1. Introduction
Heavy metal pollution, specifically by lead (Pb
2+), cadmium (Cd
2+), and copper (Cu
2+), in water bodies is a serious environmental issue that globally affects both human health and ecological balance [
1,
2]. This type of contamination mainly originates from intensive industrial activities such as mining and metallurgical processes, as well as from inadequately treated urban and industrial waste [
3]. Due to their environmental persistence, bioaccumulation potential, and high toxicity, these metals pose a significant risk to public health, affecting vital organs and often causing irreversible damage [
4,
5]. Therefore, the implementation of efficient, sustainable, and economically viable technologies for the removal of these pollutants is an urgent necessity [
6]. In this context, natural materials rich in calcium carbonate (CaCO
3), such as mollusk shells, have emerged as promising alternatives for the removal of heavy metals from aqueous solutions [
7,
8]. Several studies have reported successful results using these materials, attributed to their unique physicochemical properties, including high porosity, large surface area, and abundance of active sites capable of interacting with metallic contaminants [
9,
10,
11]. In particular, recent research has focused on the development of biocomposites through the incorporation of oyster shell powder (CaCO
3) into polymeric matrices, with polyvinyl alcohol (PVA) being especially prominent (
Figure 1) [
12,
13].
This biocomposite has demonstrated remarkable efficiency, achieving removal rates exceeding 96% in experimental trials conducted under controlled conditions, particularly for Pb
2+ and Cd
2+ ions [
14,
15]. The high efficiency is primarily attributed to the synergistic properties of CaCO
3, with its specific active sites, and the structural support provided by the PVA matrix, which facilitates more effective interaction with the metal ions [
16,
17,
18,
19].
Nevertheless, despite these experimental advances, significant knowledge gaps remain regarding the specific molecular mechanisms involved in the adsorption processes [
20]. Therefore, complementary studies are required to accurately understand the chemical and physical interactions at the molecular level between biocomposites and heavy metals. In this context, the application of computational techniques, particularly those based on Density Functional Theory (DFT), has become a key tool [
21]. DFT methodology enables detailed simulations to evaluate critical aspects such as electronic affinity, electron distribution through frontier molecular orbitals (HOMO-LUMO), and the identification of reactive sites via advanced analyses such as Natural Bond Orbital (NBO) studies and electrostatic potential maps.
The main objective of the present work is to apply DFT-based computational methods to thoroughly explore the molecular interactions between the PVA/CaCO3 biocomposite and the heavy metals Pb2+, Cd2+, and Cu2+. Specific goals include: performing precise structural optimization of molecular models; calculating key electronic descriptors to gain a deeper understanding of the composite’s adsorptive properties; and conducting spectroscopic analyses through FT-IR simulations for direct comparison with existing experimental results. Additionally, a comprehensive review of recent literature will be conducted to support and justify the computational methodology employed and to place this study within the broader context of environmental chemistry and materials science.
2. Materials and Methods
The PVA/CaCO
3 biocomposite derived from oyster shell was selected as the model adsorbent because the carbonated surface of CaCO
3 exhibits a high affinity for divalent ions, facilitating the exchange of Ca
2+ with Pb
2+, Cd
2+, or Cu
2+. Meanwhile, the hydrophilic PVA matrix prevents powder agglomeration and promotes the diffusion of contaminants toward active sites. Gaussian 16 was employed under the framework of DFT [
22], comparing the levels B3LYP/6-311++G(d,p), M06-2X/6-311++G(d,p), and M05-2X/6-311+G(d,p). Benchmark tests indicated that B3LYP/6-311++G(d,p) offered the best balance between accuracy and computational cost, and was thus selected as the reference level.
Complexes of the form [PVA–CaCO
3]···M
2+(H
2O)
5 (M = Pb, Cd, Cu) were constructed to represent the ester and hydroxyl groups of the polymer, the surface carbonates, and the pentahydrated metal ions. After geometry optimization and confirmation of the absence of imaginary frequencies, HOMO–LUMO energies were calculated to assess electronic reactivity [
23,
24], while the total dipole moment (TDM) was used to estimate overall polarity [
25]. Molecular electrostatic potential maps (MEPM) were generated to locate nucleophilic and electrophilic regions [
26]. Additionally, adsorption energies and thermodynamic parameters such as Gibbs free energy (G), enthalpy (H), and entropy (S) were computed to quantify the stability and spontaneity of ionic capture.
Fukui functions, frontier molecular orbital (FMO) analysis, and NBO analysis were applied to identify preferential reactive sites [
27,
28]. Furthermore, global reactivity descriptors including chemical potential (μ), ionization potential (I), electron affinity (A), electronegativity (χ), chemical hardness (η), and electrophilicity index (ω) were determined to provide an integrated perspective of the system’s propensity to donate or accept electronic charge. These descriptors were calculated using the following equations [
29].
In addition, a non-covalent interaction (NCI) analysis based on reduced density gradient (RDG) was performed using the Multiwfn 3.8 program [
30]. Finally, an FT-IR study was carried out to verify the interactions predicted by the computational model.
3. Results and Discussion
3.1. Choosing the Level of Computational Theory
In order to determine the most suitable level of theory for describing the interaction between the PVA/CaCO
3 biocomposite and Pb
2+, Cd
2+, and Cu
2+ ions, the methods B3LYP/6-311++G(d,p), M05-2X/6-311+G(d,p), and M06-2X/6-311++G(d,p) were compared through DFT calculations in Gaussian 16, evaluating key thermodynamic parameters: total system energy (E
system), enthalpy (H), Gibbs free energy (G), entropy (S), and total dipole moment (TDM). The results (
Table 1) show that for isolated PVA, CaCO
3, and the PVA/CaCO
3 complex, the B3LYP/6-311++G(d,p) level consistently provides the most negative values for E
system and G (−348.148 Hartree and −348.179 Hartree for PVA; −264.134 Hartree and −264.164 Hartree for CaCO
3; −875.591 Hartree and −875.654 Hartree for PVA/CaCO
3), indicating additional stabilization of between 20 and 200 kcal·mol
−1 compared to M05-2X and M06-2X. Entropy values varied by less than 10 cal·mol
−1·K
−1 across methods an insufficient difference to alter the relative stabilities while the dipole moments remained virtually unchanged (3.57–3.77 Debye for PVA, 11.30–11.31 Debye for CaCO
3, and 7.26–7.68 Debye for the complex), indicating that none of the functionals significantly distorts the charge distribution.
Consequently, B3LYP/6-311++G(d,p) offers the best compromise between energetic accuracy and computational cost and is therefore adopted as the reference level for modeling adsorbent–adsorbate interactions and for the detailed calculation of reactivity descriptors, Fukui functions, and global descriptors such as chemical potential (μ), ionization potential (I), electron affinity (A), electronegativity (χ), chemical hardness (η), and electrophilicity index (ω), which support, from a physicochemical perspective, the biocomposite’s capability to immobilize heavy metals in aqueous environments.
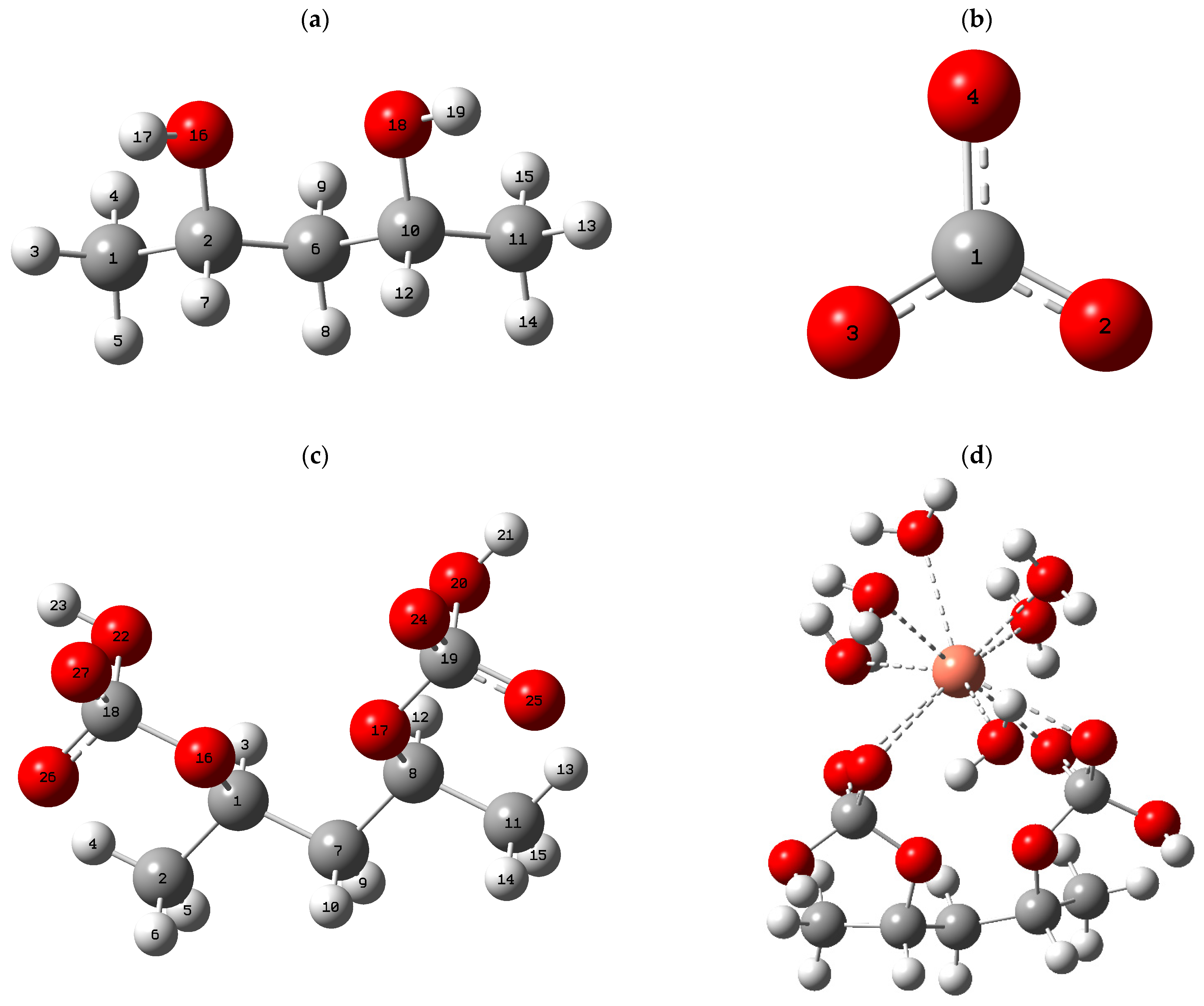
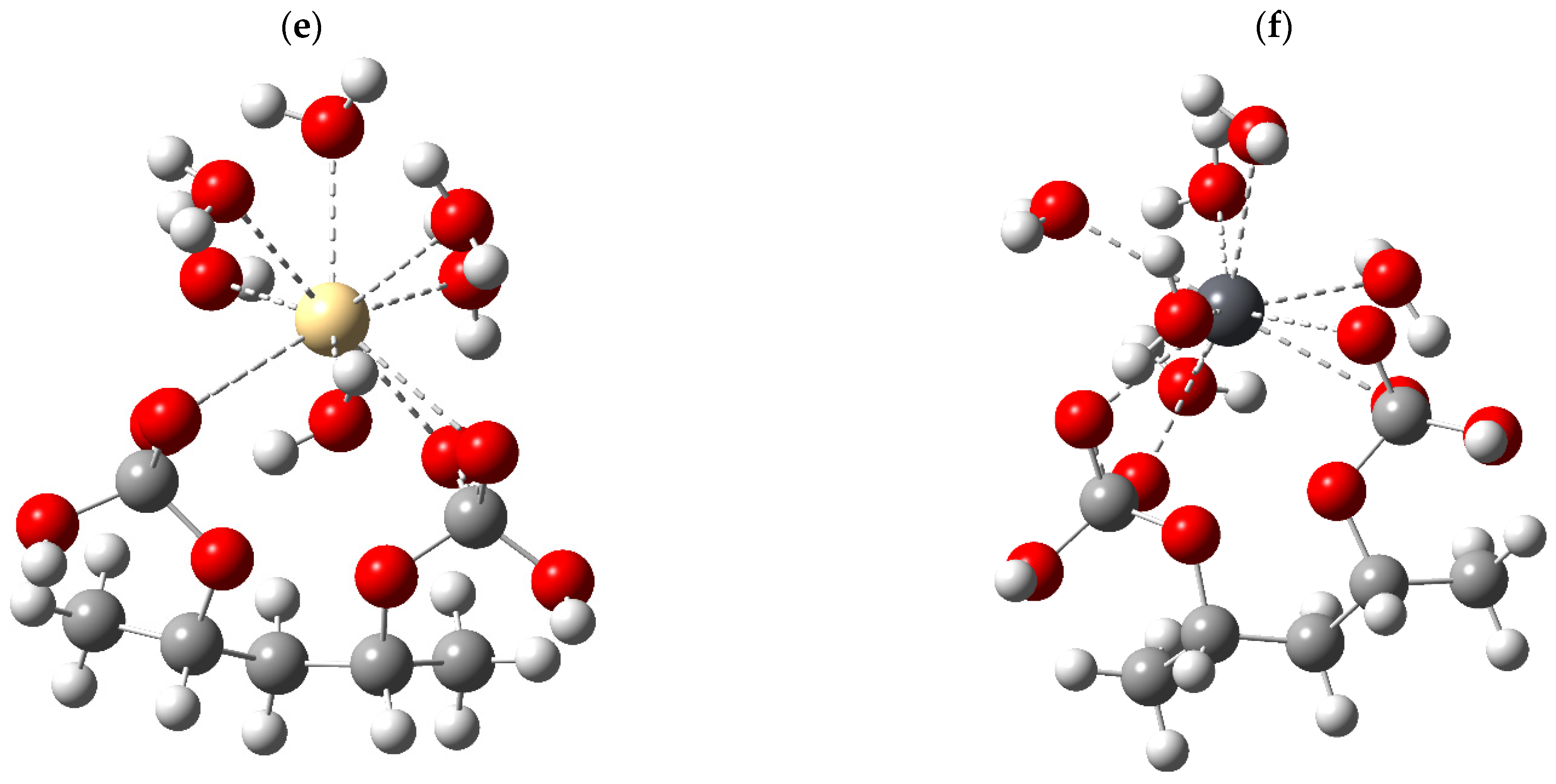
3.2. Optimization of the Structural Model
In order to assess, from an atomistic perspective, the suitability of the PVA/CaCO
3 biocomposite for heavy metal removal, representative structural models were constructed, comprising a dimer of polyvinyl alcohol (PVA) and the carbonated domains of CaCO
3, configured as an elementary interaction cell. To mimic aqueous conditions, each Pb
2+, Cd
2+, and Cu
2+ cation was represented as a pentahydrated complex, [M(H
2O)
5]
2+, whose solvent coordination exhibits labile character. After geometry optimization, the associations between the biocomposite and the hydrated metal complexes were considered adsorption states, acknowledging that the formation of metal–adsorbent bonds may induce the partial release of water molecules from the solvation shell [
31,
32]. The optimized geometries for each system are shown in
Figure 2.
Additionally, relevant electronic properties such as the molecular electrostatic potential (MPEM) and the HOMO-LUMO energy gap were determined according to the same established theoretical level. According to previous research, physical variables such as the total dipole moment, the HOMO-LUMO gap energy, and the MPEM represent essential indicators to evaluate the reactivity and adsorption capacity of the compounds under study.
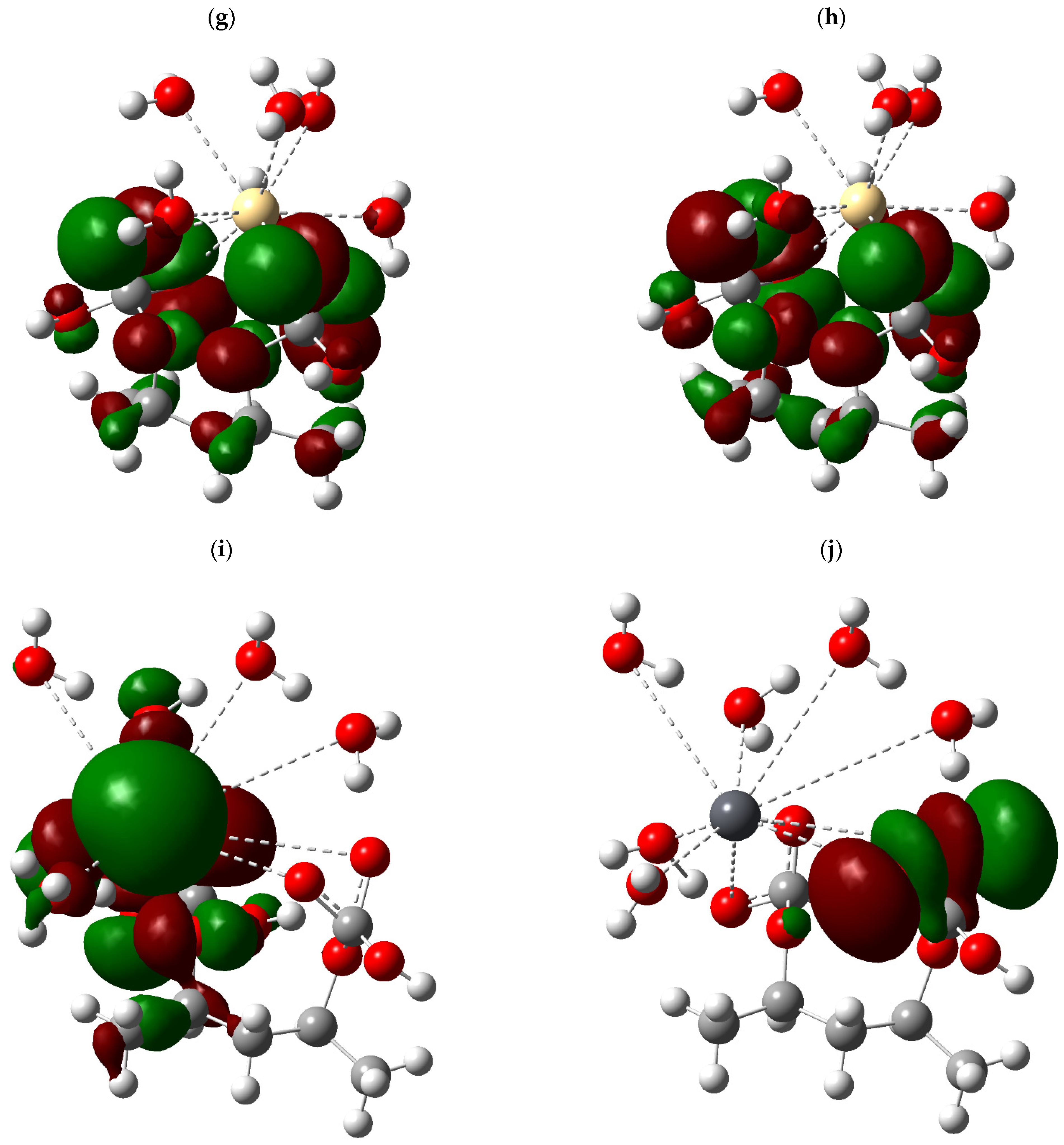
3.3. Fukui
To evaluate the impact of calcium carbonate (CaCO
3) incorporation on the electronic reactivity of polyvinyl alcohol (PVA), a frontier molecular orbital (FMO) analysis was performed based on Fukui theory, employing the indices f
+ (nucleophilic attack), f
− (electrophilic attack), the dual descriptor (f
+–f
−), as well as chemical hardness and the local work function associated with each atom in the system (
Table 2 and
Table 3). Individual profiles of pure PVA and the hybrid PVA/CaCO
3 system were compared in order to identify changes in the distribution of reactive sites and their potential implications for the adsorption of metal species.
In the case of PVA, the results indicate highly localized reactivity. A maximum f+ value of 0.3969 was observed at a carbon atom (atom 6), identifying it as a preferential electrophilic center, likely influenced by the proximity of an electronegative oxygen group. This same atom exhibited a very low f− value, resulting in a positive dual descriptor (+0.3884) that confirms its electron-accepting character. On the other hand, the oxygen atoms particularly O16 and O18 showed high f− values (0.3142 and 0.2295, respectively) and near-zero f+ values, defining them as dominant nucleophilic centers capable of engaging in interactions with metal cations through electron donation.
Following the incorporation of the inorganic CaCO3 domain, the hybrid PVA/CaCO3 system exhibits a significant redistribution of reactivity. In this new environment, the oxygen atoms associated with the carbonate anion (specifically atoms numbered 24 to 27) display elevated f+ values (up to 0.168), accompanied by moderate f− values, resulting in positive dual descriptors that indicate enhanced electronic versatility. This pattern suggests that these oxygen atoms may serve as interaction sites for both nucleophilic and electrophilic species, depending on the surrounding chemical environment.
NBO Analysis of the Hybrid PVA/CaCO3 System
To characterize the electronic distribution of the hybrid PVA–CaCO
3 system and its implications for heavy metal capture processes, a detailed NBO analysis was conducted, focusing on the most relevant orbitals for chemical reactivity (
Table 4). This study enabled the identification of both lone pairs (LP) localized on oxygen atoms and bonding orbitals (BD) with abnormally low occupancies, providing key information regarding local nucleophilicity and the susceptibility of certain bonds to polarization or cleavage.
The results reveal the presence of highly active nucleophilic centers on oxygen atoms O16 and O17, with orbital occupancies on the order of 1.954 e− and relatively high energies of −0.6005 eV. These characteristics indicate a high electron density availability and low energetic stabilization, positioning these atoms as preferential sites for coordination with metal cations such as Pb2+, Cd2+, or Cu2+. The localization of these lone pairs on both the polyvinyl alcohol chain and the carbonate domain suggests a cooperative behavior between the two fractions of the system, facilitating electron donation interactions from distinct functional environments.
Additionally, bonding orbitals with significantly lower-than-expected occupancies (<2.0 e−) were identified, such as the O16–C18 bond (0.14164 e−) and C18–O27 bond (0.06480 e−), whose positive energies (0.2328 and 0.3992 eV, respectively) denote partially vacant and electronically unstable bonds. This condition may be attributed to electronic delocalization phenomena or partial charge transfer toward more electronegative sites, generating weakened bonding regions with the potential to structurally reconfigure during adsorption. These regions may represent flexible zones within the system’s architecture, sensitive to electronic perturbations induced by the proximity of charged species.
Of particular interest is the observation of oxygen–oxygen bonds (O24–O25 and O26–O27) with intermediate occupancies (~0.47775 e−) and moderately negative energies (−0.2767 eV), suggesting an unconventional electronic dynamic within the carbonate anion. These bonds may participate in cooperative interactions, acting as electronic bridges or charge redistribution sites during the formation of metal–adsorbent complexes. Their dual involvement within the inorganic domain also reinforces the active role of CaCO3 in the capture process, beyond its passive structural function.
3.4. Evaluation of Global Reactivity Descriptors
To understand how the electronic reactivity of the PVA system evolves upon incorporation of calcium carbonate (CaCO
3) and interaction with metal ions (Cu
2+, Cd
2+, and Pb
2+), a comparative evaluation of global reactivity descriptors was conducted. The analyzed parameters include μ (chemical potential), I (ionization potential), A (electron affinity), χ (electronegativity), η (chemical hardness), and ω (electrophilicity index), which provide key insights into the system’s stability, polarizability, and its ability to donate or accept electrons in the presence of external species (
Table 5).
First, when comparing the pure PVA system with its modified version incorporating CaCO3, significant changes in its electronic profile are observed. The chemical potential of PVA, initially −0.26726 a.u., becomes less negative in the hybrid system (−0.21238 a.u.), suggesting an increased tendency of the system to engage in electron transfer processes. Electronegativity increases from 0.14322 a.u. to 0.18783 a.u., as does the electron affinity, rising from 0.00959 eV to 0.08164 eV, indicating an improved ability of the hybrid system to accept electrons. In contrast, the chemical hardness decreases from 0.13363 a.u. to 0.10619 a.u., reflecting greater electronic polarizability and, consequently, enhanced chemical reactivity. Overall, these changes reveal that the PVA/CaCO3 matrix is more versatile, adaptable, and electronically active toward external species, without compromising its structural integrity.
When analyzing the behavior of the PVA/CaCO3 system upon incorporation of metal ions, a more pronounced redistribution of global parameters is observed. In the Cu2+ complex, electronegativity reaches 0.21251 a.u., accompanied by a high electron affinity of 0.14556 eV and low chemical hardness of 0.06695 a.u., characterizing an electronically flexible system capable of stabilizing the interaction. However, its reduced electrophilicity (0.00635 a.u.) may limit its performance as a charge acceptor in certain scenarios. The Cd2+ complex, in turn, exhibits the highest electron affinity among all evaluated systems (0.16756 eV) and the lowest chemical hardness (0.01449 a.u.), making it the most polarizable and chemically reactive system, although potentially less stable under intense electronic perturbations. Finally, the Pb2+ complex displays an intermediate profile, with an electron affinity of 0.11521 eV, a hardness of 0.06675 a.u., and moderately low electrophilicity (0.00059 a.u.), suggesting a more balanced and controlled interaction suitable for applications requiring efficient retention without compromising the structural integrity of the system.
Regarding the electrophilicity descriptor, a systematic decrease is observed from the PVA system (0.00477 a.u.) toward its metal-containing derivatives, with values approaching zero. This indicates that, following coordination with the metals, the active sites of the system become electronically stabilized. Such stabilization reduces their tendency to accept additional charge, which can be interpreted as evidence of saturation at the interaction centers, thereby confirming the effective formation of coordinated complexes.
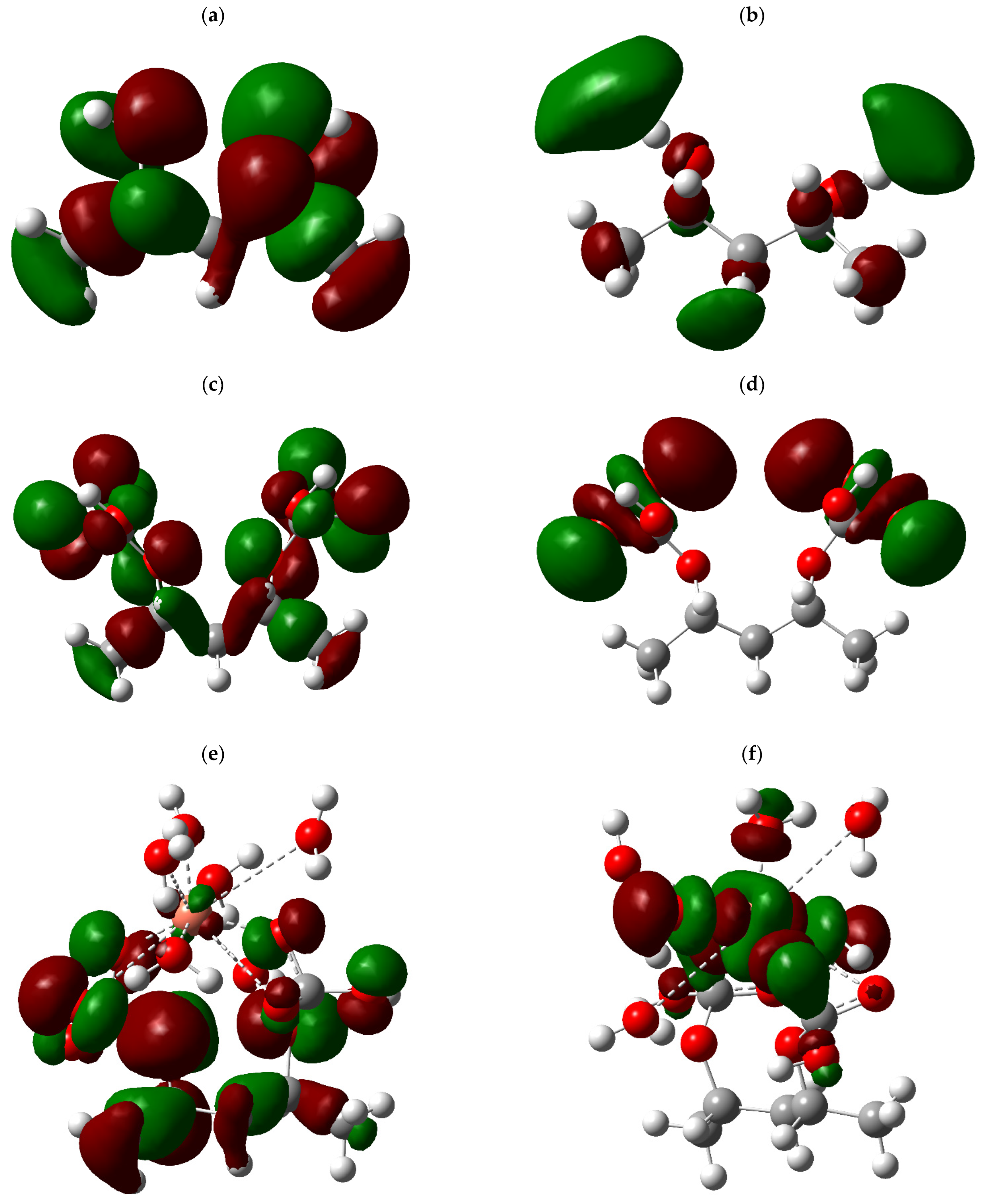
3.5. HOMO–LUMO Calculations
The energy of the difference between the HOMO and LUMO orbitals and the overall dipole moment (TDM) were studied to analyze how the biocomposite formed by PVA and oyster shell (CaCO
3) interacts with heavy metals such as Cu, Cd, and Pb, also considering coordinated water molecules [
33].
Figure 3 clearly shows the distribution of the HOMO and LUMO molecular orbitals for each structure examined, where the colors used have specific meanings: blue and red indicate areas of negative and positive electron density, respectively.
In the case of pure PVA, the color distribution is relatively homogeneous, suggesting a uniform dispersion of electron density. However, when combined with CaCO3, especially when incorporating metals, the colors concentrate significantly around the metal atoms. This concentration of coloration, particularly in intense hues, indicates regions of high chemical reactivity and enhanced interaction between the compound and the metals. These results highlight the biocomposite’s enhanced ability to adsorb heavy metals, particularly its potential in interacting with Pb and Cd.
Table 6 presents the data obtained for the HOMO–LUMO energy difference and TDM. Pure PVA showed an energy difference of 0.26726 eV and a TDM of 3.766317 Debye. Following the addition of CaCO
3, a significant decrease in the energy shift to just 0.21238 eV was observed, along with a notable increase in the total dipole moment value, reaching 7.650289 Debye. Adding metals accentuated this phenomenon, reducing the energy discrepancy to 0.13389 eV with copper and increasing the TDM to 8.859625 Debye. An even more pronounced decrease was observed, with a reduction of 0.02897 eV and a significant increase in the TDM, which reached 12.990383 Debye.
The relationship with lead also revealed a significant decrease in the discrepancy (0.13349 eV) and the maximum recorded TDM value (17.062039 Debye). The alterations in the difference between the HOMO and LUMO levels and the TDM index clearly highlight the modified biological compound’s ability to interact effectively with heavy metals, especially lead and cadmium, demonstrating its enormous potential as an absorbent agent in removing these contaminants from the aquatic environment.
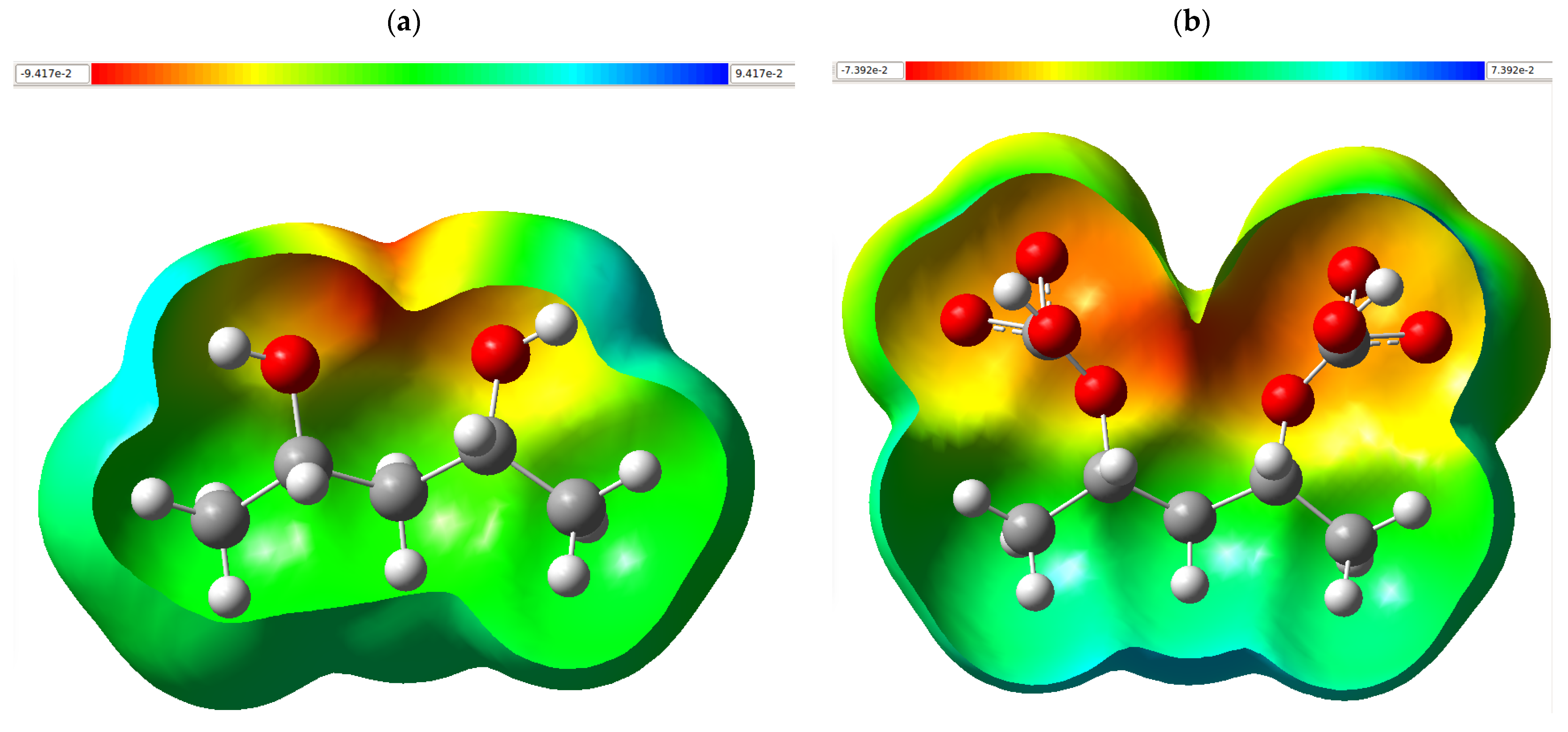
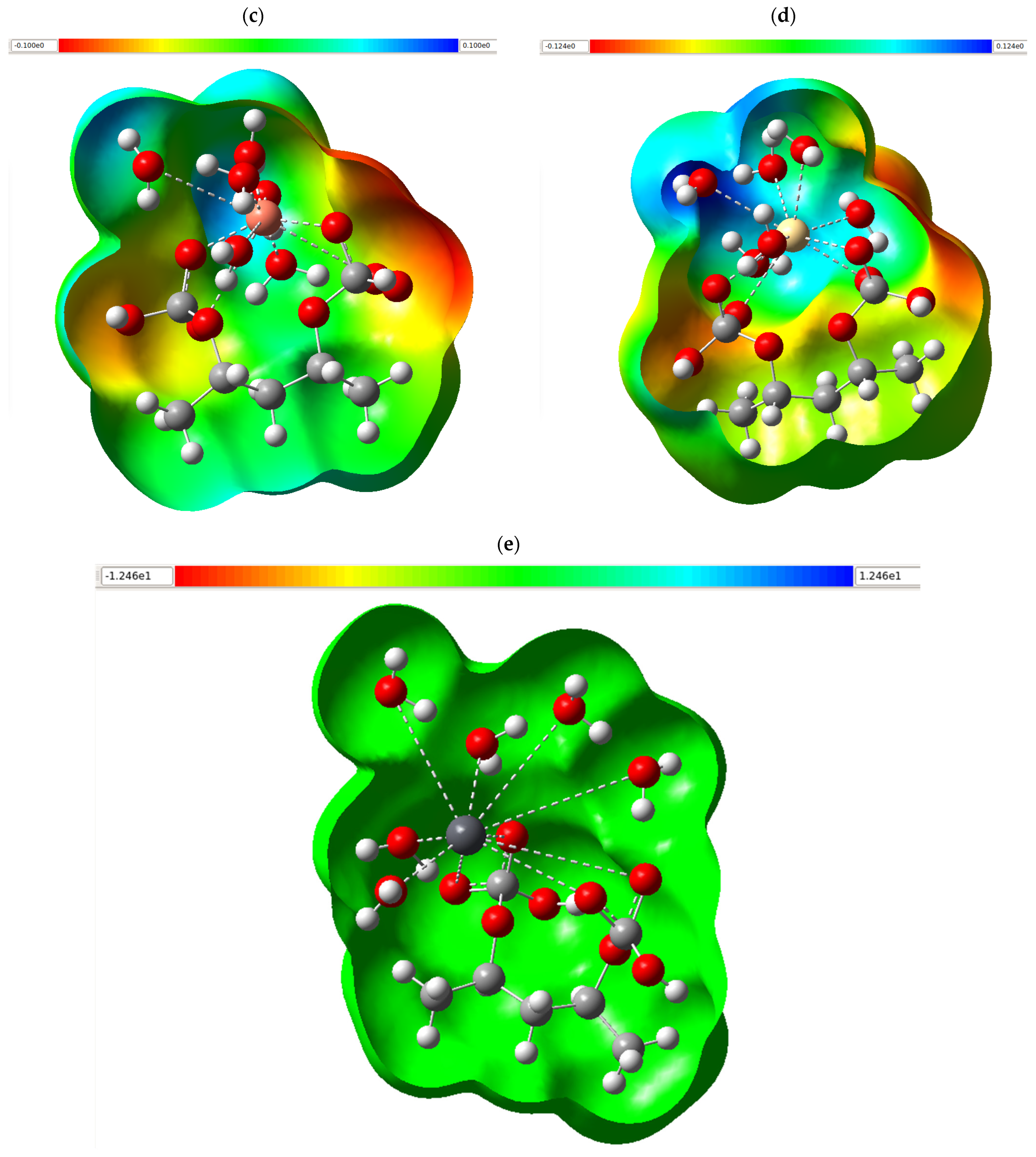
3.6. Molecular Electrostatic Potential Map
The molecular electrostatic potential map (MEPM) of the series of structures shown in
Figure 4 allows us to visualize how the surface charge distribution evolves when PVA is modified with CaCO
3 and, subsequently, when the chelating system is exposed to heavy metal ions (Cu
2+, Cd
2+, and Pb
2+). The chromatic gradient used (red (–V maximum), orange–yellow, green, and blue (+V maximum)) indicates regions increasingly richer or deficient in electron density, such that the red zones act as nucleophilic centers while the blue zones reveal electrophilic foci capable of accepting density.
In isolated PVA (
Figure 4a), a green envelope predominates, indicative of a near-neutral potential, interrupted by reddish-yellow patches located on the oxygens of the OH groups; these low-potential domains confirm that the hydroxyl sites are the most electronegative cores of the polymer and, therefore, the primary points of interaction with charged species. The incorporation of CaCO
3 (
Figure 4b) generates a two-headed lobe with extensive orange-red patches around the carbonate oxygens, while the vicinity of Ca
2+ is stained cyan-blue. This pattern denotes a more pronounced internal polarization: the calcium cation creates a positive field compensated by an electronic enrichment in the surrounding oxygens, expanding the effective surface area capable of donating electron pairs. When the composite is coordinated with Cu
2+ (
Figure 4c), the metal center appears as an intense blue nucleus (high positive potential), surrounded by a red-yellow halo in the oxygen bonds indicated by dashed bridges. The negative density shifts toward the ligands, demonstrating a charge transfer that stabilizes the complex and explains the system’s outstanding affinity for Cu
2+. The complex with Cd
2+ (
Figure 4d) maintains the multidentate geometry, but the blue around the Cd
2+ is shallower and occupies a smaller volume than in the case of copper; the lower polarization strength of the cadmium cation translates into a somewhat weaker overall electrostatic field, consistent with the expected trend of intermediate affinity. The assembly with Pb
2+ (
Figure 4e) exhibits a practically symmetrical and comparatively attenuated potential: the lead core with a larger ionic radius and lower charge density generates a moderate blue-green environment. At the same time, the oxygens still retain red-yellow zones, but without the potential concentration observed for Cu
2+. This homogeneous distribution suggests a more distributed interaction and a weaker binding. MPEMs confirm that the addition of CaCO
3 increases charge heterogeneity in the polymer matrix and creates preferential sites for metal capture; once chelation is induced, the intensity of the positive field at the metal center (Cu
2+ > Cd
2+ > Pb
2+) and the degree of neutralization of the oxygen-rich regions explain the hierarchy of affinities experienced. Thus, electrostatic analysis supports the adsorptive behavior of the PVA/CaCO
3 system and its differential effectiveness against heavy ions.
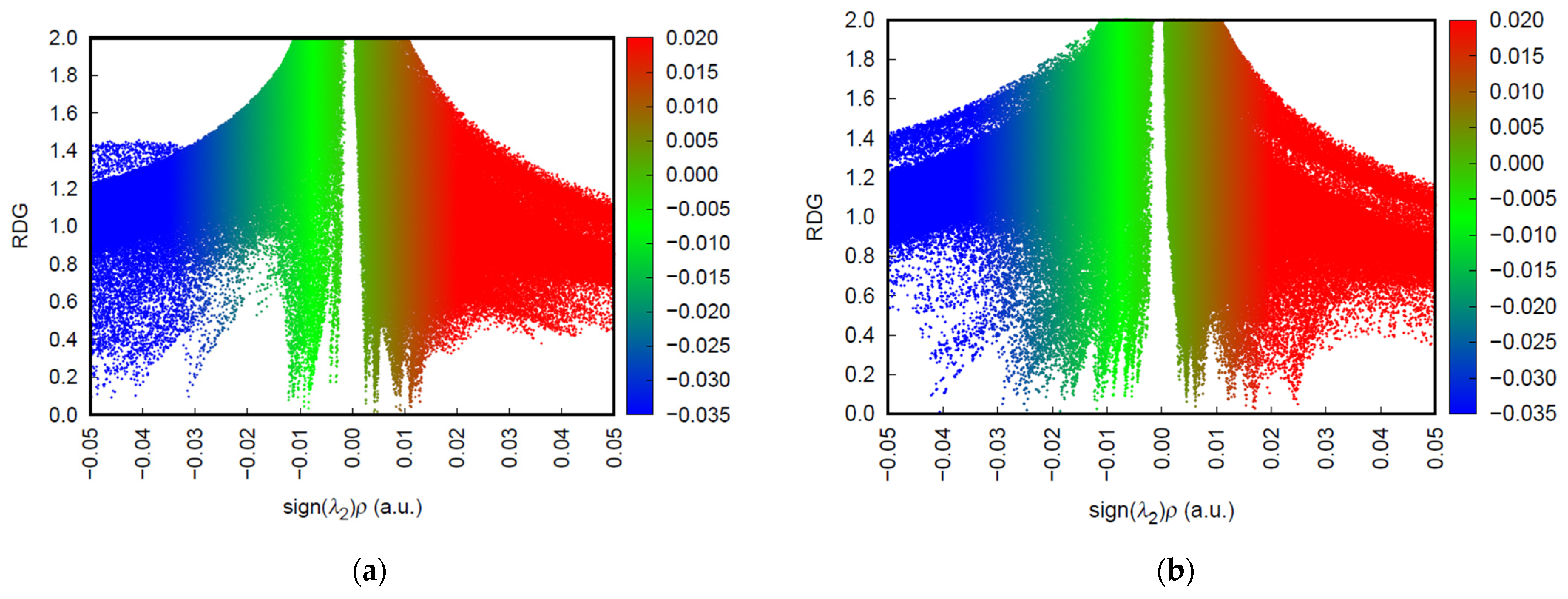
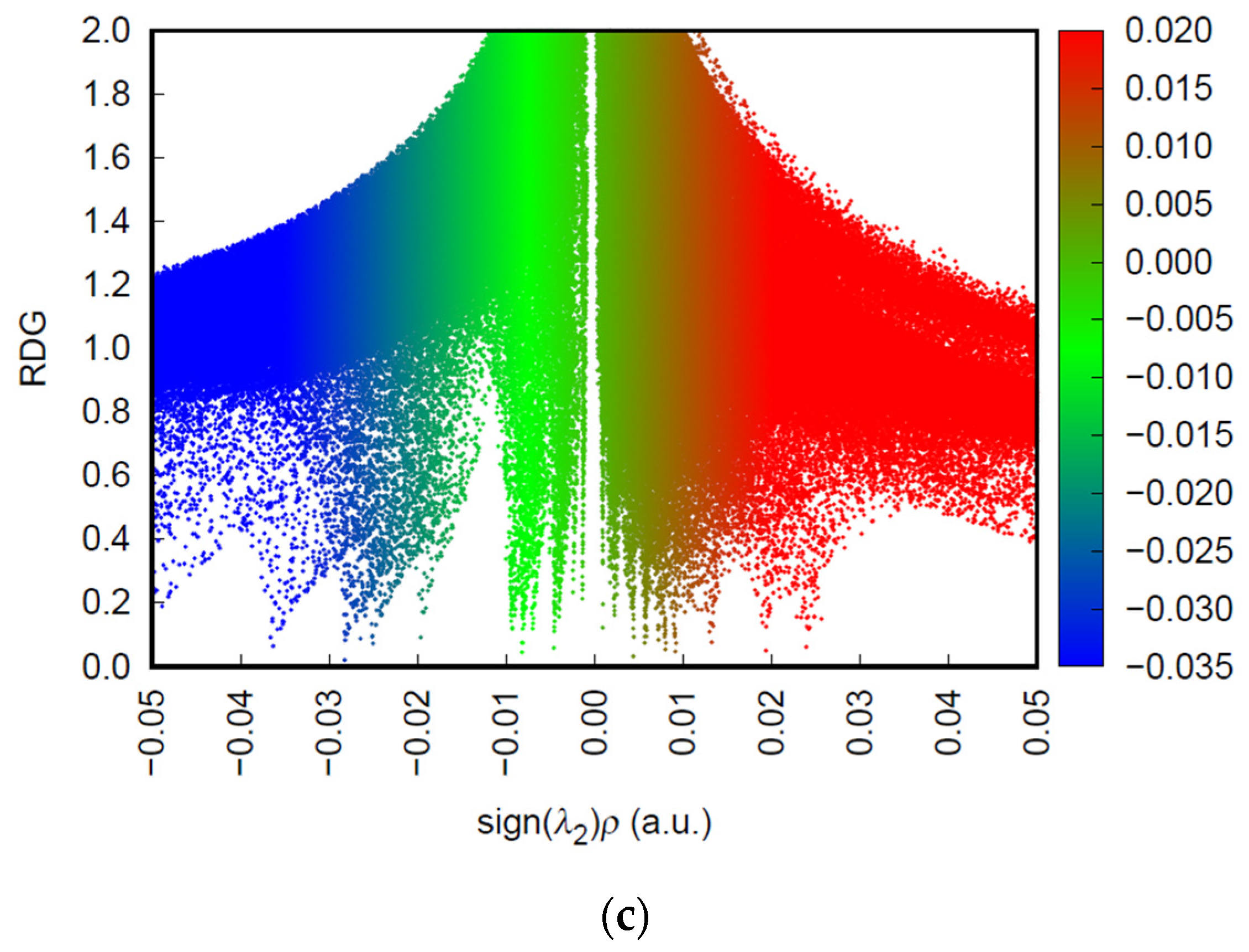
3.7. Non-Covalent Interaction (NCI/RDG) Analysis
To gain deeper insight into the adsorption mechanisms involved in the interaction between the PVA/CaCO
3 biocomposite and the metal ions Pb
2+, Cd
2+, and Cu
2+, a non-covalent interaction (NCI) analysis was performed based on reduced density gradient (RDG) theory (
Figure 5). This approach enables visualization, at the electronic surface level, of regions where attractive, van der Waals, or repulsive interactions occur between molecular fragments. The RDG vs. sign(λ
2)·ρ plots provide a quantitative map of these interactions, allowing the classification of the nature of the contacts between the adsorbent and the metal cations.
In the PVA/CaCO
3 + Cu
2+ system, an intense blue region was identified in the negative region of the horizontal axis, characteristic of strong attractive interactions, attributed to the coordination of the cupric ion with nucleophilic oxygen atoms from the carbonate or the PVA matrix. This result is consistent with the observed values for electron affinity (0.14556 eV), low chemical hardness (0.06695 a.u.), and a substantial reduction in the HOMO–LUMO gap (0.13389 eV), indicating high electronic reactivity and a flexible system in response to charged species. The visible red region at the positive end of the NCI plot indicates the presence of steric repulsion or localized electronic overlap, possibly related to the spatial arrangement of the [Cu(H
2O)
5]
2+ complex. This profile suggests a mixed interaction: predominantly chemical in nature, but with structural constraints that may limit its long-term stability (
Figure 5a). In the case of the PVA/CaCO
3 + Cd
2+ system, the RDG plot shows a less intense blue region and a broader green zone around sign (λ
2)·ρ ≈ 0, revealing an interaction dominated by van der Waals forces. This behavior is further supported by the extremely low HOMO–LUMO gap (0.02897 eV), the highest recorded electron affinity (0.16756 eV), and minimal hardness (0.01449 a.u.). While these parameters indicate a highly polarizable system with a tendency to stabilize via electronic interactions, the weak intensity of the attractive region and the lower polarization of the electrostatic field around Cd
2+ (as confirmed by MEPM maps) suggest weaker and possibly less specific adsorption. The high polarizability may result in greater overall chemical reactivity, but not necessarily in a directed or efficient interaction with the metal ion (
Figure 5b).
On the other hand, the PVA/CaCO3 + Pb2+ system exhibits a distinctive profile: the blue region in the NCI plot is broader and more intense than in the other cases, indicating a robust and well-distributed attractive interaction across the adsorbent surface. This observation is consistent with an intermediate electron affinity value (0.11521 eV), moderately low chemical hardness (0.06675 a.u.), and, notably, the highest total dipole moment in the study (17.06 Debye), which reflects significant charge separation and strong polarization induced by interaction with the plumbous ion.
Although the electrostatic field of Pb
2+ is less concentrated due to its larger ionic radius, the MEPM maps show a homogeneous distribution that favors the formation of multidentate interactions through the oxygen-rich domains of the system. This combination suggests that Pb
2+ adsorption, although less localized, is more stable and widespread, an advantage for applications involving continuous capture or treatment of contaminated media containing mixed metal species (
Figure 5c).
3.8. FT-IR Analysis
Fourier-transform infrared (FT-IR) spectroscopy is a key tool for assessing the structural modifications resulting from the incorporation of CaCO
3 into the PVA polymer matrix.
Figure 6 presents the comparative spectra corresponding to pure PVA (
Figure 6a), calcium carbonate (
Figure 6b), and the PVA/CaCO
3 biocomposite (
Figure 6c), allowing the identification of changes in characteristic bands that reflect chemical interactions between the organic and inorganic phases.
The FT-IR spectrum of PVA (
Figure 6a) exhibits a broad band centered around 3300 cm
−1, attributed to the O–H stretching vibration from hydroxyl groups in the polymer, indicating a dense network of intramolecular hydrogen bonding. Additional signals appear in the 2920–2850 cm
−1 region, corresponding to C–H stretching, as well as a composite band between 1430–1380 cm
−1 related to combined O–H and CH
2 bending vibrations. In the 1140–1085 cm
−1 region, C–O stretching bands from secondary alcohol groups are observed, further reflecting the polar character of the matrix. In turn, the spectrum of CaCO
3 (
Figure 6b) shows an intense band near 1415 cm
−1, characteristic of the asymmetric stretching mode of the carbonate ion (CO
32−), along with bending modes at 875 and 712 cm
−1, which are distinctive of the anion’s vibrational deformations. The absence of signals above 1500 cm
−1 confirms the purely inorganic nature of the compound and the lack of free hydroxyl groups.
In the FT-IR spectrum of the PVA/CaCO
3 biocomposite (
Figure 6c), representative bands from both phases can be recognized, but with shifts and modified intensities, suggesting effective interaction between the polymer and the inorganic material. The O–H stretching band is slightly shifted to ~3270 cm
−1 and exhibits increased broadness, reflecting the strengthening of hydrogen bonding between the hydroxyl groups of PVA and the carbonate oxygens. The signal in the 1430–1380 cm
−1 range is attenuated and shows slight overlap with the main carbonate absorption band, indicating possible partial coordination of OH groups with Ca
2+, which restricts vibrational freedom. Additionally, the C–O stretching band around 1090 cm
−1 becomes more intense, attributed to polarization induced by interaction with CaCO
3, while the bending mode at 875 cm
−1 becomes more pronounced, indicating improved dispersion of the mineral domain within the polymer matrix. These spectral changes confirm chemical compatibilization between PVA and CaCO
3, evidencing the formation of O–H···O–C(O) hydrogen bonds and possible Ca
2+···O coordinative interactions. Such bonding not only provides structural stabilization to the hybrid system but also enhances the availability of active sites for subsequent ionic capture processes [
34,
35,
36,
37].
4. Conclusions
In summary, the integration of computational descriptors and spectroscopic evidence clearly demonstrates that the PVA/CaCO3 biocomposite exhibits superior structural, electronic, and adsorptive characteristics compared to pristine PVA. The B3LYP/6-311++G(d,p) level of theory was identified as the most suitable, offering the lowest Gibbs free energy for the hybrid system (–875.653506 Hartree) with balanced computational efficiency. Upon CaCO3 incorporation, the HOMO-LUMO energy gap decreased by 20% (from 0.267 to 0.212 eV) and the dipole moment doubled (from 3.77 to 7.65 Debye), enhancing the overall polarity and reactivity of the matrix. Metal chelation intensified these effects: the gap dropped to 0.134 eV (Cu2+), 0.029 eV (Cd2+), and 0.133 eV (Pb2+), with the largest reduction (89%) observed in the Cd2+ system. Likewise, dipole moments increased substantially, reaching up to 17.06 Debye in the Pb2+ complex, indicating strong polarization and potential for directional electrostatic interactions. Electrostatic potential maps supported these findings, highlighting complementary electron-rich (O–H, CO32−) and electron-deficient regions, especially around the chelated metal centers, following the affinity trend Cu2+ > Cd2+ > Pb2+.
Frontier molecular orbital analysis revealed a redistribution of reactive sites in the hybrid system, with carbonate oxygens acquiring dual electrophilic/nucleophilic character, suitable for multidentate coordination. NBO analysis confirmed the presence of highly nucleophilic lone pairs (LP) and low-occupancy bonding orbitals (BD), particularly in O···C and O···O linkages, which are potentially labile and key to metal ion anchoring. Global reactivity descriptors such as chemical potential (μ), electronegativity (χ), and hardness (η) indicated enhanced electron-accepting ability and increased polarizability, especially in the PVA/CaCO3–Cd2+ complex, which displayed the lowest hardness (0.01449 a.u.) and highest electronic affinity (0.16756 eV). Noncovalent interaction (NCI/RDG) analysis further corroborated these trends, with Cu2+ and Pb2+ complexes showing robust attractive interactions, while Cd2+ exhibited weaker, dispersion-dominated behavior. FT-IR simulations validated the formation of hydrogen bonds (O–H···O=C) and Ca2+···O coordinations, evidenced by the O–H band shift (3300 → 3270 cm−1) and intensified carbonate bending at 875 cm−1.