Bentonite-Based Composites in Medicine: Synthesis, Characterization, and Applications
Abstract
1. Introduction
2. Structure and Properties of Bentonite Clays
3. Influence of Bentonite Clay Composition on Its Practical Application
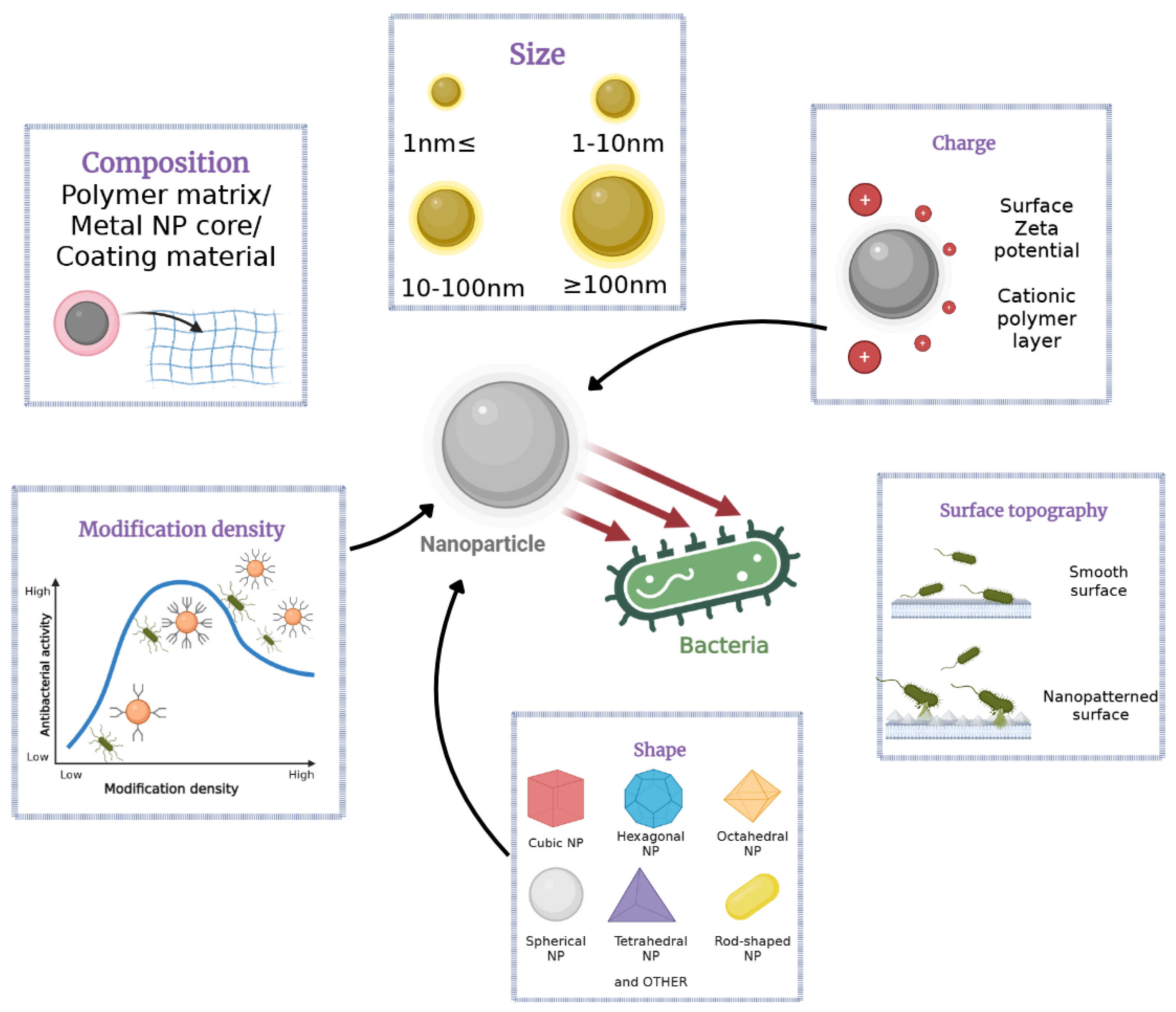
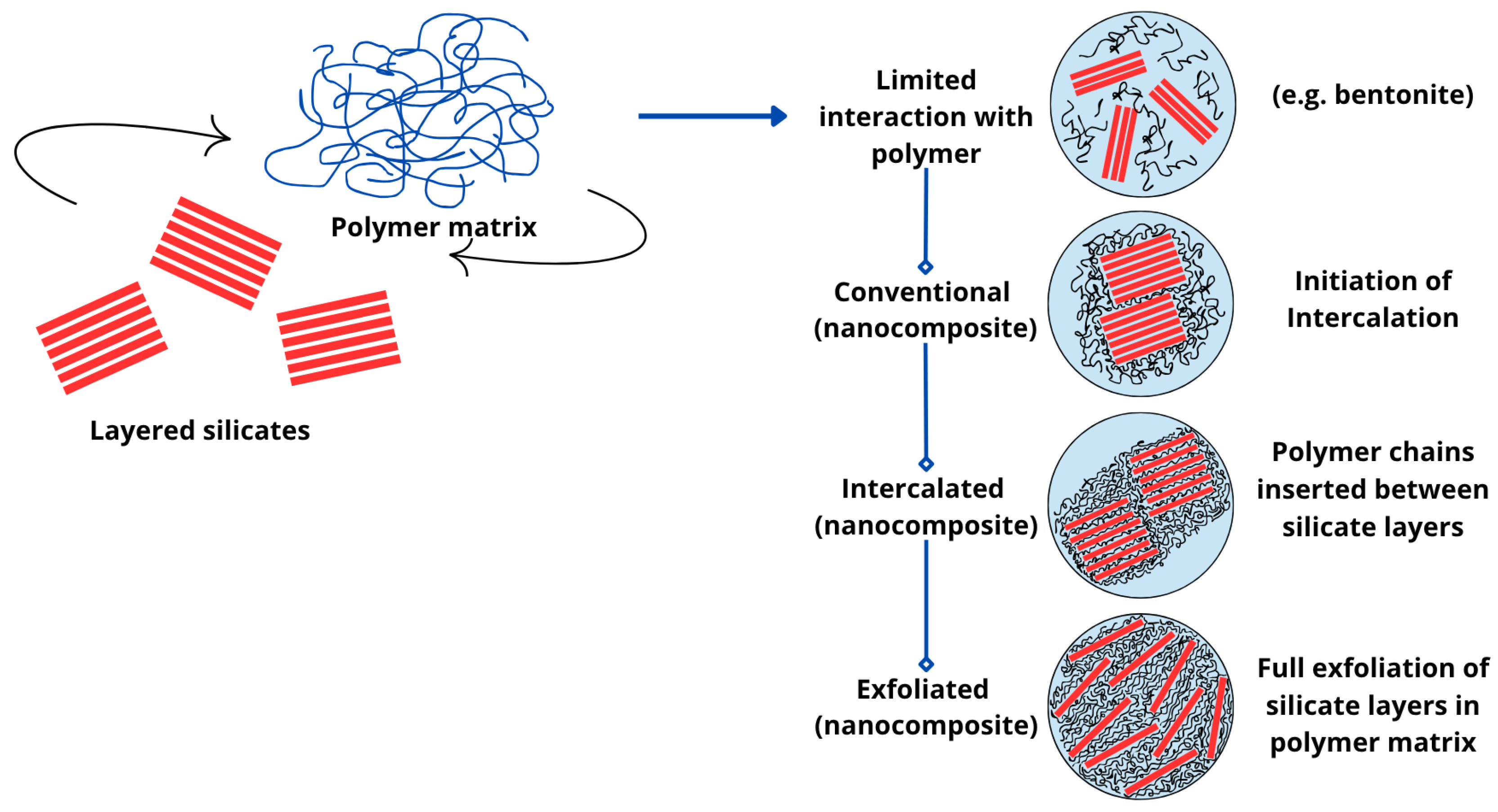
4. Modification of Bentonite Clays
- (1)
- Intercalated nanocomposites, where polymer chains are inserted into the layered silicate structure in a regular pattern, with repeating spacing of several nanometers, regardless of the polymer-to-clay ratio [80];
- (2)
- Flocculated nanocomposites, where intercalated and stacked silicate layers are flocculated to some extent due to the hydroxylated edge interactions of the silicate layers [80];
- (3)
5. Practical Use of Polymer Composite Materials in Medicine
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, L.; Chouw, N.; Jayaraman, K. Flax fibre and its composites—A review. Compos. Part B Eng. 2014, 56, 296–317. [Google Scholar] [CrossRef]
- Saba, N.; Md Tahir, P.; Jawaid, M. A review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Xu, K.; Wang, J.; Xiang, S.; Chen, Q.; Zhang, W.; Wang, P. Study on the synthesis and performance of hydrogels with ionic monomers and montmorillonite. Appl. Clay Sci. 2007, 38, 139–145. [Google Scholar] [CrossRef]
- Joseph, T.M.; Al-Hazmi, H.E.; Śniatała, B.; Esmaeili, A.; Habibzadeh, S. Nanoparticles and nanofiltration for wastewater treatment: From polluted to fresh water. Environ. Res. 2023, 238, 117114. [Google Scholar] [CrossRef]
- Nayak, B.; Samant, A.; Misra, P.K.; Saxena, M. Nanocrystalline hydroxyapatite: A potent material for adsorption, biological and catalytic studies. Mater. Today Proc. 2019, 9, 689–698. [Google Scholar] [CrossRef]
- Whitworth, T.M.; Welo Siagian, U.; Lee, R. Reverse osmosis separation of NaCl using a bentonite membrane. Sep. Sci. Technol. 2003, 38, 4009–4025. [Google Scholar] [CrossRef]
- Bangar, S.P.; Ilyas, R.; Chowdhury, A.; Navaf, M.; Sunooj, K.V.; Siroha, A.K. Bentonite clay as a nanofiller for food packaging applications. Trends Food Sci. Technol. 2023, 142, 104242. [Google Scholar] [CrossRef]
- Aga, M.B.; Dar, A.H.; Nayik, G.A.; Panesar, P.S.; Allai, F.; Khan, S.A.; Shams, R.; Kennedy, J.F.; Altaf, A. Recent insights into carrageenan-based bio-nanocomposite polymers in food applications: A review. Int. J. Biol. Macromol. 2021, 192, 197–209. [Google Scholar] [CrossRef]
- Gandhi, D.; Bandyopadhyay, R.; Soni, B. Naturally occurring bentonite clay: Structural augmentation, characterization and application as catalyst. Mater. Today Proc. 2022, 57, 194–201. [Google Scholar] [CrossRef]
- Bashiri, H.; Javanmardi, A.H.; Soltani, Z. A theoretical description for competitive adsorption at the Solid/Solution interface. Comput. Theor. Chem. 2024, 1237, 114652. [Google Scholar] [CrossRef]
- Zhu, K.-f.; Zhang, K.-n.; He, Y. Hydro-mechanical behavior and microstructure evolution of red clay-bentonite backfills. Appl. Clay Sci. 2023, 244, 107111. [Google Scholar] [CrossRef]
- Lahbabi, S.; Bouferra, R.; Saadi, L.; Khalil, A. Evaluation of the void index method on the mechanical and thermal properties of compressed earth blocks stabilized with bentonite clay. Constr. Build. Mater. 2023, 393, 132114. [Google Scholar] [CrossRef]
- Sunakbaeva, D.K. Development of nature protection measures on the basis of bentonite clays and products of utilization of sulfur-containing wastes. In Ecology; Dulaty University: Taraz, Kazakhstan, 2009; p. 20. [Google Scholar]
- Bekzhanov, M.A.; Akbasova, A.D.; Saparbayev, K.A.; Sainova, G.A.; Baikhamurova, M.O. Reclamation of Sulfur Containing Wastes of Oil and Chemical Industries. Her. Kazakh-Br. Tech. Univ. 2019, 16, 12–21. [Google Scholar]
- Panasyugin, A.S.; Tsyganov, A.R.; Masherova, N.P. Modified Bentonite Clays as Sorbents, Catalysts, Carriers of Active Catalytic Phases; BSTU: Minsk, Belarus, 2022; p. 172. [Google Scholar]
- Seredin, V.V.; Sheina, K.V.; Siteva, O.S. Regularities of zero charge formation on the surface of clay particles subjected to pressure. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2024, 335, 102–111. [Google Scholar] [CrossRef]
- Sanavada, K.; Shah, M.; Gandhi, D.; Unnarkat, A.; Vaghasiya, P. A systematic and comprehensive study of Eco-friendly bentonite clay application in esterification and wastewater treatment. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100784. [Google Scholar] [CrossRef]
- Cai, F.; Ma, F.; Zhang, X.; Reimus, P.; Qi, L.; Wang, Y.; Lu, D.; Thanh, H.V.; Dai, Z. Investigating the influence of bentonite colloids on strontium sorption in granite under various hydrogeochemical conditions. Sci. Total Environ. 2023, 900, 165819. [Google Scholar] [CrossRef]
- Vernigorova, V.N.; Sadenko, M.S. Material Science of Polymers and Composite Materials on Their Basis; Penza State University of Architecture and Construction: Penza, Russia, 2013; p. 419. [Google Scholar]
- Monteiro, M.K.S.; de Oliveira, V.R.L.; dos Santos, F.K.G.; de Barros Neto, E.L.; de Lima Leite, R.H.; Aroucha, E.M.M.; de Oliveira Silva, K.N. Influence of the ionic and nonionic surfactants mixture in the structure and properties of the modified bentonite clay. J. Mol. Liq. 2018, 272, 990–998. [Google Scholar] [CrossRef]
- Sapargaliyev, E.M.; Kravchenko, M.M. Tagan bentonite deposit in Zaisan depression. In Geology and Subsoil Protection; Bulletin of the Russian University of People’s Friendship: Moscow, Russia, 2008; Volume 4. [Google Scholar]
- Voronov, A.N.; Brodsky, A.K.; Kozlova, E.L.; Bochenska, T. Ecological and Hydrogeological Dictionary; A.N., V., Ed.; S.-Peterb.un: St. Petersburg, Russia, 2001; p. 202. [Google Scholar]
- Carmo, A.; Angélica, R.; Paz, S. Ageing characteristics related to cation exchange and interlayer spacing of some Brazilian bentonites. Heliyon 2021, 7, e06192. [Google Scholar] [CrossRef]
- Alabi, A.H.; Adekunle, V.A.; Azeez, A.A.; Akinwale, B.W.; Olanrewaju, C.A.; Oladoye, P.O.; Obayomi, K.S. Sequestration of divalent heavy metal ions from aqueous environment by adsorption using biomass-bentonite composites as potential adsorbent: Equilibrium and kinetic studies. Nano-Struct. Nano-Objects 2024, 38, 101183. [Google Scholar] [CrossRef]
- Negash, B.M.; Effiong, A.J.; Khan, H.W.; Zulkifli, N.I.B. Ion-adsorbed REE clays: Swelling challenges and future solutions. J. Mol. Liq. 2024, 403, 124849. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Gao, R.; Heinlein, J.A.; Pfefferle, L.D.; Hussain, S.; Zhang, J.; Wang, X.; An, J. Facile and green preparation of multifeatured montmorillonite-supported Fe3O4-Cu2+ hybrid magnetic nanomaterials for the selective adsorption of a high-abundance protein from complex biological matrices. Green Chem. 2023, 25, 3705–3714. [Google Scholar] [CrossRef]
- Kwolek, T.; Hodorowicz, M.; Stadnicka, K.; Czapkiewicz, J. Adsorption isotherms of homologous alkyldimethylbenzylammonium bromides on sodium montmorillonite. J. Colloid Interface Sci. 2003, 264, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Mahali, K.; Kundu, S.; Henaish, A.; Ahmed, J.; Rana, A.; Roy, S. Solubility and solvation energetics of L-histidine in aqueous NaCl/KCl electrolyte media. J. Mol. Liq. 2023, 391, 123240. [Google Scholar] [CrossRef]
- Eniola, J.O.; Sizirici, B.; Stephen, S.; Yildiz, I.; Khaleel, A.; El Fadel, M. A new synthesis route of hydrothermally carbonized Na2CO3 activated bentonite-clay as a novel adsorbent for cadmium removal from wastewater. Sep. Purif. Technol. 2024, 350, 127960. [Google Scholar] [CrossRef]
- Show, S.; Sarkhel, R.; Halder, G. Elucidating sorptive eradication of ibuprofen using calcium chloride caged bentonite clay and acid activated alginate beads in a fixed bed upward flow column reactor. Sustain. Chem. Pharm. 2022, 27, 100698. [Google Scholar] [CrossRef]
- Inoue, K.; Nishiki, Y.; Fukushi, K.; Suma, R.; Sato, T.; Sakuma, H.; Tamura, K.; Yokoyama, S.; Shimbashi, M.; Mizukami, T. Systematic comparison of Mg K-edge XANES spectra of magnesium-bearing clay minerals and magnesium silicate hydrates: A promising tool for identifying magnesium silicate hydrate in natural samples. Appl. Clay Sci. 2023, 245, 107152. [Google Scholar] [CrossRef]
- Nath, H.; Kabir, M.H.; Kafy, A.-A.; Rahaman, Z.A.; Rahman, M.T. Geotechnical properties and applicability of bentonite-modified local soil as landfill and environmental sustainability liners. Environ. Sustain. Indic. 2023, 18, 100241. [Google Scholar] [CrossRef]
- Ren, J.; Deshun, Y.; Zhai, R. Rheological behavior of bentonite-water suspension at various temperatures: Effect of solution salinity. Eng. Geol. 2021, 295, 106435. [Google Scholar] [CrossRef]
- Pavlovich, I.A.; Baraishuk, S.M.; Skripko, A. Ensuring the Reliability of Energy Systems with the Application of a New Method of Decreasing Seasonal Variations of Ground Resistance; L. A. Melentyev Institute of Energy Systems: Irkutsk, Russia, 2023. [Google Scholar]
- Waleed, M.; Alshawmar, F. Enhancing mechanical properties of low plasticity soil through coal and silica fume stabilization. Sci. Rep. 2025, 15, 9990. [Google Scholar] [CrossRef]
- Cheraghalikhani, M.; Niroumand, H.; Balachowski, L. Micro-and nano-bentonite to improve the strength of clayey sand as a nano soil-improvement technique. Sci. Rep. 2023, 13, 10913. [Google Scholar]
- Babahoum, N.; Ould Hamou, M. Characterization and purification of Algerian natural bentonite for pharmaceutical and cosmetic applications. BMC Chem. 2021, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Sommer, S.J.; Gutierrez, M. Characterization of different bentonites and their properties as a protein-fining agent in wine. Beverages 2022, 8, 31. [Google Scholar] [CrossRef]
- Crespo, E.; Martín, D.A.; Costafreda, J.L. Bentonite clays related to volcanosedimentary formations in southeastern Spain: Mineralogical, Chemical and Pozzolanic Characteristics. Minerals 2024, 14, 814. [Google Scholar] [CrossRef]
- Kadirbaeva, A.; Ishanova, M.; Bimbetova, G.Z. Features of the use of bentonite clay Darbazinskogo deposit for wastewater treatment of petrochemical industries. Neftekhimiya 2024, 2, 230–237. [Google Scholar]
- Vilarinho, F.; Vaz, M.F.; Silva, A.S. The use of montmorillonite (MMT) in food nanocomposites: Methods of incorporation, characterization of MMT/polymer nanocomposites and main consequences in the properties. Recent Pat. Food Nutr. Agric. 2020, 11, 13–26. [Google Scholar] [CrossRef]
- Behera, L.; Mohanta, M.; Thirugnanam, A. Intensification of yam-starch based biodegradable bioplastic film with bentonite for food packaging application. Environ. Technol. Innov. 2022, 25, 102180. [Google Scholar] [CrossRef]
- Heydari, A.; KhajeHassani, M.; Daneshafruz, H.; Hamedi, S.; Dorchei, F.; Kotlár, M.; Kazeminava, F.; Sadjadi, S.; Doostan, F.; Chodak, I. Thermoplastic starch/bentonite clay nanocomposite reinforced with vitamin B2: Physicochemical characteristics and release behavior. Int. J. Biol. Macromol. 2023, 242, 124742. [Google Scholar] [CrossRef]
- Adilova, N.B.; Montayev, S.A.; Montayeva, A.S.; Shakeshev, B.T.; Narikov, K.A.; Taskaliev, A.T.; Zharylgapov, S.; Usenkulov, Z. Modifying of ceramic mass by Kazakhstan bentonite for the purpose of improvement of structure and physicomechanical properties of front wall ceramics. Life Sci. J. 2014, 11, 83–89. [Google Scholar]
- Montayev, S.; Shakeshev, B.; Zharylgapov, S. Development of a technology for producing ceramic refractory material in a composition of montmorillonite clays (bentonite-like) and ferrochrome production wastes. MATEC Web Conf. 2020, 315, 07007. [Google Scholar] [CrossRef]
- Xie, H.; Sun, W.; Li, M.; Feng, X. Mechanical properties and electromagnetic wave absorption characteristics of solid waste low-carbon cementitious materials. J. Clean. Prod. 2024, 467, 142869. [Google Scholar] [CrossRef]
- Qiao, L.; Zhou, W.; Du, K. Rigid crosslink improves the surface area and porosity of β-cyclodextrin beads for enhanced adsorption of flavonoids. Food Chem. 2024, 439, 138081. [Google Scholar] [CrossRef] [PubMed]
- Dambrauskas, T.; Davidoviciene, D.; Baltakys, K.; Eisinas, A.; Jaskunas, A.; Siler, P.; Rudelis, V.; Svedaite, E. Effect of intercalated metal ions on the specific surface area and porosity of dibasic calcium silicate hydrate. Surf. Interfaces 2022, 33, 102243. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Das, S.; Sharma, P.; Kumar, M.; Gupta, R.; Sharma, H. A review on clay exfoliation methods and modifications for CO2 capture application. Mater. Today Sustain. 2023, 23, 100427. [Google Scholar] [CrossRef]
- Rivenbark, K.J.; Lilly, K.; Wang, M.; Tamamis, P.; Phillips, T.D. Green-engineered clay-and carbon-based composite materials for the adsorption of benzene from air. J. Environ. Chem. Eng. 2024, 12, 111836. [Google Scholar] [CrossRef]
- Buntin, A.; Agliullin, V. Transformation of the structure and adsorption properties of bentonite during physical and chemical treatment. J. Phys. Conf. Ser. 2022, 2373, 032006. [Google Scholar] [CrossRef]
- Parmar, S.P.; Patel, B.S.; Shukla, A.; Dixit, M. Surfactant Modified Bentonite Characterization: Effects and Comparative Analysis. Adv. Nanosci. Nanotechnol. 2022, 7, 12–25. [Google Scholar]
- Liu, P.S.; Li, L.; Zhou, N.L.; Zhang, J.; Wei, S.H.; Shen, J. Waste polystyrene foam-graft-acrylic acid/montmorillonite superabsorbent nanocomposite. J. Appl. Polym. Sci. 2007, 104, 2341–2349. [Google Scholar] [CrossRef]
- Dékány, I.; Turi, L.; Galbács, G.; Fendler, J. Cadmium ion adsorption controls the growth of CdS nanoparticles on layered montmorillonite and calumit surfaces. J. Colloid Interface Sci. 1999, 213, 584–591. [Google Scholar] [CrossRef]
- Ghasemi, H.; Afshang, M.; Gilvari, T.; Aghabarari, B.; Mozaffari, S. Rapid and effective removal of heavy metal ions from aqueous solution using nanostructured clay particles. Results Surf. Interfaces 2023, 10, 100097. [Google Scholar] [CrossRef]
- Singh, D.; Tiwari, A.; Singh, R.P.; Singh, A.K. Clove bud extract mediated green synthesis of bimetallic Ag–Fe nanoparticles: Antimicrobial, antioxidant and dye adsorption behavior and mechanistic insights of metal ion reduction. Mater. Chem. Phys. 2024, 311, 128529. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Wang, A. A Novel N-Succinylchitosan-graft-Polyacrylamide/Attapulgite Composite Hydrogel Prepared through Inverse Suspension Polymerization. Macromol. Mater. Eng. 2007, 292, 962–969. [Google Scholar] [CrossRef]
- Rodriguez Nunez, Y.A.; Castro, R.I.; Arenas, F.A.; López-Cabaña, Z.E.; Carreño, G.; Carrasco-Sánchez, V.; Marican, A.; Villaseñor, J.; Vargas, E.; Santos, L.S. Preparation of hydrogel/silver nanohybrids mediated by tunable-size silver nanoparticles for potential antibacterial applications. Polymers 2019, 11, 716. [Google Scholar] [CrossRef]
- Martsouka, F.; Papagiannopoulos, K.; Hatziantoniou, S.; Barlog, M.; Lagiopoulos, G.; Tatoulis, T.; Tekerlekopoulou, A.G.; Lampropoulou, P.; Papoulis, D. The antimicrobial properties of modified pharmaceutical bentonite with zinc and copper. Pharmaceutics 2021, 13, 1190. [Google Scholar] [CrossRef]
- Cu, B.Q.; Chao, N.H.; Vesentsev, A.I.; Buhanov, V.; Sokolovsky, P.V.; Mihaylyukova, M.O. The antibacterial properties of modified bentonite deposit tam bo. Res. Results Pharmacol. 2016, 2, 63–74. [Google Scholar]
- Obradović, M.; Daković, A.; Smiljanić, D.; Marković, M.; Ožegović, M.; Krstić, J.; Vuković, N.; Milojević-Rakić, M. Bentonite modified with surfactants—Efficient adsorbents for the removal of non-steroidal anti-inflammatory drugs. Processes 2023, 12, 96. [Google Scholar] [CrossRef]
- Topalić-Trivunović, L.; Savić, A.; Petrović, R.; Bodroža, D.; Grujić, D.; Mitrić, M.; Obrenović, Z.; Gajić, D.; Imamović, M. Antibacterial finishing of textile materials using modified bentonite. Clays Clay Miner. 2023, 71, 559–576. [Google Scholar] [CrossRef]
- Liu, P. Polymer modified clay minerals: A review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Lamrani, M.; Mouchane, M.; Taybi, H.; Mouadili, A. Comprehensive Review on the Adsorption Properties of Clay Minerals for Enhanced Removal of Toxic Dyes and Heavy Metals. J. Water Environ. Nanotechnol. 2025, 10, 85–107. [Google Scholar]
- Wang, P.; Shen, X.; Qiu, S.; Zhang, L.; Ma, Y.; Liang, J. Clay-Based Materials for Heavy Metals Adsorption: Mechanisms, Advancements, and Future Prospects in Environmental Remediation. Crystals 2024, 14, 1046. [Google Scholar] [CrossRef]
- Alomari, A.D.A. Chemically modified clay for adsorption of contaminants: Trends, advantages and limitations—A concise review. Int. J. Environ. Anal. Chem. 2024, 1–24. [Google Scholar] [CrossRef]
- Ochirkhuyag, A.; Temuujin, J. The Catalytic Potential of Modified Clays: A review. Minerals 2024, 14, 629. [Google Scholar] [CrossRef]
- Biswas, B.; Juhasz, A.L.; Mahmudur Rahman, M.; Naidu, R. Modified clays alter diversity and respiration profile of microorganisms in long-term hydrocarbon and metal co-contaminated soil. Microb. Biotechnol. 2020, 13, 522–534. [Google Scholar] [CrossRef]
- Zhang, L.; Gadd, G.M.; Li, Z. Microbial biomodification of clay minerals. Adv. Appl. Microbiol. 2021, 114, 111–139. [Google Scholar]
- Abd El-Mohdy, H. Radiation synthesis of nanosilver/poly vinyl alcohol/cellulose acetate/gelatin hydrogels for wound dressing. J. Polym. Res. 2013, 20, 177. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T.; Ebato, M. Control of cell cultivation and cell sheet detachment on the surface of polymer/clay nanocomposite hydrogels. Biomacromolecules 2006, 7, 3267–3275. [Google Scholar] [CrossRef]
- Turri, S.; Alborghetti, L.; Levi, M. Formulation and properties of a model two-component nanocomposite coating from organophilic nanoclays. J. Polym. Res. 2008, 15, 365–372. [Google Scholar] [CrossRef]
- Ninago, M.; Giaroli, M.; Passaretti, M.; Villar, M.; López, O. Polymer–clay nanocomposites for food packaging. In Nanostructured Materials for Food Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 189–213. [Google Scholar]
- Khar’Kova, E.; Mendeleev, D.; Guseva, M.; Gerasin, V. Structure and properties of polymer–polymer composites based on biopolymers and ultra-high molecular weight polyethylene obtained via ethylene in situ polymerization. J. Polym. Environ. 2019, 27, 165–175. [Google Scholar] [CrossRef]
- Narod, A.M.S.A.; Beisebekov, M.M.; Kairalapova, G.J.; Iminova, R.S.; Zhumagalieva, S.N.; Beisebekov, M.K.; Abilov, J.A. Clay polyacrylate composites as sorbents of heavy metal ions. Vestn. Chem. Ser. 2012, 3. [Google Scholar] [CrossRef]
- Firoozirad, K.; Szilágyi, B. Rapid, low-cost exploration of nucleation rates in l-glutamic acid crystallization in pure media and the presence of polymers through novel parallel experimentation. J. Cryst. Growth 2024, 642, 127786. [Google Scholar] [CrossRef]
- Naz, A.; Chowdhury, A. Pollutant extraction from water and soil using Montmorillonite clay-polymer composite: A rapid review. Mater. Today Proc. 2022, 60, 1–7. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Alateyah, A.; Dhakal, H.; Zhang, Z. Processing, properties, and applications of polymer nanocomposites based on layer silicates: A review. Adv. Polym. Technol. 2013, 32. [Google Scholar] [CrossRef]
- Das, A.; Thakur, A.K.; Kumar, K. Raman spectroscopic study of ion dissociation effect in clay intercalated polymer blend nano composite electrolyte. Vib. Spectrosc. 2017, 92, 14–19. [Google Scholar] [CrossRef]
- Das, P.; Manna, S.; Behera, A.K.; Shee, M.; Basak, P.; Sharma, A.K. Current synthesis and characterization techniques for clay-based polymer nano-composites and its biomedical applications: A review. Environ. Res. 2022, 212, 113534. [Google Scholar] [CrossRef]
- Gomes, C.F.; Gomes, J.H.; da Silva, E.F. Bacteriostatic and bactericidal clays: An overview. Environ. Geochem. Health 2020, 42, 3507–3527. [Google Scholar] [CrossRef]
- Xu, Y.; Brittain, W.J.; Xue, C.; Eby, R.K. Effect of clay type on morphology and thermal stability of PMMA–clay nanocomposites prepared by heterocoagulation method. Polymer 2004, 45, 3735–3746. [Google Scholar] [CrossRef]
- Farajzadehahary, K.; Hamzehlou, S.; Ballard, N.; Asua, J.M. The hidden secrets of the average number of radicals per particle (n¯) and their implications in control of emulsion polymerization reactors. Chem. Eng. J. 2024, 487, 150681. [Google Scholar] [CrossRef]
- Hwu, J.M.; Ko, T.H.; Yang, W.T.; Lin, J.C.; Jiang, G.J.; Xie, W.; Pan, W.P. Synthesis and properties of polystyrene–montmorillonite nanocomposites by suspension polymerization. J. Appl. Polym. Sci. 2004, 91, 101–109. [Google Scholar] [CrossRef]
- Das, S.K.; Bharatiya, D.; Parhi, B.; Pradhan, L.; Jena, B.K.; Swain, S.K. Effect of clay on TiO2 embedded PMMA nanocomposite for high-performance energy storage application. J. Energy Storage 2024, 82, 110586. [Google Scholar] [CrossRef]
- Shen, Z.; Simon, G.P.; Cheng, Y.B. Melt intercalation of PMMA into organically-modified layered silicate. MRS Online Proc. Libr. (OPL) 1999, 576, 137. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.; Yuan, L.; Gao, Q.; Yu, Q.; Chen, J.; Cheng, Y.; Sun, A.; Xu, G.; Guo, J. The effect of controlled intercalation on the mechanical performances and dimensional accuracy of material extrusion additive manufactured poly (lactic acid)/organo-montmorillonite nanocomposites. Mater. Today Commun. 2023, 37, 107208. [Google Scholar] [CrossRef]
- Alexandre, B.; Langevin, D.; Médéric, P.; Aubry, T.; Couderc, H.; Nguyen, Q.; Saiter, A.; Marais, S. Water barrier properties of polyamide 12/montmorillonite nanocomposite membranes: Structure and volume fraction effects. J. Membr. Sci. 2009, 328, 186–204. [Google Scholar] [CrossRef]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional materials in food nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef]
- Zahra, M.; Javed, M.; Iqbal, S.; Mahmood, S.; Sarfraz, S.; Yasin, M.; Bahadur, A.; Jazaa, Y.; Alshalwi, M. High-strength montmorillonite polyurethane nanocomposites with exfoliated montmorillonite: Preparation and characterization. Inorg. Chem. Commun. 2024, 162, 112262. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Gusev, A.A.; Lusti, H.R. Rational design of nanocomposites for barrier applications. Adv. Mater. 2001, 13, 1641–1643. [Google Scholar] [CrossRef]
- Momani, B.; Sen, M.; Endoh, M.; Wang, X.; Koga, T.; Winter, H.H. Temperature dependent intercalation and self–exfoliation of clay/polymer nanocomposite. Polymer 2016, 93, 204–212. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Song, Z.; Bai, Y.; Qian, W.; Wei, J.; Kanungo, D.P. Tensile behavior of polyurethane organic polymer and polypropylene fiber-reinforced sand. Polymers 2018, 10, 499. [Google Scholar] [CrossRef]
- Singh, A.K.; Bedi, R.; Kaith, B.S. Composite materials based on recycled polyethylene terephthalate and their properties—A comprehensive review. Compos. Part B Eng. 2021, 219, 108928. [Google Scholar] [CrossRef]
- Ahirwar, D.; Telang, A.; Purohit, R.; Namdev, A. A short review on polyurethane polymer composite. Mater. Today Proc. 2022, 62, 3804–3810. [Google Scholar] [CrossRef]
- Moulay, S. Functionalized polystyrene and polystyrene-containing material platforms for various applications. Polym.-Plast. Technol. Eng. 2018, 57, 1045–1092. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Pypeć, K.; Irska, I.; Piesowicz, E. Functional polymer hybrid nanocomposites based on polyolefins: A review. Processes 2020, 8, 1475. [Google Scholar] [CrossRef]
- Hernández-Hernández, K.A.; Illescas, J.; Díaz-Nava, M.; Muro-Urista, C.; Martínez-Gallegos, S.; Ortega-Aguilar, R. Polymer-clay nanocomposites and composites: Structures, characteristics, and their applications in the removal of organic compounds of environmental interest. Med. Chem. 2016, 6, 201–210. [Google Scholar] [CrossRef]
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Ingle, A.; dos Santos, C.A. An introduction to biopolymer-based nanofilms, their applications, and limitations. In Biopolymer-Based Nano Films; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–17. [Google Scholar]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Arruebo, M. Drug delivery from structured porous inorganic materials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 16–30. [Google Scholar] [CrossRef]
- Kumar, M.; Mahmood, S.; Chopra, S.; Bhatia, A. Biopolymer based nanoparticles and their therapeutic potential in wound healing—A review. Int. J. Biol. Macromol. 2024, 267, 131335. [Google Scholar] [CrossRef]
- Esquivel-Castro, T.A.; Ibarra-Alonso, M.; Oliva, J.; Martínez-Luévanos, A. Porous aerogel and core/shell nanoparticles for controlled drug delivery: A review. Mater. Sci. Eng. C 2019, 96, 915–940. [Google Scholar] [CrossRef]
- Pai, A.R.; Binumol, T.; Gopakumar, D.A.; Pasquini, D.; Seantier, B.; Kalarikkal, N.; Thomas, S. Ultra-fast heat dissipating aerogels derived from polyaniline anchored cellulose nanofibers as sustainable microwave absorbers. Carbohydr. Polym. 2020, 246, 116663. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, J.O.; Müller, C.; Laurindo, J. Influence of the simultaneous addition of bentonite and cellulose fibers on the mechanical and barrier properties of starch composite-films. Food Sci. Technol. Int. 2012, 18, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Dharini, V.; Selvam, S.P.; Jayaramudu, J.; Emmanuel, R.S. Functional properties of clay nanofillers used in the biopolymer-based composite films for active food packaging applications-Review. Appl. Clay Sci. 2022, 226, 106555. [Google Scholar] [CrossRef]
- Zakir, M.; Ashraf, U.; Tian, T.; Han, A.; Qiao, W.; Jin, X.; Zhang, M.; Tsoi, J.K.-H.; Matinlinna, J.P. The role of silane coupling agents and universal primers in durable adhesion to dental restorative materials-a review. Curr. Oral Health Rep. 2016, 3, 244–253. [Google Scholar] [CrossRef]
- Lee, J.Y.; Suh, H.N.; Choi, K.Y.; Song, C.W.; Hwang, J.H. Regenerative and anti-inflammatory effect of a novel bentonite complex on burn wounds. Vet. Med. Sci. 2022, 8, 2422–2433. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Adib-Hajbaghery, M.; Mashaiekhi, M. Comparing the effects of Bentonite & Calendula on the improvement of infantile diaper dermatitis: A randomized controlled trial. Indian J. Med. Res. 2015, 142, 742–746. [Google Scholar]
- Rana, M.S.; Kim, S. Bentonite in Korea: A Resource and Research Focus for Biomedical and Cosmetic Industries. Materials 2024, 17, 1982. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Riela, S. The use of some clay minerals as natural resources for drug carrier applications. J. Funct. Biomater. 2018, 9, 58. [Google Scholar] [CrossRef]
- Beisebekov, M.; Kairalapova, R.; Kudaibergenova, G. Organo-mineral carriers of medicinal substances. Bull. Chem. Ser. 2012, 3. [Google Scholar]
- Srasra, E.; Bekri-Abbes, I. Bentonite clays for therapeutic purposes and biomaterial design. Curr. Pharm. Des. 2020, 26, 642–649. [Google Scholar] [CrossRef]
- Bastos, C.M.; Rocha, F. Experimental peloid formulation using a Portuguese bentonite and different mineral-medicinal waters suitable for therapeutic and well-being purposes. Clays Clay Miner. 2023, 71, 684–706. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Abd Razak, S.I.; Al Arjan, W.S.; Nazir, S.; Sahaya Anand, T.J.; Mehboob, H.; Amin, R. Recent advances in biopolymeric composite materials for tissue engineering and regenerative medicines: A review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef]
- Williams, L.B. Natural antibacterial clays: Historical uses and modern advances. Clays Clay Miner. 2019, 67, 7–24. [Google Scholar] [CrossRef]
- Mokhtar, A.; Ahmed, A.B.; Asli, B.; Boukoussa, B.; Hachemaoui, M.; Sassi, M.; Abboud, M. Recent advances in antibacterial metallic species supported on montmorillonite clay mineral: A review. Minerals 2023, 13, 1268. [Google Scholar] [CrossRef]
- Kabdrakhmanova, S.; Aryp, K.; Shaimardan, E.; Kanat, E.; Selenova, B.; Nurgamit, K.; Kerimkulova, A.; Amitova, A.; Maussumbayeva, A. Acid modification of clays from the Kalzhat, Orta Tentek deposits and study their physical-chemical properties. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; An, Y.; Zhuang, G. Clay minerals stabilization by organic inhibitors. In Clay Science in Drilling and Drilling Fluids; Elsevier: Amsterdam, The Netherlands, 2024; pp. 201–222. [Google Scholar]
- Liao, Y.; Chai, S.-S.; Zhang, W.-B.; Yao, Y.; Yang, J.-L.; Yin, Y.; Li, J.-J.; Yang, Z.-Q.; Ma, X.-J.; Peng, Q. Electrochemical capacitance of clay minerals by diamine modification. Appl. Clay Sci. 2024, 250, 107296. [Google Scholar] [CrossRef]
- Kumari, N.; Mohan, C. Basics of clay minerals and their characteristic properties. In Clay and Clay Minerals; IntechOpen: London, UK, 2021; Volume 24, pp. 1–29. [Google Scholar]
- Ghosh, B.; Chakraborty, D. Clay Minerals: Their Antimicrobial and Antitoxic Applications; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Fu, Z.; Liu, G.; Qi, T.; Shen, L.; Peng, Z.; Li, X.; Zhou, Q.; Wang, Y. Efficient self-purification of anionic surfactants in Bayer liquor through a dense-ordered interfacial structure generated by trace amounts of cationic surfactants. Surf. Interfaces 2024, 51, 104723. [Google Scholar] [CrossRef]
- Bagheri, A.; Yazdani, A.; Rafati, A.A. Selection of better cationic surfactant for zeolite modification using surface studies and its application in the removal of anionic and cationic dyes. J. Mol. Liq. 2024, 403, 124881. [Google Scholar] [CrossRef]
- Du, Q.; Chen, S.; Liu, H.; Zhang, M.; Ren, S.; Luo, W. Sequential modification of montmorillonite by Al13 polycation and cationic gemini surfactant for the removal of Orange II. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133489. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X.; Lin, C.; Feng, J.; Wang, H.; Yan, W. Insight into the effect of surfactant modification on the versatile adsorption of titanate-based materials for cationic and anionic contaminants. Chemosphere 2021, 269, 129383. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Sun, Z.; Zheng, S.; Xi, Y. Structures of nonionic surfactant modified montmorillonites and their enhanced adsorption capacities towards a cationic organic dye. Appl. Clay Sci. 2017, 148, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Zaoui, A.; Sekkal, W.; Zheng, Y.-Y. Interlayer adsorption of cationic dye on cationic surfactant-modified and unmodified montmorillonite. J. Hazard. Mater. 2023, 442, 130107. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejczak, P.; Parus, A.; Balicki, S.; Kornaus, K.; Janczarek, M.; Wilk, K.A.; Jesionowski, T.; Ślosarczyk, A.; Klapiszewski, Ł. The influence of various forms of titanium dioxide on the performance of resultant cement composites with photocatalytic and antibacterial functions. Mater. Res. Bull. 2023, 160, 112139. [Google Scholar] [CrossRef]
- Cherifi, Z.; Boukoussa, B.; Zaoui, A.; Belbachir, M.; Meghabar, R. Structural, morphological and thermal properties of nanocomposites poly (GMA)/clay prepared by ultrasound and in-situ polymerization. Ultrason. Sonochem. 2018, 48, 188–198. [Google Scholar] [CrossRef]
- Dilbarkhanov, R.D. Study of antimicrobial activity of multicomponent ointment with levomycetin on the basis of bentonite clay. Pharm. Bul. 1999, 4, 20. [Google Scholar]
- L.M., S. Development of composition and technology of 5% streptocid ointment in composition with metronidazole. Pharm. Bull. 1999, 12, 16. [Google Scholar]
- Kabdrakhmanova, S.; Joshy, K.; Sathian, A.; Aryp, K.; Akatan, K.; Shaimardan, E.; Beisebekov, M.; Gulden, T.; Kabdrakhmanova, A.; Maussumbayeva, A. Anti-bacterial activity of Kalzhat clay functionalized with Ag and Cu nanoparticles. Eng. Sci. 2023, 26, 972. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Ray, S.S.; Onyango, M.S.; Momba, M.N. Preparation and antibacterial activity of chitosan-based nanocomposites containing bentonite-supported silver and zinc oxide nanoparticles for water disinfection. Appl. Clay Sci. 2015, 114, 330–339. [Google Scholar] [CrossRef]
- Devi, N.; Dutta, J. Preparation and characterization of chitosan-bentonite nanocomposite films for wound healing application. Int. J. Biol. Macromol. 2017, 104, 1897–1904. [Google Scholar] [CrossRef]
- Cabuk, M.; Alan, Y.; Unal, H.I. Enhanced electrokinetic properties and antimicrobial activities of biodegradable chitosan/organo-bentonite composites. Carbohydr. Polym. 2017, 161, 71–81. [Google Scholar] [CrossRef]
- Alkrad, J.A.; Shmeis, R.A.; Alshwabkeh, I.; Abazid, H.; Mohammad, M.A. Investigation of the potential application of sodium bentonite as an excipient in formulation of sustained release tablets. Asian J. Pharm. Sci. 2017, 12, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ara Arshavirovich, A.; Vjacheslav Ivanovich, B.; Igor Ivanovich, M.; Peter Ivanovich, M.; Vladimir Alexandrovich, S. Method of Production of Anti-Infective Agent RU 2,330,673 C1, 22 November 2006.
- Ignatieva, Y.A.; Valerievna, U.M.; Borisov, O.V. Investigation of Sorption Characteristics of Polymeric Mineral-Filled Composites for Medicine. J. Sci. Tech. Inf. Technol. Mech. Opt. 2014, 93, 52–56. [Google Scholar]
- Thomas, S.; McCubbin, P. A comparison of the antimicrobial effects of four silver-containing dressings on three organisms. J. Wound Care 2003, 12, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Tong, G.; Liu, J.; Zhang, W.; Chen, L.; Quan, C.; Jiang, Q.; Sun, H.; Zhang, C. Construction of silver nanoparticle-loaded micelles via coordinate interaction and their antibacterial activity. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 848–856. [Google Scholar] [CrossRef]
- Patil, S.; Sivaraj, R.; Rajiv, P.; Venckatesh, R.; Seenivasan, R. Green synthesis of silver nanoparticle from leaf extract of Aegle marmelos and evaluation of its antibacterial activity. Int. J. Pharm. Pharm. Sci. 2015, 7, 169–173. [Google Scholar]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, characterization, and antibacterial activity of silver nanoparticle-decorated graphene oxide nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef]
- Zhangabay, Z.; Berillo, D. Antimicrobial and antioxidant activity of AgNPs stabilized with Calendula officinalis flower extract. Results Surf. Interfaces 2023, 11, 100109. [Google Scholar] [CrossRef]
- Ermukhambetova, A.; Berillo, D. Green synthesis of silver nanoparticles using paper wasp‘s hydrolysate with antibacterial activity. Results Surf. Interfaces 2023, 11, 100114. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of silver nanoparticles using aqueous extract of medicinal plants’(Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. Int. J. Microbiol. 2019, 2019, 8642303. [Google Scholar] [CrossRef]
- Dyusebaeva, M.A.; Berillo, D.A.; Berganayeva, A.E.; Berganayeva, G.E.; Ibragimova, N.A.; Jumabayeva, S.M.; Kudaibergenov, N.Z.; Kanapiyeva, F.M.; Kirgizbayeva, A.A.; Vassilina, G.K. Antimicrobial activity of silver nanoparticles stabilized by liposoluble extract of Artemisia terrae-albae. Processes 2023, 11, 3041. [Google Scholar] [CrossRef]
- Mandal, S.K.; Brahmachari, S.; Das, P.K. In situ synthesised silver nanoparticle-infused L-lysine-based injectable hydrogel: Development of a biocompatible, antibacterial, soft nanocomposite. ChemPlusChem 2014, 79, 1733–1746. [Google Scholar] [CrossRef]
- Rattanaruengsrikul, V.; Pimpha, N.; Supaphol, P. In vitro efficacy and toxicology evaluation of silver nanoparticle-loaded gelatin hydrogel pads as antibacterial wound dressings. J. Appl. Polym. Sci. 2012, 124, 1668–1682. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef]
- Zidelkheir, B.; Boudjemaa, S.; Abdel-Goad, M.; Djellouli, B. Preparation and characterization of polystyrene/montmorillonite nanocomposite by melt intercalative compounding. Iran. Polym. J. 2006, 15, 645–653. [Google Scholar]
- Evsikova, O.; Starodubtsev, S.; Khokhlov, A. Synthesis, swelling, and adsorption properties of composites based on polyacrylamide gel and sodium bentonite. Высoкoмoлекулярные Сoединения Серия А 2002, 44, 802–808. [Google Scholar]
- Weian, Z.; Wei, L.; Yue’e, F. Synthesis and properties of a novel hydrogel nanocomposites. Mater. Lett. 2005, 59, 2876–2880. [Google Scholar] [CrossRef]
- Saravanan, P.; Raju, M.P.; Alam, S. A study on synthesis and properties of Ag nanoparticles immobilized polyacrylamide hydrogel composites. Mater. Chem. Phys. 2007, 103, 278–282. [Google Scholar] [CrossRef]
- Al, E.; Güçlü, G.; İyim, T.B.; Emik, S.; Özgümüş, S. Synthesis and properties of starch-graft-acrylic acid/Na-montmorillonite superabsorbent nanocomposite hydrogels. J. Appl. Polym. Sci. 2008, 109, 16–22. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Dutta, J. Synthesis and characterization of γ-irradiated PVA/PEG/CaCl2 hydrogel for wound dressing. Am. J. Chem. 2012, 2, 6. [Google Scholar] [CrossRef]
- Obsa, A.L.; Shibeshi, N.T.; Mulugeta, E.; Workeneh, G.A. Bentonite/amino-functionalized cellulose composite as effective adsorbent for removal of lead: Kinetic and isotherm studies. Results Eng. 2024, 21, 101756. [Google Scholar] [CrossRef]
- Hameed, A.; Tariq, M.; Sadia, S.; Alam, M.R.; Haider, A.; Wahedi, H.M. Aloesin-loaded chitosan/cellulose-based scaffold promotes skin tissue regeneration. Int. J. Biol. Macromol. 2024, 273, 133030. [Google Scholar] [CrossRef]
- Maxim, L.D.; Niebo, R.; McConnell, E.E. Bentonite toxicology and epidemiology—A review. Inhal. Toxicol. 2016, 28, 591–617. [Google Scholar] [CrossRef]
- Wang, M.; Hearon, S.E.; Phillips, T.D. A high capacity bentonite clay for the sorption of aflatoxins. Food Addit. Contam. Part A 2020, 37, 332–341. [Google Scholar] [CrossRef]
- Moosavi, M. Bentonite clay as a natural remedy: A brief review. Iran. J. Public Health 2017, 46, 1176. [Google Scholar]
- Tsiklauri, L.; Getia, M. Some Physicochemical Properties of Georgian Bentonite Clay. Georgian Sci. 2023, 5, 334–343. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Yalamarty, S.S.K.; Filipczak, N.; Jin, Y.; Li, X. Nano silver-induced toxicity and associated mechanisms. Int. J. Nanomed. 2022, 17, 1851–1864. [Google Scholar] [CrossRef]
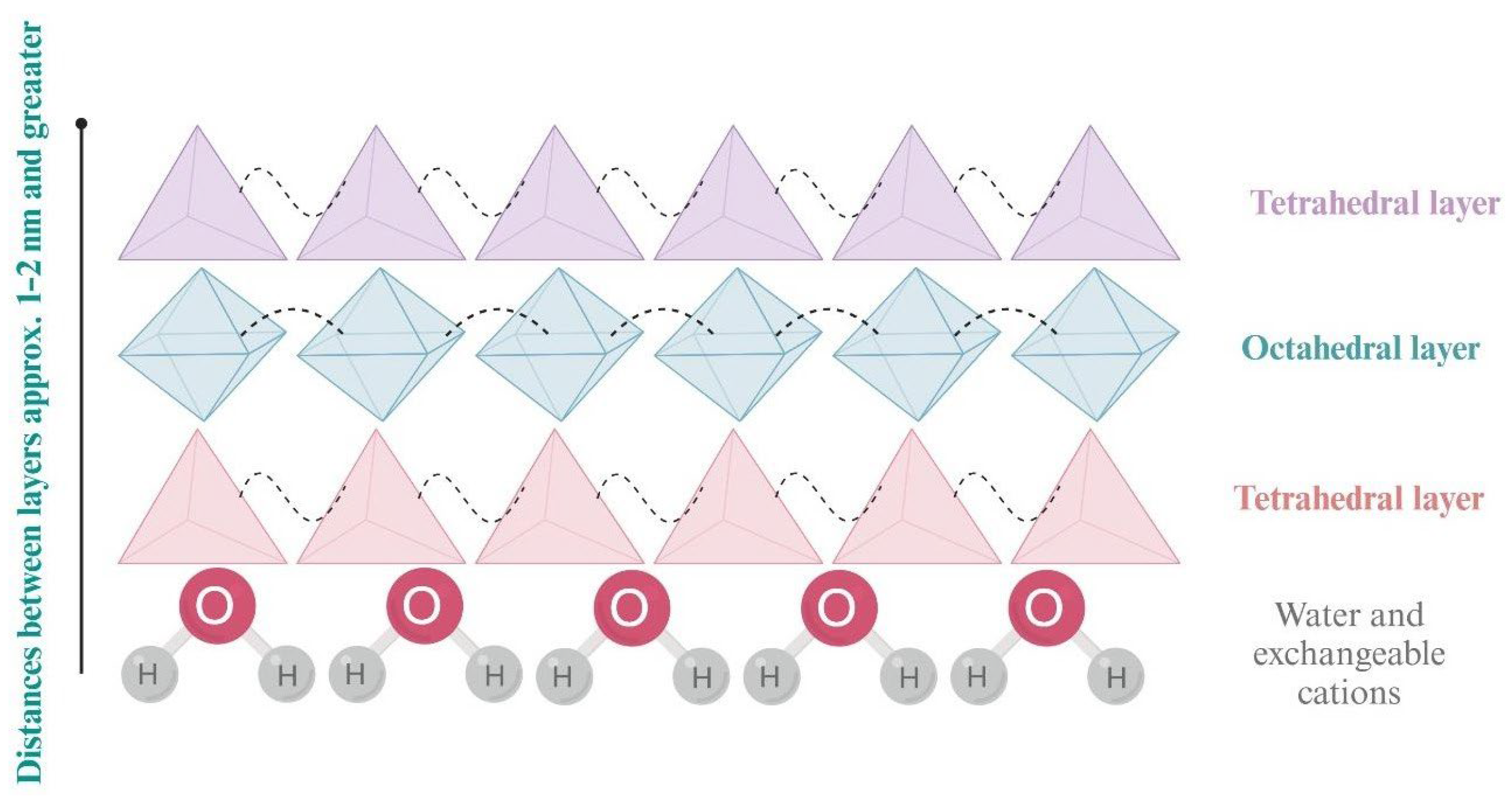
| Type of Bentonite | Chemical Composition | Physicochemical Properties | Application | Features | References |
|---|---|---|---|---|---|
| Alkaline bentonites | Main components: montmorillonite, Al2O3-4SiO2-nH2O | Ability to swell repeatedly in aqueous solution | Used in the manufacture of drilling fluids, as binders, for the production of catalysts | Used in the production of enterosorbents to remove heavy metals and radionuclides from the body | [12,18] |
| Alkaline-earth bentonites | Montmorillonite with high content of alkaline-earth cations | High adsorption and catalytic properties, high free surface energy | Used in the chemical and petrochemical industry for filtration, purification, bleaching, and refining of oils and fats | Environmentally friendly, high adsorption capacity | [12] |
| Mixed bentonites | Combination of alkaline and alkaline-earth components | May have properties characteristic of both alkaline and alkaline-earth bentonites | They are used in various fields depending on their composition | Includes properties of both categories, used in different industries | [12] |
| Scope of Application | Influence of Chemical Composition | Notes | Reference |
|---|---|---|---|
| Pharmaceutical industry | High purity of montmorillonite, high adsorption capacity | It is used in excipients (tablets, capsules) and for skincare (cleansing masks, creams). | [29] |
| Cosmetics industry | High adsorption capacity, biocompatibility | It is used to create safe and environmentally friendly cosmetic products (masks, skin creams). | [29] |
| Wastewater treatment | Large surface area, ion exchange capacity | Used for removal of heavy metals and organic pollutants. | [33] |
| Construction industry | Silica and aluminosilicate content | Improves the strength of cement and ceramic materials, reduces the ecological footprint by replacing cement clinkers. | [31] |
| Ceramics production | Increased strength, improved structure | Improves crack resistance, lowers firing temperature, increases production efficiency. | [32] |
| Manufacture of refractory materials | Interaction with industrial waste | Creation of refractory materials with improved mechanical characteristics. | [34] |
| Food industry | High adsorption capacity, interaction with polymers | Used for packaging (controlled release of bioactive components), wine clarification. | [30] |
| Nanocomposites for packaging | Improved barrier and mechanical properties, high stability | Used to create packaging with improved properties, extends the shelf life of products. | [37] |
| Modified bentonites | Organic modification (quaternized ammonium salts) | They increase the compatibility with non-polar polymers used in active packaging with antimicrobial properties. | [36] |
| Applications in bioplastics | Improved durability, reduced water absorption | The addition of bentonite improves the mechanical properties and moisture resistance in bioplastics. | [35] |
| Pharmaceutical industry | High purity, adsorption capacity, biocompatibility | Purified bentonite is used to create excipients and remove toxins and heavy metals. | [29] |
| Type of Bentonite | Chemical Composition | Physicochemical Properties | Application | Features | References |
|---|---|---|---|---|---|
| Modification with silver nanoparticles | Introduction of silver nanoparticles into the intermolecular space of clay | Improvements in physical and chemical properties, increase in specific surface area | Suppression of a wide range of pathogens, including Staphylococcus aureus and Escherichia coli | Silver is retained between the clay layers through cation exchange | [53] |
| Modification using copper and zinc ions | Introduction of copper and zinc ions into the structure of bentonite | Improvements in adsorption and mechanical properties | Increased antibacterial activity against fungi and bacteria | Used in the pharmaceutical and cosmetic industries | [52] |
| Modification with surfactants | Grafting of chemical functional groups on the surface of bentonite | Increase in specific surface area and porosity | Increased antibacterial activity, improved textural properties | Increased adsorption characteristics, broadened application areas | [54] |
| Combined methods (ultrasound, microwave treatment) | Microwave and ultrasonic treatment with the addition of nanodispersed silicon and aluminum oxides | Increased specific surface area, improved homogeneity of the structure | Improved adsorption efficiency and antibacterial properties | Used to improve the adsorption characteristics and versatility of the material | [44] |
| Chemisorption modification | Introduction of organic or inorganic compounds | Improvements in surface nature and porous structure | Increased ability to sorb heavy metals and organic pollutants, improved mechanical properties | The technique improves the sorption properties and antibacterial activity | [38,39,40] |
| Type of Modification | Additives/Methods Used | Target Bacteria/Fungi | Antibacterial Activity | Comments | References |
|---|---|---|---|---|---|
| Initial bentonite | Without modification | Staphylococcus aureus, Escherichia coli | Moderate activity against some pathogens | The main properties of bentonite are adsorption and physicochemical characteristics, without obvious antibacterial activity. | [38] |
| Silver modification | Silver nanoparticles (Ag) | Staphylococcus aureus, Escherichia coli, fungi | High activity against a wide range of bacteria and fungi | Modification with silver nanoparticles significantly improves antibacterial properties by preventing pathogen aggregation. | [47,49,50,51] |
| Copper modification | Copper nanoparticles (Cu) | Staphylococcusaureus, Escherichia coli | High activity against bacterial pathogens | Activates antibacterial activity through copper ionization mechanisms. | [52] |
| Modification with zinc | Zinc nanoparticles (Zn) | Staphylococcus aureus, Escherichia coli | Increased activity against Gram-positive and Gram-negative bacteria | Zinc improves physicochemical properties and enhances bactericidal action. | [52] |
| Surface modification (surfactants) | Surfactants | Escherichia coli, Staphylococcus aureus | Moderate activity | Modification with surfactants improves the chemical and textural properties of clays, which enhances their adsorption capacity. | [50] |
| Microwave treatment + SiO2/Al2O3 nanoparticles | Microwave processing, silicon and aluminum oxide nanoparticles | Escherichia coli, Staphylococcus aureus | Very high activity against a wide range of bacteria and fungi | Increased porosity and improved structure through microwave treatment, and the addition of nanoparticles improves the antibacterial properties. | [44] |
| Ultrasonic treatment + modification with SiO2/Al2O3 | Ultrasonic treatment, silicon and aluminum oxide nanoparticles | Staphylococcus aureus, Escherichia coli, fungi | Very high activity against various pathogens | Ultrasonic treatment increases the homogeneity of the structure and its porosity, which enhances its antibacterial activity. | [44] |
| Surfactant modification | Surfactant treatment, silver and copper nanoparticles | Escherichia coli, Staphylococcus aureus, fungi | Average activity | Treatment with surfactants improves the interaction between the clay and additives, improving its adsorption properties and antibacterial activity. | [55] |
| Study Area | Material | Modification Method and Role of Modifier | Application | Physicochemical Properties and Biological Activity | References |
|---|---|---|---|---|---|
| Application of bentonite in dentistry | Montmorillonite | Hydroxyapatite layers | Dental fillings | Resistance to exposure, biocompatibility | [23,24,70,91,149] |
| Remineralization, ion exchange with Ca2+ | Increased remineralization of teeth | ||||
| Biopolymers for drug delivery | Hydrogels, nanoparticles | Formation of hydrogels, nanocomposites | Medicines | Increased bioavailability, stability | [92,93] |
| Improving drug delivery and release | Targeted delivery of active ingredients | ||||
| Montmorillonite, kaolinite, halloysite | Thermal, acidic, organic modification | Antibacterial materials (bandages, wipes) | Increased surface area, improved porosity, thermal stability | [94,99,150] | |
| Improved bactericidal properties | Increased bactericidal activity | ||||
| Modification of bentonite clays for creation of polymer composites | Bentonite | Surfactants, crosslinking agents | Medicine, pharmaceuticals | High sorption capacity, improved mechanical properties | [105,106,107,108] |
| Increased compatibility with polymers, improved properties | Improved properties when interacting with polymers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabdrakhmanova, S.K.; Kerimkulova, A.Z.; Nauryzova, S.Z.; Aryp, K.; Shaimardan, E.; Kukhareva, A.D.; Kantay, N.; Beisebekov, M.M.; Thomas, S. Bentonite-Based Composites in Medicine: Synthesis, Characterization, and Applications. J. Compos. Sci. 2025, 9, 310. https://doi.org/10.3390/jcs9060310
Kabdrakhmanova SK, Kerimkulova AZ, Nauryzova SZ, Aryp K, Shaimardan E, Kukhareva AD, Kantay N, Beisebekov MM, Thomas S. Bentonite-Based Composites in Medicine: Synthesis, Characterization, and Applications. Journal of Composites Science. 2025; 9(6):310. https://doi.org/10.3390/jcs9060310
Chicago/Turabian StyleKabdrakhmanova, Sana K., Aigul Z. Kerimkulova, Saule Z. Nauryzova, Kadiran Aryp, Esbol Shaimardan, Anastassiya D. Kukhareva, Nurgamit Kantay, Madiar M. Beisebekov, and Sabu Thomas. 2025. "Bentonite-Based Composites in Medicine: Synthesis, Characterization, and Applications" Journal of Composites Science 9, no. 6: 310. https://doi.org/10.3390/jcs9060310
APA StyleKabdrakhmanova, S. K., Kerimkulova, A. Z., Nauryzova, S. Z., Aryp, K., Shaimardan, E., Kukhareva, A. D., Kantay, N., Beisebekov, M. M., & Thomas, S. (2025). Bentonite-Based Composites in Medicine: Synthesis, Characterization, and Applications. Journal of Composites Science, 9(6), 310. https://doi.org/10.3390/jcs9060310







