Enhanced C3H6O and CO2 Sensory Properties of Nickel Oxide-Functionalized/Carbon Nanotube Composite: A Comprehensive Theoretical Study
Abstract
1. Introductions
2. Materials and Methods
- Applicability to a wide range of systems, from small organic molecules to biologically active compounds and transition metals.
- Better accuracy than other functionals, while requiring less computational resources than, for example, high-level approximation methods such as CCSD(T).
- Possibility to describe both covalent and ionic bonds.
- The functional may be inaccurate for some systems, such as those containing transition metals or strongly polarizable atoms.
- It is not always possible to correctly describe systems with very long links using this functional because of insufficient correlational energy.
- B3LYP may give an incorrect description of donor–acceptor bonds, especially in systems with a large charge distribution.
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lennon, L.S.-X.; Swager, T.M. Chemiresistive sensing with functionalized carbon nanotubes. Nat. Rev. Methods Primers 2023, 3, 73. [Google Scholar]
- Mahmoud, N.; Sajjadi, M.; Iravani, S.; Varma, R.S. Carbon-based sustainable nanomaterials for water treatment: State-of-art and future perspectives. Chemosphere 2021, 263, 128005. [Google Scholar]
- Monika, R.; Janas, D. Carbon Nanotube Wearable Sensors for Health Diagnostics. Sensors 2021, 21, 5847. [Google Scholar] [CrossRef]
- Abdullah, A.; Wahab, N.Z.A.; Mohtar, M.N.; Hamidon, M.N.; Shafie, S.; Halin, I.A. Methods and applications of electrical conductivity enhancement of materials using carbon nanotubes. J. Electron. Mater. 2021, 50, 3207–3221. [Google Scholar]
- Negri, R.B.P.; da Silva, A.H.M.F.T.; de Sousa, A.M.F.; da Silva, A.L.N.; da Rocha, E.B.D. Improved mechanical and rheological behavior of nitrile rubber reinforced with multi-walled carbon nanotubes and carbon black dual-filler system. Mater. Today Commun. 2021, 26, 101884. [Google Scholar] [CrossRef]
- Mojtaba, A.; Zabihi, O.; Masoomi, M.; Naebe, M. Synergistic effect of MWCNTs functionalization on interfacial and mechanical properties of multi-scale UHMWPE fibre reinforced epoxy composites. Compos. Sci. Technol. 2016, 134, 1–11. [Google Scholar]
- López-Barroso, J.; Martínez-Hernández, A.L.; Rivera-Armenta, J.L.; Almendárez-Camarillo, A.; García-Casillas, P.E.; Flores-Hernández, C.G.; Velasco-Santos, C. Improvements in the thermomechanical and electrical behavior of hybrid carbon-epoxy nanocomposites. Carbon Trends 2021, 5, 100126. [Google Scholar] [CrossRef]
- Sheng, Z.; Sheng, J.; Chen, Y.; Ni, J.; Li, Y. Carbon nanotubes for flexible batteries: Recent progress and future perspective. Natl. Sci. Rev. 2021, 8, nwaa261. [Google Scholar]
- Haochuan, W.; Cao, Y.; Lo, L.-W.; Zhao, J.; Sepulveda, N.; Wang, C. Flexible carbon nanotube synaptic transistor for neurological electronic skin applications. ACS Nano 2020, 14, 10402–10412. [Google Scholar]
- Xia, S.; Qin, Z.; Ye, L.; Zhang, H.; Yu, Q.; Wu, X.; Li, J.; Yao, F. Carbon nanotubes reinforced hydrogel as flexible strain sensor with high stretchability and mechanically toughness. Chem. Eng. J. 2020, 382, 122832. [Google Scholar]
- Qu, T.; Sun, Y.; Chen, M.; Liu, Z.; Zhu, Q.; Wang, B.; Zhao, T.; Liu, C.; Tan, J.; Qiu, S.; et al. A flexible carbon nanotube sen-memory device. Adv. Mater. 2020, 32, 1907288. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Saad, M.S.I.; Piam, M.F.; Talukdar, T.A.; Shobdo, T.T.; Pritha, N.M. Carbon nanotubes: Structure, properties and applications in the aerospace industry. Results Mater. 2025, 25, 100654. [Google Scholar]
- Arash, Y.; Nanda, S.; Dalai, A.K. Carbon Nanotubes: A Review of Synthesis Methods and Applications. Reactions 2025, 5, 429–451. [Google Scholar]
- Ya, M.; Shen, X.-L.; Zeng, Q.; Wang, H.-S.; Wang, L.-S. A multi-walled carbon nanotubes based molecularly imprinted polymers electrochemical sensor for the sensitive determination of HIV. Talanta 2017, 164, 121–127. [Google Scholar]
- Chunying, J.; Mu, X.; Liu, S.; Liu, Z.; Du, B.; Wang, J.; Xu, J. A study of the detection of SARS-CoV-2 ORF1ab gene by the use of electrochemiluminescent biosensor based on dual-probe hybridization. Sensors 2022, 22, 2402. [Google Scholar]
- Wenting, S.; Shurin, M.R.; Wheeler, S.E.; He, X.; Star, A. Rapid detection of SARS-CoV-2 antigens using high-purity semiconducting single-walled carbon nanotube-based field-effect transistors. ACS Appl. Mater. Interfaces 2021, 13, 10321–10327. [Google Scholar]
- Congyu, W.; Wang, P.; Chen, J.; Zhu, L.; Zhang, D.; Wan, Y.; Ai, S. Self-powered biosensing system driven by triboelectric nanogenerator for specific detection of Gram-positive bacteria. Nano Energy 2022, 93, 106828. [Google Scholar]
- Qu, X.; Qi, P.; Wang, P.; Li, J.; Wang, C.; Zhang, D.; Wan, Y.; Ai, S.; Wang, X. A self-powered biosensing system based on triboelectric nanogenerator for rapid bacterial DNA detection. Sens. Actuators B Chem. 2023, 390, 133917. [Google Scholar] [CrossRef]
- Tahsin, E.; Dinç, B.; Üstünsoy, R.; Eraslan, H.; Ergenç, A.F.; Bektaş, M. Novel electrochemical biosensor for Escherichia coli using gold-coated tungsten wires and antibody functionalized short multiwalled carbon nanotubes. Instrum. Sci. Technol. 2024, 52, 109–124. [Google Scholar]
- Rokhsareh, A.; Raoof, J.B.; Mohseni, M.; Hashkavayi, A.B. Sandwich-type electrochemical aptasensor for highly sensitive and selective detection of Pseudomonas aeruginosa bacteria using a dual signal amplification strategy. Bioelectrochemistry 2023, 150, 108332. [Google Scholar]
- Thanattha, C.; Threrujirapapong, T.; Yordsri, V.; Treetong, A.; Inpaeng, S.; Tedsree, K.; Ayala, P.; Pichler, T.; Shi, L.; Muangrat, W. Highly Sensitive and Selective Formaldehyde Gas Sensors Based on Polyvinylpyrrolidone/Nitrogen-Doped Double-Walled Carbon Nanotubes. Sensors 2022, 22, 9329. [Google Scholar]
- Twinkle, P.; Chandnani, A.; Subbaraman, H.; Estrada, D. A review of inkjet printed graphene and carbon nanotubes based gas sensors. Sensors 2020, 20, 5642. [Google Scholar] [CrossRef] [PubMed]
- Zohauddin, A.; Manzoor, S.; Talib, M.; Islam, S.S.; Mishra, P. Self-standing MWCNTs based gas sensor for detection of environmental limit of CO2. Mater. Sci. Eng. B 2020, 255, 114528. [Google Scholar]
- Guo, S.-Y.; Hou, P.-X.; Zhang, F.; Liu, C.; Cheng, H.-M. Gas Sensors Based on Single-Wall Carbon Nanotubes. Molecules 2022, 27, 5381. [Google Scholar] [CrossRef]
- Raza, N.S.T.; Rasheed, T.; Hussain, D.; Haq, M.N.; Majeed, S.; Ahmed, N.; Nawaz, R. Modification strategies for improving the solubility/dispersion of carbon nanotubes. J. Mol. Liq. 2020, 297, 111919. [Google Scholar]
- Li, L.; Zhou, P.; Wen, J.; Sun, P.; Guo, Z. Dispersion of Single-Walled Carbon Nanotubes by Aromatic Cyclic Schiff Bases via Non-Covalent Interactions. Molecules 2024, 29, 3179. [Google Scholar] [CrossRef]
- Lisetski, L.; Bulavin, L.; Lebovka, N. Effects of Dispersed Carbon Nanotubes and Emerging Supramolecular Structures on Phase Transitions in Liquid Crystals: Physico-Chemical Aspects. Liquids 2023, 3, 246–277. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Govindhasamy, M.; Salla, S.; Kumar, M.R.; Kandhasamy, N.; Garalleh, H.A.; Garaleh, M.; Brindhadevi, K.; Pugazhendhi, A. Decoration of ZnO surface with tiny sulfide-based nanoparticles for improve photocatalytic degradation efficiency. Environ. Res. 2023, 220, 115171. [Google Scholar]
- Shadpour, M.; Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar]
- He, Y.; Jiao, M. A Mini-Review on Metal Oxide Semiconductor Gas Sensors for Carbon Monoxide Detection at Room Temperature. Chemosensors 2024, 12, 55. [Google Scholar] [CrossRef]
- Pargoletti, E.; Cappelletti, G. Breakthroughs in the Design of Novel Carbon-Based Metal Oxides Nanocomposites for VOCs Gas Sensing. Nanomaterials 2020, 10, 1485. [Google Scholar] [CrossRef]
- Nibedita, N.; Kumar, A.; Chakroborty, S.; Soren, S.; Barik, A.; Pal, K.; de Souza, F.G., Jr. Carbon nanostructure embedded novel sensor implementation for detection of aromatic volatile organic compounds: An organized review. ACS Omega 2023, 8, 4436–4452. [Google Scholar]
- Ramesh, S.; Bathula, C.; Ahmed, A.T.A.; Haldorai, Y.; Kakani, V.; Karthikeyan, C.; Selvaraj, M.; Shin, K.; Lee, Y.J.; Kim, H.-S.; et al. Nanostructurally fabrication of nickel oxide-interfaced carbon nanotubes for supercapacitors and exploration of electrochemical correlation via computer vision techniques and artificial intelligence. J. Energy Storage 2024, 82, 110429. [Google Scholar] [CrossRef]
- Babu, V.R.; Atchudan, R.; Kim, H.-J.; Yi, M.; Samyn, L.M.; de Barros, A.L.F. Fabrication of High-Performance Asymmetric Supercapacitor Consists of Nickel Oxide and Activated Carbon (NiO//AC). Catalysts 2022, 12, 375. [Google Scholar] [CrossRef]
- Aunggat, S.; Senapati, S.; Murthy, H.C.A.; Singh, L.R.; Mahato, M. Supercapacitor performance of NiO, NiO-MWCNT, and NiO–Fe-MWCNT composites. ACS Omega 2023, 8, 33380–33391. [Google Scholar]
- Boubezari, I.; Zazoua, A.; Errachid, A.; Jaffrezic-Renault, N. Sensitive Electrochemical Detection of Bioactive Molecules (Hydrogen Peroxide, Glucose, Dopamine) with Perovskites-Based Sensors. Chemosensors 2021, 9, 289. [Google Scholar] [CrossRef]
- Dereje, T.H.; Brim, E.; Rucker, K.K.; Hayes, D.; Lorenz, J.; Bisen, O.; Risch, M.; Harms, C.; Richards, R.M.; Wark, M. Influence of Annealing Temperature on the OER Activity of NiO (111) Nanosheets Prepared via Microwave and Solvothermal Synthesis Approaches. ACS Appl. Mater. Interfaces 2024, 16, 62142–62154. [Google Scholar]
- Jangra, V.; Kaur, H.; Kumar, N.; Kumar, U.; Sonkar, P.K.; Prasad, L.B. Electrochemical determination of antipsychotic drug quetiapine fumarate using hexagonal nickel oxide nanoparticle decorated functionalized multiwalled carbon nanotubes. Electrochim. Acta 2025, 531, 146432. [Google Scholar] [CrossRef]
- Moradi, S.E.; Shokrollahi, A.; Shahdost-Fard, F. Nickel cobalt oxide nanoparticles-decorated multi-walled carbon nanotubes as a high-performance sensing platform for the detection of tramadol in human biofluids. J. Alloys Compd. 2025, 1016, 178837. [Google Scholar] [CrossRef]
- Kumar, M.Q.K.P.; Khan, R.A.; Ahmad, K.; Kim, H. Fabrication of Sulfur-Doped Reduced Graphene Oxide Modified Glassy Carbon Electrode (S@rGO/GCE) Based Acetaminophen Sensor. Inorganics 2022, 10, 218. [Google Scholar]
- Zhang, X.; Wu, J.; Yan, H.; Chen, H.; Mao, W.; Dai, G. Insight into the nature of the noncovalent interactions of furan, pyridine, and pyrazine with AtX. J. Mol. Model. 2023, 29, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, H.; Chen, H.; Dai, G. Theoretical study of the nature of σ-hole regium bond between CuX/AgX/AuX and pyridine. Journal of Molecular Modeling. J. Mol. Model. 2025, 31, 134. [Google Scholar] [CrossRef]

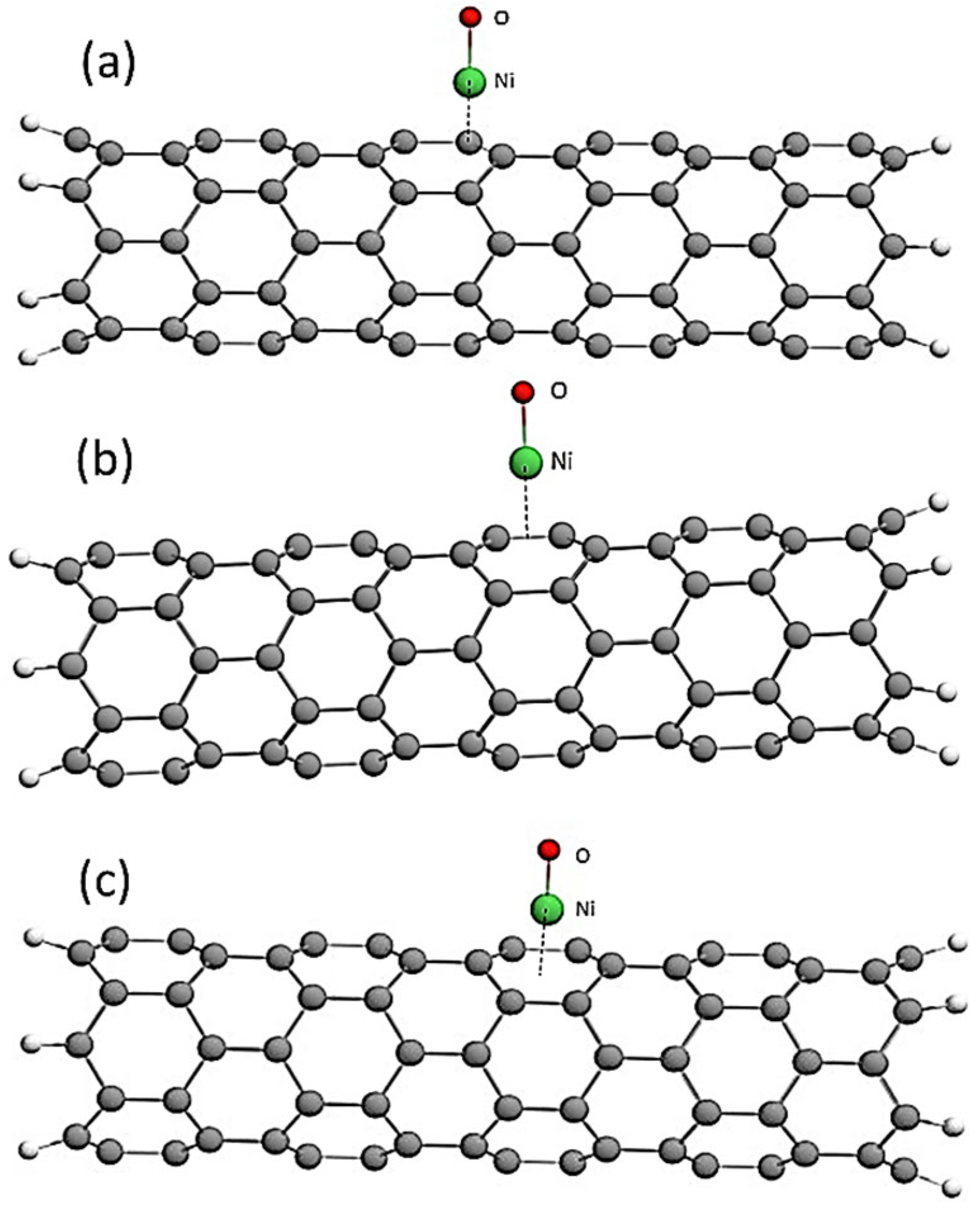
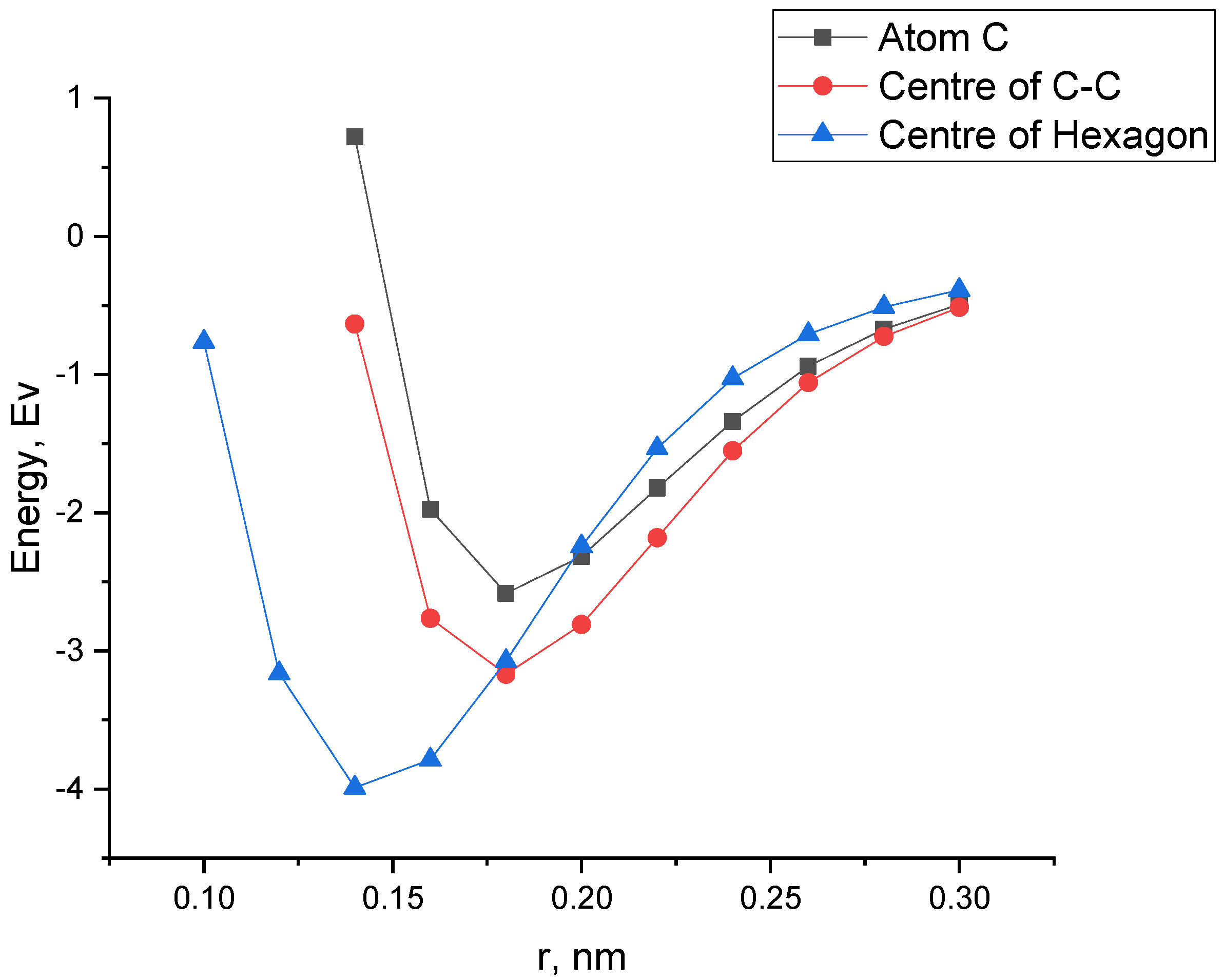
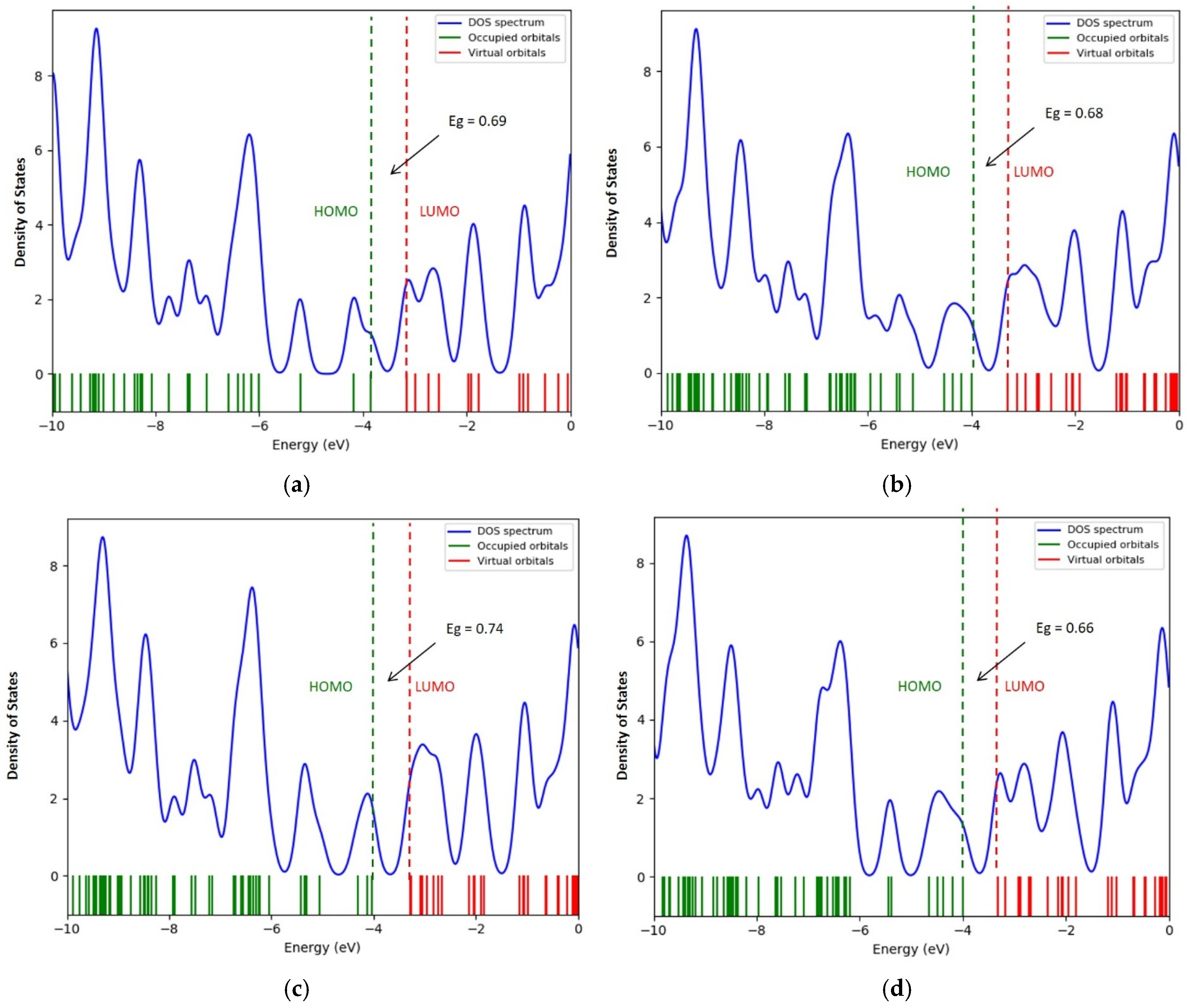

| Structure | Interaction Distance, nm | Interaction Energy, eV | ΔEg After Adsorption, eV |
|---|---|---|---|
| “Clean” nanotube type (6,0) | 0.69 | ||
| CNT (6,0)—NiO over an atom with | 0.18 | −2.58 | 0.68 |
| CNT (6,0)—NiO above the C-C communication center | 0.18 | −3.17 | 0.74 |
| CNT (6,0)—NiO above the center of the hexagon | 0.14 | −3.99 | 0.66 |
| The Variant of Adsorption of the Pt atom | The Value of the Charge on the Nickel Atom Before Joining | The Value of the Charge on the Nickel Atom After Attachment | The Average Value of the Charge of Carbon Atoms on the Surface of the Nanotube Before the Addition of the NiO Atom | The Average Value of the Charge of the Nearest Atomic Neighbors on the Surface of the Nanotube After the Addition of the NiO Atom |
|---|---|---|---|---|
| CNT (6,0)—NiO over an atom with | 0 | 0.838 | 0.01 | −0.117 |
| CNT (6,0)—NiO above the C-C communication center | 0 | 0.896 | 0.009 | −0.271 |
| CNT (6,0)—NiO above the center of the hexagon | 0 | 0.727 | 0.011 | −0.179 |
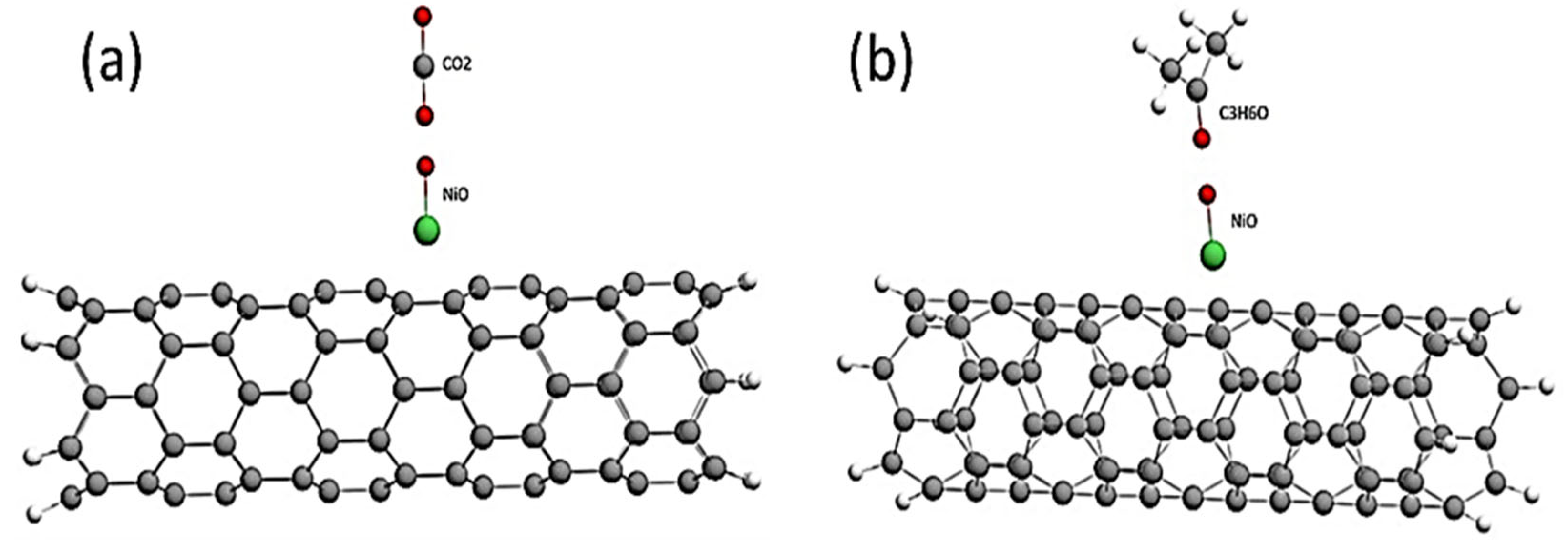
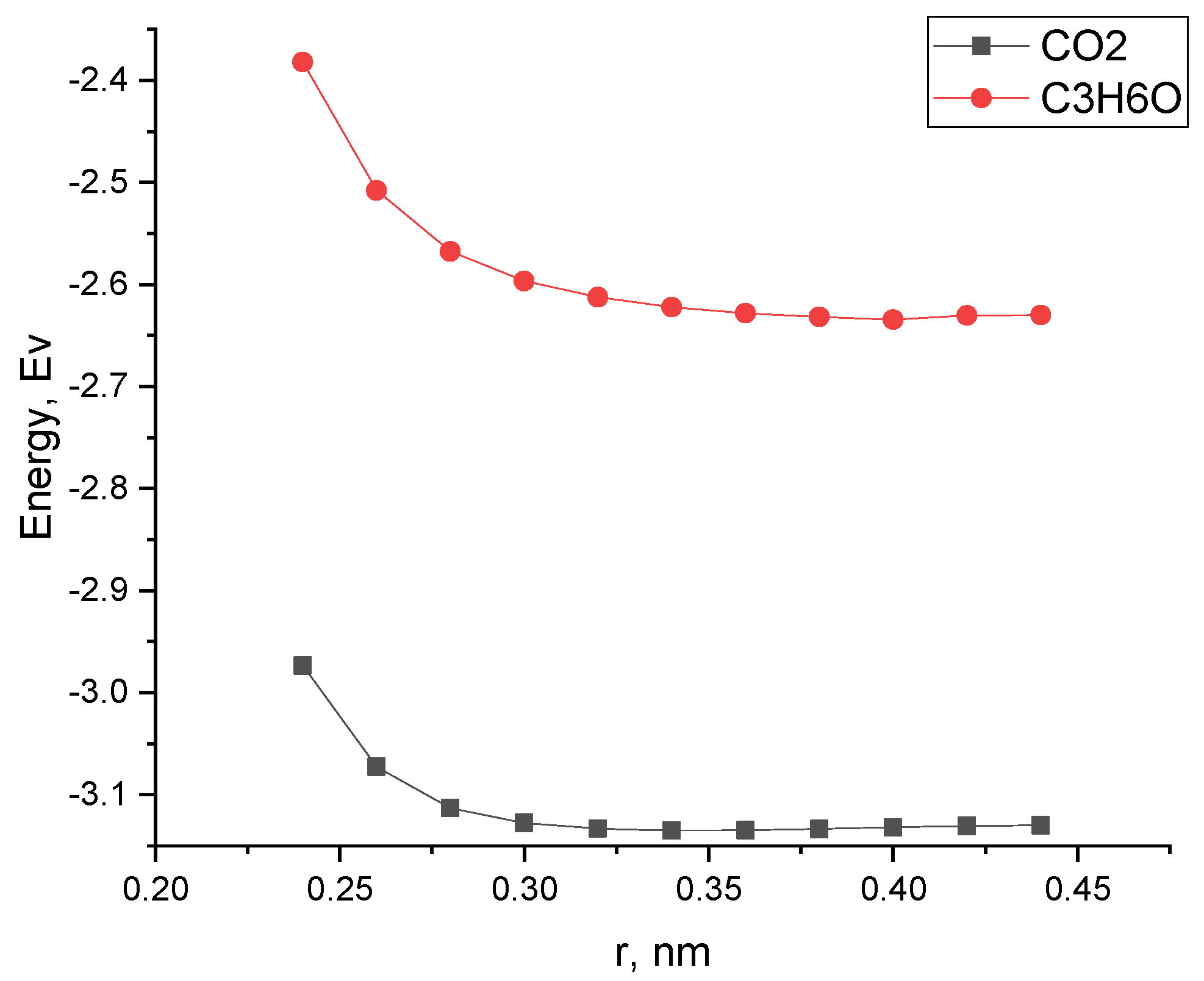
| Structure | Interaction Distance, nm | Interaction Energy, eV | ΔEg After Adsorption, eV |
|---|---|---|---|
| CNT-NiO | 0.66 | ||
| CNT-NiO + CO2 | 0.34 | −3.13 | 0.66 |
| CNT-NiO + C3H6O | 0.40 | −2.63 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dryuchkov, E.S.; Boroznin, S.V.; Zaporotskova, I.V.; Boroznina, N.P.; Murugadoss, G.; Peera, S.G. Enhanced C3H6O and CO2 Sensory Properties of Nickel Oxide-Functionalized/Carbon Nanotube Composite: A Comprehensive Theoretical Study. J. Compos. Sci. 2025, 9, 311. https://doi.org/10.3390/jcs9060311
Dryuchkov ES, Boroznin SV, Zaporotskova IV, Boroznina NP, Murugadoss G, Peera SG. Enhanced C3H6O and CO2 Sensory Properties of Nickel Oxide-Functionalized/Carbon Nanotube Composite: A Comprehensive Theoretical Study. Journal of Composites Science. 2025; 9(6):311. https://doi.org/10.3390/jcs9060311
Chicago/Turabian StyleDryuchkov, Evgeniy S., Sergey V. Boroznin, Irina V. Zaporotskova, Natalia P. Boroznina, Govindhasamy Murugadoss, and Shaik Gouse Peera. 2025. "Enhanced C3H6O and CO2 Sensory Properties of Nickel Oxide-Functionalized/Carbon Nanotube Composite: A Comprehensive Theoretical Study" Journal of Composites Science 9, no. 6: 311. https://doi.org/10.3390/jcs9060311
APA StyleDryuchkov, E. S., Boroznin, S. V., Zaporotskova, I. V., Boroznina, N. P., Murugadoss, G., & Peera, S. G. (2025). Enhanced C3H6O and CO2 Sensory Properties of Nickel Oxide-Functionalized/Carbon Nanotube Composite: A Comprehensive Theoretical Study. Journal of Composites Science, 9(6), 311. https://doi.org/10.3390/jcs9060311







