; Paul, T.; Quddus, S. Simultaneous removal of Ni2+ and Congo red from wastewater by crystalline nanocellulose—Modified coal bionano-composites: Continuous adsorption study with mathematical modeling. Groundw. Sustain. Dev. 2024, 26, 101244. [Google Scholar] [CrossRef]
- Singh, R.; Jasrotia, R.; Sharma, D.; Singh, J.; Mittal, S.; Singh, H. Recyclable magnetic nickel ferrite–carboxymethyl cellulose–sodium alginate bio-composite for efficient removal of nickel ion from water. J. Dispers. Sci. Technol. 2024, 46, 1241–1252. [Google Scholar] [CrossRef]
- Achalhi, N.; El Ouardi, Y.; Virolainen, S.; El Yousfi, R.; Lamsayah, M.; Butylina, S.; El Barkany, S.; Repo, E.; El Idrissi, A. Ionic liquids-assisted integration of biobased materials for sustainable elaboration of nanocomposites using hydroxyethyl cellulose and interlayered clay for efficient separation of Co(II) from multi-component mixtures. Cellulose 2024, 31, 9887–9906. [Google Scholar] [CrossRef]
- Reda, A.; El-Demerdash, A.-G.; Sadik, W.; El-Rafey, E.; Shoeib, T. Effectively eliminating lead and cadmium from industrial wastewater using a biowaste-based sorbent. Appl. Water Sci. 2025, 15, 25. [Google Scholar] [CrossRef]
- Njimou, J.R.; Fai, V.; Sieugaing, M.T.; Nguenamadje, D.; Godwin, J.; Genola, O.B.; Noumi, G.B.; Tripathy, B.C. Hy-drothermal synthesis of a nickel-oxide-infused orange peel nanobiocomposite for enhanced heavy metal removal from mining wastewater. Hybrid Adv. 2025, 9, 100392. [Google Scholar] [CrossRef]
- Kumar, A.; Das, T.; Thakur, R.S.; Fatima, Z.; Prasad, S.; Ansari, N.G.; Patel, D.K. Synthesis of Biomass-Derived Acti-vated Carbons and Their Immobilization on Alginate Gels for the Simultaneous Removal of Cr(VI), Cd(II), Pb(II), As(III), and Hg(II) from Water. ACS Omega 2022, 7, 41997–42011. [Google Scholar] [CrossRef] [PubMed]
- Kamaludin, N.H.I.; Ismail, H.; Rusli, A.; Ting, S.S. Thermal behavior and water absorption kinetics of polylactic acid/chitosan biocomposites. Iran. Polym. J. 2021, 30, 135–147. [Google Scholar] [CrossRef]
- Fijoł, N.; Abdelhamid, H.N.; Pillai, B.; Hall, S.A.; Thomas, N.; Mathew, A.P. 3D-printed monolithic biofilters based on a polylactic acid (PLA)—Hydroxyapatite (HAp) composite for heavy metal removal from an aqueous medium. RSC Adv. 2021, 11, 32408–32418. [Google Scholar] [CrossRef] [PubMed]
- Rekha, A.; Srinivasan, L.; Pavithra, S.; Gomathi, T.; Sudha, P.N.; Lavanya, G.; Arumugam, N.; Vidhya, A. Biosorption efficacy studies of Sargassum wightii and its biochar on the removal of chromium from aqueous solution. J. Taiwan Inst. Chem. Eng. 2023, 166, 105241. [Google Scholar] [CrossRef]
- Abugu, H.O.; Ikwelle, C.E.; Odewole, O.A.; Lawal, A.; Olaleye, A.M.; Ucheana, I.A.; Okenwa, J.C.; Egbueri, J.C. Thermal and chemical pretreatment of Ebenaeae Diospyros preusii seed for enhanced adsorptive removal of aqueous-bound Cr(VI). Discov. Water 2025, 5, 2. [Google Scholar] [CrossRef]
- Truong, T.T.; Vuong, T.X.; Chu, T.H.H.; Diep, T.H.T.; Ta, H.C.; Hoang, L.P.; Pham, T.D. Synthesis of novel hydrochar from lemongrass distillation waste and its application in individual and simultaneous adsorption of a mixture of heavy metal ions. J. Water Process Eng. 2025, 71, 107402. [Google Scholar] [CrossRef]
- Tenea, A.G.; Dinu, C.; Rus, P.A.; Ionescu, I.A.; Gheorghe, S.; Iancu, V.I.; Vasile, G.G.; Pascu, L.F.; Chiriac, F.L. Exploring adsorption dynamics of heavy metals onto varied commercial microplastic substrates: Isothermal models and kinetics analysis. Heliyon 2024, 10, e35364. [Google Scholar] [CrossRef] [PubMed]
- Oyewo, O.A.; Mutesse, B.; Leswifi, T.Y.; Onyango, M.S. Highly efficient removal of nickel and cadmium from water using sawdust-derived cellulose nanocrystals. J. Environ. Chem. Eng. 2019, 7, 103251. [Google Scholar] [CrossRef]
- Zhang, H.; Han, X.; Liu, J.; Wang, M.; Zhao, T.; Kang, L.; Zhong, S.; Cui, X. Fabrication of modified alginate-based biocomposite hydrogel microspheres for efficient removal of heavy metal ions from water. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 651, 129736. [Google Scholar] [CrossRef]
- Staroń, P.; Kuciakowski, J.; Chwastowski, J. Biocomposite of hydrochar and Lindnera jadinii with magnetic properties for adsorptive removal of cadmium ions. J. Environ. Chem. Eng. 2023, 11, 110270. [Google Scholar] [CrossRef]
- Luong, H.V.T.; Le, P.P.; Thieu, Q.Q.V.; Nguyen, V.N.H.; Nguyen, T.N.Y. Alginate functionalized sugarcane cellulose-based beads to improve methylene blue adsorption from aqueous solution. Heliyon 2024, 10, e37860. [Google Scholar] [CrossRef] [PubMed]








| Variables | Range | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Initial contaminant concentration (mg/L) | 15 | 25 | 35 |
| Amount of adsorbent (g) | - | 0.03 | - |
| Metal Ion | Optimum Conditions | Reference |
|---|---|---|
| Cd2+ | pH = 6 | [28] |
| Pb2+, Ni2+ | pH = 6 | [29] |
| Cd2+ | pH = 6–7 | [30] |
| Pb2+, Cu2+, Cd2+, Ni2+ y Cr3+ | pH = 5 | [31] |
| Ni2+ | pH = 6 | [32] |
| Pb2+ | pH = 6 | [33] |
| Kinetic Model | Parameter | Non-Linear Adjustment |
|---|---|---|
| Pseudo first order | qe | 24.7861 |
| K1 | 1.2131 | |
| R2 | 0.99996 | |
| Square Error | 0.0158 | |
| Pseudo second order | qe | 24.8081 |
| K2 | 2.2353 | |
| R2 | 0.99997 | |
| Square Error | 0.01341 | |
| Elovich | 3.7951 | |
| 7.6915 | ||
| R2 | 0.99905 | |
| Square Error | 0.4187 | |
| Intraparticle diffusion | K3 | 1.4888 |
| R2 | 0.2009 | |
| Square Error | 549.6321 |
| Isotherm Model | Parameter | Non-Linear Adjustment |
|---|---|---|
| Langmuir | qm | 35,420.125 |
| b | 2.91609 × 10−5 | |
| R2 | 0.96579 | |
| Freundlich | kf | 0.82124 |
| n | 0.85708 | |
| R2 | 0.99929 | |
| Dubinin–Radushkevich | qdr | 6.88887 |
| kdr | 9.36952 | |
| R2 | 0.95484 |
| Precursor | Modifying Agent/Reinforcing Matrix | Pollutant Removed | Removal Capacity (mg/g) | Removal (%) | pH | Adsorbent Dosage (mg) | Initial Concentration of the Contaminant (ppm) | Reference |
|---|---|---|---|---|---|---|---|---|
| Sawdust cellulose | NaNO2/NaHCO3 | Cd2+ | 99.9–2206.9 | 44.1–99.9 | 7.10 | 20 | 1–50 | [48] |
| Ni2+ | 49.98–956.6 | 38.2–99.1 | ||||||
| Brown marine algae (Phae-ophyceae) | CaCl2; activation with EDC and NHS | Pb2+ | 369.6 | 90 | 4 | 19 | 500 | [49] |
| Cu2+ | 124.1 | 5 | 200 | |||||
| Natural raffia fibres (Raphia farinifera) | CaCl2; activation with Fe3O4 | Cd2+ | 16.34 | 87.3 | 6 | 100 | 150 | [50] |
| Sugarcane cellulose | Sodium alginate | Methylene blue | 4.27 | 85.33 | 8 | 200 | 10 | [51] |
| Banana rachis (M. oranta) | Bituminous coal | Ni2+ | 328.7 | 67.95 | 4.9 | - | 25–45 | [36] |
| Congo Red | 478.3 | 76.65 | ||||||
| Sugar cane bagasse (Saccharum officinarum) | PHB | Ni2+ | 5.042 | 86.44 | 6 | 30 | 35 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Tovar, C.; Villabona-Ortíz, Á.; Toro-Madrid, O.; Ortega-Toro, R.; Bonilla Mancilla, H. Cellulose Acetate–PHB Biocomposite from Saccharum officinarum for Ni (II) Adsorption: Equilibrium and Kinetics. J. Compos. Sci. 2025, 9, 376. https://doi.org/10.3390/jcs9070376
Tejada-Tovar C, Villabona-Ortíz Á, Toro-Madrid O, Ortega-Toro R, Bonilla Mancilla H. Cellulose Acetate–PHB Biocomposite from Saccharum officinarum for Ni (II) Adsorption: Equilibrium and Kinetics. Journal of Composites Science. 2025; 9(7):376. https://doi.org/10.3390/jcs9070376
Chicago/Turabian StyleTejada-Tovar, Candelaria, Ángel Villabona-Ortíz, Oscar Toro-Madrid, Rodrigo Ortega-Toro, and Humberto Bonilla Mancilla. 2025. "Cellulose Acetate–PHB Biocomposite from Saccharum officinarum for Ni (II) Adsorption: Equilibrium and Kinetics" Journal of Composites Science 9, no. 7: 376. https://doi.org/10.3390/jcs9070376
APA StyleTejada-Tovar, C., Villabona-Ortíz, Á., Toro-Madrid, O., Ortega-Toro, R., & Bonilla Mancilla, H. (2025). Cellulose Acetate–PHB Biocomposite from Saccharum officinarum for Ni (II) Adsorption: Equilibrium and Kinetics. Journal of Composites Science, 9(7), 376. https://doi.org/10.3390/jcs9070376












