Abstract
The present work investigates the rheological behavior of ceramic slurries made of hydroxyapatite powders doped with magnesium and strontium ions and selected as particularly relevant for biomedical applications. The incorporation of doping ions into the apatite crystal structure is a well-known way to enhance the bioactivity of hydroxyapatite through compositional and structural changes, however, this also affects the rheological properties relevant to the fabrication of ceramic devices by forming techniques based on the manipulation of aqueous slurries. We analyzed the effect of different apatitic chemical compositions, powder content, and dispersant amount on the shear behavior and flowability of slurries, thus finding that the structural changes in hydroxyapatite induced by ion doping significantly affected the colloidal stability of the apatite powders and the viscoelasticity of the slurries. This leads to improved rheological behavior in the hydroxyapatite suspensions, which is suitable for the future development of ceramic slurries, particularly for achieving novel ceramic devices by extrusion-based techniques.
1. Introduction
Ceramic implants based on hydroxyapatite (Ca5(PO4)3OH, HA) have been extensively investigated for the regeneration of bone defects due to their excellent biocompatibility and osteoconductivity, which are attributed to their close compositional similarity to the mineral component of natural mammalian bone [1,2,3,4,5,6,7,8]. However, biogenic HA differs from stoichiometric HA in terms of its chemical composition and behavior in physiological environments due to the substitution of Ca2+ and PO43− with various foreign ions, including Mg2+, Sr2+, Zn2+, K+, Fe2+, Mn2+, Mo3+, Cu2+, Ni2+, and CO32− [9,10]. Ion doping alters the physicochemical properties of HA, particularly the particle size, crystallinity, thermal stability, bioresorption rate, and surface properties, all of which are relevant for the biological and mechanical performance of the final bio-devices [11,12,13,14,15,16]. In this respect, Mg2+ and Sr2+ ions were reported as being among the ones of major relevance [17,18,19,20,21]. Magnesium ions effectively promote bone mineralization and the proliferation of mesenchymal stem cells, while also limiting bacterial biofilm formation and stimulating osteogenesis and angiogenesis [22,23,24]. Strontium has been widely investigated for its anti-osteoporotic characteristics, indirectly promoting new bone formation and osseointegration [25,26], and possibly enhancing the mechanical properties of apatitic scaffolds [27,28,29,30,31].
Various processing techniques have been proposed in recent decades to prepare porous HA scaffolds from ceramic powders, such as partial sintering, replica methods, sacrificial templates, direct foaming, and 3D printing [32,33,34], all methods requiring the manipulation of powders dispersed in aqueous suspensions (slurries), which necessitates substantial optimization of the rheological properties. The rheological behavior of concentrated slurries primarily depends on the content of solid particles and their degree of agglomeration. When the slurry is subjected to shear stresses, aggregates progressively break down by increasing the shear rate, leading to apparent yield stress and resulting in time-dependent phenomena like viscoelasticity and thixotropy [35].
The agglomeration of particles is primarily governed by a subtle balance between attractive and repulsive phenomena, which arise from electrostatic forces established on the surface of electrically charged particles. Van der Waals attractive forces generally dominate over the repulsive forces, making the use of electrosteric dispersing agents essential to prevent uncontrolled agglomeration [36,37].
Despite HA slurries having been extensively developed to prepare scaffolds for bone tissue engineering, only a few studies describe the processing routes of calcium phosphate slurries [38,39,40,41]. A relevant effect of ion doping is the alteration of the surface area and surface charge of the HA [42,43,44,45], potentially affecting the affinity with water and dispersibility, which can impact the shear properties and flowability of the ceramic slurry.
Various studies have highlighted the significance of different cations and anions in modifying the surface properties of HA, which, in turn, affects their processability and performance in applications. For instance, zinc doping decreases the zeta potential of HA slurry, leading to rapid coagulation and potentially enhancing interactions with other molecules [46]. The introduction of Ag+ and K+ ions has been reported to enhance ionic conduction within HA, which may also impact its rheological properties [47]. Moreover, CO32− and HPO42− substitutions were reported to influence the solubility and surface charge of HA, and are critical factors affecting the flow behavior of HA-based slurries [48,49]. The various effects of ion doping on HA were investigated in term of crystallinity, structural, and mechanical properties, while a detailed investigation of the effect of ion doping on the rheological properties of HA slurries is still lacking.
The present study investigates the rheological behavior of hydroxyapatite doped with Mg2+ and Sr2+ ions. The Mg- and Sr-doped HA powders were obtained by wet synthesis, and aqueous slurries were prepared with increasing concentrations of a dispersant to identify the best conditions for achieving homogeneous slurries, particularly targeting future application in extrusion-based techniques such as 3D printing.
2. Materials and Methods
2.1. Synthesis of Stoichiometric and Ion-Doped HA Powders
The wet synthesis of stoichiometric (undoped) HA, taken as reference material, was carried out using calcium hydroxide (Ca(OH)2, Sigma Aldrich, St. Louis, MO, USA) and orthophosphoric acid (H3PO4, Sigma Aldrich). First, a diluted (1 mM) aqueous solution of H3PO4 (95%) was added dropwise into a stirred 3 mM calcium hydroxide solution, at a rate of 1 mL/min. The as-obtained solution was stirred for 2 h and rested overnight at room temperature before centrifugation at 10,000 rpm. The resulting precipitate was dried overnight at 40 °C in a ventilated oven. Similarly, Mg2+- and Sr2+-doped HA powders were synthesized by introducing the respective soluble salts (MgCl2∙6H2O and SrCl2·6H2O, Sigma Aldrich) in appropriate amounts to prepare Mg-doped HA (Mg/(Ca + Mg) = 6 mol%), Sr-doped HA (Sr/(Ca + Sr) = 6 mol%), and co-doped Mg-Sr-HA (Mg/(Ca + Mg + Sr) = Sr/(Ca + Mg + Sr) = 3 mol%) formulations. Finally, the powders were dried and sieved below 150 μm.
2.2. Powder Characterization
The phase composition was investigated by X-ray diffraction (XRD) (D8 Advance, Bruker, Karlsruhe, Germany, equipped with a LINXEYE detector using monochromatic CuKα1-radiation) in the 2θ range 10° to 80° with a scanning step of 0.02°. For the HA phase, the JCPDS Card No. 09–0432 was implemented. The cell parameters and geometry were evaluated by full profile Rietveld refinement by using the software TOPAS v.5, Bruker, Karlsruhe, Germany, using the space group n. 176, P63/m, typically used to describe the apatite structure.
The Fourier-transform infrared spectroscopy (FTIR) was performed by a spectrometer (Nicolet IS5, Thermo Fisher Scientific, Waltham, MA, USA) to investigate the presence of specific functional groups. Samples were ground with 5% KBr in an agate mortar, compressed into tablets, and analyzed from 500 to 4000 cm−1.
Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) 5100 (Agilent, Santa Clara, CA, USA) was used to determine the chemical composition of the powders.
The particle size distribution was calculated in the range 50–0.25 μm by SediGraph 5100, Micromeritics, Norcross, GA, USA. In particular, 3.5 g of powder was dispersed into 80 mL of aqueous solutions enriched with sodium hexametaphosphate (0.05 wt%), followed by disaggregation in ultrasound for 30 min. Finally, the obtained dispersion was analyzed with an X-ray beam, evaluating the sedimentation of the particles according to the Stokes’ law.
The specific surface area (SSA) was obtained by the Brunauer–Emmett–Teller (BET) method (Surfer, Thermo Scientific, Waltham, MA, USA). In particular, 250 mg of powder was firstly dried at 100 °C for 12 h, followed by nitrogen physical adsorption on the surface of the sample. Finally, the volume of the nitrogen adsorbed is measured to determine the amount of gas required to cover the surface of the sample.
The porosity of the powders was evaluated by Thermo Fisher Scientific Pascal 240, with intrusion of mercury into the samples with pressure in the range 0.1–200 MPa.
The morphology of the powders was investigated after gold sputter coating, by Field Emission Scanning Electron Microscopy (FE-SEM) (Zeiss-SIGMA, Carl Zeiss Microscopy GmbH, Jena, Germany).
2.3. Preparation of Water-Based Slurries
Water-based slurries were prepared by dispersing stoichiometric or ion-doped HA powders in Milli-Q water with the addition of an anionic polyelectrolyte as dispersant agent (Dolapix CA, 25 wt% of active ingredient, and density 1.1 g/mL, Zschimmer and Schwarz, Lahnstein, Germany). Slurries with different powder contents (33 and 49 wt%) and dispersant concentrations (0, 3 and 6 wt%), where the percentages were calculated with respect to the total slurry weight, were studied. In the following, the dispersant content will be sometimes referred to as the total surface area of the powder through a parameter defined as:
where is the mass of active dispersant, SSA is the specific surface area of the powder, and is the mass of powder.
2.4. Slurries Characterization
The colloidal stability of the slurries was evaluated through the measurement of the zeta potential (ζ) that is directly related to the electrostatic repulsion between the particles. The ζ potential measurements were performed using an electroacoustic instrument based on current vibration potential (DT-310, Dispersion Technology Inc.,Bedford Hills, NY, USA).
The viscosity and viscoelasticity of the slurries were measured with a controlled stress rheometer (Bohlin C-VOR 120, Bohlin Instruments Limited, Cirencester, UK) equipped with a stainless steel serrated parallel plate geometry (25 mm diameter) and setting (1 mm gap). Operating in controlled-stress mode, the shear viscosity data were recorded by applying a sequence of discrete steps, each with an equilibrium time of 60 s. The measurements were performed by increasing the shear stress in the range of 1–1000 Pa. To improve the reproducibility of the tests, the suspensions were pre-sheared at 1 Pa for 30 s before starting the measurements. The measurements were carried out at a constant temperature of 25 °C and using a solvent trap to prevent water evaporation to ensure that only rheological changes were being measured, preventing artifacts from solvent loss or drying of the sample. Dynamic small amplitude oscillatory tests were performed to study the viscoelastic behavior. The amplitude sweep tests were performed at a constant frequency of 1 Hz by increasing the shear stress in the range 1–1000 Pa to find the linear viscoelastic region (LVER), that is, the region where the stress is proportional to the strain; outside that region, the viscoelastic behavior is non-linear and microstructural breakages occur. From those tests, 10 Pa resulted as being an adequate value to prevent microstructural damages for all the tested slurries and it was used as maximum amplitude of oscillation during the frequency sweep tests in the range of 1–10 Hz.
3. Results
3.1. Characterization of the Apatite Powders
The XRD analysis of the stoichiometry and ion-doped HA powders exhibited diffraction patterns ascribable to HA, with an absence of any secondary phases (Figure 1a).
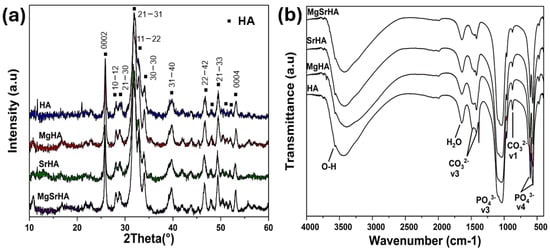
Figure 1.
XRD patterns (a) and FTIR spectra (b) for stoichiometric and ion−doped HA. The symbols (▪) and the Miller indices of XRD patterns are indicated according to JCPDS 09−0432.
Full profile analysis of the XRD patterns reported in Figure 1a (see also Figure S1 in the Supplementary Materials) showed slightly modified cell parameters for ion-doped HA compared to the stoichiometric HA (Table 1), which were ascribed to partial substitution of calcium ions with doping ions in the HA lattice [50].

Table 1.
Unit cell parameters and calculated cell volume for HA and ion-doped HA.
In particular, a slight decrease in the c-parameter was observed in Mg-doped HA, which has been previously reported as typical of Mg2+ substitution in the apatite lattice [51]; conversely, larger cell parameters and volume can be ascribed to Sr2+ doping due to its larger ionic radius compared to Ca2+ ions [52]. Larger unit cell volumes, even if lower than those previously reported [19], confirm the effective co-doping of Mg and Sr ions. No significant distortion of the HA lattice geometry was observed with ion doping, considering that the ratio between the c and a parameter (c/a) was similar in all the studied materials.
Additionally, the FTIR analysis of the as-synthesized ion-doped powders presented the typical patterns of hydroxyapatite (Figure 1b). The vibrational bands at 562 and 603 cm−1 were attributed to O-P-O bending, while peaks at 962 and 1034 cm−1 were both attributed to the stretching of PO43−. In addition, the peaks at 874, 1339, and 1456 cm−1 associated with the CO32− group were ascribed to the inclusion of atmospheric CO2 in the reaction vessel. The peak at 632 cm−1 and the broad peak at 3427 cm−1 were attributed to OH− and H2O molecules physiosorbed or incorporated into the HA structure [53,54].
The ICP-OES analysis further confirmed the presence of the doping ions in amounts very close to the nominal composition of the starting mixture. Calcium deficiency, attested by cations/phosphorous molar ratios lower than 1.67 (Table 2), confirms the effective ion substitution in the doped and co-doped HA materials.

Table 2.
Elemental composition by ICP of stoichiometric and ion-doped HA.
The particle size distribution, specific surface area (SSA), porosity, and microstructural features of stoichiometric and ion-doped HA powders were also investigated (Table 3 and Figure 2).

Table 3.
Particle size d50, specific surface area SSA, and porosity Por of stoichiometric and ion-doped HA powders.
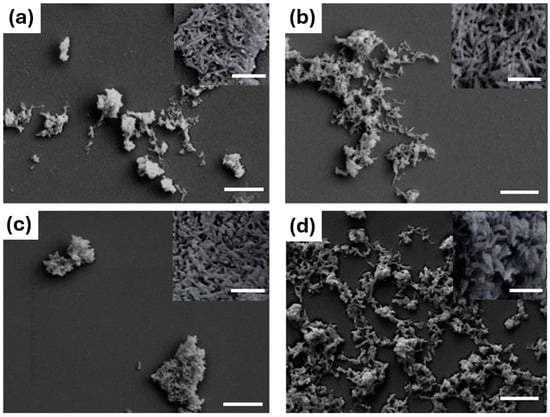
Figure 2.
FE-SEM micrographs of HA (a), MgHA (b), SrHA (c), and MgSrHA (d). Figure scale bars = 1 µm and inset scale bar = 200 nm.
The particle size, expressed as median diameter (d50), was higher for the ion-doped powders compared to the stoichiometric HA, with all average particle sizes smaller than 1 µm. Correspondingly, the SSA of ion-doped powders was lower than the stoichiometric HA. However, the decrease in SSA was not linear with the d50 values, as evidenced by the different microporosity and nanostructured roughness of the powders (Figure 2).
3.2. Characterization of the Slurries
The slurries were prepared by gradually adding the powder into water, accompanied by mechanical mixing, as previously reported [36]. In this study, the lower solid loading (33 wt%) was representative of applications requiring slurries characterized by low viscosity, such as vat photopolymerization [55,56,57,58,59], while slurries with a higher solid content (49 wt%) were representative of processing techniques involving extrusion [60] or compression molding [61]. Increasing the particle packing density up to the highest concentration is very relevant for practical uses as it yields maximal density of ceramic bodies after thermal consolidation, which is important for maximizing the mechanical strength and elastic modulus of the final device to appropriately comply with the physiological mechanical forces experienced at a correspondence of bony districts. The variation in the compositional parameter β was accomplished by increasing the amount of dispersant while maintaining a constant amount of powder.
3.2.1. Colloidal Stability
The colloidal stability of the slurries, as estimated by ζ potential measurements, increased with the amount of dispersant, particularly for the slurries containing 49 wt% of powder (Figure 3).
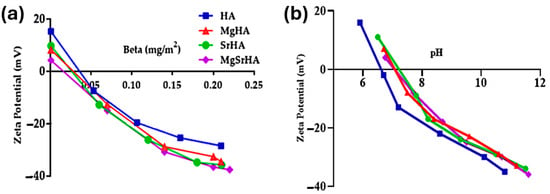
Figure 3.
Colloidal stability of the slurries containing 49 wt% powder solid loading; (a) ζ potential as function of β and (b) ζ potential as function of pH.
Positive ζ potentials were observed in the absence of dispersants (i.e., β = 0), while a significant drop was noted with the addition of even small amounts of dispersant, up to values in the range −30 to −40 mV for β of about 0.1–0.2 mg/m2, leading to a significant increase in colloidal stability. The ion doping induced the shift of the isoelectric point (IEP) towards higher pH values, slightly depending on the specific cation (Figure 3b). This phenomenon may be attributed to the segregation of a fraction of Ca2+, CO32−, and doping ions at the apatite surface, which frequently occurs in ion-substituted HA powders, as previously observed with apatites and metal oxides [62,63].
3.2.2. Flow Behavior
The viscosity–shear stress curves of the HA slurries with different solid loading (33 wt% and 49 wt%) and dispersant amount (0, 3, 6 wt%) were investigated (Figure 4).
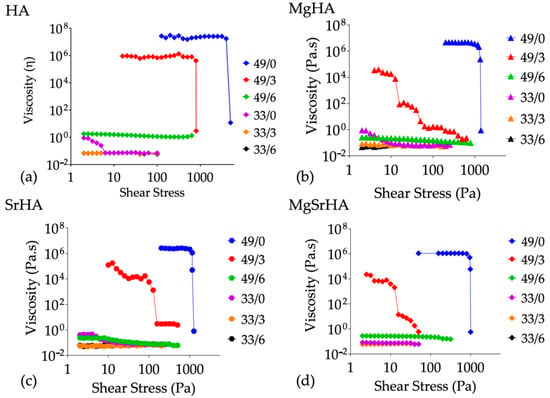
Figure 4.
Viscosity curves as a function of shear stress and the effect of solid loading and dispersant on the viscosity at a shear stress of 10 Pa: HA (a), MgHA (b), SrHA (c), and MgSrHA (d) slurries.
At low shear stress, the aggregation of the solid particles to form complex three-dimensional networks is the prevalent phenomenon for the slurries with high solid loading and zero or a low amount of dispersant, generating very high viscosities, in the order of 106 to 108 Pa·s. With increased shear stress, the viscosity of the slurries decreased with different patterns based on the progressive breaking of powder agglomerates, revealing a shear-thinning behavior eventually associated to evident yield stress.
In this respect, it can also be hypothesized that a fraction of the liquid phase remains locally immobilized within agglomerates; when the shear stress rises, it is released, decreasing the friction and, consequently, the viscosity. Instead, the slurries with low solid loading generally exhibited a Newtonian behavior with low viscosities (below 1 Pa·s) for the entire shear stress range.
It should be noted that the slurries with a high content of powder (49 wt%), but also with a high amount of dispersant (6 wt%), show an almost Newtonian behavior with comparable viscosities to slurries with low powder content (33 wt%).
This highlights the effectiveness of the dispersant in controlling the rheological behavior of concentrated slurries; the absorption of the dispersant (an anionic polyelectrolyte) on the particles’ surface prevents particle agglomeration and maintains a small mean agglomerate size.
With the highest amount of dispersant (6 wt%), the solid loading has no effect on the viscosity, but in the case of a low amount of dispersant (i.e., 3 wt% or even zero), an increase in solid loading led to increased viscosity, particularly with stoichiometric HA slurries. For the co-doped MgSrHA slurries, the increase in viscosity was the lowest.
In Figure 5, the flow curves for the slurries containing the different HA powders without any added dispersant (i.e., β = 0) are compared. It can be observed that the slurries (49 wt% of powder) with stoichiometric HA exhibited higher viscosities if compared to ion-doped HA, in accordance with the lower values of ζ potential measured in that condition.
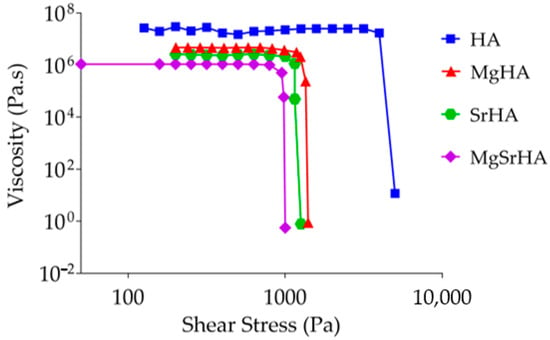
Figure 5.
Viscosity of slurries with 49 wt% solid loading and β = 0.
The MgSrHA slurries exhibited the lowest viscosity, ascribable to the higher ζ potential (in absolute value) and lower SSA, which is also associated with the higher affinity of Mg2+ and Sr2+ ions with water molecules [64,65].
On the other hand, it was found that a powder concentration of 49 wt% represents the upper solid loading limit for the preparation of slurries with stoichiometric HA, while higher solid loadings (up to 59 wt%) were achievable for ion-doped HA slurries.
The viscosity of the ion-doped HA slurries at 49 wt% with no dispersants experienced significantly lower yield stresses [66,67] if compared with stochiometric HA (Figure 5). The yield stress refers to the minimum stress required to initiate the slurry to flow and represents a rheological property strictly related to the flowability of the slurries during the applications [68,69].
3.2.3. Viscoelasticity
Small amplitude oscillatory shear (SAOS) tests were performed on stoichiometric and ion-doped HA slurries to investigate their viscoelastic behavior, starting with an amplitude sweep test to identify the linear viscoelastic region (LVER) [70,71].
The samples mostly exhibited constant storage (G′) and loss (G″) moduli up to the elastic-limit yield stress [72,73,74,75] (Figure S2 in Supplementary Materials). The stoichiometric HA slurries exhibited the widest LVER, when compared to the ones of the ion-doped HA slurries. Furthermore, a crossover of G’ and G’’ was observed at higher shear stresses, indicating the transition from elastic to viscous behavior.
For the slurries with 33 wt% solid loading, the LVER was detectable above 10 Pa, where they exhibited a significantly lower storage moduli (~1 Pa), with limited dependence on the dispersant amount.
Then, frequency sweep tests were performed in the range 1–10 Hz by keeping constant the shear stress at 10 Pa, allowing the correlation of the storage moduli with the time-dependent stiffness of the materials (Figure 6).
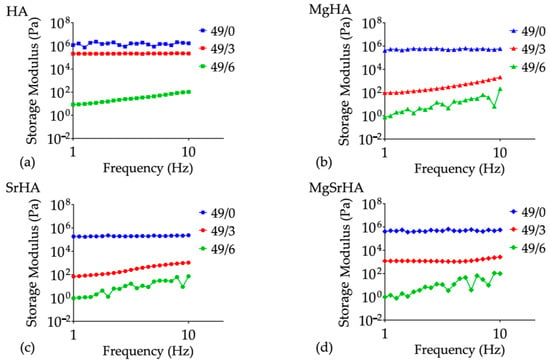
Figure 6.
Storage moduli curves as a function of frequency for the effect of solid loading and dispersant: (a) HA, (b) MgHA, (c) SrHA, and (d) MgSrHA slurries.
4. Discussion
The effect of the dispersant became prevalent with increasing powder solid loading, particularly for ion-doped HA slurries. The addition of 6 wt% dispersant resulted in a significant decrease in the storage modulus of the concentrated slurries, comparable to the ones for low solid loading slurries (33 wt%), as occurred for the viscosity.
A solid-like behavior is generally characterized by a high and constant storage modulus over most of the frequencies, while a gel-like behavior is characterized by a storage modulus that increases with frequency [76,77]. As a general behavior, we observed an increase in the storage modulus with increasing oscillation frequency, with the exception of the slurries with higher solid loading and a lower amount of dispersants exhibiting a solid-like behavior (i.e., the storage modulus nearly constant with the frequency).
To better highlight the effect of ion doping on the rheological properties of HA and ion-doped HA slurries, solid loading, and dispersant concentration, the viscosity (at 10 Pa) and the storage modulus of the slurries of the different powders were plotted against solid loading with different contents of dispersant (Figure 7).
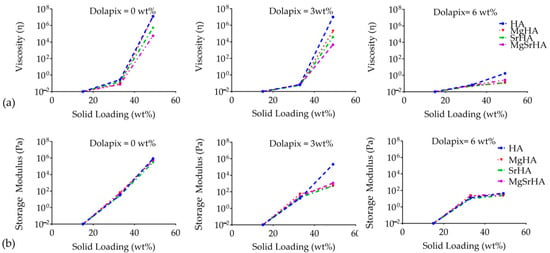
Figure 7.
(a) Viscosity at 5 Pa and (b) storage moduli at 5 Hz for stoichiometric and ion-doped HA slurries with increasing dispersant amount.
These plots represent the overlapping of the cross sections at different dispersant amounts for the four HA powders shown in Figure 4 and Figure 6. Regarding the flow behavior, a common feature for all the slurries is, as already observed, the increase in viscosity with higher solid loading, particularly for the stoichiometric HA samples (Figure 7a).
Moreover, lower viscosity values were found for ion-doped HA slurries, even in the absence of dispersants. The addition of 6 wt% of dispersant caused a significant drop in the viscosity values, regardless of solid loading and ion doping. In particular, the MgSrHA slurry showed the lowest viscosities in all cases, in accordance with the higher colloidal stability and lower SSA results.
The ion doping of HA resulted in significant changes to the physico-chemical properties of the powders, which also allowed for the preparation of higher solid loading slurries, thus, potentially acting as enhancer of the packing density of particles and, ultimately, of the mechanical performance of the final scaffold. Regarding the viscoelastic behavior, in the absence of dispersants, the elastic modulus increased with solid loading for all samples, while only 3 wt% of dispersants induced a significant drop in the elastic modulus for the ion-doped HA slurries. With 6 wt% of dispersants, the elastic modulus was the same for stoichiometric and ion-doped HA powders, regardless of the solid content in the slurry.
The results revealed that the viscosity and storage modulus of the slurries decreased with increasing the amount of dispersant, while a significant decrease in viscosity was also associated with the ion-doping process. Moreover, the effect of the dispersant was prevalent for the ion-doped HA slurries, suggesting a stronger interaction between the dispersant and the ion-doped HA surfaces. Based on the above results, the present work highlights the effect of ion doping on the surface charge of HA, particularly its effect on the electric double layer of HA particles upon dispersion in aqueous solutions and on the rheological behavior of the resulting slurries.
In this respect, the stability of the slurries was improved with ion-doped HA, as demonstrated by ζ potential measurements, while higher solid loading was achieved, reflected by the higher packing density of the particles during the processing. This results in reduced porosity and improves the mechanical performance of the final ceramic device. Furthermore, improved slurry stability contributes to more consistent shaping and sintering processes, ensuring uniform material properties throughout the ceramic structure.
The possibility of enhancing the structural cohesion of the slurry is particularly promising in view of extrusion-based applications such as 3D printing techniques, and is particularly challenging in the case of ceramic materials, which require a controlled high colloidal stability and viscosity (e.g., 3–10 Pa∙s [78]), which is associated with the easy flow of the material through a thin nozzle, which is mandatory for obtaining final devices with homogeneous structures and improved mechanical properties [79,80,81]. Additionally, the use of ion-doped HA can potentially improve the biocompatibility and bioactivity of the final ceramic material, widening its biomedical applications as bone implants and scaffolds.
5. Conclusions
The effect of single-ion (Mg2+ and Sr2+) and multiple-ion (Mg2+−Sr2+) doping on the rheological properties of HA-based aqueous slurries was evaluated in this work. The ion doping was associated with a decrease in the specific surface area and improved colloidal stability. In this way, ion doping allowed for the preparation of higher solid loading slurries. The incorporation of ions introduced lattice distortions, thus changing the surface charge, ultimately altering the electrostatic repulsion between particles upon dispersion in aqueous media, particularly in the presence of dispersant. The addition of dispersant significantly affected the rheology by increasing the solid loading. Interestingly, the ion doping proved to be a valuable factor in modulating the rheology of slurries, particularly in view of the customized and optimized viscoelastic properties for extrusion-based techniques. In this study, a suitable viscosity for extrusion was obtained by increasing the solid loading up to 49 wt% and by the addition of dispersant, up to 6 wt%. In the case of ion-doped HA slurries, the 3 wt% of dispersants can also be considered for extrusion, particularly in the case of the higher shear stresses regimes associated with the nozzle reduction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcs9040181/s1, Figure S1: Rietveld refinement of the XRD patterns. Rwp (HA) = 5.21%; Rwp (MgHA) = 6.03%; Rwp (SrHA) = 6.24%; and Rwp (MgSrHA) = 6.03% and Figure S2: Storage and loss moduli as a function of shear stress for slurries with 49 wt% solid loading and β = 0.
Author Contributions
Conceptualization, S.S. and A.T.; methodology, Z.A., D.G., A.P. and M.D.; writing—original draft preparation, Z.A. and M.D.; writing—review and editing, Z.A., D.G., M.D. and S.S.; supervision, M.D. and S.S.; and funding acquisition, S.S. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted under the industrial PhD in Chemistry program at the University of Bologna, XXXVI Cycle, and equally supported by the National Research Council of Italy and the company Finceramica S.p.A, Faenza, Italy.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohd Zaffarin, A.S.; Ng, S.-F.; Ng, M.H.; Hassan, H.; Alias, E.J.N. Nano-Hydroxyapatite as a Delivery System for Promoting Bone Regeneration In Vivo: A Systematic Review. Nanomaterials 2021, 11, 2569. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2022, 10, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Tabrizi, B.; Basirun, W.J.; Yeong, C.H.; Thein, W.M. Development of the third generation of bioceramics: Doping hydroxyapatite with s-, p-, d-, and f-blocks cations and their potential applications in bone regeneration and void filling. Ceram. Int. 2022, 49, 7142–7179. [Google Scholar] [CrossRef]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional hydroxyapatite composites for orthopedic applications: A review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar] [CrossRef]
- Rossini, Z.; Franzini, A.; Zaed, I.; Zingaretti, N.; Nicolosi, F.; Zanotti, B. Custom-made porous hydroxyapatite cranioplasty in patients with tumor versus traumatic brain injury: A single-center case series. World Neurosurg. 2020, 138, e922–e929. [Google Scholar] [CrossRef]
- Aldahak, N.; Dupre, D.; Ragaee, M.; Froelich, S.; Wilberger, J.; Aziz, K.M. Hydroxyapatite bone cement application for the reconstruction of retrosigmoid craniectomy in the treatment of cranial nerves disorders. Surg. Neurol. Int. 2017, 8, 115. [Google Scholar]
- Song, Y.; Hu, Q.; Liu, Q.; Liu, S.; Wang, Y.; Zhang, H. Design and fabrication of drug-loaded alginate/hydroxyapatite/collagen composite scaffolds for repairing infected bone defects. J. Mater. Sci. 2023, 58, 911–926. [Google Scholar] [CrossRef]
- Ozder, M.N.; Ciftci, F.; Berrak, O.; Arisan, E.D.; Ustündag, C.B. In situ synthesis and cell line studies of nano-hydroxyapatite/graphene oxide composite materials for bone support applications. Ceram. Int. 2023, 49, 14791–14803. [Google Scholar] [CrossRef]
- Yedekçi, B.; Tezcaner, A.; Alshemary, A.Z.; Yılmaz, B.; Demir, T.; Evis, Z. Synthesis and sintering of B, Sr, Mg multi-doped hydroxyapatites: Structural, mechanical and biological characterization. J. Mech. Behav. Biomed. Mater. 2021, 115, 104230. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 145–211. [Google Scholar]
- Abbas, Z.; Dapporto, M.; Tampieri, A.; Sprio, S. Toughening of bioceramic composites for bone regeneration. J. Compos. Sci. 2021, 5, 259. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Trace element doping in calcium phosphate ceramics to understand osteogenesis and angiogenesis. Trends Biotechnol. 2013, 31, 10-1016. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Alshemary, A.Z.; Evis, Z. Co-doped hydroxyapatites as potential materials for biomedical applications. Microchem. J. 2019, 144, 443–453. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Padmanabhan, V.P.; Kulandaivelu, R.; Nellaiappan, T.S.N.; Sagadevan, S.; Paiman, S.; Mohammad, F.; Al-Lohedan, H.A.; Obulapuram, P.K.; Oh, W.C. Influence of iron doping towards the physicochemical and biological characteristics of hydroxyapatite. Ceram. Int. 2021, 47, 5061–5070. [Google Scholar] [CrossRef]
- Reger, N.C.; Bhargava, A.K.; Ratha, I.; Kundu, B.; Balla, V.K. Structural and phase analysis of multi-ion doped hydroxyapatite for biomedical applications. Ceram. Int. 2019, 45, 252–263. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Nagyne-Kovacs, T.; Studnicka, L.; Kincses, A.; Spengler, G.; Molnár, M.; Tolner, M.; Lukacs, I.E.; Szilagyi, I.M.; Pokol, G. Synthesis and characterization of Sr and Mg-doped hydroxyapatite by a simple precipitation method. Ceram. Int. 2018, 44, 22976–22982. [Google Scholar] [CrossRef]
- Jin, W.; Liu, Z.; Wu, Y.; Jin, B.; Shao, C.; Xu, X.; Tang, R.; Pan, H. Synergic effect of Sr2+ and Mg2+ on the stabilization of amorphous calcium phosphate. Cryst. Growth Des. 2018, 18, 6054–6060. [Google Scholar] [CrossRef]
- Aina, V.; Lusvardi, G.; Annaz, B.; Gibson, I.R.; Imrie, F.E.; Malavasi, G.; Menabue, L.; Cerrato, G.; Martra, G. Magnesium-and strontium-co-substituted hydroxyapatite: The effects of doped-ions on the structure and chemico-physical properties. J. Mater. Sci. Mater. Med. 2012, 23, 2867–2879. [Google Scholar] [CrossRef]
- Landi, E.; Guizzardi, S.; Papa, E.; Galli, C. Mg, Sr-Cosubstituted Hydroxyapatite with Improved Structural Properties. Appl. Sci. 2021, 11, 4930. [Google Scholar] [CrossRef]
- Kadhim, M.M.; AlMashhadani, H.A.; Hashim, R.D.; Khadom, A.A.; Salih, K.A.; Salman, A.W. Effect of Sr/Mg co-substitution on corrosion resistance properties of hydroxyapatite coated on Ti–6Al–4V dental alloys. J. Phys. Chem. Solids 2022, 161, 110450. [Google Scholar] [CrossRef]
- Ciolacu, L.; Zand, E.; Negrau, C.; Jaeger, H.J.F. Bacterial attachment and biofilm formation on antimicrobial sealants and stainless steel surfaces. Foods 2022, 11, 3096. [Google Scholar] [CrossRef] [PubMed]
- Demishtein, K.; Reifen, R.; Shemesh, M.J.N. Antimicrobial properties of magnesium open opportunities to develop healthier food. Nutrients 2019, 11, 2363. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Y.; Tang, Y.; Wang, X.; Zhang, D.; Zhang, X.; Yu, Y.; Yang, X.; Cai, Q.J.B.M. A biomimetic piezoelectric scaffold with sustained Mg2+ release promotes neurogenic and angiogenic differentiation for enhanced bone regeneration. Bioact. Mater. 2023, 25, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Borciani, G.; Ciapetti, G.; Vitale-Brovarone, C.; Baldini, N. Strontium functionalization of biomaterials for bone tissue engineering purposes: A biological point of view. Materials 2022, 15, 1724. [Google Scholar] [CrossRef]
- Sprio, S.; Dapporto, M.; Montesi, M.; Panseri, S.; Lattanzi, W.; Pola, E.; Logroscino, G.; Tampieri, A. Novel osteointegrative Sr-substituted apatitic cements enriched with alginate. Materials 2016, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Besleaga, C.; Nan, B.; Popa, A.-C.; Balescu, L.M.; Nedelcu, L.; Neto, A.S.; Pasuk, I.; Leonat, L.; Popescu-Pelin, G.; Ferreira, J.M. Sr and Mg Doped Bi-Phasic Calcium Phosphate Macroporous Bone Graft Substitutes Fabricated by Robocasting: A Structural and Cytocompatibility Assessment. J. Funct. Biomater. 2022, 13, 123. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Ai, F.; Yan, J.; Zhou, K.J.J. Fabrication and characterization of Sr-doped hydroxyapatite porous scaffold. JOM 2021, 73, 1745–1753. [Google Scholar] [CrossRef]
- Hu, B.; Meng, Z.-D.; Zhang, Y.-Q.; Ye, L.-Y.; Wang, C.-J.; Guo, W.-C. Cell, Sr-HA scaffolds fabricated by SPS technology promote the repair of segmental bone defects. Tissue Cell 2020, 66, 101386. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, S.; Zhao, W.; Yang, L.; Yuan, B.; Ioan, V.S.; Iulian, A.V.; Yang, X.; Zhu, X.; Zhang, X.J.T. A bioceramic scaffold composed of strontium-doped three-dimensional hydroxyapatite whiskers for enhanced bone regeneration in osteoporotic defects. Theranostics 2020, 10, 1572. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Dapporto, M.; Tampieri, A.; Sprio, S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PLoS ONE 2017, 12, e0172100. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Avanzi, I.R.; Parisi, J.R.; Souza, A.; Cruz, M.A.; Martignago, C.C.S.; Ribeiro, D.A.; Braga, A.R.C.; Renno, A.C. 3D-printed hydroxyapatite scaffolds for bone tissue engineering: A systematic review in experimental animal studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 203–219. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Chen, S.; Xu, Z.; Wang, Q.; Yuan, P.; Zhou, Y.; Zhang, Y.; Chen, J. Heparan sulfate loaded polycaprolactone-hydroxyapatite scaffolds with 3D printing for bone defect repair. Int. J. Biol. Macromol. 2020, 148, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gardini, D.; Galassi, C.; Lapasin, R. Rheology of hydroxyapatite dispersions. J. Am. Ceram. Soc. 2005, 88, 271–276. [Google Scholar] [CrossRef]
- Amin, A.M.; Besisa, D.H.; El-Amir, A.A.; Zaki, Z.I.; Ahmed, Y.M. Role of heat treatment of hydroxyapatite powder prior to suspension preparation on the suspension flow behavior. Open Ceram. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Maas, M.; Hess, U.; Rezwan, K. The contribution of rheology for designing hydroxyapatite biomaterials. Curr. Opin. Colloid Interface Sci. 2014, 19, 585–593. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Zhang, H.; Liu, X.; Wei, Q.; Li, M.; Liu, Z.; Bao, C.; Zhang, K. Effects of dispersant concentration on the properties of hydroxyapatite slurry and scaffold fabricated by digital light processing. J. Manuf. Process. 2024, 109, 460–470. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Zhang, H.; Wei, Q.; Liu, Z.; Liu, X. Influence of particle size distribution on hydroxyapatite slurry and scaffold properties fabricated using digital light processing. J. Manuf. Process. 2024, 131, 401–411. [Google Scholar] [CrossRef]
- Navarrete-Segado, P.; Tourbin, M.; Grossin, D.; Frances, C. Tailoring hydroxyapatite suspensions by stirred bead milling. Ceram. Int. 2022, 48, 24953–24964. [Google Scholar] [CrossRef]
- Sprio, S.; Preti, L.; Montesi, M.; Panseri, S.; Adamiano, A.; Vandini, A.; Pugno, N.M.; Tampieri, A. Surface phenomena enhancing the antibacterial and osteogenic ability of nanocrystalline hydroxyapatite, activated by multiple-ion doping. ACS Biomater. Sci. Eng. 2019, 5, 5947–5959. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lam, W.; Yang, C.; Xu, B.; Ni, G.; Abbah, S.; Cheung, K.; Luk, K.; Lu, W. Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials 2007, 28, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Ignjatović, N.; Škapin, S.; Uskoković, D.P. Germanium-doped hydroxyapatite: Synthesis and characterization of a new substituted apatite. Ceram. Int. 2022, 48, 27693–27702. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Sharma, B.; Afonso, L.; Singh, M.; Soni, U.; Cahill, D. Zinc- and magnesium-doped hydroxyapatite-urea nanohybrids enhance wheat growth and nitrogen uptake. Sci. Rep. 2022, 12, 19506. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, S.; Basu, B.; Gupta, R. Conductivity Studies of Silver-, Potassium-, and Magnesium-Doped Hydroxyapatite. Int. J. Appl. Ceram. Technol 2015, 12, 319–328. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Pan, H.; Darvell, B.W. The effect of excess phosphate on the solubility of hydroxyapatite. Ceram. Int. 2014, 40, 2751–2761. [Google Scholar] [CrossRef]
- Wingender, B.; Azuma, M.; Krywka, C.; Zaslansky, P.; Boyle, J.; Deymier, A. Carbonate substitution significantly affects the structure and mechanics of carbonated apatites. Acta Biomater. 2021, 122, 377–386. [Google Scholar] [CrossRef]
- Mahanty, A.; Shikha, D. Microstructural, biocompatibility and mechanical investigation of MgHAp and AgHAp: Comparative report. J. Mater. Sci. Mater. Med. 2023, 34, 22. [Google Scholar] [CrossRef]
- Lala, S.; Ghosh, M.; Das, P.K.; Das, D.; Kar, T.; Pradhan, S.K. Magnesium substitution in carbonated hydroxyapatite: Structural and microstructural characterization by Rietveld’s refinement. Mater. Chem. Phys. 2016, 170, 319–329. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Y.; Yin, H.; Ruan, Y.; Sun, Y.; Lin, K. Effects of strontium substitution on the structural distortion of hydroxyapatite by rietveld refinement and Raman Spectroscopy. Ceram. Int. 2019, 45, 11073–11078. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhang, Y.; Liu, X.; Chen, J.; Zhang, Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 178, 222–229. [Google Scholar] [CrossRef]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koc, M.J.A.M. Vat photopolymerization of polymers and polymer composites: Processes and applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
- Shah, M.; Ullah, A.; Azher, K.; Rehman, A.U.; Juan, W.; Aktürk, N.; Tüfekci, C.S.; Salamci, M.U. Vat photopolymerization-based 3D printing of polymer nanocomposites: Current trends and applications. RSC Adv. 2023, 13, 1456–1496. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yan, Y.; Yan, H.; Liu, C.; Li, P.; Dong, P.; Zhao, Y.; Chen, J. 3D printing of hydroxyapatite scaffolds with good mechanical and biocompatible properties by digital light processing. J. Mater. Sci. 2018, 53, 6291–6301. [Google Scholar] [CrossRef]
- Mohammadi, M.; Coppola, B.; Montanaro, L.; Palmero, P. Digital light processing of high-strength hydroxyapatite ceramics: Role of particle size and printing parameters on microstructural defects and mechanical properties. J. Eur. Ceram. Soc. 2023, 43, 2761–2772. [Google Scholar] [CrossRef]
- Guo, W.; Li, B.; Li, P.; Zhao, L.; You, H.; Long, Y. Review on vat photopolymerization additive manufacturing of bioactive ceramic bone scaffolds. J. Mater. Chem. B 2023, 11, 9572–9596. [Google Scholar] [CrossRef]
- Zavřel, F.; Novák, M.; Kroupová, J.; Beveridge, C.; Štěpánek, F.; Ruphuy, G. Development of Hot-Melt Extrusion Method to Produce Hydroxyapatite/Polycaprolactone Composite Filaments. Adv. Eng. Mater. 2022, 24, 2100820. [Google Scholar] [CrossRef]
- Ramli, M.; Sulong, A.; Muhamad, N.; Muchtar, A.; Arifin, A. Stainless steel 316L–hydroxyapatite composite via powder injection moulding: Rheological and mechanical properties characterisation. Mater. Res. Innov. 2014, 18, S6-100–S6-104. [Google Scholar] [CrossRef]
- Ibrahim, D.M.; Mostafa, A.A.; Korowash, S.I. Chemical characterization of some substituted hydroxyapatites. Chem. Cent. J. 2011, 5, 74. [Google Scholar] [CrossRef]
- Kosmulski, M. Confirmation of the Differentiating Effect of Small Cations in the Shift of the Isoelectric Point of Oxides at High Ionic Strengths. Langmuir 2002, 18, 785–787. [Google Scholar] [CrossRef]
- Kolev, S.K.; St. Petkov, P.; Milenov, T.I.; Vayssilov, G.N. Sodium and Magnesium Ion Location at the Backbone and at the Nucleobase of RNA: Ab Initio Molecular Dynamics in Water Solution. ACS Omega 2022, 7, 23234–23244. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed]
- Whittingstall, P. Controlled stress rheometry as a tool to measure grease structure and yield at various temperatures. NLGI Spokesm. 1997, 61, 12. [Google Scholar]
- Semancik, J.R. Yield Stress Measurements using Controlled Stress Rheometry; TA Instruments Publication: New Castle, DE, USA, 1997; Available online: https://www.tainstruments.com/pdf/literature/RH058.pdf (accessed on 24 February 2025).
- Hu, C.; Chen, Y.; Yang, T.; Liu, H.; Huang, X.; Huo, Y.; Jia, Z.; Wang, H.; Hu, L.; Sun, H.; et al. Effect of SiC powder on the properties of SiC slurry for stereolithography. Ceram. Int. 2021, 47, 12442–12449. [Google Scholar] [CrossRef]
- Prasad, V.; Mehrotra, S.P.; Thareja, P.J.C.; Materials, B. Rheological characteristics of concentrated Indian coal ash slurries and flow through pipelines. Constr. Build. Mater. 2022, 361, 129624. [Google Scholar] [CrossRef]
- Rivas-Barbosa, R.; Escobedo-Sánchez, M.A.; Tassieri, M.; Laurati, M. i-Rheo: Determining the linear viscoelastic moduli of colloidal dispersions from step-stress measurements. Phys. Chem. Chem. Phys. 2020, 22, 3839–3848. [Google Scholar] [CrossRef]
- Chen, T. Rheological Techniques for Yield Stress Analysis; TA Instruments: New Castle, DE, USA, 2000; Volume 28, Available online: https://www.tainstruments.com/pdf/literature/RH025.pdf (accessed on 24 February 2025).
- Feilden, E. Additive Manufacturing of Ceramics and Ceramic Composites via Robocasting. Doctoral Dissertation, Imperial College London, London, UK, 2017. [Google Scholar]
- Melk, L.; Mouzon, J.; Turon, M.; Akhtar, F.; Antti, M.-L.; Anglada, M. Surface microstructural changes of spark plasma sintered zirconia after grinding and annealing. Ceram. Int. 2016, 42, 15610–15617. [Google Scholar] [CrossRef]
- Martins, S.B.; Abi-Rached, F.d.O.; Adabo, G.L.; Baldissara, P.; Fonseca, R.G. Influence of Particle and Air-Abrasion Moment on Y-TZP Surface Characterization and Bond Strength. J. Prosthodont. 2019, 28, e271–e278. [Google Scholar] [CrossRef]
- Yarahmadi, M.; Barcelona, P.; Fargas, G.; Xuriguera, E.; Roa, J. Optimization of the ceramic ink used in Direct Ink Writing through rheological properties characterization of zirconia-based ceramic materials. Ceram. Int. 2022, 48, 4775–4781. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Derkach, S.R.; Kulichikhin, V.G. Rheology of Gels and Yielding Liquids. Gels 2023, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef]
- dos Santos, V.I.; Chevalier, J.; Fredel, M.C.; Henriques, B.; Gremillard, L. Ceramics and ceramic composites for biomedical engineering applications via Direct Ink Writing: Overall scenario, advances in the improvement of mechanical and biological properties and innovations. Mater. Sci. Eng. R Rep. 2024, 161, 100841. [Google Scholar] [CrossRef]
- Altıparmak, S.C.; Yardley, V.A.; Shi, Z.; Lin, J. Extrusion-based additive manufacturing technologies: State of the art and future perspectives. J. Manuf. Process. 2022, 83, 607–636. [Google Scholar] [CrossRef]
- Rane, K.; Strano, M. A comprehensive review of extrusion-based additive manufacturing processes for rapid production of metallic and ceramic parts. Adv. Manuf. 2019, 7, 155–173. [Google Scholar] [CrossRef]
- Ravoor, J.; Elsen, S.R.; Thangavel, M.; Arumugam, D.; Karuppan, D. Development of hybrid multi-head, multi-material paste and ink extrusion type 3D printer for biomedical applications. J. Asian Ceram. Soc. 2023, 11, 437–450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).