Effect of Silane-Modified Ammonium Polyphosphate on the Mechanical, Thermal, and Flame-Retardant Properties of Rice Husk/Polylactic Acid Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
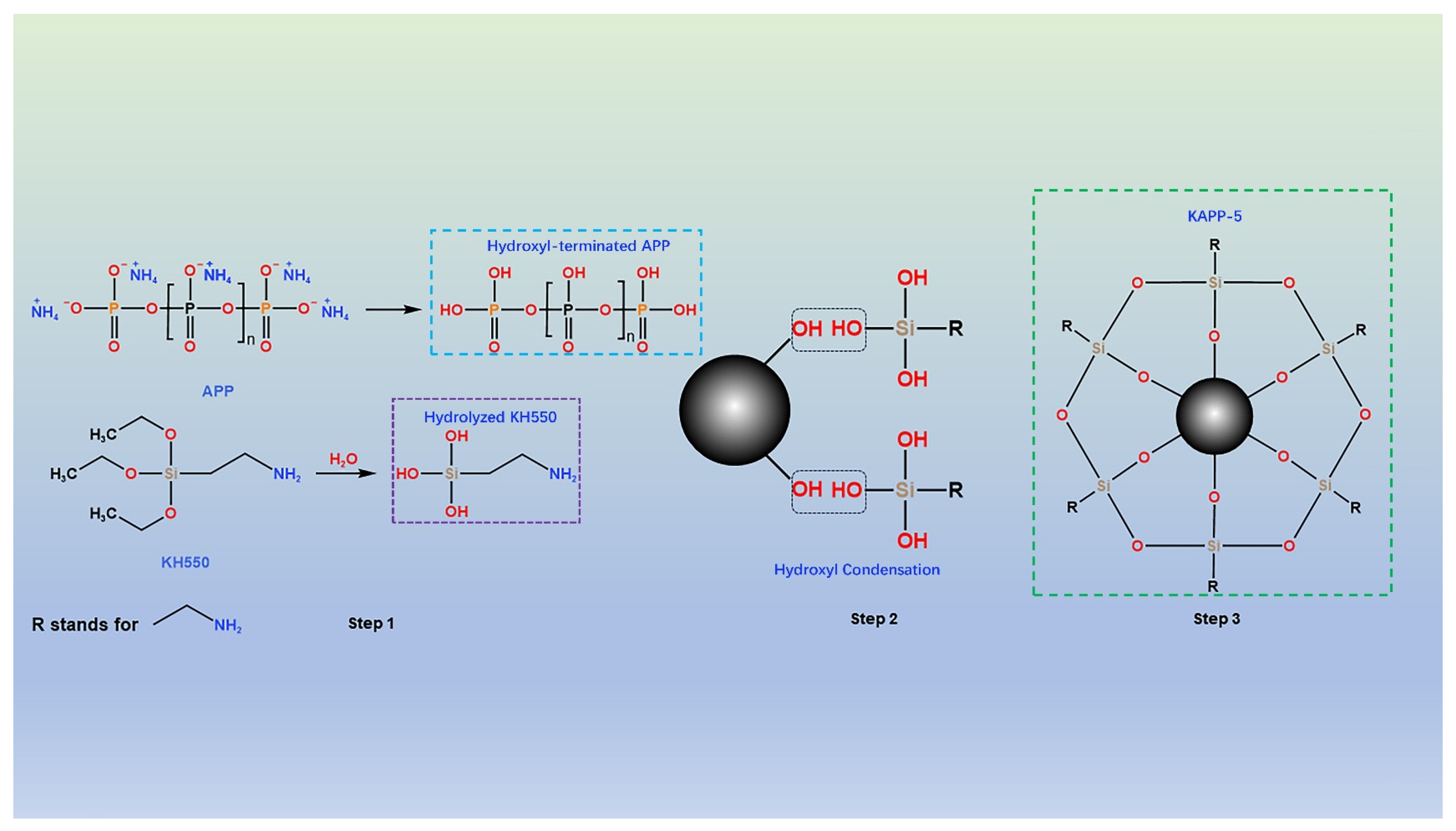
2.2. Preparation of Modified APP
2.3. Preparation of PLA Composites
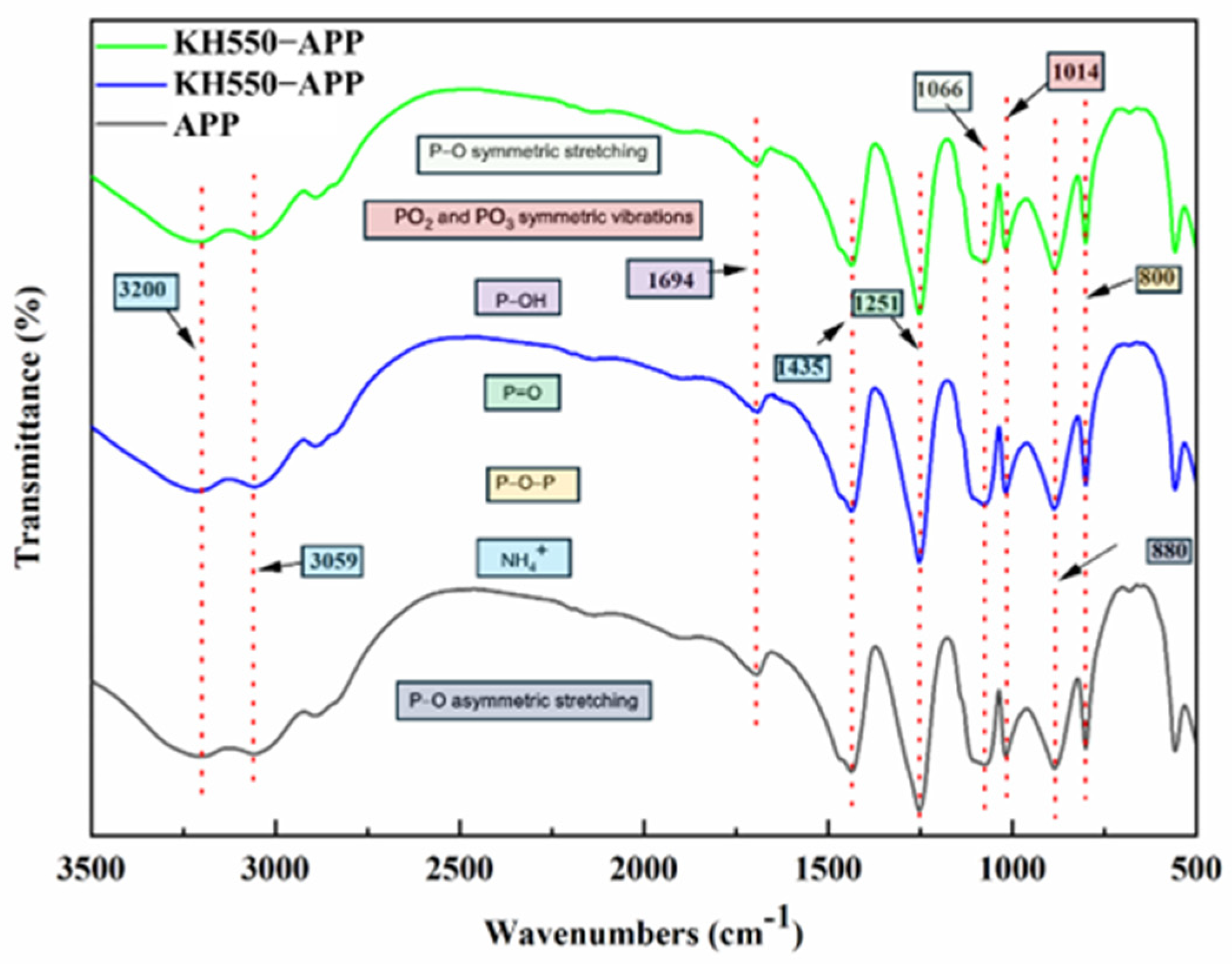
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
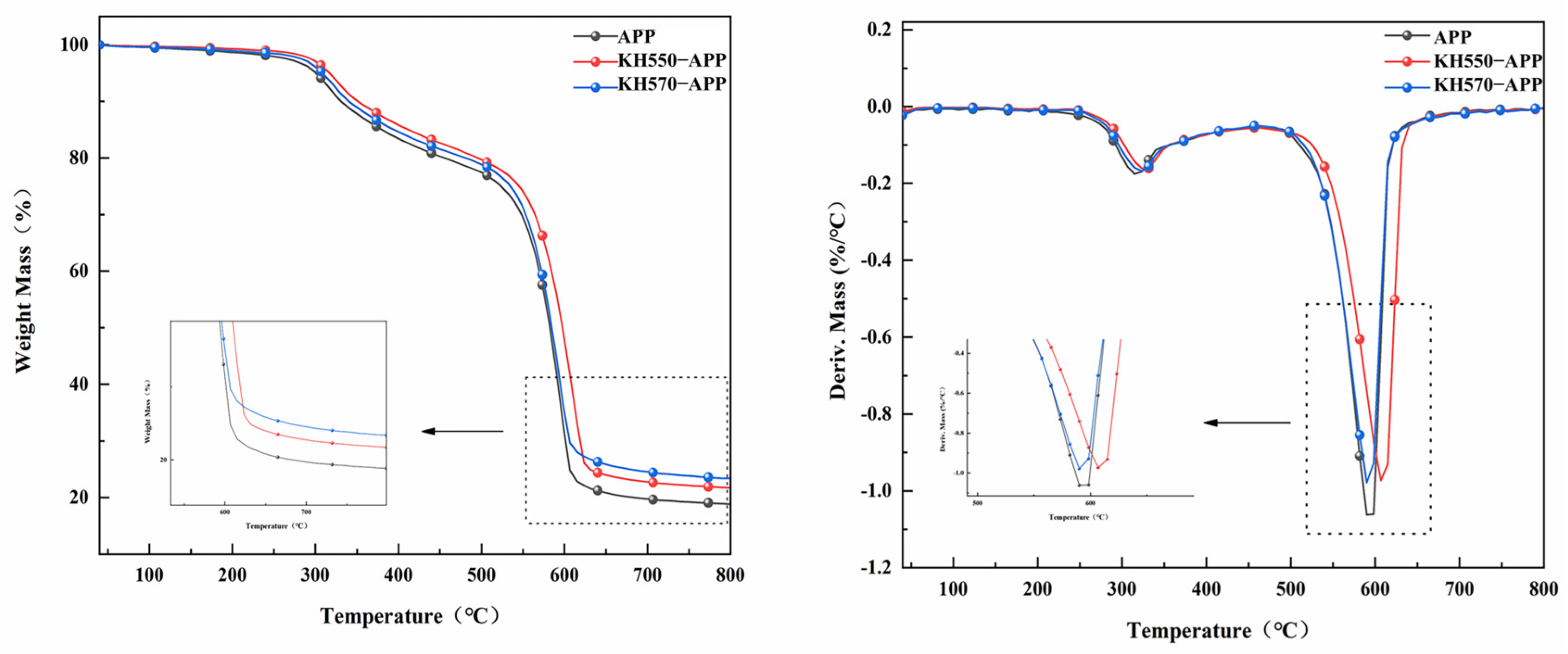
2.5. Thermogravimetric Analysis (TGA)
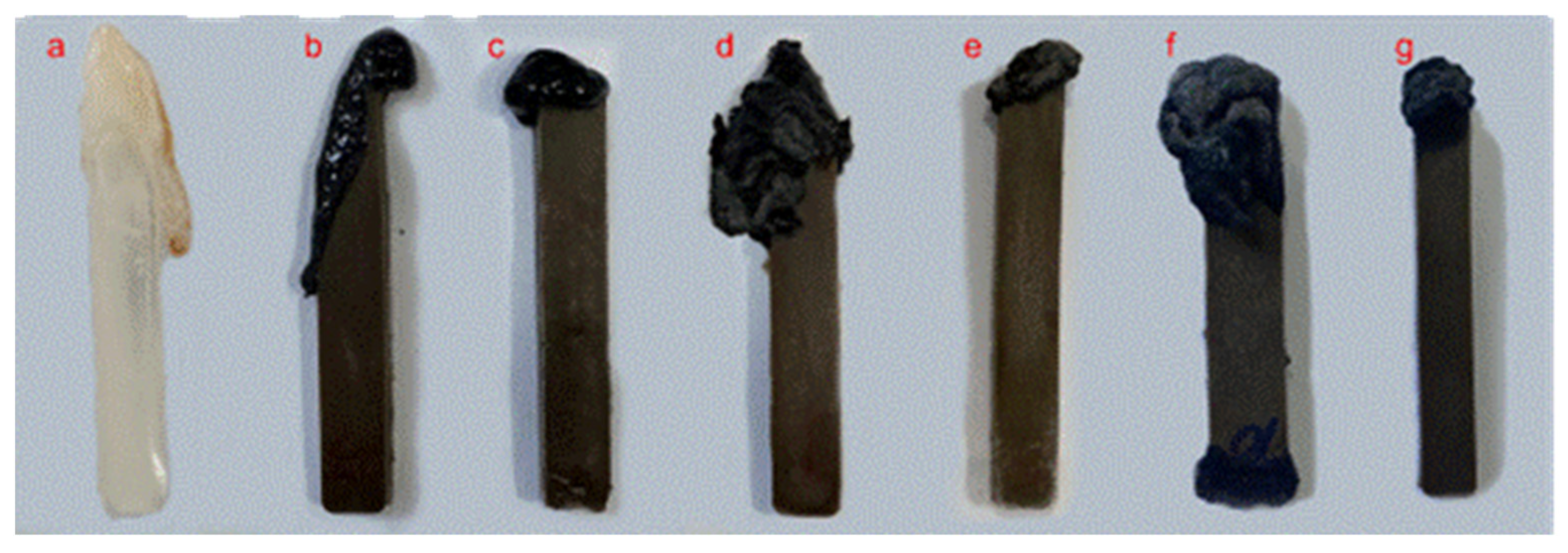
2.6. Flame Retardancy Testing
2.7. Fracture Surface Analysis
2.8. Mechanical Properties Analysis
3. Results
3.1. Characterization of APP and Silane-Modified APP
3.2. Flame-Retardant Properties
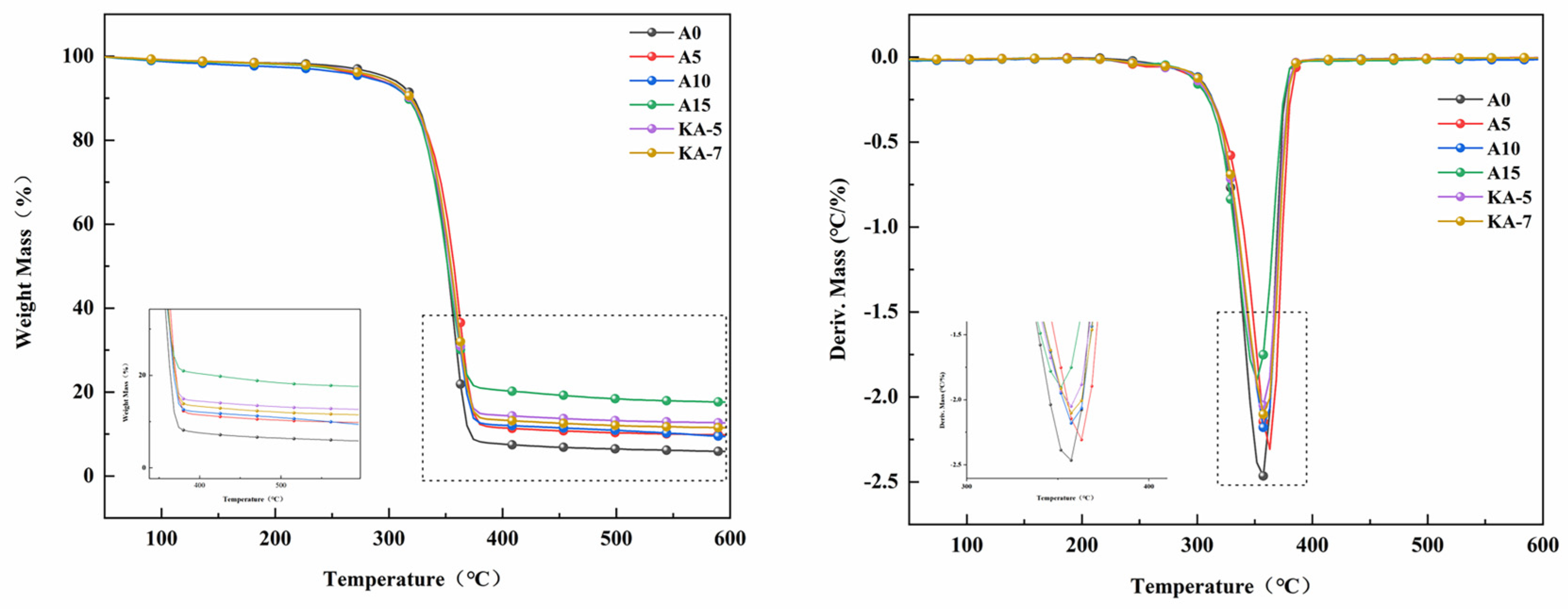
3.3. Thermal Properties
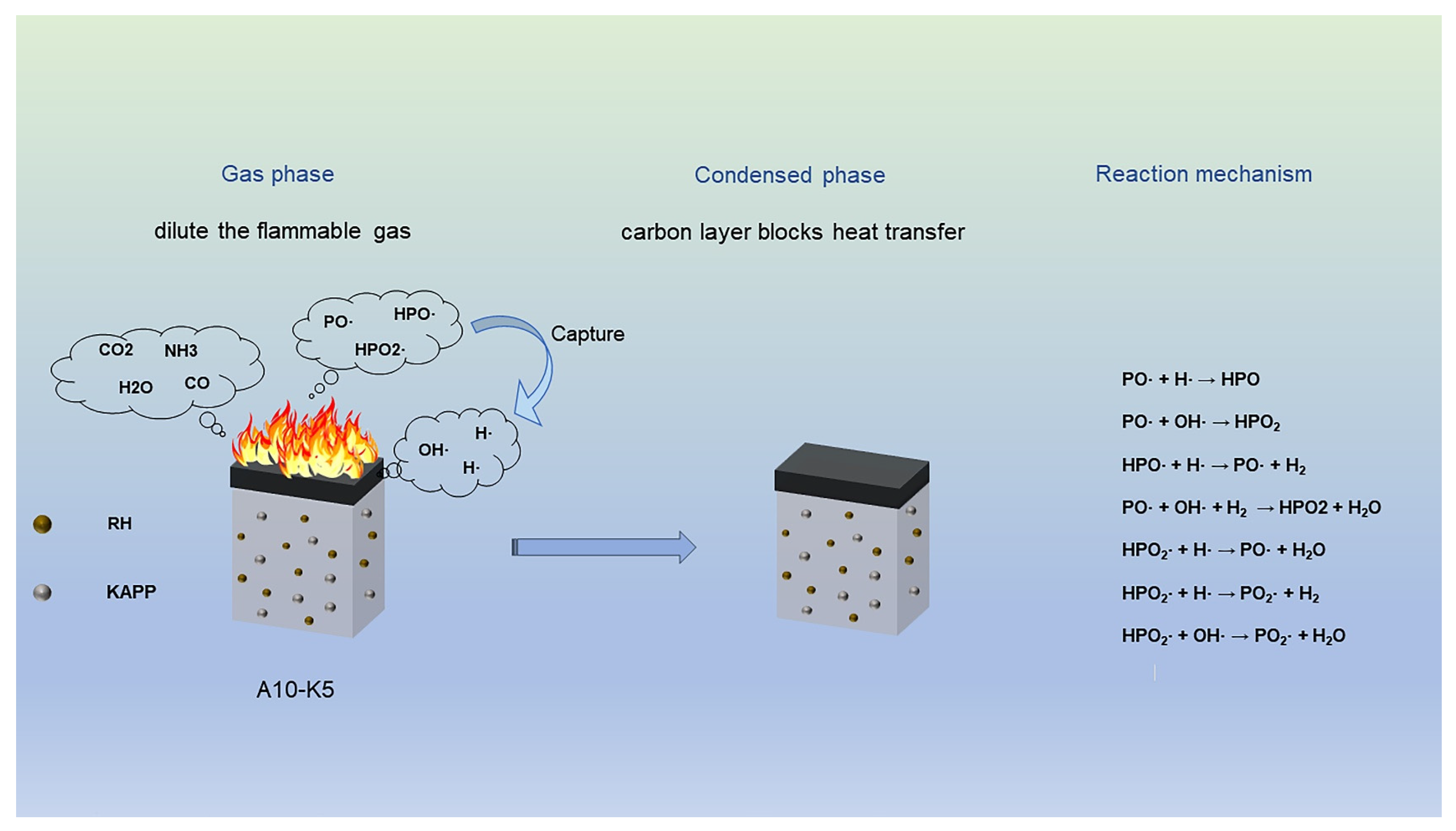
3.4. Flame-Retardant Mechanism
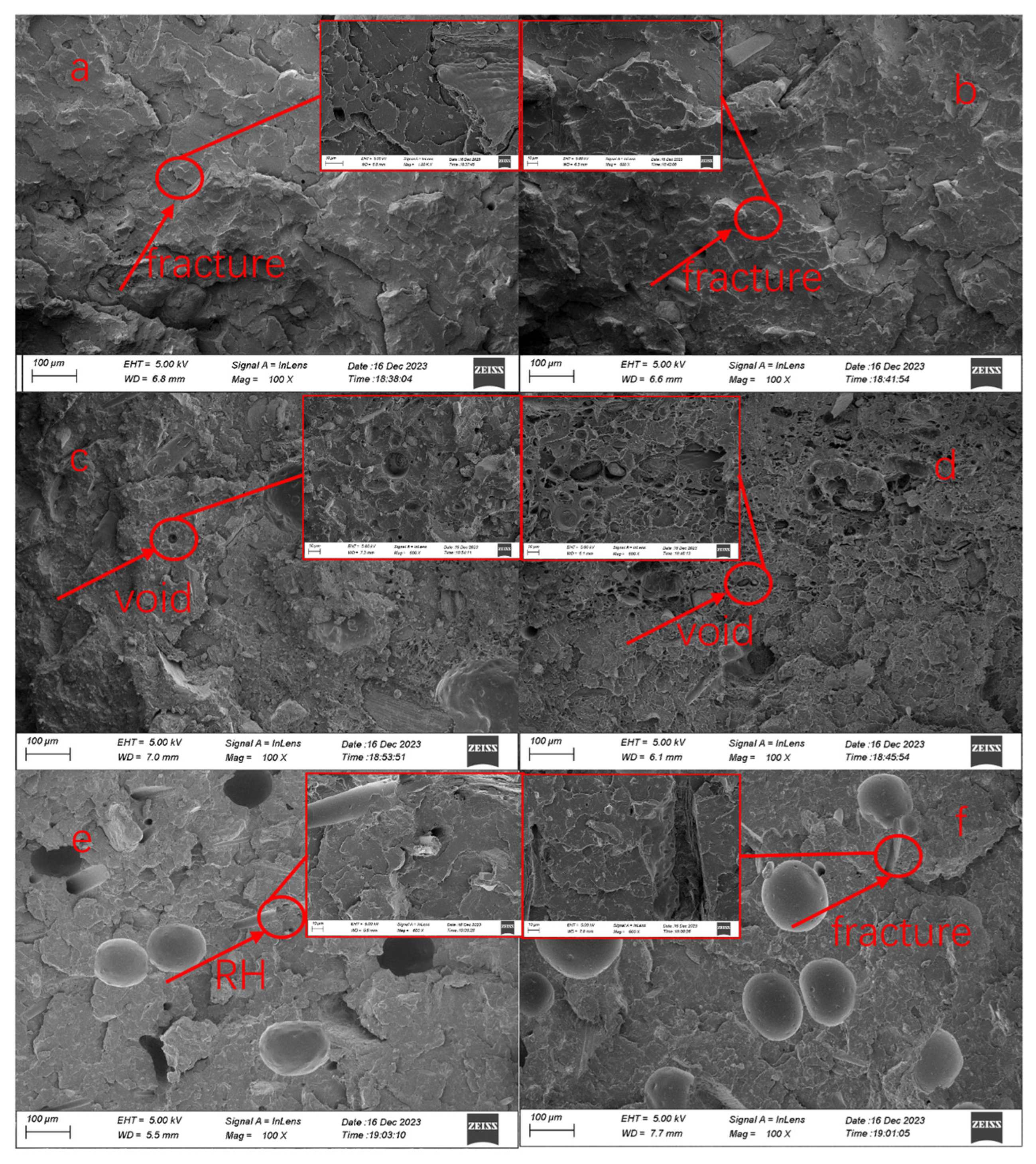
3.5. Morphology
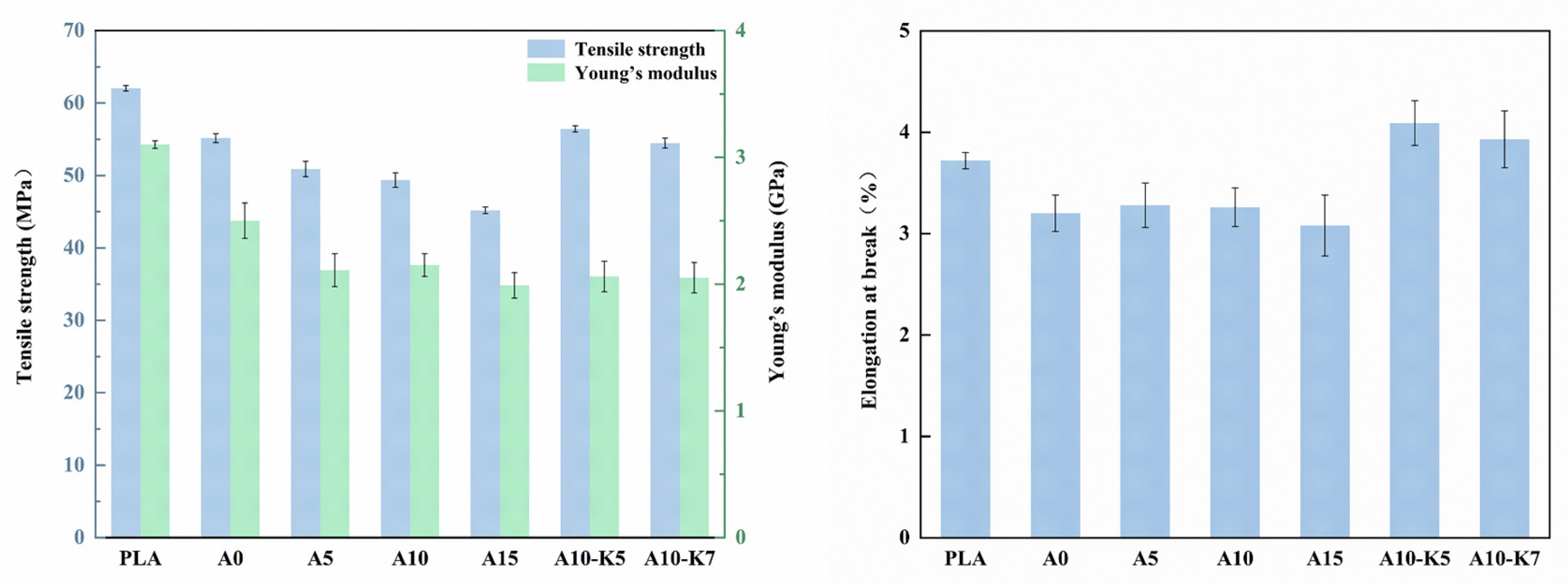
3.6. Mechanical Property
4. Conclusions
- Flame retardancy: PLA/RH-APP15% and PLA/KAPP-7 achieved UL-94 V-0 ratings with LOI values up to 29.4%, outperforming unmodified APP systems.
- Mechanical performance: Silane modification restored tensile strength to 59.92 MPa (A10-K5), mitigating the strength loss observed with unmodified APP.
- Thermal stability: Silane-modified APP increased char residue by 21.7% (KAPP-5) and delayed decomposition by 16.3 °C compared to unmodified APP.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RH | Rice husk |
| APP | Ammonium polyphosphate |
| PLA | Polylactic acid |
| LOI | Limiting oxygen index |
| IFRs | Intumescent flame retardants |
| CONE | Cone calorimetry |
| TRAPP | Novel intumescent flame retardant |
| TA | Tannic acid |
| PEI | Polyethyleneimine |
| UV | Ultraviolet |
| MCAPP | Microencapsulated APP |
| FTIR | Fourier transform infrared spectroscopy |
| TGA | Thermogravimetric analysis |
| TG | Thermogravimetric |
| DTG | Derivative thermogravimetric |
| KAPP-5 | KH550 modified APP |
| KAPP-7 | KH570 modified APP |
References
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Ouchi, T.; Ohya, Y. Design of lactide copolymers as biomaterials. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 453–462. [Google Scholar] [CrossRef]
- Masatoshi, I.J.I. Development of highly function bioplastics used for electronic products: Polylactic acid compounds and cardanol-bonded cellulose resin performing functions for electronic products. Kobunshi 2012, 61, 195–196. [Google Scholar]
- Baochai, L.; Bakar, A.A.; Mohamad, Z. An overview of the recent advances in flame retarded poly(lactic acid). Polym. Adv. Technol. 2023, 34, 1435–1450. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.; Ma, L.; Yin, X.; Zhu, Z.; Song, P. Fabrication of anti-dripping and flame-retardant polylactide modified with chitosan derivative/aluminum hypophosphite. Carbohydr. Polym. 2022, 298, 120141. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, N.; Liu, P.; Cui, H. Fabrication of highly efficient phenylphosphorylated chitosan bio-based flame retardants for flammable PLA biomaterial. Carbohydr. Polym. 2022, 287, 119317. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Liu, X.; Jiang, S.; Wang, J.; Li, H.; Bourbigot, S.; Gu, X.; Zhang, S. An effective flame retardant containing hypophosphorous acid for poly (lactic acid): Fire performance, thermal stability and mechanical properties. Polym. Test. 2019, 78, 105940. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Y.; Lu, Y.; Li, S.; Li, H.; Yan, J.; Du, S. Bio-based arginine surface-modified ammonium polyphosphate: An efficient intumescent flame retardant for epoxy resin. RSC Adv. 2022, 12, 9223–9237. [Google Scholar] [CrossRef]
- Zhang, P.; Gan, S.; Chen, L.; Chen, H.; Jia, C.; Fu, Y.; Xiong, Y. Effect of MMT on Flame Retardancy of PLA/IFR/LDH Composites. J. Renew. Mater. 2022, 10, 2937–2947. [Google Scholar] [CrossRef]
- Li, X.G.; Wu, Y.Q.; Zheng, X. Effect of Nano Anhydrous Magnesium Carbonateon Fire-Retardant Performance of Polylactic Acid/Bamboo Fibers Composites. J. Nanosci. Nanotechnol. 2011, 11, 10620–10623. [Google Scholar] [CrossRef]
- Chen, B.; Qian, L. Mechanical properties and flame retardancy of PLA composites containing zinc oxide and chain extender. J. Appl. Polym. Sci. 2021, 138, e50987. [Google Scholar] [CrossRef]
- Feng, C.; Liang, M.; Jiang, J.; Huang, J.; Liu, H. Flame retardant properties and mechanism of an efficient intumescent flame retardant PLA composites. Polym. Adv. Technol. 2016, 27, 693–700. [Google Scholar] [CrossRef]
- Kabir, I.I.; Carlos Baena, J.; Wang, W.; Wang, C.; Oliver, S.; Nazir, M.T.; Khalid, A.; Fu, Y.; Yuen, A.C.; Yeoh, G.H. Optimisation of Additives to Maximise Performance of Expandable Graphite-Based Intumescent-Flame-Retardant Polyurethane Composites. Molecules 2023, 28, 5100. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Ou, H.; Ran, Y.; Xue, H.; Zhu, F. Preparation and flame retardant properties of organic montmorillonite synergistic intumescent flame retardant polypropylene. J. Loss Prev. Process Ind. 2024, 87, 105226. [Google Scholar] [CrossRef]
- Huang, Z.; Li, S.; Tsai, L.-C.; Jiang, T.; Ma, N.; Tsai, F.-C. Flame retardant polypropylene with a single molecule intumescent flame retardant based on chitosan. Mater. Today Commun. 2022, 33, 104689. [Google Scholar] [CrossRef]
- Fang, Q.; Zhan, Y.; Chen, X.; Wu, R.; Zhang, W.; Wang, Y.; Wu, X.; He, Y.; Zhou, J.; Yuan, B. A bio-based intumescent flame retardant with biomolecules functionalized ammonium polyphosphate enables polylactic acid with excellent flame retardancy. Eur. Polym. J. 2022, 177, 111479. [Google Scholar] [CrossRef]
- Qu, Q.; Xu, J.; Wang, H.H.; Yu, Y.R.; Dong, Q.P.; Zhang, X.H.; He, Y. Carbon Nanotube-Based Intumescent Flame Retardants Achieve High-Efficiency Flame Retardancy and Simultaneously Avoid Mechanical Property Loss. Polymers 2023, 15, 1406. [Google Scholar] [CrossRef]
- Han, D.Q.; Wang, H.; Lu, T.T.; Cao, L.Y.; Dai, Y.F.; Cao, H.Z.; Yu, X.L. Scalable Manufacturing Green Core-Shell Structure Flame Retardant, with Enhanced Mechanical and Flame-Retardant Performances of Polylactic Acid. J. Polym. Environ. 2022, 30, 2516–2533. [Google Scholar] [CrossRef]
- Jin, X.; Cui, S.; Sun, S.; Gu, X.; Li, H.; Sun, J.; Zhang, S.; Bourbigot, S. The Preparation of an Intumescent Flame Retardant by Ion Exchange and Its Application in Polylactic Acid. ACS Appl. Polym. Mater. 2019, 1, 755–764. [Google Scholar] [CrossRef]
- Liao, M.; Chen, H.; Deng, L.; Wei, X.; Zou, Z.; Wang, H.; Chen, S.; Zhu, Z. Tannic acid-polyethyleneimine modified ammonium polyphosphate: For efficient flame retardant and UV resistant of polylactic acid. React. Funct. Polym. 2023, 192, 105735. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xuan, S.; Xing, W.; Bai, Z.; Lu, H. Flame Retardancy and Thermal Degradation of Intumescent Flame Retardant Poly(lactic acid)/Starch Biocomposites. Ind. Eng. Chem. Res. 2011, 50, 713–720. [Google Scholar] [CrossRef]
- Cao, C.-F.; Yu, B.; Guo, B.-F.; Hu, W.-J.; Sun, F.-N.; Zhang, Z.-H.; Li, S.-N.; Wu, W.; Tang, L.-C.; Song, P.; et al. Bio-inspired, sustainable and mechanically robust graphene oxide-based hybrid networks for efficient fire protection and warning. Chem. Eng. J. 2022, 439, 134516. [Google Scholar] [CrossRef]
- Shukor, F.; Hassan, A.; Saiful Islam, M.; Mokhtar, M.; Hasan, M. Effect of ammonium polyphosphate on flame retardancy, thermal stability and mechanical properties of alkali treated kenaf fiber filled PLA biocomposites. Mater. Des. (1980–2015) 2014, 54, 425–429. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Mohit, H.; Mavinkere Rangappa, S.; Siengchin, S.; Gorbatyuk, S.; Manimaran, P.; Alka Kumari, C.; Khan, A.; Doddamani, M. A comprehensive review on performance and machinability of plant fiber polymer composites. Polym. Compos. 2022, 43, 608–623. [Google Scholar] [CrossRef]
- Halip, J.A.; Lee, S.H.; Tahir, P.M.; Chuan, L.T.; Selimin, M.A.; Saffian, H.A. A Review: Chemical Treatments of Rice Husk for Polymer Composites. Biointerface Res. Appl. Chem. 2021, 11, 12425–12433. [Google Scholar]
- Sun, Y.; Zhang, Z.; Wang, J.; Liu, H.; Shi, D.; Liu, M.; Ying, J.; Mu, W.; Li, D.; Wu, S. Mechanical and thermal properties of rice husk/glass fiber/polylactic acid composites. Polym. Compos. 2024, 45, 15878–15890. [Google Scholar] [CrossRef]
- Wu, C.-S.; Tsou, C.-H. Fabrication, characterization, and application of biocomposites from poly (lactic acid) with renewable rice husk as reinforcement. J. Polym. Res. 2019, 26, 44. [Google Scholar] [CrossRef]
- Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Fierro, V.; Celzard, A. PLA with Intumescent System Containing Lignin and Ammonium Polyphosphate for Flame Retardant Textile. Polymers 2016, 8, 331. [Google Scholar] [CrossRef]
- Yang, H.; Yu, B.; Xu, X.; Bourbigot, S.; Wang, H.; Song, P. Lignin-derived bio-based flame retardants toward high-performance sustainable polymeric materials. Green Chem. 2020, 22, 2129–2161. [Google Scholar] [CrossRef]
- Jin, X.; Cui, S.; Sun, S.; Sun, J.; Zhang, S. The Preparation and Characterization of Polylactic Acid Composites with Chitin-Based Intumescent Flame Retardants. Polymers 2021, 13, 3513. [Google Scholar] [CrossRef]
- GB/T 2408-2008; Plastics-Determination of Burning Characteristics-Horizontal and Vertical Test. Standardization Administration of China: Beijing, China, 2008.
- GB/T 1040.1-2006; Plastics-Determination of Tensile Properties-Part 1: General Principles. Standardization Administration of China: Beijing, China, 2006.
- Sun, Y.; Yuan, B.; Shang, S.; Zhang, H.; Shi, Y.; Yu, B.; Qi, C.; Dong, H.; Chen, X.; Yang, X. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos. Part B Eng. 2020, 181, 107588. [Google Scholar] [CrossRef]
- Lin, H.; Yan, H.; Liu, B.; Wei, L.; Xu, B. The influence of KH-550 on properties of ammonium polyphosphate and polypropylene flame retardant composites. Polym. Degrad. Stab. 2011, 96, 1382–1388. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, D.; Li, W.; Cai, X. Flame retardant efficiency of KH-550 modified urea-formaldehyde resin cooperating with ammonium polyphosphate on polypropylene. Polym. Degrad. Stab. 2018, 151, 160–171. [Google Scholar] [CrossRef]
- Huang, Z.; Ruan, B.; Wu, J.; Ma, N.; Jiang, T.; Tsai, F.C. High-efficiency ammonium polyphosphate intumescent encapsulated polypropylene flame retardant. J. Appl. Polym. Sci. 2021, 138, 50413. [Google Scholar] [CrossRef]
- Tang, G.; Jiang, H.; Yang, Y.; Chen, D.; Liu, C.; Zhang, P.; Zhou, L.; Huang, X.; Zhang, H.; Liu, X. Preparation of melamine–formaldehyde resin-microencapsulated ammonium polyphosphate and its application in flame retardant rigid polyurethane foam composites. J. Polym. Res. 2020, 27, 375. [Google Scholar] [CrossRef]
- Liu, J.C.; Xu, M.J.; Lai, T.; Li, B. Effect of surface-modified ammonium polyphosphate with KH550 and silicon resin on the flame retardancy, water resistance, mechanical and thermal properties of intumescent flame retardant polypropylene. Ind. Eng. Chem. Res. 2015, 54, 9733–9741. [Google Scholar] [CrossRef]
- Yu, S.; Xiang, H.; Zhou, J.; Zhu, M. Enhanced flame-retardant performance of poly (lactic acid)(PLA) composite by using intrinsically phosphorus-containing PLA. Prog. Nat. Sci. Mater. Int. 2018, 28, 590–597. [Google Scholar] [CrossRef]
- Yiga, V.A.; Lubwama, M.; Olupot, P.W. Thermal stability of unmodified and alkali-modified rice husks for flame retardant fiber-reinforced PLA composites. J. Therm. Anal. Calorim. 2022, 147, 11049–11075. [Google Scholar] [CrossRef]
- Yu, S.; Xiang, H.; Zhou, J.; Zhu, M. Preparation and characterization of fire resistant PLA fibers with phosphorus flame retardant. Fibers Polym. 2017, 18, 1098–1105. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, W.; Li, W.; Semple, K.; Yin, N.; Zhang, W.; Dai, C. Intumescent-grafted bamboo charcoal: A natural nontoxic fire-retardant filler for polylactic acid (PLA) composites. ACS Omega 2021, 6, 26990–27006. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-S.; Bee, S.-T.; Sin, L.T.; Tee, T.-T.; Ratnam, C.; Hui, D.; Rahmat, A. A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites. Compos. Part B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Reuter, J.; Greiner, L.; Kukla, P.; Döring, M. Efficient flame retardant interplay of unsaturated polyester resin formulations based on ammonium polyphosphate. Polym. Degrad. Stab. 2020, 178, 109134. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, X.; Wang, S.; Yuan, B.; Fang, Q.; Shang, S.; Cao, C.; Chen, G. Synthesis of a bio-based flame retardant via a facile strategy and its synergistic effect with ammonium polyphosphate on the flame retardancy of polylactic acid. Polym. Degrad. Stab. 2021, 191, 109684. [Google Scholar] [CrossRef]
- Yan, M.; Pang, Y.; Shao, W.; Ma, C.; Zheng, W. Utilization of spent coffee grounds as charring agent to prepare flame retardant poly(lactic acid) composites with improved toughness. Int. J. Biol. Macromol. 2024, 264, 130534. [Google Scholar] [CrossRef]
- Zuluaga-Parra, J.; Ramos-deValle, L.; Sánchez-Valdes, S.; Torres-Lubian, R.; Pérez-Mora, R.; Ramírez-Vargas, E.; Martínez-Colunga, J.; DaSilva, L.; Vazquez-Rodriguez, S.; Lozano-Ramírez, T. Grafting of ammonium polyphosphate onto poly (lactic acid) and its effect on flame retardancy and mechanical properties. Iran. Polym. J. 2023, 32, 225–238. [Google Scholar] [CrossRef]
- Chow, W.; Teoh, E.L.; Karger-Kocsis, J. Flame retarded poly (lactic acid): A review. Express Polym. Lett. 2018, 12, 396–417. [Google Scholar] [CrossRef]
- Yang, M.; Su, J.; Zheng, Y.; Fang, C.; Lei, W.; Li, L. Effect of Different Silane Coupling Agents on Properties of Waste Corrugated Paper Fiber/Polylactic Acid Composites. Polymers 2023, 15, 3525. [Google Scholar] [CrossRef]
| Sample | PLA/% | APP/% | RH/% | KAPP-5/% | KAPP-7/% |
|---|---|---|---|---|---|
| PLA | 100 | 0 | 0 | 0 | 0 |
| A0 | 85 | 0 | 15 | 0 | 0 |
| A5 | 80 | 5 | 15 | 0 | 0 |
| A10 | 75 | 10 | 15 | 0 | 0 |
| A15 | 70 | 15 | 15 | 0 | 0 |
| A10-K5 | 75 | 0 | 15 | 10 | 0 |
| A10-K7 | 75 | 0 | 15 | 0 | 10 |
| Sample | T5% | Tmax1 | Mass Loss Rate at Tmax1 | Tmax2 | Mass Loss Rate at Tmax2 | Residual at 800 °C |
|---|---|---|---|---|---|---|
| °C | °C | wt%∙min−1 | °C | wt%∙min−1 | % | |
| APP | 300.6 | 315.3 | 1.8 | 592.6 | 12.4 | 18.9 |
| KAPP-5 | 316.9 | 323.5 | 1.6 | 606.4 | 9.7 | 21.7 |
| KAPP-7 | 308.3 | 327.4 | 1.7 | 590.5 | 9.8 | 23.4 |
| Sample | LOI (%) | UL-94 | ||
|---|---|---|---|---|
| Dripping | Ignition of Cotton | Rating | ||
| PLA | 20 | Heavy Dripping | Yes | NR |
| A0 | 19.8 | Heavy Dripping | Yes | NR |
| A5 | 23.3 | Dripping | Yes | V-2 |
| A10 | 26.6 | Slight Dripping | No | V-0 |
| A15 | 27.4 | Slight Dripping | No | V-0 |
| A10-K5 | 27.9 | Slight Dripping | No | V-0 |
| A10-K7 | 29.4 | Significantly Reduced | No | V-0 |
| Sample | T5% | Tmax | Mass Loss Rate at Tmax | Residual at 600 °C |
|---|---|---|---|---|
| °C | °C | wt%∙min−1 | % | |
| PLA | 311.0 | 352.1 | 20.8 | 1.3 |
| A0 | 298.6 | 357.4 | 16.8 | 5.8 |
| A5 | 284.3 | 363.0 | 15.7 | 9.8 |
| A10 | 279.0 | 357.5 | 14.8 | 9.4 |
| A15 | 291.6 | 351.6 | 13.0 | 17.6 |
| A10-K5 | 291.0 | 357.4 | 13.9 | 12.7 |
| A10-K7 | 291.1 | 357.5 | 14.3 | 11.5 |
| Composites | Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|
| PLA | 62.03 ± 0.38 | 3.1 ± 0.03 | 3.72 ± 0.11 |
| A0 | 55.43 ± 0.62 | 2.50 ± 0.26 | 3.20 ± 0.38 |
| A5 | 51.56 ± 1.06 | 2.11 ± 0.18 | 3.28 ± 0.37 |
| A10 | 50.46 ± 1.01 | 2.15 ± 0.09 | 3.26 ± 0.19 |
| A15 | 43.91 ± 0.45 | 1.99 ± 0.10 | 3.08 ± 0.40 |
| A10-K5 | 59.92 ± 0.42 | 2.06 ± 0.12 | 4.09 ± 0.38 |
| A10-K7 | 56.21 ± 0.68 | 2.05 ± 0.12 | 3.93 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Liu, M.; Zhang, Z.; Liu, H.; Shi, D.; Ying, J.; Mu, W.; Li, D.; Kong, I. Effect of Silane-Modified Ammonium Polyphosphate on the Mechanical, Thermal, and Flame-Retardant Properties of Rice Husk/Polylactic Acid Composites. J. Compos. Sci. 2025, 9, 251. https://doi.org/10.3390/jcs9050251
Sun Y, Liu M, Zhang Z, Liu H, Shi D, Ying J, Mu W, Li D, Kong I. Effect of Silane-Modified Ammonium Polyphosphate on the Mechanical, Thermal, and Flame-Retardant Properties of Rice Husk/Polylactic Acid Composites. Journal of Composites Science. 2025; 9(5):251. https://doi.org/10.3390/jcs9050251
Chicago/Turabian StyleSun, Yufeng, Mingyang Liu, Ziheng Zhang, Hengyu Liu, Dongming Shi, Jilai Ying, Wenlong Mu, Defeng Li, and Ing Kong. 2025. "Effect of Silane-Modified Ammonium Polyphosphate on the Mechanical, Thermal, and Flame-Retardant Properties of Rice Husk/Polylactic Acid Composites" Journal of Composites Science 9, no. 5: 251. https://doi.org/10.3390/jcs9050251
APA StyleSun, Y., Liu, M., Zhang, Z., Liu, H., Shi, D., Ying, J., Mu, W., Li, D., & Kong, I. (2025). Effect of Silane-Modified Ammonium Polyphosphate on the Mechanical, Thermal, and Flame-Retardant Properties of Rice Husk/Polylactic Acid Composites. Journal of Composites Science, 9(5), 251. https://doi.org/10.3390/jcs9050251







