Flexible Piezoresistive Sensors Based on PANI/rGO@PDA/PVDF Nanofiber for Wearable Biomonitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of Electrospinning Nanofibers Using Dopamine
2.3. Preparation of Sensing Nanofiber Materials
2.4. Fabrication of PANI/rGO@PDA/PVDF Nanofiber Piezoresistive Sensors
2.5. Characterization and Measurement
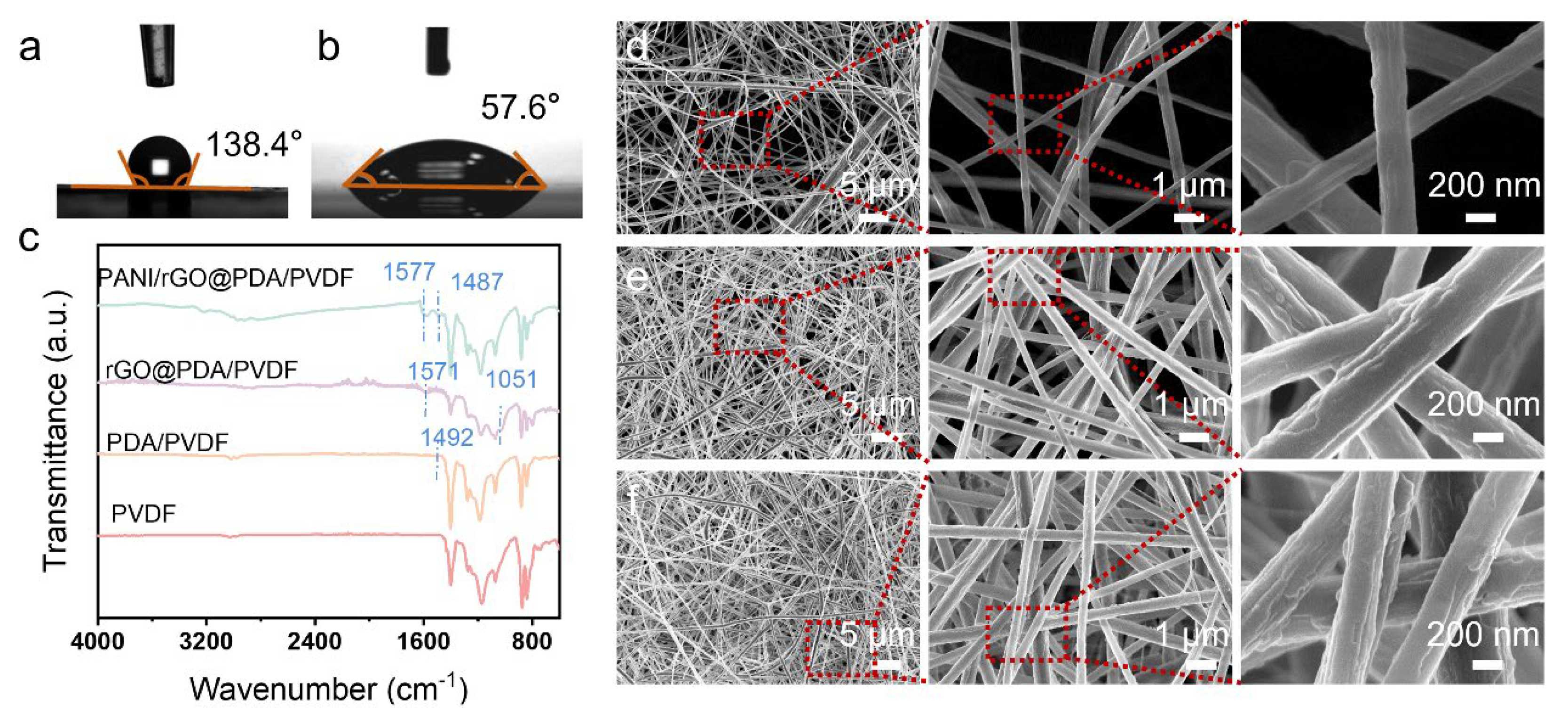
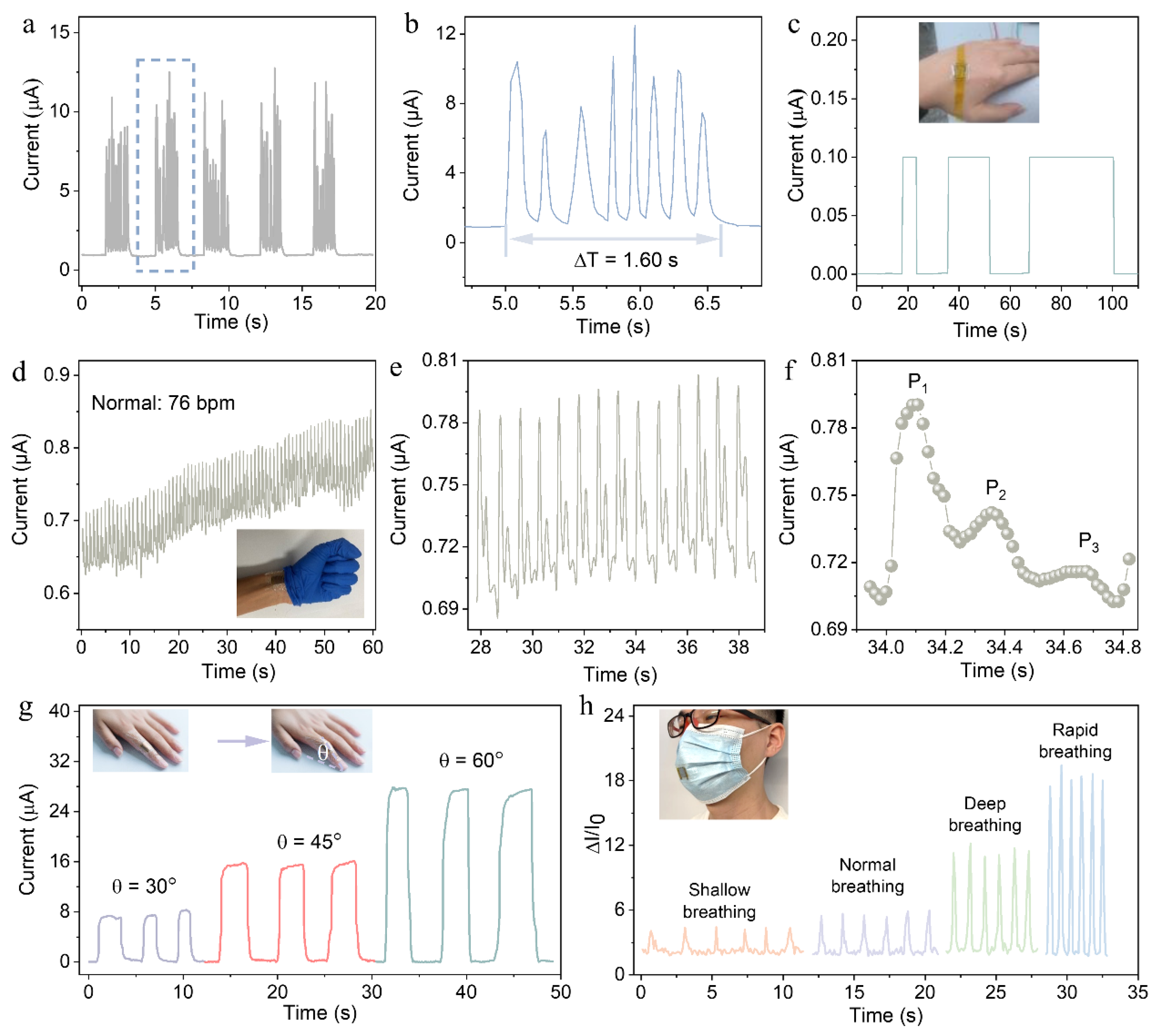
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Q.; Ding, Q.; Geng, Z.; Hu, C.; Yang, L.; Kan, Z.; Dong, B.; Won, M.; Song, H.; Xu, L.; et al. A Flexible Smart Healthcare Platform Conjugated with Artificial Epidermis Assembled by Three-Dimensionally Conductive MOF Network for Gas and Pressure Sensing. Nano-Micro Lett. 2024, 17, 50–69. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Fang, Y.; Wang, Z.; Libanori, A.; Xiao, X.; Wan, D.; Cui, X.; Sang, S.; Zhang, W.; Zhang, H.; et al. Deep Learning Assisted Body Area Triboelectric Hydrogel Sensor Network for Infant Care. Adv. Funct. Mater. 2022, 32, 2204803. [Google Scholar] [CrossRef]
- Yang, J.S.; Chung, M.K.; Yoo, J.Y.; Kim, M.U.; Kim, B.J.; Jo, M.S.; Kim, S.H.; Yoon, J.B. Interference-free nanogap pressure sensor array with high spatial resolution for wireless human-machine interfaces applications. Nat. Commun. 2025, 16, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Y Guo, Y.; Zhong, M.; Fang, Z.; Wan, P.; Yu, G. A Wearable Transient Pressure Sensor Made with MXene Nanosheets for Sensitive Broad-Range Human-Machine Interfacing. Nano Lett. 2019, 19, 1143–1150.ok. [Google Scholar] [CrossRef]
- Wei, X.; Li, H.; Yue, W.; Gao, S.; Chen, Z.; Li, Y.; Shen, G. A high-accuracy, real-time, intelligent material perception system with a machine-learning-motivated pressure-sensitive electronic skin. Matter 2022, 5, 1481–1501. [Google Scholar] [CrossRef]
- Kim, Y.; Chortos, A.; Xu, W.; Liu, Y.; Oh, J.Y.; Son, D.; Kang, J.; Foudeh, A.M.; Zhu, C.; Lee, Y.; et al. A bioinspired flexible organic artificial afferent nerve. Science 2018, 360, 998–1003. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Hou, X.; Li, G.; Wang, L.; Bai, N.; Cai, M.; Zhao, L.; Wang, Y.; Zhang, J.; et al. Highly stable flexible pressure sensors with a quasi-homogeneous composition and interlinked interfaces. Nat. Commun. 2022, 13, 1317–1328. [Google Scholar] [CrossRef]
- Niu, H.; Li, H.; Gao, S.; Li, Y.; Wei, X.; Chen, Y.; Yue, W.; Zhou, W.; Shen, G. Perception-to-cognition tactile sensing based on artificial-intelligence-motivated human full-Skin bionic electronic skin. Adv. Mater. 2022, 34, 2202622. [Google Scholar] [CrossRef]
- Pan, H.; Chen, G.; Chen, Y.; Di Carlo, A.; Mayer, M.A.; Shen, S.; Chen, C.; Li, W.; Subramaniam, S.; Huang, H.; et al. Biodegradable cotton fiber-based piezoresistive textiles for wearable biomonitoring. Biosens. Bioelectron. 2023, 222, 114999. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, W.; Hong, X.; Zou, L.; Liu, Z.; Wang, P.; Li, C. Versatile Electronic Textile Enabled by a Mixed-Dimensional Assembly Strategy. Small 2023, 19, 2208134. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Li, X.; Zeng, W.; Zhao, T.; Luo, B.; Liu, T.; Chi, M.; Cai, C.; Zhang, S.; et al. Graded Nanotexturing Architectural Wearable Triboelectric Sensor for Programmable Haptic Exploration. Nano Lett. 2024, 24, 13542–13550. [Google Scholar] [CrossRef]
- Su, Y.; Li, W.; Cheng, X.; Zhou, Y.; Yang, S.; Zhang, X.; Chen, C.; Yang, T.; Pan, H.; Xie, G.; et al. High-performance piezoelectric composites via beta phase programming. Nat. Commun. 2022, 13, 4867–4878. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Li, A.; Xu, J.; Karjagi, S.; Hahm, E.; Rulloda, L.; Li, J.; Hollister, J.; Kavehpour, P.; et al. A self-filtering liquid acoustic sensor for voice recognition. Nat. Electron. 2024, 7, 924–932. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Xu, J.; Fang, Y.; Chen, G.; Song, Y.; Li, S.; Chen, J. Giant magnetoelastic effect in soft systems for bioelectronics. Nat. Mater. 2021, 20, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Ruth, S.R.A.; Feig, V.R.; Tran, H.; Bao, Z. Microengineering Pressure Sensor Active Layers for Improved Performance. Adv. Funct. Mater. 2020, 30, 2003491. [Google Scholar] [CrossRef]
- Yang, R.; Dutta, A.; Li, B.; Tiwari, N.; Zhang, W.; Niu, Z.; Gao, Y.; Erdely, D.; Xin, X.; Li, T.; et al. Iontronic pressure sensor with high sensitivity over ultra-broad linear range enabled by laser-induced gradient micro-pyramids. Nat. Commun. 2023, 14, 2907–2918. [Google Scholar] [CrossRef]
- Bai, N.; Wang, L.; Xue, Y.; Wang, Y.; Hou, X.; Li, G.; Zhang, Y.; Cai, M.; Zhao, L.; Guan, F.; et al. Graded interlocks for iontronic pressure sensors with high sensitivity and high linearity over a broad range. ACS Nano 2022, 16, 4338–4347. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; Zhang, D.; Zhang, H.; Ma, J.; Zheng, J.; Wang, C. Dual-stage surficial microstructure to enhance the sensitivity of MXene pressure sensors for human physiological signal acquisition. ACS Appl. Mater. Interfaces 2024, 16, 1096–1106. [Google Scholar] [CrossRef]
- Wang, S.; Deng, W.; Yang, T.; Ao, Y.; Zhang, H.; Tian, G.; Deng, L.; Huang, H.; Huang, J.; Lan, B.; et al. Bioinspired MXene-based piezoresistive sensor with two-stage enhancement for motion capture. Adv. Funct. Mater. 2023, 33, 2214503. [Google Scholar] [CrossRef]
- Li, L.; Cheng, Y.; Cao, H.; Liang, Z.; Liu, Z.; Yan, S.; Li, L.; Jia, S.; Wang, J.; Gao, Y. MXene/rGO/PS spheres multiple physical networks as high-performance pressure sensor. Nano Energy 2022, 95, 106986. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Zhao, C.; Zhang, L.; Li, J.; Din, S.U.; Wang, Z.; Sun, J.; Torres, S.A.G.; Fan, Z.; et al. Aluminium surface work hardening enables multi-scale 3D lithography. Nat. Mater. 2025, 24, 39–47. [Google Scholar] [CrossRef]
- Pan, L.; Chortos, A.; Yu, G.; Wang, Y.; Isaacson, S.; Allen, R.; Shi, Y.; Dauskardt, R.; Bao, Z. An ultra-sensitive resistive pressure sensor based on hollow-sphere microstructure induced elasticity in conducting polymer film. Nat. Commun. 2014, 5, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhang, F.; Qu, S.; Yin, L.; Li, K.; Huang, Y. Marangoni-driven deterministic formation of softer, hollow microstructures for sensitivity-enhanced tactile system. Nat. Commun. 2024, 15, 5596–5608. [Google Scholar] [CrossRef]
- Wang, Q.; Jian, M.; Wang, C.; Zhang, Y. Carbonized silk nanofiber membrane for transparent and sensitive electronic skin. Adv. Funct. Mater. 2017, 27, 1605657. [Google Scholar] [CrossRef]
- Yin, R.; Yang, S.; Li, Q.; Zhang, S.; Liu, H.; Han, J.; Liu, C.; Shen, C. Flexible conductive Ag nanowire/cellulose nanofibril hybrid nanopaper for strain and temperature sensing applications. Sci. Bull. 2020, 65, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xiao, X.; Zhao, X.; Tat, T.; Bick, M.; Chen, J. Electronic textiles for wearable point-of-care systems. Chem. Rev. 2022, 122, 3259–3291. [Google Scholar] [CrossRef]
- Dai, J.; Xie, G.; Huo, X.; Li, J.; Deng, S.; Su, Y. Implantable and Biodegradable Smart Textiles for Continuous Limb and Gastrointestinal Motility Monitoring. Small 2025, 21, 2407773. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Hong, J.; Ha, M.; Jung, Y.-D.; Lim, H.; Kim, S.Y.; Ko, H. Giant tunneling piezoresistance of composite elastomers with interlocked microdome arrays for ultrasensitive and multimodal electronic skins. ACS Nano J. 2014, 8, 4689–4697. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, J.; Dai, Z.; Zang, X.; Dong, Q.; Guan, G.; Li, L.J.; Huang, W.; Dong, X. Extraordinarily stretchable all-carbon collaborative nanoarchitectures for epidermal sensors. Adv. Mater. 2017, 29, 1606411. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, L.; Wang, H.; Zhu, K.; Zhang, M. Vertical CNT–Ecoflex nanofins for highly linear broad-range-detection wearable strain sensors. J. Mater. Chem. C 2018, 6, 5132–5139. [Google Scholar] [CrossRef]
- Cheng, R.; Zeng, J.; Wang, B.; Li, J.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Ultralight, flexible and conductive silver nanowire/nanofibrillated cellulose aerogel for multifunctional strain sensor. Chem. Eng. J. 2021, 424, 130565. [Google Scholar] [CrossRef]
- Guan, F.; Xie, Y.; Wu, H.; Meng, Y.; Shi, Y.; Gao, M.; Zhang, Z.; Chen, S.; Chen, Y.; Wang, H.; et al. Silver Nanowire-Bacterial Cellulose Composite Fiber-Based Sensor for Highly Sensitive Detection of Pressure and Proximity. ACS Nano J. 2020, 14, 15428–15439. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, K.; Lou, Z.; Chen, D.; Shen, G. Recent Developments in Graphene-Based Tactile Sensors and E-Skins. Adv. Mater. Technol. 2018, 3, 1700248. [Google Scholar] [CrossRef]
- Tao, L.Q.; Zhang, K.N.; Tian, H.; Liu, Y.; Wang, D.Y.; Chen, Y.Q.; Yang, Y.; Ren, T.L. Graphene-paper pressure sensor for detecting human motions. ACS Nano J. 2017, 11, 8790–8795. [Google Scholar] [CrossRef] [PubMed]
- Bosque, A.d.; Sánchez-Romate, X.F.; Sánchez, M.; Ureña, A. Ultrasensitive and highly stretchable sensors for human motion monitoring made of graphene reinforced polydimethylsiloxane: Electromechanical and complex impedance sensing performance. Carbon 2022, 192, 234–248. [Google Scholar] [CrossRef]
- Lou, Z.; Chen, S.; Wang, L.; Shi, R.; Li, L.; Jiang, K.; Chen, D.; Shen, G. Ultrasensitive and ultraflexible e-skins with dual functionalities for wearable electronics. Nano Energy 2017, 38, 28–35. [Google Scholar] [CrossRef]
- Pan, H.; Xie, G.; Pang, W.; Wang, S.; Wang, Y.; Jiang, Z.; Du, X.; Tai, H. Surface engineering of a 3D topological network for ultrasensitive piezoresistive pressure sensors. ACS Appl. Mater. Interfaces 2020, 12, 38805. [Google Scholar] [CrossRef]
- Bi, L.; Yang, Z.; Chen, L.; Wu, Z.; Ye, C. Compressible AgNWs/Ti3C2Tx MXene aerogel-based highly sensitive piezoresistive pressure sensor as versatile electronic skins. J. Mater. Chem. A 2020, 8, 20030–20036. [Google Scholar] [CrossRef]
- Zhi, H.; Zhang, X.; Wang, F.; Wan, P.; Feng, L. Flexible Ti3C2Tx MXene/PANI/Bacterial Cellulose Aerogel for e-Skins and Gas Sensing. ACS Appl. Mater. Interfaces 2021, 13, 45987–45994. [Google Scholar] [CrossRef]
- Cao, M.; Wang, M.; Li, L.; Qiu, H.; Padhiar, M.A.; Yang, Z. Wearable rGO-Ag NW@cotton fiber piezoresistive sensor based on the fast charge transport channel provided by Ag nanowire. Nano Energy 2018, 50, 528–535. [Google Scholar] [CrossRef]
- Wardani, A.K.; Ariono, D.; Subagjo, S.; Wenten, I.G. Fouling tendency of PDA/PVP surface modified PP membrane. Surf. Interfaces. 2020, 19, 100464. [Google Scholar] [CrossRef]
- Zhu, T.; Chang, S.; Song, Y.-F.; Lahoubi, M.; Wang, W. PVP-encapsulated CoFe2O4/rGO composites with controllable electromagnetic wave absorption performance. Chem. Eng. J. 2019, 373, 755–766. [Google Scholar] [CrossRef]
- Wang, D.; Giannakis, S.; Tang, J.; Luo, K.; Tang, J.; He, Z.; Song, S.; Wang, L. Effect of rGO content on enhanced Photo-Fenton degradation of Venlafaxine using rGO encapsulated magnetic hexagonal FeTiO3 nanosheets. Chem. Eng. J. 2023, 478, 147319. [Google Scholar] [CrossRef]
- Luo, W.; Wei, Y.; Zhuang, Z.; Lin, Z.; Li, X.; Hou, C.; Li, T.; Ma, Y. Fabrication of Ti3C2Tx MXene/polyaniline composite films with adjustable thickness for high-performance flexible all-solid-state symmetric supercapacitors. Electrochim. Acta 2022, 406, 139871. [Google Scholar] [CrossRef]
- Su, Y.; Chen, C.; Pan, H.; Yang, Y.; Chen, G.; Zhao, X.; Li, W.; Gong, Q.; Xie, G.; Zhou, Y.; et al. Muscle fibers inspired high-performance piezoelectric textiles for wearable physiological monitoring. Adv. Funct. Mater. 2021, 31, 2010962. [Google Scholar] [CrossRef]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable pressure sensors for pulse wave monitoring. Adv. Mater. 2022, 34, 2109357. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Li, D.; Xu, Y.; Xu, F. Flexible and Sensitivity-Adjustable Pressure Sensors Based on Carbonized Bacterial Nanocellulose/Wood-Derived Cellulose Nanofibril Composite Aerogels. ACS Appl. Mater. Interfaces 2021, 13, 8754–8763. [Google Scholar] [CrossRef]
- Wang, B.; Shi, T.; Zhang, Y.; Chen, C.; Li, Q.; Fan, Y. Lignin-based highly sensitive flexible pressure sensor for wearable electronics. J. Mater. Chem. C 2018, 6, 6423–6428. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, R.; Zhao, Y.; Liu, H.; Zhang, D.; Shi, X.; Zhang, B.; Liu, C.; Shen, C. Conductive MXene/cotton fabric based pressure sensor with both high sensitivity and wide sensing range for human motion detection and E-skin. Chem. Eng. J. 2021, 420, 127720. [Google Scholar] [CrossRef]


| Sensing Material | Sensitivity (kPa−1)/Pressure Range | Response Time (ms) | Stability(Cycles) | Reference |
|---|---|---|---|---|
| Ti3C2Tx-paper | 3.81/0.982–10 kPa | 11 | 10,000 | [4] |
| Carbonized cellulose | 0.2/0–3 kPa 0.15/3–10 kPa | N/A | N/A | [47] |
| Graphene/paper | 17.2/0–2 kPa 0.012/2–20 kPa | 120 | 300 | [48] |
| Carbonized silk nanofiber | 34.71/0.8–400 Pa 1.16/0.4–5 kPa | 16.5 | 10,000 | [24] |
| MXene/cotton | 5.3/0–1.3 kPa | 50 | 900 | [49] |
| PANI/rGO@PDA/PVDF | 13.43/0–10 kPa | 9 | 12,000 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Wang, Y.; Xie, G.; Chen, C.; Li, H.; Wu, F.; Su, Y. Flexible Piezoresistive Sensors Based on PANI/rGO@PDA/PVDF Nanofiber for Wearable Biomonitoring. J. Compos. Sci. 2025, 9, 339. https://doi.org/10.3390/jcs9070339
Pan H, Wang Y, Xie G, Chen C, Li H, Wu F, Su Y. Flexible Piezoresistive Sensors Based on PANI/rGO@PDA/PVDF Nanofiber for Wearable Biomonitoring. Journal of Composites Science. 2025; 9(7):339. https://doi.org/10.3390/jcs9070339
Chicago/Turabian StylePan, Hong, Yuxiao Wang, Guangzhong Xie, Chunxu Chen, Haozhen Li, Fang Wu, and Yuanjie Su. 2025. "Flexible Piezoresistive Sensors Based on PANI/rGO@PDA/PVDF Nanofiber for Wearable Biomonitoring" Journal of Composites Science 9, no. 7: 339. https://doi.org/10.3390/jcs9070339
APA StylePan, H., Wang, Y., Xie, G., Chen, C., Li, H., Wu, F., & Su, Y. (2025). Flexible Piezoresistive Sensors Based on PANI/rGO@PDA/PVDF Nanofiber for Wearable Biomonitoring. Journal of Composites Science, 9(7), 339. https://doi.org/10.3390/jcs9070339






