Physicochemical Characterization and Biodegradability of Nanostructured Chitosan-Based Films Reinforced with Orange Waste
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Chitosan NPs TEM, DLS, and Z Potential Characterization
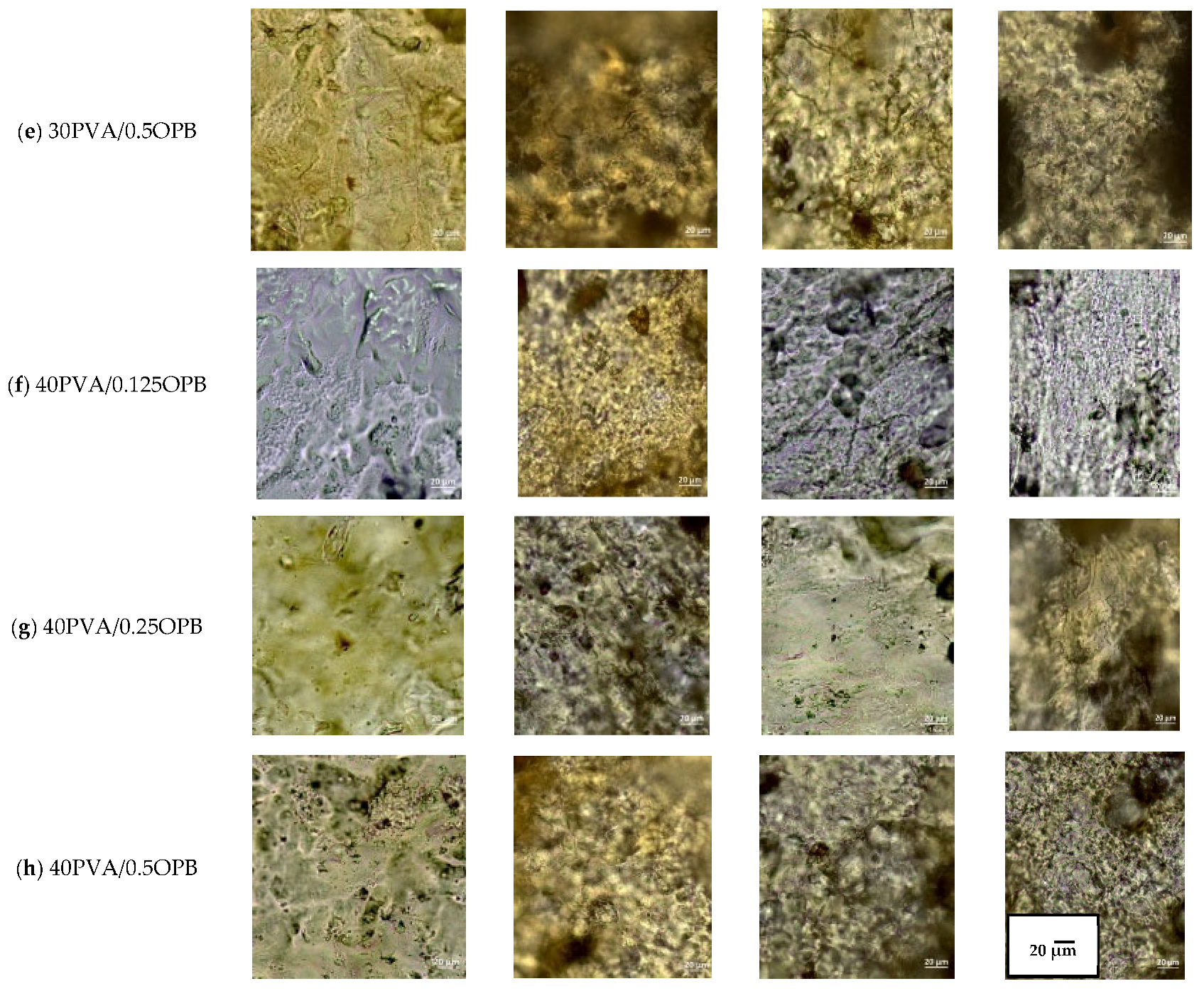
3.2. SEM and CLSM Film Characterization
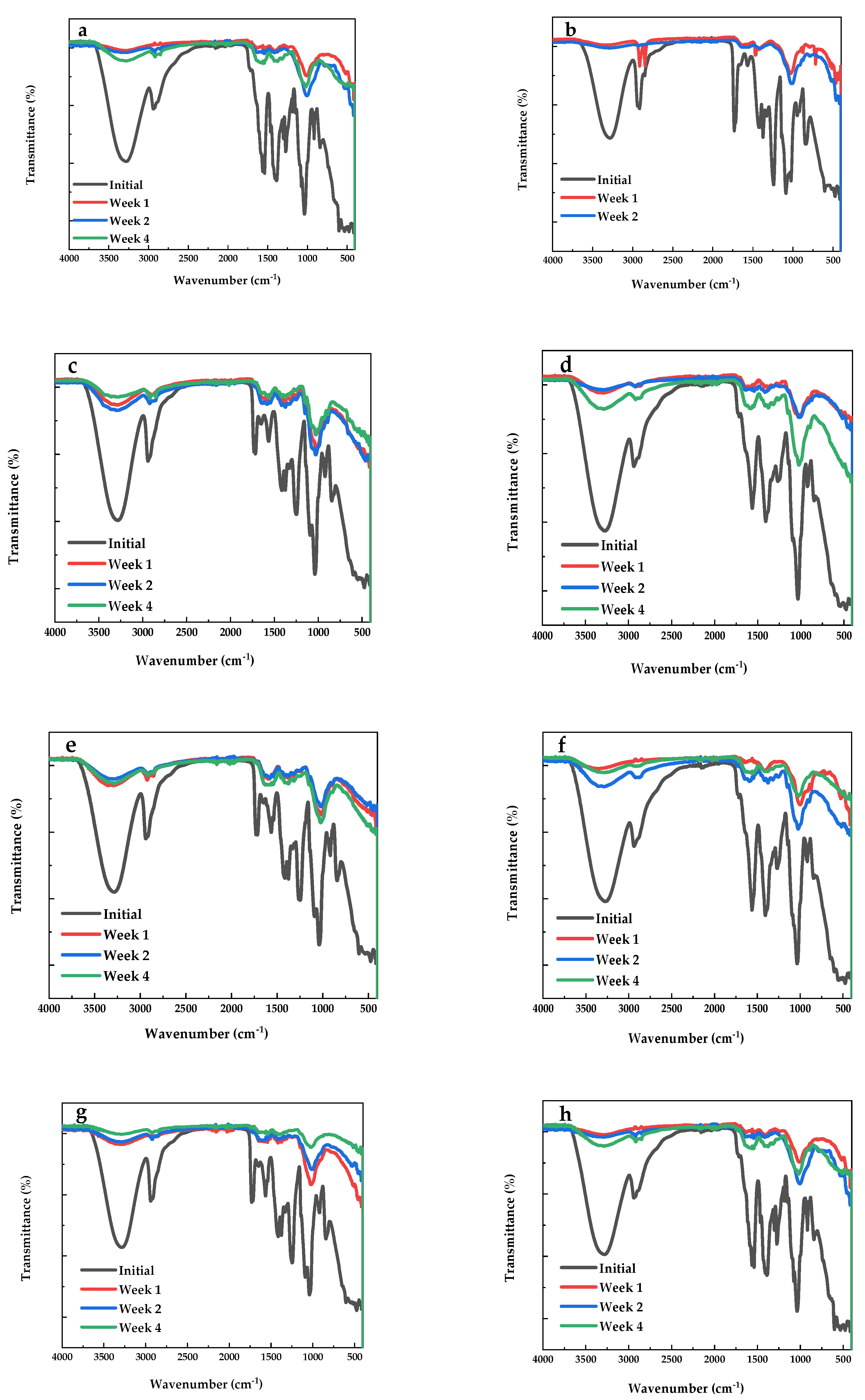
3.3. ATR-FTIR Film Characterization
3.4. MC, WVDC, and Mechanical Properties
3.5. Degradation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hameed, A.Z.; Raj, S.A.; Kandasamy, J.; Baghdadi, M.A.; Shahzad, M.A. Chitosan: A Sustainable Material for Multifarious Applications. Polymers 2022, 14, 2335. [Google Scholar] [CrossRef]
- Harutyunyan, L.R.; Lasareva, E.V. Chitosan and its derivatives: A step towards green chemistry. Biointerface Res. Appl. Chem. 2023, 13, 578. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Tang, Q.; Qiu, P.; Gou, D.; Zhao, J. Chitosan-based materials: An overview of potential applications in food packaging. Foods 2022, 11, 1490. [Google Scholar] [CrossRef]
- Dirpan, A.; Ainani, A.F.; Djalal, M. A review on biopolymer-based biodegradable film for food packaging: Trends over the last decade and future research. Polymers 2023, 15, 2781. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Güney, F.; Parın, F.N.; Şen, I.; Tuna, S. Biowaste orange peel incorporated chitosan/polyvinyl alcohol composite films for food packaging applications. Food Packag. Shelf Life 2021, 30, 100742. [Google Scholar] [CrossRef]
- Zhang, W.; Khan, A.; Ezati, P.; Priyadarshi, R.; Sani, M.A.; Rathod, N.B.; Goksen, G.; Rhim, J.W. Advances in sustainable food packaging applications of chitosan/polyvinyl alcohol blend films. Food Chem. 2024, 443, 138506. [Google Scholar] [CrossRef]
- Khezerlou, A.; Tavassoli, M.; Alizadeh Sani, M.; Mohammadi, K.; Ehsani, A.; McClements, D.J. Application of nanotechnology to improve the performance of biodegradable biopolymer-based packaging materials. Polymers 2021, 13, 4399. [Google Scholar] [CrossRef]
- Gutiérrez-Molina, J.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Barrera-Necha, L.L.; Bautista-Baños, S.; Correa-Pacheco, Z.N. Chitosan and Byrsonima crassifolia-based nanostructured coatings: Characterization and effect on tomato preservation during refrigerated storage. Food Biosci. 2021, 42, 101212. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Bautista-Baños, S.; Ventura-Aguilar, R.I. Application of natural-based nanocoatings for extending the shelf life of green bell pepper fruit. J. Food Biosci. 2021, 86, 95–102. [Google Scholar] [CrossRef]
- Istúriz-Zapata, M.A.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Acosta-Rodríguez, J.L.; Hernández-López, M.; Barrera-Necha, L.L. Efficacy of extracts of mango residues loaded in chitosan nanoparticles and their nanocoatings on in vitro and in vivo postharvest fungal. J. Phytopathol. 2022, 170, 661–674. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Guo, Z.; Feng, X.; Huang, P.; Du, M.; Zalán, Z.; Kan, J. Distribution and natural variation of free, esterified, glycosylated, and insoluble-bound phenolic compounds in brocade orange (Citrus sinensis L. Osbeck) peel. Food Res. Int. 2022, 153, 110958. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.R.; Montero, G.; Campbell, H.E.; García, C.; Coronado, M.A.; León, J.A.; Sagaste, C.A.; Pérez, L.J. Extraction and characterization of orange peel essential oil from Mexico and United States of America. J. Essent. Oil Bear. Plants 2017, 20, 897–914. [Google Scholar] [CrossRef]
- Galindo-Segura, L.A.; Pérez-Vázquez, A.; Ramírez-Martínez, A.; López-Romero, G.; Gómez-Merino, F.C. El Manejo del Bagazo de Naranja en la Zona Centro del Estado de Veracruz. Terra Lat. 2023, 41, 1–8. [Google Scholar] [CrossRef]
- De Medina-Salas, L.; Giraldi-Díaz, M.R.; Castillo-González, E.; Morales-Mendoza, L.E. Valorization of orange peel waste using precomposting and vermicomposting processes. Sustainability 2020, 12, 7626. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Sajjan, A.M.; Kamat, S.; Mujtaba, M.A.; Shettar, A.S.; Anqi, A.E.; Safaei, M.R.; et al. Bio-based material from fruit waste of orange peel for industrial applications. J. Mater. Res. Technol. 2022, 17, 3186–3197. [Google Scholar] [CrossRef]
- Yi, V.N.W.; Huey, N.C.; Peng, T.Y.; Xian, O.Z.; Hoong, S.S. Isolation of cellulose derived from orange peel and its application in biodegradable films. Cellul. Chem. Technol. 2021, 55, 311–324. [Google Scholar] [CrossRef]
- Rathinavel, S.; Saravanakumar, S.S. Development and analysis of poly vinyl alcohol/orange peel powder biocomposite films. J. Nat. Fibers 2021, 18, 2045–2054. [Google Scholar] [CrossRef]
- Sánchez-Orozco, R.; Timoteo-Cruz, B.; García- Sánchez, J.J.; Gomez-Espinosa, R.M.; Bernal-Martínez, L.A.; Torres-Blancas, T. Properties of eco-friendly orange peel-alginate-glycerol bioplastic films as potential food packaging applications. J. Macromol. Sci. Part A 2024, 61, 528–540. [Google Scholar] [CrossRef]
- Taghavi Kevij, H.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Mechanical, physical, and bio-functional properties of biopolymer films based on gelatin as affected by enriching with orange peel powder. Polym. Bull. 2021, 78, 4387–4402. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Hernández-López, M.; Tapia-Maruri, D.; Jiménez-Pérez, J.L.; Ortega-Gudiño, P.; Cruz-Miranda, L. Characterization of nanostructured chitosan-PVA films and their effects on blueberries during storage. Future Foods 2025, 11, 100571. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Jiménez-Pérez, J.L.; Cruz-Orea, A.; Fonseca-García, A.; López-Gamboa, G.; Olvera-Cano, L.I. Morphological, optical and thermal properties of bioactive-chitosan nanostructured edible films for food packaging applications. Food Biophys. 2024, 19, 207–218. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.; Moerschbacher, B.; Rivera-Rodriguez, G. Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef] [PubMed]
- Athavale, R.; Sapre, N.; Rale, V.; Tongaonkar, S.; Manna, G.; Kulkarni, A.; Shirolkar, M.M. Tuning the surface charge properties of chitosan nanoparticles. Mat. Lett. 2022, 308, 131114. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by design-driven Zeta potential optimisation study of liposomes with charge imparting membrane additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Karayianni, M.; Sentoukas, T.; Skandalis, A.; Pippa, N.; Pispas, S. Chitosan-based nanoparticles for nucleic acid delivery: Technological aspects, applications and future perspectives. Pharmaceutics 2023, 15, 1849. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant protein-based emulsifiers: Mechanisms, techniques for emulsification enhancement and applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Wan, Q.; Thompson, B.C. Control of properties through hydrogen bonding interactions in conjugated polymers. Adv. Sci. 2024, 11, 2305356. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Sitohy, M.; Alkafaas, S.S.; Dladla, M.; Ghosh, S.; Mohammed, D.M.; Solima, T.N.; Ibrahim, E.H.; Fahmy, M.A.; et al. Chitosan nanoparticles: Green synthesis, biological activities, and sustainable frontiers in targeted drug delivery and cancer nanomedicine—A comprehensive review. Mater. Today Bio 2025, 35, 102358. [Google Scholar] [CrossRef]
- Aycan, D.; Yayla, N.A.; Aydin, Y.A. Chitosan polyvinyl alcohol blend films for ibuprofen encapsulation: Fabrication, characterization and kinetics. Polym. Degrad. Stab. 2020, 181, 109346. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Li, J.; Wang, L.; Song, X. Extraction and characterization of pectin from Jerusalem artichoke residue and its application in blueberry preservation. Coatings 2022, 12, 385. [Google Scholar] [CrossRef]
- Naik, P.; Pradhan, S.; Acharya, S.K.; Sahoo, P. Effect of carbonization of orange peel particulate-reinforced polymer composites: Mechanical and morphological properties. Int. J. Surf. Eng. Interdiscip. Mater. Sci. 2022, 10, 1–20. [Google Scholar] [CrossRef]
- Tabatabaeian, A.; Ghasemi, A.R.; Shokrieh, M.M.; Marzbanrad, B.; Baraheni, M.; Fotouhi, M. Residual stress in engineering materials: A review. Adv. Eng. Mater. 2022, 24, 2100786. [Google Scholar] [CrossRef]
- Bellon, J.; Bacoup, F.; Maris, S.; Gattin, R. PLA, PBS, and PBAT biocomposites -Part A: Matrix-filler interactions with agro-industrial waste fillers (brewer’s spent grain, orange peel) and their influence on thermal mechanical and water sorption properties. Materials 2025, 18, 3867. [Google Scholar] [CrossRef]
- Pervin, R.; Ghosh, P.; Basavaraj, M.G. Tailoring pore distribution in polymer films via evaporation induced phase separation. RSC Adv. 2019, 9, 15593. [Google Scholar] [CrossRef]
- Botta, L.; Mistretta, M.C.; Lamattina, G.; Gargano, F.; Liguori, G. Opuntia-ficus indica fruit by-products as fillers for PLA-based biocomposites: A comparison between glochids and peel. Polym. Compos. 2025, 46, 12243–12256. [Google Scholar] [CrossRef]
- Shirazi, R.; Mohammadi, T.; Koupaie, E.H.; De France, K.J. Chemically crosslinked electrospun chitosan/pol(vinyl alcohol) membranes with encapsulated zeolite for organic dye removal. J. Water Process Eng. 2025, 77, 108413. [Google Scholar] [CrossRef]
- Yamala, A.; Pandit, R.; Kanparthi, R.K.; Katti, P.; Vallabhapurapu, S.; Pujala, R.K. Physically crosslinked poly (methacrylic acid-co-acrylamide)/gelatin chitosan (poly-MAGC) interpenetrating polymer network hydrogels for drug delivery and antibacterial activity. Mater. Adv. 2025, 1–16. [Google Scholar] [CrossRef]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef]
- Fang, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel forwound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar]
- Ali, M.; Gherissi, A. Synthesis and characterization of the composite material PVA/chitosan/5% sorbitol with different ratio of chitosan. Int. J. Mech. Mechatron. Eng. 2017, 17, 15–28. [Google Scholar]
- Abdelghany, A.M.; Menazea, A.A.; Ismail, A.M. Synthesis, characterization and antimicrobial activity of chitosan/polyvinyl alcohol blend doped with Hibiscus sabdariffa L. extract. J. Mol. Struct. 2019, 1197, 603–609. [Google Scholar] [CrossRef]
- Olvera Bernal, R.A.; Olekhnovich, R.O.; Uspenskaya, M.V. Chitosan/PVA nanofibers as potential material for the development of soft actuators. Polymers 2023, 15, 2037. [Google Scholar] [CrossRef] [PubMed]
- Raksa, A.; Utke, R.; Ruksakulpiwat, C.; Numpaisal, P.; Ruksakulpiwat, Y. Morphological and chemical characterization of electrospun silk fibroin/polyvinyl alcohol nanofibers. AIP Conf. Proc. 2020, 2279, 080004. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Aspanut, Z.; Majid, S.R.; Arof, A.K. FTIR studies of plasticized poly(vinyl alcohol)–chitosan blend doped with NH4NO3 polymer electrolyte membrane. Spectrochim. Acta A 2011, 78, 1068–1074. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.; Zhang, Y.; Wang, X.; Wang, J.; Ning, Z.; Ruan, Q.; Zhang, Y. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef]
- Alfuraydi, R.T.; Alminderej, F.M.; Mohamed, N.A. Evaluation of antimicrobial and anti-biofilm formation activities of novel poly(vinyl alcohol) hydrogels reinforced with crosslinked chitosan and silver nano-particles. Polymers 2022, 14, 1619. [Google Scholar] [CrossRef]
- Alfuraydi, R.T.; Al-Harby, N.F.; Alminderej, F.M.; Elmehbad, N.Y.; Mohamed, N.A. Poly (vinyl alcohol) hydrogels boosted with cross-linked chitosan and silver nanoparticles for efficient adsorption of congo red and crystal violet dyes. Gels 2023, 9, 882. [Google Scholar] [CrossRef]
- Chetouani, A.; Elkolli, M.; Bounekhel, M.; Benachour, D. Chitosan/oxidized pectin/PVA blend film: Mechanical and biological properties. Polym. Bull. 2017, 74, 4297–4310. [Google Scholar] [CrossRef]
- Cheng, Y.; Far, B.F.; Jahanbakhshi, M.; Bahrami, S.; Tamimi, P.; Sedaghatf, M.; Ghazizadehagh, E. Exploring the potential of a polyvinyl alcohol/chitosan-based nanofibrous matrix for erythromycin delivery: Fabrication, in vitro and in vivo evaluation. RSC Adv. 2023, 13, 18450. [Google Scholar] [CrossRef]
- Machado, M.I.R.; Ângelo, P.; Goncalves, Â.; Monção, R.M.; de Souza, R.R.M.; Machado, A.R. Characterization of biodegradable films applicable to agriculture with structural reinforcement. Eng. Proc. 2024, 67, 38. [Google Scholar]
- Wang, F.; Han, S.; Zhang, Y.; Gao, L.; Li, X.; Zhao, L.; Ye, H.; Li, H.; Xin, Q.; Zhang, Y. Constructing rapid water vapor transport channels within mixed matrix membranes based on two-dimensional mesoporous nanosheets. Commun. Chem. 2022, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Awaja, F.; Zhang, S.; Tripathi, M.; Nikiforov, A.; Pugno, N. Cracks, microcracks and fracture in polymer structures: Formation, detection, automatic repair. Prog. Mater. Sci. 2016, 83, 536–573. [Google Scholar] [CrossRef]
- Olonisakin, K.; Mohanty, A.K.; Thimmanagari, M.; Misra, M. Recent advances in biodegradable polymer blends and their biocomposites: A comprehensive review. Green Chem. 2025, 27, 11656–11704. [Google Scholar] [CrossRef]
- Koutoulis, A.S.; Giannakas, A.E.; Lazaridis, D.G.; Kitsios, A.P.; Karabagias, V.K.; Giannakas, A.E.; Ladavos, A.; Karabagias, I.K. Preparation and characterization of PLA-based films fabricated with different citrus species peel powder. Coatings 2024, 14, 1311. [Google Scholar] [CrossRef]
- Tone, A.M.; Herranz Solana, N.; Khan, M.R.; Borriello, A.; Torrieri, E.; Sánchez Reig, C.; Monedero Prieto, F.M. Study on the properties of PLA and PP-based films for food applications incorporating orange peel extract from agricultural by-products. Polymers 2024, 16, 1245. [Google Scholar] [CrossRef]
- Khieng, T.K.; Debnath, S.; Ting Chaw Liang, E.; Anwar, M.; Pramanik, A.; Basak, A.K. A Review on Mechanical Properties of Natural Fibre Reinforced Polymer Composites under Various Strain Rates. J. Compos. Sci. 2021, 5, 130. [Google Scholar] [CrossRef]
- Sathiya Narayanan, N.; Sai Venkat Mohan, D.; Abhinay, J.; Dinesh, T.; Satya Sai Surya Teja, V.; Praneeth, R. Effects on microhardness, tensile strength, deflection, and drop weight impact resistance with the addition of hybrid filler materials for enhancing GFRP composites. Sci. Rep. 2024, 14, 27524. [Google Scholar] [CrossRef]
- Moreno, J.; López-González, J.A.; Arcos-Nievas, M.A.; Suárez-Estrella, F.; Jurado, M.M.; Estrella-González, M.J.; López, M.J. Revisiting the succession of microbial populations throughout composting: A matter of thermotolerance. Sci. Total Environ. 2021, 773, 145587. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of orange peel waste and valorization to obtain reducing sugars. Molecules 2021, 26, 1348. [Google Scholar] [CrossRef]
- Sambudi, N.S.; Lin, W.Y.; Harun, N.Y.; Mutiari, D. Modification of poly(lactic acid) with orange peel powder as biodegradable composite. Polymers 2022, 14, 4126. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2023, 80, 7247–7312. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.S.; El Demerdash, A.M.; Sadik, W.A.; Kasaby, M.A. Enhancing the biodegradability, water solubility, and thermal properties of polyvinyl alcohol through natural polymer blending: An approach toward sustainable polymer applications. Polymers 2024, 16, 2141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Lan, W. Fabrication of antibacterial chitosan-PVA blended film using electrospray technique for food packaging applications. Int. J. Biol. Macromol. 2018, 107, 848–854. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Pinem, M.P.; Susilo, S.; Siom, B.J.; Sudrajad, A.; Pramono, A.; Meliana, Y.; Guénin, E. Modification of physio-mechanical properties of chitosan-based films via physical treatment approach. Polymers 2022, 14, 5216. [Google Scholar] [CrossRef]
- Altun, E.; Çelik, E.; Ersan, H.Y. Tailoring the Microbial Community for Improving the Biodegradation of Chitosan Films in Composting Environment. J. Polym. Environ. 2020, 28, 1548–1559. [Google Scholar] [CrossRef]
- Mahmud, M.; Talip, N.; Yacob, N.; Idris, S. Application interval and concentration effect of gamma degraded chitosan on mulberry plant. Food Res. 2023, 7, 94–100. [Google Scholar] [CrossRef]
- Westlake, J.R.; Chaloner, E.; Laabei, M.; Sgouridis, F.; Burrows, A.D.; Xie, M. Degradation investigation and active packaging performance of cross-linked chitosan film containing gallic acid. RSC Sustain. 2025, 3, 2680–2695. [Google Scholar] [CrossRef]
- Oberlintner, A.; Bajić, M.; Kalčíková, G.; Likozar, B.; Novak, U. Biodegradability study of active chitosan biopolymer films enriched with Quercus polyphenol extract in different soil types. Environ. Technol. Innov. 2021, 21, 101318. [Google Scholar] [CrossRef]
- Pantelic, B.; Ponjavic, M.; Jankovic, V.; Aleksic, I.; Stevanovic, S.; Murray, J.; Fournet, M.B.; Nikodinovic-Runic, J. Upcycling biodegradable PVA/starch film to a bacterial biopigment and biopolymer. Polymers 2021, 13, 3692. [Google Scholar] [CrossRef]
- Chin, K.; Sam, S.T.; Ong, H.L.; Wong, Y.S.; Tan, W.K. Biodegradation improvement of bioinspired crosslinked and noncrosslinked polyvinyl alcohol nanocomposites with cellulose nanocrystals extracted from rice straw through natural soil burial exposure. Polym. Compos. 2022, 43, 6955–6965. [Google Scholar] [CrossRef]
- Gao, Q.; Guo, L.; Li, S.; Wu, W.; Ding, J.; Xu, H.; Luo, C.; Li, J.; Li, D.; Liu, Z. Biodegradation mechanism of cellulose, hemicellulose, and lignin in bacteria-dominant aerobic composting from agricultural biomass waste: A review. Carbohydr. Polym. Tech. Appl. 2025, 11, 100879. [Google Scholar]






| Formulation | Components (%) | |||||
|---|---|---|---|---|---|---|
| PVA | NP | OPB | CS | Gly | CA | |
| CSNP | - | 33 | - | 67 | - | - |
| PVA | 100 | - | - | - | - | - |
| 30PVA/0.125OPB | 30 | 33 | 0.125 | 34.875 | 1 | 1 |
| 30PVA/0.25OPB | 30 | 33 | 0.250 | 34.750 | 1 | 1 |
| 30PVA/0.5OPB | 30 | 33 | 0.500 | 34.500 | 1 | 1 |
| 40PVA/0.125OPB | 40 | 33 | 0.125 | 24.875 | 1 | 1 |
| 40PVA/0.25OPB | 40 | 33 | 0.250 | 24.750 | 1 | 1 |
| 40PVA/0.5OPB | 40 | 33 | 0.500 | 24.500 | 1 | 1 |
| Film | MC (%) * | WVDC (×10−8 cm2/s) * | E (MPa) * | σ (MPa) * | ɛ (%) * |
|---|---|---|---|---|---|
| CSNP | 30 ± 2 c | 0.152 ± 0.020 a | 51.85 ± 8.72 b | 2.88 ± 0.86 b | 6.95 ± 0.88 a |
| PVA | 4 ± 1 a | 23.101 ± 0.804 f | 67.44 ± 12.11 c | 24.68 ± 1.15 c | 124.66 ± 7.75 d |
| 30PVA/0.125OPB | 7 ± 1 bd | 7.668 ± 0.107 d | 11.26 ± 0.29 a | 1.32 ± 0.23 a | 17.37 ± 2.81 bc |
| 30PVA/0.25OPB | 7 ± 1 bd | 8.272 ± 0.268 d | 10.52 ± 1.03 a | 1.21 ± 0.12 a | 11.97 ± 2.88 ab |
| 30PVA/0.5OPB | 7 ± 1 b | 9.654 ± 0.242 e | 17.34 ± 2.54 a | 1.11 ± 0.07 a | 11.50 ± 1.50 ab |
| 40PVA/0.125OPB | 6 ± 1 b | 1.431 ± 0.319 b | 8.84 ± 0.37 a | 1.21 ± 0.26 a | 22.37 ± 4.32 c |
| 40PVA/0.25OPB | 7 ± 2 b | 2.686 ± 0.154 c | 10.86 ± 1.10 a | 0.81 ± 0.15 a | 11.58 ± 1.83 ab |
| 40PVA/0.5OPB | 9 ± 2 d | 3.585 ± 0.249 c | 12.34 ± 0.62 a | 0.72 ± 0.05 a | 9.49 ± 1.04 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa-Pacheco, Z.N.; Bautista-Baños, S.; Ortega-Gudiño, P.; Cisneros-López, E.O.; Tapia-Maruri, D.; Jiménez-Pérez, J.L. Physicochemical Characterization and Biodegradability of Nanostructured Chitosan-Based Films Reinforced with Orange Waste. J. Compos. Sci. 2025, 9, 627. https://doi.org/10.3390/jcs9110627
Correa-Pacheco ZN, Bautista-Baños S, Ortega-Gudiño P, Cisneros-López EO, Tapia-Maruri D, Jiménez-Pérez JL. Physicochemical Characterization and Biodegradability of Nanostructured Chitosan-Based Films Reinforced with Orange Waste. Journal of Composites Science. 2025; 9(11):627. https://doi.org/10.3390/jcs9110627
Chicago/Turabian StyleCorrea-Pacheco, Zormy Nacary, Silvia Bautista-Baños, Pedro Ortega-Gudiño, Erick Omar Cisneros-López, Daniel Tapia-Maruri, and José Luis Jiménez-Pérez. 2025. "Physicochemical Characterization and Biodegradability of Nanostructured Chitosan-Based Films Reinforced with Orange Waste" Journal of Composites Science 9, no. 11: 627. https://doi.org/10.3390/jcs9110627
APA StyleCorrea-Pacheco, Z. N., Bautista-Baños, S., Ortega-Gudiño, P., Cisneros-López, E. O., Tapia-Maruri, D., & Jiménez-Pérez, J. L. (2025). Physicochemical Characterization and Biodegradability of Nanostructured Chitosan-Based Films Reinforced with Orange Waste. Journal of Composites Science, 9(11), 627. https://doi.org/10.3390/jcs9110627







