Immunomodulatory Potential and Biocompatibility of Chitosan–Hydroxyapatite Biocomposites for Tissue Engineering
Abstract
1. Introduction
- -
- it supports hemostasis, aiding in the rapid cessation of bleeding by promoting platelet aggregation and clot formation.
- -
- it enhances angiogenesis, the formation of new blood vessels, which is critical for supplying nutrients and oxygen to regenerating tissue.
- -
- it can activate macrophages and influence their polarization toward a regenerative (M2) phenotype, thereby contributing to immune modulation and wound healing.
- -
- its intrinsic antimicrobial activity provides a barrier against bacterial colonization, reducing the risk of infection during tissue repair.
Novelty
2. Materials and Composition of Biocomposites
3. Fabrication and Characterization
4. Immunomodulatory Properties: In Vitro and In Vivo Evidence
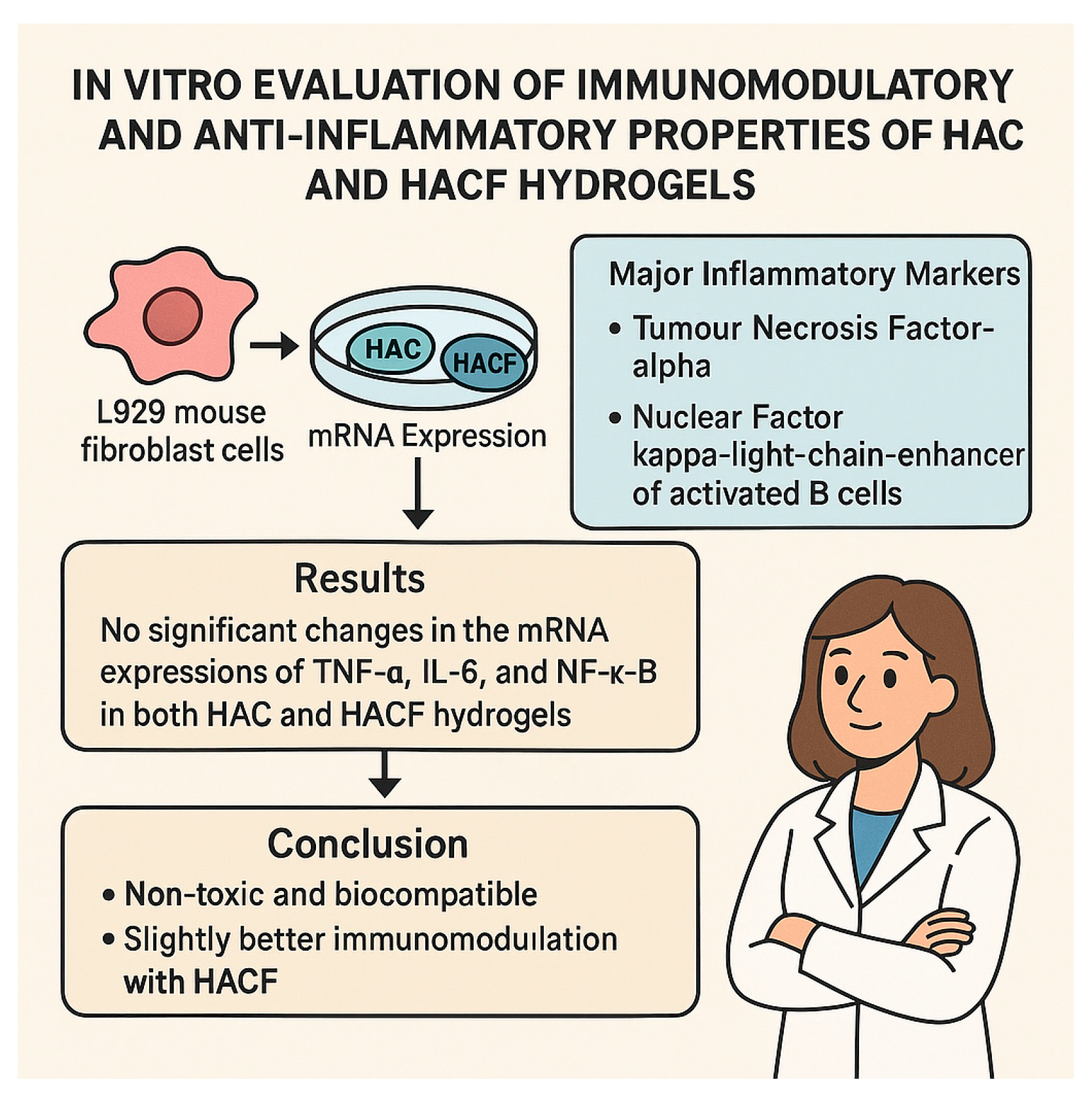
4.1. In Vitro Immunomodulatory Assessment (HAC and HACF Scaffolds)
- -
- Negative Control (Untreated Cells): Cells cultured without any treatment to establish baseline gene expression levels.
- -
- Positive Control (Pro-inflammatory Stimulus): Cells treated with known inducers of inflammation, such as lipopolysaccharide (LPS), to confirm the assay’s sensitivity and the cells’ responsiveness to inflammatory stimuli. For instance, LPS has been used to activate macrophages and fibroblasts, leading to increased production of cytokines like TNF-α and IL-6.
4.2. In Vivo Assessment in a Dermatitis Model (Chitosan–Xanthan–Hydroxyapatite Composite)
5. Mechanisms Contributing to Immunomodulation
- Intrinsic Properties of Chitosan: Chitosan is described as non-antigenic and biocompatible. It is also stated to have significant biochemical importance in processes like macrophage activation and recruitment of neutrophils to sites of infection. While macrophages are inflammatory cells, their activation phenotype can shift from pro-inflammatory (M1 macrophages) to pro-resolving/regenerative (M2 macrophages). Its role in enhancing the function of inflammatory cells suggests a potential to guide the inflammatory response towards a regenerative phase rather than simply suppressing it [60,61,62].
- Synergistic Effects in Composites: The combination of components like chitosan, alginate, hydroxyapatite, and fucoidan may lead to synergistic actions that enhance the overall immunomodulatory effect. The negatively charged sulphated fucoidan in HACF, for instance, was suggested to make it more effective than HAC. While not detailed in the sources, complex molecular interactions (electrostatic, hydrogen bonding) within the composite structure could influence the release of components or the material’s surface properties, affecting cell interactions [65,66].
- Modulation of Inflammatory Cytokine Expression: The in vitro study showed no increase in TNF-α, IL-6, and NF-κB expression by fibroblasts in contact with the scaffolds. The in vivo study showed a reduction in TNF-α, IL-1β, and IL-6 levels in the skin tissue. This suggests the biomaterials can directly or indirectly downregulate the production or activity of key pro-inflammatory mediators [67,68].
- Influence on Mast Cells and Associated Pathways: The reduction in mast cells and plasma histamine levels observed in vivo is a direct anti-inflammatory effect. The reduced expression of JAK1 and JAK3, components of the IL-31 pathway involved in itching and inflammation, further points to the material’s ability to interfere with key inflammatory signaling cascades in dermatitis [69,70]. The anti-allergic actions of AnxA1, which were shown to be modulated by the biomaterial treatment (reduced expression compared to controls, though the meaning of reduced expression vs. increased expression in controls is complex based solely on the provided text structure) [64], also indicate an interaction with allergy/inflammatory mediators.
- Controlled Degradation and Non-Toxic Products: A suitable degradation rate ensures the scaffold is replaced by new tissue without causing chronic inflammation due to persistent foreign material. The biodegradation products should also be non-toxic and biocompatible. The studies indicate the scaffolds are non-toxic in vitro and their degradation products did not induce significant inflammatory marker expression [73,74].
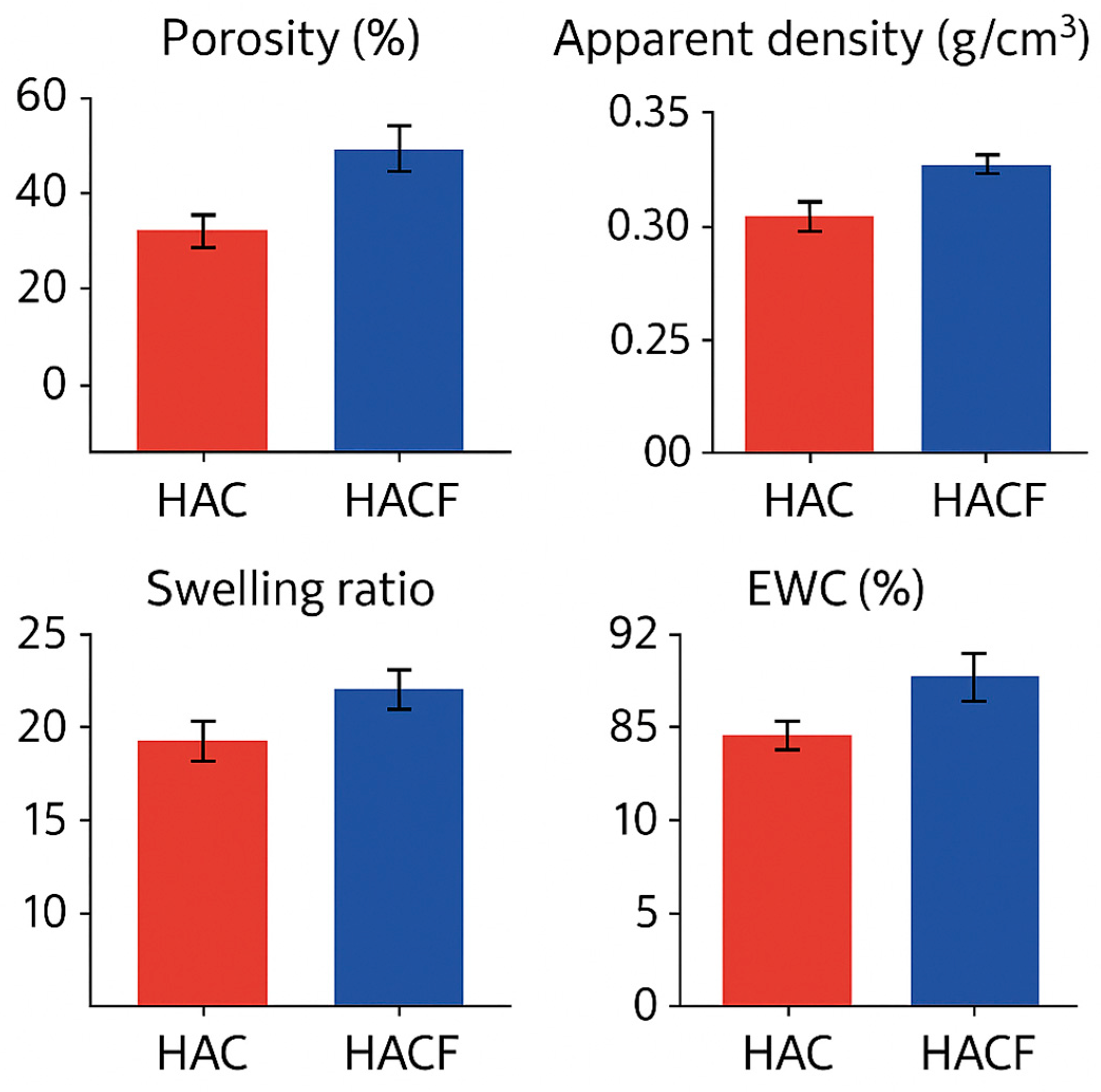
- Support for Tissue Regeneration: By providing a favorable microenvironment (porosity, water content) for cell adhesion, proliferation, and migration, and by potentially attracting key cells involved in healing (fibroblasts, keratinocytes, potentially macrophages), the biomaterials facilitate tissue repair [75].
6. Other Biocompatibility Aspects
- Hemolysis: Both HAC and HACF hydrogels exhibited very low hemolysis rates, well within the acceptable range for biomaterials. This indicates minimal damage to red blood cells upon contact [78].
- RBC Aggregation: Microscopic analysis showed no aggregation of red blood cells in contact with the hydrogel extracts, demonstrating no adverse effect on blood fluidity [79].
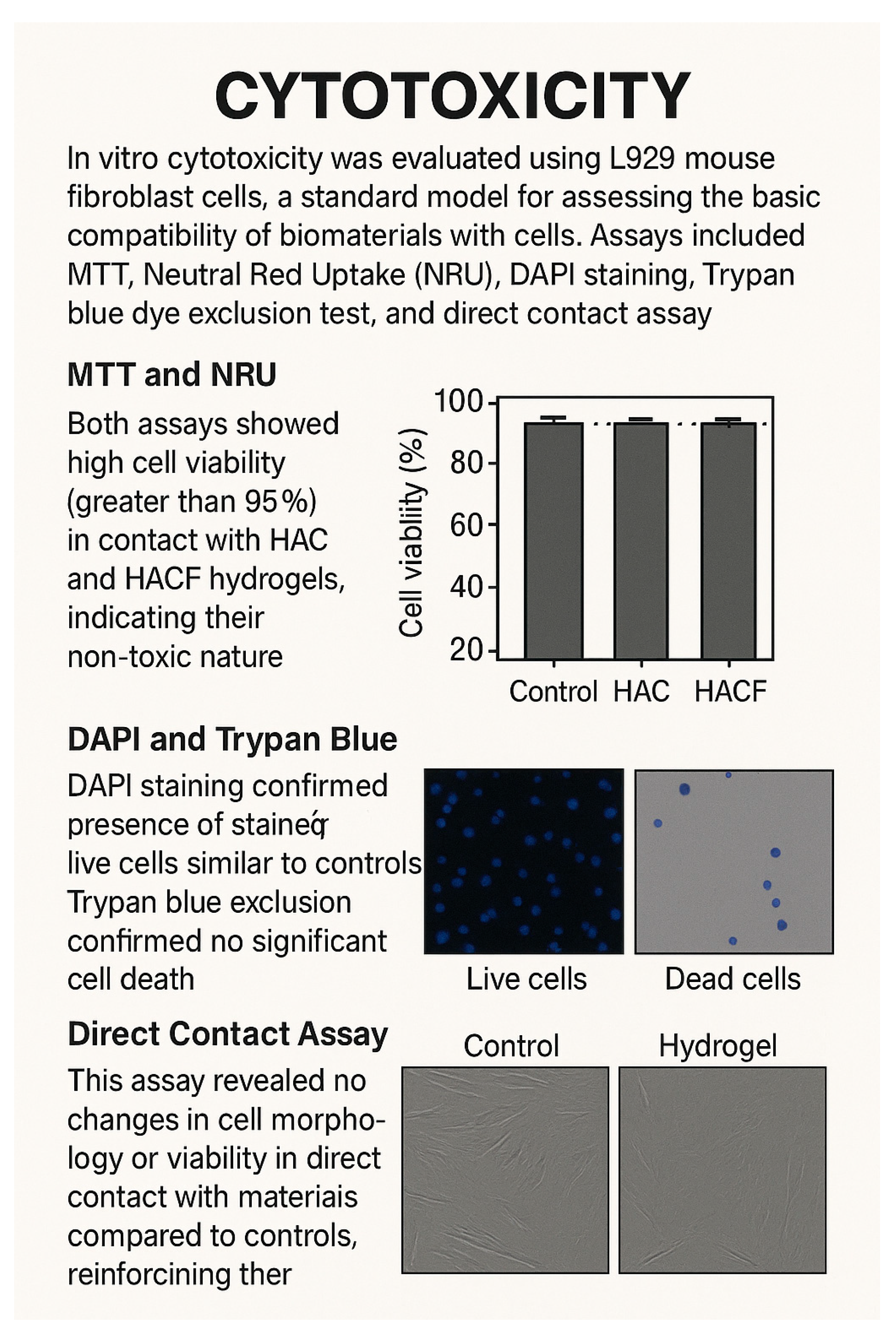
- Cytotoxicity: In vitro cytotoxicity was evaluated using L929 mouse fibroblast cells, a standard model for assessing the basic compatibility of biomaterials with cells. Assays included MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide), Neutral Red Uptake (NRU), DAPI (4′,6-Diamidino-2-Phenylindole) staining, Trypan blue dye exclusion test, and direct contact assay [82,83].
- MTT and NRU: Both assays showed high cell viability (greater than 95%) in contact with HAC and HACF hydrogels, indicating their non-toxic nature and that of their degradation products [84].
- Direct Contact Assay: This assay revealed no changes in cell morphology or viability in direct contact with the materials compared to controls, reinforcing their biocompatibility. These comprehensive in vitro cytotoxicity evaluations demonstrate that the HAC and HACF hydrogels are cytocompatible and non-toxic [87,88].
7. Potential Applications
- -
- Tissue Engineering Scaffolds: Their porous architecture, suitable pore size, swelling capacity, biocompatibility, and controlled degradation make them promising candidates as scaffolds for regenerating various tissues. The absence of significant inflammatory response observed in vitro is critical for the success of tissue regeneration, as chronic inflammation can impede healing and lead to scar tissue formation [89].
- -
- Treatment of Inflammatory Skin Diseases: The in vivo findings in the dermatitis model are particularly relevant [90]. The ability of the chitosan–xanthan–hydroxyapatite composite to reduce inflammation, decrease mast cell numbers, lower pro-inflammatory cytokine levels, and improve tissue regeneration suggests its potential as a therapeutic dressing or material for managing inflammatory skin conditions like dermatitis [90,91]. This approach offers an alternative to conventional therapies that primarily suppress inflammation. The enhanced effect when combined with conditioned medium also points to strategies combining these biomaterials with bioactive molecules for improved outcomes [92,93].
- -
- Regenerative Dentistry and Bone Regeneration: Although not the primary focus on immunomodulation in this paper, the sources also mention the traditional association of hydroxyapatite–chitosan composites with bone and periodontal tissue engineering [94]. The HAC and HACF compositions were investigated partly based on previous favorable results for adhesion and growth of mesenchymal cells from dental pulp and bone tissue engineering applications [95]. The immunomodulatory and biocompatibility profiles are essential for these applications to ensure successful integration and remodeling of bone tissue without adverse immune reactions.
8. Limitations
9. Protocols
9.1. HAC Formulation: Chitosan–Hydroxyapatite Composite Preparation Protocol
- -
- Chitosan Solution: Dissolve 1.5% (w/v) medium molecular weight chitosan (degree of deacetylation: 75–85%) in 0.1 M acetic acid at 40 °C.
- -
- Hydroxyapatite Precursors: Prepare separate aqueous solutions of calcium nitrate tetrahydrate [Ca(NO3)2·4H2O] and diammonium hydrogen phosphate [(NH4)2HPO4].
- -
- Mixing: Add the calcium solution to the chitosan solution and stir at 250 rpm for 2 h at 40 °C. Then, introduce the phosphate solution into the mixture.
- -
- pH Adjustment: Adjust the pH to an alkaline range using ammonium hydroxide (NH4OH), monitoring with a pH meter.
- -
- Aging and Sonication: Maintain the mixture at 40 °C with stirring at 85 rpm for 24 h, followed by sonication for 3 h to ensure homogeneity.
- -
- Scaffold Formation: Pour the resulting gel into Teflon molds (5 × 10 mm), freeze at −80 °C, and lyophilize for 48 h to obtain 3D scaffolds.
Characterization
- -
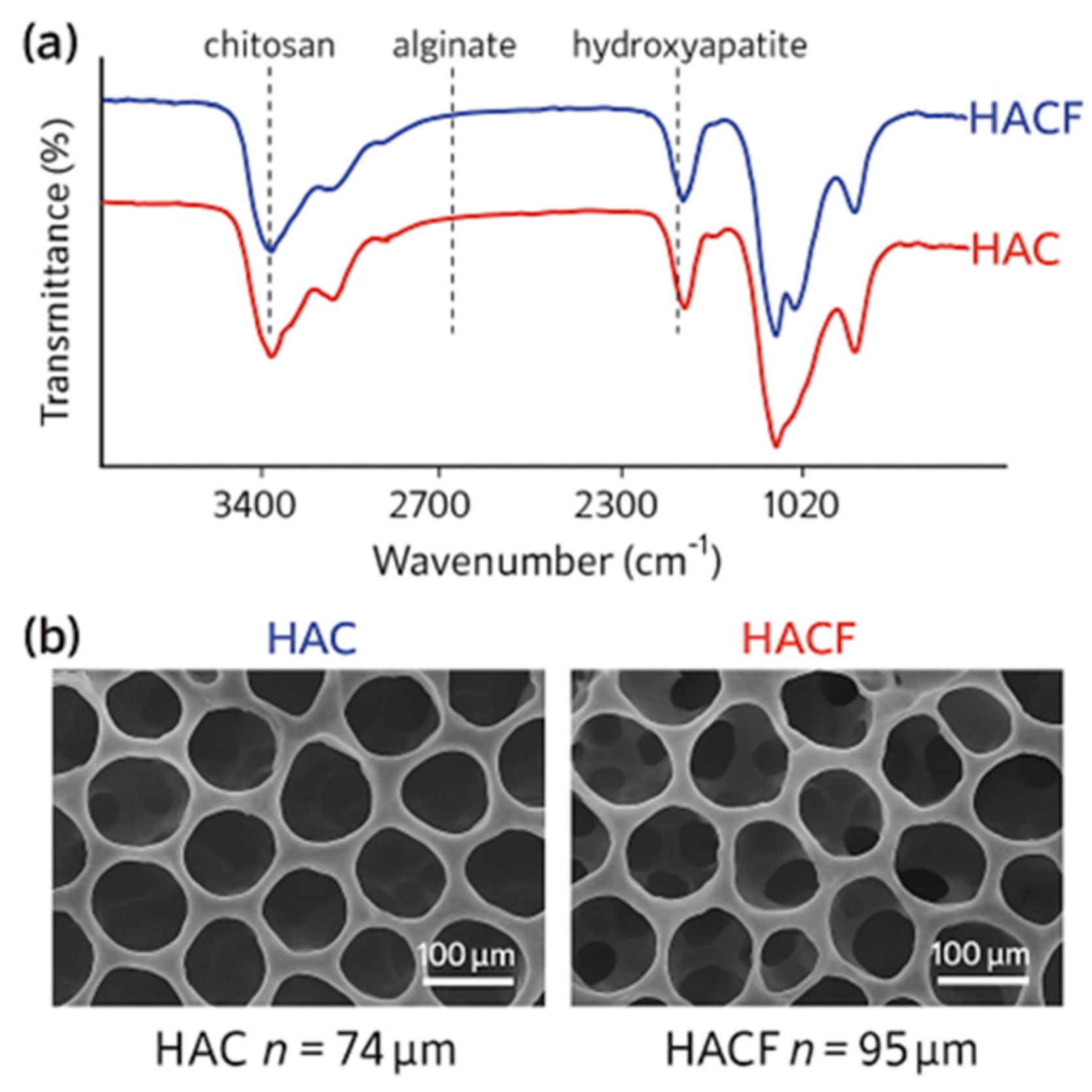
- Morphology: Scanning Electron Microscopy (SEM) was used to assess the porous structure and distribution of hydroxyapatite within the chitosan matrix.
- -
- Structural Analysis: Fourier Transform-Infrared Spectroscopy (FTIR) and X-ray Diffraction (XRD) analyses confirmed the formation of hydroxyapatite and its interaction with chitosan.
- -
- Mechanical Properties: Compression testing evaluated the mechanical strength suitable for bone tissue engineering applications [100].
9.2. HACF Formulation: Cross-Linked Chitosan–Hydroxyapatite Composite
- -
- Chitosan Solution: Dissolve 2.0 g of chitosan in 50 mL of 2% (v/v) acetic acid, stirring for 6 h.
- -
- Hydroxyapatite Addition: Add a specific amount of hydroxyapatite powder to the chitosan solution and stir for 1 h at room temperature to achieve a homogeneous mixture.
- -
- Molding and Freezing: Transfer the mixture into molds and freeze at −18 °C for 24 h.
- -
- Freeze-Drying: Lyophilize the frozen samples at −50 °C for 72 h to create porous scaffolds.
- -
- Neutralization: Immerse the scaffolds in a 10 wt% sodium hydroxide (NaOH) solution for 12 h to neutralize residual acetic acid, followed by washing with deionized water.
- -
- Final Freeze-Drying: Freeze-dry the neutralized scaffolds to obtain the final product.
Characterization
- -
- Microstructure: Field Emission Scanning Electron Microscopy (FESEM) and Transmission Electron Microscopy (TEM) analyzed the scaffold’s morphology and hydroxyapatite distribution.
- -
- Phase Composition: X-ray Diffraction (XRD) assessed the crystalline phases present in the composite.
- -
- Chemical Interactions: Fourier Transform-Infrared Spectroscopy (FT-IR) identified functional groups and interactions between chitosan and hydroxyapatite [101].
10. Batch-to-Batch Variability and Potential Immunogenicity
11. Future Approaches
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AnxA1 | Annexin A1 |
| BMP-2 | Bone morphogenetic protein-2 |
| CD40 and CD86 | Cluster of Differentiation 40 and Cluster of Differentiation 86 |
| CS | Chitosan |
| CS-HAp | Chitosan–hydroxyapatite |
| CM | Conditioned medium |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| ECM | Native extracellular matrix |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EWC | Equilibrium water content |
| FESEM | Field Emission Scanning Electron Microscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| G1 | Induced without treatment |
| G2 | Induced and treated with standard hydrocortisone ointment |
| G3 | Induced and treated with the biomaterial without conditioned medium |
| G4 | Induced and treated with the biomaterial with conditioned medium |
| HAC | Hydroxyapatite, alginate, and chitosan |
| HACF | Hydroxyapatite, alginate, chitosan, and fucoidan |
| HAp | Hydroxyapatite |
| IL-10 and IL-4 | Anti-inflammatory cytokines |
| IL-1β and IL-6 | Interleukin-1 beta and Interleukin-6 |
| IGFBPs | Insulin-like Growth Factor Binding Proteins |
| JAKs | Janus kinase |
| JAK1/3 | Itching/inflammation pathways |
| LPS | Lipopolysaccharide |
| mRNA | Messenger RNA |
| M1 | Pro-inflammatory M1 macrophages |
| M2 | Pro-resolving/regenerative M2 macrophages |
| MSCs | Mesenchymal stem cells |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NRU | Neutral Red Uptake |
| PBMCs | Peripheral blood mononuclear cells |
| qPCR | Quantitative polymerase chain reaction |
| RBC | Red blood cells |
| SEM | Scanning electron microscopy |
| STAT | Signal Transducer and Activator of Transcription |
| TEM | Transmission Electron Microscopy |
| TGF-β | Pro-inflammatory cytokine |
| TNF-α | Tumour Necrosis Factor-alpha |
| VEGF | Vascular endothelial growth factor |
| XRD | X-ray Diffraction |
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 44, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Noro, J.; Vilaça-Faria, H.; Reis, R.L.; Pirraco, R.P. Extracellular matrix-derived materials for tissue engineering and regenerative medicine: A journey from isolation to characterization and application. Bioact. Mater. 2024, 34, 494–519. [Google Scholar]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–123. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef]
- de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Bai, J.; Zhang, H.; Long, H.; Jiang, B.; Wang, J.; Huang, X.; Zhang, H.; Zhao, J. Prospective applications of bioactive materials in orthopedic therapies: A review. Heliyon 2024, 10, e36152. [Google Scholar] [CrossRef]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, R.B.; Yoshikawa, A.H.; Sant’Ana, M.; Souza, H.R.; Possebon, L.; Navarro da Rocha, D.; Ferreira, J.R.M.; Vidotti, G.A.G.; Girol, A.P. Reduction of inflammation and improvement of skin tissue repair using biomaterials composed of hydroxyapatite and chitosan associated to conditioned media derived from dental pulp stem cells. Int. J. Biol. Macromol. 2025, 308, 142353. [Google Scholar] [CrossRef] [PubMed]
- Sumayya, A.S.; Muraleedhara Kurup, G. Marine macromolecules cross-linked hydrogel scaffolds as physiochemically and biologically favorable entities for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2017, 28, 807–825. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P.; et al. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Kumar, B.; Singh, N.; Kumar, P. A review on sources, modification techniques, properties and potential applications of alginate-based modified polymers. Eur. Polym. J. 2024, 213, 113078. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.M.; da Rocha, D.N.; Bombaldi de Souza, R.F.; Santos, J.L.; Ferreira, J.R.M.; Moraes, Â.M. Cell-Friendly Chitosan-Xanthan Gum Membranes Incorporating Hydroxyapatite Designed for Periodontal Tissue Regeneration. Pharmaceutics 2023, 15, 705. [Google Scholar] [CrossRef]
- Elkhenany, H.; Soliman, M.W.; Atta, D.; El-Badri, N. Innovative Marine-Sourced Hydroxyapatite, Chitosan, Collagen, and Gelatin for Eco-Friendly Bone and Cartilage Regeneration. J. Biomed. Mater. Res. A 2025, 113, e37833. [Google Scholar] [CrossRef]
- San, H.H.M.; Alcantara, K.P.; Bulatao, B.P.I.; Chaichompoo, W.; Nalinratana, N.; Suksamrarn, A.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Development of Turmeric Oil-Loaded Chitosan/Alginate Nanocapsules for Cytotoxicity Enhancement against Breast Cancer. Polymers 2022, 14, 1835. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Zhang, X.; Liang, B.; Li, X.; Li, Y.; Sun, C. Double-crosslinked pea protein isolation-sodium alginate composite microgels embedding Lactobacillus plantarum: Effect of calcium chloride concentration. LWT 2025, 222, 117670. [Google Scholar] [CrossRef]
- Devi, G.V.Y.; Nagendra, A.H.; Shenoy, P.S.; Chatterjee, K.; Venkatesan, J. Fucoidan-Incorporated Composite Scaffold Stimulates Osteogenic Differentiation of Mesenchymal Stem Cells for Bone Tissue Engineering. Mar. Drugs 2022, 20, 589. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Jiao, G.; Kermanshahi-Pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Moeinzadeh, A.; Ashtari, B.; Garcia, H.; Koruji, M.; Velazquez, C.A.; Bagher, Z.; Barati, M.; Shabani, R.; Davachi, S.M. The Effect of Chitosan/Alginate/Graphene Oxide Nanocomposites on Proliferation of Mouse Spermatogonial Stem Cells. J. Funct. Biomater. 2023, 14, 556. [Google Scholar] [CrossRef]
- Jangra, N.; Singla, A.; Puri, V.; Dheer, D.; Chopra, H.; Malik, T.; Sharma, A. Herbal bioactive-loaded biopolymeric formulations for wound healing applications. RSC Adv. 2025, 15, 12402–12442. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zeng, Z.; Geng, Z.; Guo, C.; Pei, D.; Zhang, J.; Yu, S. Macroporous methacrylated hyaluronic acid hydrogel with different pore sizes forin vitroandin vivoevaluation of vascularization. Biomed. Mater. 2022, 17, 025006. [Google Scholar] [CrossRef]
- Leal-Egaña, A.; Braumann, U.D.; Díaz-Cuenca, A.; Nowicki, M.; Bader, A. Determination of pore size distribution at the cell-hydrogel interface. J. Nanobiotechnol. 2011, 9, 24. [Google Scholar] [CrossRef]
- Kedzierski, A.; Kheirabadi, S.; Jaberi, A.; Ataie, Z.; Mojazza, C.L.; Williamson, M.L.; Hjaltason, A.M.; Risbud, A.; Xiang, Y.; Sheikhi, A. Engineering the Hierarchical Porosity of Granular Hydrogel Scaffolds Using Porous Microgels to Improve Cell Recruitment and Tissue Integration. Adv. Funct. Mater. 2025, 35, 2417704. [Google Scholar] [CrossRef]
- Jastram, A.; Lindner, T.; Luebbert, C.; Sadowski, G.; Kragl, U. Swelling and Diffusion in Polymerized Ionic Liquids-Based Hydrogels. Polymers 2021, 13, 1834. [Google Scholar] [CrossRef]
- Thankam, F.G.; Muthu, J. Influence of physical and mechanical properties of amphiphilic biosynthetic hydrogels on long-term cell viability. J. Mech. Behav. Biomed. Mater. 2014, 35, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium Biomaterials-Road to the Clinic. Bioengineering 2022, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Restu, W.K.; Yamamoto, S.; Nishida, Y.; Ienaga, H.; Aoi, T.; Maruyama, T. Hydrogel formation by short D-peptide for cell-culture scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110746. [Google Scholar] [CrossRef]
- Nelson, M.T.; Johnson, J.; Lannutti, J. Media-based effects on the hydrolytic degradation and crystallization of electrospun synthetic-biologic blends. J. Mater. Sci. Mater. Med. 2014, 25, 297–309. [Google Scholar] [CrossRef]
- Horner, C.B.; Ico, G.; Johnson, J.; Zhao, Y.; Nam, J. Microstructure-dependent mechanical properties of electrospun core-shell scaffolds at multi-scale levels. J. Mech. Behav. Biomed. Mater. 2016, 59, 207–219. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Jiang, L.; Huang, A.; Wang, X.F.; Li, Q.; Turng, L.S. Electrospun polycaprolactone/gelatin composites with enhanced cell-matrix interactions as blood vessel endothelial layer scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for drug delivery and tissue engineering: The role of genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Conte, R.; Margarucci, S.; Peluso, G.; Calarco, A. Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds. Int. J. Mol. Sci. 2025, 26, 764. [Google Scholar] [CrossRef] [PubMed]
- Omoigui, S. The Interleukin-6 inflammation pathway from cholesterol to aging--role of statins, bisphosphonates and plant polyphenols in aging and age-related diseases. Immun. Ageing 2007, 4, 1. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Kong, L.; Xue, Y.; Tang, M. Threshold. Dose of Three Types of Quantum Dots (QDs) Induces Oxidative Stress. Triggers DNA Damage and Apoptosis in Mouse Fibroblast L929 Cells. Int. J. Environ. Res. Public Health 2015, 12, 13435–13454. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Lock, A.; Cornish, J.; Musson, D.S. The Role of In Vitro Immune Response Assessment for Biomaterials. J. Funct. Biomater. 2019, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Chihara, T.; Dong, H.; Kagami, H. Effect of TNF-α and IL-6 on Compact Bone-Derived Cells. Tissue Eng. Regen. Med. 2021, 18, 441–451. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, X.; Huang, Y.; Gong, Y.; He, Y.; Xiao, D.; Wang, S.; Zhao, C. Oxidized fucoidan-based nanocomposite hydrogel for cryptotanshinone delivery and prevention of postoperative abdominal adhesions. J. Control. Release 2025, 382, 113733. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-based biomaterials for pharmaceutical and biomedical applications: A focus on topical drug administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; He, X.; Cong, J.; Zhao, W.; Fu, Y.; Lu, C.; Wu, C.; Pan, X.; Quan, G. Biomineralized in situ catalytic nanoreactor integrated microneedle patch for on demand immunomodulator supply to combat psoriasis. Theranostics 2024, 14, 6571–6586. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Parham, M.M.; Krishnan, B.; Huttenbach, Y.T. A comparison of mast cells in skin biopsies of cutaneous mastocytosis with other inflammatory dermatoses: A study of 33 cases. J. Cutan. Pathol. 2024, 51, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Ozpinar, E.W.; Frey, A.L.; Cruse, G.; Freytes, D.O. Mast Cell-Biomaterial Interactions and Tissue Repair. Tissue Eng. Part B Rev. 2021, 27, 590–603. [Google Scholar] [CrossRef]
- Parisi, J.D.S.; Corrêa, M.P.; Gil, C.D. Lack of Endogenous Annexin A1 Increases Mast Cell Activation and Exacerbates Experimental Atopic Dermatitis. Cells 2019, 8, 51. [Google Scholar] [CrossRef]
- Pupjalis, D.; Goetsch, J.; Kottas, D.J.; Gerke, V.; Rescher, U. Annexin A1 released from apoptotic cells acts through formyl peptide receptors to dampen inflammatory monocyte activation via JAK/STAT/SOCS signalling. EMBO Mol. Med. 2011, 3, 102–114. [Google Scholar] [CrossRef]
- Ogulur, I.; Mitamura, Y.; Yazici, D.; Pat, Y.; Ardicli, S.; Li, M.; D’Avino, P.; Beha, C.; Babayev, H.; Zhao, B.; et al. Type 2 immunity in allergic diseases. Cell Mol. Immunol. 2025, 22, 211–242. [Google Scholar] [CrossRef]
- Reay, S.L.; Marina Ferreira, A.; Hilkens, C.M.U.; Novakovic, K. The Paradoxical Immunomodulatory Effects of Chitosan in Biomedicine. Polymers 2024, 17, 19. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.J.; He, C.H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Liu, J.C.; Trujillo-de Santiago, G.; Cha, B.H.; Vishwakarma, A.; Ghaemmaghami, A.M.; Khademhosseini, A. Delivery strategies to control inflammatory response: Modulating M1-M2 polarization in tissue engineering applications. J. Control. Release 2016, 240, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, O.; Yanovska, A.; Kosinov, O.; Maksymov, D.; Moskalenko, R.; Ramanavicius, A.; Pogorielov, M. Synthetic Calcium-Phosphate Materials for Bone Grafting. Polymers 2023, 15, 3822. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Ciobanu, C.; Predoi, S.-A.; Țălu, Ș. Hydroxyapatite: Innovations in Synthesis, Properties, Nanocomposites, Biomedical Applications, and Technological Developments; Napoca Star Publishing House: Cluj-Napoca, Romania, 2024; pp. 65–72. ISBN 978-606-062-901-6. [Google Scholar]
- Zhu, T.; Jiang, J.; Zhao, J.; Chen, S.; Yan, X. Regulating Preparation Of Functional Alginate-Chitosan Three-Dimensional Scaffold For Skin Tissue Engineering. Int. J. Nanomed. 2019, 14, 8891–8903. [Google Scholar] [CrossRef]
- Ţălu, Ş.; Matos, R.S.; Da Fonseca Filho, H.D.; Predoi, D.; Iconaru, S.L.; Ciobanu, C.S.; Ghegoiu, L. Morphological and Fractal Features of cancer cells anchored on Composite Layers based on Magnesium-Doped Hydroxyapatite loaded in Chitosan Matrix. Micron 2024, 176, 103548. [Google Scholar] [CrossRef]
- Htwe, S.S.; Harrington, H.; Knox, A.; Rose, F.; Aylott, J.; Haycock, J.W.; Ghaemmaghami, A.M. Investigating NF-κB signaling in lung fibroblasts in 2D and 3D culture systems. Respir. Res. 2015, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Cristinziano, L.; Criscuolo, G.; Strisciuglio, C.; Palestra, F.; Lagnese, G.; Di Salvatore, A.; Marone, G.; Spadaro, G.; Loffredo, S.; et al. The JAK1/JAK2 inhibitor ruxolitinib inhibits mediator release from human basophils and mast cells. Front. Immunol. 2024, 15, 1443704. [Google Scholar] [CrossRef]
- Koumaki, D.; Gregoriou, S.; Evangelou, G.; Krasagakis, K. Pruritogenic Mediators and New Antipruritic Drugs in Atopic Dermatitis. J. Clin. Med. 2023, 12, 2091. [Google Scholar] [CrossRef]
- Honkisz-Orzechowska, E.; Łażewska, D.; Baran, G.; Kieć-Kononowicz, K. Uncovering the Power of GPR18 Signalling: How RvD2 and Other Ligands Could Have the Potential to Modulate and Resolve Inflammation in Various Health Disorders. Molecules 2024, 29, 1258. [Google Scholar] [CrossRef]
- Fernandez-Medina, T.; Vaquette, C.; Gomez-Cerezo, M.N.; Ivanovski, S. Characterization of the Protein Corona of Three Chairside Hemoderivatives on Melt Electrowritten Polycaprolactone Scaffolds. Int. J. Mol. Sci. 2023, 24, 6162. [Google Scholar] [CrossRef]
- Sirous, S.; Aghamohseni, M.M.; Farhad, S.Z.; Beigi, M.; Ostadsharif, M. Mesenchymal stem cells in PRP and PRF containing poly(3-caprolactone)/gelatin Scaffold: A comparative in-vitro study. Cell Tissue Bank. 2024, 25, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Sindhi, K.; Pingili, R.B.; Beldar, V.; Bhattacharya, S.; Rahaman, J.; Mukherjee, D. The role of biomaterials-based scaffolds in advancing skin tissue construct. J. Tissue Viabil. 2025, 34, 100858. [Google Scholar] [CrossRef]
- Donmazov, S.; Saruhan, E.N.; Pekkan, K.; Piskin, S. Review of Machine Learning Techniques in Soft Tissue Biomechanics and Biomaterials. Cardiovasc. Eng. Technol. 2024, 15, 522–549. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Tayebi, L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J. Tissue Eng. Regen. Med. 2021, 15, 747–762. [Google Scholar] [CrossRef]
- de la Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell-Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Amarnath, L.P.; Srinivas, A.; Ramamurthi, A. In vitro hemocompatibility testing of UV-modified hyaluronan hydrogels. Biomaterials 2006, 27, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.H.; Piety, N.Z.; Shevkoplyas, S.S. Influence of red blood cell aggregation on perfusion of an artificial microvascular network. Microcirculation 2017, 24, 12317. [Google Scholar] [CrossRef]
- Ji, S.; Chiniforooshan Esfahani, I.; Yang, R.; Sun, H. Adsorption and Morphology Analysis of Bovine Serum Albumin on a Micropillar-Enhanced Quartz Crystal Microbalance. J. Phys. Chem. B 2024, 128, 10247–10257. [Google Scholar] [CrossRef]
- Kuten Pella, O.; Hornyák, I.; Horváthy, D.; Fodor, E.; Nehrer, S.; Lacza, Z. Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 10557. [Google Scholar] [CrossRef]
- Ozdemir, K.G.; Yilmaz, H.; Yilmaz, S. In vitro evaluation of cytotoxicity of soft lining materials on L929 cells by MTT assay. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Etheridge, J.M.; Thompson, J.A.; Vorwald, C.E.; Dean, D.; Fisher, J.P. Evaluation of the in vitro cytotoxicity of cross-linked biomaterials. Biomacromolecules 2013, 14, 1321–1329. [Google Scholar] [CrossRef]
- Dominijanni, A.J.; Devarasetty, M.; Forsythe, S.D.; Votanopoulos, K.I.; Soker, S. Cell Viability Assays in Three-Dimensional Hydrogels: A Comparative Study of Accuracy. Tissue Eng. Part C Methods 2021, 27, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Isaja, L.; Mucci, S.; Vera, J.; Rodríguez-Varela, M.S.; Marazita, M.; Morris-Hanon, O.; Videla-Richardson, G.A.; Sevlever, G.E.; Scassa, M.E.; Romorini, L. Chemical hypoxia induces apoptosis of human pluripotent stem cells by a NOXA-mediated HIF-1α and HIF-2α independent mechanism. Sci. Rep. 2020, 10, 20653. [Google Scholar] [CrossRef]
- Kerschbaum, H.H.; Tasa, B.A.; Schürz, M.; Oberascher, K.; Bresgen, N. Trypan Blue–Adapting a Dye Used for Labelling Dead Cells to Visualize Pinocytosis in Viable Cells. Cell Physiol. Biochem. 2021, 55, 171–184. [Google Scholar]
- Margot, A.M.; Engels, A.; Sittinger, M.; Dehne, T.; Hemmati-Sadeghi, S. Quantitatively measuring the cytotoxicity of viscous hydrogels with direct cell sampling in a micro scale format “MicroDrop” and its comparison to CCK8. J. Mater. Sci. Mater. Med. 2024, 35, 34. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.; Thom, H.; Sheppard, A.L.; Keeney, E.; O’Donnell, R.; Jackson, J.; Roadevin, C.; Dawson, S.; Lane, D.; Stubbs, J.; et al. Defining the optimum strategy for identifying adults and children with coeliac disease: Systematic review and economic modelling. Health Technol. Assess. 2022, 26, 1–310. [Google Scholar] [CrossRef]
- Khan, A.R.; Gholap, A.D.; Grewal, N.S.; Jun, Z.; Khalid, M.; Zhang, H. Advances in smart hybrid scaffolds: A strategic approach for regenerative clinical applications. Eng. Regen. 2025, 6, 85–110. [Google Scholar] [CrossRef]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Souza, A.P.C.; Neves, J.G.; Navarro da Rocha, D.; Lopes, C.C.; Moraes, Â.M.; Correr-Sobrinho, L.; Correr, A.B. Chitosan/Xanthan membrane containing hydroxyapatite/Graphene oxide nanocomposite for guided bone regeneration. J. Mech. Behav. Biomed. Mater. 2022, 136, 105464. [Google Scholar] [CrossRef]
- Nag, S.; Mohanto, S.; Ahmed, M.G.; Subramaniyan, V. “Smart” stimuli-responsive biomaterials revolutionizing the theranostic landscape of inflammatory arthritis. Mater. Today Chem. 2024, 39, 102178. [Google Scholar] [CrossRef]
- Zarubova, J.; Hasani-Sadrabadi, M.M.; Ardehali, R.; Li, S. Immunoengineering strategies to enhance vascularization and tissue regeneration. Adv. Drug Deliv. Rev. 2022, 184, 114233. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Leyendecker Junior, A.; Gomes Pinheiro, C.C.; Lazzaretti Fernandes, T.; Franco Bueno, D. The use of human dental pulp stem cells for in vivo bone tissue engineering: A systematic review. J. Tissue Eng. 2018, 9, 2041731417752766. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hong, Y.; Fu, X.; Sun, X. Advances and applications of biomimetic biomaterials for endogenous skin regeneration. Bioact. Mater. 2024, 39, 492–520. [Google Scholar] [CrossRef]
- Barreto, M.E.V.; Medeiros, R.P.; Shearer, A.; Fook, M.V.L.; Montazerian, M.; Mauro, J.C. Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. J. Funct. Biomater. 2022, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, S.; Ionita, M.; Ignat, S.R.; Costache, M.; Hermenean, A. Graphene Oxide Enhances Chitosan-Based 3D Scaffold Properties for Bone Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5077. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J. Biomed. Mater. Res. A 2022, 110, 266–272. [Google Scholar] [CrossRef]
- Wang, H.; Sun, R.; Huang, S.; Wu, H.; Zhang, D. Fabrication and properties of hydroxyapatite/chitosan composite scaffolds loaded with periostin for bone regeneration. Heliyon 2024, 10, e25832. [Google Scholar] [CrossRef]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent Advances of Chitosan-Based Injectable Hydrogels for Bone and Dental Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 587658. [Google Scholar] [CrossRef] [PubMed]
- Sinani, G.; Sessevmez, M.; Gök, M.K.; Özgümüş, S.; Alpar, H.O.; Cevher, E. Modified chitosan-based nanoadjuvants enhance immunogenicity of protein antigens after mucosal vaccination. Int. J. Pharm. 2019, 569, 118592. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Gao, Y.; Shu, J.; Zhang, C.; Zhao, K. Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines. Vaccines 2022, 10, 1906. [Google Scholar] [CrossRef]
- Predoi, D.; Ţălu, Ş.; Ciobanu, S.C.; Iconaru, S.L.; Matos, R.S.; Da Fonseca Filho, H.D. Exploring the Physicochemical Traits, Antifungal Capabilities, and 3D spatial complexity of Hydroxyapatite with Ag+‒Mg2+ Substitution in the Biocomposite Thin Films. Micron 2024, 184, 103661. [Google Scholar] [CrossRef]
- Liuyun, J.; Yubao, L.; Chengdong, X. Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J. Biomed. Sci. 2009, 16, 65. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, S.; Kiryk, J.; Horodniczy, T.; Struzik, N.; Wiśniewska, K.; Matys, J.; Dobrzyński, M. Bone Regeneration Capabilities of Scaffolds Containing Chitosan and Nanometric Hydroxyapatite-Systematic Review Based on In Vivo Examinations. Biomimetics 2024, 9, 503. [Google Scholar] [CrossRef]
- Fendi, F.; Abdullah, B.; Suryani, S.; Usman, A.N.; Tahir, D. Development and application of hydroxyapatite-based scaffolds for bone tissue regeneration: A systematic literature review. Bone 2024, 183, 117075. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Ţălu, Ş.; Predoi, S.-A.; Buton, N.; Ramos, G.Q.; Da Fonseca Filho, H.D.; Matos, R.S. Synthesis, Characterization, and Antifungal properties of Chrome-Doped Hydroxyapatite thin films. Mater. Chem. Physics 2024, 324, 129690. [Google Scholar] [CrossRef]
- Dou, D.D.; Zhou, G.; Liu, H.W.; Zhang, J.; Liu, M.L.; Xiao, X.F.; Fei, J.J.; Guan, X.L.; Fan, Y.B. Sequential releasing of VEGF and BMP-2 in hydroxyapatite collagen scaffolds for bone tissue engineering: Design and characterization. Int. J. Biol. Macromol. 2019, 123, 622–628. [Google Scholar] [CrossRef]
- Cao, H.; He, S.; Wu, M.; Hong, L.; Feng, X.; Gao, X.; Li, H.; Liu, M.; Lv, N. Cascaded controlled delivering growth factors to build vascularized and osteogenic microenvironment for bone regeneration. Mater. Today Bio 2024, 25, 101015. [Google Scholar] [CrossRef]
| Assessment | Test/Method | Result |
|---|---|---|
| Hemolysis | Hemolysis assay | Very low hemolysis rates; within acceptable biomaterial range |
| RBC Aggregation | Microscopic analysis | No red blood cell aggregation; normal blood fluidity |
| Plasma Protein Adsorption | Protein adsorption assay | High albumin adsorption; favorable for biocompatibility |
| General Cytotoxicity | L929 fibroblast cell model | Comprehensive in vitro evaluation using multiple assays |
| MTT and NRU | MTT and Neutral Red Uptake assays | Cell viability >95%; non-toxic |
| DAPI and Trypan Blue | DAPI staining and Trypan Blue dye exclusion | Viable cells observed; minimal cell death |
| Direct Contact Assay | Direct material–cell contact | No changes in morphology or viability; confirms cytocompatibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frumento, D.; Țălu, Ș. Immunomodulatory Potential and Biocompatibility of Chitosan–Hydroxyapatite Biocomposites for Tissue Engineering. J. Compos. Sci. 2025, 9, 305. https://doi.org/10.3390/jcs9060305
Frumento D, Țălu Ș. Immunomodulatory Potential and Biocompatibility of Chitosan–Hydroxyapatite Biocomposites for Tissue Engineering. Journal of Composites Science. 2025; 9(6):305. https://doi.org/10.3390/jcs9060305
Chicago/Turabian StyleFrumento, Davide, and Ștefan Țălu. 2025. "Immunomodulatory Potential and Biocompatibility of Chitosan–Hydroxyapatite Biocomposites for Tissue Engineering" Journal of Composites Science 9, no. 6: 305. https://doi.org/10.3390/jcs9060305
APA StyleFrumento, D., & Țălu, Ș. (2025). Immunomodulatory Potential and Biocompatibility of Chitosan–Hydroxyapatite Biocomposites for Tissue Engineering. Journal of Composites Science, 9(6), 305. https://doi.org/10.3390/jcs9060305









