Enhanced Photoelectrochemical Water Splitting Using a NiFe2O4/NG@MIL-100(Fe)/TiO2 Composite Photoanode: Synthesis, Characterization, and Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NiFe2O4
2.3. Synthesis of Nitrogen-Doped Graphene
2.4. Synthesis of NiFe2O4/NG
2.5. Synthesis of MIL-100(Fe)
2.6. Synthesis of NiFe2O4/NG@MIL-100(Fe) Nanocomposite
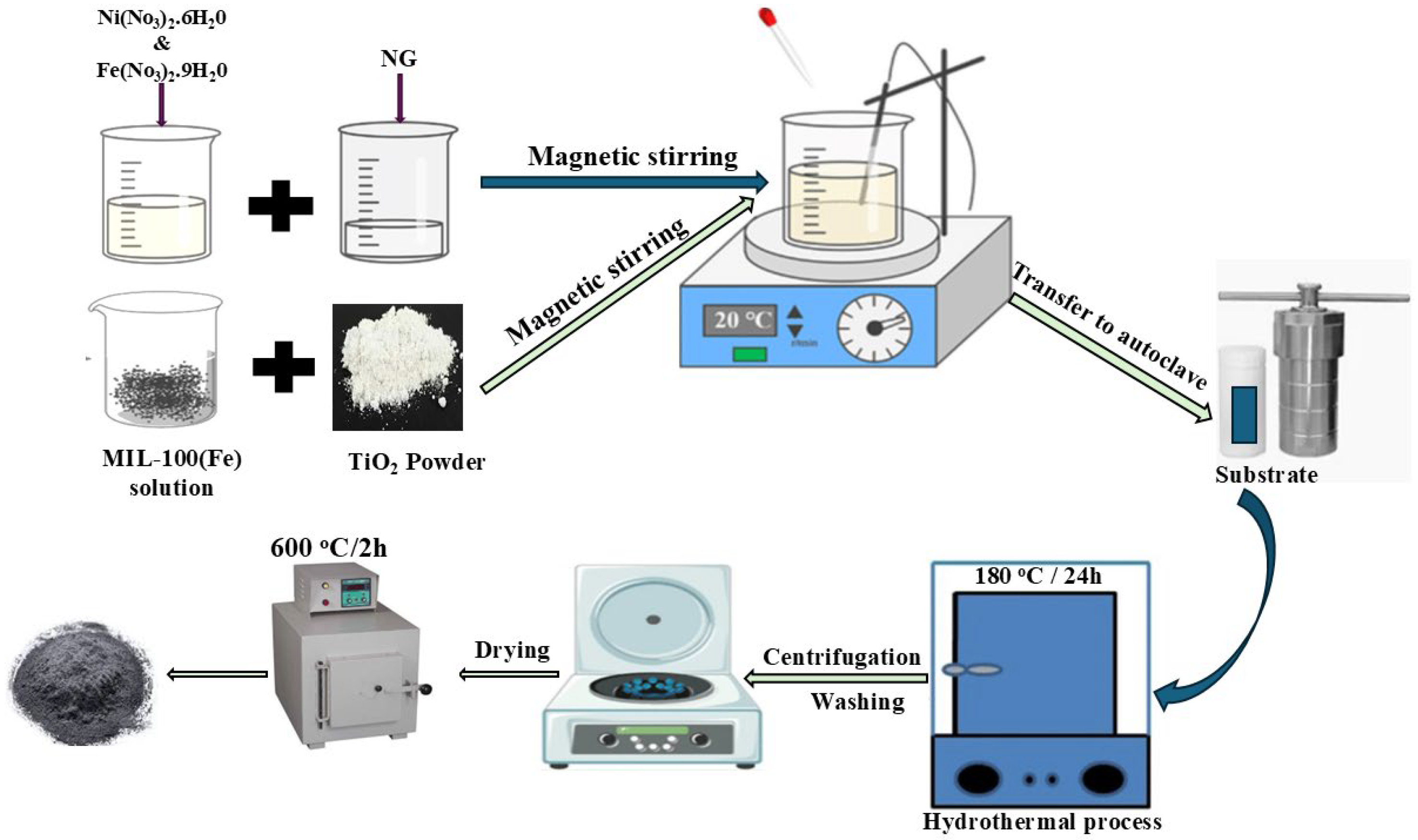
2.7. Synthesis and Fabrication of NiFe2O4/NG@MIL-100(Fe)/TiO2 Nanocomposite
2.8. Characterization
2.9. Photoelectrochemical Water Splitting
3. Results and Discussions
3.1. Physiochemical Characterizations of NiFe2O4/NG@MIL-100(Fe)/TiO2
3.1.1. XRD Analysis
3.1.2. Morphology Studies
3.1.3. FTIR Analysis
3.1.4. XPS Analysis
3.1.5. BET Measurement
3.1.6. Williamson–Hall Plot
3.2. Optoelectronic Analysis
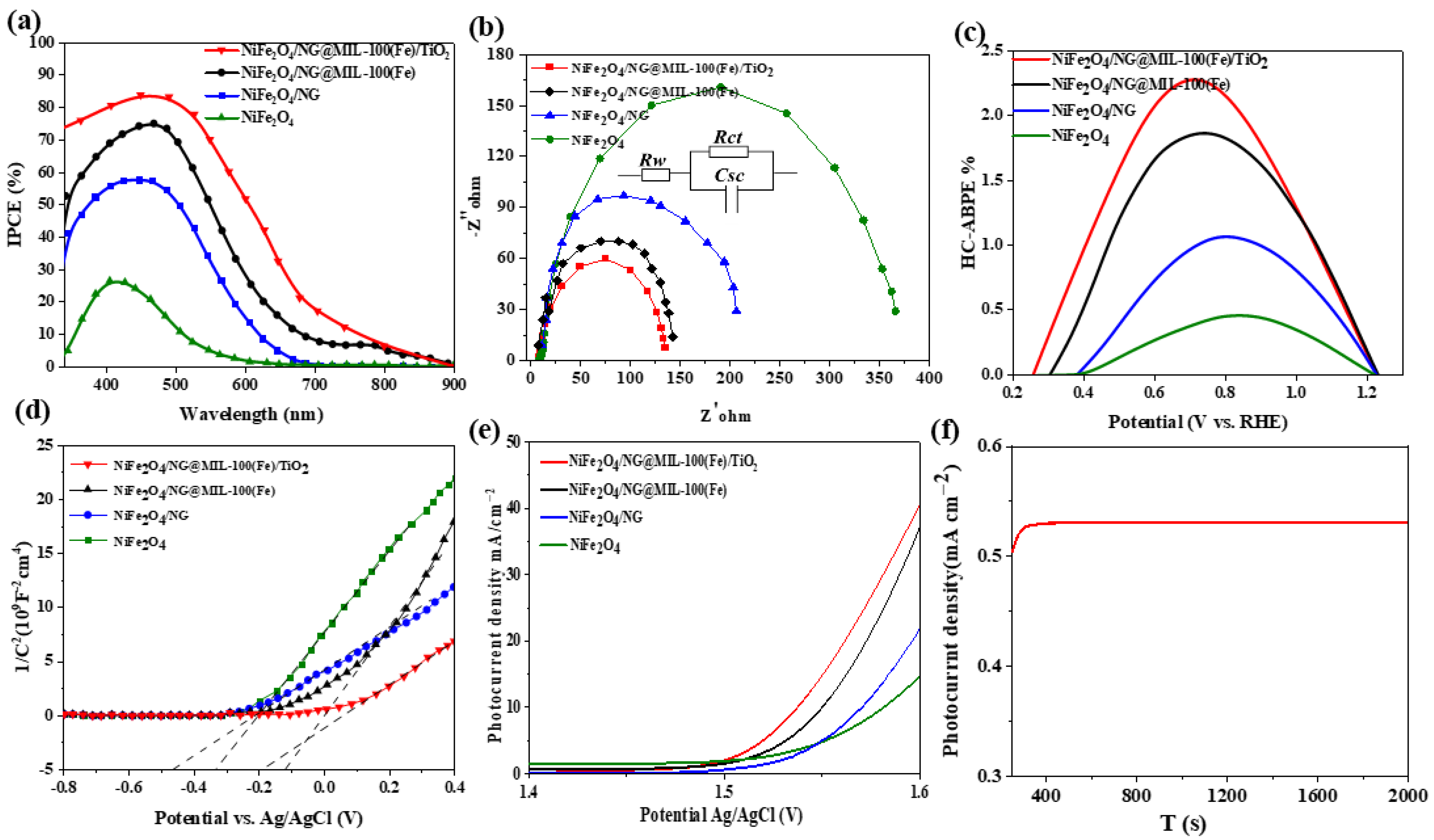
3.3. Electrochemical Characterizations and Photoelectrochemical Water Splitting
3.4. Photoelectrochemical Mechanism for Water Splitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qi, Y.; Zhang, B.; Zhang, G.; Zheng, Z.; Xie, T.; Chen, S.; Ma, G.; Li, C.; Domen, K.; Zhang, F. Efficient overall water splitting of a suspended photocatalyst boosted by metal-support interaction. Joule 2024, 8, 193–203. [Google Scholar] [CrossRef]
- Ling, L.; Liu, L.; Feng, Y.; Zhu, J.; Bian, Z. Synthesis of TiO2 mesocrystal film with enhanced photocatalytic activity. Chin. J. Catal. 2018, 39, 639–645. [Google Scholar] [CrossRef]
- Abarca González, J.A.; Díaz Sainz, G.; Merino García, I.; Irabien Gulías, Á.; Albo Sánchez, J. Photoelectrochemical CO2 electrolyzers: From photoelectrode fabrication to reactor configuration. J. Energy Chem. 2023, 85, 455–480. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, G.; Liu, T. Controllable Synthesis Heterojunction of g-C3N4 and BiVO4 to Enhance the Photocatalytic Oxygen Evolution Activity. ACS Sustain. Chem. Eng. 2024, 12, 5675–5684. [Google Scholar] [CrossRef]
- She, H.; Zhou, H.; Li, L.; Zhao, Z.; Jiang, M.; Huang, J.; Wang, L.; Wang, Q. Construction of a two-dimensional composite derived from TiO2 and SnS2 for enhanced photocatalytic reduction of CO2 into CH4. ACS Sustain. Chem. Eng. 2018, 7, 650–659. [Google Scholar] [CrossRef]
- Yin, D.; Ning, X.; Zhang, Q.; Du, P.; Lu, X. Dual modification of BiVO4 photoanode for synergistically boosting photoelectrochemical water splitting. J. Colloid Interface Sci. 2023, 646, 238–244. [Google Scholar] [CrossRef]
- Feng, C.; Liu, L.; Fu, H.; Zhan, F.; Jia, H.; Cheng, X.; Yu, F.; Zhou, Q.; Bian, Z.; Zhang, B. Structural tuning of BiVO4/MnFe-MOF photoanodes boosts hole extraction for photoelectrochemical water splitting. Catal. Sci. Technol. 2024, 14, 4860–4868. [Google Scholar] [CrossRef]
- Li, R.; Zhang, C.; You, K.; Li, B.; Bu, W.; Meng, X.; Ma, B.; Ding, Y. Molecular confined synthesis of magnetic CoOx/Co/C hybrid catalyst for photocatalytic water oxidation and CO2 reduction. Chin. Chem. Lett. 2023, 34, 108801. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Richards, B.S.; Hudry, D.; Busko, D.; Turshatov, A.; Howard, I.A. Photon upconversion for photovoltaics and photocatalysis: A critical review: Focus review. Chem. Rev. 2021, 121, 9165–9195. [Google Scholar] [CrossRef]
- Mary, A.S.; Murugan, C.; Mahendiran, D.; Murugan, P.; Pandikumar, A. Investigation of Mn incorporation into NiOOH electrocatalyst loaded on BiVO4 photoanode for enhanced photoelectrochemical water splitting: Experimental and theoretical approach. Mater. Today Energy 2024, 41, 101541. [Google Scholar] [CrossRef]
- Shao, J.; Liu, H.; Jiang, X.; Wang, Y.; Luo, L.; Li, Y.; Zhang, Z.; Lu, B. High Performance Ferroelectric-modulated Photoelectrodes by Using Grid Charge Collector. Adv. Funct. Mater. 2024, 34, 2316409. [Google Scholar] [CrossRef]
- Bie, C.; Yu, H.; Cheng, B.; Ho, W.; Fan, J.; Yu, J. Design, fabrication, and mechanism of nitrogen-doped graphene-based photocatalyst. Adv. Mater. 2021, 33, 2003521. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yao, D.; Chu, C.; Mao, S. Photocatalytic H2O2 production systems: Design strategies and environmental applications. Chem. Eng. J. 2023, 451, 138489. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Haji Jumali, M.H.; Yap, C.C.; Mat Salleh, M. Influence of TiO2 Nanoparticles on Enhancement of Optoelectronic Properties of PFO-Based Light Emitting Diode. J. Nanomater. 2013, 2013, 561534. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Liu, M.; Liao, R.; Zhan, H.; Qi, X.; Wang, Y.; Huang, Y. The enhanced photocatalytic activity of TiO2 (B)/MIL-100 (Fe) composite via Fe–O clusters. New J. Chem. 2022, 46, 739–746. [Google Scholar] [CrossRef]
- Rashid, T.; Raza, A.; Saleh, H.A.; Khan, S.; Rahaman, S.; Pandey, K.; AlDamen, M.A.; Sama, F.; Ahmad, A.; Shahid, M. Green Synthesis of Ni/Fe3O4/rGO Nanocomposites for Desulfurization of Fuel. ACS Appl. Nano Mater. 2023, 6, 18905–18917. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Chang, C.-L.; Tseng, Z.-C.; Hsiao, V.K.; Huang, C.-Y. Enhancing the Photoelectrochemical Performance of a Nanoporous Silicon Photocathode through Electroless Nickel Deposition. Nanomaterials 2023, 13, 2552. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Rajan, A.; Rugma, T.; Bernaurdshaw, N. A review on particulate photocatalytic hydrogen production system: Progress made in achieving high energy conversion efficiency and key challenges ahead. Renew. Sustain. Energy Rev. 2021, 152, 111694. [Google Scholar] [CrossRef]
- Dou, Y.; Zhou, J.; Zhou, A.; Li, J.-R.; Nie, Z. Visible-light responsive MOF encapsulation of noble-metal-sensitized semiconductors for high-performance photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5, 19491–19498. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, D.H.; Kim, J.-H.; Jang, H.W.; Lee, J.-H. NH2-MIL-125(Ti)/TiO2 nanorod heterojunction photoanodes for efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2019, 244, 511–518. [Google Scholar] [CrossRef]
- Ali, M.; Pervaiz, E.; Noor, T.; Rabi, O.; Zahra, R.; Yang, M. Recent advancements in MOF-based catalysts for applications in electrochemical and photoelectrochemical water splitting: A review. Int. J. Energy Res. 2021, 45, 1190–1226. [Google Scholar] [CrossRef]
- Li, C.F.; Zhao, J.W.; Xie, L.J.; Wu, J.Q.; Ren, Q.; Wang, Y.; Li, G.R. Surface-adsorbed carboxylate ligands on layered double hydroxides/metal–organic frameworks promote the electrocatalytic oxygen evolution reaction. Angew. Chem. 2021, 133, 18277–18285. [Google Scholar] [CrossRef]
- Jia, M.; Xiong, W.; Yang, Z.; Cao, J.; Zhang, Y.; Xiang, Y.; Xu, H.; Song, P.; Xu, Z. Metal-organic frameworks and their derivatives-modified photoelectrodes for photoelectrochemical applications. Coord. Chem. Rev. 2021, 434, 213780. [Google Scholar] [CrossRef]
- Jia, L.; Arain, M.; Ahmed, A.; Yikai, F.; Zhenda, C.; Hussain, I.; Ashraf, G.A.; Ahmed, S.B.; Dai, H. Emerging trends in metal-organic framework (MOFs) photocatalysts for hydrogen energy using water splitting: A state-of-the-art review. J. Ind. Eng. Chem. 2024, 131, 54–135. [Google Scholar]
- Shateran, F.; Ghasemzadeh, M.A.; Aghaei, S.S. Preparation of NiFe2O4@MIL-101 (Fe)/GO as a novel nanocarrier and investigation of its antimicrobial properties. RSC Adv. 2022, 12, 7092–7102. [Google Scholar] [CrossRef]
- Alves, R.C.; Schulte, Z.M.; Luiz, M.T.; Bento da Silva, P.c.; Frem, R.C.; Rosi, N.L.; Chorilli, M. Breast cancer targeting of a drug delivery system through postsynthetic modification of curcumin@ N3-bio-MOF-100 via click chemistry. Inorg. Chem. 2021, 60, 11739–11744. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Q.; Zhang, T.; Yan, X.; Duan, R. Analysis of chemical-looping hydrogen production and power generation system driven by solar energy. Renew. Energy 2020, 154, 863–874. [Google Scholar] [CrossRef]
- Alhabradi, M.; Yang, X.; Alruwaili, M.; Tahir, A.A. Nano multi-layered HfO2/α-Fe2O3 nanocomposite photoelectrodes for photoelectrochemical water splitting. Heliyon 2024, 10, e27078. [Google Scholar] [CrossRef]
- Chnani, A.; Strehle, S. Hematite nanowire and nanoflake-decorated photoelectrodes: Implications for photoelectrochemical water splitting. ACS Appl. Nano Mater. 2021, 5, 1016–1022. [Google Scholar] [CrossRef]
- Kavitha, S.; Ranjith, R.; Jayamani, N.; Vignesh, S.; Palanivel, B.; Djellabi, R.; Bianchi, C.; Alharthi, F.A. Fabrication of visible-light-responsive TiO2/α-Fe 2O3-heterostructured composite for rapid photo-oxidation of organic pollutants in water. J. Mater. Sci. Mater. Electron. 2022, 33, 8906–8919. [Google Scholar] [CrossRef]
- Guesh, K.; Caiuby, C.A.; Mayoral, A.l.; Díaz-García, M.; Díaz, I.; Sanchez-Sanchez, M. Sustainable preparation of MIL-100 (Fe) and its photocatalytic behavior in the degradation of methyl orange in water. Cryst. Growth Des. 2017, 17, 1806–1813. [Google Scholar] [CrossRef]
- Majumder, S.; Yadav, A.A.; Hunge, Y.M.; Jeffery, A.A.; Thomas, N.; Alshgari, R.A.; Chicardi, E.; Mushab, M.; Kim, K.H. Exploring FeVO4/ZnCo2O4 np heterojunctions for superior photoelectrochemical performance. J. Alloys Compd. 2025, 1010, 177281. [Google Scholar] [CrossRef]
- Majumder, S.; Yadav, A.A.; Palanisamy, A.K.; Hunge, Y.M.; KN, S.; Jeffery, A.A.; Tighezza, A.M.; Srinivasan, R.; Somu, P.; Kim, K.H. Design and optimization of FeVO4/Fe2TiO5 heterojunction photoanode for efficient photoelectrochemical water splitting. Ceram. Int. 2025, 51, 2536–2546. [Google Scholar] [CrossRef]
- Yadav, A.; Hunge, Y.; Kang, S.-W. Ultrasound assisted synthesis of highly active nanoflower-like CoMoS4 electrocatalyst for oxygen and hydrogen evolution reactions. Ultrason. Sonochemistry 2021, 72, 105454. [Google Scholar] [CrossRef] [PubMed]
- Babu Naidu, K.C.; Madhuri, W. Hydrothermal synthesis of NiFe2O4 nano-particles: Structural, morphological, optical, electrical and magnetic properties. Bull. Mater. Sci. 2017, 40, 417–425. [Google Scholar] [CrossRef]
- Yang, J.; Guo, D.; Zhao, S.; Lin, Y.; Yang, R.; Xu, D.; Shi, N.; Zhang, X.; Lu, L.; Lan, Y.Q. Cobalt phosphides nanocrystals encapsulated by P-doped carbon and married with P-doped graphene for overall water splitting. Small 2019, 15, 1804546. [Google Scholar] [CrossRef]
- Soysal, F. Nitrogen-doped reduced graphene oxide decorated with silver nanoparticles for photoelectrochemical water splitting. J. Mater. Sci. Mater. Electron. 2023, 34, 259. [Google Scholar] [CrossRef]
- Chandel, N.; Sharma, S.; Dutta, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Kumar, R.; Gupta, V.K.; Agarwal, S.; Singh, P. Adsorption and photocatalysis compiled toxic dyes mineralization using graphitic carbon nitride modified ZnFe2O4 and CoFe2O4 photocatalysts supported onto N-doped graphene. Desalination Water Treat. 2020, 191, 381–399. [Google Scholar] [CrossRef]
- He, X.; Fang, H.; Gosztola, D.J.; Jiang, Z.; Jena, P.; Wang, W.-N. Mechanistic insight into photocatalytic pathways of MIL-100 (Fe)/TiO2 composites. ACS Appl. Mater. Interfaces 2019, 11, 12516–12524. [Google Scholar] [CrossRef]
- Simon, M.A.; Anggraeni, E.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Thanh, T.C.; Hartono, S.B.; Yuliana, M.; Ismadji, S. Hydrothermal synthesize of HF-free MIL-100 (Fe) for isoniazid-drug delivery. Sci. Rep. 2019, 9, 16907. [Google Scholar] [CrossRef]
- Sivakumar, P.; Ramesh, R.; Ramanand, A.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis, Studies and Growth Mechanism of Ferromagnetic NiFe2O4 Nanosheet. Appl. Surf. Sci. 2012, 258, 6648–6652. [Google Scholar] [CrossRef]
- Sanadi, P.D.; Chougale, R.K.; Malavekar, D.B.; Kim, J.H.; Masimukku, S.; Chang-Chien, G.P.; Kamble, G.S. Efficient hydrogen evolution via neutral water electrolysis using nanocrystalline TiO2 electrocatalyst. Scientific Reports 2025, 15, 16074. [Google Scholar] [CrossRef]
- Hamedi, A.; Trotta, F.; Borhani Zarandi, M.; Zanetti, M.; Caldera, F.; Anceschi, A.; Nateghi, M.R. In Situ Synthesis of MIL-100(Fe) at the Surface of Fe(3)O(4)@AC as Highly Efficient Dye Adsorbing Nanocomposite. Int. J. Mol. Sci. 2019, 20, 5612. [Google Scholar] [CrossRef]
- Wu, X.-L.; Jiang, L.-Y.; Cao, F.-F.; Guo, Y.-G.; Wan, L.-J. LiFePO4 nanoparticles embedded in a nanoporous carbon matrix: Superior cathode material for electrochemical energy-storage devices. Adv. Mater. 2009, 21, 2710–2714. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, A.; Masa, J.; Rösler, C.; Xia, W.; Weide, P.; Botz, A.J.; Fischer, R.A.; Schuhmann, W.; Muhler, M. Co@Co3O4 encapsulated in carbon nanotube-grafted nitrogen-doped carbon polyhedra as an advanced bifunctional oxygen electrode. Angew. Chem. Int. Ed. 2016, 55, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Descostes, M.; Mercier, F.; Thromat, N.; Beaucaire, C.; Gautier-Soyer, M. Use of XPS in the determination of chemical environment and oxidation state of iron and sulfur samples: Constitution of a data basis in binding energies for Fe and S reference compounds and applications to the evidence of surface species of an oxidized pyrite in a carbonate medium. Appl. Surf. Sci. 2000, 165, 288–302. [Google Scholar]
- Kang, B.K.; Im, S.Y.; Lee, J.; Kwag, S.H.; Kwon, S.B.; Tiruneh, S.; Kim, M.-J.; Kim, J.H.; Yang, W.S.; Lim, B. In-situ formation of MOF derived mesoporous Co3N/amorphous N-doped carbon nanocubes as an efficient electrocatalytic oxygen evolution reaction. Nano Res. 2019, 12, 1605–1611. [Google Scholar] [CrossRef]
- Zhang, Y.; Rui, K.; Ma, Z.; Sun, W.; Wang, Q.; Wu, P.; Zhang, Q.; Li, D.; Du, M.; Zhang, W. Cost-effective vertical carbon nanosheets/iron-based composites as efficient electrocatalysts for water splitting reaction. Chem. Mater. 2018, 30, 4762–4769. [Google Scholar] [CrossRef]
- Saeed, F.; Ahmad, M.; Zada, A.; Qi, D.; Wang, Y. Phosphorus-doped CoFe2O4 nanoparticles decorated nitrogen-doped graphene for efficient and stable electrocatalytic water splitting. Int. J. Hydrogen Energy 2024, 59, 1196–1204. [Google Scholar] [CrossRef]
- Li, X.; Niu, Z.; Jiang, J.; Ai, L. Cobalt nanoparticles embedded in porous N-rich carbon as an efficient bifunctional electrocatalyst for water splitting. J. Mater. Chem. A 2016, 4, 3204–3209. [Google Scholar] [CrossRef]
- Saeed, F.; Samia; Ahmad, M.; Rehman, W.; Sana, Y.; Ameen, S.; Altowyan, A.S.; Zada, A. Sulfur-Doped Zinc Oxide-Nikel Oxide as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Electrocatalysis 2024, 16, 15–27. [Google Scholar] [CrossRef]
- Feng, H.; Yanan, D.; Ting, X.; Yongzhi, L.; Xin, Z.; Jianyi, X.; Guofang, Z.; Ying, C.; Yanghuan, Z. Influence of adding graphene on the hydrogen storage thermodynamics and kinetics of as-milled CeMg12–Ni alloy. Int. J. Hydrogen Energy 2023, 48, 13213–13226. [Google Scholar] [CrossRef]
- Li, R.; Xu, J.; Ba, J.; Li, Y.; Liang, C.; Tang, T. Facile synthesis of nanometer-sized NiFe layered double hydroxide/nitrogen-doped graphite foam hybrids for enhanced electrocatalytic oxygen evolution reactions. Int. J. Hydrogen Energy 2018, 43, 7956–7963. [Google Scholar] [CrossRef]
- Long, Y.; Zhao, G.; Yang, L.; An, Y.; Xu, Y.; Xu, C. Ultralow Ru loaded CoNi bimetallic phosphides nanorods as efficient electrocatalyst for overall water splitting. Int. J. Hydrogen Energy 2024, 51, 336–348. [Google Scholar] [CrossRef]
- Wang, H.; Lee, H.-W.; Deng, Y.; Lu, Z.; Hsu, P.-C.; Liu, Y.; Lin, D.; Cui, Y. Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nat. Commun. 2015, 6, 7261. [Google Scholar] [CrossRef]
- Dionigi, F.; Strasser, P. NiFe-based (oxy) hydroxide catalysts for oxygen evolution reaction in non-acidic electrolytes. Adv. Energy Mater. 2016, 6, 1600621. [Google Scholar] [CrossRef]
- Görlin, M.; Chernev, P.; Ferreira de Araújo, J.; Reier, T.; Dresp, S.r.; Paul, B.; Krähnert, R.; Dau, H.; Strasser, P. Oxygen evolution reaction dynamics, faradaic charge efficiency, and the active metal redox states of Ni–Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc. 2016, 138, 5603–5614. [Google Scholar] [CrossRef]
- Peng, P.; Lin, X.-M.; Liu, Y.; Filatov, A.S.; Li, D.; Stamenkovic, V.R.; Yang, D.; Prakapenka, V.B.; Lei, A.; Shevchenko, E.V. Binary transition-metal oxide hollow nanoparticles for oxygen evolution reaction. ACS Appl. Mater. Interfaces 2018, 10, 24715–24724. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional heterostructure assembly of NiFe LDH nanosheets on NiCoP nanowires for highly efficient and stable overall water splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Rehman, W.; Saeed, F.; Zhao, Y.; Maryam, B.; Arain, S.; Ayaz, M.; Jamil, A.; Liu, X. Boosted Photoelectrochemical Water Oxidation Performance with a Quaternary Heterostructure: CoFe2O4/MWCNT-Doped MIL-100 (Fe)/TiO2. Catalysts 2024, 14, 901. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Xiu, H.; Zhuang, H.; Li, J.; Zhou, Y.; Liu, D.; Kuang, Y. General in situ photoactivation route with IPCE over 80% toward CdS photoanodes for photoelectrochemical applications. Small 2021, 17, 2104307. [Google Scholar] [CrossRef] [PubMed]
- Hahn, N.T.; Mullins, C.B. Photoelectrochemical performance of nanostructured Ti-and Sn-doped α-Fe2O3 photoanodes. Chem. Mater. 2010, 22, 6474–6482. [Google Scholar] [CrossRef]
- Tamgadge, R.M.; Shukla, A. Fluorine-doped anatase for improved supercapacitor electrode. Electrochim. Acta 2018, 289, 342–353. [Google Scholar] [CrossRef]
- Moto, K.; Yoshimine, R.; Suemasu, T.; Toko, K. Improving carrier mobility of polycrystalline Ge by Sn doping. Sci. Rep. 2018, 8, 14832. [Google Scholar] [CrossRef]
- Paul Inbaraj, C.R.; Gudelli, V.K.; Mathew, R.J.; Ulaganathan, R.K.; Sankar, R.; Lin, H.Y.; Lin, H.-I.; Liao, Y.-M.; Cheng, H.-Y.; Lin, K.-H. Sn-Doping Enhanced Ultrahigh Mobility In1–x Sn x Se Phototransistor. ACS Appl. Mater. Interfaces 2019, 11, 24269–24278. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, J.-Y.; Kim, Y.; Kim, H.S.; Sung, Y.-E. Surface Modification of Stretched TiO2 Nanotubes for Solid-State Dye-Sensitized Solar Cells. J. Phys. Chem. C 2007, 111, 9614–9623. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Mizukoshi, Y.; Maeda, Y.; Ince, N.H. Supporting of Pristine TiO2 with Noble Metals to Enhance the Oxidation and Mineralization of Paracetamol by Sonolysis and Sonophotolysis. Appl. Catal. B Environ. 2015, 172–173, 7–17. [Google Scholar] [CrossRef]
- Malara, F.; Minguzzi, A.; Marelli, M.; Morandi, S.; Psaro, R.; Dal Santo, V.; Naldoni, A. α-Fe2O3/NiOOH: An Effective Heterostructure for Photoelectrochemical Water Oxidation. ACS Cat. 2015, 5, 5292–5300. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhao, G.; Tian, H.; Shi, H.; Zhou, T. Design of a Novel Cu2O/TiO2/Carbon Aerogel Electrode and Its Efficient Electrosorption-Assisted Visible Light Photocatalytic Degradation of 2,4,6-Trichlorophenol. ACS Appl. Mat. Inter. 2012, 4, 3965–3972. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.-Y.; Guo, R.-T.; Pan, W.-G.; Huang, C.-Y.; Tang, J.-Y.; Liu, X.-Y.; Qin, H.; Xu, Q.-Y. The MoS2/TiO2 heterojunction composites with enhanced activity for CO2 photocatalytic reduction under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 306–316. [Google Scholar] [CrossRef]
- Nayak, S.; Mohapatra, L.; Parida, K. Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction. J. Mat. Chem. 2015, 3, 18622–18635. [Google Scholar] [CrossRef]
- Guo, J.; Yang, X.; Bai, S.; Xiang, X.; Luo, R.; He, J.; Chen, A. Effect of Mo doping and NiFe-LDH cocatalyst on PEC water oxidation efficiency. J. Colloid Interface Sci. 2019, 540, 9–19. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Xiao, Y.; Jia, D.; Li, C.; Zheng, J.; Liu, X. Nanoporous Fe-doped BiVO4 Modified with MIL-53 (Fe) for Enhanced Photoelectrochemical Stability and Water Splitting Perfromances. Catal. Lett. 2018, 149, 870–875. [Google Scholar] [CrossRef]
- Tian, K.; Wu, L.; Han, T.; Gao, L.; Wang, P.; Chai, H.; Jin, J. Dual modification of BiVO4 photoanode by rare earth element neodymium doping and further NiFe2O4 co-catalyst deposition for efficient photoelectrochemical water oxidation. J. Alloy. Compd. 2022, 923, 166352. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, W.; Saeed, F.; Arain, S.; Usman, M.; Maryam, B.; Liu, X. Enhanced Photoelectrochemical Water Splitting Using a NiFe2O4/NG@MIL-100(Fe)/TiO2 Composite Photoanode: Synthesis, Characterization, and Performance. J. Compos. Sci. 2025, 9, 250. https://doi.org/10.3390/jcs9050250
Rehman W, Saeed F, Arain S, Usman M, Maryam B, Liu X. Enhanced Photoelectrochemical Water Splitting Using a NiFe2O4/NG@MIL-100(Fe)/TiO2 Composite Photoanode: Synthesis, Characterization, and Performance. Journal of Composites Science. 2025; 9(5):250. https://doi.org/10.3390/jcs9050250
Chicago/Turabian StyleRehman, Waheed, Faiq Saeed, Samia Arain, Muhammad Usman, Bushra Maryam, and Xianhua Liu. 2025. "Enhanced Photoelectrochemical Water Splitting Using a NiFe2O4/NG@MIL-100(Fe)/TiO2 Composite Photoanode: Synthesis, Characterization, and Performance" Journal of Composites Science 9, no. 5: 250. https://doi.org/10.3390/jcs9050250
APA StyleRehman, W., Saeed, F., Arain, S., Usman, M., Maryam, B., & Liu, X. (2025). Enhanced Photoelectrochemical Water Splitting Using a NiFe2O4/NG@MIL-100(Fe)/TiO2 Composite Photoanode: Synthesis, Characterization, and Performance. Journal of Composites Science, 9(5), 250. https://doi.org/10.3390/jcs9050250






