Rheological Behavior of Ion-Doped Hydroxyapatite Slurries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Stoichiometric and Ion-Doped HA Powders
2.2. Powder Characterization
2.3. Preparation of Water-Based Slurries
2.4. Slurries Characterization
3. Results
3.1. Characterization of the Apatite Powders
3.2. Characterization of the Slurries
3.2.1. Colloidal Stability
3.2.2. Flow Behavior
3.2.3. Viscoelasticity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohd Zaffarin, A.S.; Ng, S.-F.; Ng, M.H.; Hassan, H.; Alias, E.J.N. Nano-Hydroxyapatite as a Delivery System for Promoting Bone Regeneration In Vivo: A Systematic Review. Nanomaterials 2021, 11, 2569. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2022, 10, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Tabrizi, B.; Basirun, W.J.; Yeong, C.H.; Thein, W.M. Development of the third generation of bioceramics: Doping hydroxyapatite with s-, p-, d-, and f-blocks cations and their potential applications in bone regeneration and void filling. Ceram. Int. 2022, 49, 7142–7179. [Google Scholar] [CrossRef]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional hydroxyapatite composites for orthopedic applications: A review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar] [CrossRef]
- Rossini, Z.; Franzini, A.; Zaed, I.; Zingaretti, N.; Nicolosi, F.; Zanotti, B. Custom-made porous hydroxyapatite cranioplasty in patients with tumor versus traumatic brain injury: A single-center case series. World Neurosurg. 2020, 138, e922–e929. [Google Scholar] [CrossRef]
- Aldahak, N.; Dupre, D.; Ragaee, M.; Froelich, S.; Wilberger, J.; Aziz, K.M. Hydroxyapatite bone cement application for the reconstruction of retrosigmoid craniectomy in the treatment of cranial nerves disorders. Surg. Neurol. Int. 2017, 8, 115. [Google Scholar]
- Song, Y.; Hu, Q.; Liu, Q.; Liu, S.; Wang, Y.; Zhang, H. Design and fabrication of drug-loaded alginate/hydroxyapatite/collagen composite scaffolds for repairing infected bone defects. J. Mater. Sci. 2023, 58, 911–926. [Google Scholar] [CrossRef]
- Ozder, M.N.; Ciftci, F.; Berrak, O.; Arisan, E.D.; Ustündag, C.B. In situ synthesis and cell line studies of nano-hydroxyapatite/graphene oxide composite materials for bone support applications. Ceram. Int. 2023, 49, 14791–14803. [Google Scholar] [CrossRef]
- Yedekçi, B.; Tezcaner, A.; Alshemary, A.Z.; Yılmaz, B.; Demir, T.; Evis, Z. Synthesis and sintering of B, Sr, Mg multi-doped hydroxyapatites: Structural, mechanical and biological characterization. J. Mech. Behav. Biomed. Mater. 2021, 115, 104230. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 145–211. [Google Scholar]
- Abbas, Z.; Dapporto, M.; Tampieri, A.; Sprio, S. Toughening of bioceramic composites for bone regeneration. J. Compos. Sci. 2021, 5, 259. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Trace element doping in calcium phosphate ceramics to understand osteogenesis and angiogenesis. Trends Biotechnol. 2013, 31, 10-1016. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Alshemary, A.Z.; Evis, Z. Co-doped hydroxyapatites as potential materials for biomedical applications. Microchem. J. 2019, 144, 443–453. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Padmanabhan, V.P.; Kulandaivelu, R.; Nellaiappan, T.S.N.; Sagadevan, S.; Paiman, S.; Mohammad, F.; Al-Lohedan, H.A.; Obulapuram, P.K.; Oh, W.C. Influence of iron doping towards the physicochemical and biological characteristics of hydroxyapatite. Ceram. Int. 2021, 47, 5061–5070. [Google Scholar] [CrossRef]
- Reger, N.C.; Bhargava, A.K.; Ratha, I.; Kundu, B.; Balla, V.K. Structural and phase analysis of multi-ion doped hydroxyapatite for biomedical applications. Ceram. Int. 2019, 45, 252–263. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Nagyne-Kovacs, T.; Studnicka, L.; Kincses, A.; Spengler, G.; Molnár, M.; Tolner, M.; Lukacs, I.E.; Szilagyi, I.M.; Pokol, G. Synthesis and characterization of Sr and Mg-doped hydroxyapatite by a simple precipitation method. Ceram. Int. 2018, 44, 22976–22982. [Google Scholar] [CrossRef]
- Jin, W.; Liu, Z.; Wu, Y.; Jin, B.; Shao, C.; Xu, X.; Tang, R.; Pan, H. Synergic effect of Sr2+ and Mg2+ on the stabilization of amorphous calcium phosphate. Cryst. Growth Des. 2018, 18, 6054–6060. [Google Scholar] [CrossRef]
- Aina, V.; Lusvardi, G.; Annaz, B.; Gibson, I.R.; Imrie, F.E.; Malavasi, G.; Menabue, L.; Cerrato, G.; Martra, G. Magnesium-and strontium-co-substituted hydroxyapatite: The effects of doped-ions on the structure and chemico-physical properties. J. Mater. Sci. Mater. Med. 2012, 23, 2867–2879. [Google Scholar] [CrossRef]
- Landi, E.; Guizzardi, S.; Papa, E.; Galli, C. Mg, Sr-Cosubstituted Hydroxyapatite with Improved Structural Properties. Appl. Sci. 2021, 11, 4930. [Google Scholar] [CrossRef]
- Kadhim, M.M.; AlMashhadani, H.A.; Hashim, R.D.; Khadom, A.A.; Salih, K.A.; Salman, A.W. Effect of Sr/Mg co-substitution on corrosion resistance properties of hydroxyapatite coated on Ti–6Al–4V dental alloys. J. Phys. Chem. Solids 2022, 161, 110450. [Google Scholar] [CrossRef]
- Ciolacu, L.; Zand, E.; Negrau, C.; Jaeger, H.J.F. Bacterial attachment and biofilm formation on antimicrobial sealants and stainless steel surfaces. Foods 2022, 11, 3096. [Google Scholar] [CrossRef] [PubMed]
- Demishtein, K.; Reifen, R.; Shemesh, M.J.N. Antimicrobial properties of magnesium open opportunities to develop healthier food. Nutrients 2019, 11, 2363. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Y.; Tang, Y.; Wang, X.; Zhang, D.; Zhang, X.; Yu, Y.; Yang, X.; Cai, Q.J.B.M. A biomimetic piezoelectric scaffold with sustained Mg2+ release promotes neurogenic and angiogenic differentiation for enhanced bone regeneration. Bioact. Mater. 2023, 25, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Borciani, G.; Ciapetti, G.; Vitale-Brovarone, C.; Baldini, N. Strontium functionalization of biomaterials for bone tissue engineering purposes: A biological point of view. Materials 2022, 15, 1724. [Google Scholar] [CrossRef]
- Sprio, S.; Dapporto, M.; Montesi, M.; Panseri, S.; Lattanzi, W.; Pola, E.; Logroscino, G.; Tampieri, A. Novel osteointegrative Sr-substituted apatitic cements enriched with alginate. Materials 2016, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Besleaga, C.; Nan, B.; Popa, A.-C.; Balescu, L.M.; Nedelcu, L.; Neto, A.S.; Pasuk, I.; Leonat, L.; Popescu-Pelin, G.; Ferreira, J.M. Sr and Mg Doped Bi-Phasic Calcium Phosphate Macroporous Bone Graft Substitutes Fabricated by Robocasting: A Structural and Cytocompatibility Assessment. J. Funct. Biomater. 2022, 13, 123. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Ai, F.; Yan, J.; Zhou, K.J.J. Fabrication and characterization of Sr-doped hydroxyapatite porous scaffold. JOM 2021, 73, 1745–1753. [Google Scholar] [CrossRef]
- Hu, B.; Meng, Z.-D.; Zhang, Y.-Q.; Ye, L.-Y.; Wang, C.-J.; Guo, W.-C. Cell, Sr-HA scaffolds fabricated by SPS technology promote the repair of segmental bone defects. Tissue Cell 2020, 66, 101386. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, S.; Zhao, W.; Yang, L.; Yuan, B.; Ioan, V.S.; Iulian, A.V.; Yang, X.; Zhu, X.; Zhang, X.J.T. A bioceramic scaffold composed of strontium-doped three-dimensional hydroxyapatite whiskers for enhanced bone regeneration in osteoporotic defects. Theranostics 2020, 10, 1572. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Dapporto, M.; Tampieri, A.; Sprio, S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PLoS ONE 2017, 12, e0172100. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Avanzi, I.R.; Parisi, J.R.; Souza, A.; Cruz, M.A.; Martignago, C.C.S.; Ribeiro, D.A.; Braga, A.R.C.; Renno, A.C. 3D-printed hydroxyapatite scaffolds for bone tissue engineering: A systematic review in experimental animal studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 203–219. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Chen, S.; Xu, Z.; Wang, Q.; Yuan, P.; Zhou, Y.; Zhang, Y.; Chen, J. Heparan sulfate loaded polycaprolactone-hydroxyapatite scaffolds with 3D printing for bone defect repair. Int. J. Biol. Macromol. 2020, 148, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gardini, D.; Galassi, C.; Lapasin, R. Rheology of hydroxyapatite dispersions. J. Am. Ceram. Soc. 2005, 88, 271–276. [Google Scholar] [CrossRef]
- Amin, A.M.; Besisa, D.H.; El-Amir, A.A.; Zaki, Z.I.; Ahmed, Y.M. Role of heat treatment of hydroxyapatite powder prior to suspension preparation on the suspension flow behavior. Open Ceram. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Maas, M.; Hess, U.; Rezwan, K. The contribution of rheology for designing hydroxyapatite biomaterials. Curr. Opin. Colloid Interface Sci. 2014, 19, 585–593. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Zhang, H.; Liu, X.; Wei, Q.; Li, M.; Liu, Z.; Bao, C.; Zhang, K. Effects of dispersant concentration on the properties of hydroxyapatite slurry and scaffold fabricated by digital light processing. J. Manuf. Process. 2024, 109, 460–470. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Zhang, H.; Wei, Q.; Liu, Z.; Liu, X. Influence of particle size distribution on hydroxyapatite slurry and scaffold properties fabricated using digital light processing. J. Manuf. Process. 2024, 131, 401–411. [Google Scholar] [CrossRef]
- Navarrete-Segado, P.; Tourbin, M.; Grossin, D.; Frances, C. Tailoring hydroxyapatite suspensions by stirred bead milling. Ceram. Int. 2022, 48, 24953–24964. [Google Scholar] [CrossRef]
- Sprio, S.; Preti, L.; Montesi, M.; Panseri, S.; Adamiano, A.; Vandini, A.; Pugno, N.M.; Tampieri, A. Surface phenomena enhancing the antibacterial and osteogenic ability of nanocrystalline hydroxyapatite, activated by multiple-ion doping. ACS Biomater. Sci. Eng. 2019, 5, 5947–5959. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lam, W.; Yang, C.; Xu, B.; Ni, G.; Abbah, S.; Cheung, K.; Luk, K.; Lu, W. Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials 2007, 28, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Ignjatović, N.; Škapin, S.; Uskoković, D.P. Germanium-doped hydroxyapatite: Synthesis and characterization of a new substituted apatite. Ceram. Int. 2022, 48, 27693–27702. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Sharma, B.; Afonso, L.; Singh, M.; Soni, U.; Cahill, D. Zinc- and magnesium-doped hydroxyapatite-urea nanohybrids enhance wheat growth and nitrogen uptake. Sci. Rep. 2022, 12, 19506. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, S.; Basu, B.; Gupta, R. Conductivity Studies of Silver-, Potassium-, and Magnesium-Doped Hydroxyapatite. Int. J. Appl. Ceram. Technol 2015, 12, 319–328. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Pan, H.; Darvell, B.W. The effect of excess phosphate on the solubility of hydroxyapatite. Ceram. Int. 2014, 40, 2751–2761. [Google Scholar] [CrossRef]
- Wingender, B.; Azuma, M.; Krywka, C.; Zaslansky, P.; Boyle, J.; Deymier, A. Carbonate substitution significantly affects the structure and mechanics of carbonated apatites. Acta Biomater. 2021, 122, 377–386. [Google Scholar] [CrossRef]
- Mahanty, A.; Shikha, D. Microstructural, biocompatibility and mechanical investigation of MgHAp and AgHAp: Comparative report. J. Mater. Sci. Mater. Med. 2023, 34, 22. [Google Scholar] [CrossRef]
- Lala, S.; Ghosh, M.; Das, P.K.; Das, D.; Kar, T.; Pradhan, S.K. Magnesium substitution in carbonated hydroxyapatite: Structural and microstructural characterization by Rietveld’s refinement. Mater. Chem. Phys. 2016, 170, 319–329. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Y.; Yin, H.; Ruan, Y.; Sun, Y.; Lin, K. Effects of strontium substitution on the structural distortion of hydroxyapatite by rietveld refinement and Raman Spectroscopy. Ceram. Int. 2019, 45, 11073–11078. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhang, Y.; Liu, X.; Chen, J.; Zhang, Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 178, 222–229. [Google Scholar] [CrossRef]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koc, M.J.A.M. Vat photopolymerization of polymers and polymer composites: Processes and applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
- Shah, M.; Ullah, A.; Azher, K.; Rehman, A.U.; Juan, W.; Aktürk, N.; Tüfekci, C.S.; Salamci, M.U. Vat photopolymerization-based 3D printing of polymer nanocomposites: Current trends and applications. RSC Adv. 2023, 13, 1456–1496. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yan, Y.; Yan, H.; Liu, C.; Li, P.; Dong, P.; Zhao, Y.; Chen, J. 3D printing of hydroxyapatite scaffolds with good mechanical and biocompatible properties by digital light processing. J. Mater. Sci. 2018, 53, 6291–6301. [Google Scholar] [CrossRef]
- Mohammadi, M.; Coppola, B.; Montanaro, L.; Palmero, P. Digital light processing of high-strength hydroxyapatite ceramics: Role of particle size and printing parameters on microstructural defects and mechanical properties. J. Eur. Ceram. Soc. 2023, 43, 2761–2772. [Google Scholar] [CrossRef]
- Guo, W.; Li, B.; Li, P.; Zhao, L.; You, H.; Long, Y. Review on vat photopolymerization additive manufacturing of bioactive ceramic bone scaffolds. J. Mater. Chem. B 2023, 11, 9572–9596. [Google Scholar] [CrossRef]
- Zavřel, F.; Novák, M.; Kroupová, J.; Beveridge, C.; Štěpánek, F.; Ruphuy, G. Development of Hot-Melt Extrusion Method to Produce Hydroxyapatite/Polycaprolactone Composite Filaments. Adv. Eng. Mater. 2022, 24, 2100820. [Google Scholar] [CrossRef]
- Ramli, M.; Sulong, A.; Muhamad, N.; Muchtar, A.; Arifin, A. Stainless steel 316L–hydroxyapatite composite via powder injection moulding: Rheological and mechanical properties characterisation. Mater. Res. Innov. 2014, 18, S6-100–S6-104. [Google Scholar] [CrossRef]
- Ibrahim, D.M.; Mostafa, A.A.; Korowash, S.I. Chemical characterization of some substituted hydroxyapatites. Chem. Cent. J. 2011, 5, 74. [Google Scholar] [CrossRef]
- Kosmulski, M. Confirmation of the Differentiating Effect of Small Cations in the Shift of the Isoelectric Point of Oxides at High Ionic Strengths. Langmuir 2002, 18, 785–787. [Google Scholar] [CrossRef]
- Kolev, S.K.; St. Petkov, P.; Milenov, T.I.; Vayssilov, G.N. Sodium and Magnesium Ion Location at the Backbone and at the Nucleobase of RNA: Ab Initio Molecular Dynamics in Water Solution. ACS Omega 2022, 7, 23234–23244. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed]
- Whittingstall, P. Controlled stress rheometry as a tool to measure grease structure and yield at various temperatures. NLGI Spokesm. 1997, 61, 12. [Google Scholar]
- Semancik, J.R. Yield Stress Measurements using Controlled Stress Rheometry; TA Instruments Publication: New Castle, DE, USA, 1997; Available online: https://www.tainstruments.com/pdf/literature/RH058.pdf (accessed on 24 February 2025).
- Hu, C.; Chen, Y.; Yang, T.; Liu, H.; Huang, X.; Huo, Y.; Jia, Z.; Wang, H.; Hu, L.; Sun, H.; et al. Effect of SiC powder on the properties of SiC slurry for stereolithography. Ceram. Int. 2021, 47, 12442–12449. [Google Scholar] [CrossRef]
- Prasad, V.; Mehrotra, S.P.; Thareja, P.J.C.; Materials, B. Rheological characteristics of concentrated Indian coal ash slurries and flow through pipelines. Constr. Build. Mater. 2022, 361, 129624. [Google Scholar] [CrossRef]
- Rivas-Barbosa, R.; Escobedo-Sánchez, M.A.; Tassieri, M.; Laurati, M. i-Rheo: Determining the linear viscoelastic moduli of colloidal dispersions from step-stress measurements. Phys. Chem. Chem. Phys. 2020, 22, 3839–3848. [Google Scholar] [CrossRef]
- Chen, T. Rheological Techniques for Yield Stress Analysis; TA Instruments: New Castle, DE, USA, 2000; Volume 28, Available online: https://www.tainstruments.com/pdf/literature/RH025.pdf (accessed on 24 February 2025).
- Feilden, E. Additive Manufacturing of Ceramics and Ceramic Composites via Robocasting. Doctoral Dissertation, Imperial College London, London, UK, 2017. [Google Scholar]
- Melk, L.; Mouzon, J.; Turon, M.; Akhtar, F.; Antti, M.-L.; Anglada, M. Surface microstructural changes of spark plasma sintered zirconia after grinding and annealing. Ceram. Int. 2016, 42, 15610–15617. [Google Scholar] [CrossRef]
- Martins, S.B.; Abi-Rached, F.d.O.; Adabo, G.L.; Baldissara, P.; Fonseca, R.G. Influence of Particle and Air-Abrasion Moment on Y-TZP Surface Characterization and Bond Strength. J. Prosthodont. 2019, 28, e271–e278. [Google Scholar] [CrossRef]
- Yarahmadi, M.; Barcelona, P.; Fargas, G.; Xuriguera, E.; Roa, J. Optimization of the ceramic ink used in Direct Ink Writing through rheological properties characterization of zirconia-based ceramic materials. Ceram. Int. 2022, 48, 4775–4781. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Derkach, S.R.; Kulichikhin, V.G. Rheology of Gels and Yielding Liquids. Gels 2023, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef]
- dos Santos, V.I.; Chevalier, J.; Fredel, M.C.; Henriques, B.; Gremillard, L. Ceramics and ceramic composites for biomedical engineering applications via Direct Ink Writing: Overall scenario, advances in the improvement of mechanical and biological properties and innovations. Mater. Sci. Eng. R Rep. 2024, 161, 100841. [Google Scholar] [CrossRef]
- Altıparmak, S.C.; Yardley, V.A.; Shi, Z.; Lin, J. Extrusion-based additive manufacturing technologies: State of the art and future perspectives. J. Manuf. Process. 2022, 83, 607–636. [Google Scholar] [CrossRef]
- Rane, K.; Strano, M. A comprehensive review of extrusion-based additive manufacturing processes for rapid production of metallic and ceramic parts. Adv. Manuf. 2019, 7, 155–173. [Google Scholar] [CrossRef]
- Ravoor, J.; Elsen, S.R.; Thangavel, M.; Arumugam, D.; Karuppan, D. Development of hybrid multi-head, multi-material paste and ink extrusion type 3D printer for biomedical applications. J. Asian Ceram. Soc. 2023, 11, 437–450. [Google Scholar] [CrossRef]

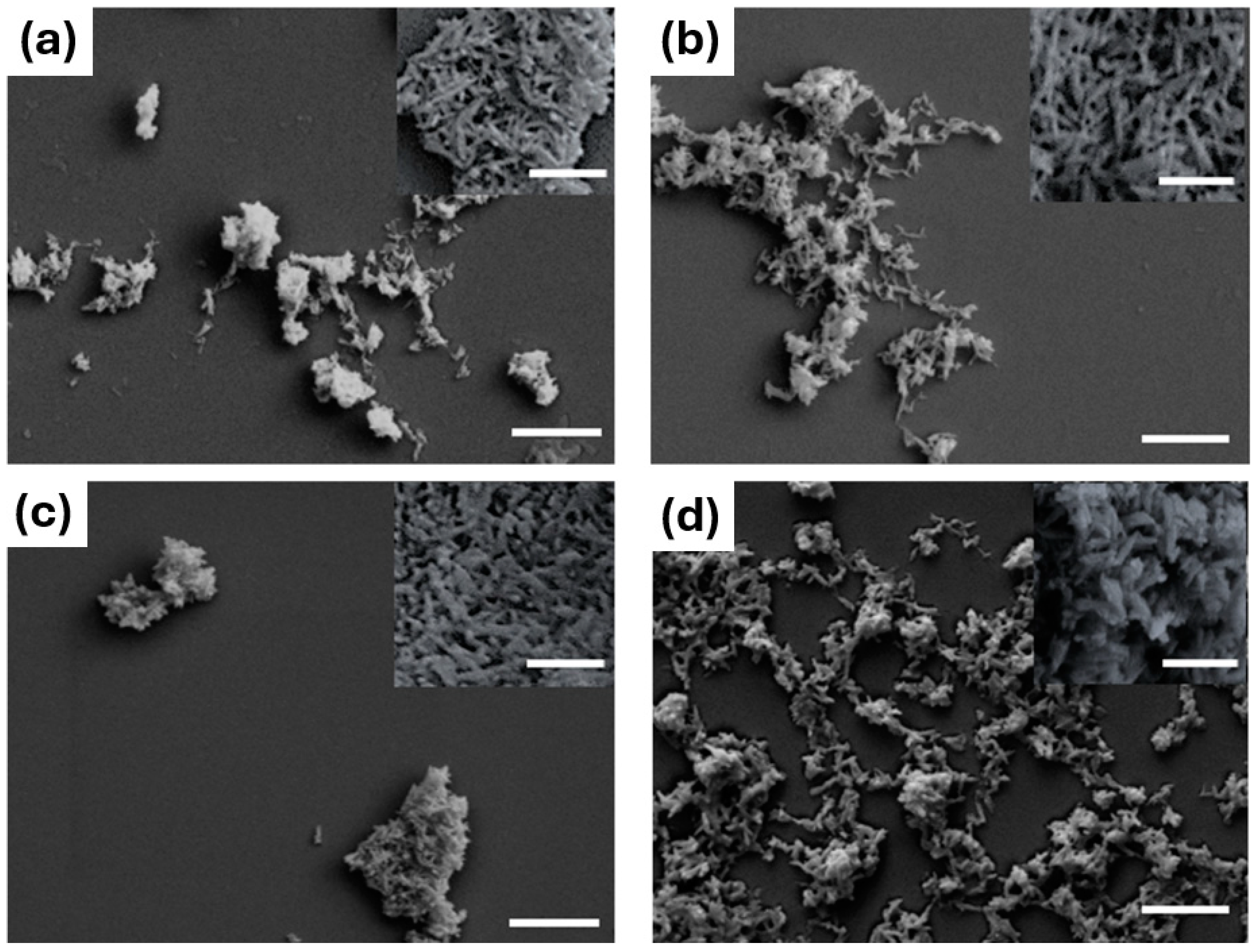

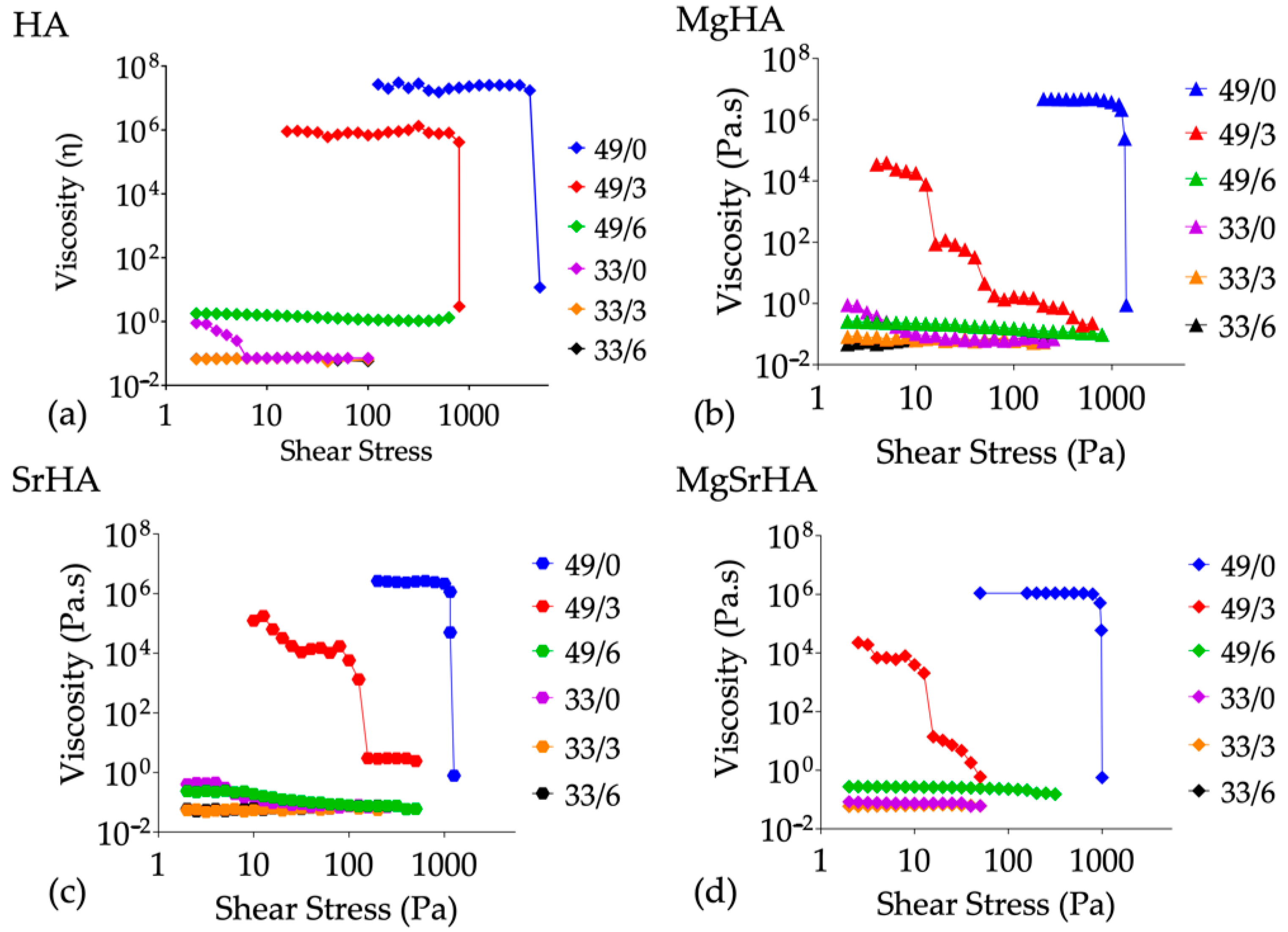


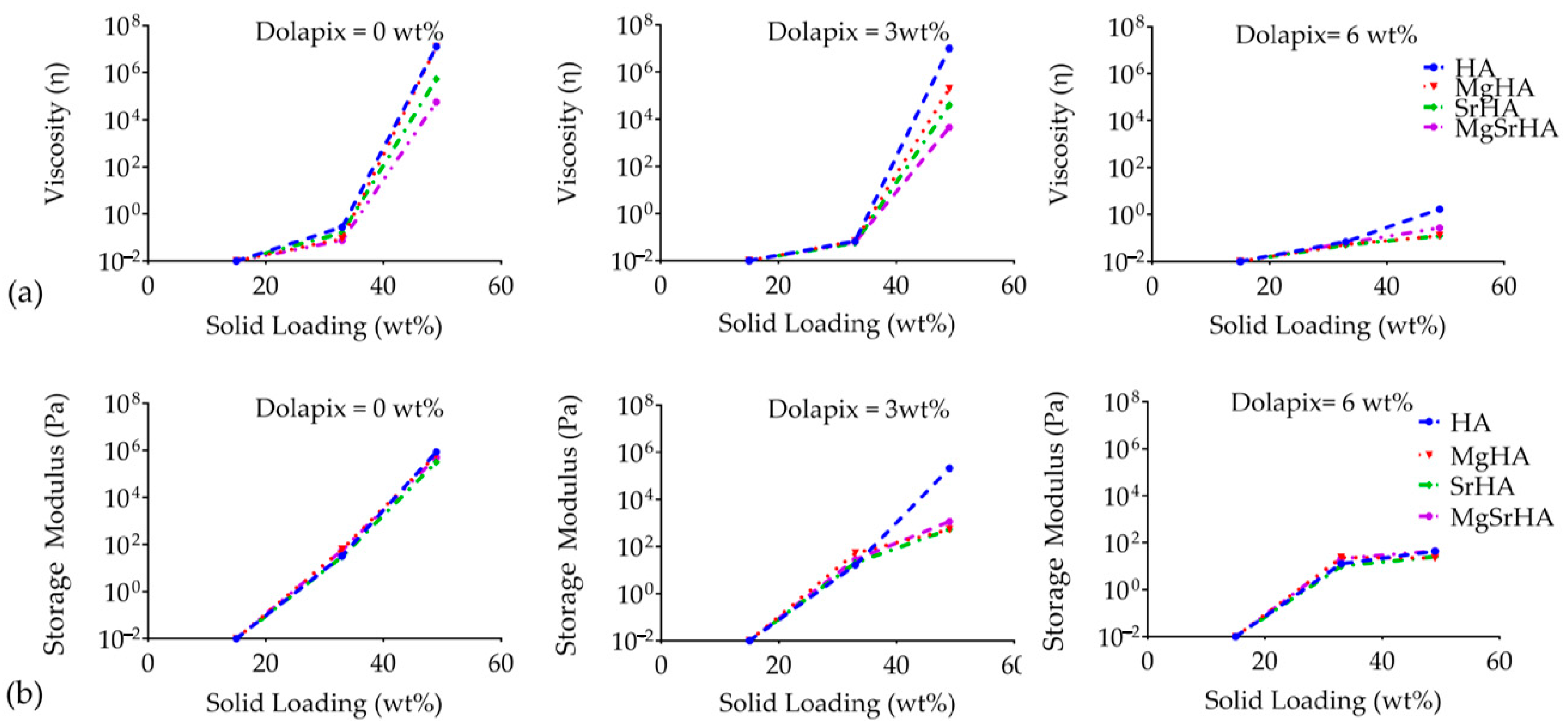
| Sample | a (Å) | c (Å) | c/a | Vol (Å3) |
|---|---|---|---|---|
| HA | 9.4297 ± 0.0015 | 6.8953 ± 0.0011 | 0.731 | 530.99 ± 0.18 |
| MgHA | 9.4357 ± 0.0011 | 6.8860 ± 0.0008 | 0.730 | 530.94 ± 0.14 |
| SrHA | 9.4496 ± 0.0013 | 6.9159 ± 0.0009 | 0.732 | 534.83 ± 0.16 |
| MgSrHA | 9.4521 ± 0.0009 | 6.9000 ± 0.0007 | 0.730 | 533.88 ± 0.12 |
| Sample | Ca/P (mol%) | (Ca + Mg + Sr)/P (mol%) | Mg/(Ca + Mg) (mol%) | Sr/(Ca + Sr) (mol%) |
|---|---|---|---|---|
| HA | 1.64 ± 0.01 | 1.64 ± 0.01 | - | - |
| MgHA | 1.54 ± 0.01 | 1.61 ± 0.01 | 4.91 ± 0.02 | - |
| SrHA | 1.52 ± 0.00 | 1.61 ± 0.00 | - | 5.29 ± 0.03 |
| MgSrHA | 1.53 ± 0.01 | 1.57 ± 0.01 | 2.41 ± 0.07 | 2.67 ± 0.07 |
| Sample | d50 (μm) | SSA (m2/g) | Por (%) |
|---|---|---|---|
| HA | 0.75 ± 0.01 | 115.95 ± 2.32 | 40.35 ± 0.81 |
| MgHA | 0.81 ± 0.02 | 87.29 ± 1.74 | 60.91 ± 1.21 |
| SrHA | 0.92 ± 0.02 | 94.06 ± 1.88 | 51.50 ± 1.03 |
| MgSrHA | 0.82 ± 0.02 | 85.86 ± 1.71 | 44.77 ± 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, Z.; Dapporto, M.; Piancastelli, A.; Gardini, D.; Tampieri, A.; Sprio, S. Rheological Behavior of Ion-Doped Hydroxyapatite Slurries. J. Compos. Sci. 2025, 9, 181. https://doi.org/10.3390/jcs9040181
Abbas Z, Dapporto M, Piancastelli A, Gardini D, Tampieri A, Sprio S. Rheological Behavior of Ion-Doped Hydroxyapatite Slurries. Journal of Composites Science. 2025; 9(4):181. https://doi.org/10.3390/jcs9040181
Chicago/Turabian StyleAbbas, Zahid, Massimiliano Dapporto, Andreana Piancastelli, Davide Gardini, Anna Tampieri, and Simone Sprio. 2025. "Rheological Behavior of Ion-Doped Hydroxyapatite Slurries" Journal of Composites Science 9, no. 4: 181. https://doi.org/10.3390/jcs9040181
APA StyleAbbas, Z., Dapporto, M., Piancastelli, A., Gardini, D., Tampieri, A., & Sprio, S. (2025). Rheological Behavior of Ion-Doped Hydroxyapatite Slurries. Journal of Composites Science, 9(4), 181. https://doi.org/10.3390/jcs9040181






