Composite Membrane Based on Graphene Oxide and Carboxymethylcellulose from Local Kazakh Raw Materials for Possible Applications in Electronic Devices
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
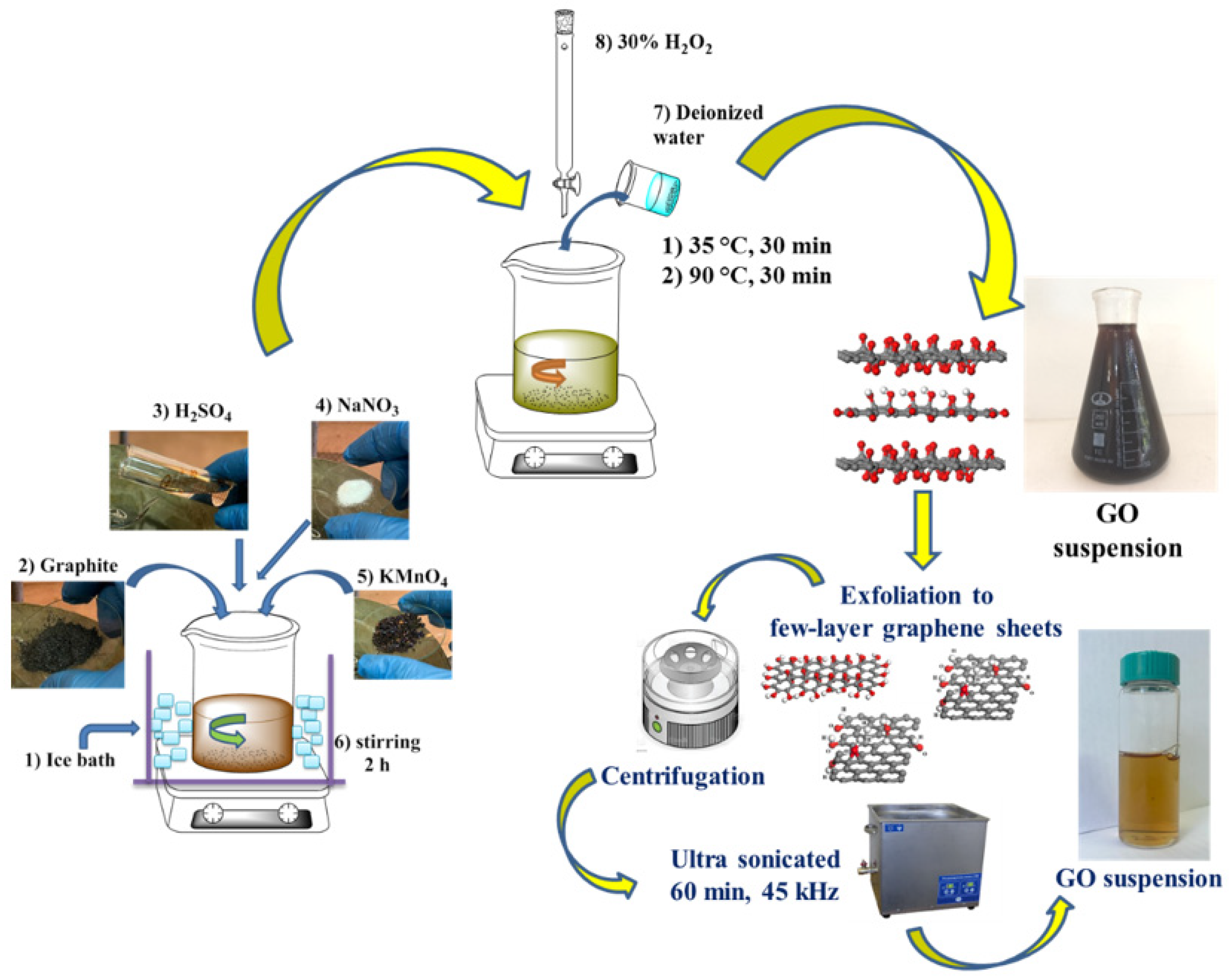
2.2.1. Synthesis of GO
2.2.2. Synthesis of CMC
2.2.3. Synthesis of Composite Membrane Based on GO/CMC
2.2.4. SEM Analysis
2.2.5. FTIR Analysis
2.2.6. X-ray Diffraction
2.2.7. Mechanical Characterization
2.2.8. Electrical Characterization
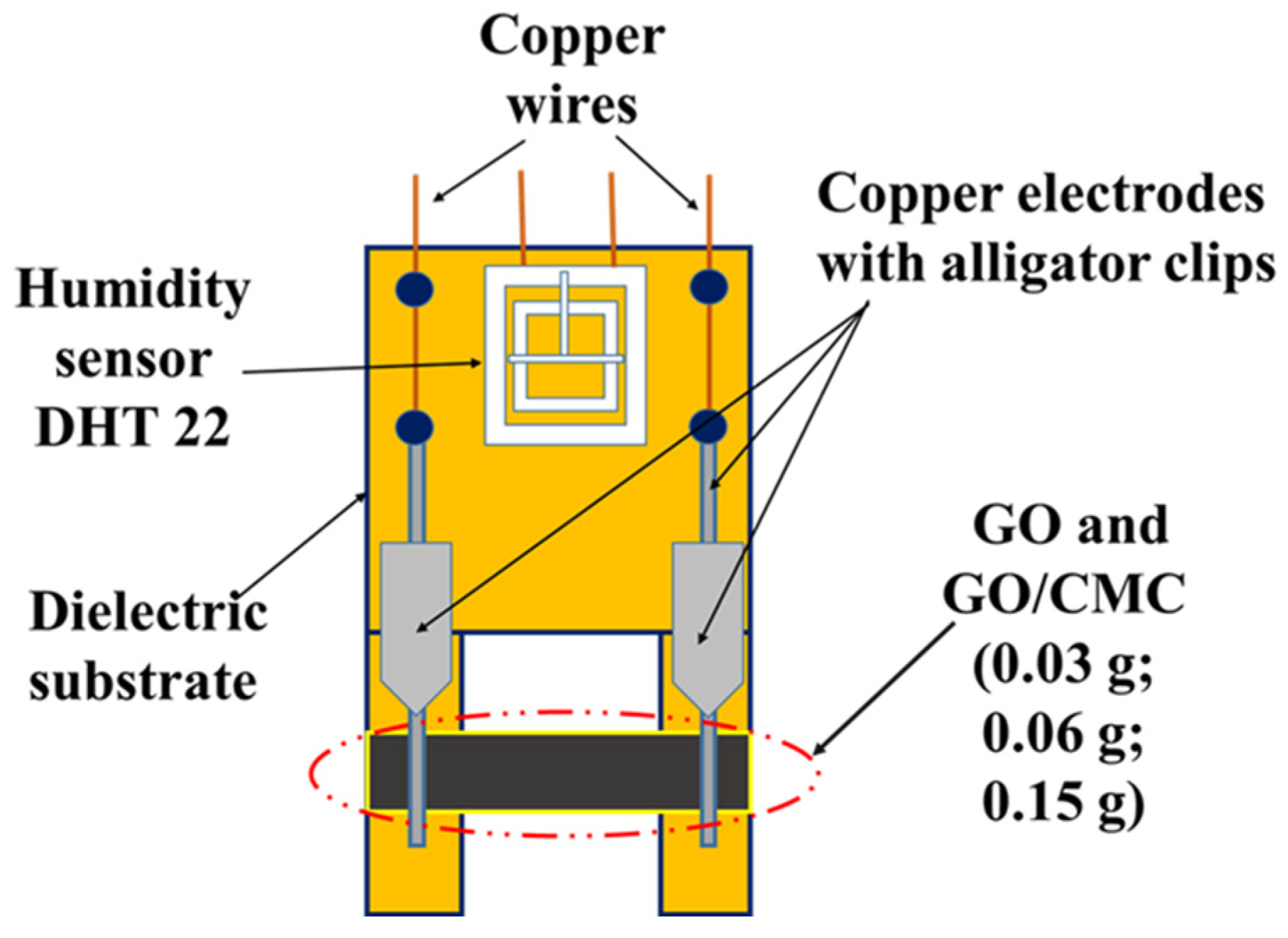
2.2.9. Creation of a Humidity Sensor Based on GO and GO/CMC
3. Results and Discussion
3.1. SEM Analysis
3.2. X-ray Diffraction
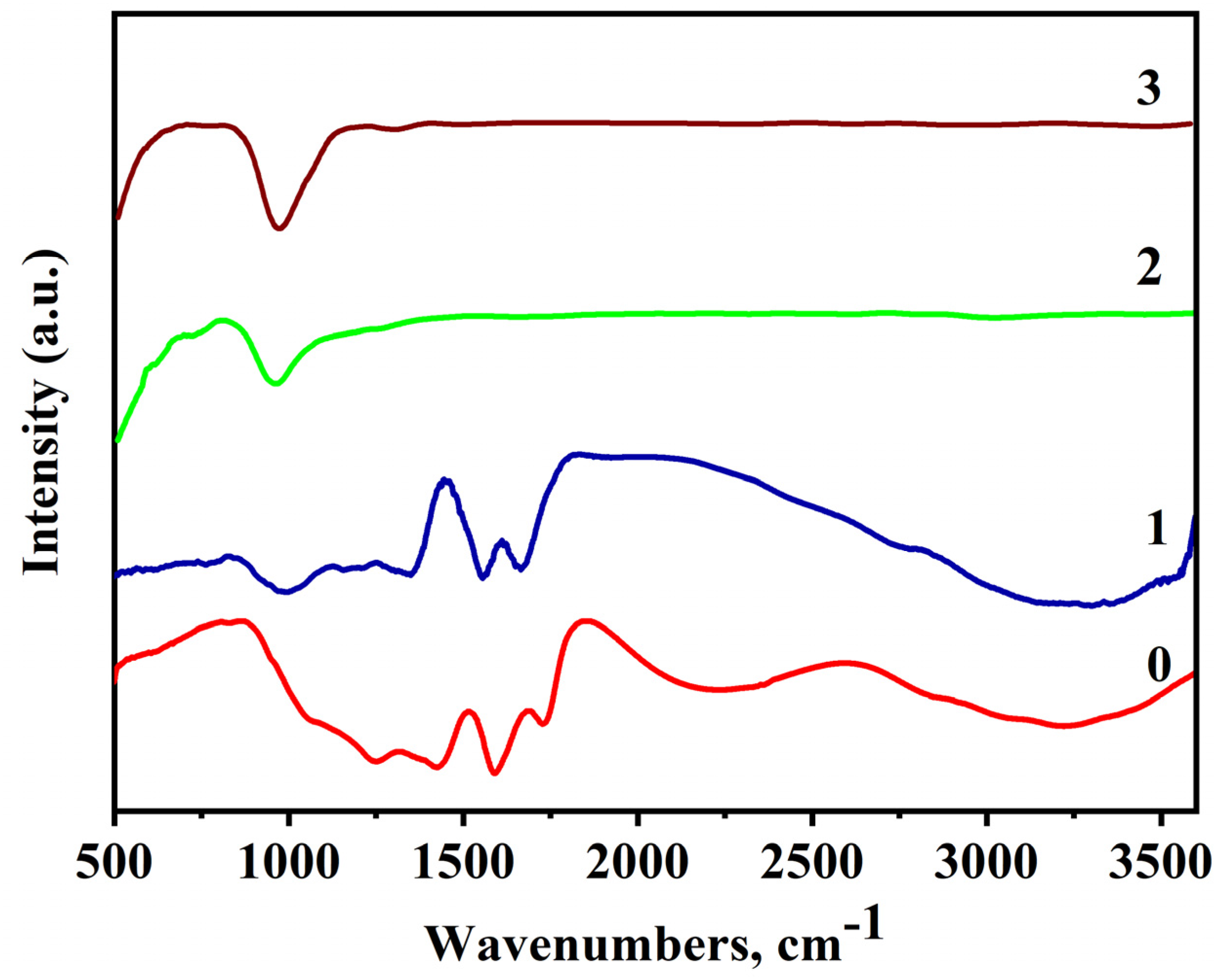
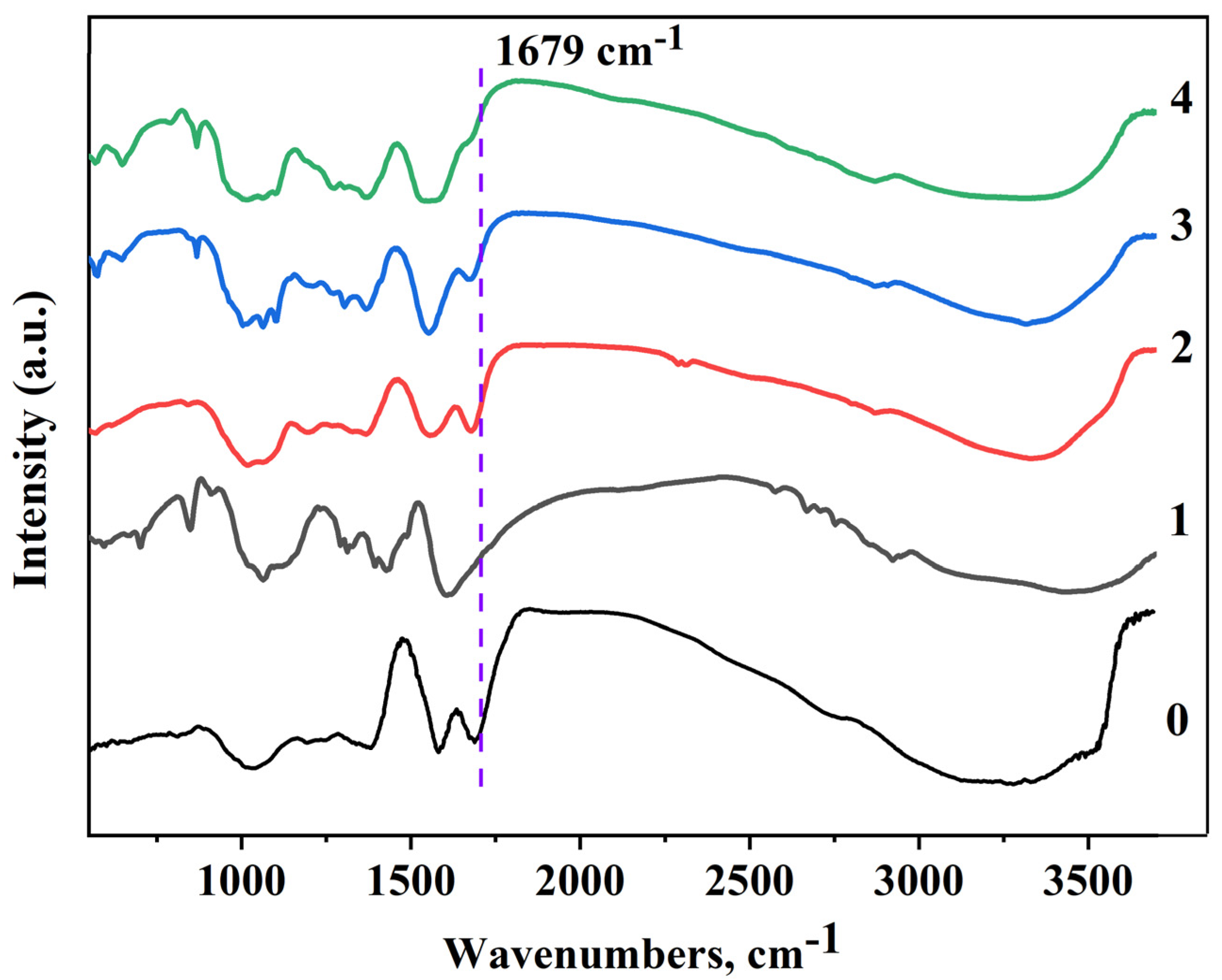
3.3. FTIR Analysis
3.4. Mechanical Characterization
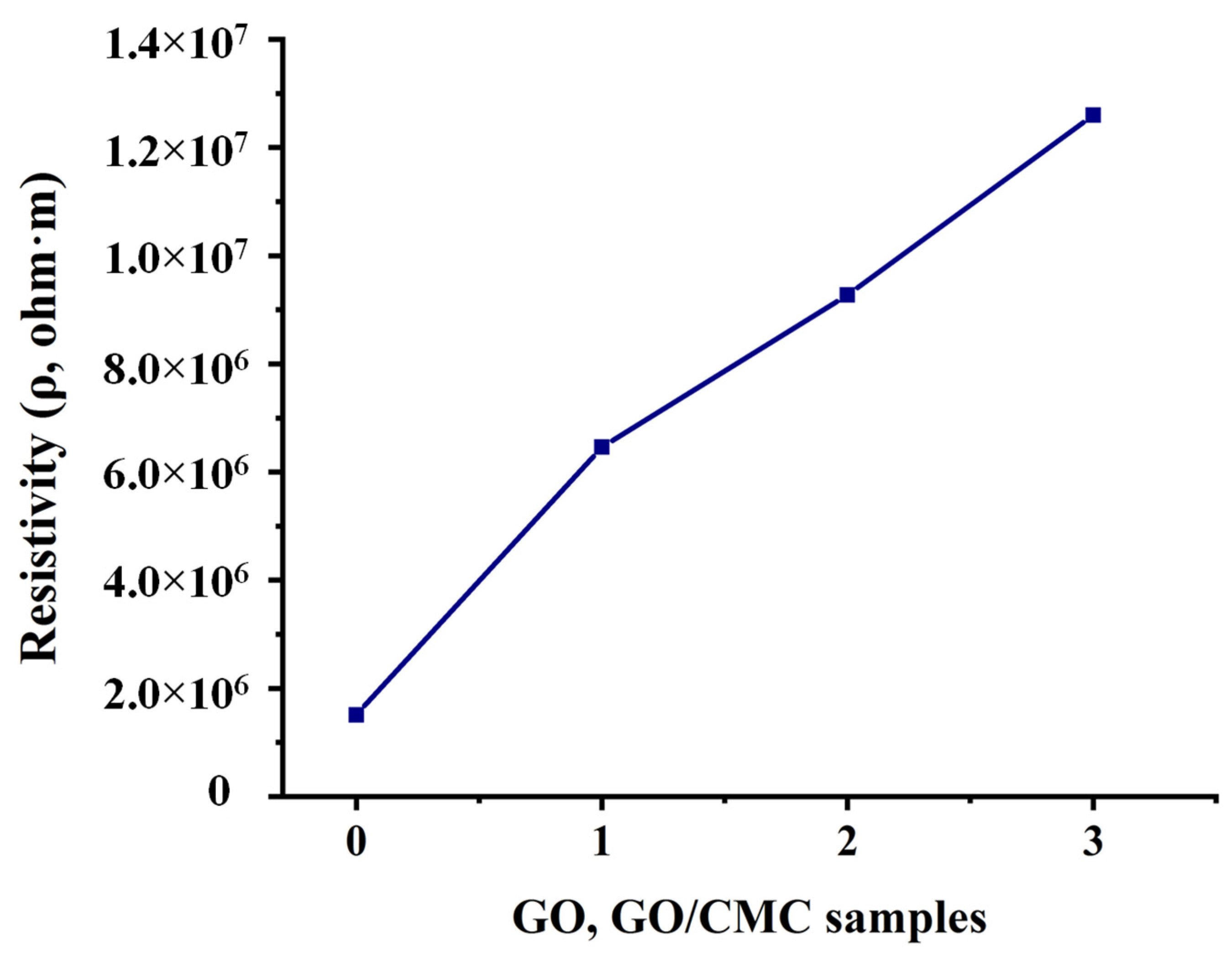
3.5. Electrical Characterization
3.6. Electrophysical Characteristics of the Humidity Sensor Based on GO and GO/CMC (0.03 g; 0.06 g; 0.15 g)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maria, L.S. Graphene Oxide Carboxymethylcellulose Nanocomposite for Dressing Materials. J. Mater. 2020, 13, 1980. [Google Scholar]
- Maio, D.; Giallombardo, R.; Scaaro, P.A.; Palumbo, I. Pibiri: Synthesis of a fluorinated grapheme oxide–silica nanohybrid: Im-proving oxygen a_nity. J. RSC Adv. 2016, 6, 46037–46047. [Google Scholar] [CrossRef]
- Mansurov, Z.; Jandosov, J.; Kerimkulova, A.; Azat, S.; Zhubanova, A.; Digel, I.; Savistkaya, I.; Akimbekov, N.; Kistaubaeva, A. Nanostructured Carbon Materials for Biomedical Use. J. Eurasian Chem. Tecnol. 2013, 15, 209–217. [Google Scholar] [CrossRef]
- Azat, S.; Rosa, B.; Pavlenko, V.V.; Kerimkulova, A.R.; Raymond, L.D.; Mansurov, Z.A. Applications of activated carbon sorbents based on greek walnut. J. Appl. Mech. Mater. 2014, 467, 49–51. [Google Scholar] [CrossRef]
- Azat, S.; Arkhangelsky, E.; Papathanasiou, T.; Zorpas, A.A.; Abirov, A.; Inglezakis, V.J. Synthesis of biosourced silica-Ag nano-composites and amalgamation reaction with mercury in aqueous solutions. J.ComptesRendusChim. 2020, 23, 77–92. [Google Scholar]
- Kuanyshbekov, T.K.; Ilyin, A.M.; Beall, G.W.; Guseinov, N.R. Creation of a Humidity Sensor Based on Functionalized Graphene Nanostructures. J. Sens. Transducers 2019, 229, 39–46. [Google Scholar]
- Ilyin, A.M.; Guseinov, N.R.; Kuanyshbekov, T.K.; Beall, G.W.; Tulegenova, M.A. Computer Simulation and First Principles Study of Ga-Doped Graphene Nanostructures. J. Comput. Theor. Nanosci. 2019, 16, 39–41. [Google Scholar] [CrossRef]
- Tulegenova, M.; Ilyin, A.; Guseinov, N.; Beall, G.; Kuanyshbekov, T. Computer Simulation of the Effect of Structural Defects on the Effectiveness of the Graphene’s Protective Properties. J. Comput. Theor. Nanosci. 2019, 16, 351–354. [Google Scholar] [CrossRef]
- Kuanyshbekov, T.K.; Ak, K.; Kabdrakhmanova, S.K.; Nemkaeva, R.; Aitzhanov, M.; Imasheva, A. Kairatuly Synthesis of Graphene Oxide from Graphite by the Hummers Method. J. Oxid. Commun. 2021, 44, 356–365. [Google Scholar]
- Akatan, K.; Kuanyshbekov, T.K.; Kabdrakhmanova, S.K.; Imasheva, A.A.; Battalova, A.K.; Abylkalykova, R.B.; Nasyrova, A.K.; Ibraeva, Z.E. Synthesis of nanocomposite material through modification of graphene oxide by nanocellulose. Chem. Bull. Kazakh. Natl. Univ. 2021, 102, 14–20. [Google Scholar] [CrossRef]
- Akatan, K.; Kabdrakhmanova, S.; Kuanyshbekov, T.; Ibraeva, Z.; Battalova, A.; Joshy, K.S.; Thomas, S. Highly-efficient isolation of mi-crocrystalline cellulose and nanocellulose from sunflower seed waste via environmentally benign method. J. Cellul. 2022, 29, 3787–3802. [Google Scholar] [CrossRef]
- Maio, R.; Scaffaro, R.; Lentini, L.; Piccionello, A.P. Perfluorocarbons–graphene oxide nanoplatforms as biocompatible oxygen reservoirs. J. Anal. Chim. Acta 2018, 1037, 1–380. [Google Scholar] [CrossRef]
- Scaaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Pro-cessing-morphology-properties relationships. J. Compos. Part S Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Scaaro, R.; Maio, A.; Presti, F.L.; Giallombardo, D.; Botta, L.; Bondì, M.L.; Agnello, S. Synthesis and self-assembly of a PEGylat-ed-graphene aerogel. J. Compos. Sci. Technol. 2016, 128, 193–200. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. TrendsBiotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, D.; He, Y. Graphene oxide/polyacrylamide/carboxymethylcellulose sodium nanocomposite hydrogel with enhanced mechanical strength: Preparation, characterization and the swelling behavior. J. RSC Adv. 2014, 4, 44600–44609. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Gao, Z.; Fang, L. Toxicology of graphene oxide nanosheets against paecilomyces catenlannulatus. J.Bull.Environ.Contam. Toxicol. 2015, 95, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. J. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Song, Z.; Xu, Y.; Yang, W.; Cui, L.; Zhang, J.; Liu, J. Graphene/tri-block copolymer composites prepared via RAFT polymerizations for dual controlled drug delivery via pH stimulation and biodegradation. Eur. Polym. J. 2015, 69, 559–572. [Google Scholar] [CrossRef]
- Jiao, Z. Carboxymethyl cellulose-grafted graphene oxide for efficient antitumor drug delivery. J. Nanotechnol. Rev. 2018, 7, 291–301. [Google Scholar] [CrossRef]
- Ampaiwong, J. Reduced Graphene Oxide/Carboxymethyl Cellulose Nanocomposites Novel Conductive Films. J. Nanosci. Nanotechnol. 2019, 19, 3544–3550. [Google Scholar] [CrossRef]
- Son, Y.-R.; Yop, R.K.; Park, S.-J. Influence of reduced graphene oxide on mechanical behaviors of sodium carboxymethyl cellulose. J. Compos. Part B Eng. 2015, 83, 36–42. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Li, G.H.; Liu, K.; Ma, Z.W. Flexible Solid-State Supercapacitors Based on Carbon Nanoparticles/MnO2Nanorods Hybrid Structure. J. ACS Nano 2012, 6, 656–661. [Google Scholar]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Starch-based biomaterials for wound-dressing applications. J. Starch 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Carboxymethyl cellulose-based oral delivery systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef]
- Ge, X.; Shan, Y.; Wu, L.; Mu, X.; Peng, H.; Jiang, Y. High-strength and morphology-controlled aerogel based on carboxymethyl cellulose and graphene oxide. Carbohydr. Polym. 2018, 197, 277–283. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, X.; Jiang, K.; Liu, F.; You, F.; Yao, C. Fabrication of Reusable Carboxymethyl Cellulose/Graphene Oxide Composite Aerogel with Large Surface Area for Adsorption of Methylene Blue. Nanomaterials 2021, 11, 1609. [Google Scholar] [CrossRef]
- Seidi, F.; Salimi, H.; Shamsabadi, A.A.; Shabanian, M. Synthesis of hybrid materials using graft copolymerization on non-cellulosic polysaccharides via homogenous ATRP. Prog. Polym. Sci. 2018, 76, 1–39. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Avérous, L. Progress in nano-biocomposites based on polysaccharides and nanoclays. Mater. Sci. Eng. R Rep. 2009, 67, 1–17. [Google Scholar] [CrossRef]
- Imre, B.; García, L.; Puglia, D.; Vilaplana, F. Reactive compatibilization of plant polysaccharides and biobased polymers: Review on current strategies, expectations and reality. Carbohydr. Polym. 2019, 209, 20–37. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; Ibrahim, M.M.; Abdelfatah, M.; Ramadan, M.S.; El-Mehasseb, I.M. Synthesis, characterization, and cytotoxicity of selfassembly of hybrid nanocomposite modified membrane of carboxymethyl cellulose/graphene oxide for photocatalytic antifouling, energy storage, and supercapacitors ap-plication. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127035. [Google Scholar] [CrossRef]
- Shakeri, A.; Salehi, H.; Nakhjiri, M.T.; Shakeri, E.; Khankeshipour, N. Carboxymethylcellulose-quaternary graphene oxide nanocomposite polymer hydrogel as a biode-gradable draw agent for osmotic water treatment process. Cellulose 2019, 26, 1841–1853. [Google Scholar] [CrossRef]
- Maslennikov, A.; Peretz, R.; Vadivel, V.K.; Mamane, H. Recycled Paper Sludge (RPS)-Derived Nanocellulose: Production, Detection and Water Treatment Application. Appl. Sci. 2022, 12, 3077. [Google Scholar] [CrossRef]
- Omar, A.; Badry, R.; Hegazy, M.A.; Yahia, I.S.; Elhaes, H.; Zahran, H.Y.; Ibrahim, M.A.; Refaat, A. Enhancing the optical properties of chitosan, carboxymethyl cellulose, sodium alginate modified with nano metal oxide and graphene oxide. Opt. Quantum Electron. 2022, 54, 1–15. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Nikitha, M.R.; Karthikeyan, P.; Meenakshi, S. Perceptive removal of toxic azo dyes from water using magnetic Fe3O4 reinforced graphene ox-ide–carboxymethyl cellulose recyclable composite: Adsorption investigation of parametric studies and their mechanisms. Surf. Interfaces 2020, 21, 100648. [Google Scholar] [CrossRef]
- Lim, D.J.; Marks, N.A.; Rowles, M.R. Universal Scherrer equation for graphene fragments. Carbon 2020, 162, 475–480. [Google Scholar] [CrossRef]
- Hen, J.; Li, H.; Zhang, L.; Du, C.; Fang, T.; Hu, J. Direct Reduction of Graphene Oxide/Nanofbrillated Cellulose Composite Film and its Electrical Conductivity Research. J. Sci. Rep. 2020, 10, 124. [Google Scholar]
- Zhao, M.; Zhang, S.; Fang, G.; Huang, C.; Wu, T. Directionally-Grown Carboxymethyl Cellulose/Reduced Graphene Oxide Aerogel with Excellent Structure Stability and Adsorption Capacity. J. Polym. 2020, 12, 2219. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.E.; Suflet, D.M. Cellulose-Based Hydrogels for Medical/Pharmaceutical Applications in Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Yu, H.; Hong, H.-J.; Kim, S.M.; Ko, H.C.; Jeong, H.S. Mechanically enhanced graphene oxide/carboxymethyl cellulose nanofibril composite fiber as a scalable adsorbent for heavy metal removal. Carbohydr. Polym. 2020, 240, 116348. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Harun, M.M.; Changamu, E.O.; Maingi, F.M. Microwave synthesis of Carboxymethylcellulose (CMC) from Rice Husk. IOSR J. Appl. Chem. 2019, 12, 33–42. [Google Scholar]
- Kumar, B.; Deeba, F.; Priyadarshi, R.; Sauraj; Bano, S.; Kumar, A.; Negi, Y.S. Development of novel cross-linked carboxymethyl cellulose/poly(potassium 1-hydroxy acrylate): Synthesis, characterization and properties. Polym. Bull. 2019, 77, 4555–4570. [Google Scholar] [CrossRef]
- Mekkaoui, A.A.; Orfi, H.; Bejtka, K.; Laayati, M.; Labyad, S.A.; El Firdoussi, L.; Pirri, C.F.; Chiodoni, A.; El Houssame, S. Carboxymethyl cellulose nanocolloids anchored Pd(0) nanoparticles (CMC@Pd NPs): Synthesis, characterization, and catalytic application in transfer hydrogenation. Environ. Sci. Pollut. Res. 2022, 30, 81619–81634. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Wang, X.; Liu, Y.; Liu, Z.; Chen, W. Preparation of Graphene Oxide/Cellulose Composites with Microcrystalline Cellulose Acid Hydrolysis Using the Waste Acids Generated by the Hummers Method of Graphene Oxide Synthesis. Polymers 2021, 13, 4453. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wan, K.; Xie, P.; Miao, Y.; Liu, Z. Ultralight, High Capacitance, Mechanically Strong Graphene-Cellulose Aerogels. Molecules 2021, 26, 4891. [Google Scholar] [CrossRef]
- Miao, Y.Y.; Zhang, C.Y.; Huang, D.J.; Tian, L.; Zhao, T.J.; Zhai, X.Y.; Liu, Z.B. Preparation of graphene oxide /cellulose composite in mixed acid solution derived from Hummers method. J. For. Eng. 2018, 3, 97–102. [Google Scholar]
- Priya, V.N.; Rajkumar, M.; Mobika, J.; Sibi, S.L. Adsorption of As (V) ions from aqueous solution by carboxymethyl cellulose incorporated layered double hydroxide/reduced graphene oxide nanocomposites: Isotherm and kinetic studies. Environ. Technol. Innov. 2022, 26, 102268. [Google Scholar] [CrossRef]
- Nayak, J.; Vashishtha, A. Synthesis, Characterization and Preparation of Nanocellulose and different Cellu-lose/Nanocellulose/Graphene Composites from Cassava root and its Potential Application for Pesticide Adsorption from water. J. IJRAR 2018, 5, 513–524. [Google Scholar]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- He, S.; Wang, G.S.; Lu, C.; Luo, X.; Wen, B.; Guo, L.; Cao, M.S. Enhanced wave absorption of nanocomposites based on the synthesized complex symmetrical CuS nanostructure and poly (vinylidene fluoride). J. Mater Chem. A 2013, 1, 4685–4692. [Google Scholar] [CrossRef]
- Song, W.-L.; Cao, M.-S.; Lu, M.-M.; Liu, J.; Yuan, J.; Fan, L.-Z. Improved dielectric properties and highly efficient and broadened bandwidth electromagnetic attenuation of thickness-decreased carbon nanosheet/wax composites. J. Mater. Chem. C 2012, 1, 1846–1854. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X. Conducting polymers directly coated on reduced grapheme oxide sheets as high-performance supercapacitor electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Lim, W.H.; Yap, Y.K.; Chong, W.Y.; Ahmad, H. All-Optical Graphene Oxide Humidity Sensors. Sensors 2014, 14, 24329–24337. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, R.; Guha, P.K.; Bhattacharyya, T.K. Humidity Sensor Based on High Proton Conductivity of Graphene Oxide. IEEE Trans. Nanotechnol. 2015, 14, 931–937. [Google Scholar] [CrossRef]
- Hernández-Rivera, D.; Rodríguez-Roldán, G.; Mora-Martínez, R.; Suaste-Gómez, E. A Capacitive Humidity Sensor Based on an Electrospun PVDF/Graphene Membrane. Sensors 2017, 17, 1009. [Google Scholar] [CrossRef]
- Anderson, J.H.; Parks, G.A. Electrical conductivity of silica gel in the presence of adsorbed water. J. Phys. Chem. 1968, 72, 3662–3668. [Google Scholar] [CrossRef]



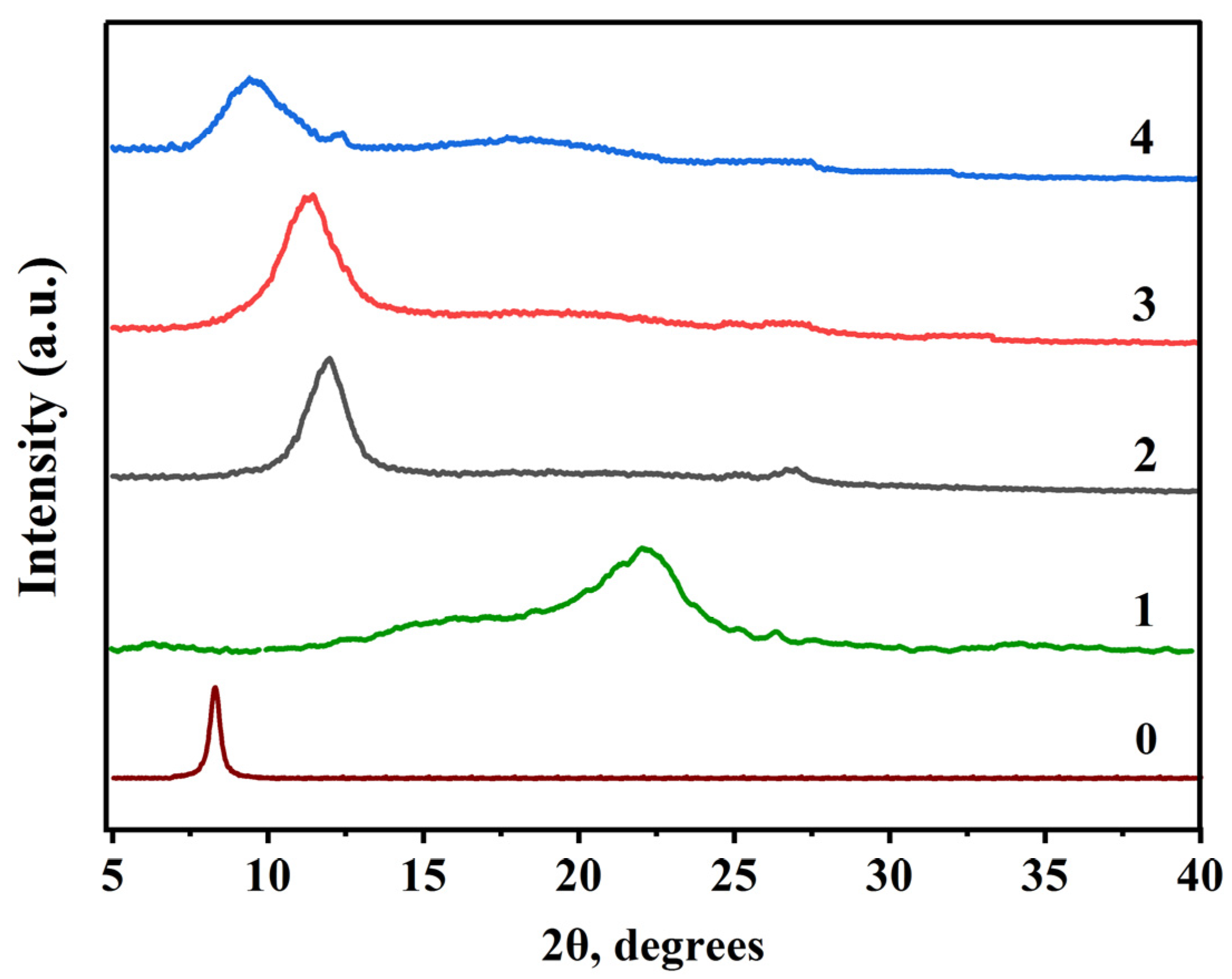


| Samples | Parameters | Peak Position | θ (°) (2θ(°)/2) | β | cos θ (°) | Crystallite Size (Ǻ) D | Crystallite Size (nm) D | ||
|---|---|---|---|---|---|---|---|---|---|
| K [38] | Λ (Ǻ) | 2θ (°) | FWHM (In Degree) | FWHM (In Radian) | |||||
| 0 | 0.94 | 1.5418 | 7.05 | 3.52 | 0.27 | 0.0048075 | 0.92 | 279.31 | 27.93 |
| 2 | 0.94 | 1.5418 | 11.89 | 5.94 | 1.77 | 0.031041 | 0.94 | 44.05 | 4.40 |
| 3 | 0.94 | 1.5418 | 11.35 | 5.67 | 2.49 | 0.0435557 | 0.82 | 27.31 | 2.73 |
| 4 | 0.94 | 1.5418 | 9.70 | 4.85 | 3.78 | 0.0660983 | 0.13 | 3.05 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuanyshbekov, T.; Sagdollin, Z.; Zhasasynov, E.; Akatan, K.; Kurbanova, B.; Guseinov, N.; Tolepov, Z.; Kantay, N.; Beisebekov, M. Composite Membrane Based on Graphene Oxide and Carboxymethylcellulose from Local Kazakh Raw Materials for Possible Applications in Electronic Devices. J. Compos. Sci. 2023, 7, 342. https://doi.org/10.3390/jcs7080342
Kuanyshbekov T, Sagdollin Z, Zhasasynov E, Akatan K, Kurbanova B, Guseinov N, Tolepov Z, Kantay N, Beisebekov M. Composite Membrane Based on Graphene Oxide and Carboxymethylcellulose from Local Kazakh Raw Materials for Possible Applications in Electronic Devices. Journal of Composites Science. 2023; 7(8):342. https://doi.org/10.3390/jcs7080342
Chicago/Turabian StyleKuanyshbekov, Tilek, Zhandos Sagdollin, Elzhas Zhasasynov, Kydyrmolla Akatan, Bayan Kurbanova, Nazim Guseinov, Zhandos Tolepov, Nurgamit Kantay, and Madyar Beisebekov. 2023. "Composite Membrane Based on Graphene Oxide and Carboxymethylcellulose from Local Kazakh Raw Materials for Possible Applications in Electronic Devices" Journal of Composites Science 7, no. 8: 342. https://doi.org/10.3390/jcs7080342
APA StyleKuanyshbekov, T., Sagdollin, Z., Zhasasynov, E., Akatan, K., Kurbanova, B., Guseinov, N., Tolepov, Z., Kantay, N., & Beisebekov, M. (2023). Composite Membrane Based on Graphene Oxide and Carboxymethylcellulose from Local Kazakh Raw Materials for Possible Applications in Electronic Devices. Journal of Composites Science, 7(8), 342. https://doi.org/10.3390/jcs7080342






