1. Introduction
Tissue-engineered scaffolds are three-dimensional, biofunctional structures designed to support the regeneration or repair of damaged tissues by providing a temporary framework for cell attachment, proliferation, differentiation, and Extracellular Matrix (ECM) deposition. They serve as artificial environments that mimic the natural ECM, promoting tissue integration and eventual scaffold degradation in vivo [
1,
2]. To function effectively, these scaffolds must be biocompatible, mechanically appropriate for the target tissue, and possess a porous architecture that facilitates cell infiltration, vascularization, and nutrient exchange [
3,
4].
Materials used in TE scaffolds range from metals, ceramics, and synthetic polymers to naturally derived polymers and decellularized extracellular matrices, and functional additives such as nanoparticles, each selected based on specific mechanical and biological requirements [
5,
6]. Recent advances emphasize the design of biofunctional scaffolds that not only resemble native tissue architecture but also actively interact with the host environment to enhance regeneration [
7].
Histology has long been considered the traditional gold standard for assessing the biological functionality and biocompatibility of tissue-engineered constructs. However, this method presents several significant drawbacks; it is invasive, causes tissue destruction, provides only semi-quantitative data, often requires the use of multiple animal subjects, can alter the physical structure of constructs during processing, fails to represent the entire tissue volume, and does not directly assess functional outcomes. These limitations make it poorly suited for dynamic, in vivo studies. As a result, there has been a growing shift toward advanced medical imaging techniques that allow non-destructive, longitudinal, and 3D assessment of TE constructs in living systems. These modalities enable researchers to monitor how constructs integrate with host tissues and how they influence physiological processes, without harming the specimen or relying on large numbers of animals [
8].
While previous reviews have examined specific imaging techniques in TE, most have focused on a single modality, specific tissue type, or preclinical applications. In contrast, this review offers an integrative and comparative analysis of multiple non-invasive imaging methods, including ultrasound, CT, MRI, optical imaging, and PAI, with a special emphasis on their use with scaffold-based constructs. It not only addresses the technical and translational challenges of each modality but also highlights emerging dual-modality approaches that combine structural and functional information. By linking preclinical imaging capabilities with clinical needs, this review provides a thorough framework that supports both scaffold optimization and clinical translation, an aspect not fully covered in earlier literature.
2. Role of Medical Imaging in Scaffold Evaluation
Medical imaging plays a pivotal role in TE and regenerative medicine by enabling the non-invasive evaluation of scaffold constructs, tissue formation, vascularization, and functional integration with host tissues. As scaffold complexity grows, incorporating cells, growth factors, and responsive biomaterials, imaging modalities can provide detailed morphological, functional, and molecular information [
9,
10].
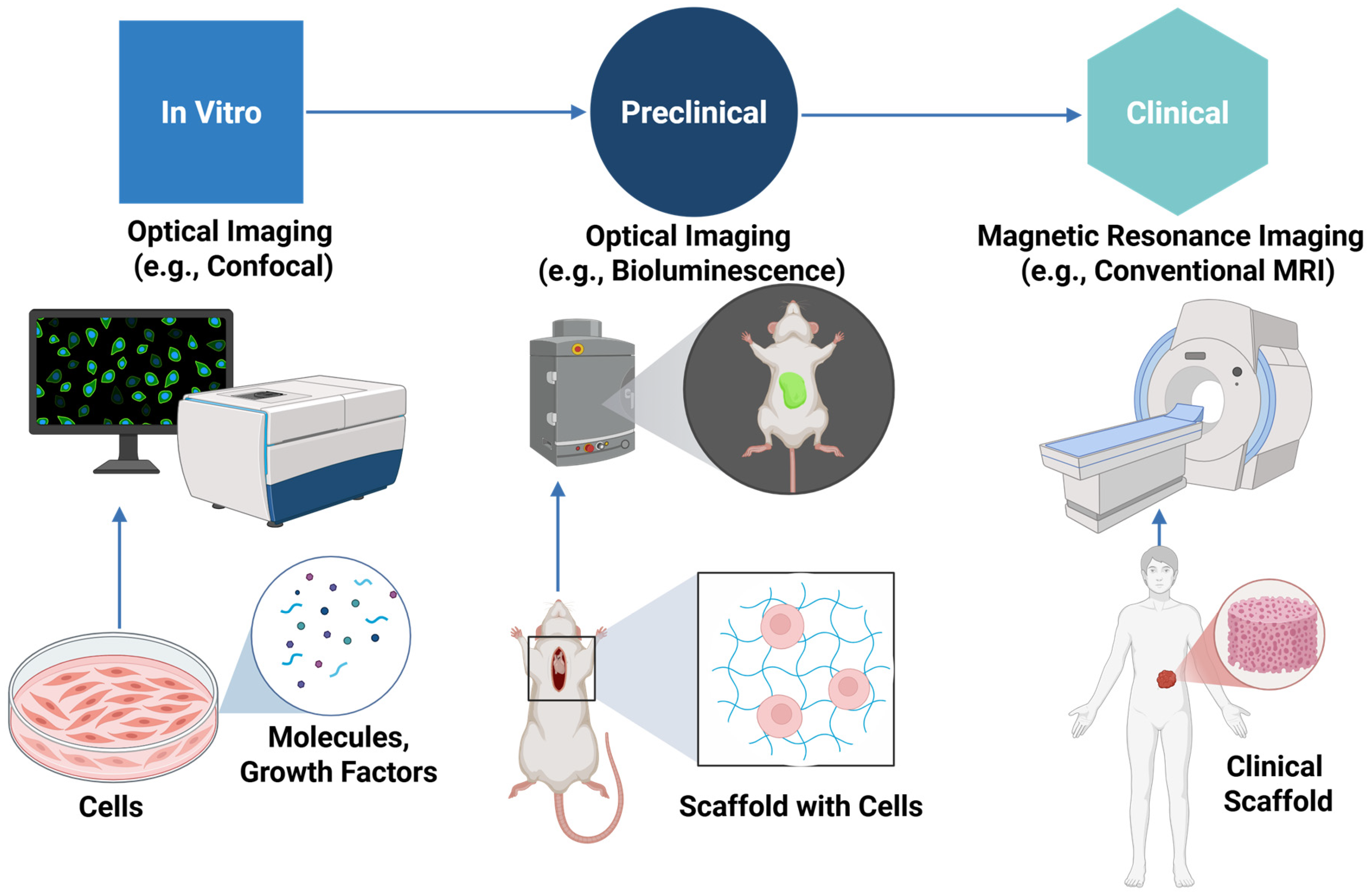
Figure 1 illustrates the comprehensive imaging process of scaffolds, beginning with in vitro assessments, advancing through preclinical models for functional and biological evaluation, and ultimately translating to clinical applications for monitoring integration, performance, and safety in patients. These techniques span cellular and molecular analysis in vitro to non-invasive scaffold imaging in animal and human studies.
Table 1 summarizes key features of these imaging modalities, comparing their resolution, depth penetration, scaffold compatibility, and functional capabilities, thereby guiding selection based on the specific requirements of scaffold evaluation in TE.
No single imaging technique fulfills all requirements, and the choice of imaging modality depends on factors such as penetration depth, spatial and temporal resolution, contrast mechanisms, and safety considerations [
10]. Recent advances have expanded the range of available imaging techniques, broadly categorized into non-optical methods (such as X-ray/CT, MRI, ultrasound, and Single-photon emission computed tomography (SPECT), optical methods (such as OCT and multiphoton microscopy), and hybrid techniques like PAI [
11]. Each method presents unique advantages and limitations; for example, MRI offers high soft tissue contrast without ionizing radiation, μ-CT provides high spatial resolution for bone and vascular structures, and PAI bridges optical and ultrasound properties to capture functional information at greater depths [
11,
12]. This continuum from microscopy to clinical imaging supports comprehensive scaffold assessment from in vitro validation of cell behavior to in vivo monitoring in animal models and human trials.
Table 1.
Overview of Non-Invasive Imaging Modalities for Tissue-Engineered Scaffold Evaluation.
Table 1.
Overview of Non-Invasive Imaging Modalities for Tissue-Engineered Scaffold Evaluation.
| Imaging Modality | Resolution | Depth Penetration | Strengths | Limitations | Challenges in Composite Scaffolds | Refs. |
|---|
| Optical Imaging | - Sub-micron lateral resolution
- Depth sectioning | Up to ~200 µm in highly scattering 3D engineered tissues (limited by NIR excitation and tissue optical properties) | Real-time, label-free, repeatable in live tissues; detects metabolic/structural changes; bioreactor-compatible; high resolution; histology-aligned. | Shallow depth (≤1 mm); affected by scattering/absorption; needs advanced optics; possible signal overlap; risk of photodamage at high power. | Difficulty in monitoring specific scaffold components (e.g., distinguishing gelatin from HA); risk of dye leakage and photobleaching; potential for limited penetration depth in tissue in NIR Imaging (Two-Color NIR Fluorescence). | [13,14] |
| MRI | 125–288 µm in-plane; ~0.8 mm slice (3T MRI) | Deep (whole-tissue/organ imaging; suitable for large animal and clinical translation). | Multiparametric, non-invasive tracking of scaffold and tissue; high soft-tissue contrast; USPIO-enabled degradation mapping. | USPIO (Ultra-Small SuperParamagnetic Iron Oxide nanoparticles) labeling may interfere with some advanced MRI sequences, high cost and limited availability, motion sensitivity, and difficulty distinguishing between closely overlapping soft tissue components. | Difficulty in non-invasive, label-free, longitudinal monitoring of scaffold degradation; distinguishing between multiple scaffold components; conventional MRI lacks molecular specificity. | [14,15] |
| X-ray/CT | Down to 1.6–2 μm (high-resolution micro-CT) | High
| - Non-destructive, high spatial resolution;
- 3D visualization, compatible with large and small animal models;
- Suitable for calcified tissues. | - Limited soft tissue contrast without contrast agents;
- limiting frequent in vivo imaging;
- Susceptibility to metal artifacts in some implants;
- Requires long scanning and processing times for high-resolution 3D reconstruction;
- Poor sensitivity to early mineralization stages and non-mineralized tissues. | Beam hardening artifacts due to differential X-ray attenuation between polymer and ceramic phases; obscures polymer phase and complicates distinguishing polymer degradation from ceramic resorption. | [16,17,18] |
| Ultrasound | ~40 µm axial resolution (via high-frequency probe at 32 MHz) | Moderate | Low cost
Portable
Real-time imaging
Functional assessment | Operator-dependent
Limited soft tissue contrast. | Difficulty in visualizing microvessels and quantifying microvascularization in bioabsorbable scaffolds. | [19,20] |
3. Non-Invasive Imaging Techniques for Scaffold Assessment
Non-invasive evaluations have gained increasing importance in TE, particularly for monitoring scaffold performance, tissue growth, and construct degradation over time. Advancements in non-invasive imaging technologies, such as ultrasound elasticity imaging, near-infrared fluorescence (NIR-FL) imaging, and PAI, offer promising opportunities for real-time and longitudinal monitoring without damaging the scaffold or surrounding tissues [
9,
21]. For instance, the use of alkaline phosphatase (ALP)-responsive scaffolds combined with NIR-FL and PAI allows for visualization of early-stage bone regeneration processes, overcoming the limitations of conventional CT and MRI techniques in detecting newly formed tissue [
22].
Moreover, non-invasive imaging plays a crucial role in bridging laboratory research with clinical applications by providing reliable, FDA-compliant monitoring tools for scaffold validation and tissue integration [
9]. By offering real-time feedback on scaffold performance and tissue regeneration, non-invasive imaging strategies not only reduce experimental variability but also accelerate the optimization and clinical translation of tissue-engineered therapies [
21].
A wide range of non-invasive imaging modalities is available for scaffold evaluation, each offering unique strengths depending on the application. These techniques can be broadly grouped into optical, magnetic resonance-based, X-ray-based, and ultrasound-based approaches, as outlined below [
23].
3.1. Optical Imaging Techniques
Optical imaging techniques offer detailed insights into cell viability, distribution, and scaffold integration without the need for destructive processing. Traditional optical microscopy methods, such as confocal and nonlinear microscopy, have been used for live-cell imaging and scaffold monitoring [
24]. However, their utility is constrained by limited penetration depth and short working distances [
25].
To overcome these limitations, Hofmann et al. [
26] introduced a fiber-optic-based (FOB) imaging system that enables non-destructive, high-resolution imaging of fluorescently labeled cells through thick tissue-engineered scaffolds. This system employs microimaging channels embedded within the scaffold, which guide excitation light from an external source into the interior of the construct. The emitted fluorescence, collected through the exterior scaffold surface, allows for precise mapping of cell distribution with up to 20–30 µm resolution, exceeding the penetration depth of standard confocal microscopy by over twofold. This method supports integration with bioreactors and real-time imaging across working distances as long as 8 cm, enabling longitudinal studies of cellular processes such as migration and cytokinesis during scaffold maturation [
26].
Complementing these developments in optical detection, the integration of optical 3D printing methods has revolutionized scaffold fabrication itself. Techniques such as stereolithography (SLA), two-photon polymerization (TPP), and laser-assisted direct ink writing (DIW) provide ultra-high-resolution control over scaffold architecture, often down to the sub-micron level [
27,
28]. These approaches allow for the simultaneous patterning of scaffold geometry and functional components, such as growth factors or even living cells, with precise spatial fidelity. For instance, stereolithographic systems using digital light processing (DLP) can fabricate complex, multiscale structures with both speed and biocompatibility, aligning well with the imaging requirements for functional scaffold assessment [
29].
3.1.1. Near-Infrared Fluorescence Imaging
Near-infrared fluorescence imaging (NIR-FL) advantages, such as reduced light scattering, deep tissue penetration, low autofluorescence, and minimal photodamage, make it particularly well-suited for in vivo applications, especially in preclinical and translational studies [
30,
31]. NIR-FL systems rely on two main types of fluorescent probes: “always-on” probes, such as FDA-approved indocyanine green (ICG), and “activatable” probes, which emit fluorescence only in response to specific biological cues such as enzyme activity, pH shifts, or reactive oxygen species. While always-on probes are widely used due to their simplicity, they often suffer from limited specificity and high background signal. In contrast, activatable probes offer improved signal-to-background and enhanced specificity within the complex biological environments [
31].
In bone TE, ALP-responsive activatable probes have proven particularly useful for detecting early-stage osteogenic differentiation. One notable example is the use of the LET-3 probe, which was conjugated to 3D-printed polycaprolactone/calcium silicate (PCL/CS) composite scaffolds. This platform enabled dual-mode NIR-FL and PAI, allowing for non-invasive and dynamic visualization of ALP activity and new bone formation over multiple weeks in vivo. Beyond osteogenesis, NIR-FL has also been applied to monitor scaffold degradation, inflammatory responses, and stem cell migration [
22,
30].
A particularly promising approach involves the design of bone-targeted NIR probes, achieved by conjugating fluorophores with bone-seeking ligands such as bisphosphonates. These probes facilitate prolonged imaging of scaffold–bone interface integration and support real-time visualization of tissue–material interactions during regeneration [
32]. Despite its broad potential, NIR-FL still faces several limitations. These include suboptimal fluorescence intensity, limited photostability, and nonspecific signal interference in vivo. Current research efforts are focused on developing next-generation fluorophores in the NIR-II region (1000–1700 nm), offering deeper tissue penetration, greater imaging precision, and improved safety profiles. Such innovations are expected to enhance the clinical applicability of NIR-FL in scaffold-based regenerative medicine [
30].
3.1.2. Photoacoustic Imaging (PAI)
PAI, referred to as optoacoustic imaging, merges the superior contrast of optical imaging with the deep penetration and high spatial resolution provided by ultrasound [
33,
34]. In this hybrid method, pulsed or modulated laser light is absorbed by tissue chromophores such as hemoglobin, leading to rapid thermal expansion and the generation of ultrasonic waves, which are then captured to reconstruct high-resolution images of internal structures [
35,
36].
A unique advantage of PAI, especially in the form of photoacoustic microscopy (PAM), is its ability to deliver high-contrast, label-free imaging of blood-rich structures like the vascular networks surrounding implanted scaffolds. This is particularly important for evaluating neovascularization, which plays a critical role in scaffold integration and long-term functionality [
33,
36]. Among PAM types, optical-resolution PAM (OR-PAM) offers lateral resolution as fine as 2.6–5 µm, while acoustic-resolution PAM (AR-PAM) enables imaging at depths of 2–3 mm with slightly reduced resolution [
37,
38].
The effectiveness of PAM in tracking vascularization and scaffold degradation has been demonstrated in various preclinical studies. For example, PLGA inverse opal scaffolds implanted in mouse ears were monitored over six weeks using both OR-PAM and AR-PAM. The PAI revealed dynamic vascular changes over time linked to scaffold pore size (80 vs. 200 µm), which correlated well with histological findings [
37]. The spatial distribution of cells within tissue-engineered constructs was successfully visualized over time in vitro using cell-tracking dyes, gold nanoparticles, or the intrinsic optical absorption of melanoma cells (
Figure 2A,B) [
39]. The coronal and sagittal PAM images (
Figure 2A) demonstrate melanoma cell infiltration throughout the entire 1.2 mm-thick scaffold. A 3D reconstruction (
Figure 2B) further visualizes individual cells and clusters as black dots or patches. This imaging depth exceeds that of conventional microscopy techniques, highlighting the advantage of PAM for volumetric scaffold analysis.
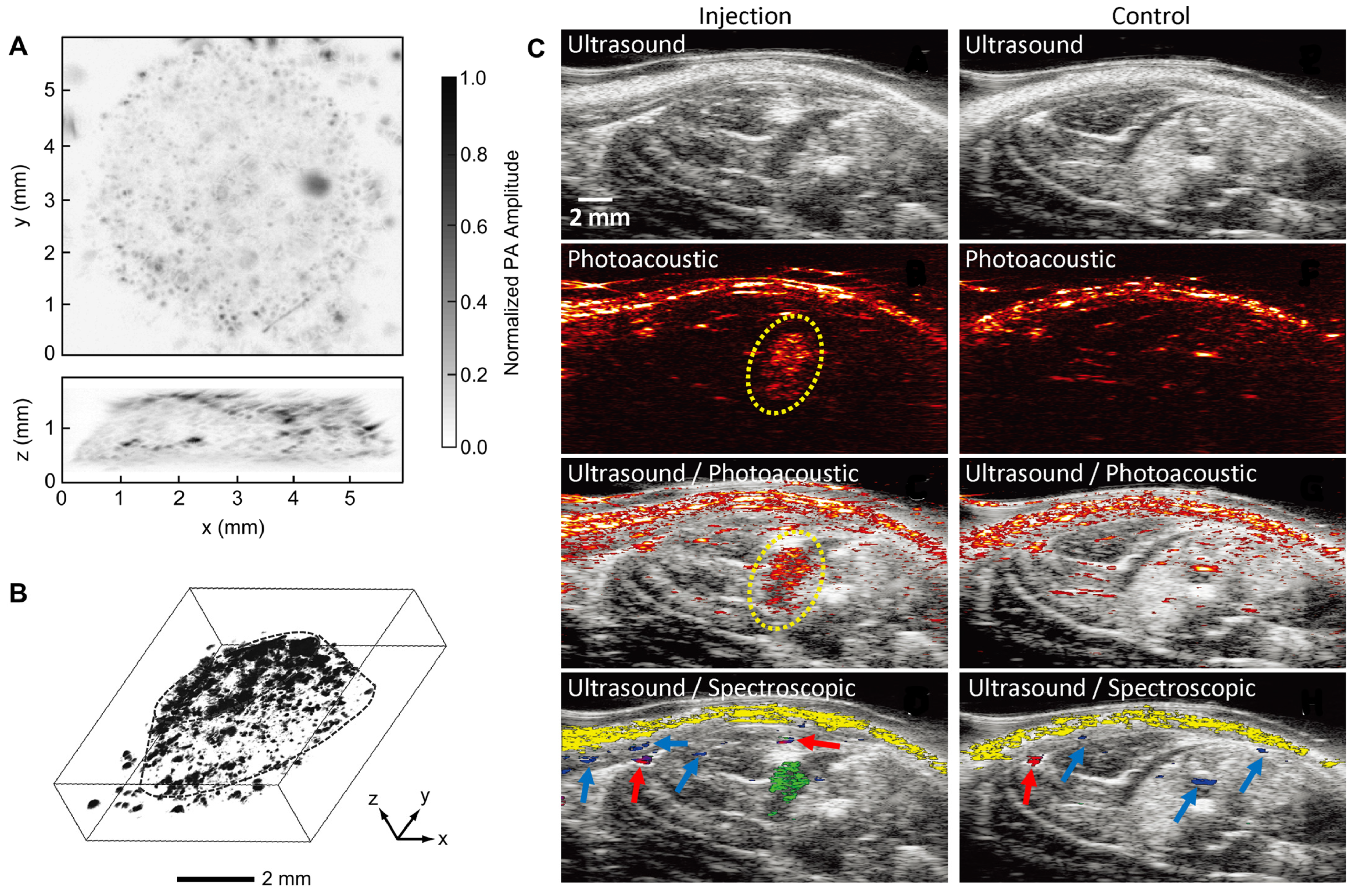
Linear-array ultrasound-based PAI enables deeper tissue visualization than photoacoustic (PA) microscopy, making it suitable for in vivo monitoring in rat models commonly used in tissue regeneration studies. For instance, Mesenchymal stem cells (MSCs) labeled with Au nanotracer (Au NT) and delivered into ischemic muscle within a PEGylated fibrin gel were successfully tracked over time using a combination of ultrasound, PA, and spectroscopic imaging [
40]. As shown in
Figure 2C, the ultrasound image provides structural details of the lower limb but cannot identify the MSCs in both the injected and control groups. However, PA and combined US/PA images reveal the presence of nanotracer-labeled MSCs within the gel (outlined in yellow). To further differentiate the MSCs from surrounding tissues, spectral analysis was employed. Multiwavelength PA imaging distinguishes the unique optical signature of gold nanospheres from that of oxygenated and deoxygenated hemoglobin, enabling precise identification of MSCs (green) versus oxygenated hemoglobin (red), deoxygenated hemoglobin (blue), and skin (yellow) in the injected group.
In contrast, the control images show PA signals only from background tissues, confirming that the observed MSC signals are specific to gold nanotracer labeling. These results highlight the capability of US/PA imaging, enhanced by spectral analysis, for noninvasive and longitudinal tracking of MSCs in vivo. This technique can be used to track cells in a scaffold in vivo if the cells are loaded with Au nanotracers (Au NT).
PAI has also been integrated with other modalities. Notably, when combined with OCT, PAM adds vascular contrast to OCT’s structural imaging, enabling simultaneous assessment of scaffold morphology and perfusion [
37]. PA contrast agents—such as single-walled carbon nanotubes (SWCNTs)—have enhanced signal strength and enabled detailed tracking of scaffold degradation and porosity [
37,
38]. Furthermore, spectroscopic PAI, which uses multiple wavelengths (e.g., 750 and 850 nm), can differentiate between oxygenated and deoxygenated hemoglobin, offering functional insight into oxygen saturation in regenerating tissue [
35].
Ogunlade et al. [
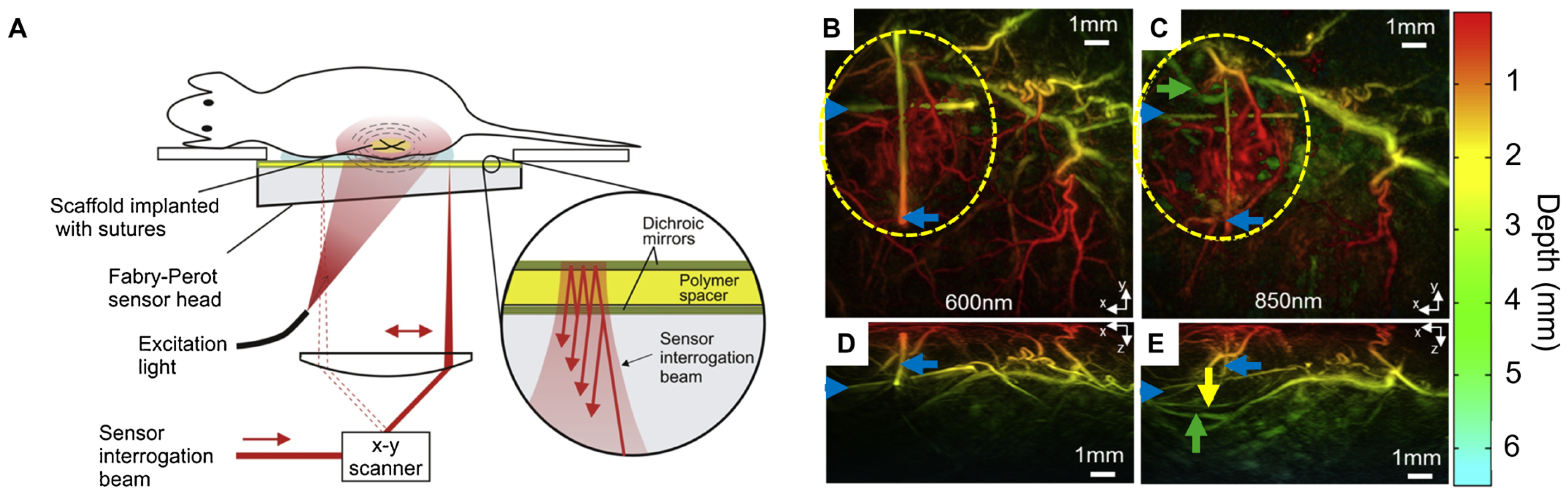
36] used wide-field tomographic PAI to monitor the vascular integration of decellularized human tracheal scaffolds implanted in murine models. This non-invasive technique enabled longitudinal monitoring of scaffold performance over 15 weeks, with imaging results correlating strongly with post-explant histological analysis. As illustrated in
Figure 3A, the experimental setup uses a Fabry–Perot (FP) sensor optimized for wide-field tissue visualization. An all-optical preclinical photoacoustic tomography (PAT) scanner was used to generate and detect PA signals. The system operates in tomography mode, utilizing a wide-field excitation beam to illuminate a large tissue volume, allowing for deeper penetration (millimeters to centimeters) compared to the limited sub-millimeter depth of OR-PAM. To evaluate imaging performance and detect neovascularization around implanted scaffolds, PAIs were acquired at wavelengths between 600–850 nm at 15 weeks post-implantation (
Figure 3B–E). Maximum intensity projections revealed clearer visualization of deeper vessels at 850 nm compared to 600 nm. Neovascular regions were highlighted around the scaffold, with vessel depth color-coded (red for superficial, green to turquoise for deeper vessels). Yellow and green arrows indicated deeper vessels, while blue arrows marked cross-shaped sutures used as fiducial markers to assist in image alignment and anatomical reference.
Additionally, ultrasound–PA systems have shown great potential for tracking scaffold placement in deeper tissues such as bone and muscle. These multimodal platforms integrate anatomical imaging from ultrasound with functional mapping from PAI, enabling real-time monitoring of scaffold location, vascularization, and oxygenation [
38].
Despite these advantages, PAI has limitations, including relatively shallow imaging depth (<3 mm in high-resolution PAM), signal interference from highly scattering tissues, and the need for exogenous contrast agents in scaffold materials lacking intrinsic absorption. Nevertheless, its high resolution, real-time capability, sensitivity, and non-ionizing nature make PAI a promising complementary tool for non-invasive evaluation of scaffold integration and tissue regeneration [
38].
Although the clinical translation of optical imaging modalities such as NIR-FL and PAI for scaffold monitoring in tissue engineering is still limited, promising preclinical studies suggest future potential. For instance, NIR-FL has been successfully employed to non-invasively track scaffold degradation and stem cell fate in a rat calvarial bone defect model, demonstrating its applicability for longitudinal monitoring of composite scaffolds during bone regeneration [
33]. Similarly, PAI has emerged as a powerful tool in tissue engineering, enabling non-invasive, real-time evaluation of scaffold vascularization, cellular infiltration, and structural integration in preclinical models [
41]. With ongoing technological advancements and increasing commercial availability of PAI systems, the pathway for clinical translation of these modalities in regenerative medicine and scaffold assessment is becoming increasingly feasible. Clinical translation of PAI has seen notable progress, particularly in oncology. Zare et al. [
42] reviewed multiple clinical trials that applied PAI for cancer detection and monitoring, including a study registered as ClinicalTrials.gov Identifier NCT04110249, which evaluates PAI for imaging tumors and normal tissues in head and neck cancer patients. While these applications focus on oncology, they demonstrate the modality’s advancing clinical relevance. Such progress underscores the translational potential of PAI for broader biomedical applications, and with further development, its application in scaffold monitoring for tissue engineering may be realized. These advances, alongside ongoing clinical trials of PAI for cancer diagnostics, underscore their potential translational relevance to regenerative applications [
34].
3.1.3. Optical Coherence Tomography (OCT)
OCT is a non-invasive imaging method with high resolution that is extensively utilized to evaluate the microstructural features and biological responses of bioresorbable and 3D-bioprinted scaffolds. OCT allows detailed visualization of scaffold morphology and tissue-scaffold interactions in both vascular and TE contexts [
43].
Brugaletta et al. [
44] used OCT to investigate the circumferential neointimal healing after implantation of ABSORB
TM bioresorbable vascular scaffolds. The study revealed that neointimal thickness increased symmetrically over time, mimicking the structure of a fibrous cap, with significant tissue growth observed around the scaffold struts at 6- and 12-month post-implantation. This demonstrates OCT’s capacity to monitor dynamic healing responses with high precision. The study found reduced shrinkage and neointimal hyperplasia in the second-generation devices, reflecting improvements in design and manufacturing. OCT enabled classification of strut degradation stages based on reflectivity, offering insights into the scaffold’s bioresorption behavior [
44]. Beyond vascular applications, Wang et al. [
45] demonstrated the use of swept-source OCT for non-invasive, three-dimensional evaluation of bioprinted hydrogel scaffolds. Their automated analysis provided high-resolution measurements of pore size, shape, interconnectivity, and fabrication defects. Importantly, OCT helped visualize differences between designed and actual printed geometries, supporting its role in scaffold quality control and design optimization in TE.
3.1.4. Bioluminescence Imaging
Bioluminescence Imaging (BLI) facilitates dynamic, non-invasive visualization of biological processes such as cell survival, proliferation, differentiation, and oxygen availability, without the need for external light excitation or biopsies. Its high sensitivity, lack of autofluorescence, and low cost make it advantageous over fluorescence and radiological imaging modalities. BLI detects light emitted through luciferase-catalyzed oxidation of luciferin, which is typically introduced via genetic constructs [
45].
A significant development in this field is the use of bioluminescence resonance energy transfer (BRET) systems, such as the GpNLuc reporter. This system merges eGFP with an ATP-independent NanoLuc luciferase, generating signals more than 150 times brighter than traditional luciferases. This increased brightness allows for more sensitive, real-time imaging in deeper and more complex environments, including mineralized and vascularized tissues [
45].
Figure 4 schematically outlines the use of GpNLuc-transduced MSCs, scaffold integration, and in vivo IVIS imaging [
45].
In bone TE, this system has been applied to monitor GpNLuc-labeled MSCs in both scaffold-free spheroids and 3D-printed scaffolds. In rat calvarial defect models, persistent bioluminescent signals correlated with cell viability and distribution [
45]. BLI is used to assess differentiation and gene expression using promoter-linked luciferase reporters, such as PECAM-1 and osteocalcin, in engineered fibrin gels and PLA/calcium phosphate composite scaffolds. This enabled longitudinal tracking of endothelial and osteogenic processes [
45].
3.2. Magnetic Resonance Imaging (MRI)
Magnetic Resonance Imaging (MRI) is a non-invasive imaging technique that detects signals from hydrogen protons in tissue water using strong magnetic and radiofrequency fields. Unlike imaging methods that rely on external light or sound reflection, MRI generates signals directly from tissues, making it particularly effective for visualizing soft tissue [
8].
MRI provides detailed 3D imaging with superior soft tissue contrast and avoids exposure to ionizing radiation [
46]. MRI encompasses a broad range of techniques, each offering unique insights into scaffold behavior and tissue regeneration. These include conventional MRI, primarily used for structural evaluation [
15], T1/T2 relaxation mapping to assess tissue composition and water content [
47], magnetic resonance spectroscopy (MRS) for metabolic profiling, and chemical exchange saturation transfer (CEST) for molecular-level imaging [
12]. Each technique serves a distinct role in characterizing scaffold–host interactions and monitoring scaffold performance over time.
While conventional MRI lacks the molecular sensitivity of advanced modalities like spectroscopy or CEST, it remains a fundamental technique for anatomical and gross functional assessment of scaffolds in soft TE. Its clinical availability and safety profile further support its use in translational research and human applications [
10].
MRI applications in TE span from in vitro scaffold characterization to longitudinal imaging in small and large animal models. It has been widely used in musculoskeletal TE, particularly for cartilage repair, intervertebral disc regeneration, and muscle tissue reconstruction [
28].
3.2.1. Conventional MRI
Conventional MRI, particularly using T1- and T2-weighted sequences, is commonly employed for non-invasive evaluation of tissue-engineered scaffolds, offering excellent soft tissue contrast, multi-planar imaging, and radiation-free [
46]. These sequences allow for longitudinal evaluation of scaffold–host interactions, morphological changes, and general tissue remodeling in vivo.
T1-weighted imaging provides high-resolution anatomical detail and is particularly suited for imaging fat-containing tissues. T2-weighted imaging, by contrast, is sensitive to water content and can reveal dynamic changes in ECM composition, edema, and inflammatory responses [
48]. For instance, decreasing T2 signal intensity over time has been associated with tissue maturation events such as proteoglycan accumulation in engineered cartilage and mineral deposition in bone [
49].
In engineered cartilage and bone constructs, reductions in T2-weight signal intensity have been associated with ECM production and mineral deposition, respectively [
49]. Xu et al. [
46] observed a reduction of over 50% in T2 values during osteogenic differentiation in MSC-embedded gelatin-based scaffolds, which correlated well with calcium accumulation and ALP expression.
These qualitative assessments can be performed without the use of contrast agents and are often applied in early-phase or preclinical studies. While they provide essential information on scaffold performance and general tissue response, T1- and T2-weighted images do not yield direct quantitative data. This limitation has led to the development of advanced quantitative MRI methods, which allow for a more detailed and objective analysis of tissue composition and structure.
3.2.2. Quantitative MRI
Quantitative MRI (qMRI), particularly T1 and T2 mapping, enhances traditional MRI techniques by generating voxel-wise numerical maps that reflect specific tissue characteristics such as water content, collagen fiber orientation, and Glycosaminoglycan (GAG) distribution [
50]. Unlike conventional weighted imaging, which offers qualitative contrast, qMRI provides reproducible, quantifiable biomarkers of tissue development within engineered constructs. Moreover, delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) improves the visualization and assessment of cartilage composition by increasing T1 and T2 relaxation rates following the injection of a paramagnetic contrast agent into the articular cartilage.
Despite its lower sensitivity to ECM composition, T1 mapping reliably reflects tissue hydration and remains stable across MRI field strengths, serving as an effective tool for monitoring water content changes during scaffold remodeling and cell-mediated ECM formation [
50].
T2 mapping is especially effective in assessing collagen architecture and hydration in engineered cartilage. Studies have shown that T2 values can distinguish between hyaline cartilage and fibrocartilage following repair and have been correlated with mechanical properties such as the Young’s modulus and dynamic modulus. Furthermore, depth-dependent T2 profiles reflect the zonal structure of native cartilage, aiding in the evaluation of laminar organization and matrix maturation [
50].
Despite being sensitive to motion artifacts and technical variability, qMRI is highly valuable when integrated with complementary techniques such as dGEMRIC. These multiparametric strategies allow for more comprehensive characterization of engineered tissues in both preclinical and clinical settings [
50].
In composite scaffolds containing polymer and ceramic or metallic phases, MRI can be significantly affected by magnetic susceptibility differences between the phases. These variations often lead to signal voids or distortions, especially in T2-weighted images, complicating the visualization of soft tissue formation and scaffold degradation. Additionally, the presence of calcium in hydrogels, as reported in previous studies, has been shown to shorten relaxation times, further affecting image contrast [
12]. Therefore, careful selection of scaffold materials and MRI parameters is essential to mitigate these artifacts and to achieve accurate assessment of composite scaffold performance.
3.2.3. Magnetic Resonance Spectroscopy (MRS)
MRS is a powerful modality that complements conventional MRI by providing biochemical and metabolic information from tissue-engineered scaffolds. Unlike traditional MRI, which offers structural and anatomical detail, MRS enables the detection and quantification of specific metabolites such as choline, creatine, lactate, and N-acetylaspartate, making it highly useful for evaluating cell activity, inflammation, and scaffold integration at a molecular level [
10,
50].
In TE applications, MRS has been employed to monitor metabolic shifts associated with tissue repair and regeneration. For example, alterations in choline and creatine levels within implanted scaffolds have been linked to changes in cellular density and membrane turnover, key indicators of scaffold bioactivity [
10]. Additionally,
1H MRS has been used to assess cartilage and meniscal constructs, enabling discrimination between tissue types and the progression of healing or degeneration in vivo [
12]. Despite its utility, MRS faces certain limitations, such as lower spatial resolution compared to conventional MRI and sensitivity to motion artifacts. However, its capacity to provide real-time, non-destructive metabolic insight makes it a valuable complement in scaffold evaluation, particularly when used alongside anatomical imaging methods [
12].
3.2.4. Magnetic Resonance Elastography (MRE)
MRE enables non-invasive mapping of soft tissue stiffness through visualization of propagating shear waves. This method is highly promising for TE, as scaffold stiffness often reflects maturation and functional integration [
51]. MRE involves coupling a mechanical actuator to the construct to generate shear waves, which are captured using motion-encoded MR sequences. The resulting displacement data are processed via inversion algorithms to produce elastograms—quantitative stiffness maps [
51,
52,
53].
In engineered cartilage models, MRE has enabled in vivo longitudinal tracking of scaffold stiffness and its correlation with matrix deposition. For example, Khalilzad-Sharghi et al. [
53] monitored human MSC-seeded cartilage constructs in mice over 8 weeks, observing increased stiffness in silk scaffolds (~7.6 to ~17.2 kPa) alongside upregulation of cartilage genes (aggrecan, type II collagen, cartilage oligomeric matrix protein) and decreased T
2 values, indicating matrix maturation. The study also reported scaffold-specific differences, with gelatin scaffolds showing the greatest stiffness gains, aligned with histological and gene expression data, supporting MRE as a biomarker for chondrogenic differentiation [
53].
Microscopic MRE (μMRE) has been demonstrated using high-field MRI (9.4–11.7 T), suitable for small samples and high-resolution needs in TE. Proper actuator setup and parameter optimization are essential for accurate wave propagation, especially in heterogeneous constructs [
53]. Othman et al. [
51] showed that μMRE could detect mechanical differences missed by conventional MRI, distinguishing soft adipogenic constructs (~1–2 kPa) from stiffer osteogenic ones (~20–25 kPa), highlighting its versatility in scaffold-cell systems. MRE offers early, spatially resolved, and quantitative feedback on construct mechanics. Its application may reduce animal use and facilitate clinical translation by non-invasively identifying functionally viable scaffolds.
3.2.5. Chemical Exchange Saturation Transfer (CEST)
As a sensitive molecular imaging modality, CEST MRI allows for the non-invasive detection of target biomolecules in scaffolds without the use of external contrast agents. Unlike T1- or T2-weighted imaging, CEST leverages the exchange of saturable protons in molecules, such as hydroxyl, amide, or amine groups, with bulk water protons to generate signal contrast. These chemical exchanges are detected as changes in the water signal intensity, allowing for indirect, label-free imaging of tissue microenvironments [
53].
CEST imaging has proven particularly useful in evaluating biodegradable scaffolds, especially hydrogel-based systems. Because many hydrogels inherently contain exchangeable protons or can be engineered to include CEST-active compounds, they can be monitored in vivo over time. For example, studies have demonstrated the use of CEST to non-invasively assess the degradation of Hyaluronic Acid (HA)-based hydrogels implanted in mice, showing correlation with histological findings and scaffold clearance [
54].
Compared to traditional contrast-enhanced MRI, CEST avoids toxicity concerns linked to gadolinium or iron-based agents. It provides highly specific image contrast tied to functional groups in scaffold materials, making it suitable for longitudinal tracking of scaffold fate, integration, and microenvironmental changes such as pH variation or enzyme-mediated degradation [
55].
Despite these advantages, several technical challenges remain, including signal interpretation complexity, sensitivity to field inhomogeneities, and limited clinical implementation. Nonetheless, ongoing development in acquisition protocols and validation in preclinical models indicates promising future applications of CEST in both scaffold monitoring and targeted drug delivery [
56].
3.2.6. Contrast-Enhanced MRI (SPIONs, Gadolinium)
Contrast-enhanced MRI greatly improves non-invasive scaffold evaluation in TE. Two widely used agents are Superparamagnetic Iron Oxide Nanoparticles (SPIONs) and gadolinium-based compounds, both enhancing visualization of scaffold architecture and cell dynamics in vivo [
57]. SPIONs are commonly used to label cells, such as astrocytes, chondrocytes, and MSCs, for MRI tracking within scaffolds [
58]. For example, SPION-labeled chondrocytes embedded in polyvinylidene fluoride (PVDF) hydrogels retained MRI signal for up to 30 days post-implantation, enabling longitudinal monitoring [
10].
Gadolinium-Based Contrast Agents (GBCAs) have also proven valuable for tracking endothelial progenitor cells and monitoring their homing to ischemic tissues [
59]. Contrast agents can also be integrated directly into scaffolds. Calcium phosphate cements, for instance, have been functionalized with gadopentetate dimeglumine and SPIONs to enhance visibility in mineralized, hydrogen-poor regions [
10]. Similarly, gadolinium-functionalized single-walled carbon nanotubes have provided strong, sustained contrast after incorporation into scaffolds [
58].
These agents also applied for scaffold degradation tracking and image-guided interventions. SPIONs embedded in hydrogel embolic systems enabled real-time monitoring of scaffold delivery and breakdown, while gadolinium-containing hydrogels supported MRI-guided embolization procedures [
10]. Despite these innovations, concerns remain regarding gadolinium toxicity and long-term retention. Consequently, research has focused on developing biodegradable, biocompatible MRI-visible nanostructures that combine safety with high imaging performance [
58].
3.3. X-Ray-Based Imaging Techniques
X-ray-based imaging techniques are widely applied in the evaluation of tissue-engineered scaffolds due to their non-invasive nature and ability to capture high-resolution structural information. These modalities rely on differential X-ray attenuation across tissues and biomaterials, making them particularly valuable for assessing mineralized constructs such as bone scaffolds [
60,
61]. Depending on the resolution and contrast mechanisms required, different X-ray-based techniques, including conventional computed tomography (CT), micro-CT (μ-CT), and phase-contrast CT (PC-CT), can be applied [
62,
63]. While each approach offers distinct advantages, their effectiveness varies depending on scaffold composition, imaging depth, and the need for contrast agents.
3.3.1. CT
CT is a well-established non-invasive imaging technique widely used to assess scaffold morphology, degradation, and integration, particularly in preclinical bone regeneration studies. Conventional CT provides detailed cross-sectional and volumetric visualization of mineralized tissue, allowing the evaluation of scaffold–host interface, overall shape retention, and structural remodeling over time [
64,
65].
One of CT’s primary applications in scaffold research is the assessment of mineral deposition and bone regeneration. High-density scaffold materials, such as calcium phosphate or HA-based composites, are easily visualized due to their high X-ray attenuation, which makes CT particularly useful for evaluating mineralized scaffold performance in orthotopic sites [
62,
63].
In addition to structural analysis, CT has also been used in angiogenesis studies through contrast-enhanced imaging, particularly in combination with agents like Microfil or barium sulfate. These agents permit 3D visualization of vascular networks inside and around the scaffold, which is critical for evaluating the success of vascularized grafts [
64].
However, CT has important limitations when applied to polymeric or soft tissue-based scaffolds due to their inherently low radiodensity. In such cases, the addition of contrast agents or the use of phase-contrast CT may be necessary to improve image clarity [
66,
67]. Moreover, CT resolution is often insufficient for fine tissue differentiation without complementary modalities such as histology or MRI, especially when newly formed tissue and scaffold materials have overlapping attenuation values [
67]. These challenges are further complicated when dealing with composite scaffolds composed of both polymer and ceramic or metallic phases. In composite scaffolds containing ceramic or metallic phases, X-ray CT imaging is challenged by differential X-ray attenuation, which can lead to significant beam hardening artifacts. These artifacts obscure the polymer phase and make it difficult to distinguish between polymer degradation and ceramic resorption, especially over prolonged implantation periods. Using phase contrast CT or appropriate staining agents can partially overcome these issues, but may introduce other limitations such as artifact generation or sample alteration [
18].
Despite these challenges, CT remains a powerful tool for scaffold evaluation in mineralized environments due to its speed, wide availability, and compatibility. When appropriately combined with contrast strategies or complementary modalities, it offers meaningful insights into scaffold morphology, bone integration, and vascularization [
64,
65,
66].
3.3.2. µ-CT
µ-CT has been especially useful for analyzing scaffold fidelity post-fabrication, comparing actual printed geometries with CAD designs. These comparisons help identify deviations such as filament fusion or unintended porosity, which directly impact mechanical integrity and biological performance [
61]. High-resolution scans also support the creation of accurate finite element (FE) models for simulating scaffold behavior under physiological loading conditions [
61].
Phase-contrast µ-CT has emerged as a powerful solution for visualizing soft tissue and low-density polymers, enabling imaging of hydrated scaffolds and cell-laden constructs with minimal staining [
68]. This technique enhances contrast by detecting X-ray phase shifts rather than absorption, making it particularly valuable in hydrogel-based or soft-tissue scaffolds [
61].
3.3.3. Phase-Contrast CT (PC-CT)
PC-CT is an advanced X-ray imaging technique that enhances soft tissue contrast beyond what conventional CT can offer [
67]. By exploiting phase shifts in X-rays as they pass through biological tissues, PC-CT enables the generation of high-resolution, three-dimensional images of scaffold structures. This is particularly valuable for delicate or low-attenuation constructs such as tissue-engineered esophageal scaffolds [
68,
69].
Recent studies have demonstrated that this technique can be used to monitor decellularized and recellularized scaffolds. In these studies, PC-CT was applied to examine tissue layer organization, quantify scaffold density, and assess cellular repopulation in implanted constructs [
70,
71]. Its ability to perform repeated imaging without damaging the sample makes it a promising tool for longitudinal assessment and quality control of soft scaffolds in preclinical models [
71].
3.4. Ultrasound-Based Techniques
3.4.1. Conventional Ultrasound (B-Mode, Doppler)
Ultrasound enables volumetric, real-time, and longitudinal monitoring without damaging constructs [
14,
72]. B-mode ultrasound is the most widely used technique, offering grayscale visualization of engineered tissues. It has been applied to assess ECM deposition, scaffold structure, and cell-mediated remodeling [
14,
72,
73]. For instance, image attenuation and grayscale intensity changes have correlated with ECM content in fibrin and polymeric scaffolds seeded with fibroblasts or stem cells. However, B-mode imaging is sensitive to system settings and operator variability and may not reliably capture compositional changes [
14].
To overcome these limitations, Quantitative Ultrasound (QUS) techniques such as the integrated backscatter coefficient (IBC) and spectral analysis analyze radiofrequency (RF) data to provide system-independent metrics reflecting microstructure, scatterer size, and density [
14,
72]. IBC has shown strong linear correlations with cell density and collagen content in engineered hydrogels, while spectral parameters (e.g., mid-band fit, spectral slope) have been used to differentiate mineral types and map mineralization in osteogenic constructs [
14,
74].
Doppler ultrasound enables non-invasive evaluation of blood flow in tissue-engineered vascular grafts, shedding light on perfusion dynamics and vessel integrity. While sensitivity for microvasculature is limited, it can be enhanced using contrast agents like microbubbles [
9,
14]. Recently, nanobubbles have emerged as a promising platform for gene delivery [
73], also demonstrating contrast-enhancing properties [
75]. This dual functionality makes them suitable for both therapeutic and diagnostic applications, classifying them as a theranostic agent. In a notable study, Gessner et al. employed acoustic angiography and contrast-enhanced ultrasound to generate high-resolution maps of vascular networks in decellularized liver scaffolds, offering a valuable pre-implantation evaluation tool [
76].
Overall, ultrasound—especially when combined with QUS and Doppler techniques—is a versatile, scalable, and clinically relevant method for assessing scaffold structure, function, and integration. Remaining challenges include limited depth at higher frequencies, inter-platform variability, and the need for multimodal approaches for comprehensive evaluation.
3.4.2. Ultrasound Elastography (Strain or Shear Wave)
Ultrasound elastography has emerged as a valuable method for evaluating the mechanical properties of tissue-engineered scaffolds. Two primary forms—strain imaging and shear wave elastography—have been applied to map scaffold stiffness, elasticity distribution, and mechanical changes during degradation under both in vitro and in vivo conditions [
77].
In one study, ultrasonic shear wave imaging (USWI) was used by Yu et al. [
78] to assess Poly (Carbonate Urethane) Urea (PCUU) scaffolds embedded in a Poly (Vinyl Alcohol) (PVA) phantom. Shear wave propagation was first modeled numerically, followed by in vitro experiments. Elasticity maps generated using the Helmholtz inversion algorithm successfully identified scaffold boundaries and provided spatially resolved stiffness measurements, demonstrating the feasibility of USWI for non-destructive, real-time imaging of elastomeric constructs.
Acoustic radiation force-based methods, including supersonic shear wave imaging, have also been successfully employed to estimate the shear modulus of engineered tissues. These techniques use focused ultrasound pulses to generate localized displacements and monitor shear wave propagation through the scaffold. This allows for rapid, localized, and contact-free stiffness mapping, which is particularly suitable for sterile environments in TE research [
14].
Strain imaging elastography has also been extended to stiffer scaffolds. Zhou et al. [
20] applied it to Polydimethylsiloxane (PDMS) constructs with elastic moduli ranging from 47 kPa to 4 MPa, achieving sensitivity to stiffness differences as small as 157 kPa. This demonstrates its applicability to harder materials, including those used in bone and load-bearing TE.
Despite its utility, ultrasound elastography faces limitations. Strain imaging yields relative rather than absolute stiffness values and is influenced by boundary conditions and applied stress. Additionally, scaffold heterogeneity and complex geometries may interfere with shear wave propagation and affect the accuracy of modulus reconstruction.
Recognizing the challenges caused by scaffold heterogeneity, we believe that future research could benefit from using standardized phantoms with controlled mechanical gradients, as well as adding elastography data into computational models like inverse finite element methods. These approaches may help enhance the spatial resolution and reliability of stiffness mapping in complex, clinically relevant scaffolds.
To address these limitations, several advanced ultrasound elastography techniques have been developed, significantly enhancing their relevance and utility for mechanical characterization of engineered scaffolds in tissue engineering applications.
Beyond conventional strain imaging, acoustic radiation force-based methods—such as acoustic radiation force impulse (ARFI) imaging, supersonic shear wave imaging, and spatially modulated ultrasound radiation force (SMURF), enable non-invasive, real-time quantification of scaffold stiffness and viscoelastic properties with spatial resolution suitable for both in vitro and in vivo studies [
14,
72].
For instance, dual-mode ultrasound elastography (DUE) has been applied to 3D hydrogels to map localized strain and derive viscoelastic parameters like elasticity and viscosity, offering insights into cell-matrix mechanobiology [
14]. Such techniques have proven effective even in stiffer constructs like polyglycolic acid (PGA) scaffolds with elastic moduli in the MPa range [
72]. Moreover, composite scaffolds comprising polymer-ceramic phases introduce acoustic impedance mismatches that can complicate elastography assessments. These mismatches lead to heterogeneous strain distributions and complex shear wave behavior, potentially reducing measurement accuracy in multiphasic materials. DUE and ARFI imaging help overcome these challenges by decoupling deformation excitation and imaging acquisition, improving the reliability of stiffness mapping in heterogeneous scaffolds.
Additionally, viscoelastic modeling approaches, such as the application of Burger’s model, have been utilized to interpret elastography data from scaffolds exhibiting both elastic and viscous behaviors. This modeling is particularly valuable for degradable or bioactive composites where mechanical properties evolve due to tissue ingrowth and scaffold resorption [
14].
Recognizing the challenges caused by scaffold heterogeneity, future research could benefit from using standardized phantoms with controlled mechanical gradients and integrating elastography data into computational models like inverse finite element analysis. These strategies, combined with multimodal imaging approaches, may help enhance the spatial resolution, quantitative accuracy, and clinical translatability of scaffold mechanical assessments [
14,
72].
Furthermore, a recent review by Quijano et al. [
79] specifically emphasized the clinical potential of ultrasound elastography in the non-invasive assessment of biomaterials and engineered tissues. This work highlighted the applicability of both strain imaging and shear wave elastography for quantifying scaffold stiffness, viscoelasticity, and mechanical integrity in vivo. Additionally, the review compared ultrasound elastography with other advanced modalities such as MR elastography and optical coherence elastography, outlining their respective advantages and challenges for clinical translation. These insights underscore the role of ultrasound elastography not only as a research tool but also as a promising modality for longitudinal, real-time mechanical evaluation of scaffolds in clinical regenerative medicine applications [
79].
3.4.3. High-Frequency Ultrasound Microscopy
High-frequency ultrasound microscopy (HFUM), typically in the 30–55 MHz range, offers non-destructive, micrometer-scale imaging of scaffold microstructure. It enables volumetric and quantitative assessment of scaffold composition, fiber architecture, and cell infiltration—critical for evaluating remodeling and integration [
80,
81].
Mercado et al. [
80] utilized the Integrated Backscatter Coefficient (IBC) with a 38 MHz transducer to detect spatial heterogeneity in collagen fiber density and size within 3D hydrogel constructs. IBC-based parametric imaging revealed regional heterogeneity and correlated with cell accumulation, making it a powerful tool for monitoring scaffold architecture and cell-matrix dynamics. Bushnell et al. [
81] employed spectral ultrasound imaging with a 55 MHz transducer to track micrometastatic recruitment in scaffolds implanted in murine cancer models. By analyzing spectral parameters like Mid-Band Fit (MBF), slope, average scatterer diameter, and acoustic concentration, spectral ultrasound imaging distinguished tumor-bearing from healthy scaffolds with high sensitivity, capturing microenvironmental changes beyond B-mode imaging.
Importantly, HFUM combined with spectral ultrasound imaging (SUSI) has demonstrated efficacy in evaluating composite scaffolds incorporating mineral phases such as hydroxyapatite [
14]. Gudur et al. [
82] applied SUSI to collagen hydrogels doped with hydroxyapatite, enabling quantitative assessment of mineral content, particle size, and spatial distribution via spectral parameters like MBF and spectral slope. This approach effectively captured compositional heterogeneity, offering insights into the microstructural evolution of polymer-ceramic composites during tissue regeneration. Such capabilities underscore the potential of HFUM and SUSI for non-invasive, real-time characterization of composite scaffolds, although acoustic impedance mismatches in mineralized matrices may still pose challenges.
These findings support HFUM’s utility for real-time, non-invasive, and quantitative scaffold evaluation. However, challenges include limited penetration depth, acoustic mismatch sensitivity in mineralized scaffolds, and the need for standardized acquisition and interpretation protocols for clinical translation.
3.5. Multimodal Imaging
Multimodal imaging has emerged as a transformative strategy for evaluating tissue-engineered constructs by integrating complementary strengths of individual imaging modalities. Since no single technique can fully capture the complex structural, functional, and molecular dynamics of scaffold-based regeneration, combining approaches—such as CT/SPECT, MRI/CT, MRI/fluorescence, ultrasound/PA, and PA/OCT—offers synergistic advantages [
10,
83]. These hybrid systems have been explored in both preclinical and clinical settings to enhance scaffold visualization, monitor degradation, track cell behavior, and assess host integration. The advancement of multifunctional contrast agents—such as gadolinium-conjugated gold nanoparticles (MRI/X-ray), and fluorescently tagged iron oxide nanoparticles (MRI/optical)—has further enabled real-time, non-invasive, and multi-parameter imaging of regenerative constructs [
10].
Another promising hybrid technique is SPECT/CT, which merges high-resolution anatomical CT with molecular-level SPECT imaging [
84,
85]. Kempen et al. [
86] demonstrated the utility of SPECT/CT by tracking in vivo release of radiolabeled BMP-2 from PLGA scaffolds. SPECT confirmed sustained release over eight weeks, while μ-CT simultaneously validated bone formation, enabling non-destructive, dual-parameter evaluation within a single animal model.
While SPECT effectively detects bone remodeling activity, its spatial resolution is inferior to that of MRI and CT. Additionally, it lacks the specificity to distinguish scaffold-derived tissue from host bone without supplementary histological analysis. Additionally, the presence of radiopaque materials like calcium phosphate in scaffolds can obscure anatomical landmarks in CT, complicating image interpretation [
85].
Altogether, multimodal imaging offers a comprehensive toolkit for scaffold evaluation, integrating spatial, temporal, mechanical, and molecular data. As technology and contrast agent development continue to advance, multimodal systems will likely play an increasingly critical role in reducing animal use, accelerating clinical translation, and enabling more precise engineering of functional tissue constructs.
4. Challenges and Future Directions in Scaffold Imaging
The evaluation of tissue-engineered scaffolds remains a critical step in advancing regenerative medicine, requiring accurate, non-invasive, and longitudinal assessment methods [
86]. This review has highlighted that while numerous imaging modalities have shown promise, each technique presents unique strengths and limitations that must be considered when selecting the appropriate strategy.
One of the primary technical challenges is the trade-off between imaging depth and spatial resolution, particularly in optical and PAI. For example, PAI offers high-resolution vascular imaging but suffers from shallow penetration depths (~3 mm in high-resolution configurations) [
33]. This limitation restricts its applicability for monitoring scaffold integration in deep-seated tissues such as bone or large muscle masses, which are often the targets of regenerative therapies [
33]. Without reliable deep tissue imaging, clinicians cannot fully assess scaffold vascularization or tissue maturation in vivo, creating a translational gap.
MRI offers detailed imaging with strong soft tissue contrast, without exposing the subject to ionizing radiation, making it particularly advantageous for longitudinal studies of scaffold integration in musculoskeletal and soft TE [
9,
25]. However, its limited sensitivity to early metabolic changes and structural detail within hard tissues necessitates integration with complementary techniques such as MRS [
10], CEST imaging [
56], or contrast-enhanced MRI using SPIONs or gadolinium-based agents [
10,
57]. MRI, while providing superior soft tissue contrast, is susceptible to magnetic susceptibility artifacts when applied to composite scaffolds containing metallic or ceramic phases. These artifacts can obscure critical regions of scaffold-tissue integration or mask subtle changes such as polymer degradation versus ceramic resorption [
15]. This constraint not only complicates scaffold monitoring but also hinders the design of composites optimized for both biological performance and imaging compatibility.
CT and µ-CT remain essential tools for analyzing mineralized scaffold structure and bone formation due to their high spatial resolution and compatibility with a variety of contrast agents [
69]. Nevertheless, their inability to differentiate soft tissues or low-density materials without specialized agents limits their use in non-mineralized environments [
66,
67]. Emerging solutions, such as PC-CT, have improved visualization of soft tissue scaffolds, but their clinical accessibility is still limited [
71]. CT and micro-CT, despite their high resolution, are limited by their poor soft tissue contrast. When applied to polymer-ceramic composite scaffolds, differential X-ray attenuation can induce beam hardening artifacts, complicating the interpretation of scaffold degradation dynamics. These artifacts obscure the polymer matrix, making it difficult to distinguish between polymer degradation and ceramic resorption—an essential distinction for evaluating composite scaffold performance in vivo [
18].
Ultrasound-based techniques, particularly QUS and Doppler imaging, offer real-time feedback and portability features that make them attractive for both research and bedside applications [
72]. Their efficacy is highly dependent on operator expertise, and they face challenges with depth penetration and tissue-specific resolution, especially in mineralized scaffolds [
14].
Hybrid modalities such as PAI have bridged some of these limitations by combining optical contrast with ultrasound penetration, enabling label-free visualization of vascularization and scaffold degradation [
87]. However, PAI still suffers from shallow imaging depth in high-resolution configurations and may require exogenous contrast agents for non-chromophoric scaffolds [
38].
Optical imaging modalities, including NIR-FL and OCT, have demonstrated exceptional capabilities in visualizing cellular dynamics and scaffold microarchitecture [
88]. Innovations such as activatable fluorophores and swept-source OCT systems have enhanced their specificity and depth resolution, though optical scattering and limited penetration continue to constrain in vivo applications [
30].
BLI represents a powerful tool for tracking cellular activities within scaffolds non-destructively and over extended periods [
42,
89]. Its high sensitivity and low background noise are valuable in preclinical models, yet BLI is limited by the need for genetic labeling and challenges in signal attenuation within dense or mineralized tissues [
90].
Multimodal imaging strategies, such as MRI/optical, or ultrasound/PA, have emerged to address these limitations by combining the strengths of different modalities. For instance, MRI offers anatomical detail, while optical methods provide sensitive molecular tracking. Nam et al. [
10] highlighted how dual-labeled nanoparticles, like SPIONs tagged with fluorescent dyes, enable concurrent MRI and optical imaging, enhancing scaffold visualization in both deep tissue and cellular contexts. Nevertheless, several challenges hinder the clinical translation of these technologies. One is the need for biocompatible, target-specific contrast agents that provide a clear signal without long-term toxicity. Another is imaging standardization, as differences in imaging protocols and interpretation can reduce reproducibility across studies. Techniques like ultrasound elastography and MR elastography are promising for mechanical property assessment but require refinement in spatial resolution and validation in clinical trials.
Future research should focus on optimizing multimodal imaging systems, improving biocompatibility of contrast agents, and integrating AI-based analytics to enhance data interpretation and predictive modeling of scaffold behavior. The integration of imaging with computational simulations and “digital twin” models also holds great potential for guiding scaffold design and personalized therapy.
While current non-invasive imaging technologies provide powerful insights into scaffold structure and function, there remains a pressing need for innovation in depth penetration, multi-parametric analysis, and clinical translation [
91]. By advancing these areas, medical imaging can become an even more integral part of the regenerative medicine workflow, enhancing both the scientific understanding and therapeutic success of tissue-engineered constructs.
Additionally, it is essential to align the choice of imaging modalities with the specific scaffold composition and the target tissue. For instance, CT and micro-CT are optimal for mineralized scaffolds such as bone constructs, providing high-resolution structural information [
63,
66]. MRI is particularly advantageous for soft tissue-engineered scaffolds, including cartilage and muscle, due to its excellent soft tissue contrast without ionizing radiation [
54,
56]. Optical imaging techniques, including NIR-FL and OCT, are well-suited for superficial tissues and small animal models due to their high resolution but limited penetration depth [
25,
29]. Ultrasound-based modalities, like elastography, offer real-time mechanical property assessment, beneficial for load-bearing scaffolds [
84]. PAI effectively monitors vascularization and oxygenation in moderately deep tissues [
36,
87]. Understanding these specific features facilitates the tailored application of imaging strategies according to the scaffold type and clinical requirements.
5. Conclusions
Non-invasive medical imaging has emerged as a cornerstone in the evaluation of tissue-engineered scaffolds, offering essential tools for monitoring scaffold structure, integration, degradation, and regenerative performance over time. From high-resolution modalities like µ-CT and MRI to functionally informative techniques such as PAI, bioluminescence, and NIR-FL, each method contributes uniquely to scaffold characterization in both preclinical and translational research.
Despite these advances, no single imaging modality can yet provide a complete picture across all scaffold types and clinical scenarios. Technical limitations, including depth penetration, resolution, contrast specificity, and biocompatibility of imaging agents, continue to challenge widespread adoption. Moreover, the integration of these technologies into clinical workflows requires further validation, standardization, and regulatory alignment. In particular, despite extensive progress in preclinical studies, the clinical translation of NIR-FL and PAI for scaffold imaging remains limited. Future research should prioritize scaffold-specific human studies and incorporate these modalities into clinical trial designs for regenerative medicine.
Looking ahead, the development of multimodal imaging platforms, tissue-specific contrast agents, and machine learning–driven image analysis holds great promise. Such innovations will not only improve the precision of scaffold evaluation but also accelerate the translation of engineered tissues into clinical therapies. Ultimately, the synergy between imaging science and TE will be key to realizing the full potential of regenerative medicine. In addition to the biomedical imaging applications discussed for scaffolds, it is worthwhile to explore and integrate imaging techniques that enable the detection of bacterial infections, immune responses, or inflammation. This could involve combining existing imaging methods or engineering scaffold composites with materials that support the visualization of pathogen-specific or immune-related signals.