1. Introduction
Ionizing radiation—
γ and X-rays—is commonly utilized in various areas of our every-day life [
1], and to maintain safe working conditions, protective shields should be used to minimize the exposure dose. While lead is well established as an effective shielding material, its toxicity [
2] should be pointed out, in addition to other discouraging factors such as heaviness and limited adaptability to body contours (as in the case with lead aprons [
3]). Therefore, the scientific community has been redirected towards nano- and microcomposites—a class of relatively novel materials combining a basic component (continuous phase; matrix) and nanoparticles [
1,
4,
5,
6]. Developed composites are tailored to meet requirements in various sectors. Nevertheless, flexible/wearable shields appear to receive particular attention [
7,
8,
9]. For instance, Wang et al. [
10] proposed flexible material using silicone, tungsten, and nano-titanium oxide (20 silicone–(80−
x) W–(
x) TiO
2, where
x are 0.1, 0.5, 1.0, and 2.0 wt.%), which could potentially be used in robots working in modern nuclear power plants, while the combination of polyvinyl alcohol with polypyrrole and various amounts of bismuth oxide (Bi
2O
3) nanoparticles might be used in nuclear medicine [
11]. Sheela et al. [
12] fabricated high-density polyethylene with various additions of bismuth powder, where the authors concluded that the sample with 40 wt.% of Bi could be utilized as flexible coats for staff or flexible covers in space applications.
Composites are evolving through different modifications, such as the selection of components of the continuous phase or alterations at the nanoparticle level. In relation to the latter, it is important to note that nanotechnology offers modifications and various functionalizations of pure particles, such as core–shell particles. Such particles, as the term suggests, include one material (core), which is encapsulated by the outer material (shell). Usually, core–shell nanoparticles appear in a concentric spherical form, however, the morphology of these forms varies, including, for instance, several small core materials surrounded by one outer layer [
13]. These core–shell particles allow, among other things, greater stability and dispersibility, and above all, they serve as an economical solution, since the valuable compound is covered with the inexpensive layer, thus preventing the core consumption phenomenon [
13]. The applications of core–shell particles can be found in various fields [
14] (e.g., in biomedical applications [
15]), where the area of radiological protection has not been omitted. Indeed, the literature reports analysis of core–shell structures in terms of shielding properties. For instance, the proper selection of the inner compound and outer layer could be used to suppress two various radiations (co-absorbers). Zhang et al. [
16] studied Gd
2O
3@W-Al composites regarding their ability to attenuate neutron and gamma radiation with the MCNP5 simulation program. Proceeding further, Hao et al. [
17] explored the effect of tungsten layer thickness in Gd
2O
3@W core–shell powder (again with MCNP5 software). They concluded that increasing the shell thickness could enhance the shielding effect. Some proposals also include tungsten as the core compound. As an example, in Ref. [
18] the core–shell structures were tungsten and boron nitride (W@BN) obtained with the arc discharge technique. According to the authors, tungsten encapsulation was conducted in order to prevent tungsten oxidation (which could result in a decrement in shielding effectiveness). The composite samples were prepared utilizing biphenyl epoxy and cationic initiator (N-benzylpyrazinium hexafluoroantimonate), where the addition of core–shell particles (W@BN) were 10 and 20 wt.%. The samples were studied using thermal neutron and gamma radiations (
241Am–Be neutron source and with
137Cs), where the outcomes showed that greater filler improved shielding ability. Additionally, it was stated that the developed core–shell particles can serve as a tool in the attenuation of secondary gamma rays. Other proposed co-absorbers against neutron and gamma rays were lead borate@polydopamine (PBO@PDA) core–shell particles bonded with silicone rubber [
19]. The composites had various filler amounts (from 5 wt.% to 40 wt.%), and the results showed that with a greater filler amount, the shielding properties were enhanced. Organic materials have also been considered in the development of core–shell particles. Lou and co-workers [
20] studied phase change microcapsules with paraffin as the core and lead tungstate (PbWO
4) as the shell, aiming to provide gamma-ray shielding properties (outer layer) along with thermal energy storage (paraffin). Similarly, Cai et al. [
21] encapsulated a paraffin compound with lead tungstate (PbWO
4), bearing in mind areas where simultaneous gamma radiation attenuating ability along with thermal regulation would be essential. The study included an exploration of morphology alterations (spindle, spherical microcapsules) by controlling the growth of the PbWO
4 shell, which in turn was related to the change in trisodium citrate dihydrate content. Based on this study, it was concluded that the spindle-shaped Pn@PbWO
4 microcapsules exhibited a greater encapsulation ratio and a reduced degree of supercooling in comparison to spherical microcapsules.
Generally, lead particles (along with other high-density metals) are widely recognized as effective shielding components in developed composites. However, other metal particles (with lower atomic numbers) should not be underestimated. Thus, iron seems to be a promising filler, taking into consideration its cost and popularity. Particularly, its distinctive feature (in the comparison with lead) is its safety for the human body, as this microelement (in appropriate amounts) is essential for the oxygen transport process [
22]. However, it is relevant to emphasize that iron is chemically reactive, which causes the unfavorable phenomenon of corrosion. Consequently, in addition to the literature that incorporates pure iron particles in shielding composites [
23,
24,
25], scientific attention has focused on developing more complex forms (i.e., coatings) in order to enhance stability [
26].
Recently, we explored composites, where iron-encapsulated multi-walled carbon nanotubes (Fe@MWCNTs) were used as fillers [
27]. In addition to the determination of the shielding properties (gamma and X-rays), the research involved investigation with an aggressive medium (H
2O
2(aq) (30%), CH
3COOH (99.5%), and H
2SO
4(aq) (96%);
v/
v/
v = 5:5:0.1), where treated samples included an Fe@MWCNTs–paraffin composite and an additional composite with pure iron particles as fillers from our prior paper [
23]. The study confirmed that composites with incorporated pure iron particles exhibited increasing digestibility, which did not occur for the iron-encapsulated multi-walled carbon nanotubes [
27]. Nonetheless, it is essential to note that the mentioned carbon nanotubes were synthesized in the lab (via catalytic chemical vapor deposition (c-CVD) method), while considering various facilities’ conditions (e.g., diversity in terms of laboratory equipment, qualifications of technical staff, etc.) scientific efforts should include both synthesized and widely available (economic) options.
The overall goal of this study was to develop (independently from laboratory conditions) gamma-ray shields that are comfortable in shape alteration while also exhibiting corrosion resistance through nanoparticle morphology adaptation. Therefore, paraffin-based composites were filled with 10 wt.% of iron particles with carbon shells (hydrophobic and hydrophilic, respectively), improving the composites’ durability by eliminating direct contact between iron and external conditions. The composite samples were prepared with a non-complex manufacturing procedure, employing commercially available and ready-for-use materials (encompassing both matrices and particles). The composite samples were experimentally studied in terms of gamma-ray shielding properties with a 60Co source. Apart from shielding characteristics, the composites were examined in terms of thermal properties with a temperature-modulated differential scanning calorimetry technique, where the determination of melting points (falling within ca. 310–325 K range) indicated easiness in the plasticization of the proposed composite samples.
2. Materials and Methods
The following sections include the information on sample preparation process, particle characterization (scanning and transmission electron microscope pictures and energy-dispersive X-ray spectroscopy analysis), along with the methods that allowed the determination of shielding and thermal properties.
2.1. Sample Preparation
The preparation process for the shielding composites included several and simple steps, using commercially purchased materials. The main component—paraffin—was purchased from Orlen Południe (Trzebinia, Poland), with the ability to effortlessly adapt to any shape with the force and warmth of hands, while iron particles with both hydrophilic (>67% Fe; 25–30% C) and hydrophobic (>83% Fe; <14% C, APS < 60 nm) carbon shells were purchased from PlasmaChem GmbH (Berlin, Germany). Detailed data concerning paraffin is included elsewhere [
23], while the characterization of the particles is presented in the following section. Firstly, the particles and paraffin were weighed using an analytical balance WTC 2000 (Radwag, Radom, Poland), which was followed by placement in the vacuum dryer Goldbrunn 450 (Goldbrunn, Germany) at ca. 373.15 K. The next step was cold mixing with hand press for c.a. 50 min to ensure uniform particles distribution. As the gamma-ray shielding measurement was carried out based on the sample thickness, the composites were put in rings (2 cm high) with material surplus, which were subsequently trimmed with a three-plate system. A comprehensive description, including graphical representation, is presented in Ref. [
23].
2.2. Scanning Electron Microscope (SEM) and Transmission Electron Microscopy (TEM) Pictures Along with Energy-Dispersive X-Ray Spectroscopy Analysis of Particles
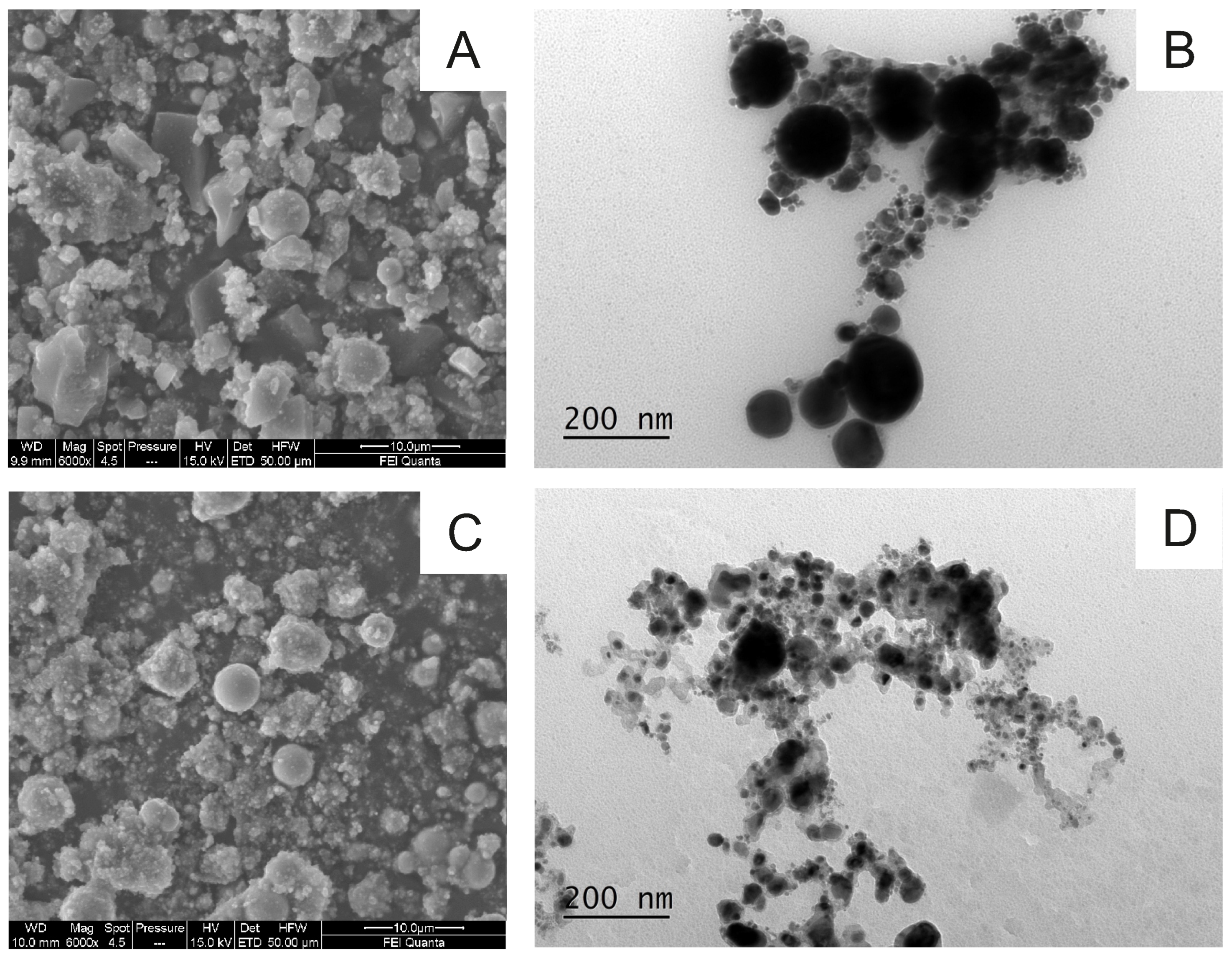
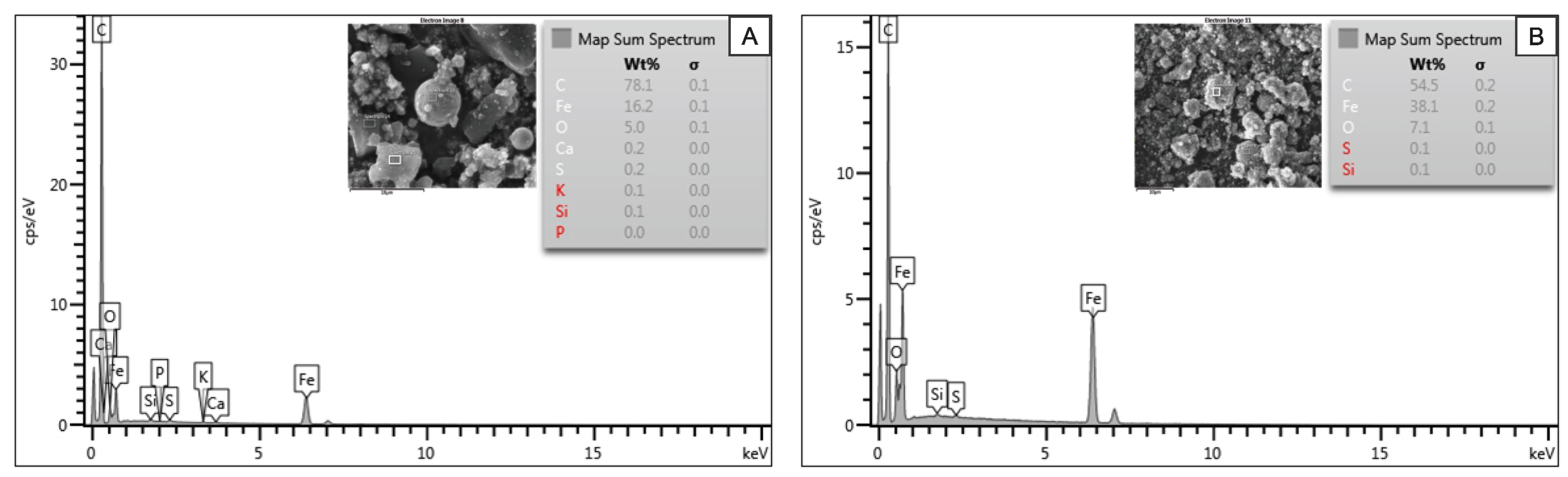
The morphological and structural characterization of the iron nanoparticles was performed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM images were obtained using a JEOL JSM-6700F field emission scanning electron microscope (Tokyo, Japan), while TEM images were obtained using a JEM-2010F microscope (JEOL, Tokyo, Japan). Energy-dispersive X-ray spectroscopy (EDS) analysis was carried out using an Oxford Instruments INCA Energy 300 spectrometer (Oxford, UK), integrated into the SEM system.
For SEM and EDS analysis, the nanoparticles were deposited on silica substrates, while copper grids were used for TEM studies. The samples were prepared by dispersing the nanoparticles in methanol, followed by drop-casting and drying at room temperature.
Figure 1 shows representative SEM and TEM images of the iron nanoparticles functionalized with hydrophilic (
Figure 1A,B) and hydrophobic (
Figure 1C,D) carbon shells.
The SEM micrographs reveal irregular morphologies, particularly for the hydrophilic samples (A), while a near-to-spherical shape is observed for the hydrophobic ones (C). Hydrophilic particles tend to display angular and less uniform surfaces, whereas hydrophobic particles present a higher proportion of rounded geometries.
TEM analysis confirmed the nanometric range of the particles and exhibited a well-defined core–shell structure. The hydrophilic samples showed an average particle diameter of ∼90 nm, whereas the hydrophobic ones exhibited slightly smaller particles, with an average of ∼60 nm. In both cases, the carbon coating around the metal cores had an average thickness of ∼10 nm. These values were obtained by measuring 50 particles per sample using ImageJ 1.54k software, based on calibrated TEM images. The EDS spectra in
Figure 2 reveal that the samples are composed mostly of iron (Fe) and carbon (C), with a minor oxygen content. These additional signals were likely due to superficial oxidation or to residual elements from the substrate materials used during analysis.
2.3. Gamma-Ray Shielding Measurements
The composites were examined with 60Co and a Geiger-Müller counter (3B Scientific Physics, Hamburg, Germany) with 1 h as the acquisition time. The shielding measurement included three series and was conducted for various thickness of composite samples, i.e., from 2 cm to 26 cm with 4 cm steps, beginning with collecting the number of counts coming from 60Co (i.e., no layer). The obtained number of counts, , decreased with the background value, , according to the following formula: .
The uncertainty estimation of the gamma-ray shielding measurements included a total of 100 measurements of the counts directly from the
60Co (again, with G-M counter as detector and 1 h acquisition time) and the determination of the background level value (100 repetitions). The
60Co-counts were reduced by the background value; thus, the calculated relative uncertainty (a standard deviation divided by the mean value) was 5% [
27]. More detailed information including schematic images (along with pictures) of the gamma-ray measuring station together with the explanation of the 1 h acquisition time for the detector can be found in Ref. [
23].
2.4. Differential Scanning Calorimetry Measurements
The specific isobaric heat capacity () of the samples was determined using temperature-modulated differential scanning calorimetry (TMDSC) using a TA Instruments Q2000 system (New Castle, DE, USA). All measurements, including conventional DSC scans for the determination of phase transition temperatures and latent heat of fusion, were performed using the same instrument and under the same conditions. The measurements were performed under a high-purity nitrogen atmosphere (50 mL min−1, ≥99.999%) to ensure thermal stability and prevent oxidative effects. The temperature range covered from 253 K to 353 K, including both solid and liquid states of the materials.
To guarantee measurement reproducibility and minimize uncertainty, each sample was analyzed in triplicate. The DSC instrument was calibrated using high-purity indium as the reference material, in accordance with the protocol described by Cabaleiro et al. [
28], which included temperature and enthalpy calibration, as well as sapphire-based sensitivity checks for modulated heat flow analysis. This methodology allowed for accurate
determination under quasi-isothermal TMDSC conditions, with reported uncertainties below 3% across the entire temperature range.
3. Results and Discussion
The fundamental law of the absorption of gamma radiation by shields is expressed as
In Equation (
1),
N and
stand for the number of counts for the placed shield and without any absorber, respectively, while
is linear attenuation coefficient and
x is the thickness of the mentioned absorber. In this study, in order to experimentally designate the shielding effectiveness of composites, Equation (
1) was adjusted as follows:
where,
represents the number of counts for the applied thickness of the composite, while
refers to number of counts for the source (both results were suitable reduced by the background value, as mentioned in the Materials and Methods Section). To determine the
-values for the paraffin-based composite samples, Equation (
2) was fitted to the experimental data, summarized in
Table 1.
The linear attenuation coefficient value,
, allowed for the designation of other shielding parameters, such as the half-value layer (HVL), in accordance with the following equation:
where
represents the linear attenuation coefficient value.
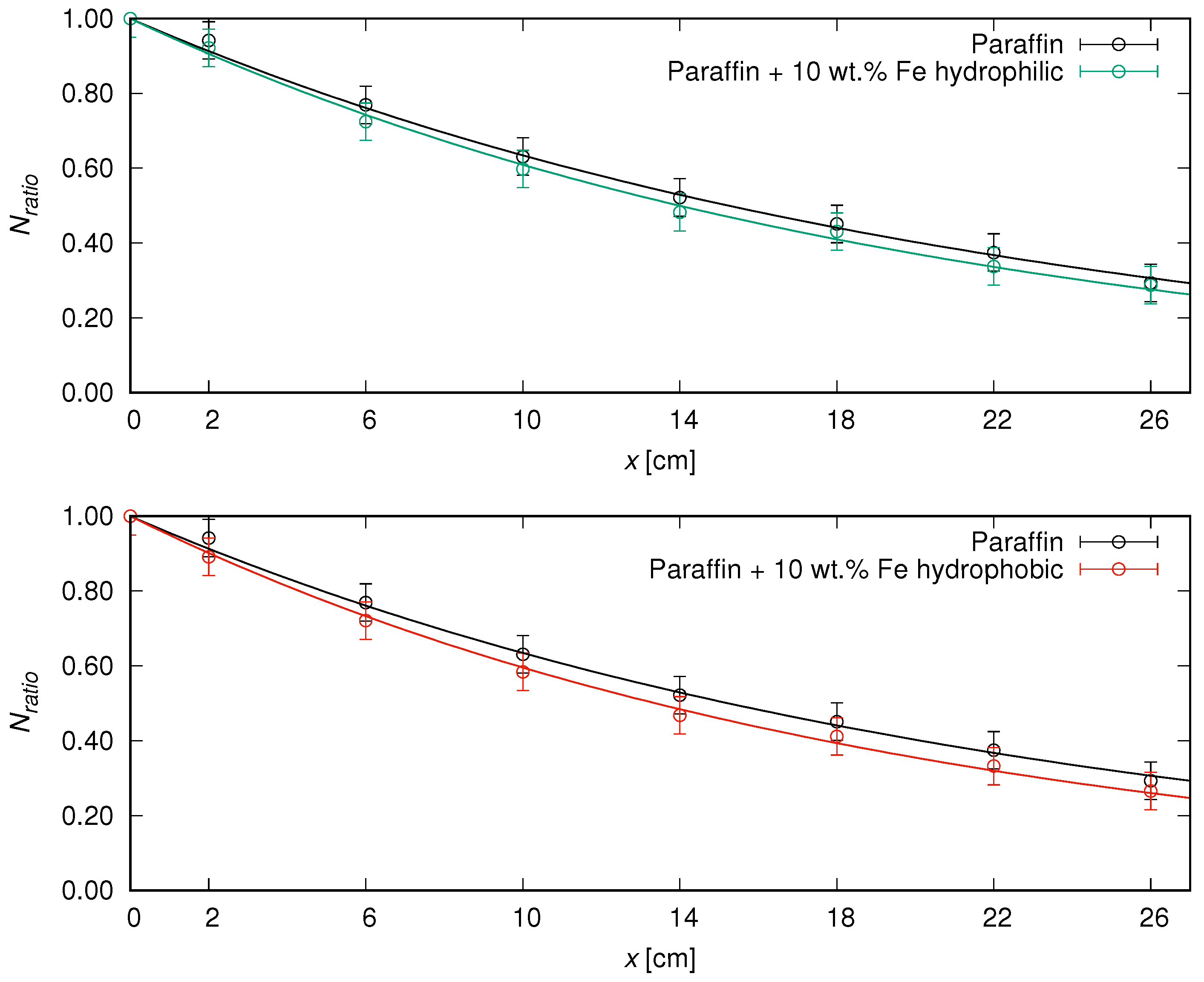
The summarized shielding parameters, i.e., the
-values with theirs uncertainties designated by fitting Equation (
2) to numerical values, accompanied by the HVL values (with calculated uncertainty values), are included in
Table 2, while the graphical representation of the results is shown in
Figure 3.
The gathered data indicate that the addition of iron particles subtly enhanced the shielding properties (in comparison to pure matrix), while the difference between two types of iron particles (with hydrophobic or hydrophilic shells) was also slight. The half-value layer values (the thickness of a material which reduced by half the incident radiation) were ca. 15 cm and 14 cm for the paraffin and developed composites, respectively. Nevertheless, it should be noted that the development of novel shields involves a selection of materials (type, quantity, and manufacturing process), taking into account overall functionality in real-life use. In this study, the shields easily adjusted with the force of hands provided radiological protection with full customization in various areas. The free possibility of shape alteration outside laboratory conditions paves the way for the greater mobility of such composites, which is a key factor, e.g., in considering transport of radioactive materials. Iron—among various fillers—is already taken into account when creating new composites [
23,
29,
30], taking into consideration its attributes such as availability and natural resource commonness [
31] or possibilities in obtaining nanoparticles form with green synthesis [
32], such as with tea (
Camellia sinensis) polyphenols [
33].
The unfavorable phenomenon of iron corrosion could result in the overall weakening of the shielding material. The prevention of eventual degradation could be achieved by encapsulating iron with an outer layer, thus preventing iron reactions with corrosive conditions. Indeed, in our previous study [
27], the developed composites included iron-encapsulated multi-walled carbon nanotubes (Fe@MWCNTs), which showed corrosion resistance. The designated ultraviolet–visible light (UV-Vis) spectra exhibited Fe
3+ complexes for the composite with the addition of the pure iron particles from the study [
23], which was not observed for paraffin-based composites filled with Fe@MWCNTs [
27]. Additionally, it is worth highlighting that the aforementioned iron-encapsulated multi-walled carbon nanotubes were synthesized via catalytic chemical vapor deposition [
27], while commercial Fe@C particles—considering facilities with poor laboratory conditions, which thus do not allow the
in situ synthesis of particles/nanotubes—could be perceived as more available substitutes. Finally, the shell of iron particles (hydrophobic/hydrophilic carbon shell) could be recognized as compatible with the used paraffin matrix.
The
of pure paraffin and two nanocomposites containing 10 wt.% iron nanoparticles, functionalized with either hydrophilic or hydrophobic surface groups, was evaluated over the temperature range from 253 K to 353 K. This analysis aimed to assess the influence of nanoparticle inclusion and surface chemistry on the thermal behavior of the composites, with particular attention to both solid and liquid states of the paraffin matrix, given their critical roles in thermal energy storage applications [
34].
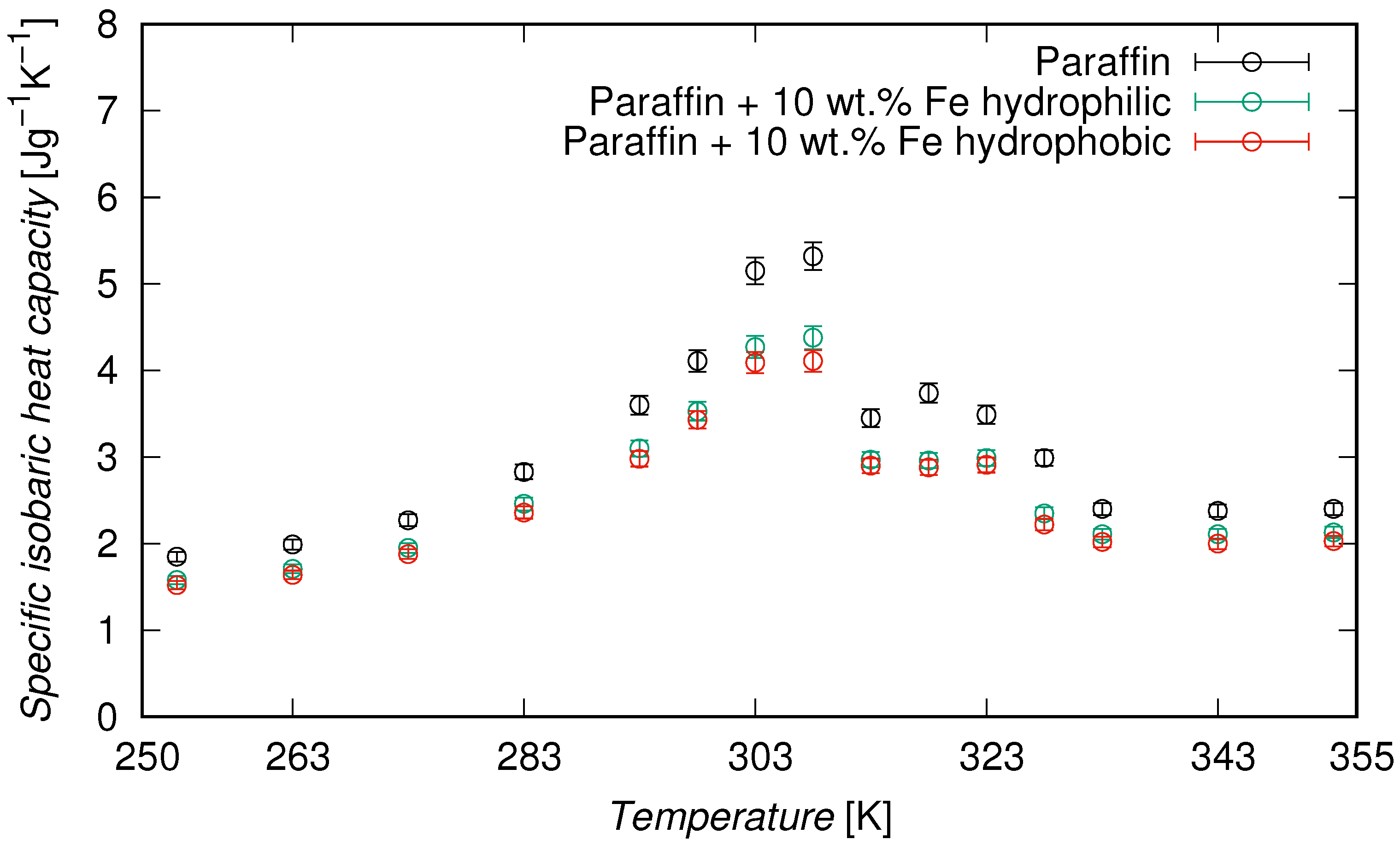
DSC measurements confirmed that the paraffin used in this study exhibited two distinct melting peaks, centered at 310.5 K and 325.5 K, corresponding to the melting of different paraffin fractions present in the technical-grade blend. This dual melting behavior is characteristic of technical-grade paraffin and was preserved in the composite formulations, indicating that the presence of iron nanoparticles did not interfere with the thermal phase transition of the matrix. The evolution of
as a function of temperature is shown in
Figure 4, while representative values are provided in
Table 3. In all cases,
increased with temperature and reached a maximum near the melting point of paraffin, typically between 303 K and 323 K. This peak corresponds to the latent heat absorption associated with the phase transition, as is characteristic of phase change materials. At 308 K, the pure paraffin exhibited a
of 5.32 J·g
−1·K
−1. In comparison, the composites showed lower peak values, with 4.38 J·g
−1·K
−1 for the hydrophilic shell and 4.11 J·g
−1·K
−1 for the hydrophobic one, with reductions of 17.7% and 22.7%, respectively, in comparison to the pure paraffin. On average, over the full temperature range (253–353 K), the specific heat was reduced by 14.6% and 18.2% for the hydrophilic and the hydrophobic samples, respectively. A more detailed analysis revealed that in the solid phase (253–273 K),
decreased by 14.2% and 17.5%, respectively, while in the liquid phase (333–353 K), the reductions were 11.6% and 15.7%. These results confirm that the presence of iron nanoparticles led to a systematic reduction in
, with a more pronounced effect for the hydrophobic surface functionalization.
The higher reduction observed in the hydrophobic system supports the hypothesis of lower interfacial compatibility with the paraffin matrix, leading to diminished cooperative molecular rearrangements. This interpretation is consistent with the results reported by Nam et al. [
35], who demonstrated that the interaction between nanoparticle surfaces and the matrix plays a key role in the thermal behavior of nano-enhanced phase change materials (PCMs). Furthermore, similar reductions in isobaric heat capacity have also been reported for paraffin-based systems. For example, Jesumathy et al. [
36] observed an 11% reduction in isobaric heat capacity for paraffin containing 10 wt% CuO nanoparticles, which is consistent with the values obtained in this study.
In addition, the latent heat of fusion of the paraffin phase was determined by integrating the endothermic peaks from DSC thermograms obtained at different heating rates. A representative average value of 60 ± 1 J/g was adopted to characterize the phase change enthalpy. Particularly, the presence of nanoparticles in the composite formulations did not significantly affect the latent heat, which remained within the same range. This indicates that the thermal storage capacity of the paraffin is largely preserved despite the incorporation of additives.
4. Conclusions
This study presents gamma-ray shielding covers characterized by their ease of shape customization. The desired shape of manufactured composites could be achieved without laboratory conditions (e.g., no need to reprocess the sample) as their morphology could be manually adjusted, thus enhancing their versatility for various applications. The composites can be potentially used as anatomically fitted patients’ shields, as additional passive cover during radioactive waste transport, or, generally, in radiation-exposure fields that require complex shields. The developed paraffin-based composites were filled with core–shell particles—with iron as the core and carbon shells with varied hydrophobic and hydrophilic properties—which paves the way of their application in environments with more stringent conditions. Indeed, the coating of iron with carbon shells potentially prevents the corrosion phenomenon of this metal, resulting in the higher stability of the composites. Given the selected ingredients, the developed samples could be classified as non-toxic, ensuring safe-in-contact working conditions, in which direct (manual) contact with metal (Fe) is limited due to the carbon shell and chemically inert paraffin. The manufacturing process could be considered relatively quick, utilizing ease-in-use equipment. The composites were examined in terms of shielding efficiency (with
60Co) and thermal properties (determination of isobaric heat capacity values). The half-value layers were ∼15 cm and ∼14 cm for the paraffin and the manufactured composites, accordingly. Despite the fact that the developed composites are inferior to traditional Pb-covers (according to study [
37], HVL value for lead considering
60Co is ∼1.11 cm), it is important to highlight that paraffin composites are non-toxic and susceptible to shape change outside laboratory conditions. Regarding thermal properties, the composites with hydrophobic shells showed lower
cp values than those with hydrophilic shells. Thus, at 308 K, reductions of 22.7% and 17.7%, respectively, compared to the pure paraffin were reported. Further studies could be extended, taking into account the development of samples with various mass fractions and measurements with other gamma-ray sources. Additionally, the composites should be examined in terms of durability, including performance under corrosive conditions, or gamma-ray induced changes in the structure.