There Are over 60 Ways to Produce Biocompatible Calcium Orthophosphate (CaPO4) Deposits on Various Substrates
Abstract
1. Introduction
2. General Knowledge, Terminology and Definitions
- The kernel contains substances that are toxic, cause adverse or allergic reactions, or have a bitter or unpleasant odor;
- The coating protects the core material from its environment and increases its stability and shelf life;
- The coating improves mechanical integrity, which means that coated products are more resistant to misuse (e.g., wear and tear);
- Modification of the surface properties of the core, such as biocompatibility, light reflection, electrical conductivity, color, etc.;
- Decoration (in cases where the core alone is tasteless);
- The core contains material that can easily migrate and stain hands, clothes, etc.;
- Changing the release profile of active ingredients, such as pharmaceuticals, from the core.
3. Brief Knowledge on the Important Pre- and Post-Deposition Treatments
- Part 1.
- Methods to Produce Biocompatible CaPO4 Deposits
4. CaPO4 Deposited on Various Substrates
4.1. Thermal Spraying Deposition Techniques
4.1.1. Plasma Spraying
4.1.2. High Velocity Oxy-Fuel (HVOF) Spraying
4.2. Vapor Deposition Techniques
4.2.1. Ion- and Electron-Beam Assisted (IBAD and EBAD) Depositions
4.2.2. Pulsed Laser Deposition (PLD)
4.2.3. Magnetron Sputtering
4.2.4. Electron-Cyclotron-Resonance (ECR) Plasma Sputtering
4.2.5. Metalorganic Chemical Vapor Deposition (MOCVD)
4.2.6. Molecular Precursor and Thermal Decomposition Techniques
4.3. Wet Techniques
4.3.1. Electrophoretic Deposition (EPD)
4.3.2. Electrochemical (ECD) or Cathodic Deposition
4.3.3. Sol–Gel Deposition
4.3.4. Wet-Chemical and Biomimetic Deposition Techniques
4.3.5. Dip Coating Technique
4.3.6. Spin Coating Technique
4.3.7. Hydrothermal Deposition Method
4.3.8. Thermal Substrate Deposition Technique
4.3.9. Alternate Soaking Deposition
4.3.10. Micro-Arc Oxidation (MAO) Technique
4.4. Other CaPO4 Deposition Techniques: Miscellaneous
4.4.1. Hot Isostatic Pressing (HIP)
4.4.2. Implantation into the Surface of Superplastic Alloys
4.4.3. A Double Layered Capsule Hydrothermal Hot Pressing
4.4.4. Detonation Gun (D-Gun) Spraying
4.4.5. Aerosol–Gel Deposition
4.4.6. Aerosol Deposition (AD)
4.4.7. Cold Spraying (CS)
4.4.8. Blast Coating
4.4.9. Direct Laser Melting
4.4.10. Transmission Laser Coating
4.4.11. Laser Cladding
4.4.12. Laser-Engineered Net Shaping (LENS™)
4.4.13. Matrix Assisted Pulsed Laser Evaporation (MAPLE)
4.4.14. Liquid Phase Laser Deposition
4.4.15. Laser-Induced Forward Transfer with Optical Stamp (LIFTOP)
4.4.16. Laser-Induced Single-Step Coating (LISSC)
4.4.17. Electrostatic Spray Deposition (ESD) Technique
4.4.18. Spray Pyrolysis (Pyrosol) Technique
4.4.19. Polymeric Deposition Route
4.4.20. Atomic Layer Deposition (ALD)
4.4.21. Drop-On-Demand (DOD) Micro-Dispensing Technique
4.4.22. Vapor Diffusion Sitting Drop Micro-Method (VDSDM)
4.4.23. Mechanochemical Synthesis or Ball Impact Method
4.4.24. Mechanofusion
4.4.25. Autocatalytic Deposition
4.4.26. Galvanic Deposition
4.4.27. Anodization Technique
4.4.28. Simultaneous Precipitation and Electrodeposition
4.4.29. Electrical Stimulation
4.4.30. Cyclic Electrodeposition
4.4.31. Cyclic Spin Coating
4.4.32. Biomediated Deposition Technique (Biosynthesis)
4.4.33. Emulsion Route
4.4.34. Slurry Processing Technique
4.4.35. Slip Coating Technique
4.4.36. Deposition by Solvent Evaporation
4.4.37. Discrete Crystalline Deposition
4.4.38. Powder Mixed Electrical Discharge Machining (PMEDM)
4.4.39. Investment Casting
4.4.40. Brush Painting
4.4.41. Photocatalytic Deposition
4.4.42. Adsorption
4.4.43. Sonocoating
4.4.44. Ultrasonic Mechanical Coating and Armoring (UMCA)
4.4.45. Osteomimetic Deposition
4.4.46. Surface-Induced Mineralization (SIM)
4.4.47. Ionized Jet Deposition (IJD)
4.4.48. Undisclosed Proprietary Deposition Techniques
4.4.49. Additive Manufacturing Techniques
5. Deposition of Ion-Substituted CaPO4 and CaPO4-Containing Biocomposites
6. Conversion-Formed CaPO4 Deposits
- Part 2.
- Properties and Applications
7. A Brief Description of the Most Important Properties
7.1. Introduction
7.2. Elastic Modulus and Hardness
7.3. Fatigue Properties
7.4. Thickness
7.5. Adhesion and Cohesion
7.6. Surface Characteristics: Crystallinity, Morphology and Roughness
7.7. Biodegradation
7.8. Interaction with Cells and Tissue Responses
8. Biomedical Applications of CaPO4 Deposits
9. Future Directions
10. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: https://en.wikipedia.org/wiki/Surface_engineering (accessed on 20 April 2023).
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.H.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Wang, R. Surface modifications of bone implants through wet chemistry. J. Mater. Chem. 2006, 16, 2309–2321. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium Phosphates in Oral Biology and Medicine, Monographs in Oral Science; Myers, H.M., Ed.; Karger: Basel, Switzerland, 1991; Volume 15, 201p. [Google Scholar]
- Dorozhkin, S.V. Calcium Orthophosphates: Applications in Nature, Biology, and Medicine; Pan Stanford: Singapore, 2012; 854p. [Google Scholar]
- Dorozhkin, S.V. Calcium Orthophosphate-Based Bioceramics and Biocomposites; Wiley-VCH: Weinheim, Germany, 2016; 405p. [Google Scholar]
- Ong, J.L.; Chan, D.C.N. Hydroxyapatite and their use as coatings in dental implants: A review. Crit. Rev. Biomed. Eng. 1999, 28, 667–707. [Google Scholar] [CrossRef] [PubMed]
- de Groot, K.; Wolke, J.G.C.; Jansen, J.A. Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 137–147. [Google Scholar] [CrossRef]
- Onoki, T.; Hashida, T. New method for hydroxyapatite coating of titanium by the hydrothermal hot isostatic pressing technique. Surf. Coat. Tech. 2006, 200, 6801–6807. [Google Scholar] [CrossRef]
- Kobayashi, T.; Itoh, S.; Nakamura, S.; Nakamura, M.; Shinomiya, K.; Yamashita, K. Enhanced bone bonding of hydroxyapatite-coated titanium implants by electrical polarization. J. Biomed. Mater. Res. A 2007, 82A, 145–151. [Google Scholar] [CrossRef]
- Epinette, J.A.M.D.; Geesink, R.G.T. Hydroxyapatite Coated Hip and Knee Arthroplasty; Elsevier: Amsterdam, The Netherlands, 1995; 394p. [Google Scholar]
- Willmann, G. Coating of implants with hydroxyapatite–material connections between bone and metal. Adv. Eng. Mater. 1999, 1, 95–105. [Google Scholar] [CrossRef]
- Epinette, J.A.; Manley, M.T. (Eds.) Fifteen Years of Clinical Experience with Hydroxyapatite Coatings in Joint Arthroplasty; Springer: Paris, France, 2004; 452p. [Google Scholar]
- Habibovic, P.; Li, J.; van der Valk, C.M.; Meijer, G.; Layrolle, P.; van Blitterswijk, C.A.; de Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 2005, 26, 23–36. [Google Scholar] [CrossRef]
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Kim, K.H.; Park, C.; Kim, H.E. Dense nanostructured hydroxyapatite coating on titanium by aerosol deposition. J. Am. Ceram. Soc. 2009, 92, 683–687. [Google Scholar] [CrossRef]
- Callahan, T.J.; Gantenberg, J.B.; Sands, B.E. Calcium phosphate (Ca-P) coating draft guidance for preparation of Food and Drug Administration (FDA) submissions for orthopedic and dental endosseous implants. In Characterization and Performance of Calcium Phosphate Coatings for Implants; Horowitz, E., Parr, J.E., Eds.; ASTM STP 1196: Philadelphia, PA, USA, 1994; pp. 185–197. [Google Scholar]
- Implants for Surgery: Coating for Hydroxyapatite Ceramics; ISO: Geneva, Switzerland, 1996; pp. 1–8.
- 510(K) Information Needed for Hydroxyapatite Coated Orthopedic Implants. March 10, 1995 (revised 2/20/97). Available online: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm080224.htm (accessed on 20 April 2023).
- ISO 13779-2:2000; Implants for Surgery—Hydroxyapatite—Part 2: Coatings of Hydroxyapatite. ISO: Geneva, Switzerland, 2000. Available online: https://www.iso.org/standard/26841.html (accessed on 20 April 2023).
- ISO 13779-2:2008; Implants for Surgery—Hydroxyapatite—Part 2: Coatings of Hydroxyapatite. ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/43827.html (accessed on 20 April 2023).
- ISO 13779-2:2018; Implants for Surgery—Hydroxyapatite—Part 2: Thermally Sprayed Coatings of Hydroxyapatite. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/64617.html (accessed on 20 April 2023).
- ISO 13779-4:2002; Implants for Surgery—Hydroxyapatite—Part 4: Determination of Coating Adhesion Strength. ISO: Geneva, Switzerland, 2002. Available online: https://www.iso.org/standard/30723.html (accessed on 20 April 2023).
- ISO 13779-4:2018; Implants for Surgery—Hydroxyapatite—Part 4: Determination of Coating Adhesion Strength. ISO: Geneva, Switzerland, 2002. Available online: https://www.iso.org/standard/64619.html (accessed on 20 April 2023).
- Available online: https://www.wikiwand.com/en/Coating (accessed on 20 April 2023).
- Ohring, M. Materials Science of Thin Films, 2nd ed.; Academic Press: San Diego, CA, USA, 2002; 794p. [Google Scholar]
- Kibardin, S.A.; Lazurkin, V.B. Thin-layer chromatography of proteins on plates coated with hydroxylapatite. Biochemistry 1965, 30, 483–487. [Google Scholar]
- McHugh, T.H. Protein-lipid interactions in edible films and coatings. Nahrung 2000, 44, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Tech. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Cabañas, M.V. Bioceramic coatings for medical implants. In Bio-Ceramics with Clinical Applications; Vallet-Regí, M., Ed.; Wiley: Chichester, UK, 2014; pp. 249–289. [Google Scholar]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium phosphate-based coatings on titanium and its alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85B, 279–299. [Google Scholar] [CrossRef]
- Narayanan, R.; Kim, K.H.; Rautray, T.R. Surface Modification of Titanium for Biomaterial Applications; Nova Science: Hauppauge, NY, USA, 2010; p. 352. [Google Scholar]
- Tsui, Y.C.; Doyle, C.; Clyne, T.W. Plasma sprayed hydroxyapatite coatings on titanium substrates: Part 1: Mechanical properties and residual stress levels. Biomaterials 1998, 19, 2015–2029. [Google Scholar] [CrossRef]
- Oyane, A.; Uchida, M.; Choong, C.; Triffitt, J.; Jones, J.; Ito, A. Simple surface modification of poly(ε-caprolactone) for apatite deposition from simulated body fluid. Biomaterials 2005, 26, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Lakstein, D.; Kopelovitch, W.; Barkay, Z.; Bahaa, M.; Hendel, D.; Eliaz, N. Enhanced osseointegration of grit-blasted, NaOH-treated and electrochemically hydroxyapatite-coated Ti–6Al–4V implants in rabbits. Acta Biomater. 2009, 5, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Ritman-Hertz, O.; Aronov, D.; Weinberg, E.; Shenhar, Y.; Rosenman, G.; Weinreb, M.; Ron, E. The effect of surface treatments on the adhesion of electrochemically deposited hydroxyapatite coating to titanium and on its interaction with cells and bacteria. J. Mater. Sci. Mater. Med. 2011, 22, 1741–1752. [Google Scholar] [CrossRef]
- Pattanayak, D.K.; Yamaguchi, S.; Matsushita, T.; Nakamura, T.; Kokubo, T. Apatite-forming ability of titanium in terms of pH of the exposed solution. J. R. Soc. Interface 2012, 9, 2145–2155. [Google Scholar] [CrossRef]
- El-Rab, S.M.F.G.; Fadl-allah, S.A.; Montser, A.A. Improvement in antibacterial properties of Ti by electrodeposition of biomimetic Ca–P apatite coat on anodized titania. Appl. Surf. Sci. 2012, 261, 1–7. [Google Scholar] [CrossRef]
- Ajami, E.; Aguey-Zinsou, K.F. Calcium phosphate growth at electropolished titanium surfaces. J. Funct. Biomater. 2012, 3, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, H.; Chen, M.; Li, K.; Wang, B.; Xu, Z.; Cao, S.; Zhang LDeng, H.; Lu, J. Strong-bonding calcium phosphate coatings on carbon/carbon composites by ultrasound-assisted anodic oxidation treatment and electrochemical deposition. Appl. Surf. Sci. 2012, 258, 5117–5125. [Google Scholar] [CrossRef]
- Ágreda, C.G.; Mendes, M.W.D.; Bressiani, J.C.; Bressiani, A.H.A. Apatite coating on titanium samples obtained by powder metallurgy. Adv. Sci. Technol. 2013, 86, 28–33. [Google Scholar]
- Xiao, X.; Yu, J.; Tang, H.; Mao, D.; Wang, C.; Liu, R. TiO2 nanotube arrays induced deposition of hydroxyapatite coating by hydrothermal treatment. Mater. Chem. Phys. 2013, 138, 695–702. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Lu, Y.P.; Xiao, G.Y.; Li, S.T.; Sun, R.X.; Li, M.S. Microstructural inhomogeneity in plasma-sprayed hydroxyapatite coatings and effect of post-heat treatment. Appl. Surf. Sci. 2006, 252, 2412–2421. [Google Scholar] [CrossRef]
- Khalili, V.; Naji, H. Developing a mechanochemical surface pretreatment to increase the adhesion strength of hydroxyapatite electrophoretic coating on the NiTi alloy as a bone implant. Surf. Coat. Tech. 2020, 397, 125985. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Ziaei-Moayyed, A.A.; Mesgar, A.S.M. Adhesive and cohesive properties by indentation method of plasma-sprayed hydroxyapatite coatings. Appl. Surf. Sci. 2007, 253, 4960–4965. [Google Scholar] [CrossRef]
- Lu, Y.P.; Chen, Y.M.; Li, S.T.; Wang, J.H. Surface nanocrystallization of hydroxyapatite coating. Acta Biomater. 2008, 4, 1865–1872. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.Y.; Liu, B.; Jiang, C.C.; Li, N.B.; Lu, Y.P. The formation of hydroxyapatite layer onto hopeite coating on stainless steel substrate. Corr. Sci. 2016, 111, 216–229. [Google Scholar] [CrossRef]
- Leonor, I.B.; Reis, R.L. An innovative auto-catalytic deposition route to produce calcium-phosphate coatings on polymeric biomaterials. J. Mater. Sci. Mater. Med. 2003, 14, 435–441. [Google Scholar] [CrossRef]
- Bunker, B.C.; Rieke, P.C.; Tarasevich, B.J.; Campbell, A.A.; Fryxell, G.E.; Graff, G.L.; Song, L.; Liu, J.; Virden, J.W.; McVay, G.L. Ceramic thin-film formation on functionalized interfaces through biomimetic processing. Science 1994, 264, 48–55. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiong, C.; Zhang, S.; Li, X.; Zhang, L. Bone-like apatite coating on functionalized poly(etheretherketone) surface via tailored silanization layers technique. Mater. Sci. Eng. C 2015, 55, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Le, V.Q.; Pourroy, G.; Cochis, A.; Rimondini, L.; Abdel-Fattah, W.I.; Mohammed, H.I.; Carradò, A. Alternative technique for calcium phosphate coating on titanium alloy implants. Biomatter 2014, 4, e28534. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Will, J.; Detsch, R.; Boccaccini, A.R.; Greil, P. Formation and in vitro biocompatibility of biomimetic hydroxyapatite coatings on chemically treated carbon substrates. J. Biomed. Mater. Res. A 2014, 102A, 193–203. [Google Scholar] [CrossRef]
- Tanahashi, M.; Yao, T.; Kokubo, T.; Minoda, M.; Miyamoto, T.; Nakamura, T.; Yamamuro, T. Apatite coated on organic polymers by biomimetic process: Improvement in its adhesion to substrate by NaOH treatment. J. Appl. Biomater. 1994, 5, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Tang, A.; Wang, R. Accelerating calcium phosphate growth on NaOH-treated poly-(lactic-co-glycolic acid) by evaporation-induced surface crystallization. Appl. Surf. Sci. 2008, 255, 2442–2448. [Google Scholar] [CrossRef]
- Okada, M.; Furuzono, T. Hydroxyapatite nanocrystal coating on biodegradable microspheres. Mater. Sci. Eng. B 2010, 173, 199–203. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Liu, X.; Liu, H. Biomimetic synthesis and characterization of carbon nanofiber/hydroxyapatite composite scaffolds. Carbon 2013, 51, 335–345. [Google Scholar] [CrossRef]
- Peng, F.; Shaw, M.T.; Olson, J.R.; Wei, M. Influence of surface treatment and biomimetic hydroxyapatite coating on the mechanical properties of hydroxyapatite/poly(L-lactic acid) fibers. J. Biomater. Appl. 2013, 27, 641–649. [Google Scholar] [CrossRef]
- Hashizume, M.; Maeda, M.; Iijima, K. Biomimetic calcium phosphate coating on polyimide films by utilizing surface-selective hydrolysis treatments. J. Ceram. Soc. Jpn. 2013, 121, 816–818. [Google Scholar] [CrossRef]
- Kramer, E.; Kunkemoeller, B.; Wei, M. Evaluation of alkaline pre-treatment of PLLA fibers for biomimetic hydroxyapatite coating. Surf. Coat. Tech. 2014, 244, 23–28. [Google Scholar] [CrossRef]
- Rajesh, P.; Mohan, N.; Yokogawa, Y.; Varma, H. Pulsed laser deposition of hydroxyapatite on nanostructured titanium towards drug eluting implants. Mater. Sci. Eng. C 2013, 33, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Riau, A.K.; Mondal, D.; Setiawan, M.; Palaniappan, A.; Yam, G.H.F.; Liedberg, B.; Venkatraman, S.S.; Mehta, J.S. Functionalization of the polymeric surface with bioceramic nanoparticles via a novel, nonthermal dip coating method. ACS Appl. Mater. Interfaces 2016, 8, 35565–35577. [Google Scholar] [CrossRef]
- Xiong, X.B.; Zeng, X.R.; Zou, C.L.; Zhou, J.Z. Strong bonding strength between HA and (NH4)2S2O8-treated carbon/carbon composite by hydrothermal treatment and induction heating. Acta Biomater. 2009, 5, 1785–1790. [Google Scholar] [PubMed]
- Mucalo, M.R.; Yokogawa, Y.; Toriyama, M.; Suzuki, T.; Kawamoto, Y.; Nagata, F.; Nishizawa, K. Growth of calcium phosphate on surface-modified cotton. J. Mater. Sci. Mater. Med. 1995, 6, 597–605. [Google Scholar] [CrossRef]
- Yokogawa, Y.; Paz Reyes, J.; Mucalo, M.R.; Toriyama, M.; Kawamoto, Y.; Suzuki, T.; Nishizawa, K.; Nagata, F.; Kamayama, T. Growth of calcium phosphate on phosphorylated chitin fibres. J. Mater. Sci. Mater. Med. 1997, 8, 407–412. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Liu, X.; Xiong, X.; Liu, H. Biomimetic growth of hydroxyapatite on phosphorylated electrospun cellulose nanofibers. Carbohyd. Polym. 2012, 90, 1573–1581. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Kato, K.; Ikada, Y. In vitro hydroxyapatite deposition onto a film surface-grated with organophosphate polymer. J. Biomed. Mater. Res. 1994, 28, 1365–1373. [Google Scholar] [CrossRef]
- Kim, H.M.; Uenoyama, M.; Kokubo, T.; Minoda, M.; Miyamoto, T.; Nakamura, T. Biomimetic apatite formation on polyethylene photografted with vinyltrimethoxysilane and hydrolyzed. Biomaterials 2001, 22, 2489–2494. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Wang, W.; Zhang, J.; Zhang, L.; He, F.; Yang, J. Controllable preparation of a nano-hydroxyapatite coating on carbon fibers by electrochemical deposition and chemical treatment. Mater. Sci. Eng. C 2016, 63, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gong, T.; Zhou, S. The functionalization of multi-walled carbon nanotubes by in situ deposition of hydroxyapatite. Biomaterials 2010, 31, 5182–5190. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. In situ deposition of hydroxyapatite on graphene nanosheets. Mater. Res. Bull. 2013, 48, 175–179. [Google Scholar] [CrossRef]
- Waterman, J.; Pietak, A.; Birbilis, N.; Woodfield, T.; Dias, G.; Staiger, M.P. Corrosion resistance of biomimetic calcium phosphate coatings on magnesium due to varying pretreatment time. Mater. Sci. Eng. B 2011, 176, 1756–1760. [Google Scholar] [CrossRef]
- Tanahashi, M.; Yao, T.; Kokubo, T.; Minoda, M.; Miyamoto, T.; Nakamura, T.; Yamamuro, T. Apatite coated on organic polymers by biomimetic process: Improvement in its adhesion to substrate by glow-discharge treatment. J. Biomed. Mater. Res. 1995, 29, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Yabutsuka, T.; Fukushima, K.; Kidokoro, Y.; Matsunaga, T.; Takai, S.; Yao, T. Fabrication of bioactive fiber reinforced polyetheretherketone by the function of apatite nuclei. Key Eng. Mater. 2017, 720, 246–251. [Google Scholar] [CrossRef]
- Baker, K.C.; Drelich, J.; Miskioglu, I.; Israel, R.; Herkowitz, H.N. Effect of polyethylene pretreatments on the biomimetic deposition and adhesion of calcium phosphate films. Acta Biomater. 2007, 3, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Miyaji, F.; Kokubo, T.; Takadama, H.; Nakamura, T.; Murakami, A. Apatite-organic polymer composites prepared by a biomimetic process: Improvement in adhesion of the apatite layer to the substrate by ultraviolet irradiation. J. Mater. Sci. Mater. Med. 1998, 9, 285–290. [Google Scholar] [CrossRef]
- Suzuki, N.; Umeda, T.; Sumi, T.; Horikoshi, S.; Kuwahara, H.; Toyama, T.; Musha, Y.; Itatani, K. Rapid formation of hydroxyapatite layer on polyetheretherketone by vacuum ultraviolet irradiation and microwave heating techniques. J. Ceram. Soc. Jpn. 2016, 124, 49–54. [Google Scholar] [CrossRef]
- Gopi, D.; Sherif, E.S.M.; Rajeswari, D.; Kavitha, L.; Pramod, R.; Dwivedi, J.; Polaki, S.R. Evaluation of the mechanical and corrosion protection performance of electrodeposited hydroxyapatite on the high energy electron beam treated titanium alloy. J. Alloys Compd. 2014, 616, 498–504. [Google Scholar] [CrossRef]
- Gopi, D.; Karthika, A.; Rajeswari, D.; Kavitha, L.; Pramod, R.; Dwivedi, J. Investigation on corrosion protection and mechanical performance of minerals substituted hydroxyapatite coating on HELCDEB-treated titanium using pulsed electrodeposition method. RSC Adv. 2014, 4, 34751–34759. [Google Scholar] [CrossRef]
- Orii, Y.; Masumoto, H.; Honda, Y.; Anada, T.; Goto, T.; Sasaki, K.; Suzuki, O. Enhancement of octacalcium phosphate deposition on a titanium surface activated by electron cyclotron resonance plasma oxidation. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93B, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Mutsuzaki, H.; Yokoyama, Y.; Ito, A.; Oyane, A. Formation of apatite coatings on an artificial ligament using a plasma- and precursor-assisted biomimetic process. Int. J. Mol. Sci. 2013, 14, 19155–19168. [Google Scholar] [CrossRef] [PubMed]
- Faria, D.; Henriques, B.; Souza, A.C.; Silva, F.S.; Carvalho, O. Laser-assisted production of HAp-coated zirconia structured surfaces for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 112, 104049. [Google Scholar] [CrossRef]
- Roguska, A.; Hiromoto, S.; Yamamoto, A.; Woźniak, M.J.; Pisarek, M.; Lewandowska, M. Collagen immobilization on 316L stainless steel surface with cathodic deposition of calcium phosphate. Appl. Surf. Sci. 2011, 257, 5037–5045. [Google Scholar] [CrossRef]
- Jo, J.H.; Kang, B.G.; Shin, K.S.; Kim, H.E.; Hahn, B.D.; Park, D.S.; Koh, Y.H. Hydroxyapatite coating on magnesium with MgF₂ interlayer for enhanced corrosion resistance and biocompatibility. J. Mater. Sci. Mater. Med. 2011, 22, 2437–2447. [Google Scholar] [CrossRef]
- Chi, M.H.; Tsou, H.K.; Chung, C.J.; He, J.L. Biomimetic hydroxyapatite grown on biomedical polymer coated with titanium dioxide interlayer to assist osteocompatible performance. Thin Solid Films 2013, 549, 98–102. [Google Scholar] [CrossRef]
- Wu, J.; Hirata, I.; Zhao, X.; Gao, B.; Okazaki, M.; Kato, K. Influence of alkyl chain length on calcium phosphate deposition onto titanium surfaces modified with alkylphosphonic acid monolayers. J. Biomed. Mater. Res. A 2013, 101A, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiong, C.; Zhang, L. Formation of bone-like apatite on plasma-carboxylated poly(etheretherketone) surface. Mater. Lett. 2014, 126, 147–150. [Google Scholar] [CrossRef]
- Chen, W.; Long, T.; Guo, Y.J.; Zhu, Z.A.; Guo, Y.P. Hydrothermal synthesis of hydroxyapatite coatings with oriented nanorod arrays. RSC Adv. 2014, 4, 185–191. [Google Scholar] [CrossRef]
- Chen, W.; Tian, B.; Lei, Y.; Ke, Q.F.; Zhu, Z.A.; Guo, Y.P. Hydroxyapatite coatings with oriented nanoplate and nanorod arrays: Fabrication, morphology, cytocompatibility and osteogenic differentiation. Mater. Sci. Eng. C 2016, 67, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Kizuki, T.; Matsushita, T.; Kokubo, T. Apatite-forming PEEK with TiO2 surface layer coating. J. Mater. Sci. Mater. Med. 2015, 26, 5359. [Google Scholar] [CrossRef] [PubMed]
- Sidane, D.; Chicot, D.; Yala, S.; Ziani, S.; Khireddine, H.; Iost, A.; Decoopman, X. Study of the mechanical behavior and corrosion resistance of hydroxyapatite sol-gel thin coatings on 316 L stainless steel pre-coated with titania film. Thin Solid Films 2015, 593, 71–80. [Google Scholar] [CrossRef]
- Ulasevich, S.A.; Poznyak, S.K.; Kulak, A.I.; Lisenkov, A.D.; Starykevich, M.; Skorb, E.V. Photocatalytic deposition of hydroxyapatite onto a titanium dioxide nanotubular layer with fine tuning of layer nanoarchitecture. Langmuir 2016, 32, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Azem, F.A.; Birlik, I.; Braic, V.; Toparli, M.; Celik, E.; Parau, A.; Kiss, A.; Titorencu, I.; Vladescu, A. Effect of SiC interlayer between Ti6Al4V alloy and hydroxyapatite films. Proc. Inst. Mech. Eng. H 2015, 229, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.A.E.; Ruiz, G.C.M.; Faria, A.N.; Zancanela, D.C.; Pereira, L.S.; Ciancaglini, P.; Ramos, A.P. Calcium carbonate hybrid coating promotes the formation of biomimetic hydroxyapatite on titanium surfaces. Appl. Surf. Sci. 2016, 370, 459–468. [Google Scholar] [CrossRef]
- Yang, Y.C.; Yang, C.Y. Mechanical and histological evaluation of a plasma sprayed hydroxyapatite coating on a titanium bond coat. Ceram. Int. 2013, 39, 6509–6516. [Google Scholar] [CrossRef]
- Sawaguchi, H.; Xu, J.; Kawai, T.; Mineta, T.; Nonomura, Y. Formation process of apatite layer on titanium-coated silicon wafer surfaces. J. Ceram. Soc. Jpn. 2016, 124, 753–756. [Google Scholar] [CrossRef]
- Strąkowska, P.; Beutner, R.; Gnyba, M.; Zielinski, A.; Scharnweber, D. Electrochemically assisted deposition of hydroxyapatite on Ti6Al4V substrates covered by CVD diamond films—coating characterization and first cell biological results. Mater. Sci. Eng. C 2016, 59, 624–635. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, D.; Zhou, J.; Huang, M.; Zhang, X. Construction of different morphology of calcium phosphate film on titanium base oxidation layer superhydrophobic-superhydrophilic pattern. Mater. Lett. 2016, 180, 309–312. [Google Scholar] [CrossRef]
- Oyane, A.; Kawashita, M.; Nakanishi, K.; Kokubo, T.; Minoda, M.; Miyamoto, T.; Nakamura, T. Bonelike apatite formation on ethylene-vinyl alcohol copolymer modified with silane coupling agent and calcium silicate solutions. Biomaterials 2003, 24, 1729–1735. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Kubo, M.; Takashima, S.; Tsuru, K.; Hayakawa, S.; Osaka, A. In vitro apatite formation on organic polymers modified with a silane coupling reagent. J. R. Soc. Interface 2005, 22, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Balas, F.; Kawashita, M.; Nakamura, T.; Kokubo, T. Formation of bone-like apatite on organic polymers treated with a silane-coupling agent and a titania solution. Biomaterials 2006, 27, 1704–1710. [Google Scholar] [CrossRef]
- Rakngarm, A.; Mutoh, Y. Characterization and fatigue damage of plasma sprayed HAp top coat with Ti and HAp/Ti bond coat layers on commercially pure titanium substrate. J. Mech. Behav. Biomed. Mater. 2009, 2, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Laonapakul, T.; Otsuka, Y.; Nimkerdphol, A.R.; Mutoh, Y. Acoustic emission and fatigue damage induced in plasma-sprayed hydroxyapatite coating layers. J. Mech. Behav. Biomed. Mater. 2012, 8, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Nimkerdphol, A.R.; Otsuka, Y.; Mutoh, Y. Effect of dissolution/precipitation on the residual stress redistribution of plasma-sprayed hydroxyapatite coating on titanium substrate in simulated body fluid (SBF). J. Mech. Behav. Biomed. Mater. 2014, 36, 98–108. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, S.Y.; Bae, C.J.; Noh, Y.J.; Kim, H.E.; Kim, H.M.; Ko, J.S. Porous ZrO2 bone scaffold coated with hydroxyapatite with fluorapatite intermediate layer. Biomaterials 2003, 24, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Yoon, B.H.; Koh, Y.H.; Kim, H.E. Processing and performance of hydroxyapatite/fluorapatite double layer coating on zirconia by the powder slurry method. J. Am. Ceram. Soc. 2006, 89, 2466–2472. [Google Scholar] [CrossRef]
- Pham, M.T.; Reuther, H.; Matz, W.; Mueller, R.; Steiner, G.; Oswald, S.; Zyganov, I. Surface induced reactivity for titanium by ion implantation. J. Mater. Sci. Mater. Med. 2000, 11, 383–391. [Google Scholar] [CrossRef]
- Baumann, H.; Bethge, K.; Bilger, G.; Jones, D.; Symietz, I. Thin hydroxyapatite surface layers on titanium produced by ion implantation. Nucl. Instrum. Meth. B 2002, 196, 286–292. [Google Scholar] [CrossRef]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Biliński, A.; Lewandowska-Szumieł, M.; Rajchel, B. Effect of dual ion implantation of calcium and phosphorus on the properties of titanium. Biomaterials 2005, 26, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Rautray, T.R.; Narayanan, R.; Kwon, T.Y.; Kim, K.H. Accelerator based synthesis of hydroxyapatite by MeV ion implantation. Thin Solid Films 2010, 518, 3160–3163. [Google Scholar] [CrossRef]
- Coreño-Alonsoa, J.; Coreño-Alonsob, O.; Martínez-Rosalesc, J.M. Apatite formation on alumina: The role of the initial adsorption of calcium and phosphate ions. Ceram. Int. 2014, 40, 4909–4915. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S.; Bianchi, C.L.; Cassinelli, C.; Vernè, E. Surface modification of Ti-6Al-4 V alloy for biomineralization and specific biological response: Part II, alkaline phosphatase grafting. J. Mater. Sci. Mater. Med. 2011, 22, 1835–1842. [Google Scholar] [CrossRef]
- Aminian, A.; Pardun, K.; Volkmann, E.; Li Destri, G.; Marletta, G.; Treccani, L.; Rezwan, K. Enzyme-assisted calcium phosphate biomineralisation on an inert alumina surface. Acta Biomater. 2015, 13, 335–343. [Google Scholar] [CrossRef]
- Jaroszewicz, J.; Idaszek, J.; Choinska, E.; Szlazak, K.; Hyc, A.; Osiecka-Iwan, A.; Swieszkowski, W.; Moskalewski, S. Formation of calcium phosphate coatings within polycaprolactone scaffolds by simple, alkaline phosphatase based method. Mater. Sci. Eng. C 2019, 96, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, Z.E. The effect of heat treatment on the morphology of D-gun sprayed hydroxyapatite coatings. J. Biomed. Mater. Res. Appl. Biomater. 1999, 48, 861–868. [Google Scholar] [CrossRef]
- Lynn, A.K.; DuQuesnay, D.L. Hydroxyapatite-coated Ti–6Al–4V. Part 2: The effects of post-deposition heat treatment at low temperatures. Biomaterials 2002, 23, 1947–1953. [Google Scholar] [CrossRef]
- Yang, Y.; Agarwal, C.M.; Kim, K.H.; Martin, H.; Schul, K.; Bumgardner, J.M.; Ong, J.L. Characterization and dissolution behavior of sputtered calcium phosphate coatings after different postdeposition heat treatment temperatures. J. Oral Implant. 2003, 29, 270–277. [Google Scholar] [CrossRef]
- Lee, Y.P.; Wang, C.K.; Huang, T.H.; Chen, C.C.; Kao, C.T.; Ding, S.J. In vitro characterization of post heat-treated plasma-sprayed hydroxyapatite coatings. Surf. Coat. Tech. 2005, 197, 367–374. [Google Scholar] [CrossRef]
- Johnson, S.; Haluska, M.; Narayan, R.J.; Snyder, R.L. In situ annealing of hydroxyapatite thin films. Mater. Sci. Eng. C 2006, 26, 1312–1316. [Google Scholar] [CrossRef]
- Cannillo, V.; Lusvarghi, L.; Sola, A.; Barletta, M. Post-deposition laser treatment of plasma sprayed titania-hydroxyapatite functionally graded coatings. J. Eur. Ceram. Soc. 2009, 29, 3147–3158. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Thermal treatment optimization of electrodeposited hydroxyapatite coatings on Ti6Al4V substrate. Adv. Eng. Mater. 2012, 14, 377–382. [Google Scholar] [CrossRef]
- Berkin, A.B.; Deryabina, V.V.; Sharafutdinov, M.R.; Karmanov, N.S. Structural changes in calcium phosphate thin films on titanium during heat treatment. Russ. Phys. J. 2014, 56, 1124–1129. [Google Scholar] [CrossRef]
- Azis, S.A.A.; Kennedy, J.; Cao, P. Effect of annealing on microstructure of hydroxyapatite coatings and their behaviours in simulated body fluid. Adv. Mater. Res. 2014, 922, 657–662. [Google Scholar] [CrossRef]
- Shirdar, M.R.; Izman, S.; Taheri, M.M.; Assadian, M.; Kadir, M.R.A. Effect of post-treatment techniques on corrosion and wettability of hydroxyapatite-coated Co–Cr–Mo alloy. Arab. J. Sci. Eng. 2015, 40, 1197–1203. [Google Scholar] [CrossRef]
- Jaber, N.B.; Drevet, R.; Fauré, J.; Demangel, C.; Potiron, S.; Tara, A.; Larbi, A.B.C.; Benhayoune, H. A new process for the thermal treatment of calcium phosphate coatings electrodeposited on Ti6Al4V substrate. Adv. Eng. Mater. 2015, 17, 1608–1615. [Google Scholar] [CrossRef]
- Hontsu, S.; Nakamori, M.; Tabata, H.; Ishii, J.; Kawai, T. Pulsed laser deposition of bioceramic hydroxyapatite thin films on polymer materials. Jpn. J. Appl. Phys. 1996, 35, L1208–L1210. [Google Scholar] [CrossRef]
- Hontsu, S.; Nakamori, M.; Kato, N.; Tabata, H.; Ishii, J.; Matsumoto, T.; Kawai, T. Formation of hydroxyapatite thin films on surface-modified polytetrafluoroethylene substrates. Jpn. J. Appl. Phys. 1998, 37, L1169–L1171. [Google Scholar] [CrossRef]
- Chen, C.; Wang, D.; Bao, Q.; Zhang, L.; Lei, T. Influence of laser remelting on the microstructure and phases constitution of plasma sprayed hydroxyapatite coatings. Appl. Surf. Sci. 2005, 250, 98–103. [Google Scholar] [CrossRef]
- Feddes, B.; Vredenberg, A.M.; Wehner, M.; Wolke, J.C.G.; Jansen, J.A. Laser-induced crystallization of calcium phosphate coatings on polyethylene (PE). Biomaterials 2005, 26, 1645–1651. [Google Scholar] [CrossRef]
- dos Santos, E.A.; Moldovan, S.; Mateescu, M.; Faerber, J.; Acosta, M.; Pelletier, H.; Anselme, K.; Werckmann, J. Physical–chemical and biological behavior of an amorphous calcium phosphate thin film produced by RF-magnetron sputtering. Mater. Sci. Eng. C 2012, 32, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Weng, J.; Chen, J.; Feng, J.; Yang, Z.; Zhang, X. Water vapor-treated hydroxyapatite coatings after plasma spraying and their characteristics. Biomaterials 1996, 17, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, K.H.; Agarwal, C.M.; Ong, J.L. Effect of post-deposition heating temperature and the presence of water vapor during heat treatment on crystallinity of calcium phosphate coatings. Biomaterials 2003, 24, 5131–5137. [Google Scholar] [CrossRef]
- Yang, C.W.; Lee, T.M.; Lui, T.S.; Chang, E. A comparison of the microstructural feature and bonding strength of plasma-sprayed hydroxyapatite coatings with hydrothermal and vacuum post-heat treatment. Mater. Trans. 2005, 46, 709–715. [Google Scholar] [CrossRef]
- Li, H.; Khor, K.A.; Cheang, P. Effect of steam treatment during plasma spraying on the microstructure of hydroxyapatite (HA) splats and coatings. J. Therm. Spray Tech. 2006, 15, 610–616. [Google Scholar] [CrossRef]
- Yang, C.W.; Lui, T.S. Microstructural self-healing effect of hydrothermal crystallization on bonding strength and failure mechanism of hydroxyapatite coatings. J. Eur. Ceram. Soc. 2008, 28, 2151–2159. [Google Scholar] [CrossRef]
- Yang, C.W.; Lui, T.S. Kinetics of hydrothermal crystallization under saturated steam pressure and the self-healing effect by nanocrystallite for hydroxyapatite coatings. Acta Biomater. 2009, 5, 2728–2737. [Google Scholar] [CrossRef]
- Huang, Y.; Qu, Y.; Yang, B.; Li, W.; Zhang, B.; Zhang, X. In vivo biological responses of plasma sprayed hydroxyapatite coatings with an electric polarized treatment in alkaline solution. Mater. Sci. Eng. C 2009, 29, 2411–2416. [Google Scholar] [CrossRef]
- Chen, H.T.; Wang, M.C.; Chang, K.M.; Wang, S.H.; Shih, W.J.; Li, W.L. Phase transformation and morphology of calcium phosphate prepared by electrochemical deposition process through alkali treatment and calcinations. Metall. Mater. Trans. A 2014, 45, 2260–2269. [Google Scholar] [CrossRef]
- Wang, C.; Li, K.Z.; Zhai, Y.Q.; Li, H.J.; Wang, J.L.; Jiao, G.S. Study of fluorhydroxyapatite coatings on carbon/carbon composites. Surf. Coat. Tech. 2009, 203, 1771–1775. [Google Scholar] [CrossRef]
- Su, Y.; Lu, Y.; Su, Y.; Hu, J.; Lian, J.; Li, G. Enhancing the corrosion resistance and surface bioactivity of a calcium-phosphate coating on a biodegradable AZ60 magnesium alloy via a simple fluorine post-treatment method. RSC Adv. 2015, 5, 56001–56010. [Google Scholar] [CrossRef]
- Kato, R.; Nakamura, S.; Katayama, K.; Yamashita, K. Electrical polarization of plasma-spray-hydroxyapatite coatings for improvement of osteoconduction of implants. J. Biomed. Mater. Res. A 2005, 74A, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Lui, T.S.; Chen, L.H. Hydrothermal crystallization effect on the improvement of erosion resistance and reliability of plasma-sprayed hydroxyapatite coatings. Thin Solid Films 2009, 517, 5380–5385. [Google Scholar] [CrossRef]
- Saju, K.K.; Reshmi, R.; Jayadas, N.H.; James, J.; Jayaraj, M.K. Polycrystalline coating of hydroxyapatite on TiAl6V4 implant material grown at lower substrate temperatures by hydrothermal annealing after pulsed laser deposition. Proc. Inst. Mech. Eng. H 2009, 223, 1049–1057. [Google Scholar] [CrossRef]
- Ozeki, K.; Aoki, H.; Masuzawa, T. Influence of the hydrothermal temperature and pH on the crystallinity of a sputtered hydroxyapatite film. Appl. Surf. Sci. 2010, 256, 7027–7031. [Google Scholar] [CrossRef]
- Hahn, B.D.; Lee, J.M.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Lee, B.K.; Kim, J.W.; Kim, H.E.; et al. Enhanced bioactivity and biocompatibility of nanostructured hydroxyapatite coating by hydrothermal annealing. Thin Solid Films 2011, 519, 8085–8090. [Google Scholar] [CrossRef]
- Ubele, D.; Pluduma, L.; Gross, K.A.; Viksna, A. Hydrothermal processing for increasing the hydroxyl ion concentration in hydroxyl depleted hydroxyapatite. Key Eng. Mater. 2018, 762, 42–47. [Google Scholar] [CrossRef]
- Tian, Y.S.; Qian, X.L.; Chen, M.Q. Effect of saturated steam treatment on the crystallinity of plasma-sprayed hydroxyapatite coatings. Surf. Coat. Tech. 2015, 266, 38–41. [Google Scholar] [CrossRef]
- Han, Y.; Xu, K.; Lu, J. Morphology and composition of hydroxyapatite coatings prepared by hydrothermal treatment on electrodeposited brushite coatings. J. Mater. Sci. Mater. Med. 1999, 10, 243–248. [Google Scholar] [CrossRef]
- Kumar, M.; Dasarathy, H.; Riley, C. Electrodeposition of brushite coatings and their transformation to hydroxyapatite in aqueous solutions. J. Biomed. Mater. Res. 1999, 45, 302–310. [Google Scholar] [CrossRef]
- Han, Y.; Fu, T.; Lu, J.; Xu, K. Characterization and stability of hydroxyapatite coatings prepared by an electrodeposition and alkaline-treatment process. J. Biomed. Mater. Res. 2001, 54, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, G.; Lian, J. A chemical conversion hydroxyapatite coating on AZ60 magnesium alloy and its electrochemical corrosion behavior. Int. J. Electrochem. Sci. 2012, 7, 11497–11511. [Google Scholar] [CrossRef]
- da Rocha, D.N.; de Oliveira Cruz, L.R.; de Campos, J.B.; Marçal, R.L.S.B.; Mijares, D.Q.; Coelho, P.G.; da Silva, M.H.P. Mg substituted apatite coating from alkali conversion of acidic calcium phosphate. Mater. Sci. Eng. C 2017, 70, 408–417. [Google Scholar] [CrossRef]
- Prezas, P.R.; Soares, M.J.; Borges, J.P.; Silva, J.C.; Oliveira, F.J.; Graça, M.P.F. Bioactivity enhancement of plasma-sprayed hydroxyapatite coatings through non-contact corona electrical charging. Nanomaterials 2023, 13, 1058. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Z.; Hu, J. A study of bionics micro-textures on the surface of HA bio-coatings prepared by nanosecond laser. Ceram. Int. 2023, 49, 11999–12011. [Google Scholar] [CrossRef]
- Eliaz, N.; Shmueli, S.; Shur, I.; Benayahu, D.; Aronov, D.; Rosenman, G. The effect of surface treatment on the surface texture and contact angle of electrochemically deposited hydroxyapatite coating and on its interaction with bone-forming cells. Acta Biomater. 2009, 5, 3178–3191. [Google Scholar] [CrossRef]
- Gregor, M.; Plecenik, T.; Tofail, S.A.M.; Zahoran, M.; Truchly, M.; Vargova, M.; Laffir, F.; Plesch, G.; Kus, P.; Plecenik, A. Hydrophobicity of electron beam modified surface of hydroxyapatite films. Appl. Surf. Sci. 2015, 337, 249–253. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Ivanov, K.V.; Ugodchikova, A.V.; Kashin, A.D.; Uvarkin, P.V.; Sharkeev, Y.P.; Tolkacheva, T.V.; Tolmachev, A.I.; Schmidt, J.; Egorkin, V.S.; et al. The effect of pulsed electron irradiation on the structure, phase composition, adhesion and corrosion properties of calcium phosphate coating on Mg0.8Ca alloy. Mater. Chem. Phys. 2023, 294, 126996. [Google Scholar] [CrossRef]
- Kostuchenko, A.V.; Kannykin, S.V.; Kuschev, S.B.; Dybov, V.A. Synthesis of composite calcium-phosphate coatings via pulsed photon processing. Bull. Russ. Acad. Sci. Phys. 2016, 80, 1161–1164. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Zai, W.; Li, G.; Liu, S.; Lian, J.; Jiang, Z. Fabrication of superhydrophobic calcium phosphate coating on Mg-Zn-Ca alloy and its corrosion resistance. J. Mater. Eng. Perform. 2017, 26, 6117–6129. [Google Scholar] [CrossRef]
- Yoshinari, M.; Oda, Y.; Inoue, T.; Matsuzaka, K.; Shimono, M. Bone response to calcium phosphate-coated and bisphosphonate-immobilized titanium implants. Biomaterials 2002, 23, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Cattini, A.; Bellucci, D.; Sola, A.; Pawłowski, L.; Cannillo, V. Functional bioactive glass topcoats on hydroxyapatite coatings: Analysis of microstructure and in-vitro bioactivity. Surf. Coat. Tech. 2014, 240, 110–117. [Google Scholar] [CrossRef]
- Yanovska, A.; Kuznetsov, V.; Stanislavov, A.; Danilchenko, S.; Sukhodub, L. Calcium–phosphate coatings obtained biomimetically on magnesium substrates under low magnetic field. Appl. Surf. Sci. 2012, 258, 8577–8584. [Google Scholar] [CrossRef]
- Liu, C.; Tian, A.; Yang, H.; Xu, Q.; Xue, X. Electrodeposited hydroxyapatite coatings on the TiO2 nanotube in static magnetic field. Appl. Surf. Sci. 2013, 287, 218–222. [Google Scholar] [CrossRef]
- Nelea, V.; Pelletier, H.; Iliescu, M.; Werckmann, J.; Craciun, V.; Mihailescu, I.N.; Ristoscu, C.; Ghica, C. Calcium phosphate thin film processing by pulsed laser deposition and in situ assisted ultraviolet pulsed laser deposition. J. Mater. Sci. Mater. Med. 2002, 13, 1167–1173. [Google Scholar] [CrossRef]
- Xiong, X.B.; Zeng, X.R.; Zou, J.Z.; Xie, S.H. Preparation of improved hydroxyapatite coating on HT-C/C by modified induction heating deposition/hydrothermal treatment technologies. Surf. Eng. 2011, 27, 591–594. [Google Scholar] [CrossRef]
- Zhao, X.; Li, H.; Chen, M.; Li, K.; Lu, J.; Zhang, L.; Cao, S. Nano/micro-sized calcium phosphate coating on carbon/carbon composites by ultrasonic assisted electrochemical deposition. Surf. Interface Anal. 2012, 44, 21–28. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kato, N.; Hatoko, Y.; Hontsu, S. Optimization of humid conditions using an ultrasonic nebulizer for the fabrication of hydroxyapatite film with the Er:YAG laser deposition method. Key Eng. Mater. 2017, 720, 269–274. [Google Scholar] [CrossRef]
- Mostaghimi, J.; Passandideh-Fard, M.; Chandra, S. Dynamics of splat formation in plasma spray coating process. Plasma Chem. Plasma P 2002, 22, 59–84. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, A.; Dussoubs, B. Quo Vadis thermal spraying? J. Therm. Spray Tech. 2001, 10, 44–66. [Google Scholar] [CrossRef]
- Aoyagi, M.; Hayashi, M.; Yoshida, Y.; Yao, Y. Implants for Bones, Joints and Tooth Roots. U.S. Patent US4146936, 3 April 1979. [Google Scholar]
- Zhao, G.L.; Wen, G.; Song, Y.; Wu, K. Near surface martensitic transformation and recrystallization in a Ti–24Nb–4Zr–7.9Sn alloy substrate after application of a HA coating by plasma spraying. Mater. Sci. Eng. C 2011, 31, 106–113. [Google Scholar] [CrossRef]
- Gligorijević, B.R.; Vilotijević, M.; Šćepanović, M.; Vuković, N.S.; Radović, N. Substrate preheating and structural properties of power plasma sprayed hydroxyapatite coatings. Ceram. Int. 2016, 42, 411–420. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Alamara, K.; Saber-Samandari, S.; Gross, K.A. Micro-Raman spectroscopy shows how the coating process affects the characteristics of hydroxylapatite. Acta Biomater. 2013, 9, 9538–9546. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Alamara, K.; Saber-Samandari, S. Calcium phosphate coatings: Morphology, micro-structure and mechanical properties. Ceram. Int. 2014, 40, 563–572. [Google Scholar] [CrossRef]
- Freidberg, J.P. Plasma Physics and Fusion Energy; Cambridge University Press: Cambridge, UK, 2007; 692p. [Google Scholar]
- Herman, H. Plasma-sprayed coatings. Sci. Am. 1988, 9, 112–117. [Google Scholar] [CrossRef]
- Fauchais, P. Understanding plasma spraying. J. Phys. D Appl. Phys. 2004, 37, R86–R108. [Google Scholar] [CrossRef]
- Quek, C.H.; Khor, K.A.; Cheang, P. Influence of processing parameters in the plasma spraying of hydroxyapatite/Ti–6Al–4V composite coatings. J. Mater. Process. Tech. 1999, 89–90, 550–555. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Layrolle, P. 1.16 Calcium phosphate coatings. In Comprehensive Biomaterials II; Ducheyne, P., Healy, K., Hutmacher, D.W., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 360–367. [Google Scholar]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Tech. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Heimann, R.B. Thermal spraying of biomaterials. Surf. Coat. Tech. 2006, 201, 2012–2019. [Google Scholar] [CrossRef]
- Xu, J.L.; Joguet, D.; Cizek, J.; Khor, K.A.; Liao, H.L.; Coddet, C.; Chen, W.N. Synthesis and characterization on atmospheric plasma sprayed amorphous silica doped hydroxyapatite coatings. Surf. Coat. Tech. 2012, 206, 4659–4665. [Google Scholar] [CrossRef]
- Su, Y.; Li, K.; Guan, K.; Zhu, X.; Sun, J. Mechanical properties of the supersonic atmospheric plasma sprayed Ca–P coating post-processed by a microwave-hydrothermal method. Mater. Sci. Eng. C 2019, 95, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, H.C.; Turner, I.G.; Doyle, C. Direct morphological comparison of vacuum plasma sprayed and detonation gun sprayed hydroxyapatite coatings. Biomaterials 1999, 20, 315–322. [Google Scholar] [CrossRef]
- Gledhill, H.C.; Turner, I.G.; Doyle, C. In vitro fatigue behavior of vacuum plasma and detonation gun sprayed hydroxyapatite coatings. Biomaterials 2001, 22, 1233–1240. [Google Scholar] [CrossRef]
- Ganvir, A.; Nagar, S.; Markocsan, N.; Balani, K. Deposition of hydroxyapatite coatings by axial plasma spraying: Influence of feedstock characteristics on coating microstructure, phase content and mechanical properties. J. Eur. Ceram. Soc. 2021, 41, 4637–4649. [Google Scholar] [CrossRef]
- Gross, K.A.; Saber-Samandari, S. Revealing mechanical properties of a suspension plasma sprayed coating with nanoindentation. Surf. Coat. Tech. 2009, 203, 2995–2999. [Google Scholar] [CrossRef]
- Pawłowski, L. Suspension and solution thermal spray coatings. Surf. Coat. Tech. 2009, 203, 2807–2829. [Google Scholar] [CrossRef]
- Podlesak, H.; Pawłowski, L.; D’Haese, R.; Laureyns, J.; Lampke, T.; Bellayer, S. Advanced microstructural study of suspension plasma sprayed hydroxyapatite coatings. J. Therm. Spray Tech. 2010, 19, 657–664. [Google Scholar] [CrossRef]
- Łatka, L.; Pawłowski, L.; Chicot, D.; Pierlot, C.; Petit, F. Mechanical properties of suspension plasma sprayed hydroxyapatite coatings submitted to simulated body fluid. Surf. Coat. Tech. 2010, 205, 954–960. [Google Scholar] [CrossRef]
- d’Haese, R.; Pawłowski, L.; Bigan, M.; Jaworski, R.; Martel, M. Phase evolution of hydroxapatite coatings suspension plasma sprayed using variable parameters in simulated body fluid. Surf. Coat. Tech. 2010, 204, 1236–1246. [Google Scholar] [CrossRef]
- Pateyron, B.; Pawłowski, L.; Calve, N.; Delluc, G.; Denoirjean, A. Modeling of phenomena occurring in plasma jet during suspension spraying of hydroxyapatite coatings. Surf. Coat. Tech. 2013, 214, 86–90. [Google Scholar] [CrossRef]
- Xu, H.; Geng, X.; Liu, G.; Xiao, J.; Li, D.; Zhang, Y.; Zhu, P.; Zhang, C. Deposition, nanostructure and phase composition of suspension plasma-sprayed hydroxyapatite coatings. Ceram. Int. 2016, 42, 8684–8690. [Google Scholar] [CrossRef]
- Huang, Y.; Song, L.; Liu, X.; Xiao, Y.; Wu, Y.; Chen, J.; Wu, F.; Gu, Z. Hydroxyapatite coatings deposited by liquid precursor plasma spraying: Controlled dense and porous micorstructures and osteoblastic cell responses. Biofabrication 2010, 2, 045003. [Google Scholar] [CrossRef] [PubMed]
- Candidato, R.T.; Sokołowski, P.; Pawłowski, L.; Denoirjean, A. Preliminary study of hydroxyapatite coatings synthesis using solution precursor plasma spraying. Surf. Coat. Tech. 2015, 277, 242–250. [Google Scholar] [CrossRef]
- Candidato, R.T.; Sokołowski, P.; Pawłowski, L.; Lecomte-Nana, G.; Constantinescu, C.; Denoirjean, A. Development of hydroxyapatite coatings by solution precursor plasma spray process and their microstructural characterization. Surf. Coat. Tech. 2017, 318, 39–49. [Google Scholar] [CrossRef]
- Morks, M.F.; Kobayashi, A. Effect of gun current on the microstructure and crystallinity of plasma sprayed hydroxyapatite coatings. Appl. Surf. Sci. 2007, 253, 7136–7142. [Google Scholar] [CrossRef]
- Wu, G.M.; Hsiao, W.D.; Kung, S.F. Investigation of hydroxyapatite coated polyether ether ketone composites by gas plasma sprays. Surf. Coat. Tech. 2009, 203, 2755–2758. [Google Scholar] [CrossRef]
- Morks, M.F.; Kobayashi, A.; Fahim, N.F. Abrasive wear behavior of sprayed hydroxyapitite coatings by gas tunnel type plasma spraying. Wear 2007, 262, 204–209. [Google Scholar] [CrossRef]
- Kobayashi, A.; Subramanian, B. Hydroxyapatite and YSZ reinforced hydroxyapatite coatings by gas tunnel type plasma spraying. Key Eng. Mater. 2013, 529–530, 213–216. [Google Scholar] [CrossRef]
- Nakamura, M.; Kobayashi, A.; Nozaki, K.; Horiuchi, N.; Nagai, A.; Yamashita, K. Improvement of osteoblast adhesion through polarization of plasma-sprayed hydroxyapatite coatings on metal. J. Med. Biol. Eng. 2014, 34, 44–48. [Google Scholar] [CrossRef]
- Inagaki, M.; Yokogawa, Y.; Kameyama, T. Formation of highly oriented hydroxyapatite in hydroxyapatite/titanium composite coating by radio-frequency thermal plasma spraying. J. Mater. Sci. Mater. Med. 2003, 14, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Kameyama, T. Phase transformation of plasma-sprayed hydroxyapatite coating with preferred crystalline orientation. Biomaterials 2007, 28, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Demnati, I.; Parco, M.; Grossin, D.; Fagoaga, I.; Drouet, C.; Barykin, G.; Combes, C.; Braceras, I.; Goncalves, S.; Rey, C. Hydroxyapatite coating on titanium by a low energy plasma spraying mini-gun. Surf. Coat. Tech. 2012, 206, 2346–2353. [Google Scholar] [CrossRef]
- Gligorijević, B.R.; Vilotijević, M.; Šćepanović, M.; Vidović, D.; Radović, N.A. Surface structural heterogeneity of high power plasma-sprayed hydroxyapatite coatings. J. Alloys Compd. 2016, 687, 421–430. [Google Scholar] [CrossRef]
- Boi, M.; Bianchi, M.; Gambardella, A.; Liscio, F.; Kaciulis, S.; Visani, A.; Barbalinardo, M.; Valle, F.; Iafisco, M.; Lungaro, L.; et al. Tough and adhesive nanostructured calcium phosphate thin films deposited by the pulsed plasma deposition method. RSC Adv. 2015, 5, 78561–78571. [Google Scholar] [CrossRef]
- Gambardella, A.; Bianchi, M.; Kaciulis, S.; Mezzi, A.; Brucale, M.; Cavallini, M.; Herrmannsdoerfer, T.; Chanda, G.; Uhlarz, M.; Cellini, A.; et al. Magnetic hydroxyapatite coatings as a new tool in medicine: A scanning probe investigation. Mater. Sci. Eng. C 2016, 62, 444–449. [Google Scholar] [CrossRef]
- Jinawath, S.; Hengst, M.; Heimann, R.B. Plasma-sprayed DCPD/CaCO3 coatings on Ti6Al4V substrates. J. Sci. Res. Chula Univ. 2004, 29, 33–43. [Google Scholar]
- Chahal, H.K.; Matthews, S.; Jones, M.I. Fabrication of calcium phosphate coatings by the inflight reaction of precursor feedstocks using plasma spraying. J. Therm. Spray Tech. 2023, 32, 1465–1481. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K. Anisotropy in nanohardness of microplasma sprayed hydroxyapatite coating. Adv. Appl. Ceram. 2010, 109, 346–354. [Google Scholar] [CrossRef]
- Dey, A.; Nandi, S.K.; Kundu, B.; Kumar, C.; Mukherjee, P.; Roy, S.; Mukhopadhyay, A.K.; Sinha, M.K.; Basu, D. Evaluation of hydroxyapatite and β-tri calcium phosphate microplasma spray coated pin intra-medullary for bone repair in a rabbit model. Ceram. Int. 2011, 37, 1377–1391. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K. Evaluation of residual stress in microplasma sprayed hydroxyapatite coating by nanoindentation. Ceram. Int. 2014, 40, 1263–1272. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K. Microplasma Sprayed Hydroxyapatite Coatings; CRC Press: Boca Raton, FL, USA, 2015; 234p. [Google Scholar]
- Liu, X.; He, D.; Zhou, Z.; Wang, G.; Wang, Z.; Guo, X. Effect of post-heat treatment on the microstructure of micro-plasma sprayed hydroxyapatite coatings. Surf. Coat. Tech. 2019, 367, 225–230. [Google Scholar] [CrossRef]
- Lu, Y.P.; Jiao, Y.; Wang, J.H.; Xu, W.H.; Xiao, G.Y.; Zhu, R.F. A further insight into pores in plasma sprayed hydroxyapatite coating. Surf. Coat. Tech. 2012, 206, 3550–3553. [Google Scholar] [CrossRef]
- de Groot, K.; Geesink, R.G.T.; Klein, C.P.A.T.; Serekian, P. Plasma sprayed coatings of hydroxylapatite. J. Biomed. Mater. Res. 1987, 21, 1375–1381. [Google Scholar] [CrossRef]
- Gross, K.A.; Berndt, C.C. Thermal processing of hydroxyapatite for coating production. J. Biomed. Mater. Res. 1998, 39, 580–587. [Google Scholar] [CrossRef]
- Gross, K.A.; Berndt, C.C.; Stephens, P.; Dinnebier, R. Oxyapatite in hydroxyapatite coatings. J. Mater. Sci. 1998, 33, 3985–3991. [Google Scholar] [CrossRef]
- Gross, K.A.; Berndt, C.C.; Herman, H. Amorphous phase formation in plasma-sprayed hydroxyapatite coatings. J. Biomed. Mater. Res. 1998, 39, 407–414. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. Appl. Biomater. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Grey, C.P. Phase, structural and microstructural investigations of plasma sprayed hydroxyapatite coatings. Mater. Sci. Eng. A 2003, 360, 70–84. [Google Scholar] [CrossRef]
- Li, H.; Ng, B.S.; Khor, K.A.; Cheang, P.; Clyne, T.W. Raman spectroscopy determination of phases within thermal sprayed hydroxyapatite splats and subsequent in vitro dissolution examination. Acta Mater. 2004, 52, 445–453. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf. Coat. Tech. 2011, 205, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Demnati, I.; Grossin, D.; Combes, C.; Rey, C. Plasma-sprayed apatite coatings: Review of physical-chemical characteristics and their biological consequences. J. Med. Biol. Eng. 2014, 34, 1–7. [Google Scholar] [CrossRef]
- Heimann, R.B. Structural changes of hydroxylapatite during plasma spraying: Raman and NMR spectroscopy results. Coatings 2021, 11, 987. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chang, E. Influence of residual stress on bonding strength and fracture of plasma-sprayed hydroxyapatite coatings on Ti–6Al–4V substrate. Biomaterials 2001, 22, 1827–1836. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chang, E.; Lee, S.Y. Mechanical properties and Young’s modulus of plasma-sprayed hydroxyapatite coating on Ti substrate in simulated body fluid. J. Biomed. Mater. Res. A 2003, 67A, 886–899. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, E. Measurements of residual stresses in plasma-sprayed hydroxyapatite coatings on titanium alloy. Surf. Coat. Tech. 2005, 190, 122–131. [Google Scholar] [CrossRef]
- Carradò, A. Structural, microstructural, and residual stress investigations of plasma-sprayed hydroxyapatite on Ti–6Al–4V. ACS Appl. Mater. Interf. 2010, 2, 561–565. [Google Scholar] [CrossRef]
- Yang, Y.C. Investigation of residual stress generation in plasma-sprayed hydroxyapatite coatings with various spraying programs. Surf. Coat. Tech. 2011, 205, 5165–5171. [Google Scholar] [CrossRef]
- Cizek, J.; Khor, K.A.; Prochazka, Z. Influence of spraying conditions on thermal and velocity properties of plasma sprayed hydroxyapatite. Mater. Sci. Eng. C 2007, 27, 340–344. [Google Scholar] [CrossRef]
- Cizek, J.; Khor, K.A. Role of in-flight temperature and velocity of powder particles on plasma sprayed hydroxyapatite coating characteristics. Surf. Coat. Tech. 2012, 206, 2181–2191. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Ardhaoui, M.; Benyounis, K.; Looney, L.; Stokes, J.T. Plasma sprayed hydroxyapatite coatings: Understanding process relationships using design of experiment analysis. Surf. Coat. Tech. 2015, 283, 29–36. [Google Scholar] [CrossRef]
- Hasan, M.F.; Wang, J.; Berndt, C.C. Effect of power and stand-off distance on plasma sprayed hydroxyapatite coatings. Mater. Manuf. Process. 2013, 28, 1279–1285. [Google Scholar] [CrossRef]
- Cheang, P.; Khor, K.A. Thermal spraying of hydroxyapatite (HA) coatings: Effects of powder feedstock. J. Mater. Process. Tech. 1995, 48, 429–436. [Google Scholar] [CrossRef]
- Kweh, S.W.K.; Khor, K.A.; Cheang, P. Plasma-sprayed hydroxyapatite (HA) coatings with flame-spheroidized feedstock: Microstructure and mechanical properties. Biomaterials 2000, 21, 1223–1234. [Google Scholar] [CrossRef]
- Singh, T.P.; Singh, H.; Singh, H. Characterization of thermal sprayed hydroxyapatite coatings on some biomedical implant materials. J. Appl. Biomater. Funct. Mater. 2014, 12, 48–56. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Wang, Z.; Wang, G. The influence of process parameters on the structure, phase composition, and texture of micro-plasma sprayed hydroxyapatite coatings. Coatings 2018, 8, 106. [Google Scholar] [CrossRef]
- Gross, K.A.; Petzold, C.; Pluduma-LaFarge, L.; Kumermanis, M.; Haugen, H.J. Structural and chemical hierarchy in hydroxyapatite coatings. Materials 2020, 13, 4447. [Google Scholar] [CrossRef]
- Khor, K.A.; Cheang, P. Characterization of plasma sprayed hydroxyapatite powders and coatings. In Thermal Spray Coatings: Research, Design and Applications; Berndt, C.C., Bernecki, T.F., Eds.; ASM International: Novelty, OH, USA, 1993; pp. 347–352. [Google Scholar]
- Dyshlovenko, S.; Pateyron, B.; Pawłowski, L.; Murano, D. Numerical simulation of hydroxyapatite powder behaviour in plasma jet. Surf. Coat. Tech. 2004, 179, 110–117, Erratum in Surf. Coat. Tech. 2004, 187, 408–409. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-sprayed hydroxylapatite-based coatings: Chemical, mechanical, microstructural, and biomedical properties. J. Therm. Spray Tech. 2016, 25, 827–850. [Google Scholar] [CrossRef]
- Jacquot, L.; Bonnin, M.P.; Machenaud, A.; Chouteau, J.; Saffarini, M.; Vidalain, J.P. Clinical and radiographic outcomes at 25–30 years of a hip stem fully coated with hydroxylapatite. J. Arthroplast. 2018, 33, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.V.; Story, B.J.; La, D.; Wagner, W.R.; LeGeros, J.P. Highly crystalline MP-1™ hydroxylapatite coating. Part I: In vitro characterization and comparison to other plasma-sprayed hydroxylapatite coatings. Clin. Oral Implant. Res. 1999, 10, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, H.; Ishikawa, K.; Ojima, S.; Hirayama, Y.; Seto, K.; Eguchi, G. Evaluation of a high-velocity flame-spraying technique for hydroxyapatite. Biomaterials 1992, 13, 471–477. [Google Scholar] [CrossRef]
- Sobolev, V.V.; Guilemany, J.M. Dynamic processes during high velocity oxyfuel spraying. Int. Mater. Rev. 1996, 41, 13–32. [Google Scholar] [CrossRef]
- Khor, K.A.; Li, H.; Cheang, P. Processing-microstructure-property relations in HVOF sprayed calcium phosphate based bioceramic coatings. Biomaterials 2003, 24, 2233–2243. [Google Scholar] [CrossRef]
- Khor, K.A.; Li, H.; Cheang, P. Significance of melt-fraction in HVOF sprayed hydroxyapatite particles, splats and coatings. Biomaterials 2004, 25, 1177–1186. [Google Scholar] [CrossRef]
- Li, H.; Khor, K.A.; Cheang, P. Adhesive and bending failure of thermal sprayed hydroxyapatite coatings: Effect of nanostructures at interface and crack propagation phenomenon during bending. Eng. Fract. Mech. 2007, 74, 1894–1903. [Google Scholar] [CrossRef]
- Ramdan, R.D.; Prawara, B.; Suratman, R.; Pradana, I.A.; Rinaldi, A. Development of HVOF coating of hydroxy apatite on titanium alloy with carbon nano tube intermediate layer. Appl. Mech. Mater. 2014, 660, 937–941. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Gross, K.A. The use of thermal printing to control the properties of calcium phosphate deposits. Biomaterials 2010, 31, 6386–6393. [Google Scholar] [CrossRef]
- Stiegler, N.; Bellucci, D.; Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Sola, A. High-velocity suspension flame sprayed (HVSFS) hydroxyapatite coatings for biomedical applications. J. Therm. Spray Tech. 2012, 21, 275–287. [Google Scholar] [CrossRef]
- Bolelli, G.; Bellucci, D.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Müller, P.; Sola, A. Comparison between suspension plasma sprayed and high velocity suspension flame sprayed bioactive coatings. Surf. Coat. Technol. 2015, 280, 232–249. [Google Scholar] [CrossRef]
- Blum, M.; Sayed, M.; Mahmoud, E.M.; Killinger, A.; Gadow, R.; Naga, S.M. In vitro evaluation of biologically derived hydroxyapatite coatings manufactured by high velocity suspension spraying. J. Therm. Spray Tech. 2021, 30, 1891–1904. [Google Scholar] [CrossRef]
- Tiwari, S.; Mishra, S.B. Post annealing effect on corrosion behavior, bacterial adhesion, and bioactivity of LVOF sprayed hydroxyapatite coating. Surf. Coat. Tech. 2021, 405, 126500. [Google Scholar] [CrossRef]
- Taqriban, R.B.; Fitriyana, D.F.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Flame spray coating of α-tricalcium phosphate on AISI 316L alloy. Cogent Eng. 2022, 9, 2113014. [Google Scholar] [CrossRef]
- Cuerno, R.; Barabási, A.L. Dynamic scaling of ion-sputtered surfaces. Phys. Rev. Lett. 1995, 74, 4746–4749. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, K.; Verhoeven, J.; Marée, C.H.M.; Habraken, F.H.P.M.; Jansen, J.A. Study of the influence of oxygen on the composition of thin films obtained by r.f. sputtering from a Ca5(PO4)3OH target. Thin Solid Films 1997, 304, 191–195. [Google Scholar] [CrossRef]
- Barthell, B.L.; Archuleta, T.A.; Kossowsky, R. Ion beam deposition of calcium hydroxyapatite. Mater. Res. Soc. Symp. Proc. 1989, 110, 709–715. [Google Scholar] [CrossRef]
- Ong, J.L.; Harris, L.A.; Lucas, L.C.; Lacefield, W.R.; Rigney, D. X-ray photoelectron spectroscopy characterization of ion beam sputter deposited calcium phosphate coatings. J. Am. Ceram. Soc. 1991, 74, 2301–2304. [Google Scholar] [CrossRef]
- Choi, J.M.; Kim, H.E.; Lee, I.S. Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials 2000, 21, 469–473. [Google Scholar] [CrossRef]
- Wang, C.X.; Chen, Z.Q.; Guan, L.M.; Wang, M.; Liu, Z.Y.; Wang, P.L. Fabrication and characterization of graded calcium phosphate coatings produced by ion beam sputtering/mixing deposition. Nucl. Instr. Methods Phys. Res. B 2001, 179, 364–372. [Google Scholar] [CrossRef]
- Lee, I.S.; Whang, C.N.; Lee, G.H.; Cui, F.Z.; Ito, A. Effects of ion beam assist on the formation of calcium phosphate film. Nucl. Instr. Methods Phys. Res. B 2003, 206, 522–526. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, S.H.; Kim, H.W.; Kong, Y.M.; Kim, H.E. Fluoridated apatite coatings on titanium obtained by electron-beam deposition. Biomaterials 2005, 26, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, A.; Thomas, B.; Jin, C.; Narayan, R.; Cuomo, J.; Yang, Y.; Ong, J.L. A study on functionally graded HA coatings processed using ion beam assisted deposition with in situ heat treatment. Surf. Coat. Tech. 2006, 200, 6111–6116. [Google Scholar] [CrossRef]
- Blalock, T.; Bai, X.; Rabiei, A. A study on microstructure and properties of calcium phosphate coatings processed using ion beam assisted deposition on heated substrates. Surf. Coat. Tech. 2007, 201, 5850–5858. [Google Scholar] [CrossRef]
- Bai, X.; Sandukas, S.; Appleford, M.R.; Ong, J.L.; Rabiei, A. Deposition and investigation of functionally graded calcium phosphate coatings on titanium. Acta Biomater. 2009, 5, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Choe, H.C.; Brantley, W.A.; Sohn, I.B. Hydroxyapatite thin film coatings on nanotube-formed Ti–35Nb–10Zr alloys after femtosecond laser texturing. Surf. Coat. Tech. 2013, 217, 13–22. [Google Scholar] [CrossRef]
- Cooley, D.R.; van Dellen, A.F.; Burgess, J.O.; Windeler, S. The advantages of coated titanium implants prepared by radiofrequency sputtering from hydroxyapatite. Prosthet. Dent. 1992, 67, 93–100. [Google Scholar] [CrossRef]
- van Dijk, K.; Schaeken, H.G.; Wolke, J.G.C.; Jansen, J.A. Influence of annealing temperature on RF magnetron sputtered calcium phosphate coatings. Biomaterials 1996, 17, 405–410. [Google Scholar] [CrossRef]
- Nelea, V.; Morosanu, C.; Iliescu, M.; Mihailescu, I.N. Microstructure and mechanical properties of hydroxyapatite thin films grown by RF magnetron sputtering. Surf. Coat. Tech. 2003, 173, 315–322. [Google Scholar] [CrossRef]
- Wan, T.; Aoki, H.; Hikawa, J.; Lee, J.H. RF-magnetron sputtering technique for producing hydroxyapatite coating film on various substrates. Bio-Med. Mater. Eng. 2007, 17, 291–297. [Google Scholar]
- Ozeki, K.; Fukui, Y.; Aoki, H. Influence of the calcium phosphate content of the target on the phase composition and deposition rate of sputtered films. Appl. Surf. Sci. 2007, 253, 5040–5044. [Google Scholar] [CrossRef]
- Ueda, K.; Narushima, T.; Goto, T.; Taira, M.; Katsube, T. Fabrication of calcium phosphate films for coating on titanium substrates heated up to 773 K by RF magnetron sputtering and their evaluations. Biomed. Mater. 2007, 2, S160–S166. [Google Scholar] [CrossRef]
- Pichugin, V.F.; Surmenev, R.A.; Shesterikov, E.V.; Ryabtseva, M.A.; Eshenko, E.V.; Tverdokhlebov, S.I.; Prymak, O.; Epple, M. The preparation of calcium phosphate coatings on titanium and nickel-titanium by RF-magnetron sputtered deposition: Composition, structure and micromechanical properties. Surf. Coat. Tech. 2008, 202, 3913–3920. [Google Scholar] [CrossRef]
- Snyders, R.; Bousser, E.; Music, D.; Jensen, J.; Hocquet, S.; Schneider, J.M. Influence of the chemical composition on the phase constitution and the elastic properties of RF-sputtered hydroxyapatite coatings. Plasma Process. Polym. 2008, 5, 168–174. [Google Scholar] [CrossRef]
- O’Kane, C.; Duffy, H.; Meenan, B.J.; Boyd, A.R. The influence of target stoichiometry on the surface properties of sputter deposited calcium phosphate thin films. Surf. Coat. Tech. 2008, 203, 121–128. [Google Scholar] [CrossRef]
- Boyd, A.R.; O’Kane, C.; Meenan, B.J. Control of calcium phosphate thin film stoichiometry using multi-target sputter deposition. Surf. Coat. Tech. 2013, 233, 131–139. [Google Scholar] [CrossRef]
- López, E.O.; Mello, A.; Sendão, H.; Costa, L.T.; Rossi, A.L.; Ospina, R.O.; Borghi, F.F.; Filho, J.G.S.; Rossi, A.M. Growth of crystalline hydroxyapatite thin films at room temperature by tuning the energy of the RF-magnetron sputtering plasma. ACS Appl. Mater. Interfaces 2013, 5, 9435–9445. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Surmenev, R.A.; Nikonova, Y.A.; Selezneva, I.I.; Ivanova, A.A.; Putlyaev, V.I.; Prymak, O.; Epple, M. Fabrication, ultra-structure characterization and in vitro studies of RF magnetron sputter deposited nano-hydroxyapatite thin films for biomedical applications. Appl. Surf. Sci. 2014, 317, 172–180. [Google Scholar] [CrossRef]
- Bramowicz, M.; Braic, L.; Azem, F.A.; Kulesza, S.; Birlik, I.; Vladescu, A. Mechanical properties and fractal analysis of the surface texture of sputtered hydroxyapatite coatings. Appl. Surf. Sci. 2016, 379, 338–346. [Google Scholar] [CrossRef]
- Cotell, C.M.; Chrisey, D.B.; Grabowski, K.S.; Sprague, J.A.; Gosset, C.R. Pulsed laser deposition of hydroxylapatite thin films on Ti–6Al–4V. J. Appl. Biomater. 1992, 3, 87–93. [Google Scholar] [CrossRef]
- Baeri, P.; Torrisi, L.; Marino, N.; Foti, G. Ablation of hydroxyapatite by pulsed laser irradiation. Appl. Surf. Sci. 1992, 54, 210–214. [Google Scholar] [CrossRef]
- Cleries, L.; Martinez, E.; Fernandez-Pradas, J.M.; Sardin, G.; Esteve, J.; Morenza, J.L. Mechanical properties of calcium phosphate coatings deposited by laser ablation. Biomaterials 2000, 21, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Nelea, V.; Ristoscu, C.; Chiritescu, C.; Ghica, C.; Mihailescu, I.N.; Pelletier, H.; Mille, P.; Cornet, A. Pulsed laser deposition of hydroxyapatite thin films on Ti–5Al–2.5Fe substrates with and without buffer layers. Appl. Surf. Sci. 2000, 168, 127–131. [Google Scholar] [CrossRef]
- Fernández-Pradas, J.M.; Clèries, L.; Martinez, E.; Sardin, G.; Esteve, J.; Morenza, J.L. Influence of thickness on the properties of hydroxyapatite coatings deposited by KrF laser ablation. Biomaterials 2001, 22, 2171–2175. [Google Scholar] [CrossRef]
- Fernández-Pradas, J.M.; Clèries, L.; Sardin, G.; Morenza, J.L. Characterization of calcium phosphate coatings deposited by Nd:YAG laser ablation at 355 nm: Influence of thickness. Biomaterials 2002, 23, 1989–1994. [Google Scholar] [CrossRef]
- Socol, G.; Torricelli, P.; Bracci, B.; Iliescu, M.; Miroiu, F.; Bigi, A.; Werckmann, J.; Mihailescu, I.N. Biocompatible nanocrystalline octacalcium phosphate thin films obtained by pulsed laser deposition. Biomaterials 2004, 25, 2539–2545. [Google Scholar] [CrossRef]
- Kim, H.; Vohra, Y.K.; Louis, P.J.; Lacefield, W.R.; Lemons, J.E.; Camata, R.P. Biphasic and preferentially oriented microcrystalline calcium phosphate coatings: In vitro and in vivo studies. Key Eng. Mater. 2005, 284–286, 207–210. [Google Scholar] [CrossRef]
- Bigi, A.; Bracci, B.; Cuisinier, F.; Elkaim, R.; Fini, M.; Mayer, I.; Mihailescu, I.N.; Socol, G.; Sturba, L.; Torricelli, P. Human osteoblast response to pulsed laser deposited calcium phosphate coatings. Biomaterials 2005, 26, 2381–2389. [Google Scholar] [CrossRef]
- Koch, C.F.; Johnson, S.; Kumar, D.; Jelinek, M.; Chrisey, D.B.; Doraiswamy, A.; Jin, C.; Narayan, R.J.; Mihailescu, I.N. Pulsed laser deposition of hydroxyapatite thin films. Mater. Sci. Eng. C 2007, 27, 484–494. [Google Scholar] [CrossRef]
- Kim, H.; Camata, R.P.; Lee, S.; Rohrer, G.S.; Rollett, A.D.; Vohra, Y.K. Crystallographic texture in pulsed laser deposited hydroxyapatite bioceramic coatings. Acta Mater. 2007, 55, 131–139. [Google Scholar] [CrossRef]
- Dinda, G.P.; Shin, J.; Mazumder, J. Pulsed laser deposition of hydroxyapatite thin films on Ti–6Al–4V: Effect of heat treatment on structure and properties. Acta Biomater. 2009, 5, 1821–1830. [Google Scholar] [CrossRef]
- Tri, L.Q.; Chua, D.H.C. An investigation into the effects of high laser fluence on hydroxyapatite/calcium phosphate films deposited by pulsed laser deposition. Appl. Surf. Sci. 2009, 256, 76–80. [Google Scholar] [CrossRef]
- Rau, J.V.; Generosi, A.; Laureti, S.; Komlev, V.S.; Ferro, D.; Cesaro, S.N.; Paci, B.; Albertini, V.R.; Agostinelli, E.; Barinov, S.M. Physicochemical investigation of pulsed laser deposited carbonated hydroxyapatite films on titanium. ACS Appl. Mater. Interfaces 2009, 1, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.C.; Borghi, F.F.; Ospina, R.O.; López, E.O.; Borges, F.O.; Mello, A. Nd:YAG (532 nm) pulsed laser deposition produces crystalline hydroxyapatite thin coatings at room temperature. Surf. Coat. Tech. 2017, 329, 174–183. [Google Scholar] [CrossRef]
- González-Estrada, O.A.; Comas, A.D.P.; Ospina, R. Characterization of hydroxyapatite coatings produced by pulsed-laser deposition on additive manufacturing Ti6Al4V ELI. Thin Solid Films 2022, 763, 139592. [Google Scholar] [CrossRef]
- Akazawa, H.; Ueno, Y. Growth of preferentially c-axis oriented hydroxyapatite thin films on Si(100) substrate by electron-cyclotron-resonance plasma sputtering. Appl. Surf. Sci. 2013, 276, 217–222. [Google Scholar] [CrossRef]
- Akazawa, H.; Ueno, Y. Control of composition and crystallinity in hydroxyapatite films deposited by electron cyclotron resonance plasma sputtering. J. Phys. Chem. Solids 2014, 75, 94–99. [Google Scholar] [CrossRef]
- Akazawa, H.; Ueno, Y. Distinct crystallinity and orientations of hydroxyapatite thin films deposited on C- and A-plane sapphire substrates. J. Cryst. Growth 2014, 404, 241–245. [Google Scholar] [CrossRef]
- Akazawa, H.; Ueno, Y. Low-temperature crystallization and high-temperature instability of hydroxyapatite thin films deposited on Ru, Ti, and Pt metal substrates. Surf. Coat. Tech. 2015, 266, 42–48. [Google Scholar] [CrossRef]
- Massaro, C.; Baker, M.A.; Cosentino, F.; Ramires, P.A.; Klose, S.; Milella, E. Surface and biological evaluation of hydroxyapatite-based coatings on titanium deposited by different techniques. J. Biomed. Mater. Res. 2001, 58, 651–657. [Google Scholar] [CrossRef]
- León, B.; Jansen, J.A. (Eds.) Thin Calcium Phosphate Coatings for Medical Implants; Springer: New York, NY, USA, 2009; 326p. [Google Scholar]
- Zalm, P.C. Quantitative sputtering. In Handbook of Ion Beam Processing Technology; Cuomo, J.J., Rossnagel, S.M., Kaufman, H.R., Eds.; Noyes Publications: Park Ridge, NJ, USA, 1989; pp. 78–111. [Google Scholar]
- Elayaraja, K.; Chandra, V.S.; Joshy, M.I.A.; Suganthi, R.V.; Asokan, K.; Kalkura, S.N. Nanocrystalline biphasic resorbable calcium phosphate (HAp/β-TCP) thin film prepared by electron beam evaporation technique. Appl. Surf. Sci. 2013, 274, 203–209. [Google Scholar] [CrossRef]
- Hamdi, M.; Hakamata, S.; Ektessabi, A.M. Coating of hydroxyapatite thin film by simultaneous vapor deposition. Thin Solid Films 2000, 377–378, 484–489. [Google Scholar] [CrossRef]
- Hamdi, M.; Ide-Ektessabi, A. Preparation of hydroxyapatite layer by ion beam assisted simultaneous vapor deposition. Surf. Coat. Tech. 2003, 163–164, 362–367. [Google Scholar] [CrossRef]
- Hamdi, M.; Ide-Ektessabi, A. Calcium phosphate coatings: A comparative study between simultaneous vapor deposition and electron beam deposition techniques. Surf. Coat. Tech. 2006, 201, 3123–3128. [Google Scholar] [CrossRef]
- Hamdi, M.; Ide-Ektessabi, A. Dissolution behavior of simultaneous vapor deposited calcium phosphate coatings in vitro. Mater. Sci. Eng. C 2007, 27, 670–674. [Google Scholar] [CrossRef]
- Ali, M.Y.; Hung, W.; Yongqi, F. A review of focused ion beam sputtering. Int. J. Precision Eng. Manuf. 2010, 11, 157–170. [Google Scholar] [CrossRef]
- Yoshinari, M.; Ohtsuka, Y.; Dérand, T. Thin hydroxyapatite coating produced by the ion beam dynamic mixing method. Biomaterials 1994, 15, 529–535. [Google Scholar] [CrossRef]
- Yoshinari, M.; Ohtsuka, Y.; Dérand, T. The biocomatibility (cell culture and histologic study) of hydroxyapatite-coated implants created by ion beam dynamic mixing method. Clin. Oral Impl. Res. 1996, 7, 96–100. [Google Scholar] [CrossRef]
- Amy, R.L.; Storb, R. Selective mitochondrial damage by a ruby laser microbeam: An electron microscopic study. Science 1955, 122, 756–758. [Google Scholar] [CrossRef]
- Singh, R.K.; Narayan, J. Pulsed-laser evaporation technique for deposition of thin films: Physics and theoretical model. Phys. Rev. B 1990, 41, 8843–8859. [Google Scholar] [CrossRef]
- Jedynski, M.; Hoffman, J.; Mroz, W.; Szymanski, Z. Plasma plume induced during ArF laser ablation of hydroxyapatite. Appl. Surf. Sci. 2008, 255, 2230–2236. [Google Scholar] [CrossRef]
- Katayama, H.; Katto, M.; Nakayama, T. Laser-assisted laser ablation method for high-quality hydroxyapatite coating onto titanium substrate. Surf. Coat. Tech. 2009, 204, 135–140. [Google Scholar] [CrossRef]
- Bao, Q.; Chen, C.; Wang, D.; Lei, T.; Liu, J. Pulsed laser deposition of hydroxyapatite thin films under Ar atmosphere. Mater. Sci. Eng. A 2006, 429, 25–29. [Google Scholar] [CrossRef]
- Fernandez-Pradas, J.M.; Cleries, L.; Martinez, E.; Sardin, G.; Esteve, J.; Morenza, J.L. Calcium phosphate coatings deposited by laser ablation at 355 nm under different substrate temperatures and water vapour pressures. Appl. Phys. A 2000, 71, 37–42. [Google Scholar] [CrossRef]
- Ievlev, V.M. Coatings based on calcium phosphates for metallic medical implants. Russ. Chem. Rev. 2013, 82, 131–149. [Google Scholar] [CrossRef]
- Rau, J.V.; Smirnov, V.V.; Laureti, S.; Generosi, A.; Varvaro, G.; Fosca, M.; Ferro, D.; Cesaro, S.N.; Albertini, V.R.; Barinov, S.M. Properties of pulsed laser deposited fluorinated hydroxyapatite films on titanium. Mater. Res. Bull. 2010, 45, 1304–1310. [Google Scholar] [CrossRef]
- Koch, B.; Wolke, J.G.C.; de Groot, K. X-ray diffraction studies on plasma-sprayed calcium phosphate-coated implants. J. Biomed. Mater. Res. 1990, 24, 655–667. [Google Scholar] [CrossRef]
- Duta, L.; Oktar, F.N.; Stan, G.E.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I.N. Novel doped hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Kim, H.; Camata, R.P.; Vohra, Y.K.; Lacefield, W.R. Control of phase composition in hydroxyapatite/tetracalcium phosphate biphasic thin coatings for biomedical applications. J. Mater. Sci. Mater. Med. 2005, 16, 961–966. [Google Scholar] [CrossRef]
- Kim, H.; Camata, R.P.; Chowdhury, S.; Vohra, Y.K. In vitro dissolution and mechanical behavior of c-axis preferentially oriented hydroxyapatite thin films fabricated by pulsed laser deposition. Acta Biomater. 2010, 6, 3234–3241. [Google Scholar] [CrossRef]
- Jiménez, E.; Arias, J.L.; León, B.; Pérez-Amor, M. Electric discharge assisted pulsed laser deposition of hydroxylapatite. Thin Solid Films 2004, 453–454, 422–426. [Google Scholar] [CrossRef]
- Solla, E.L.; Borrajo, J.P.; González, P.; Serra, J.; Liste, S.; Chiussi, S.; León, B.; Pérez-Amor, M. Plasma assisted pulsed laser deposition of hydroxylapatite thin films. Appl. Surf. Sci. 2005, 248, 360–364. [Google Scholar] [CrossRef]
- Yashiro, H.; Kakehata, M. High crystalline hydroxyapatite coating by eclipse type pulsed-laser deposition for low annealing temperature. Appl. Phys. Lett. 2022, 120, 131602. [Google Scholar] [CrossRef]
- Duta, L.; Popescu, A.C. Current status on pulsed laser deposition of coatings from animal-origin calcium phosphate sources. Coatings 2019, 9, 335. [Google Scholar] [CrossRef]
- Shaw, B.J.; Miller, R.P. Sputtering of Bone on Prostheses. U.S. Patent US3918100, 11 October 1975. [Google Scholar]
- Gill, W.D.; Kay, E. Efficient low pressure sputtering in a large inverted magnetron suitable for film synthesis. Rev. Scientif. Instrum. 1965, 36, 277–282. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Hong, Z.; Luan, L.; Paik, S.B.; Deng, B.; Ellis, D.E.; Ketterson, J.B.; Mello, A.; Eon, J.G.; Terra, J.; Rossi, A. Crystalline hydroxyapatite thin films produced at room temperature—An opposing radio frequency magnetron sputtering approach. Thin Solid Films 2007, 515, 6773–6780. [Google Scholar] [CrossRef]
- Lai, H.C.; Tsai, H.H.; Hung, K.Y.; Feng, H.P. Fabrication of hydroxyapatite targets in radio frequency sputtering for surface modification of titanium dental implants. J. Intell. Mater. Syst. Struct. 2015, 26, 1050–1058. [Google Scholar] [CrossRef]
- Boyd, A.R.; Duffy, H.; McCann, R.; Cairns, M.L.; Meenan, B.J. The influence of argon gas pressure on co-sputtered calcium phosphate thin films. Nucl. Instrum. Methods Phys. Res. B 2007, 258, 421–428. [Google Scholar] [CrossRef]
- Long, J.; Sim, L.; Xu, S.; Ostrikov, K. Reactive plasma-aided RF sputtering deposition of hydroxyapatite bio-implant coatings. Chem. Vapor Depos. 2007, 13, 299–306. [Google Scholar] [CrossRef]
- Marchenko, E.S.; Dubovikov, K.M.; Baigonakova, G.A.; Gordienko, I.I.; Volinsky, A.A. Surface structure and properties of hydroxyapatite coatings on niti substrates. Coatings 2023, 13, 722. [Google Scholar] [CrossRef]
- Kozelskaya, A.; Dubinenko, G.; Vorobyev, A.; Fedotkin, A.; Korotchenko, N.; Gigilev, A.; Shesterikov, E.; Zhukov, Y.; Tverdokhlebov, S. Porous CaP coatings formed by combination of plasma electrolytic oxidation and RF-magnetron sputtering. Coatings 2020, 10, 1113. [Google Scholar] [CrossRef]
- Shi, J.Z.; Chen, C.Z.; Yu, H.J.; Zhang, S.J. Application of magnetron sputtering for producing bioactive ceramic coatings on implant materials. Bull. Mater. Sci. 2008, 31, 877–884. [Google Scholar] [CrossRef]
- Zhang, S. (Ed.) Hydroxyapatite Coatings for Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2013; 469p. [Google Scholar]
- Matsuo, S.; Kiuchi, M. Low temperature chemical vapor deposition method utilizing an electron cyclotron resonance plasma. Jpn. J. Appl. Phys. 1983, 22, 210–212. [Google Scholar] [CrossRef]
- Akazawa, H. Highly conductive, undoped ZnO thin films deposited by electron-cyclotron-resonance plasma sputtering on silica glass substrate. Thin Solid Films 2009, 518, 22–26. [Google Scholar] [CrossRef]
- Tracton, A.A. Coatings Technology Handbook, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005; 936p. [Google Scholar]
- Allen, G.C.; Ciliberto, E.; Fragala, I.; Spoto, G. Surface and bulk study of calcium phosphate bioceramics obtained by Metal Organic Chemical Vapor Deposition. Nucl. Instrum. Methods Phys. Res. B 1996, 116, 457–460. [Google Scholar] [CrossRef]
- Darr, J.A.; Guo, Z.X.; Raman, V.; Bououdina, M.; Rehman, I.U. Metal organic chemical vapour deposition (MOCVD) of bone mineral like carbonated hydroxyapatite coatings. Chem. Commun. 2004, 6, 696–697. [Google Scholar] [CrossRef]
- Hartshorn, R.; Stockwell, S.; Lebedev, M.; Krumdieck, S. Precursor system for bio-integration ceramics and deposition onto tantala scaffold bone interface surfaces. Surf. Coat. Tech. 2007, 201, 9413–9416. [Google Scholar] [CrossRef]
- Sato, M.; Tu, R.; Goto, T. Preparation of hydroxyapatite and calcium phosphate films by MOCVD. Mater. Trans. 2007, 48, 3149–3153. [Google Scholar] [CrossRef]
- Clearwater, D.J.; Hartshorn, R.M.; Krumdieck, S.P. Exploring multiple precursors in pulsed pressure-MOCVD. ECS Transact. 2009, 25, 973–977. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Niinomi, M.; Nakai, M.; Gozawa, T.; Akahori, T.; Saito, K.; Tu, R.; Goto, T. Fabrication of hydroxyapatite film on Ti–29Nb–13Ta–4.6Zr using a MOCVD technique. Mater. Trans. 2010, 51, 2277–2283. [Google Scholar] [CrossRef]
- Krumdieck, S.P.; Reyngoud, B.P.; Barnett, A.D.; Clearwater, D.J.; Hartshorn, R.M.; Bishop, C.M.; Redwood, B.P.; Palmer, E.L. Deposition of bio-integration ceramic hydroxyapatite by pulsed-pressure MOCVD using a single liquid precursor solution. Chem. Vap. Deposit. 2010, 16, 55–63. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Tsutsumi, H.; Saito, K.; Goto, T. Calcium phosphate coating of biomedical titanium alloys using metal-organic chemical vapour deposition. Mater. Tech. 2015, 30, B8–B12. [Google Scholar] [CrossRef]
- Jiang, G.; Shi, D. Coating of hydroxyapatite on porous alumina substrate through a thermal decomposition method. J. Biomed. Mater. Res. 1999, 48, 117–120. [Google Scholar] [CrossRef]
- Takahashi, K.; Hayakawa, T.; Yoshinari, M.; Hara, H.; Mochizuki, C.; Sato, M.; Nemoto, K. Molecular precursor method for thin calcium phosphate coating on titanium. Thin Solid Films 2005, 484, 1–9. [Google Scholar] [CrossRef]
- Hayakawa, T.; Takahashi, K.; Yoshinari, M.; Okada, H.; Yamamoto, H.; Sato, M.; Nemoto, K. Trabecular bone response to titanium implants with a thin carbonate-containing apatite coating applied using the molecular precursor method. Int. J. Oral Maxillofac. Implant. 2006, 21, 851–858. [Google Scholar]
- Ueno, D.; Sato, M.; Hayakawa, T. Guided bone regeneration using hydroxyapatite-coated titanium fiber web in rabbit mandible: Use of molecular precursor method. J. Hard Tiss. Biol. 2013, 22, 329–336. [Google Scholar] [CrossRef]
- Hirota, M.; Hayakawa, T.; Ohkubo, C.; Sato, M.; Hara, H.; Toyama, T.; Tanaka, Y. Bone responses to zirconia implants with a thin carbonate-containing hydroxyapatite coating using a molecular precursor method. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102B, 1277–1288. [Google Scholar] [CrossRef]
- Hayakawa, T.; Sato, M. Molecular precursor method for thin carbonate-containing apatite coating on dental implants. Dent. Mater. J. 2020, 39, 181–186. [Google Scholar] [CrossRef]
- Kaneko, H.; Sasaki, H.; Honma, S.; Hayakawa, T.; Sato, M.; Yajima, Y.; Yoshinari, M. Influence of thin carbonate-containing apatite coating with molecular precursor method to zirconia on osteoblast-like cell response. Dent. Mater. J. 2014, 33, 39–47. [Google Scholar] [CrossRef]
- Nijhuis, A.W.G.; Leeuwenburgh, S.C.G.; Jansen, J.A. Wet-chemical deposition of functional coatings for bone implantology. Macromol. Biosci. 2010, 10, 1316–1329. [Google Scholar] [CrossRef]
- Eliaz, N.; Kopelovitch, W.; Burstein, L.; Kobayashi, E.; Hanawa, T. Electrochemical processes of nucleation and growth of calcium phosphate on titanium supported by real-time quartz crystal microbalance measurements and X-ray photoelectron spectroscopy analysis. J. Biomed. Mater. Res. A 2009, 89A, 270–280. [Google Scholar] [CrossRef]
- Metoki, N.; Baik, S.I.; Isheim, D.; Mandler, D.; Seidman, D.N.; Eliaz, N. Atomically resolved calcium phosphate coating on a gold substrate. Nanoscale 2018, 10, 8451–8458. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Himeno, T.; Kokubo, T.; Nakamura, T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 2005, 26, 4366–4373. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Wang, L.J.; Lu, J.W.; Xu, F.S.; Zhang, F.S. Dynamics of crystallization and dissolution of calcium orthophosphates at the near-molecular level. Chin. Sci. Bull. 2011, 56, 713–721. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, Y.C.; Lin, J.G.; Hodgson, P.D.; Wen, C.E. Apatite-inducing ability of titanium oxide layer on titanium surface: The effect of surface energy. J. Mater. Res. 2008, 23, 1682–1688. [Google Scholar] [CrossRef]
- Gu, Y.W.; Tay, B.Y.; Lim, C.S.; Yong, M.S. Biomimetic deposition of apatite coating on surface-modified NiTi alloy. Biomaterials 2005, 26, 6916–6923. [Google Scholar] [CrossRef]
- Liang, F.; Zhou, L.; Wang, K. Apatite formation on porous titanium by alkali and heat-treatment. Surf. Coat. Tech. 2003, 165, 133–139. [Google Scholar] [CrossRef]
- Wang, X.X.; Hayakawa, S.; Tsuru, K.; Osaka, A. A comparative study of in vitro apatite deposition on heat-, H2O2-, and NaOH-treated titanium surfaces. J. Biomed. Mater. Res. 2000, 52, 172–178. [Google Scholar]
- Song, W.H.; Jun, Y.K.; Han, Y.; Hong, S.H. Biomimetic apatite coatings on micro-arc oxidized titania. Biomaterials 2004, 25, 3341–3349. [Google Scholar] [CrossRef]
- Wen, H.B.; Wolke, J.G.C.; de Wijn, J.R.; Liu, Q.; Cui, F.Z. Fast precipitation of calcium phosphate layers on titanium induced by simple chemical treatments. Biomaterials 1997, 18, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Li, Y.C.; Plessis, J.D.; Hodgson, P.D.; Wen, C. Influence of calcium ion deposition on apatite-inducing ability of porous titanium for biomedical applications. Acta Biomater. 2009, 5, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Dinçer, M.; Teker, D.; Sağ, C.P.; Öztürk, K. Enhanced bonding of biomimetic apatite coatings on surface-modified titanium substrates by hydrothermal pretreatment. Surf. Coat. Tech. 2013, 226, 27–33. [Google Scholar] [CrossRef]
- Feng, B.; Chen, Y.; Zhang, X.D. Effect of water vapor treatment on apatite formation on precalcified titanium and bond strength of coatings to substrates. J. Biomed. Mater. Res. 2002, 59, 12–17. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Nishimura, D.; Doi, H.; Nomura, N.; Hanawa, T. Cathodic alkaline treatment of zirconium to give the ability to form calcium phosphate. Acta Biomater. 2010, 6, 4161–4166. [Google Scholar] [CrossRef] [PubMed]
- Yabutsuka, T.; Fukushima, K.; Hiruta, T.; Takai, S.; Yao, T. Effect of pores formation process and oxygen plasma treatment to hydroxyapatite formation on bioactive PEEK prepared by incorporation of precursor of apatite. Mater. Sci. Eng. C 2017, 81, 349–358. [Google Scholar] [CrossRef]
- Masamoto, K.; Fujibayashi, S.; Yabutsuka, T.; Hiruta, T.; Otsuki, B.; Okuzu, Y.; Goto, K.; Shimizu, T.; Shimizu, Y.; Ishizaki, C.; et al. In vivo and in vitro bioactivity of a “precursor of apatite” treatment on polyetheretherketone. Acta Biomater. 2019, 91, 48–59. [Google Scholar] [CrossRef]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; de Groot, K. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J. Biomed. Mater. Res. 1994, 28, 7–15. [Google Scholar] [CrossRef]
- Pham, M.T.; Matz, W.; Grambole, D.; Herrmann, F.; Reuther, H.; Richter, E.; Steiner, G. Solution deposition of hydroxyapatite on titanium pre-treated with a sodium ion implantation. J. Biomed. Mater. Res. 2002, 59, 716–724. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Malafaya, P.B.; Reis, R.L. Sodium silicate gel as a precursor for the in vitro nucleation and growth of a bone-like apatite coating in compact and porous polymeric structures. Biomaterials 2003, 24, 2575–2584. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Variola, F.; Brunski, J.B.; Orsini, G.; de Oliveira, T.P.; Wazen, R.; Nanci, A. Nanoscale surface modifications of medically relevant metals: State-of-the art and perspectives. Nanoscale 2011, 3, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Yoshimura, M. Biocompatible hydroxyapatite ceramic coating on titanium alloys by electrochemical methods via Growing Integration Layers [GIL] strategy: A review. Ceram Int. 2023, 49, 24532–24540. [Google Scholar] [CrossRef]
- Available online: https://www.wikiwand.com/en/Electrophoretic_deposition (accessed on 20 April 2023).
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Phillips, D.C.; Shaw, B.J. Electrochemical Deposition of Bone. U.S. Patent US3892648, 1 June 1975. [Google Scholar]
- Han, Y.; Xu, K.W.; Lu, J.; Wu, Z. The structural characteristics and mechanical behaviors of nonstoichiometric apatite coatings sintered in air atmosphere. J. Biomed. Mater. Res. 1999, 45, 198–203. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Electrophoretic hydroxyapatite coatings and fibers. Mater. Lett. 2000, 42, 262–271. [Google Scholar] [CrossRef]
- Wang, C.; Ma, J.; Cheng, W.; Zhang, R. Thick hydroxyapatite coatings by electrophoretic deposition. Mater. Lett. 2002, 57, 99–105. [Google Scholar] [CrossRef]
- Mondragón-Cortez, P.; Vargas-Gutiérrez, G. Electrophoretic deposition of hydroxyapatite submicron particles at high voltages. Mater. Lett. 2004, 58, 1336–1339. [Google Scholar] [CrossRef]
- Meng, X.; Kwon, T.Y.; Kim, K.H. Different morphology of hydroxyapatite coatings on titanium by electrophoretic deposition. Key Eng. Mater. 2006, 309–311, 639–642. [Google Scholar] [CrossRef]
- Meng, X.; Kwon, T.Y.; Kim, K.H. Hydroxyapatite coating by electrophoretic deposition at dynamic voltage. Dent. Mater. J. 2008, 27, 666–671. [Google Scholar] [CrossRef]
- Kollatha, V.O.; Chen, Q.; Closset, R.; Luyten, J.; Traina, K.; Mullens, S.; Boccaccini, A.R.; Cloots, R. AC vs. DC electrophoretic deposition of hydroxyapatite on titanium. J. Eur. Ceram. Soc. 2013, 33, 2715–2721. [Google Scholar] [CrossRef]
- Rad, A.T.; Solati-Hashjin, M.; Osman, N.A.A.; Faghihi, S. Improved bio-physical performance of hydroxyapatite coatings obtained by electrophoretic deposition at dynamic voltage. Ceram. Int. 2014, 40, 12681–12691. [Google Scholar]
- Kumar, R.M.; Kuntal, K.K.; Singh, S.; Gupta, P.; Bhushan, B.; Gopinath, P.; Lahiri, D. Electrophoretic deposition of hydroxyapatite coating on Mg-3Zn alloy for orthopaedic application. Surf. Coat. Tech. 2016, 287, 82–92. [Google Scholar] [CrossRef]
- Muthaiah, V.M.S.; Rajput, M.; Tripathi, A.; Suwas, S.; Chatterjee, K. Electrophoretic deposition of nanocrystalline calcium phosphate coating for augmenting bioactivity of additively manufactured Ti-6Al-4V. ACS Mater. Au 2022, 2, 132–142. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; Peng, K.W. Electrophoretic deposition of porous hydroxyapatite scaffold. Biomaterials 2003, 24, 3505–3510. [Google Scholar] [CrossRef]
- Ustundag, C.B.; Kaya, F.; Kamitakahara, M.; Kaya, C.; Ioku, K. Production of tubular porous hydroxyapatite using electrophoretic deposition. J. Ceram. Soc. Jpn. 2012, 120, 569–573. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Loghmani, S.K.; Shahrabi, T.; Khanmohammadi, S. Electrophoretic deposition of hydroxyapatite nanostructured coatings with controlled porosity. J. Eur. Ceram. Soc. 2014, 34, 97–106. [Google Scholar] [CrossRef]
- Nie, X.; Leyland, A.; Matthews, A.; Jiang, J.C.; Meletis, E.I. Effects of solution pH and electrical parameters on hydroxyapatite coatings deposited by a plasma-assisted electrophoresis technique. J. Biomed. Mater. Res. 2001, 57, 612–618. [Google Scholar] [CrossRef]
- Nie, X.; Leyland, A.; Matthews, A. Deposition of layered bioceramic hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis. Surf. Coat. Tech. 2000, 125, 407–414. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef] [PubMed]
- Pani, R.; Behera, R.R.; Roy, S. Electrophoretic deposition of hydroxyapatite coating: A state of art. Mater. Today Proc. 2022, 62, 4086–4093. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Bogunia, S.; Ratajczak, P.; Aniołek, K. Effect of temperature on electrochemically assisted deposition and bioactivity of CaP coatings on CpTi grade 4. Materials 2021, 14, 5081. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.C.; Yen, S.K. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater. Sci. Eng. C 2002, 20, 153–160. [Google Scholar] [CrossRef]
- Yen, S.K.; Lin, C.M. Cathodic reactions of electrolytic hydroxyapatite coating on pure titanium. Mater. Chem. Phys. 2002, 77, 70–76. [Google Scholar] [CrossRef]
- Mokabber, T.; Lu, L.Q.; van Rijn, P.; Vakis, A.I.; Pei, Y.T. Crystal growth mechanism of calcium phosphate coatings on titanium by electrochemical deposition. Surf. Coat. Tech. 2018, 334, 526–535. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Electrodeposition of calcium phosphate coatings on metallic substrates for bone implant applications: A review. Coatings 2022, 12, 539. [Google Scholar] [CrossRef]
- Manso, M.; Jimenez, C.; Morant, C.; Herrero, P.; Martinez-Duart, J.M. Electrodeposiiton of hydroxyapatite coatings in basic conditions. Biomaterials 2000, 21, 1755–1761. [Google Scholar] [CrossRef]
- Duan, K.; Fan, Y.; Wang, R. Electrochemical deposition and patterning of calcium phosphate bioceramic coating. Ceram. Trans. 2003, 147, 53–61. [Google Scholar]
- Lu, X.; Zhao, Z.; Leng, Y. Calcium phosphate crystal growth under controlled atmosphere in electrochemical deposition. J. Cryst. Growth 2005, 284, 506–516. [Google Scholar] [CrossRef]
- Wang, S.H.; Shih, W.J.; Li, W.L.; Hon, M.H.; Wang, M.C. Morphology of calcium phosphate coatings deposited on a Ti–6Al–4V substrate by an electrolytic method under 80 Torr. J. Eur. Ceram. Soc. 2005, 25, 3287–3292. [Google Scholar] [CrossRef]
- Lin, S.; LeGeros, R.Z.; LeGeros, J.P. Adherent octacalciumphosphate coating on titanium alloy using modulated electrochemical deposition method. J. Biomed. Mater. Res. A 2003, 66A, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Drevet, R.; Velard, F.; Potiron, S.; Laurent-Maquin, D.; Benhayoune, H. In vitro dissolution and corrosion study of calcium phosphate coatings elaborated by pulsed electrodeposition current on Ti6Al4V substrate. J. Mater. Sci. Mater. Med. 2011, 22, 753–761. [Google Scholar] [CrossRef]
- Utku, F.S.; Seckin, E.; Goller, G.; Tamerler, C.; Urgen, M. Carbonated hydroxyapatite deposition at physiological temperature on ordered titanium oxide nanotubes using pulsed electrochemistry. Ceram. Int. 2014, 40, 15479–15487. [Google Scholar] [CrossRef]
- Utku, F.S.; Seckin, E.; Goller, G.; Tamerler, C.; Urgen, M. Electrochemically designed interfaces: Hydroxyapatite coated macro-mesoporous titania surfaces. Appl. Surf. Sci. 2015, 350, 62–68. [Google Scholar] [CrossRef]
- Etminanfar, M.R.; Khalil-Allafi, J.; Parsa, A.B. On the electrocrystallization of pure hydroxyapatite nanowalls on Nitinol alloy using a bipolar pulsed current. J. Alloys Compd. 2016, 678, 549–555. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sengupta, S.; Saha, P.; Das, K.; Das, S. Synthesis of calcium hydrogen phosphate and hydroxyapatite coating on SS316 substrate through pulsed electrodeposition. Mater. Sci. Eng. C 2016, 69, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Drevet, R.; Fauré, J.; Sayen, S.; Marle-Spiess, M.; El Btaouri, H.; Benhayoune, H. Electrodeposition of biphasic calcium phosphate coatings with improved dissolution properties. Mater. Chem. Phys. 2019, 236, 121797. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, C. Calcium phosphate based bioactive ceramic layers on implant materials preparation, properties, and biological performance. Coatings 2020, 10, 823. [Google Scholar] [CrossRef]
- Zai, W.; Sun, S.; Man, H.C.; Lian, J.; Zhang, Y. Preparation and anticorrosion properties of electrodeposited calcium phosphate (CaP) coatings on Mg–Zn–Ca metallic glass. Mater. Chem. Phys. 2022, 290, 126532. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Manna, J.S.; Saha, P.; Dhara, S. Synthesis, characterization and cytocompatibility assessment of hydroxyapatite-polypyrrole composite coating synthesized through pulsed reverse electrochemical deposition. Mater. Sci. Eng. C 2019, 94, 597–607. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, Y.H.; Choe, H.C.; Brantley, W.A. Surface characteristics of hydroxyapatite coatings on nanotubular Ti–25Ta–xZr alloys prepared by electrochemical deposition. Surf. Coat. Tech. 2014, 259B, 274–280. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Seah, K.H.W. Electrochemical cathodic deposition of hydroxyapatite: Improvements in adhesion and crystallinity. Mater. Sci. Eng. C 2009, 29, 1233–1238. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Cathodic electrodeposition of ceramic and organoceramic materials: Fundamental aspects. Adv. Coll. Interf. Sci. 2002, 97, 277–315. [Google Scholar] [CrossRef] [PubMed]
- Shirkhanzadeh, M. Bioactive calcium phosphate coatings prepared by electrodeposition. J. Mater. Sci. Lett. 1991, 10, 1415–1417. [Google Scholar] [CrossRef]
- Lee, K.; Jeong, Y.H.; Ko, Y.M.; Choe, H.C.; Brantley, W.A. Hydroxyapatite coating on micropore-formed titanium alloy utilizing electrochemical deposition. Thin Solid Films 2013, 549, 154–158. [Google Scholar] [CrossRef]
- Katić, J.; Metikoš-Huković, M.; Babić, R. Nitinol modified by calcium phosphate coatings prepared by sol-gel and electrodeposition methods. ECS Trans. 2013, 53, 83–93. [Google Scholar] [CrossRef]
- Katić, J.; Metikoš-Huković, M.; Škapin, S.D.; Petravić, M.; Varašanec, M. The potential-assisted deposition as valuable tool for producing functional apatite coatings on metallic materials. Electrochim. Acta 2014, 127, 173–179. [Google Scholar] [CrossRef]
- Metoki, N.; Leifenberg-Kuznits, L.; Kopelovich, W.; Burstein, L.; Gozin, M.; Eliaz, N. Hydroxyapatite coatings electrodeposited at near-physiological conditions. Mater. Lett. 2014, 119, 24–27. [Google Scholar] [CrossRef]
- Eliaz, N.; Sridhar, T.M. Electrocrystallization of hydroxyapatite and its dependence on solution conditions. Cryst. Growth Des. 2008, 8, 3965–3977. [Google Scholar] [CrossRef]
- Zogbi, M.M., Jr.; Saito, E.; Zanin, H.; Marciano, F.R.; Lobo, A.O. Hydrothermal–electrochemical synthesis of nano-hydroxyapatite crystals on superhydrophilic vertically aligned carbon nanotubes. Mater. Lett. 2014, 132, 70–74. [Google Scholar] [CrossRef]
- Wang, J.; Chao, Y.; Wan, Q.; Zhu, Z.; Yu, H. Fluoridated hydroxyapatite coatings on titanium obtained by electrochemical deposition. Acta Biomater. 2009, 5, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Matsuo, K.; Mizutani, N.; Hasegawa, J. Hydrothermal-electrochemical deposition of calcium phosphates on various metals. Dent. Mater. J. 1999, 18, 259–270. [Google Scholar] [CrossRef]
- He, D.; Liu, P.; Liu, X.; Ma, F.; Chen, X.; Li, W.; Du, J.; Wang, P.; Zhao, J. Characterization of hydroxyapatite coatings deposited by hydrothermal electrochemical method on NaOH immersed Ti6Al4V. J. Alloys Compd. 2016, 672, 336–343. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, X.B.; Ma, J.; Ni, X.Y.; Zeng, X.R.; Muhammad, A.Z.G.S.; Chen, D.Z. Post-hydrothermal treatment of hydrothermal electrodeposited CaHPO4 on C/C composites in sodium silicate-containing solution at various temperatures. Ceram. Int. 2019, 45, 5894–5903. [Google Scholar]
- Xiong, X.B.; Liu, L.; Ma, J.; Ni, X.Y.; Li, Y.Y.; Zeng, X.R. A simplified process for preparing adhesive hydroxyapatite coatings on carbon/carbon composites. Surf. Coat. Tech. 2019, 377, 124925. [Google Scholar] [CrossRef]
- Wang, J.; Layrolle, P.; Stigter, M.; de Groot, K. Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: Physicochemical characteristics and cell attachment. Biomaterials 2004, 25, 583–592. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Weiss, P.; Layrolle, P. An electrodeposition method of calcium phosphate coatings on titanium alloy. J. Mater. Sci. Mater. Med. 2007, 18, 381–390. [Google Scholar] [CrossRef]
- Peng, P.; Kumar, S.; Voelcker, N.H.; Szili, E.; Smart, R.S.C.; Griesser, H.J. Thin calcium phosphate coatings on titanium by electrochemical deposition in modified simulated body fluid. J. Biomed. Mater. Res. A 2006, 76A, 347–355. [Google Scholar] [CrossRef]
- Yousefpour, M.; Afshar, A.; Yang, X.; Li, X.; Yang, B.; Wu, Y.; Chen, J.; Zhang, X. Nano-crystalline growth of electrochemically deposited apatite coating on pure titanium. J. Electroanal. Chem. 2006, 589, 96–105. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Electrochemical nano-grained calcium phosphate coatings on Ti–6Al–4V for biomaterial applications. Scripta Mater. 2007, 56, 229–232. [Google Scholar] [CrossRef]
- Narayanan, R.; Kim, S.Y.; Kwon, T.Y.; Kim, K.H. Nanocrystalline hydroxyapatite coatings from ultrasonated electrolyte: Preparation, characterization and osteoblast responses. J. Biomed. Mater. Res. A 2008, 87A, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Kwon, T.Y.; Kim, K.H. Preparation and characteristics of nano-grained calcium phosphate coatings on titanium from ultrasonated bath at acidic pH. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85B, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, B.; Ai, S.; Wan, D. Electroplating of HAp-brushite coating on metallic bioimplants with advanced hemocompatibility and osteocompatibility properties. J. Appl. Biomater. Function. Mater. 2022, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Dutta, S.; Seshadri, S.K. Hydroxy apatite coatings on Ti–6Al–4V from seashell. Surf. Coat. Tech. 2006, 200, 4720–4730. [Google Scholar] [CrossRef]
- Daltin, A.L.; Chopart, J.P. Influence of a constant perpendicular high magnetic field on the electrodeposition of calcium phosphate coating. Magnetochemistry 2022, 8, 62. [Google Scholar] [CrossRef]
- Eliaz, N.; Eliyahu, M. Electrochemical processes of nucleation and growth of hydroxyapatite on titanium supported by real-time electrochemical atomic force microscopy. J. Biomed. Mater. Res. A 2007, 80A, 621–634. [Google Scholar] [CrossRef]
- Wang, H.; Eliaz, N.; Hobbs, L.W. The nanostructure of an electrochemically deposited hydroxyapatite coating. Mater. Lett. 2011, 65, 2455–2457. [Google Scholar] [CrossRef]
- Shirkhanzadeh, M. Electrochemical preparation of bioactive calcium phosphate coatings on porous substrates by the periodic pulse technique. J. Mater. Sci. Lett. 1993, 12, 16–19. [Google Scholar] [CrossRef]
- Liu, D.; Savino, K.; Yates, M.Z. Microstructural engineering of hydroxyapatite membranes to enhance proton conductivity. Adv. Funct. Mater. 2009, 19, 3941–3947. [Google Scholar] [CrossRef]
- Liu, D.; Savino, K.; Yates, M.Z. Coating of hydroxyapatite films on metal substrates by seeded hydrothermal deposition. Surf. Coat. Tech. 2011, 205, 3975–3986. [Google Scholar] [CrossRef]
- Fu, C.; Savino, K.; Gabrys, P.; Zeng, A.; Guan, B.; Olvera, D.; Wang, C.; Song, B.; Awad, H.; Gao, Y.; et al. Hydroxyapatite thin films with giant electrical polarization. Chem. Mater. 2015, 27, 1164–1171. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol–gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef]
- Li, T.T.; Ling, L.; Lin, M.C.; Peng, H.K.; Ren, H.T.; Lou, C.W.; Lin, J.H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited hydroxyapatite-based biocoatings: Recent progress and future challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Dev, P.R.; Anand, C.P.; Michael, D.S.; Wilson, P. Hydroxyapatite coatings: A critical review on electrodeposition parametric variations influencing crystal facet orientation towards enhanced electrochemical sensing. Mater. Adv. 2022, 3, 7773–7809. [Google Scholar] [CrossRef]
- Schnelleres Einheilen Durch Die Elektrochemische Calciumphosphat-Beschichtung BONIT®. Available online: https://www.dot-coating.de/images/pdf/beschichtungen/ortho/Elektrochemische-Calciumphosphat-Beschichtung-BONIT.pdf (accessed on 20 April 2023).
- Sassoni, E.; Masi, G.; Bignozzi, M.C.; Franzoni, E. Electrodeposition of hydroxyapatite coatings for marble protection: Preliminary results. Coatings 2019, 9, 207. [Google Scholar] [CrossRef]
- Ebelmen, J. Untersuchungen über die Verbindung der Borsaure und Kieselsaure mit Aether. Ann. Chim. Phys. Ser. 3 1846, 57, 319–355. [Google Scholar]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-gel derived hydroxyapatite coatings for titanium implants: A review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Morris, R.E. (Ed.) The Sol-Gel Process: Uniformity, Polymers and Applications; Nova Science: Hauppauge, NY, USA, 2011; 887p. [Google Scholar]
- Weng, W.; Baptisa, J.L. Alkoxide route for preparing hydroxyapatite and its coatings. Biomaterials 1998, 19, 125–131. [Google Scholar] [CrossRef]
- Liu, D.; Yang, Q.; Troczynski, T. Sol-gel hydroxyapatite coatings on stainless steel substrates. Biomaterials 2002, 23, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Metikoš-Huković, M.; Tkalacec, E.; Kwokal, A.; Piljac, J. An in vitro study of Ti and Ti-alloys coated with sol-gel derived hydroxyapatite coatings. Surf. Coat. Tech. 2003, 165, 40–50. [Google Scholar] [CrossRef]
- Kim, H.W.; Kong, Y.M.; Bae, C.J.; Noh, Y.J.; Kim, H.E. Sol–gel derived fluor-hydroxyapatite biocoatings on zirconia substrate. Biomaterials 2004, 25, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Pilliar, R. Calcium phosphate sol-gel-derived thin films on porous surfaced implants for enhanced osteoconductivity. Part I: Synthesis and characterization. Biomaterials 2004, 25, 5303–5312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xianting, Z.; Yongsheng, W.; Kui, C.; Wenjian, W. Adhesion strength of sol-gel derived fluoridated hydroxyapatite coatings. Surf. Coat. Tech. 2006, 200, 6350–6354. [Google Scholar] [CrossRef]
- Cheng, K.; Zhang, S.; Weng, W.; Khor, K.A.; Miao, S.; Wang, Y. The adhesion strength and residual stress of colloidal-sol gel derived β-tricalcium-phosphate/fluoridated-hydroxyapatite biphasic coatings. Thin Solid Films 2008, 516, 3251–3255. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.S.; Zeng, X.T.; Khor, K.A.; Weng, W.; Sun, D.E. Evaluation of adhesion strength and toughness of fluoridated hydroxyapatite coatings. Thin Solid Films 2008, 516, 5162–5167. [Google Scholar] [CrossRef]
- Aksakal, B.; Gavgali, M.; Dikici, B. The effect of coating thickness on corrosion resistance of hydroxyapatite coated Ti6Al4V and 316L SS implants. J. Mater. Eng. Perform. 2010, 19, 894–899. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K. Controlling the degradation rate of AZ91 magnesium alloy via sol-gel derived nanostructured hydroxyapatite coating. Mater. Sci. Eng. C 2013, 33, 3817–3825. [Google Scholar] [CrossRef]
- Romonţi, D.C.; Iskra, J.; Bele, M.; Demetrescu, I.; Milošev, I. Elaboration and characterization of fluorohydroxyapatite and fluoroapatite sol-gel coatings on CoCrMo alloy. J. Alloys Compd. 2016, 665, 355–364. [Google Scholar] [CrossRef]
- Jaafar, A.; Schimpf, C.; Mandel, M.; Hecker, C.; Rafaja, D.; Krüger, L.; Arki, P.; Joseph, Y. Sol-gel derived hydroxyapatite coating on titanium implants: Optimization of sol–gel process and engineering the interface. J. Mater. Res. 2022, 37, 2558–2570. [Google Scholar] [CrossRef]
- Malakauskaite-Petruleviciene, M.; Stankeviciute, Z.; Beganskiene, A.; Kareiva, A. Sol-gel synthesis of calcium hydroxyapatite thin films on quartz substrate using dip-coating and spin-coating techniques. J. Sol-Gel Sci. Tech. 2014, 71, 437–446. [Google Scholar] [CrossRef]
- Carradò, A.; Viart, N. Nanocrystalline spin coated sol-gel hydroxyapatite thin films on Ti substrate: Towards potential applications for implants. Solid State Sci. 2010, 12, 1047–1050. [Google Scholar] [CrossRef]
- Roest, R.; Latella, B.A.; Heness, G.; Ben-Nissan, B. Adhesion of sol-gel derived hydroxyapatite nanocoatings on anodised pure titanium and titanium (Ti6Al4V) alloy substrates. Surf. Coat. Tech. 2011, 205, 3520–3529. [Google Scholar] [CrossRef]
- Malakauskaite-Petruleviciene, M.; Stankeviciute, Z.; Niaura, G.; Prichodko, A.; Kareiva, A. Synthesis and characterization of sol-gel derived calcium hydroxyapatite thin films spin-coated on silicon substrate. Ceram. Int. 2015, 41, 7421–7428. [Google Scholar] [CrossRef]
- Malakauskaite-Petruleviciene, M.; Stankeviciute, Z.; Niaura, G.; Garskaite, E.; Beganskiene, A.; Kareiva, A. Characterization of sol-gel processing of calcium phosphate thin films on silicon substrate by FTIR spectroscopy. Vib. Spectrosc. 2016, 85, 16–21. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Khan, A.S.; Zeeshan, R.; Akhtar, S.; Chaudhry, A.A.; Matinlinna, J.P. An antibacterial porous calcium phosphate bilayer functional coatings on titanium dental implants. Ceram. Int. 2023, 49, 2401–2409. [Google Scholar] [CrossRef]
- Kim, H.W.; Koh, Y.H.; Li, L.H.; Lee, S.; Kim, H.E. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol-gel method. Biomaterials 2004, 25, 2533–2538. [Google Scholar] [CrossRef]
- Esfahani, H.; Dabir, F.; Taheri, M.; Sohrabi, N.; Toroghinejad, M.R. Sol-gel derived hydroxyapatite coating on TiB2/TiB/Ti substrate. Surf. Eng. 2012, 28, 526–531. [Google Scholar] [CrossRef]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca,P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef]
- Wang, X.X.; Yan, W.; Hayakawa, S.; Tsuru, K.; Osaka, A. Apatite deposition on thermally and anodically oxidized titanium surfaces in a simulated body fluid. Biomaterials 2003, 24, 4631–4637. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.O.; Allo, B.A.; Klassen, R.; Hutter, J.L.; Dixon, S.J.; Rizkalla, A.S. Control of surface topography in biomimetic calcium phosphate coatings. Langmuir 2012, 28, 3871–3880. [Google Scholar] [CrossRef] [PubMed]
- Ballo, A.M.; Xia, W.; Palmquist, A.; Lindahl, C.; Emanuelsson, L.; Lausmaa, J.; Engqvist, H.; Thomsen, P. Bone tissue reactions to biomimetic ion-substituted apatite surfaces on titanium implants. J. R. Soc. Interface 2012, 9, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Xiao, G.Y.; Xu, W.H.; Zhu, R.F.; Lu, Y.P. Factors influencing the deposition of hydroxyapatite coating onto hollow glass microspheres. Mater. Sci. Eng. C 2013, 33, 2744–2751. [Google Scholar] [CrossRef]
- Barrére, F.; Layrolle, P.; van Blitterswijk, C.A.; de Groot, K. Biomimetic calcium phosphate coatings on Ti6AI4V: A crystal growth study of octacalcium phosphate and inhibition by Mg2+ and HCO3−. Bone 1999, 25 (Suppl. 2), 107S–111S. [Google Scholar] [CrossRef]
- Barrere, F.; Layrolle, P.; van Blitterswijk, C.A.; de Groot, K. Biomimetic coatings on titanium: A crystal growth study of octacalcium phosphate. J. Mater. Sci. Mater. Med. 2001, 12, 529–534. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, D.N.; de Oliveira Cruz, L.R.; Mijares, D.Q.; Marçal, R.L.S.B.; de Campos, J.B.; Coelho, P.G.; da Silva, M.H.P. Temperature influence on the calcium phosphate coatings by chemical method. Key Eng. Mater. 2017, 720, 197–200. [Google Scholar] [CrossRef]
- Stefanic, M.; Krnel, K.; Pribosic, I.; Kosmac, T. Rapid biomimetic deposition of octacalcium phosphate coatings on zirconia ceramics (Y-TZP) for dental implant applications. Appl. Surf. Sci. 2012, 258, 4649–4656. [Google Scholar] [CrossRef]
- Stefanic, M.; Krnel, K.; Kosmac, T. Novel method for the synthesis of a β-tricalcium phosphate coating on a zirconia implant. J. Eur. Ceram. Soc. 2013, 33, 3455–3465. [Google Scholar] [CrossRef]
- Zan, Q.; Zhuang, Y.; Dong, L.; Wang, C.; Wen, N.; Xu, G. Improving bioactivity of porous β-TCP ceramics by forming bone-like apatite layer on the surfaces of pore walls. Key Eng. Mater. 2012, 512–515, 1815–1820. [Google Scholar] [CrossRef]
- Takadama, H.; Kim, H.M.; Kokubo, T.; Nakamura, T. TEM-EDX study of mechanism of bonelike apatite formation on bioactive titanium metal in simulated body fluid. J. Biomed. Mater. Res. 2001, 57, 441–448. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. A 2003, 64A, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Nishimura, D.; Doi, H.; Nomura, N.; Hanawa, T. Difference in surface reactions between titanium and zirconium in Hanks’ solution to elucidate mechanism of calcium phosphate formation on titanium using XPS and cathodic polarization. Mater. Sci. Eng. C 2009, 29, 1702–1708. [Google Scholar] [CrossRef]
- Bianchi, M.; Edreira, E.R.U.; Wolke, J.G.C.; Birgani, Z.T.; Habibovic, P.; Jansen, J.A.; Tampieri, A.; Marcacci, M.; Leeuwenburgh, S.C.G.; van den Beucken, J.J.J.P. Substrate geometry directs the in vitro mineralization of calcium phosphate ceramics. Acta Biomater. 2014, 10, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Yamaguchi, S. 1.13 Bioactive layer formation on metals and polymers. In Comprehensive Biomaterials II; Ducheyne, P., Healy, K., Hutmacher, D.W., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 311–332. [Google Scholar]
- Sun, T.; Wang, M. Electrochemical deposition of apatite/collagen composite coating on NiTi shape memory alloy and coating properties. Mater. Res. Soc. Symp. Proc. 2010, 1239, 141–146. [Google Scholar]
- Miyaji, F.; Kim, H.M.; Handa, S.; Kokubo, T.; Nakamura, T. Bonelike apatite coating on organic polymers: Novel nucleation process using sodium silicate solution. Biomaterials 1999, 20, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kishimoto, K.; Miyaji, F.; Kokubo, T.; Yao, T.; Suetsugu, Y.; Tanaka, J.; Nakamura, T. Composition and structure of apatite formed on organic polymer in simulated body fluid with a high content of carbonate ion. J. Mater. Sci. Mater. Med. 2000, 11, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Influence of ionic strength and carbonate on the Ca-P coating formation from SBF×5 solution. Biomaterials 2002, 23, 1921–1930. [Google Scholar] [CrossRef]
- Barrere, F.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Nucleation of biomimetic Ca-P coatings on Ti6Al4V from a SBF×5 solution: Influence of magnesium. Biomaterials 2002, 23, 2211–2220. [Google Scholar] [CrossRef]
- Barrere, F.; Snel, M.M.E.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Nano-scale study of the nucleation and growth of calcium phosphate coating on titanium implants. Biomaterials 2004, 25, 2901–2910. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, G.; Wang, M.; Hunziker, E.B.; Liu, Y. Crystalline biomimetic calcium phosphate coating on mini-pin implants to accelerate osseointegration and extend drug release duration for an orthodontic application. Nanomaterials 2022, 12, 2439. [Google Scholar] [CrossRef] [PubMed]
- Taş, A.C.; Bhaduri, S.B. Rapid coating of Ti6Al4V at room temperature with a calcium phosphate solution similar to 10x simulated body fluid. J. Mater. Res. 2004, 19, 2742–2749. [Google Scholar] [CrossRef]
- Vaquette, C.; Ivanovski, S.; Hamlet, S.M.; Hutmacher, D.W. Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials 2013, 34, 5538–5551. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, G.K.; Demirtaş, T.T.; Gümüşderelioğlu, M. Microwave-induced biomimetic approach for hydroxyapatite coatings of chitosan scaffolds. Carbohyd. Polym. 2017, 157, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Namlı, E.M.; Dolaz, M.; Aygan, A. 10XSBF bone-like hydroxyapatite coating of Ti–6Al–4V with microwave irradiation and its antibacterial properties. Trans. Indian I. Metals 2023, 76, 1281–1290. [Google Scholar] [CrossRef]
- Nijhuis, A.W.G.; Nejadnik, M.R.; Nudelman, F.; Walboomers, X.F.; te Riet, J.; Habibovic, P.; Birgani, Z.T.; Li, Y.; Bomans, P.H.H.; Jansen, J.A.; et al. Enzymatic pH control for biomimetic deposition of calcium phosphate coatings. Acta Biomater. 2014, 10, 931–939. [Google Scholar] [CrossRef]
- Li, F.; Feng, Q.L.; Cui, F.Z.; Li, H.D.; Schubert, H. A simple biomimetic method for calcium phosphate coating. Surf. Coat. Tech. 2002, 154, 88–93. [Google Scholar] [CrossRef]
- Dorozhkina, E.I.; Dorozhkin, S.V. Structure and properties of the precipitates formed from condensed solutions of the revised simulated body fluid. J. Biomed. Mater. Res. A 2003, 67A, 578–581. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Bracci, B.; Facchini, A.; Panzavolta, S.; Segatti, F.; Struba, L. Nanocrystalline hydroxyapatite coatings on titanium: A new fast biomimetic method. Biomaterials 2005, 26, 4085–4089. [Google Scholar] [CrossRef]
- Bracci, B.; Panzavolta, S.; Bigi, A. A new simplified calcifying solution to synthesize calcium phosphate coatings. Surf. Coat. Tech. 2013, 232, 13–21. [Google Scholar] [CrossRef]
- Zheng, Y.; Dong, G.; He, L.; Wu, G.; Zheng, H.; Deng, C. In vitro study of calcium phosphate layers on hydroxyapatite ceramics surface mineralized in different solutions. Ceram. Int. 2016, 42, 1660–1665. [Google Scholar] [CrossRef]
- Morejón-Alonso, L.; Bussulo, M.A.; Debone, R.; González-Martínez, E.; González, J.E. Apatite coatings on chemically modified titanium using a new accelerated biomimetic route. Mater. Lett. 2020, 280, 128576. [Google Scholar] [CrossRef]
- Zhou, H.; Nabiyouni, M.; Bhaduri, S.B. Microwave assisted apatite coating deposition on Ti6Al4V implants. Mater. Sci. Eng. C 2013, 33, 4435–4443. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhou, H.; Nabiyouni, M.; Bhaduri, S.B. Rapid coating of AZ31 magnesium alloy with calcium deficient hydroxyapatite using microwave energy. Mater. Sci. Eng. C 2015, 49, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Guo, Y.; Huang, Z.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Preparation and corrosion behaviors of calcium phosphate conversion coating on magnesium alloy. Surf. Coat. Tech. 2016, 307, 99–108. [Google Scholar] [CrossRef]
- Shen, S.; Cai, S.; Li, Y.; Ling, R.; Zhang, F.; Xu, G.; Wang, F. Microwave aqueous synthesis of hydroxyapatite bilayer coating on magnesium alloy for orthopedic application. Chem. Eng. J. 2017, 309, 278–287. [Google Scholar] [CrossRef]
- Chandanshive, B.; Dyondi, D.; Ajgaonkar, V.R.; Banerjee, R.; Khushalani, D. Biocompatible calcium phosphate based tubes. J. Mater. Chem. 2010, 20, 6923–6928. [Google Scholar] [CrossRef]
- Brinker, C.J.; Frye, G.C.; Hurd, A.J.; Ashley, C.S. Fundamentals of sol-gel dip coating. Thin Solid Films 1991, 201, 97–108. [Google Scholar] [CrossRef]
- Lacefield, W.R. Hydroxyapatite coatings. Ann. N. Y. Acad. Sci. 1988, 523, 72–80. [Google Scholar] [CrossRef]
- Duan, K.; Tang, A.; Wang, R. A new evaporation-based method for the preparation of biomimetic calcium phosphate coatings on metals. Mater. Sci. Eng. C 2009, 29, 1334–1337. [Google Scholar] [CrossRef]
- Pontin, M.G.; Lange, F.F.; Sanchez-Herencia, A.J.; Moreno, R. Effect of unfired tape porosity on surface film formation by dip coating. J. Am. Ceram. Soc. 2005, 88, 2945–2948. [Google Scholar] [CrossRef]
- Gu, Y.; Meng, G. A model for ceramic membrane formation by dip-coating. J. Eur. Ceram. Soc. 1999, 19, 1961–1966. [Google Scholar] [CrossRef]
- Li, T.; Lee, J.; Kobayashi, T.; Aoki, H. Hydroxyapatite coating by dipping method, and bone bonding strength. J. Mater. Sci. Mater. Med. 1996, 7, 355–357. [Google Scholar] [CrossRef]
- Mavis, B.; Taş, A.C. Dip coating of calcium hydroxyapatite on Ti–6Al–4V substrates. J. Am. Ceram. Soc. 2000, 83, 989–991. [Google Scholar] [CrossRef]
- Amiruddin, A.M.H.; Shamsudin, R.; Jalar, A.; Hamid, M.A.A. Single layer brushite coating onto stainless steel substrate using dip-coating technique. Adv. Mater. Res. 2012, 399–401, 2004–2007. [Google Scholar] [CrossRef]
- Albano, M.P.; Garrido, L.B. Effect of zirconia tape porosity on fluorapatite surface film formation by dip coating. Ceram. Int. 2013, 39, 29–37. [Google Scholar] [CrossRef]
- Albano, M.P.; Garrido, L.B.; Teixeira, L.N.; Rosa, A.L.; Oliveira, P.T. Comparison of different fluorapatite dip coated layers on porous zirconia tapes. Ceram. Int. 2014, 40, 12509–12517. [Google Scholar] [CrossRef]
- Sonmez, S.; Aksakal, B.; Dikici, B. Influence of hydroxyapatite coating thickness and powder particle size on corrosion performance of MA8M magnesium alloy. J. Alloys Compd. 2014, 596, 125–131. [Google Scholar] [CrossRef]
- You, C.; Yeo, I.S.; Kim, M.D.; Eom, T.K.; Lee, J.Y.; Kim, S. Characterization and in vivo evaluation of calcium phosphate coated cp-titanium by dip-spin method. Curr. Appl. Phys. 2005, 5, 501–506. [Google Scholar] [CrossRef]
- Yuan, Q.; Sahu, L.K.; D’Souza, N.A.; Golden, T.D. Synthesis of hydroxyapatite coatings on metal substrates using a spincasting technique. Mater. Chem. Phys. 2009, 116, 523–526. [Google Scholar] [CrossRef]
- Mennicke, U.; Salditt, T. Preparation of solid-supported lipid bilayers by spin-coating. Langmuir 2002, 18, 8172–8177. [Google Scholar] [CrossRef]
- Tunkara, E.; Dag, Ö. Salt-acid-surfactant lyotropic liquid crystalline mesophases: Synthesis of highly transparent mesoporous calcium hydroxyapatite thin films. Eur. J. Inorg. Chem. 2016, 2016, 2114–2121. [Google Scholar] [CrossRef]
- Fujishiro, Y.; Fuiimoto, A.; Sato, T.; Okuwaki, A. Coating of hydroxyapatite on titanium plates using thermal dissociation of calcium-EDTA chelate complex in phosphate solutions under hydrothermal conditions. J. Coll. Interf. Sci. 1995, 173, 119–127. [Google Scholar] [CrossRef]
- Najdoski, M.Z.; Majhi, P.; Grozdanov, I.S. A simple chemical method for preparation of hydroxyapatite coatings on Ti6Al4V substrate. J. Mater. Sci. Mater. Med. 2001, 12, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Hiromoto, S.; Tomozawa, M. Corrosion behavior of magnesium with hydroxyapatite coatings formed by hydrothermal treatment. Mater. Trans. 2010, 51, 2080–2087. [Google Scholar] [CrossRef]
- Tomozawa, M.; Hiromoto, S. Microstructure of hydroxyapatite- and octacalcium phosphate-coatings formed on magnesium by a hydrothermal treatment at various pH values. Acta Mater. 2011, 59, 355–363. [Google Scholar] [CrossRef]
- Kim, S.M.; Jo, J.H.; Lee, S.M.; Kang, M.H.; Kim, H.E.; Estrin, Y.; Lee, J.H.; Lee, J.W.; Koh, Y.H. Hydroxyapatite-coated magnesium implants with improved in vitro and in vivo biocorrosion, biocompatibility, and bone response. J. Biomed. Mater. Res. A 2014, 102A, 429–441. [Google Scholar] [CrossRef]
- Li, J.L.; Wang, S.; Cao, F.; Lin, X.; Wei, X.W.; Zhao, Z.H.; Dou, X.J.; Yu, W.T.; Yang, K.; Zhao, D.W. Fabrication and characterization of nanopillar-like HA coating on porous Ti6Al4V scaffold by a combination of alkali–acid-heat and hydrothermal treatments. Acta Metall. Sin. Eng. 2019, 32, 1075–1088. [Google Scholar] [CrossRef]
- Suchanek, K.; Perzanowski, M.; Lekki, J.; Strąg, M.; Marszałek, M. Ammonium hydroxide mediated hydrothermal crystallization of hydroxyapatite coatings on titanium substrate. Ceramics 2019, 2, 180–189. [Google Scholar] [CrossRef]
- Kien, P.; Quan, T.; Anh, L.T. Coating characteristic of hydroxyapatite on titanium substrates via hydrothermal treatment. Coatings 2021, 11, 1226. [Google Scholar] [CrossRef]
- Santiago, E.; Martin, V.; Colaço, B.; Fernandes, M.H.; Santos, C.; Gomes, P.S. Hydrothermal synthesis of fluorapatite coatings over titanium implants for enhanced osseointegration—An in vivo study in the rabbit. J. Funct. Biomater. 2022, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, J.; Chen, G.; Zhu, P. Convenient synthesis of hydroxyapatite-coated ferroferric oxide microspheres by hydrothermal method. Mater. Lett. 2019, 253, 218–221. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Hamdy, A.S.; Khalil, K.A.; Lim, J.H. A novel simple in situ biomimetic and simultaneous deposition of bonelike apatite within biopolymer matrix as bone graft substitutes. Mater. Lett. 2014, 137, 260–264. [Google Scholar] [CrossRef]
- Mishima, F.D.; Louro, L.H.L.; Moura, F.N.; Gobbo, L.A.; da Silva, M.H.P. Hydroxyapatite scaffolds produced by hydrothermal deposition of monetite on polyurethane sponges substrates. Key Eng. Mater. 2012, 493–494, 820–825. [Google Scholar] [CrossRef]
- Xiao, D.; Guo, T.; Yang, F.; Feng, G.; Shi, F.; Li, J.; Wang, D.; Duan, K.; Weng, J. In situ formation of nanostructured calcium phosphate coatings on porous hydroxyapatite scaffolds using a hydrothermal method and the effect on mesenchymal stem cell behavior. Ceram. Int. 2017, 43, 1588–1596. [Google Scholar] [CrossRef]
- Sasaki, M.; Inoue, M.; Katada, Y.; Hiromoto, S.; Taguchi, T. Formation of hydroxyapatite on nickel-free high-nitrogen stainless steel by chemical solution deposition method in neutral/alkaline solution. Key Eng. Mater. 2013, 529–530, 237–242. [Google Scholar] [CrossRef]
- Hiromoto, S.; Tomozawa, M.; Maruyama, N. Fatigue property of a bioabsorbable magnesium alloy with a hydroxyapatite coating formed by a chemical solution deposition. J. Mech. Behav. Biomed. Mater. 2013, 25, 1–10. [Google Scholar] [CrossRef]
- Ohtsu, N.; Hiromoto, S.; Yamane, M.; Satoh, K.; Tomozawa, M. Chemical and crystallographic characterizations of hydroxyapatite- and octacalcium phosphate-coatings on magnesium synthesized by chemical solution deposition using XPS and XRD. Surf. Coat. Tech. 2013, 218, 114–118. [Google Scholar] [CrossRef]
- Lee, L.H.; Han, J.S. Deposition behavior and characteristics of hydroxyapatite coatings on Al2O3, Ti, and Ti6Al4V formed by a chemical bath method. Ceram. Int. 2014, 40, 5321–5326. [Google Scholar] [CrossRef]
- Asl, S.K.F.; Nemeth, S.; Tan, M.J. Hydrothermally deposited protective and bioactive coating for magnesium alloys for implant application. Surf. Coat. Tech. 2014, 258, 931–937. [Google Scholar]
- Asl, S.K.F.; Nemeth, S.; Tan, M.J. Mechanism of calcium phosphate deposition in a hydrothermal coating process. Surf. Coat. Tech. 2015, 270, 197–205. [Google Scholar]
- Morales, J.G.; Clemente, R.R.; Armas, B.; Combescure, C.; Berjoan, R.; Cubo, J.; Martínez, E.; Carmona, J.G.; Garelik, S.; Murtra, J.; et al. Controlled nucleation and growth of thin hydroxyapatite layers on titanium implants by using induction heating technique. Langmuir 2004, 20, 5174–5178. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.B.; Zeng, X.R.; Zou, C.L. Preparation of enhanced HA coating on H2O2-treated carbon/carbon composite by induction heating and hydrothermal treatment methods. Mater. Chem. Phys. 2009, 114, 434–438. [Google Scholar]
- Xiong, X.B.; Zou, C.L.; Zeng, X.R.; Li, P.; Fa, Y.B.; Tang, H.L.; Xie, S.H. Transformation of induction heating deposited monetite coating to hydroxyapatite coating on HT-C/C composites by hydrothermal treatment in two types of solution. Mater. Sci. Eng. C 2009, 29, 2019–2023. [Google Scholar]
- Xiong, X.B.; Ni, X.Y.; Zeng, X.R.; Zou, J.Z. A study of monetite precipitation on HT-C/C composites by induction heating method at different substrate temperatures. Surf. Coat. Tech. 2013, 223, 6–10. [Google Scholar] [CrossRef]
- Ni, X.Y.; Chu, C.C.; Xiong, X.B.; Li, A.J.; Bai, R.C. Preparation of hydroxyapatite coating using chemical liquid vaporization deposition on carbon/carbon composites. RSC Adv. 2014, 4, 41129–41134. [Google Scholar]
- Ziani-Cherif, H.; Abe, Y.; Imachi, K.; Matsuda, T. Hydroxyapatite coating on titanium by thermal substrate method in aqueous solution. J. Biomed. Mater. Res. 2002, 59, 390–397. [Google Scholar]
- Kuroda, K.; Miyashita, Y.; Ichino, R.; Okido, M.; Takai, O. Preparation of calcium phosphate coatings on titanium using the thermal substrate method and their in vitro evaluation. Mater. Trans. 2002, 43, 3015–3019. [Google Scholar] [CrossRef]
- Kuroda, K.; Nakamoto, S.; Ichino, R.; Okido, M.; Pilliar, R.M. Hydroxyapatite coatings on a 3D porous surface using thermal substrate method. Mater. Trans. 2005, 46, 1633–1635. [Google Scholar] [CrossRef]
- Miyazaki, H.; Maeda, H.; Yoshida, S.; Suzuki, H.; Ota, T. Deposition of hydroxyapatite thin films from saturated calcium phosphate solution by controlling the substrate temperature. J. Ceram. Soc. Jpn. 2014, 122, 835–837. [Google Scholar] [CrossRef]
- Taguchi, T.; Kishida, A.; Akashi, M. Hydroxyapatite formation on/in poly(vinyl alcohol) hydrogel matrices using a novel alternate soaking process. Chem. Lett. 1998, 27, 711–712. [Google Scholar] [CrossRef]
- Yang, H.; Masse, S.; Zhang, H.; Hélary, C.; Li, L.; Coradin, T. Surface reactivity of hydroxyapatite nanocoatings deposited on iron oxide magnetic spheres toward toxic metals. J. Coll. Interf. Sci. 2014, 417, 1–8. [Google Scholar] [CrossRef]
- Furuzono, T.; Taguchi, T.; Kishida, A.; Akashi, M.; Tamada, Y. Preparation and characterization of apatite deposited on silk fabric using an alternate soaking process. J. Biomed. Mater. Res. 2000, 50, 344–352. [Google Scholar] [CrossRef]
- Góes, J.C.; Figueiró, S.D.; Oliveira, A.M.; Macedo, A.A.; Silva, C.C.; Ricardo, N.M.; Sombra, A.S. Apatite coating on anionic and native collagen films by an alternate soaking process. Acta Biomater. 2007, 3, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Miyamoto, M.; Ban, S. Bioactive apatite coating on titanium using an alternate soaking process. Dent. Mater. J. 2007, 26, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Onomoto, H.; Kashiwazaki, H.; Inoue, N.; Koyama, Y.; Takakuda, K.; Tanaka, J. Improvement of biocompatibility of chitosan fiber modified by Ca-phosphate deposition through an alternate soaking process. Mater. Trans. 2009, 50, 1269–1272. [Google Scholar] [CrossRef]
- Chang, W.; Mu, X.; Zhu, X.; Ma, G.; Li, C.; Xu, F.; Nie, J. Biomimetic composite scaffolds based mineralization of hydroxyapatite on electrospun calcium-containing poly(vinyl alcohol) nanofibers. Mater. Sci. Eng C 2013, 33, 4369–4376. [Google Scholar] [CrossRef] [PubMed]
- Izawa, H.; Nishino, S.; Maeda, H.; Morita, K.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Kadokawa, J.I. Mineralization of hydroxyapatite upon a unique xanthan gum hydrogel by an alternate soaking process. Carbohyd. Polym. 2014, 102, 846–851. [Google Scholar] [CrossRef]
- Harding, J.L.; Krebs, M.D. Bioinspired deposition-conversion synthesis of tunable calcium phosphate coatings on polymeric hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 2024–2032. [Google Scholar] [CrossRef]
- Chatelain, G.; Bourgeois, D.; Ravaux, J.; Averseng, O.; Vidaud, C.; Meyer, D. Alternate dipping preparation of biomimetic apatite layers in the presence of carbonate ions. Biomed. Mater. 2014, 9, 015003. [Google Scholar] [CrossRef]
- Anawati, A.H.; Ono, S. Enhanced uniformity of apatite coating on a PEO film formed on AZ31 Mg alloy by an alkali pretreatment. Surf. Coat. Tech. 2015, 272, 182–189. [Google Scholar] [CrossRef]
- Strangem, D.G.T.; Oyen, M.L. Biomimetic bone-like composites fabricated through an automated alternate soaking process. Acta Biomater. 2011, 7, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liao, J.; Tian, T.; Zhang, Q.; Cai, X. A potential flower-like coating consisting of calcium-phosphate nanosheets on titanium surface. Chin. Chem. Lett. 2017, 28, 1893–1896. [Google Scholar] [CrossRef]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Gofer, Y.; Borodianskiy, K.; Sobolev, A. Hydroxyapatite coating on Ti-6Al-7Nb alloy by plasma electrolytic oxidation in salt-based electrolyte. Materials 2022, 15, 7374. [Google Scholar] [CrossRef]
- Kazantseva, E.A.; Komarova, E.G. Microstructure and elemental composition of UMAO calcium phosphate coatings. Procedia Struct. Integr. 2022, 40C, 207–213. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Komarova, E.G.; Kazantseva, E.A.; Lozhkomoev, A.S.; Kazantsev, S.O.; Bakina, O.V.; Mishina, M.V.; Zima, A.P.; Krivoshchekov, S.V.; Khlusov, I.A.; et al. UMAOH calcium phosphate coatings designed for drug delivery: Vancomycin, 5-fluorouracil, interferon α-2b case. Materials 2022, 15, 4643. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A. Characterisation of oxide films produced by plasma electrolytic oxidation of a Ti–6Al–4V alloy. Surf. Coat. Tech. 2000, 130, 195–206. [Google Scholar] [CrossRef]
- Legostaeva, E.V.; Sharkeev, Y.P.; Epple, M.; Prymak, O. Structure and properties of microarc calcium phosphate coatings on the surface of titanium and zirconium alloys. Russ. Phys. J. 2014, 56, 1130–1136. [Google Scholar] [CrossRef]
- Sun, J.; Han, Y.; Huang, X. Hydroxyapatite coatings prepared by micro-arc oxidation in Ca- and P-containing electrolyte. Surf. Coat. Tech. 2007, 201, 5655–5658. [Google Scholar] [CrossRef]
- Yang, X.; Yu, S.; Li, W. Preparation of bioceramic films containing hydroxyapatites on Ti–6Al–4V alloy surfaces by the micro-arc oxidation technique. Mater. Res. Bull. 2009, 44, 947–949. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Zhao, T.G. Effects of phosphates on microstructure and bioactivity of micro-arc oxidized calcium phosphate coatings on Mg–Zn–Zr magnesium alloy. Colloid Surf. B 2013, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharkeev, Y.P.; Kulyashova, K.S. Regularities of forming calcium phosphate coatings on zirconium from electrolytes based on synthesized and biological hydroxyapatite. Russ. Phys. J. 2014, 56, 1170–1175. [Google Scholar] [CrossRef]
- Seyfoori, A.; Mirdamadi, S.; Seyedraoufi, Z.S.; Khavandi, A.; Aliofkhazraei, M. Synthesis of biphasic calcium phosphate containing nanostructured films by micro arc oxidation on magnesium alloy. Mater. Chem. Phys. 2013, 142, 87–94. [Google Scholar] [CrossRef]
- Bai, Y.; Kim, K.A.; Park, I.S.; Lee, S.J.; Bae, T.S.; Lee, M.H. In situ composite coating of titania-hydroxyapatite on titanium substrate by micro-arc oxidation coupled with electrophoretic deposition processing. Mater. Sci. Eng. B 2011, 176, 1213–1221. [Google Scholar] [CrossRef]
- Rojaeea, R.; Fathia, M.; Raeissi, K. Electrophoretic deposition of nanostructured hydroxyapatite coating on AZ91 magnesium alloy implants with different surface treatments. Appl. Surf. Sci. 2013, 285B, 664–673. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. Biodegradable magnesium alloy coated by fluoridated hydroxyapatite using MAO/EPD technique. Surf. Eng. 2014, 30, 545–551. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, Y.; Wang, Y.; Jia, D. Characteristic of microarc oxidized coatings on titanium alloy formed in electrolytes containing chelate complex and nano-HA. Appl. Surf. Sci. 2007, 253, 5045–5050. [Google Scholar] [CrossRef]
- Matykina, E.; Montuori, M.; Gough, J.; Monfort, F.; Berkani, A.; Skeldon, P.; Thompson, G.E.; Habazaki, H. Spark anodising of titanium for biomedical applications. T. I. Met. Finish. 2006, 84, 125–133. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Matthews, A. Deposition and evaluation of duplex hydroxyapatite and plasma electrolytic oxidation coatings on magnesium. Surf. Coat. Tech. 2015, 269, 170–182. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.; Wang, F.; Zhao, L.; Shimizu, T. Biomimetic deposition of apatite coatings on micro-arc oxidation treated biomedical NiTi alloy. Surf. Coat. Tech. 2010, 204, 3294–3299. [Google Scholar] [CrossRef]
- Teng, H.P.; Yang, C.J.; Lin, J.F.; Huang, Y.H.; Lu, F.H. A simple method to functionalize the surface of plasma electrolytic oxidation produced TiO2 coatings for growing hydroxyapatite. Electrochim. Acta 2016, 193, 216–224. [Google Scholar] [CrossRef]
- Li, Y.; Lee, I.S.; Cui, F.Z.; Choi, S.H. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium. Biomaterials 2008, 29, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liang, C.; Huang, N.; Duan, F.; Wang, L. Formation of hydroxyapatite produced by microarc oxidation coupled with sol-gel technology. Mater. Manuf. Process. 2014, 29, 1085–1094. [Google Scholar] [CrossRef]
- Liu, F.; Song, Y.; Wang, F.; Shimizu, T.; Igarashi, K.; Zhao, L. Formation characterization of hydroxyapatite on titanium by microarc oxidation and hydrothermal treatment. J. Biosci. Bioeng. 2005, 100, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ryu, J.J.; Sung, Y.M. One-step approach for nano-crystalline hydroxyapatite coating on titanium via micro-arc oxidation. Electrochem. Commun. 2007, 9, 1886–1891. [Google Scholar] [CrossRef]
- Han, Y.; Sun, J.; Huang, X. Formation mechanism of HA-based coatings by micro-arc oxidation. Electrochem. Comm. 2008, 10, 510–513. [Google Scholar] [CrossRef]
- Ou, S.F.; Lin, C.S.; Pan, Y.N. Microstructure and surface characteristics of hydroxyapatite coating on titanium and Ti-30Nb-1Fe-1Hf alloy by anodic oxidation and hydrothermal treatment. Surf. Coat. Tech. 2011, 205, 2899–2906. [Google Scholar] [CrossRef]
- Durdu, S.; Deniz, Ö.F.; Kutbay, I.; Usta, M. Characterization and formation of hydroxyapatite on Ti6Al4V coated by plasma electrolytic oxidation. J. Alloys Compd. 2013, 551, 422–429. [Google Scholar] [CrossRef]
- Mohedano, M.; Guzman, R.; Arrabal, R.; Lacomba, J.L.L.; Matykina, E. Bioactive plasma electrolytic oxidation coatings—the role of the composition, microstructure, and electrochemical stability. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101B, 1524–1537. [Google Scholar] [CrossRef]
- Tang, H.; Gao, Y. Preparation and characterization of hydroxyapatite containing coating on AZ31 magnesium alloy by micro-arc oxidation. J. Alloys Compd. 2016, 688, 699–708. [Google Scholar] [CrossRef]
- Shin, K.R.; Yoon, S.I.; Ko, Y.G.; Shin, D.H. Deposition of hydroxyl-apatite on titanium subjected to electrochemical plasma coating. Electrochim. Acta 2013, 109, 173–180. [Google Scholar] [CrossRef]
- Lugovskoy, A.; Lugovskoy, S. Production of hydroxyapatite layers on the plasma electrolytically oxidized surface of titanium alloys. Mater. Sci. Eng. C 2014, 43, 527–532. [Google Scholar] [CrossRef]
- Rafieerad, A.R.; Ashra, M.R.; Mahmoodian, R.; Bushroa, A.R. Surface characterization and corrosion behavior of calcium phosphate-base composite layer on titanium and its alloys via plasma electrolytic oxidation: A review paper. Mater. Sci. Eng. C 2015, 57, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Li, Y.; Munir, K.; Wen, C. Calcium phosphate-based composite coating by micro-arc oxidation (MAO) for biomedical application: A review. Crit. Rev. Solid State Mater. Sci. 2018, 43, 392–416. [Google Scholar] [CrossRef]
- Bocanegra-Bernal, M.H. Hot isostatic pressing (HIP) technology and its applications to metals and ceramics. J. Mater. Sci. 2004, 39, 6399–6420. [Google Scholar] [CrossRef]
- Khor, K.; Yip, C.; Cheang, P. Post-spray hot isostatic pressing of plasma sprayed Ti–6Al–4V/hydroxyapatite composite coatings. J. Mater. Process. Tech. 1997, 71, 280–287. [Google Scholar] [CrossRef]
- Herø, H.; Wie, H.; Jorgensen, R.B.; Ruyter, I.E. Hydroxyapatite coatings on Ti produced by hot isostatic pressing. J. Biomed. Mater. Res. 1994, 28, 343–348. [Google Scholar] [CrossRef]
- Wie, H.; Hero, H.; Solheim, T. Hot isostatic pressing-processed hydroxyapatite-coated titanium implants: Light microscopic and scanning electron microscopy investigations. Int. J. Oral. Maxillofac. Implant. 1998, 13, 837–844. [Google Scholar]
- Fu, Y.; Batchelor, A.W.; Khor, K.A. Hot isostatic pressing of hydroxyapatite coating for improved fretting wear resistance. J. Mater. Sci. Lett. 1998, 17, 1695–1696. [Google Scholar] [CrossRef]
- Nonami, T.; Kamiya, A.; Naganuma, K.; Kameyana, T. Implantation of hydroxyapatite granules into superplastic titanium alloy for biomaterials. Mater. Sci. Eng. C 1998, 6, 281–284. [Google Scholar] [CrossRef]
- Nonami, T.; Kamiya, A.; Naganuma, K.; Kameyana, T. Preparation of hydroxyapatite-granule-implanted superplastic titanium-alloy. J. Mater. Sci. Mater. Med. 1998, 9, 203–206. [Google Scholar] [CrossRef]
- Parast, S.Y.; Jauhari, I.; Zaeem, M.A. Implantation of HA into superplastic Ti–6Al–4V: Kinetics and mechanical behaviors of implanted layer. Metall. Mater. Trans. A 2011, 42A, 219–226. [Google Scholar] [CrossRef]
- Khalid, H.M.; Jauhari, I.; Dom, A.H.M. Development of nanolayer hydroxyapatite (HA) on titanium alloy via superplastic deformation method. Metall. Mater. Trans. A 2012, 43A, 3776–3785. [Google Scholar] [CrossRef]
- Khalid, H.M.; Jauhari, I.; Jamlus, S.A.; Dom, A.H.M. High temperature deformation of Ti alloy superplastically embedded with HA. Mater. Sci. Eng. A 2012, 534, 37–42. [Google Scholar] [CrossRef]
- Khalid, H.M.; Hasan, S.N.; Jauhari, I. Mechanical behaviour of nanolayer hydroxyapatite (HA) coating developed through superplastic embedment of titanium (Ti) alloy (Ti–6Al–4V). Mater. Chem. Phys. 2016, 177, 314–321. [Google Scholar] [CrossRef]
- Onoki, T.; Yamamoto, S.Y. Hydroxyapatite ceramics coating on magnesium alloy via a double layered capsule hydrothermal hot-pressing. J. Ceram. Soc. Jpn. 2010, 118, 749–752. [Google Scholar] [CrossRef]
- Onoki, T.; Tanaka, M.A.; Hashida, T. New processing method for hydroxyapatite coating by hydrothermal techniques. J. Jpn. Soc. Powder Powder Metall. 2005, 52, 861–864. [Google Scholar] [CrossRef]
- Onoki, T.; Kuno, T.; Nakahira, A.; Hashida, T. Effects of titanium surface treatment on adhesive properties of hydroxyapatite ceramics coating to titanium substrates by double layered capsule hydrothermal hot-pressing. J. Ceram. Soc. Jpn. 2010, 118, 530–534. [Google Scholar] [CrossRef]
- Klyui, N.I.; Temchenko, V.P.; Gryshkov, A.P.; Dubok, V.A.; Shynkaruk, A.V.; Lyashenko, B.A.; Barynov, S.M. Properties of the hydroxyapatite coatings obtained by gas-detonation deposition onto titanium substrates. Funct. Mater. 2011, 18, 285–292. [Google Scholar]
- Klyui, M.I.; Temchenko, V.P.; Gryshkov, O.P.; Dubok, V.A.; Kladko, V.P.; Kuchuk, A.V.; Dzhagan, V.M.; Yukhymchuk, V.O.; Kiselov, V.S. Bio-SiC ceramics coated with hydroxyapatite using gas-detonation deposition: An alternative to titanium-based medical implants. Funct. Mater. 2013, 20, 163–171. [Google Scholar] [CrossRef]
- Zatovsky, I.V.; Nikolenko, I.O.; Slobodyanyk, M.S.; Klyui, M.I.; Temchenko, V.P. Interaction of acid calcium phosphates with CaO (CaCO3) during their deposition onto titanium surface under conditions of gas detonation deposition. Rep. NAS Ukr. 2017, 5, 66–72. [Google Scholar]
- Bulina, N.V.; Rybin, D.K.; Makarova, S.V.; Dudina, D.V.; Batraev, I.S.; Utkin, A.V.; Prosanov, I.Y.; Khvostov, M.V.; Ulianitsky, V.Y. Detonation spraying of hydroxyapatite on a titanium alloy implant. Materials 2021, 14, 4852. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, A.S.; Tsygankov, P.A.; Vesnin, V.R.; Parshin, B.A.; Zaitsev, V.V.; Lukina, Y.S. Physicochemical properties and osseointegration of titanium implants with bioactive calcium phosphate coatings produced by detonation spraying. Inorg. Mater. 2022, 58, 71–77. [Google Scholar] [CrossRef]
- Manso, M.; Langletm, M.; Jimenezm, C.; Martinez-Duart, J.M. Microstructural study of aerosol-gel derived hydroxyapatite coatings. Biomol. Eng. 2002, 19, 63–66. [Google Scholar] [CrossRef]
- Manso, M.; Ogueta, S.; Herrero-Fernández, P.; Vázquez, L.; Langlet, M.; García-Ruiz, J.P. Biological evaluation of aerosol-gel-derived hydroxyapatite coatings with human mesenchymal stem cells. Biomaterials 2002, 23, 3985–3990. [Google Scholar] [CrossRef]
- Manso, M.; Martínez-Duart, J.M.; Langlet, M.; Jiménez, C.; Herrero, P.; Millon, E. Aerosol-gel-derived microcrystalline hydroxyapatite coatings. J. Mater. Res. 2002, 17, 1482–1489. [Google Scholar] [CrossRef]
- Manso-Silván, M.; Langlet, M.; Jiménez, C.; Fernández, M.; Martínez-Duart, J.M. Calcium phosphate coatings prepared by aerosol-gel. J. Eur. Ceram. Soc. 2003, 23, 243–246. [Google Scholar] [CrossRef]
- Seo, D.S.; Chae, H.C.; Lee, J.K. Fabrication and microstructure of hydroxyapatite coatings on zirconia by room temperature spray process. J. Nanosci. Nanotech. 2015, 15, 6039–6043. [Google Scholar] [CrossRef]
- Seo, D.S.; Lee, J.K.; Hwang, K.H.; Hahn, B.D.; Yoon, S.Y. Influence of starting powders on hydroxyapatite coatings fabricated by room temperature spraying method. J. Nanosci. Nanotech. 2015, 15, 6032–6038. [Google Scholar] [CrossRef]
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Kim, J.W.; Cho, Y.L.; Park, C.; Kim, H.E.; et al. Preparation and in vitro characterization of aerosol-deposited hydroxyapatite coatings with different surface roughnesses. Appl. Surf. Sci. 2011, 257, 7792–7799. [Google Scholar] [CrossRef]
- Hahn, B.D.; Cho, Y.L.; Park, D.S.; Choi, J.J.; Ryu, J.; Kim, J.W.; Ahn, C.W.; Park, C.; Kim, H.E.; Kim, S.G. Effect of fluorine addition on the biological performance of hydroxyapatite coatings on Ti by aerosol deposition. J. Biomater. Appl. 2013, 27, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Seo, D.S.; Lee, J.K. Fabrication of xenogeneic bone-derived hydroxyapatite thin film by aerosol deposition method. Appl. Surf. Sci. 2008, 255, 388–390. [Google Scholar] [CrossRef]
- Jo, J.H.; Li, Y.; Kim, S.M.; Kim, H.E.; Koh, Y.H. Hydroxyapatite/poly(ε-caprolactone) double coating on magnesium for enhanced corrosion resistance and coating flexibility. J. Biomater. Appl. 2013, 28, 617–625. [Google Scholar] [CrossRef]
- Kitajima, A.; Tsukamoto, M.; Akedo, J. Hydroxyapatite film coated on poly-L-lactic acid by aerosol deposition method. J. Ceram. Soc. Jpn. 2010, 118, 417–420. [Google Scholar] [CrossRef]
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Kim, J.W.; Ahn, C.W.; Kim, H.E.; Yoon, B.H.; et al. Osteoconductive hydroxyapatite coated PEEK for spinal fusion surgery. Appl. Surf. Sci. 2013, 283, 6–11. [Google Scholar] [CrossRef]
- Hahn, B.D.; Lee, J.M.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Lee, B.K.; Shin, D.S.; Kim, H.E. Mechanical and in vitro biological performances of hydroxyapatite-carbon nanotube composite coatings deposited on Ti by aerosol deposition. Acta Biomater. 2009, 5, 3205–3214. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, K.Y.; Hahn, B.D.; Choi, J.J.; Yoon, W.H.; Lee, B.K.; Park, D.S.; Park, C. Photocatalytic nanocomposite thin films of TiO2-β-calcium phosphate by aerosol-deposition. Cat. Comm. 2009, 10, 596–599. [Google Scholar] [CrossRef]
- Kim, S.G.; Hahn, B.D.; Park, D.S.; Lee, Y.C.; Choi, E.J.; Chae, W.S.; Baek, D.H.; Choi, J.Y. Aerosol deposition of hydroxyapatite and 4-hexylresorcinol coatings on titanium alloys for dental implants. J. Oral Maxillofac. Surg. 2011, 69, e354–e363. [Google Scholar] [CrossRef]
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Kim, H.E.; Kim, S.G. Aerosol deposition of hydroxyapatite–chitosan composite coatings on biodegradable magnesium alloy. Surf. Coat. Tech. 2011, 205, 3112–3118. [Google Scholar] [CrossRef]
- Snyder, K.L.; Holmes, H.R.; McCarthy, C.; Rajachar, R.M. Bioactive vapor deposited calcium-phosphate silica sol-gel particles for directing osteoblast behavior. J. Biomed. Mater. Res. A 2016, 104A, 2135–2148. [Google Scholar] [CrossRef]
- Taira, Y.; Kaida, K.; Egoshi, T. Ceramic coating on hydroxyapatite by aerosol deposition. J. Prosthodont. Res. 2022, 66, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Cinca, N.; Vilardell, A.M.; Dosta, S.; Concustell, A.; Cano, I.G.; Guilemany, J.M.; Estradé, S.; Ruiz, A.; Peiró, F. A new alternative for obtaining nanocrystalline bioactive coatings: Study of hydroxyapatite deposition mechanisms by cold gas spraying. J. Am. Ceram. Soc. 2016, 99, 1420–1428. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Cinca, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M. Feasibility of using low pressure cold gas spray for the spraying of thick ceramic hydroxyapatite coatings. Int. J. Appl. Ceram. Technol. 2019, 16, 221–229. [Google Scholar] [CrossRef]
- Chatelain, D.; Denoirjean, A.; Guipont, V.; Rossignol, F.; Tessier-Doyen, N. Influence of the thermal treatment of a hydroxyapatite powder on the characteristics of coatings deposited by cold gas spraying. Surf. Coat. Tech. 2022, 446, 128697. [Google Scholar] [CrossRef]
- Gärtner, F.; Stoltenhoff, T.; Schmidt, T.; Kreye, H. The cold spray process and its potential for industrial applications. J. Therm. Spray Tech. 2006, 15, 223–232. [Google Scholar] [CrossRef]
- Paterlini, A.; Alexis, J.; Balcaen, Y.; Bertrand, G. Cold spraying of thick biomimetic and stoichiometric apatite coatings for orthopaedic implants. Coatings 2022, 12, 722. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Li, H.; Geng, W.; Bao, Y. Development of a cold spraying system for fabricating hydroxyapatite coating. Appl. Mech. Mater. 2012, 151, 300–304. [Google Scholar] [CrossRef]
- Singh, R.P.; Batra, U. Effect of cold spraying parameters and their interaction an hydroxyapatite deposition. J. Appl. Fluid Mech. 2013, 6, 555–561. [Google Scholar]
- Lee, J.H.; Jang, H.L.; Lee, K.M.; Baek, H.R.; Jin, K.; Noh, J.H. Cold-spray coating of hydroxyapatite on a three-dimensional polyetheretherketone implant and its biocompatibility evaluated by in vitro and in vivo minipig model. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105B, 647–657. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Barakat, N.A.M.; Lim, J.K. Hydroxyapatite-doped poly(lactic acid) porous film coating for enhanced bioactivity and corrosion behavior of AZ31 Mg alloy for orthopedic applications. Ceram. Int. 2013, 39, 183–195. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.; Wang, Y.; Huang, J.; Li, H. Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: Inherited nanostructures and enhanced properties. Carbon 2014, 67, 250–259. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Li, H. Nanostructural characteristics of vacuum cold-sprayed hydroxyapatite/graphene-nanosheet coatings for biomedical applications. J. Therm. Spray Technol. 2014, 23, 1149–1156. [Google Scholar] [CrossRef]
- Kubicki, G.; Leshchynsky, V.; Elseddawy, A.; Wiśniewska, M.; Maev, R.G.; Jakubowicz, J.; Sulej-Chojnacka, J. Microstructure and properties of hydroxyapatite coatings made by aerosol cold spraying-sintering technology. Coatings 2022, 12, 535. [Google Scholar] [CrossRef]
- Kern, M.; Thompson, V.P. Effects of sandblasting and silica-coating procedures on pure titanium. J. Dent. 1994, 22, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Miyamoto, Y.; Nagayama, M.; Asaoka, K. Blast coating method: New method of coating titanium surface with hydroxyapatite at room temperature. J. Biomed. Mater. Res. Appl. Biomater. 1997, 38, 129–134. [Google Scholar] [CrossRef]
- Mano, T.; Ueyama, Y.; Ishikawa, K.; Matsumura, T.; Suzuki, K. Initial tissue response to a titanium implant coated with apatite at room temperature using a blast coating method. Biomaterials 2002, 23, 1931–1936. [Google Scholar] [CrossRef]
- Gbureck, U.; Masten, A.; Probst, J.; Thull, R. Tribochemical structuring and coating of implant metal surfaces with titanium oxide and hydroxyapatite layers. Mater. Sci. Eng. C 2003, 23, 461–465. [Google Scholar] [CrossRef]
- O’Hare, P.; Meenan, B.J.; Burke, G.A.; Byrne, G.; Dowling, D.; Hunt, J.A. Biological responses to hydroxyapatite surfaces deposited via a co-incident microblasting technique. Biomaterials 2010, 31, 515–522. [Google Scholar] [CrossRef]
- O’Sullivan, C.; O’Hare, P.; O’Leary, N.D.; Crean, A.M.; Ryan, K.; Dobson, A.D.; O’Neill, L. Deposition of substituted apatites with anticolonizing properties onto titanium surfaces using a novel blasting process. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95B, 141–149. [Google Scholar] [CrossRef]
- Dunne, C.F.; Twomey, B.; O’Neill, L.; Stanton, K.T. Co-blasting of titanium surfaces with an abrasive and hydroxyapatite to produce bioactive coatings: Substrate and coating characterisation. J. Biomater. Appl. 2014, 28, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Dunne, C.F.; Twomey, B.; Kelly, C.; Simpson, J.C.; Stanton, K.T. Hydroxyapatite and fluorapatite coatings on dental screws: Effects of blast coating process and biological response. J. Mater. Sci. Mater. Med. 2015, 26, 5347. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.D.; O’Neill, L.; Twomey, B.; Dowling, D.P. Comparison between shot peening and abrasive blasting processes as deposition methods for hydroxyapatite coatings onto a titanium alloy. Surf. Coat. Tech. 2013, 216, 224–231. [Google Scholar] [CrossRef]
- Jung, U.W.; Hwang, J.W.; Choi, D.Y.; Hu, K.S.; Kwon, M.K.; Choi, S.H.; Kim, H.J. Surface characteristics of a novel hydroxyapatite-coated dental implant. J. Periodontal Implant. Sci. 2012, 42, 59–63. [Google Scholar] [CrossRef]
- Goyenvalle, E.; Aguado, E.; Cognet, R.; Bourges, X.; Daculsi, G. Calcium phosphate ceramic blasting on titanium surface improve bone ingrowth. Key Eng. Mater. 2008, 361–363, 1351–1354. [Google Scholar]
- Kurella, A.K.; Dahotre, N.B. A multi-textured calcium phosphate coating for hard tissue via laser surface engineering. JOM 2006, 58, 64–66. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Laser surface treatment for porous and textured Ca-P bio-ceramic coating on Ti–6Al–4V. Biomed. Mater. 2007, 2, 274–281. [Google Scholar] [CrossRef]
- Santhanakrishnan, S.; Ho, Y.H.; Dahotre, N.B. Laser coating of hydroxyapatite on Mg for enhanced physiological corrosion resistance and biodegradability. Mater. Tech. 2012, 27, 273–277. [Google Scholar] [CrossRef]
- Nag, S.; Paital, S.R.; Nandawana, P.; Mahdak, K.; Ho, Y.H.; Vora, H.; Banerjee, R.; Dahotre, N.B. Laser deposited biocompatible Ca–P coatings on Ti–6Al–4V: Microstructural evolution and thermal modeling. Mater. Sci. Eng. C 2013, 33, 165–173. [Google Scholar] [CrossRef]
- Tlotleng, M.; Akinlabi, E.; Shukla, M.; Pityana, S. Microstructures, hardness and bioactivity of hydroxyapatite coatings deposited by direct laser melting process. Mater. Sci. Eng. C 2014, 43, 189–198. [Google Scholar] [CrossRef]
- Cheng, G.J.; Ye, C. Experiment, thermal simulation, and characterizations on transmission laser coating of hydroxyapatite on metal implant. J. Biomed. Mater. Res. A 2010, 92A, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lusquiños, F.; Pou, J.; Arias, J.L.; Boutinguiza, M.; Léon, B.; Pérez-Amor, M.; Driessens, F.C.M.; Merry, J.C.; Gibson, I.; Best, S.; et al. Production of calcium phosphate coatings on Ti6Al4V obtained by Nd:Yttrium-aluminum-garnet laser cladding. J. Appl. Phys. 2001, 90, 4231–4236. [Google Scholar] [CrossRef]
- Lusquiños, F.; de Carlos, A.; Pou, J.; Arias, J.L.; Boutinguiza, M.; León, B.; Pérez-Amor, M.; Driessens, F.C.M.; Hing, K.; Gibson, I.; et al. Calcium phosphate coatings obtained by Nd:YAG laser cladding: Physicochemical and biologic properties. J. Biomed. Mater. Res. A 2003, 64A, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Lusquiños, F.; Pou, J.; Boutinguiza, M.; Quintero, F.; Soto, R.; León, B.; Pérez-Amor, M. Main characteristics of calcium phosphate coatings obtained by laser cladding. Appl. Surf. Sci. 2005, 247, 486–492. [Google Scholar] [CrossRef]
- Lusquiños, F.; Pou, J.; Arias, J.L.; Boutinguiza, M.; León, B.; Pérez-Amor, M.; Driessens, F.C.M. Alloying of hydroxyapatite onto Ti6Al-4V by high power laser irradiation. J. Mater. Sci. Mater. Med. 2002, 13, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Paital, S.R.; He, W.; Dahotre, N.B. Laser pulse dependent micro textured calcium phosphate coatings for improved wettability and cell compatibility. J. Mater. Sci. Mater. Med. 2010, 21, 2187–2200. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, Y.M.; Yu, H.L.; Ding, J.J.; Tang, X.H.; Li, J.G.; Zhou, Y.H. In situ fabrication of bioceramic composite coatings by laser cladding. Surf. Coat. Tech. 2005, 491, 47–54. [Google Scholar] [CrossRef]
- Wang, D.G.; Chen, C.Z.; Ma, J.; Zhang, G. In situ synthesis of HA coating by laser cladding. Colloid Surf. B 2008, 66, 155–162. [Google Scholar] [CrossRef]
- Lü, X.; Lin, X.; Guan, T.; Gao, B.; Huang, W. Effect of the mass ratio of CaCO3 to CaHPO4·2H2O on in situ synthesis of hydroxyapatite coating by laser cladding. Rare Metal Mater. Eng. 2011, 40, 22–27. [Google Scholar]
- Lü, X.; Lin, X.; Cao, Y.; Hu, J.; Gao, B.; Huang, W. Effects of processing parameters and heat treatment on phase structure of the hydroxyapatite coating on pure Ti surface by laser cladding in-situ synthesis. Rare Metal Mater. Eng. 2011, 40, 714–717. [Google Scholar]
- Lv, X.; Lin, X.; Hu, J.; Gao, B.; Huang, W. Phase evolution in calcium phosphate coatings obtained by in situ laser cladding. Mater. Sci. Eng. C 2012, 32, 872–877. [Google Scholar] [CrossRef]
- Yang, G.; Hu, S.H.; Liu, Y.; Huang, A.G. Laser cladding of fluoridated hydroxyapatite coatings on titanium alloy: Preparation and characterization. Appl. Mech. Mater. 2014, 487, 204–209. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Q.; Xu, P.; Li, L.; Jiang, H.; Bai, Y. Bioactivity of calcium phosphate bioceramic coating fabricated by laser cladding. Laser Phys. Lett. 2016, 13, 055601. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, J.; Guan, T.H.; Wu, J.; Zhang, C.B.; Gao, B. Physical properties and cellular responses to calcium phosphate coating produced by laser rapid forming on titanium. Laser Med. Sci. 2014, 29, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, M.; Mousavi, S.; Nikzamir, S.; Milan, P.B.; Mozafari, M. Effect of laser cladded co-doped strontium fluorapatite nanopowder coating on the antibacterial and cell attachment of Ti-6Al-4V implants for bone applications. Mater. Technol. 2022, 37, 829–841. [Google Scholar] [CrossRef]
- Pei, X.; Wang, J.; Wan, Q.; Kang, L.; Xiao, M.; Bao, H. Functionally graded carbon nanotubes/hydroxyapatite composite coating by laser cladding. Surf. Coat. Tech. 2011, 205, 4380–4387. [Google Scholar] [CrossRef]
- Griffith, M.L.; Keicher, D.L.; Romero, J.T.; Smugeresky, J.E.; Atwood, C.L.; Harwell, L.D.; Greene, D.L. Laser engineered net shaping (LENS™) for the fabrication of metallic components. Am. Soc. Mech. Eng. Mater. Div. 1996, 74, 175–176. [Google Scholar]
- Roy, M.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Laser processing of bioactive tricalcium phosphate coating on titanium for load-bearing implants. Acta Biomater. 2008, 4, 324–333. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. In vitro antimicrobial and biological properties of laser assisted tricalcium phosphate coating on titanium for load bearing implant. Mater. Sci. Eng. C 2009, 29, 1965–1968. [Google Scholar] [CrossRef]
- Balla, V.K.; Das, M.; Bose, S.; Ram, G.D.J.; Manna, I. Laser surface modification of 316 L stainless steel with bioactive hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4594–4598. [Google Scholar] [CrossRef]
- Roy, M.; Balla, V.K.; Bandyopadhyay, A.; Bose, S. Compositionally graded hydroxyapatite/tricalcium phosphate coating on Ti by laser and induction plasma. Acta Biomater. 2011, 7, 866–873. [Google Scholar] [CrossRef]
- Piqué, A. The Matrix-Assisted Pulsed Laser Evaporation (MAPLE) process: Origins and future directions. Appl. Phys. A 2011, 105, 517–528. [Google Scholar] [CrossRef]
- Visan, A.; Grossin, D.; Stefan, N.; Duta, L.; Miroiu, F.M.; Stan, G.E.; Sopronyi, M.; Luculescu, C.; Freche, M.; Marsan, O.; et al. Biomimetic nanocrystalline apatite coatings synthesized by Matrix Assisted Pulsed Laser Evaporation for medical applications. Mater. Sci. Eng. B 2014, 181, 56–63. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Fini, M.; Sima, F.; Serban, N.; Mihailescu, I.N.; Bigi, A. Magnesium and strontium doped octacalcium phosphate thin films by matrix assisted pulsed laser evaporation. J. Inorg. Biochem. 2012, 107, 65–72. [Google Scholar] [CrossRef]
- Negroiu, G.; Piticescu, R.M.; Chitanu, G.C.; Mihailescu, I.N.; Zdrentu, L.; Miroiu, M. Biocompatibility evaluation of a novel hydroxyapatite-polymer coating for medical implants (in vitro tests). J. Mater. Sci. Mater. Med. 2008, 19, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Boanini, E.; Capuccini, C.; Fini, M.; Mihailescu, I.N.; Ristoscu, C.; Sima, F.; Torricelli, P. Biofunctional alendronate-hydroxyapatite thin films deposited by matrix assisted pulsed laser evaporation. Biomaterials 2009, 30, 6168–6177. [Google Scholar] [CrossRef]
- Miroiu, F.M.; Socol, G.; Visan, A.; Stefan, N.; Craciun, D.; Craciun, V.; Dorcioman, G.; Mihailescu, I.N.; Sima, L.E.; Petrescu, S.M.; et al. Composite biocompatible hydroxyapatite-silk fibroin coatings for medical implants obtained by matrix assisted pulsed laser evaporation. Mater. Sci. Eng. B 2010, 169, 151–158. [Google Scholar] [CrossRef]
- Visan, A.; Stan, G.E.; Ristoscu, C.; Popescu-Pelin, G.; Sopronyi, M.; Besleaga, C.; Luculescu, C.; Chifiriuc, M.C.; Hussien, M.D.; Marsan, O.; et al. Combinatorial MAPLE deposition of antimicrobial orthopedic maps fabricated from chitosan and biomimetic apatite powders. Int. J. Pharm. 2016, 511, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Saito, N.; Sakamaki, I.; Koga, K.; Nakamura, M.; Nathanael, J.A.; Yoshizawa, N.; Shitomi, K.; Mayumi, K.; Miyaji, H. Laser-assisted biomineralization on human dentin for tooth surface functionalization. Mater. Sci. Eng. C 2019, 105, 110061. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, H.; Oyane, A.; Narazaki, A. Biological modification of tooth surface by laser-based apatite coating techniques. J. Oral Biosci. 2022, 64, 217–221. [Google Scholar] [CrossRef]
- Pramatarova, L.; Pecheva, E.; Dimova-Malinovska, D.; Presker, R.; Stutzmann, M.; Schwarz, U.; Kniep, R. A novel laser-liquid-solid interaction process for hydroxyapatite formation on porous silicon. Proc. SPIE Int. Soc. Optical Eng. 2005, 5830, 110–114. [Google Scholar]
- Pecheva, E.; Petrov, T.; Lungu, C.; Montgomery, P.; Pramatarova, L. Stimulated in vitro bone-like apatite formation by a novel laser processing technique. Chem. Eng. J. 2008, 137, 144–153. [Google Scholar] [CrossRef]
- Lee, B.H.; Oyane, A.; Tsurushima, H.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. A new approach for hydroxyapatite coating on polymeric materials using laser-induced precursor formation and subsequent aging. ACS Appl. Mater. Interfaces 2009, 1, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Sakamaki, I.; Pyatenko, A.; Nakamura, M.; Ishilawa, Y.; Shimizu, Y.; Kawaguchi, K.; Koshizaki, N. Laser-assisted calcium phosphate deposition on polymer substrates in supersaturated solutions. RSC Adv. 2014, 4, 53645–53648. [Google Scholar] [CrossRef]
- Oyane, A.; Nakamura, M.; Sakamaki, I.; Shimizu, Y.; Miyata, S.; Miyaji, H. Laser-assisted wet coating of calcium phosphate for surface-functionalization of PEEK. PLoS ONE 2018, 13, e0206524. [Google Scholar] [CrossRef]
- Oyane, A.; Matsuoka, N.; Koga, K.; Shimizu, Y.; Nakamura, M.; Kawaguchi, K.; Koshizaki, N.; Sogo, Y.; Ito, A.; Unuma, H. Laser-assisted biomimetic process for surface functionalization of titanium metal. Colloid Interface Sci. Commun. 2015, 4, 5–9. [Google Scholar] [CrossRef]
- Mahanti, M.; Nakamura, M.; Pyatenko, A.; Sakamaki, I.; Koga, K.; Oyane, A. The mechanism underlying calcium phosphate precipitation on titanium via ultraviolet, visible, and near infrared laser-assisted biomimetic process. J. Phys. D: Appl. Phys. 2016, 49, 304003. [Google Scholar] [CrossRef]
- Oyane, A.; Sakamaki, I.; Koga, K.; Nakamura, M. Formation of a calcium phosphate layer with immobilized cobalt chromite nanoparticles on cobalt−chromium alloy by a laser-assisted biomimetic process. Appl. Sci. 2020, 10, 5584. [Google Scholar] [CrossRef]
- Oyane, A.; Sakamaki, I.; Shimizu, Y.; Kawaguchi, K.; Sogo, Y.; Ito, A.; Koshizaki, N. Laser-assisted biomimetic process for calcium phosphate coating on a hydroxyapatite ceramic. Key Eng. Mater. 2013, 529–530, 217–222. [Google Scholar] [CrossRef]
- Nakamura, M.; Oyane, A. Physicochemical fabrication of calcium phosphate-based thin layers and nanospheres using laser processing in solutions. J. Mater. Chem. B 2016, 4, 6289–6301. [Google Scholar] [CrossRef]
- Bohandy, J.; Kim, B.F.; Adrian, F.J. Metal deposition from a supported metal film using an excimer laser. J. Appl. Phys. 1986, 60, 1538–1539. [Google Scholar] [CrossRef]
- Narazaki, A.; Oyane, A.; Miyaji, H. Laser-induced forward transfer with optical stamp of a protein-immobilized calcium phosphate film prepared by biomimetic process to a human dentin. Appl. Sci. 2020, 10, 7984. [Google Scholar] [CrossRef]
- Um, S.H.; Chung, Y.W.; Seo, Y.; Seo, H.; Ok, M.R.; Kim, Y.C.; Han, H.S.; Chung, J.J.; Edwards, J.R.; Jeon, H. Robust hydroxyapatite coating by laser-induced hydrothermal synthesis. Adv. Funct. Mater. 2020, 30, 2005233. [Google Scholar] [CrossRef]
- Park, J.; Um, S.H.; Seo, Y.; Lee, J.; Kim, Y.C.; Ok, M.R.; Hwang, S.W.; Sun, J.Y.; Han, H.S.; Jeon, H. Improving hydroxyapatite coating ability on biodegradable metal through laser-induced hydrothermal coating in liquid precursor: Application in orthopedic implants. Bioact. Mater. 2023, 25, 796–806. [Google Scholar] [CrossRef]
- van Zomeren, A.; Kelder, E.M.; Marijnissen, J.C.M.; Schoonman, J. The production of thin films of LiMn2O4 by electrospraying. J. Aerosol Sci. 1994, 25, 1229–1235. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.; Wolke, J.; Schoonman, J.; Jansen, J. Electrostatic spray deposition (ESD) of calcium phosphate coatings. J. Biomed. Mater. Res. A 2003, 66A, 330–334. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Schoonman, J.; Jansen, J.A. Influence of precursor solution parameters on chemical properties of calcium phosphate coatings prepared using electrostatic spray deposition (ESD). Biomaterials 2004, 25, 641–649. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Schoonman, J.; Jansen, J.A. Influence of deposition parameters on morphological properties of biomedical calcium phosphate coatings prepared using electrostatic spray deposition. Thin Solid Films 2005, 472, 105–113. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Schoonman, J.; Jansen, J.A. Influence of deposition parameters on chemical properties of calcium phosphate coatings prepared by using electrostatic spray deposition. J. Biomed. Mater. Res. A 2005, 74A, 275–284. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Schoonman, J.; Jansen, J.A. Deposition of calcium phosphate coatings with defined chemical properties using the electrostatic spray deposition technique. J. Eur. Ceram. Soc. 2006, 26, 487–493. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.G.; Heine, M.C.; Wolke, J.G.C.; Pratsinis, S.E.; Schoonman, J.; Jansen, J.A. Morphology of calcium phosphate coatings for biomedical applications deposited using electrostatic spray deposition. Thin Solid Films 2006, 503, 69–78. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, Y.H.; Oh, N.H.; Cheon, Y.W.; Cho, Y.J.; Lee, C.M.; Kim, K.B.; Lee, N.S. A study of hydroxyapatite coating on porous Ti compact by electrostatic spray deposition. Solid State Phenom. 2007, 124–126, 1789–1792. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, L.; Nyandoto, G.; Malshe, A.P. Electrostatic spray deposition of nanostructured hydroxyapatite coating for biomedical applications. J. Manufact. Sci. Eng. Trans. ASME 2008, 130, 0210011–0210017. [Google Scholar] [CrossRef]
- Li, X.; Koller, G.; Huang, J.; di Silvio, L.; Renton, T.; Esat, M.; Bonfield, W.; Edirisinghe, M. A novel jet-based nano-hydroxyapatite patterning technique for osteoblast guidance. J. R. Soc. Interface 2010, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Ahmad, Z.; Huang, J.; Edirisinghe, M.J.; Jayasinghe, S.N.; Ireland, D.C.; Brooks, R.A.; Rushton, N.; Bonfield, W.; Best, S.M. The role of surface wettability and surface charge of electrosprayed nanoapatites on the behaviour of osteoblasts. Acta Biomater. 2010, 6, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Bosco, R.; Leeuwenburgh, S.C.G.; van den Beucken, J.J.J.P.; Jansen, J.A.; Prat, M.; Roveri, N. Electrostatic spray deposition of biomimetic nanocrystalline apatite coatings onto titanium. Adv. Eng. Mater. 2012, 14, B13–B20. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Xu, G.; Ye, X.; He, D.; Zhong, J. Micropattern of nano-hydroxyapatite/silk fibroin composite onto Ti alloy surface via template-assisted electrostatic spray deposition. Mater. Sci. Eng. C 2012, 32, 390–394. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Edirisinghe, M.J. Novel patterning of nano-bioceramics: Template-assisted electrohydrodynamic atomization spraying. J. R. Soc. Interface 2008, 5, 253–257. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Edirisinghe, M.J.; Bonfield, W. An electrically driven jetting technique for diverse high-resolution surface structures of nanometre hydroxyapatite crystals. Colloid Surf. B 2011, 82, 562–570. [Google Scholar] [CrossRef]
- Kim, B.H.; Jeong, J.H.; Jeon, Y.S.; Jeon, K.O.; Hwang, K.S. Hydroxyapatite layers prepared by sol-gel assisted electrostatic spray deposition. Ceram. Int. 2007, 33, 119–122. [Google Scholar] [CrossRef]
- Hou, X.; Choy, K.L.; Leach, S.E. Processing and in vitro behavior of hydroxyapatite coatings prepared by electrostatic spray assisted vapor deposition method. J. Biomed. Mater. Res. A 2007, 83A, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, W.; Malshe, A. Novel nanostructured hydroxyapatite coating for dental and orthopedic implants. JOM 2009, 61, 67–69. [Google Scholar] [CrossRef]
- Aizawa, M.; Itatani, K.; Howell, F.S.; Kishioka, A.; Kinoshita, M. Formation of porous calcium phosphate films on α-Al2O3 substrates by spray-pyrolysis technique. J. Ceram. Soc. Jpn. 1994, 102, 732–736. [Google Scholar] [CrossRef]
- Aizawa, M.; Itatani, K.; Howell, F.S.; Kishioka, A.; Kinoshita, M. Formation of porous calcium phosphate films on partially stabilized zirconia substrates by the spray-pyrolysis technique. J. Mater. Sci. 1995, 30, 4936–4945. [Google Scholar] [CrossRef]
- Aizawa, M.; Yamamoto, T.; Itatani, K.; Suemasu, H.; Mozue, A.; Okada, I. Formation of calcium-phosphate films with gradient composition on alumina ceramics by spray-pyrolysis technique and their biocompatibilities by cell-culture tests. Key Eng. Mater. 2001, 192–195, 103–106. [Google Scholar] [CrossRef]
- Cabaňas, M.V.; Vallet-Regí, M. Calcium phosphate coatings deposited by aerosol chemical vapour deposition. J. Mater. Chem. 2003, 13, 1104–1107. [Google Scholar] [CrossRef]
- Aizawa, M.; Itatani, K.; Okada, I. Characterization of porous β-tricalcium phosphate films formed on alumina ceramics by spray-pyrolysis technique and their in vitro evaluations using osteoblasts. J. Ceram. Soc. Jpn. 2005, 113, 245–251. [Google Scholar] [CrossRef]
- Jokanovic, V.; Uskokovic, D. Calcium hydroxyapatite thin films on titanium substrates prepared by ultrasonic spray pyrolysis. Mater. Trans. 2005, 46, 228–235. [Google Scholar] [CrossRef]
- Ye, G.; Troczynski, T. Hydroxyapatite coatings by pulsed ultrasonic spray pyrolysis. Ceram. Int. 2008, 34, 511–516. [Google Scholar] [CrossRef]
- Aguilar-Frutis, M.; Kumar, S.; Falcony, C. Spray-pyrolyzed hydroxyapatite thin-film coatings. Surf. Coat. Tech. 2009, 204, 1116–1120. [Google Scholar] [CrossRef]
- Rajapakse, R.M.G.; Wijesinghe, W.P.S.L.; Mantilaka, M.M.M.G.P.G.; Senarathna, K.G.C.; Herath, H.M.T.U.; Premachandra, T.N.; Ranasinghe, C.S.K.; Rajapakse, R.P.V.J.; Edirisinghe, M.; Mahalingam, S.; et al. Preparation of bone-implants by coating hydroxyapatite nanoparticles on self-formed titanium dioxide thin-layers on titanium metal surfaces. Mater. Sci. Eng. C 2016, 63, 172–184. [Google Scholar]
- Brendel, T.; Engel, A.; Rüssel, C. Hydroxyapatite coatings by a polymeric route. J. Mater. Sci. Mater. Med. 1992, 3, 175–179. [Google Scholar] [CrossRef]
- Putkonen, M.; Sajavaara, T.; Rahkila, P.; Xu, L.; Cheng, S.; Niinistö, L.; Whitlow, H.J. Atomic layer deposition and characterization of biocompatible hydroxyapatite thin films. Thin Solid Films 2009, 517, 5819–5824. [Google Scholar] [CrossRef]
- Holopainen, J.; Kauppinen, K.; Mizohata, K.; Santala, E.; Mikkola, E.; Heikkilä, M.; Kokkonen, H.; Leskelä, M.; Lehenkari, P.; Tuukkanen, J.; et al. Preparation and bioactive properties of nanocrystalline hydroxyapatite thin films obtained by conversion of atomic layer deposited calcium carbonate. Biointerphases 2014, 9, 031008. [Google Scholar] [CrossRef] [PubMed]
- Avila, I.; Pantchev, K.; Holopainen, J.; Ritala, M.; Tuukkanen, J. Adhesion and mechanical properties of nanocrystalline hydroxyapatite coating obtained by conversion of atomic layer-deposited calcium carbonate on titanium substrate. J. Mater. Sci. Mater. Med. 2018, 29, 111. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.E.; Bower, K.W. Review of the drop on-demand ink jet with primary emphasis on the Gould jet concept. J. Appl. Photo. Eng. 1979, 5, 174–178. [Google Scholar]
- Sun, J.; Thian, E.S.; Fuh, J.Y.H.; Chang, L.; Hong, G.S.; Wang, W.; Tay, B.Y.; Wong, Y.S. Fabrication of bio-inspired composite coatings for titanium implants using the micro-dispensing technique. Microsyst. Tech. 2012, 18, 2041–2051. [Google Scholar] [CrossRef]
- Thian, E.S.; Chang, L.; Lim, P.N.; Gurucharan, B.; Sun, J.; Fuh, J.Y.H.; Ho, B.; Tay, B.Y.; Teo, E.Y.; Wang, W. Chemically-modified calcium phosphate coatings via drop-on-demand micro-dispensing technique. Surf. Coat. Tech. 2013, 231, 29–33. [Google Scholar] [CrossRef]
- Chang, L.; Sun, J.; Fuh, J.Y.H.; Thian, E.S. Deposition and characterization of a dual-layer silicon- and silver-containing hydroxyapatite coating via a drop-on-demand technique. RSC Adv. 2013, 3, 11162–11168. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Verdugo-Escamilla, C.; Gavira, J.A. Bioinspired calcium phosphate coated mica sheets by vapour diffusion and its effects on lysozyme assembly and crystallization. Cryst. Growth Des. 2016, 16, 5150–5158. [Google Scholar] [CrossRef]
- Hannora, A.E.; Mukasyan, A.S.; Mansurov, Z.A. Mechanochemical synthesis of nanocrystalline hydroxyapatite coating. Eurasian Chem.Tech. J. 2010, 12, 79–95. [Google Scholar] [CrossRef]
- Hayashi, N.; Ueno, S.; Komarov, S.V.; Kasai, E.; Oki, T. Fabrication of hydroxyapatite coatings by the ball impact process. Surf. Coat. Tech. 2012, 206, 3949–3954. [Google Scholar] [CrossRef]
- Alonso, M.; Satoh, M.; Miyanami, K. Mechanism of the combined coating-mechanofusion processing of powders. Powder Technol. 1989, 59, 45–52. [Google Scholar] [CrossRef]
- Sugo, K.; Kato, M.; Ishikawa, T.; Yamamoto, A.; Ogawa, T. Hydroxyapatite microcarrier (1). Tiss. Cult. Res. Commun. 2006, 25, 105–111. [Google Scholar]
- Oliveira, J.M.; Leonor, I.B.; Reis, R. Preparation of bioactive coatings on the surface of bioinert polymers through an innovative “auto-catalytic” electroless route. Key Eng. Mater. 2005, 284–286, 203–207. [Google Scholar] [CrossRef]
- Le, V.Q.; Cochis, A.; Rimondini, L.; Pourroy, G.; Stanic, V.; Palkowski, H.; Carrado, A. Biomimetic calcium–phosphates produced by an autocatalytic route on stainless steel 316 L and bio-inert polyolefin. RSC Adv. 2013, 3, 11255–11262. [Google Scholar] [CrossRef]
- Aguilar-Reyes, E.A.; León Patiño, C.A.; Jacinto-Díaz, B. Formation of calcium-phosphate coatings on Ti6Al4V substrates by an autocatalytic deposition route. Key Eng. Mater. 2015, 631, 247–252. [Google Scholar] [CrossRef]
- Chen, B.; Liang, C. Preparation of hydroxyapatite coating by the use of a sacrificial Mg anode method. Ceram. Int. 2007, 33, 701–703. [Google Scholar] [CrossRef]
- Blanda, G.; Brucato, V.; Pavia, F.C.; Greco, S.; Piazza, S.; Sunseri, C.; Inguanta, R. Galvanic deposition and characterization of brushite/hydroxyapatite coatings on 316L stainless steel. Mater. Sci. Eng. C 2016, 64, 93–101. [Google Scholar] [CrossRef]
- Mousa, H.M.; Lee, D.H.; Park, C.H.; Kim, C.S. A novel simple strategy for in situ deposition of apatite layer on AZ31B magnesium alloy for bone tissue regeneration. Appl. Surf. Sci. 2015, 351, 55–65. [Google Scholar] [CrossRef]
- Mousa, H.M.; Hussein, K.H.; Raslan, A.A.; Lee, J.; Woo, H.M.; Park, C.H.; Kim, C.S. Amorphous apatite thin film formation on a biodegradable Mg alloy for bone regeneration: Strategy, characterization, biodegradation, and in vitro cell study. RSC Adv. 2016, 6, 22563–22574. [Google Scholar] [CrossRef]
- Bucur, A.I.; Linul, E.; Taranu, B.O. Hydroxyapatite coatings on Ti substrates by simultaneous precipitation and electrodeposition. Appl. Surf. Sci. 2020, 527, 146820. [Google Scholar] [CrossRef]
- Taranu, B.O.; Ianasi, P.; Rus, S.F.; Bucur, A.I. Simultaneous precipitation and electrodeposition of hydroxyapatite coatings at different temperatures on various metal substrates. Coatings 2022, 12, 288. [Google Scholar] [CrossRef]
- Ota, F.; Fujisaki, K.; Osanai, K.; Sasagawa, K. A novel method for coating calcium phosphate with trace elements extracted from bone using electrical stimulation. J. Mech. Behav. Biomed. Mater. 2022, 133, 105330. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montelongo, J.; Muñoz-Noval, A.; Torres-Costa, V.; Martín-Palma, R.J.; Manso-Silvan, M. Cyclic calcium phosphate electrodeposition on porous silicon. Int. J. Electrochem. Sci. 2012, 7, 1840–1851. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Gallach, D.; Naveas, N.; Torres-Costa, V.; Climent-Font, A.; García-Ruiz, J.P.; Manso-Silvan, M. Calcium phosphate/porous silicon biocomposites prepared by cyclic deposition methods: Spin coating vs. electrochemical activation. Mater. Sci. Eng. C 2014, 34, 245–251. [Google Scholar] [CrossRef]
- Subramanian, B.; Dhandapani, P.; Maruthamuthu, S.; Jayachandran, M. Biosynthesis of calcium hydroxylapatite coating on sputtered Ti/TiN nano multilayers and their corrosion behavior in simulated body solution. J. Biomater. Appl. 2012, 26, 687–705. [Google Scholar] [CrossRef]
- Fujii, S.; Oleada, M.; Sawa, H.; Furuzono, T.; Nakamura, Y. Hydroxyapatite nanoparticles as particulate emulsifier: Fabrication of hydroxyapatite-coated biodegradable microspheres. Langmuir 2009, 25, 9759–9766. [Google Scholar] [CrossRef]
- Fujii, S.; Okada, M.; Nishimura, T.; Sugimoto, T.; Maeda, H.; Hamasaki, H.; Furuzono, T.; Nakamura, Y. Hydroxyapatite-coated poly(ε-caprolactone) microspheres fabricated via a Pickering emulsion route: Effect of fabrication parameters on diameter and chemical composition. Compos. Interf. 2013, 20, 45–56. [Google Scholar] [CrossRef]
- Okada, M.; Fujii, S.; Nishimura, T.; Nakamura, Y.; Takeda, S.; Furuzono, T. Solvent-free formation of hydroxyapatite coated biodegradable particles via nanoparticle-stabilized emulsion route. Appl. Surf. Sci. 2012, 262, 39–44. [Google Scholar] [CrossRef]
- Okada, M.; Takeda, S.; Furuzono, T. A novel approach to prepare hydroxyapatite-coated biodegradable polymer microspheres loaded with magnetic Fe3O4 via nanoparticle-stabilized emulsions. Key Eng. Mater. 2013, 529–530, 223–228. [Google Scholar]
- Ohtsu, N.; Nakamura, Y.; Semboshi, S. Thin hydroxyapatite coating on titanium fabricated by chemical coating process using calcium phosphate slurry. Surf. Coat. Tech. 2012, 206, 2616–2621. [Google Scholar] [CrossRef]
- Ohtsu, N.; Takahara, T.; Hirano, M.; Arai, H. Effect of treatment temperature on the biocompatibility and mechanical strength of hydroxyapatite coating formed on titanium using calcium phosphate slurry. Surf. Coat. Tech. 2014, 239, 185–190. [Google Scholar] [CrossRef]
- Yang, J.Z.; Sultana, R.; Hu, X.Z.; Huang, Z.H. Porous hydroxyapatite coating on strong ceramic substrate fabricated by low density slip coating-deposition and coating-substrate co-sintering. J. Eur. Ceram. Soc. 2011, 31, 2065–2071. [Google Scholar] [CrossRef]
- Sultana, R.; Yang, J.; Hu, X. Deposition of micro-porous hydroxyapatite/tri-calcium phosphate coating on zirconia-based substrate. J. Am. Ceram. Soc. 2012, 95, 1212–1215. [Google Scholar] [CrossRef]
- Yang, J.Z.; Sultana, R.; Ichim, P.; Hu, X.Z.; Huang, Z.H.; Yi, W.; Jiang, B.; Xu, Y. Micro-porous calcium phosphate coatings on load-bearing zirconia substrate: Processing, property and application. Ceram. Int. 2013, 39, 6533–6542. [Google Scholar] [CrossRef]
- Banba, Y.; Umeda, T.; Kuroe, H.; Toyama, T.; Musha, Y.; Itatani, K. Formation of hydroxyapatite layer on graphite sheet immersed in calcium phosphate solution by microwave heating. J. Ceram. Soc. Jpn. 2013, 121, 901–906. [Google Scholar] [CrossRef]
- Mendes, V.C.; Moineddin, R.; Davies, J.E. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials 2007, 28, 4748–4755. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.C.; Moineddin, R.; Davies, J.E. Discrete calcium phosphate nanocrystalline deposition enhances osteoconduction on titanium-based implant surfaces. J. Biomed. Mater. Res. A 2009, 90A, 577–585. [Google Scholar] [CrossRef]
- Kansal, H.K.; Singh, S.; Kumar, P. Technology and research developments in powder mixed electric discharge machining (PMEDM). J. Mater. Proc. Technol. 2007, 184, 32–41. [Google Scholar] [CrossRef]
- Ekmekci, N.; Ekmekci, B. Hydroxyapatite deposition onto Ti–6Al–4V surface in powder mixed electrical discharge machining. Adv. Mater. Res. 2014, 856, 205–209. [Google Scholar] [CrossRef]
- Ou, S.F.; Wang, C.Y. Fabrication of a hydroxyapatite-containing coating on Ti–Ta alloy by electrical discharge coating and hydrothermal treatment. Surf. Coat. Tech. 2016, 302, 238–243. [Google Scholar] [CrossRef]
- Prakash, C.; Uddin, M.S. Surface modification of β-phase Ti implant by hydroaxyapatite mixed electric discharge machining to enhance the corrosion resistance and in-vitro bioactivity. Surf. Coat. Tech. 2017, 326, 134–145. [Google Scholar] [CrossRef]
- Aliyu, A.A.A.; Abdul-Rani, A.M.; Rao, T.V.V.L.N.; Axinte, E.; Hastuty, S.; Parameswari, R.P.; Subramaniam, J.R.; Thyagarajan, S.P. Characterization, adhesion strength and in-vitro cytotoxicity investigation of hydroxyapatite coating synthesized on Zr-based BMG by electro discharge process. Surf. Coat. Technol. 2019, 370, 213–226. [Google Scholar] [CrossRef]
- Al-Amin, M.; Rani, A.M.A.; Aliyu, A.A.A.; Bryant, M.G.; Danish, M.; Ahmad, A. Bio-ceramic coatings adhesion and roughness of biomaterials through PM-EDM: A comprehensive review. Mater. Manuf. Process. 2020, 35, 1157–1180. [Google Scholar] [CrossRef]
- Yu, Y.T.; Hsieh, S.F.; Lin, M.H.; Huang, J.W.; Ou, S.F. Improvement in Ca and P incorporation in the coating on Ti by gas-assisted electrical discharge coating. Surf. Coat. Tech. 2020, 400, 126120. [Google Scholar] [CrossRef]
- Sohmura, T.; Tamasaki, H.; Ohara, T.; Takahashi, J. Calcium-phosphate surface coating by casting to improve bioactivity of titanium. J. Biomed. Mater. Res. Appl. Biomater. 2001, 58, 478–485. [Google Scholar] [CrossRef]
- Escobedo, J.C.; Ortiz, J.C.; Almanza, J.M.; Cortés, D.A. Hydroxyapatite coating on a cobalt base alloy by investment casting. Scr. Mater. 2006, 54, 1611–1615. [Google Scholar] [CrossRef]
- Minouei, H.; Meratian, M.; Fathi, M.H.; Ghazvinizadeh, H. Biphasic calcium phosphate coating on cobalt-base surgical alloy during investment casting. J. Mater. Sci. Mater. Med. 2011, 22, 2449–2455. [Google Scholar] [CrossRef]
- Arafat, A.; Idris, M.H.; Kadir, M.R.A.; Jafari, H. Characterisation of calcium phosphate coating on investment cast 316 L stainless steel. Mater. Res. Inn. 2014, 18 (Suppl. 2), 886–891. [Google Scholar]
- Rodrigues, L.F., Jr.; Tronco, M.C.; Escobar, C.F.; Rocha, A.S.; Santos, L.A.L. Painting method for hydroxyapatite coating on titanium substrate. Ceram. Int. 2019, 45, 14806–14815. [Google Scholar] [CrossRef]
- Woźniak, B.; Szałaj, U.; Chodara, A.; Mizeracki, J.; Łojkowski, M.; Myszka, D.; Łojkowski, W. Mechanism for sonocoating a polymer surface with nano-hydroxyapatite. Mater. Lett. 2019, 249, 155–159. [Google Scholar] [CrossRef]
- Rogowska-Tylman, J.; Locs, J.; Salma, I.; Woźniak, B.; Pilmane, M.; Zalite, V.; Wojnarowicz, J.; Kędzierska-Sar, A.; Chudoba, T.; Szlązak, K.; et al. In vivo and in vitro study of a novel nanohydroxyapatite sonocoated scaffolds for enhanced bone regeneration. Mater. Sci. Eng. C 2019, 99, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Chen, Y.C.; Liao, C.C.; Lin, L.W.; Chen, C.F.; Wang, K.K.; Chen, S.T.; Hsueh, Y.H.; Wu, C.H.; Ou, S.F. Improvement in bioactivity and corrosion resistance of Ti by hydroxyapatite deposition using ultrasonic mechanical coating and armoring. Ceram. Int. 2022, 48, 4999–5008. [Google Scholar] [CrossRef]
- Lin, M.H.; Fan, F.Y.; Kuo, C.H.; Lin, L.W.; Wang, K.K.; Chen, C.F.; Wang, Y.H.; Ou, S.F. Nanostructured hydroxyapatite coatings on NiTi shape memory alloys by ultrasonic mechanical coating and armouring. Surf. Coat. Tech. 2022, 431, 127998. [Google Scholar] [CrossRef]
- Idaszek, J.; Jaroszewicz, J.; Choińska, E.; Górecka, Ż.; Hyc, A.; Osiecka-Iwan, A.; Wielunska-Kuś, B.; Święszkowski, W.; Moskalewski, S. Toward osteomimetic formation of calcium phosphate coatings with carbonated hydroxyapatite. Biomater. Adv. 2023, 149, 213403. [Google Scholar] [CrossRef]
- Campbell, A.A.; Fryxell, G.E.; Linehan, J.C.; Graff, G.L. Surface-induced mineralization: A new method for producing calcium phosphate coatings. J. Biomed. Mater. Res. 1996, 32, 111–118. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Campbell, A.A.; Graff, G.L.; Miller, G.J. Histological and biomechanical evaluation of calcium phosphate coatings applied through surface-induced mineralization to porous titanium implants. J. Biomed. Mater. Res. 1997, 34, 539–543. [Google Scholar] [CrossRef]
- Campbell, A.A.; Song, L.; Li, X.S.; Nelson, B.J.; Bottoni, C.; Brooks, D.E.; Dejong, E.S. Development, characterization, and anti-microbial efficacy of hydroxyapatite-chlorhexidine coatings produced by surface-induced mineralization. J. Biomed. Mater. Res. 2000, 53, 400–407. [Google Scholar] [CrossRef]
- Montesissa, M.; Borciani, G.; Rubini, K.; Valle, F.; Boi, M.; Baldini, N.; Boanini, E.; Graziani, G. Ionized jet deposition of calcium phosphates-based nanocoatings: Tuning coating properties and cell behavior by target composition and substrate heating. Nanomaterials 2023, 13, 1758. [Google Scholar] [CrossRef]
- Sato, M.; Aslani, A.; Sambito, M.A.; Kalkhoran, N.M.; Slamovich, E.B.; Webster, T.J. Nanocrystalline hydroxyapatite/titania coatings on titanium improves osteoblast adhesion. J. Biomed. Mater. Res. A 2008, 84A, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sambito, M.A.; Aslani, A.; Kalkhoran, N.M.; Slamovich, E.B.; Webster, T.J. Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials 2006, 27, 2358–2369. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, M.E.; Aslani, A.; Liu, H. The effects of nanostructured hydroxyapatite coating on the biodegradation and cytocompatibility of magnesium implants. J. Biomed. Mater. Res. A 2013, 101A, 2340–2354. [Google Scholar] [CrossRef] [PubMed]
- Chinellato, F.; Wilbig, J.; Al-Sabbagh, D.; Colombo, P.; Günster, J. Gas flow assisted powder deposition for enhanced flowability of fine powders: 3D printing of α-tricalcium phosphate. Open Ceram. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, S.; Zeng, X.; Qian, M.; Sun, D.; Weng, W. Interfacial study of magnesium-containing fluoridated hydroxyapatite coatings. Thin Solid Films 2011, 519, 4629–4633. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Cacciotti, I. Multisubstituted hydroxyapatite powders and coatings: The influence of the codoping on the hydroxyapatite performances. Int. J. Appl. Ceram. Technol. 2019, 16, 1864–1884. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: A review. Ceram. Int. 2021, 47, 4426–4445. [Google Scholar] [CrossRef]
- Li, H.; Khor, K.A.; Cheang, P. Titanium dioxide reinforced hydroxyapatite coatings deposited by high velocity oxy-fuel (HVOF) spray. Biomaterials 2002, 23, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, T.; Li, H.; Chen, M.; Cao, S.; Zhang, L.; Hou, X. Electrochemically assisted co-deposition of calcium phosphate/collagen coatings on carbon/carbon composites. Appl. Surf. Sci. 2011, 257, 3612–3619. [Google Scholar] [CrossRef]
- Singh, G.; Singh, S.; Prakash, S. Surface characterization of plasma sprayed pure and reinforced hydroxyapatite coating on Ti6Al4V alloy. Surf. Coat. Tech. 2011, 205, 4814–4820. [Google Scholar] [CrossRef]
- Ciobanu, G.; Ciobanu, O. Investigation on the effect of collagen and vitamins on biomimetic hydroxyapatite coating formation on titanium surfaces. Mater. Sci. Eng. C 2013, 33, 1683–1688. [Google Scholar] [CrossRef]
- Mittal, M.; Nath, S.K.; Prakash, S. Improvement in mechanical properties of plasma sprayed hydroxyapatite coatings by Al2O3 reinforcement. Mater. Sci. Eng. C 2013, 33, 2838–2845. [Google Scholar] [CrossRef]
- Ustundag, C.B.; Avciata, O.; Kaya, F.; Kaya, C. Hydrothermally mixed hydroxyapatite-multiwall carbon nanotubes composite coatings on biomedical alloys by electrophoretic deposition. J. Phys. Chem. B 2013, 117, 1571–1576. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Kui, C.; Lin, J.; Weng, W. Preparation of collagen/calcium phosphate coatings and evaluation of their biological performances. In Developments and Applications of Calcium Phosphate Bone Cements; Liu, C., He, H., Eds.; Springer: Singapore, 2018; Volume 9, pp. 547–596. [Google Scholar]
- Safavi, M.S.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. RF-magnetron sputter deposited hydroxyapatite-based composite & multilayer coatings: A systematic review from mechanical, corrosion, and biological points of view. Ceram. Int. 2021, 47, 3031–3053. [Google Scholar]
- Demnati, I.; Grossin, D.; Combes, C.; Parco, M.; Braceras, I.; Rey, C. A comparative physico-chemical study of chlorapatite and hydroxyapatite: From powders to plasma sprayed thin coatings. Biomed. Mater. 2012, 7, 054101. [Google Scholar] [CrossRef]
- Chen, W.; Oh, S.; Ong, A.P.; Oh, N.; Liu, Y.; Courtney, H.S.; Appleford, M.; Ong, J.L. Antibacterial and osteogenic properties of silver-containing hydroxyapatite coatings produced using a sol gel process. J. Biomed. Mater. Res. A 2007, 82A, 899–906. [Google Scholar] [CrossRef]
- Qu, J.; Lu, X.; Li, D.; Ding, Y.; Leng, Y.; Weng, J.; Qu, S.; Feng, B.; Watari, F. Silver/hydroxyapatite composite coatings on porous titanium surfaces by sol-gel method. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97B, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Furko, M.; Balázsi, C. Morphological, chemical, and biological investigation of ionic substituted, pulse current deposited calcium phosphate coatings. Materials 2020, 13, 4690. [Google Scholar] [CrossRef] [PubMed]
- Eraković, S.; Janković, A.; Veljović, D.; Palcevskis, E.; Mitrić, M.; Stevanović, T.; Janaćković, D.; Miskovic-Stankovic, V. Corrosion stability and bioactivity in simulated body fluid of silver/hydroxyapatite and silver/hydroxyapatite/lignin coatings on titanium obtained by electrophoretic deposition. J. Phys. Chem. B 2013, 117, 1633–1643. [Google Scholar] [CrossRef]
- Mirzaee, M.; Vaezi, M.; Palizdar, Y. Synthesis and characterization of silver doped hydroxyapatite nanocomposite coatings and evaluation of their antibacterial and corrosion resistance properties in simulated body fluid. Mater. Sci. Eng. C 2016, 69, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Syromotina, D.S.; Surmeneva, M.A.; Gorodzha, S.N.; Pichugin, V.F.; Ivanova, A.A.; Grubova, I.Y.; Kravchuk, K.S.; Gogolinskii, K.V.; Prymak, O.; Epple, M.; et al. Physical-mechanical characteristics of RF magnetron sputter-deposited coatings based on silver-doped hydroxyapatite. Russ. Phys. J. 2014, 56, 1198–1205. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Tyurin, A.I.; Pirozhkova, T.S.; Shuvarin, I.A.; Prymak, O.; Epple, M.; Chaikina, M.V.; Surmenev, R.A. Fabrication and physico-mechanical properties of thin magnetron sputter deposited silver-containing hydroxyapatite films. Appl. Surf. Sci. 2016, 360, 929–935. [Google Scholar] [CrossRef]
- Noda, I.; Miyaji, F.; Ando, Y.; Miyamoto, H.; Shimazaki, T.; Yonekura, Y.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. Development of novel thermal sprayed antibacterial coating and evaluation of release properties of silver ions. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89B, 456–465. [Google Scholar] [CrossRef]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef]
- Guimond-Lischer, S.; Ren, Q.; Braissant, O.; Gruner, P.; Wampfler, B.; Maniura-Weber, K. Vacuum plasma sprayed coatings using ionic silver doped hydroxyapatite powder to prevent bacterial infection of bone implants. Biointerphases 2016, 11, 011012. [Google Scholar] [CrossRef]
- Sanpo, N.; Tan, M.L.; Cheang, P.; Khor, K.A. Antibacterial property of cold-sprayed HA-Ag/PEEK coating. J. Therm. Spray Tech. 2009, 18, 10–15. [Google Scholar] [CrossRef]
- Bai, X.; More, K.; Rouleau, C.M.; Rabiei, A. Functionally graded hydroxyapatite coatings doped with antibacterial components. Acta Biomater. 2010, 6, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Kocourek, T.; Remsa, J.; Weiserová, M.; Jurek, K.; Mikšovský, J.; Strnad, J.; Galandáková, A.; Ulrichová, J. Antibacterial, cytotoxicity and physical properties of laser–silver doped hydroxyapatite layers. Mater. Sci. Eng. C 2013, 33, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Yanovska, A.A.; Stanislavov, A.S.; Sukhodub, L.B.; Kuznetsov, V.N.; Illiashenko, V.Y.; Danilchenko, S.N.; Sukhodub, L.F. Silver-doped hydroxyapatite coatings formed on Ti–6Al–4V substrates and their characterization. Mater. Sci. Eng. C 2014, 36, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Lindahl, C.; Lausmaa, J.; Borchardt, P.; Ballo, A.; Thomsen, P.; Engqvist, H. Biomineralized strontium-substituted apatite/titanium dioxide coating on titanium surfaces. Acta Biomater. 2010, 6, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ito, A.; Sogo, Y.; Li, X.; Oyane, A. Zinc-containing apatite layers on external fixation rods promoting cell activity. Acta Biomater. 2010, 6, 962–968. [Google Scholar] [CrossRef]
- Wang, X.; Ito, A.; Sogo, Y.; Li, X.; Oyane, A. Silicate-apatite composite layers on external fixation rods and in vitro evaluation using fibroblast and osteoblast. J. Biomed. Mater. Res. A 2010, 92A, 1181–1189. [Google Scholar]
- Hijón, N.; Cabañas, M.V.; Peña, J.; Vallet-Regí, M. Dip coated silicon-substituted hydroxyapatite films. Acta Biomater. 2006, 2, 567–574. [Google Scholar] [CrossRef]
- Bir, F.; Khireddine, H.; Touati, A.; Sidane, D.; Yala, S.; Oudadesse, H. Electrochemical depositions of fluorohydroxyapatite doped by Cu2+, Zn2+, Ag+ on stainless steel substrates. Appl. Surf. Sci. 2012, 258, 7021–7030. [Google Scholar] [CrossRef]
- Singh, G.; Singh, H.; Sidhu, B.S. Corrosion behavior of plasma sprayed hydroxyapatite and hydroxyapatite-silicon oxide coatings on AISI 304 for biomedical application. Appl. Surf. Sci. 2013, 284, 811–818. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, S.; Zhangd, M.; Yang, M.; Deng, L.; Xiong, X.; Ni, X. Deposition of calcium phosphate coatings using condensed phosphates (P2O74- and P3O105-) as phosphate source through induction heating. Mater. Sci. Eng. C 2016, 69, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Drevet, R.; Lemelle, A.; Untereiner, V.; Manfait, M.; Sockalingum, G.D.; Benhayoune, H. Morphological modifications of electrodeposited calcium phosphate coatings under amino acids effect. Appl. Surf. Sci. 2013, 268, 343–348. [Google Scholar] [CrossRef]
- Yusoff, M.F.M.; Kadir, M.R.A.; Iqbal, N.; Hassan, M.A.; Hussain, R. Dipcoating of poly (ε-caprolactone)/hydroxyapatite composite coating on Ti6Al4V for enhanced corrosion protection. Surf. Coat. Tech. 2014, 245, 102–107. [Google Scholar] [CrossRef]
- Oyane, A.; Wang, X.; Sogo, Y.; Ito, A.; Tsurushima, H. Calcium phosphate composite layers for surface-mediated gene transfer. Acta Biomater. 2012, 8, 2034–2046. [Google Scholar] [CrossRef]
- Xiao, X.F.; Liu, R.F.; Zheng, Y.Z. Characterization of hydroxyapatite/titania composite coatings codeposited by a hydrothermal–electrochemical method on titanium. Surf. Coat. Tech. 2006, 200, 4406–4413. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Zhang, L.; Hu, D.; Liu, Q. Preparation and characterization of implanted Fe catalyst in hydroxyapatite layer for uniformly dispersion carbon nanotube growth. Appl. Surf. Sci. 2018, 455, 75–83. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.; Kim, H.E.; Koh, Y.H.; Kim, H.W.; Jang, J.H. Formation of hydroxyapatite within porous TiO2 layer by micro-arc oxidation coupled with electrophoretic deposition. Acta Biomater. 2009, 5, 2196–2205. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Tayebi, L.; Vashaee, D. Biodegradable magnesium bone implants coated with a novel bioceramic nanocomposite. Materials 2020, 13, 1315. [Google Scholar] [CrossRef]
- Jagadeeshanayaka, N.; Awasthi, S.; Jambagi, S.C.; Srivastava, C. Bioactive surface modifications through thermally sprayed hydroxyapatite composite coatings: A review of selective reinforcements. Biomater. Sci. 2022, 10, 2484–2523. [Google Scholar] [CrossRef]
- Shirkhanzadeh, M.; Azadegan, M.; Liu, G.Q. Bioactive delivery systems for the slow-release of antibiotics–incorporation of Ag+ ions into micro-porous hydroxyapatite coatings. Mater. Lett. 1995, 24, 7–12. [Google Scholar] [CrossRef]
- Lee, I.S.; Whang, C.N.; Ohm, K.S.; Parkm, J.C.; Leem, K.Y.; Lee, G.H.; Chung, S.M.; Sun, X.D. Formation of silver incorporated calcium phosphate film for medical applications. Nucl. Instrum. Meth. B 2006, 242, 45–47. [Google Scholar] [CrossRef]
- Ciobanu, G.; Ilisei, S.; Luca, C. Hydroxyapatite-silver nanoparticles coatings on porous polyurethane scaffold. Mater. Sci. Eng. C 2014, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Chen, W.; Dong, Y.; Marymont, J.V.; Lei, Y.; Ke, Q.; Guo, Y.; Zhu, Z. Silver nanoparticle-loaded hydroxyapatite coating: Structure, antibacterial properties, and capacity for osteogenic induction in vitro. RSC Adv. 2016, 6, 8549–8562. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, X.; Savino, K.; Gabrys, P.; Gao, Y.; Chaimayo, W.; Miller, B.L.; Yates, M.Z. Antimicrobial silver-hydroxyapatite composite coatings through two-stage electrochemical synthesis. Surf. Coat. Tech. 2016, 301, 13–19. [Google Scholar] [CrossRef]
- Funao, H.; Nagai, S.; Sasaki, A.; Hoshikawa, T.; Tsuji, T.; Okada, Y.; Koyasu, S.; Toyama, Y.; Nakamura, M.; Aizawa, M.; et al. A novel hydroxyapatite film coated with ionic silver via inositol hexaphosphate chelation prevents implant-associated infection. Sci. Rep. 2016, 6, 23238. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y. Artificial hydroxyapatite film for the conservation of outdoor marble artworks. Mater. Lett. 2014, 124, 201–203. [Google Scholar] [CrossRef]
- Sassoni, E.; Naidu, S.; Scherer, G.W. The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J. Cult. Herit. 2011, 12, 346–355. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Zuo, G.; Wanga, X.; Hua, P.; Ma, Q.; Dong, G.; Yue, Y.; Zhang, B. Hydroxyapatite conversion layer for the preservation of surface gypsification marble relics. Corros. Sci. 2014, 88, 6–9. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E.; Graziani, G. Brushing, poultice or immersion? The role of the application technique on the performance of a novel hydroxyapatite-based consolidating treatment for limestone. J. Cult. Herit. 2015, 16, 173–184. [Google Scholar] [CrossRef]
- Sassoni, E. Hydroxyapatite and other calcium phosphates for the conservation of cultural heritage: A review. Materials 2018, 11, 557. [Google Scholar] [CrossRef]
- Naidu, S.; Scherer, G.W. Nucleation, growth and evolution of calcium phosphate films on calcite. J. Coll. Interf. Sci. 2014, 435, 128–137. [Google Scholar] [CrossRef]
- Possenti, E.; Colombo, C.; Conti, C.; Gigli, L.; Merlini, M.; Plaisier, J.R.; Realini, M.; Sali, D.; Gatta, G.D. Diammonium hydrogenphosphate for the consolidation of building materials. Investigation of newly-formed calcium phosphates. Constr. Build. Mater. 2019, 195, 557–563. [Google Scholar] [CrossRef]
- Huang, W.; Day, D.E.; Kittiratanapiboon, K.; Rahaman, M.N. Kinetics and mechanism of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006, 17, 583–596. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, W.; Rahaman, M.N.; Day, D.E.; Wang, D. Mechanism for converting Al2O3-containing borate glass to hydroxyapatite in aqueous phosphate solution. Acta Biomater. 2009, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rahaman, M.N.; Fu, Q.; Bal, B.S.; Yao, A.; Day, D.E. Conversion of bioactive borosilicate glass to multilayered hydroxyapatite in dilute phosphate solution. J. Am. Ceram. Soc. 2007, 90, 3804–3810. [Google Scholar] [CrossRef]
- Roy, D.M.; Linnehan, S.K. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature 1974, 247, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, D.; Yang, L.; Tang, H. Hydrothermal conversion of coral into hydroxyapatite. Mater. Charact. 2001, 47, 83–87. [Google Scholar] [CrossRef]
- Greiner, M.; Férnandez-Díaz, L.; Griesshaber, E.; Zenkert, M.N.; Yin, X.; Ziegler, A.; Veintemillas-Verdaguer, S.; Schmahl, W.W. Biomineral reactivity: The kinetics of the replacement reaction of biological aragonite to apatite. Minerals 2018, 8, 315. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Sato, M.; Takahashi, I.; Ishikawa, K. Fabrication of apatite-coated gypsum granules and histological evaluation using rabbits. Ceram. Int. 2018, 44, 20330–20336. [Google Scholar] [CrossRef]
- Dong, T.; Liu, Y.; He, L.; Yang, F.; Zhang, K. Preparation of hydroxyapatite coating for the conservation of gypsum crust on historic limestones. Mater. Lett. X 2021, 12, 100103. [Google Scholar] [CrossRef]
- Tang, S.; Tian, B.; Guo, Y.J.; Zhu, Z.A.; Guo, Y.P. Chitosan/carbonated hydroxyapatite composite coatings: Fabrication, structure and biocompatibility. Surf. Coat. Tech. 2014, 251, 210–216. [Google Scholar] [CrossRef]
- Karalkeviciene, R.; Briedyte, G.; Popov, A.; Tutliene, S.; Zarkov, A.; Kareiva, A. Low-temperature synthesis approach for calcium hydroxyapatite coatings on titanium substrate. Inorganics 2023, 11, 33. [Google Scholar] [CrossRef]
- Tamura, A.; Asaoka, T.; Furukawa, K.; Ushida, T.; Tateishi, T. Application of α-TCP/HAp functionally graded porous beads for bone regenerative scaffold. Adv. Sci. Technol. 2013, 86, 63–69. [Google Scholar]
- Doi, K.; Abe, Y.; Kobatake, R.; Okazaki, Y.; Oki, Y.; Naito, Y.; Prananingrum, W.; Tsuga, K. Novel development of phosphate treated porous hydroxyapatite. Materials 2017, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Nikolaev, A.L.; Melikhov, I.V.; Saparin, G.V.; Bliadze, V.G. Chemical preparation of dielectrics for studying their microtopography by the SEM. Scanning 1992, 14, 112–117. [Google Scholar] [CrossRef]
- Aubry, C.; Drouet, C.; Azaïs, T.; Kim, H.J.; Oh, J.M.; Karacan, I.; Chou, J.; Ben-Nissan, B.; Camy, S.; Cazalbou, S. Bio-activation of HA/β-TCP porous scaffolds by high-pressure CO2 surface remodeling: A novel “coating-from” approach. Materials 2022, 15, 7306. [Google Scholar] [CrossRef]
- Polikreti, K.; Maniatis, Y. Micromorphology, composition and origin of the orange patina on the marble surfaces of Propylaea (Acropolis, Athens). Sci. Total Environ. 2003, 308, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Maravelaki-Kalaitzaki, P. Black crusts and patinas on Pentelic marble from the Parthenon and Erechtheum (Acropolis, Athens): Characterization and origin. Anal. Chim. Acta 2005, 532, 187–198. [Google Scholar] [CrossRef]
- Nakhaei, M.; Jirofti, N.; Ebrahimzadeh, M.H.; Moradi, A. Different methods of hydroxyapatite-based coatings on external fixator pin with high adhesion approach. Plasma Process. Polym. 2023, 20, e2200219. [Google Scholar] [CrossRef]
- Bral, A.; Mommaerts, M.Y. In vivo biofunctionalization of titanium patient-specific implants with nano hydroxyapatite and other nano calcium phosphate coatings: A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Desante, G.; Pudełko, I.; Krok-Borkowicz, M.; Pamuła, E.; Jacobs, P.; Kazek-Kęsik, A.; Nießen, J.; Telle, R.; Gonzalez-Julian, J.; Schickle, K. Surface multifunctionalization of inert ceramic implants by calcium phosphate biomimetic coating doped with nanoparticles encapsulating antibiotics. ACS Appl. Mater. Interfaces 2023, 15, 21699–21718. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Peraire, C.; Arias, J.L.; Bernal, D.; Pou, J.; León, B.; Arañó, A.; Roth, W. Biological stability and osteoconductivity in rabbit tibia of pulsed laser deposited hydroxylapatite coatings. J. Biomed. Mater. Res. A 2006, 77A, 370–379. [Google Scholar] [CrossRef]
- ASTM F1609-08(2014); Standard Specification for Calcium Phosphate Coatings for Implantable Materials. ASTM International: West Conshohocken, PA, USA, 2014. Available online: https://www.astm.org/f1609-08r14.html (accessed on 20 April 2023).
- Arias, J.L.; Mayor, M.B.; Pou, J.; Leng, Y.; León, B.; Pérez-Amor, M. Micro- and nano-testing of calcium phosphate coatings produced by pulsed laser deposition. Biomaterials 2003, 24, 3403–3408. [Google Scholar] [CrossRef] [PubMed]
- Saber-Samandari, S.; Gross, K.A. Nanoindentation reveals mechanical properties within thermally sprayed hydroxyapatite coatings. Surf. Coat. Tech. 2009, 203, 1660–1664. [Google Scholar] [CrossRef]
- McManamon, C.; de Silva, J.P.; Power, J.; Ramirez-Garcia, S.; Morris, M.A.; Cross, G.L. Interfacial characteristics and determination of cohesive and adhesive strength of plasma-coated hydroxyapatite via nanoindentation and microscratch techniques. Langmuir 2014, 30, 11412–11420. [Google Scholar] [CrossRef]
- Hasan, F.; Wang, J.; Berndt, C. Evaluation of the mechanical properties of plasma sprayed hydroxyapatite coatings. Appl. Surf. Sci. 2014, 303, 155–162. [Google Scholar] [CrossRef]
- Hasan, M.F.; Wang, J.; Berndt, C.C. Determination of the mechanical properties of plasma-sprayed hydroxyapatite coatings using the Knoop indentation technique. J. Therm. Spray Tech. 2015, 24, 865–877. [Google Scholar] [CrossRef]
- Ohki, M.; Takahashi, S.; Jinnai, R.; Hoshina, T. Interfacial strength of plasma-sprayed hydroxyapatite coatings. J. Therm. Spray Tech. 2020, 29, 1119–1133. [Google Scholar] [CrossRef]
- Lemoine, P.; Acheson, J.; McKillop, S.; van den Beucken, J.J.J.P.; Ward, J.; Boyd, A.; Meenan, B. Nanoindentation and nano-scratching of hydroxyapatite coatings for resorbable magnesium alloy bone implant applications. J. Mech. Behav. Biomed. Mater. 2022, 133, 105306. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, B.; Choi, A.H.; Latella, B.A.; Bendavid, A. 3.8 Biomedical thin films: Mechanical properties. In Comprehensive Biomaterials II; Ducheyne, P., Healy, K., Hutmacher, D.W., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 3, pp. 128–143. [Google Scholar]
- Kummer, F.J.; Jaffe, W.L. Stability of a cyclically loaded hydroxylapatite coating: Effect of substrate material, surface, preparation and testing environment. J. Appl. Mater. 1992, 3, 211–215. [Google Scholar]
- Reis, R.L.; Monteiro, F.J.; Hastings, G.W. Stability of hydroxylapatite plasma-sprayed coated Ti–6Al–4V under cyclic bending in simulated physiological solution. J. Mater. Sci. Mater. Med. 1994, 5, 457–462. [Google Scholar] [CrossRef]
- Wolke, J.G.C.; van der Waerden, J.P.C.M.; de Groot, K.; Jansen, J.A. Stability of radiofrequency magnetron sputtered calcium phosphate coatings under cyclically loaded conditions. Biomaterials 1997, 18, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.A.M.; Wolke, J.G.C.; Klein, C.P.A.T.; de Groot, K. Fatigue behavior of calcium phosphate coatings with different stability under dry and wet conditions. J. Biomed. Mater. Res. 1999, 48, 741–748. [Google Scholar]
- Loanapakul, T.; Nimkerdphol, A.R.; Otsuka, Y.; Mutoh, Y. Fatigue behavior and apatite precipitation of plasma-sprayed HAp coating on commercially pure titanium substrate in simulated body fluid (SBF). Adv. Mater. Res. 2012, 506, 66–69. [Google Scholar] [CrossRef]
- Nimkerdphol, A.R.; Loanapakul, T.; Otsuka, Y.; Mutoh, Y. Effect of apatite precipitation on failure behavior of hydroxyapatite coating layer on titanium substrate. Adv. Mater. Res. 2012, 506, 61–65. [Google Scholar] [CrossRef]
- Otsuka, Y.; Kojima, D.; Mutoh, Y. Prediction of cyclic delamination lives of plasma-sprayed hydroxyapatite coating on Ti–6Al–4V substrates with considering wear and dissolutions. J. Mech. Behav. Biomed. Mater. 2016, 64, 113–124. [Google Scholar] [CrossRef]
- Haynes, J.A.; Rigney, E.D.; Janowski, G.M. Effects of cyclic bending and physiological solution on plasma-sprayed hydroxylapatite coatings of varying crystallinity. J. Biomed. Mater. Res. 1999, 48, 403–410. [Google Scholar] [CrossRef]
- Ashroff, S.; Napper, S.A.; Hale, P.N., Jr.; Siriwardane, U.; Mukherjee, D.P. Cyclic fatigue of hydroxyapatite-coated titanium alloy implant material-effect of crystallinity. J. Long-Term Eff. Med. Implant. 1996, 6, 143–155. [Google Scholar]
- Zhang, C.; Leng, Y.; Zhang, X. In vitro stability of plasma-sprayed hydroxyapatite coatings on Ti–6Al–4V implants under cyclic loading. J. Biomed. Mater. Res. 2000, 50, 267–275. [Google Scholar] [CrossRef]
- Gineste, L.; Gineste, M.; Ranz, X.; Ellefterion, A.; Guilhem, A.; Rouquet, N.; Frayssinet, P. Degradation of hydroxylapatite, fluorapatite, and fluorhydroxyapatite coatings of dental implants in dogs. J. Biomed. Mater. Res. 1999, 48, 224–234. [Google Scholar] [CrossRef]
- Wang, B.C.; Lee, T.M.; Chang, E.; Yang, C.Y. The shear strength and the failure mode of plasma-sprayed hydroxyapatite coating to bone: The effect of coating thickness. J. Biomed. Mater. Res. 1993, 27, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Wang, B.C.; Lee, T.M.; Chang, E.; Chang, G.L. Intramedullary implant of plasma-sprayed hydroxyapatite coating: An interface study. J. Biomed. Mater. Res. 1997, 36, 39–48. [Google Scholar] [CrossRef]
- Vercaigne, S.; Wolke, J.G.C.; Naert, I.; Jansen, J.A. A mechanical evaluation of TiO2-gritblasted and Ca-P magnetron sputter coated implants placed into the trabecular bone of the goat: Part 1. Clin. Oral Implant. Res. 2000, 11, 305–313. [Google Scholar] [CrossRef]
- Lynn, A.K.; DuQuesnay, D.L. Hydroxyapatite-coated Ti–6Al–4V Part 1: The effect of coating thickness on mechanical fatigue behavior. Biomaterials 2002, 23, 1937–1946. [Google Scholar] [CrossRef]
- Svehla, M.; Morberg, P.; Bruce, W.; Zicat, B.; Walsh, W.R. The effect of substrate roughness and hydroxyapatite coating thickness on implant shear strength. J. Arthroplast. 2002, 17, 304–311. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.G.; Lim, S.C. Histomorphometric study of bone reactions with different hydroxyapatite coating thickness on dental implants in dogs. Thin Solid Films 2011, 519, 4618–4622. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Alias, J.; Zulkifli, F.H.; Kadirgama, K.; Ghani, S.A.C.; Shariffuddin, J.H.M. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Reikeras, O.; Gunderson, R.B. Failure of HA coating on a gritblasted acetabular cup: 155 Patients followed for 7–10 years. Acta Orthop. Scand. 2002, 73, 104–108. [Google Scholar] [CrossRef]
- Pawłowski, L. The Science and Engineering of Thermal Spray Coatings, 2nd ed.; Wiley: New York, NY, USA, 2008; 691p. [Google Scholar]
- Guipont, V.; Jeandin, M.; Bansard, S.; Khor, K.A.; Nivard, M.; Berthe, L.; Cuq-Lelandais, J.P.; Boustie, M. Bond strength determination of hydroxyapatite coatings on Ti–6Al–4V substrates using the LAser Shock Adhesion Test (LASAT). J. Biomed. Mater. Res. A 2010, 95A, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Nimb, L.; Gotfredsen, K.; Steen, J.J. Mechanical failure of hydroxyapatite-coated titanium and cobalt-chromium-molybdenum alloy implants. An animal study. Acta Orthop. Belg. 1993, 59, 333–338. [Google Scholar] [PubMed]
- ASTM C633–01(2008); Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings. ASTM International: West Conshohocken, PA, USA, 2008. Available online: https://www.astm.org/c0633-13r21.html (accessed on 20 April 2023).
- ASTM F1147–05(2011); Standard Test Method for Tension Testing of Calcium Phosphate and Metallic Coatings. ASTM International: West Conshohocken, PA, USA, 2011. Available online: https://www.astm.org/f1147-05r17e01.html (accessed on 20 April 2023).
- Mukherjee, D.P.; Dorairaj, N.R.; Mills, D.K.; Graham, D.; Krauser, J.T. Fatigue properties of hydroxyapatite-coated dental implants after exposure to a periodontal pathogen. J. Biomed. Mater. Res. 2000, 53, 467–474. [Google Scholar] [CrossRef]
- Cheng, K.; Ren, C.; Weng, W.; Du, P.; Shen, G.; Han, G.; Zhang, S. Bonding strength of fluoridated hydroxyapatite coatings: A comparative study on pull-out and scratch analysis. Thin Solid Films 2009, 517, 5361–5364. [Google Scholar] [CrossRef]
- Toque, J.A.; Herliansyah, M.K.; Hamdi, M.; Ide-Ektessabi, A.; Sopyan, I. Adhesion failure behavior of sputtered calcium phosphate thin film coatings evaluated using microscratch testing. J. Mech. Behav. Biomed. Mater. 2010, 3, 324–330. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Zou, Y.L.; Fu, W.; Bai, X.B.; Ji, G.C.; Yao, H.L.; Wang, H.T.; Wang, F. Wear behavior of plasma sprayed hydroxyapatite bioceramic coating in simulated body fluid. Ceram. Int. 2019, 45, 4526–4534. [Google Scholar] [CrossRef]
- Barnes, D.; Johnson, S.; Snell, R.; Best, S. Using scratch testing to measure the adhesion strength of calcium phosphate coatings applied to poly(carbonate urethane) substrates. J. Mech. Behav. Biomed. Mater. 2012, 6, 128–138. [Google Scholar] [CrossRef]
- Roland, T.; Pelletier, H.; Krier, J. Scratch resistance and electrochemical corrosion behavior of hydroxyapatite coatings on Ti6Al4V in simulated physiological media. J. Appl. Electrochem. 2013, 43, 53–63. [Google Scholar] [CrossRef]
- Kulyashova, K.; Sharkeev, Y.; Sainova, A. Mechanical properties of calcium phosphate coatings produced by method of RF-magnetron sputtering on bioinert alloys. Adv. Mater. Res. 2014, 1013, 188–193. [Google Scholar] [CrossRef]
- ISO 20502:2005; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Adhesion of Ceramic Coatings by Scratch Testing. ISO: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/34189.html (accessed on 20 April 2023).
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Vasanthan, A.; Kim, H.; Drukteinis, S.; Lacefield, W. Implant surface modification using laser guided coatings: In vitro comparison of mechanical properties. J. Prosthodont. 2008, 17, 357–364. [Google Scholar] [CrossRef]
- Filiaggi, M.J.; Coombs, N.A.; Pilliar, R.M. Characterization of the interface in plasma-sprayed HA coating/Ti–6Al–4V implant system. J. Biomed. Mater. Res. 1991, 25, 1211–1229. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Lin, J.; Hodgson, P.D.; Wen, C. Effect of heat-treatment atmosphere on the bond strength of apatite layer on Ti substrate. Dent. Mater. 2008, 24, 1549–1555. [Google Scholar] [CrossRef]
- Rajesh, P.; Muraleedharan, C.V.; Komath, M.; Varma, H. Pulsed laser deposition of hydroxyapatite on titanium substrate with titania interlayer. J. Mater. Sci. Mater. Med. 2011, 22, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Man, H.C.; Xing, W.; Zheng, X. Adhesion strength of plasma-sprayed hydroxyapatite coatings on laser gas-nitrided pure titanium. Surf. Coat. Tech. 2009, 203, 3116–3122. [Google Scholar] [CrossRef]
- Yang, S.; Xing, W.; Man, H.C. Pulsed laser deposition of hydroxyapatite film on laser gas nitriding NiTi substrate. Appl. Surf. Sci. 2009, 255, 9889–9892. [Google Scholar] [CrossRef]
- Man, H.C.; Chiu, K.Y.; Cheng, F.T.; Wong, K.H. Adhesion study of pulsed laser deposited hydroxyapatite coating on laser surface nitrided titanium. Thin Solid Films 2009, 517, 5496–5501. [Google Scholar] [CrossRef]
- Nelea, V.; Morosanu, C.; Bercu, M.; Mihailescu, I.N. Interfacial titanium oxide between hydroxyapatite and TiAlFe substrate. J. Mater. Sci. Mater. Med. 2007, 18, 2347–2354. [Google Scholar] [CrossRef]
- Berezhnaya, A.Y.; Mittova, V.O.; Kukueva, E.V.; Mittova, I.Y. Effect of high-temperature annealing on solid-state reactions in hydroxyapatite/TiO2 films on titanium substrates. Inorg. Mater. 2010, 46, 971–977. [Google Scholar] [CrossRef]
- Ehlert, M.; Radtke, A.; Bartmański, M.; Piszczek, P. Evaluation of the cathodic electrodeposition effectiveness of the hydroxyapatite layer used in surface modification of Ti6Al4V-based biomaterials. Materials 2022, 15, 6925. [Google Scholar] [CrossRef]
- Yang, J.X.; Jiao, Y.P.; Cui, F.Z.; Lee, I.S.; Yin, Q.S.; Zhang, Y. Modification of degradation behavior of magnesium alloy by IBAD coating of calcium phosphate. Surf. Coat. Tech. 2008, 202, 5733–5736. [Google Scholar] [CrossRef]
- Kokubo, T.; Miyaji, F.; Kim, H.M. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Bonding strength of bonelike apatite layer to Ti metal substrate. J. Biomed. Mater. Res. 1997, 38, 121–127. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Hodgson, P.D.; Wen, C. Microstructures and bond strengths of the calcium phosphate coatings formed on titanium from different simulated body fluids. Mater. Sci. Eng. C 2009, 29, 165–171. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Cazalbou, S.; Grossin, D.; Brouillet, F.; Sarda, S. Surface properties of biomimetic nanocrystalline apatites; applications in biomaterials. Prog. Cryst. Growth Charact. Mater. 2014, 60, 63–73. [Google Scholar] [CrossRef]
- Xue, W.; Liu, X.; Zheng, X.; Ding, C. Effect of hydroxyapatite coating crystallinity on dissolution and osseointegration in vivo. J. Biomed. Mater. Res. A 2005, 74A, 553–561. [Google Scholar] [CrossRef]
- ISO 13779-3:2018; Implants for Surgery—Hydroxyapatite—Part 3: Chemical Analysis and Characterization of Crystallinity Ratio and Phase Purity. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/64618.html (accessed on 20 April 2023).
- Ducheyne, P.; Qiu, Q. Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999, 20, 2287–2303. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hieda, Y.; Kogai, Y. Effect of hydroxyapatite surface morphology on cell adhesion. Mater. Sci. Eng. C 2016, 69, 1263–1267. [Google Scholar] [CrossRef]
- Lee, W.K.; Lee, S.M.; Kim, H.M. Effect of surface morphology of calcium phosphate on osteoblast-like HOS cell responses. J. Ind. Eng. Chem. 2009, 15, 677–682. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Wu, V.M.; Uskoković, V. Is there a relationship between solubility and resorbability of different calcium phosphate phases in vitro? Biochim. Biophys. Acta 2016, 1860, 2157–2768. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Dissolution mechanism of calcium apatites in acids: A review of literature. World J. Methodol. 2012, 2, 1. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate (CaPO4)-based bioceramics: Preparation, properties, and applications. Coatings 2022, 12, 1380. [Google Scholar] [CrossRef]
- Tucker, B.E.; Cottel, C.M.; Auyeung, R.C.Y.; Spector, M.; Nancollas, G.H. Pre-conditioning and dual constant composition dissolution kinetics of pulsed laser deposited hydroxyapatite thin films on silicon substrates. Biomaterials 1996, 17, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Khor, K.A.; Li, H.; Cheang, P.; Boey, S.Y. In vitro behavior of HVOF sprayed calcium phosphate splats and coatings. Biomaterials 2003, 24, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Verestiuc, L.; Morosanu, C.; Bercu, M.; Pasuk, I.; Mihailescu, I.N. Chemical growth of calcium phosphate layers on magnetron sputtered HA films. J. Cryst. Growth 2004, 264, 483–491. [Google Scholar] [CrossRef]
- Heimann, R.B. Characterization of as-plasma-sprayed and incubated hydroxyapatite coatings with high resolution techniques. Materialwiss. Werkst. 2009, 40, 23–30. [Google Scholar] [CrossRef]
- Ntsoane, T.P.; Topic, M.; Bucher, R. Near-surface in vitro studies of plasma sprayed hydroxyapatite coatings. Powder Diffr. 2011, 26, 138–143. [Google Scholar] [CrossRef]
- Choudhary, L.; Raman, R.K.S.; Nie, J.F. In vitro evaluation of degradation of a calcium phosphate coating on a Mg-Zn-Ca alloy in a physiological environment. Corrosion 2012, 68, 499–506. [Google Scholar] [CrossRef]
- Edreira, E.R.U.; Wolke, J.G.C.; Aldosari, A.A.F.; Al-Johany, S.S.; Anil, S.; Jansen, J.A.; van den Beucken, J.J.J.P. Effects of calcium phosphate composition in sputter coatings on in vitro and in vivo performance. J. Biomed. Mater. Res. A 2015, 103A, 300–310. [Google Scholar] [CrossRef]
- Luo, Z.S.; Cui, F.Z.; Feng, Q.L.; Li, H.D.; Zhu, X.D.; Spector, M. In vitro and in vivo evaluation of degradability of hydroxyapatite coatings synthesized by ion beam-assisted deposition. Surf. Coat. Tech. 2000, 131, 192–195. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Ryabtseva, M.A.; Shesterikov, E.V.; Pichugin, V.F.; Peitsch, T.; Epple, M. The release of nickel from nickel-titanium (NiTi) is strongly reduced by a sub-micrometer thin layer of calcium phosphate deposited by RF-magnetron sputtering. J. Mater. Sci. Mater. Med. 2010, 21, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Kawasaki, Y.; Narushima, T.; Goto, T.; Kurihara, J.; Nakagawa, H.; Kawamura, H.; Taira, M. Calcium phosphate films with/without heat treatments fabricated using RF magnetron sputtering. J. Biomech. Sci. Eng. 2009, 4, 392–403. [Google Scholar] [CrossRef]
- Gross, K.A.; Berndt, C.C. In vitro testing of plasma-sprayed hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 1994, 5, 219–224. [Google Scholar] [CrossRef]
- Gross, K.A.; Berndt, C.C.; Goldschlag, D.D.; Iacono, V.J. In vitro changes of hydroxyapatite coatings. Int. J. Oral Max. Impl. 1997, 12, 589–597. [Google Scholar]
- Boyd, A.R.; Meenan, B.J.; Leyland, N.S. Surface characterisation of the evolving nature of radio frequency (RF) magnetron sputter deposited calcium phosphate thin films after exposure to physiological solution. Surf. Coat. Tech. 2006, 200, 6002–6013. [Google Scholar] [CrossRef]
- Coelho, P.G.; de Assis, S.L.; Costa, I.; Thompson, V.P. Corrosion resistance evaluation of a Ca- and P-based bioceramic thin coating in Ti–6Al–4V. J. Mater. Sci. Mater. Med. 2009, 20, 215–222. [Google Scholar] [CrossRef]
- Lim, Y.M.; Kim, B.H.; Jeon, Y.S.; Jeon, K.O.; Hwang, K.S. Calcium phosphate films deposited by electrostatic spray deposition and an evaluation of their bioactivity. J. Ceram. Process. Res. 2005, 6, 255–258. [Google Scholar]
- Long, T.; Hong, F.; Shen, S.; Wang, L.; Wang, Y.; Wang, J. In vitro degradation of electrodeposited calcium phosphate coatings by osteoclast-like cells. Biomed. Mater. 2012, 7, 045012. [Google Scholar] [CrossRef]
- Klein, C.P.A.T.; Patka, P.; Wolke, J.G.C.; de Blieck-Hogervorst, J.M.A.; de Groot, K. Long-term in vivo study of plasma-sprayed coatings on titanium alloys of tetracalcium phosphate, hydroxyapatite and α-tricalcium phosphate. Biomaterials 1994, 15, 146–150. [Google Scholar] [CrossRef]
- Klein, C.P.A.T.; Patka, P.; van der Lubbe, H.B.M.; Wolcke, J.G.C.; de Groot, K. Plasma-sprayed coatings of tetracalcium phosphate, hydroxylapatite, and α-TCP on titanium alloy: An interface study. J. Biomed. Mater. Res. 1991, 25, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, P.; Lugowski, S.; Davies, J.E. Dissolution behavior of calcium phosphate nanocrystals deposited on titanium alloy surfaces. J. Biomed. Mater. Res. A 2010, 94A, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.P.A.T.; Wolke, J.G.C.; de Blieck-Hogervorst, J.M.A.; de Groot, K. Calcium phosphate plasma-sprayed coatings and their stability: An in vivo study. J. Biomed. Mater. Res. 1994, 28, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Dhert, W.J.A.; Klein, C.P.A.T.; Wolke, J.G.C.; van der Velde, E.A.; de Groot, K.; Rozing, P.M. A mechanical investigation of fluorapatite, magnesium whitlockite and hydroxylapatite plasma-sprayed coatings in goats. J. Biomed. Mater. Res. 1992, 25, 1183–1200. [Google Scholar] [CrossRef]
- Dhert, W.J.A.; Klein, C.P.A.T.; Jansen, J.A.; van der Velde, E.A.; Vriesde, R.C.; de Groot, K.; Rozing, P.M. A histological and histomorphometrical investigation of fluorapatite, magnesium whitlockite and hydroxylapatite plasma-sprayed coatings in goats. J. Biomed. Mater. Res. 1993, 27, 127–138. [Google Scholar] [CrossRef]
- Caulier, H.; van der Waerden, J.P.C.M.; Paquay, Y.C.G.J.; Wolke, J.G.C.; Kalk, W.; Naert, I.; Jansen, J.A. Effect of calcium phosphate (Ca-P) coatings on trabecular bone response: A histological study. J. Biomed. Mater. Res. 1995, 29, 1061–1069. [Google Scholar] [CrossRef]
- de Bruijn, J.D.; Bovell, Y.P.; van Blitterswijk, C.A. Structural arrangements at the interface between plasma sprayed calcium phosphates and bone. Biomaterials 1994, 15, 543–550. [Google Scholar] [CrossRef]
- Cleries, L.; Fernandez-Pradas, J.M.; Morenza, J.L. Behaviour in simulated body fluid of calcium phosphate coatings obtained by laser ablation. Biomaterials 2000, 21, 1861–1865. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.H.; Raeissi, K.; Sharifnabi, A. Biodegradation assessment of nanostructured fluoridated hydroxyapatite coatings on biomedical grade magnesium alloy. Ceram. Int. 2014, 40, 15149–15158. [Google Scholar] [CrossRef]
- Hulshoff, J.E.G.; van Dijk, K.; van der Waerden, J.P.C.M.; Kalk, W.; Jansen, J.A. A histological and histomorphometrical evaluation of screw-type calciumphosphate (Ca-P) coated implants; an in vivo experiment in maxillary cancellous bone of goats. J. Mater. Sci. Mater. Med. 1996, 7, 603–609. [Google Scholar] [CrossRef]
- Caulier, H.; van der Waerden, J.P.C.M.; Wolke, J.G.C.; Kalk, W.; Naert, I.; Jansen, J.A. A histological and histomorphometrical evaluation of the application of screw-designed calciumphosphate (Ca-P)-coated implants in the cancellous maxillary bone of the goat. J. Biomed. Mater. Res. 1997, 35, 19–30. [Google Scholar] [CrossRef]
- Caulier, H.; Hayakawa, T.; Naert, I.; van der Waerden, J.P.C.M.; Wolke, J.G.C.; Jansen, J.A. An animal study on the bone behaviour of Ca-P-coated implants: Influence of implant location. J. Mater. Sci. Mater. Med. 1997, 8, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Simank, H.G.; Stuber, M.; Frahm, R.; Helbig, L.; van Lenthe, H.; Müller, R. The influence of surface coatings of dicalcium phosphate (DCPD) and growth and differentiation factor-5 (GDF-5) on the stability of titanium implants in vivo. Biomaterials 2006, 27, 3988–3994. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kawashima, M.; Hatanaka, R.; Kusunoki, M.; Nishikawa, H.; Hontsu, S.; Nakamura, M. Cytocompatibility of calcium phosphate coatings deposited by an ArF pulsed laser. J. Mater. Sci. Mater. Med. 2008, 19, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sima, L.E.; Stan, G.E.; Morosanu, C.O.; Melinescu, A.; Ianculescu, A.; Melinte, R.; Neamtu, J.; Petrescu, S.M. Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films. J. Biomed. Mater. Res. A 2010, 95A, 1203–1214. [Google Scholar] [CrossRef]
- Hong, Z.; Mello, A.; Yoshida, T.; Luan, L.; Stern, P.H.; Rossi, A.; Ellis, D.E.; Ketterson, J.B. Osteoblast proliferation on hydroxyapatite coated substrates prepared by right angle magnetron sputtering. J. Biomed. Mater. Res. A 2010, 93A, 878–885. [Google Scholar] [CrossRef]
- Cairns, M.L.; Meenan, B.J.; Burke, G.A.; Boyd, A.R. Influence of surface topography on osteoblast response to fibronectin coated calcium phosphate thin films. Colloid Surface B 2010, 78, 283–290. [Google Scholar] [CrossRef]
- Gomes, A.M.; da Silva, D.F.; Bezerra, F.J.; Zambuzzi, W.F. Nanohydroxyapatite-coated titanium surface increases vascular endothelial cells distinct signaling responding to high glucose concentration. J. Funct. Biomater. 2023, 14, 188. [Google Scholar] [CrossRef]
- Duta, L. In vivo assessment of synthetic and biological-derived calcium phosphate-based coatings fabricated by pulsed laser deposition: A review. Coatings 2021, 11, 99. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Komarova, E.G.; Chebodaeva, V.V.; Sedelnikova, M.B.; Zakharenko, A.M.; Golokhvast, K.S.; Litvinova, L.S.; Khaziakhmatova, O.G.; Malashchenko, V.V.; Yurova, K.A.; et al. Amorphous–crystalline calcium phosphate coating promotes in vitro growth of tumor-derived Jurkat T cells activated by anti-CD2/CD3/CD28 antibodies. Materials 2021, 14, 3693. [Google Scholar] [CrossRef]
- Eggert, A.; Buhren, B.A.; Schrumpf, H.; Haversath, M.; Ruppert, M.; Jäger, M.; Krauspe, R.; Zilkens, C. Influence of a calcium phosphate coating (BONIT®) on the proliferation and differentiation potential of human mesenchymal stroma cells in the early phase of bone healing. J. Funct. Biomater. 2022, 13, 176. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Lin, X.; Li, H. Macrophage polarization related to biomimetic calcium phosphate coatings: A preliminary study. Materials 2023, 16, 332. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Yoshinari, M.; Kiba, H.; Yamamoto, H.; Nemoto, K.; Jansen, J.A. Trabecular bone response to surface roughened and calcium phosphate (Ca-P) coated titanium implants. Biomaterials 2002, 23, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.L.; Bessho, K.; Cavin, R.; Carnes, D.L. Bone response to radio frequency sputtered calcium phosphate implants and titanium implants in vivo. J. Biomed. Mater. Res. 2002, 59, 184–190. [Google Scholar] [CrossRef]
- Dalton, J.E.; Cook, S.D. In vivo mechanical and histological characteristics of HA-coated implants vary with coating vendor. J. Biomed. Mater. Res. 1995, 29, 239–245. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Deporter, D.A.; Pilliar, R.M.; Valiquette, N.; Yakubovich, R. The effect of sol-gel-formed calcium phosphate coatings on bone ingrowth and osteoconductivity of porous-surfaced Ti alloy implants. Biomaterials 2004, 25, 865–876. [Google Scholar] [CrossRef]
- Yan, Y.; Wolke, J.G.C.; de Ruijter, A.; Li, Y.; Jansen, J.A. Growth behavior of rat bone marrow cells on RF magnetron sputtered hydroxyapatite and dicalcium pyrophosphate coatings. J. Biomed. Mater. Res. A 2006, 78A, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.H.; Lee, I.S.; Bai, W.; Cui, F.Z.; Feng, H.L. Improvement of fibroblast adherence to titanium surface by calcium phosphate coating formed with IBAD. Surf. Coat. Tech. 2005, 193, 366–371. [Google Scholar] [CrossRef]
- Pareta, R.A.; Taylor, E.; Webster, T.J. Increased osteoblast density in the presence of novel calcium phosphate coated magnetic nanoparticles. Nanotechnology 2008, 19, 265101. [Google Scholar] [CrossRef]
- Drevet, R.; Viteaux, A.; Maurin, J.C.; Benhayoune, H. Human osteoblast-like cells response to pulsed electrodeposited calcium phosphate coatings. RSC Adv. 2013, 3, 11148–11154. [Google Scholar] [CrossRef]
- Ball, M.D.; Downes, S.; Scotchford, C.A.; Antonov, E.N.; Bagratashvili, V.N.; Popov, V.K.; Lo, W.J.; Grant, D.M.; Howdle, S.M. Osteoblast growth on titanium foils coated with hydroxyapatite by pulsed laser ablation. Biomaterials 2001, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Bucci-Sabattini, V.; Cassinelli, C.; Coelho, P.G.; Minnici, A.; Trani, A.; Ehrenfest, D.M.D. Effect of titanium implant surface nanoroughness and calcium phosphate low impregnation on bone cell activity in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wang, J.; Tache, A.; Valiquette, N.; Deporter, D.; Pilliar, R. Calcium phosphate sol-gel-derived thin films on porous-surfaced implants for enhanced osteoconductivity: Part II: Short-term in vivo studies. Biomaterials 2004, 25, 5313–5321. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A. A critical review of decades of research on calcium phosphate–based coatings: How far are we from their widespread clinical application? Curr. Opin. Biomed. Eng. 2019, 10, 35–44. [Google Scholar] [CrossRef]
- Lavos-Valereto, I.C.; Wolynec, S.; Deboni, M.C.Z.; Knig, B., Jr. In vitro and in vivo biocompatibility testing of Ti–6Al–7Nb alloy with and without plasma-sprayed hydroxyapatite coating. J. Biomed. Mater. Res. 2001, 58, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, C.W. Comparison of hydroxyapatite-coated and non-hydroxyapatite-coated noncemented total hip arthroplasty in same patients. J. Arthroplast. 2007, 22, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Goosen, J.H.; Kums, A.J.; Kollen, B.J.; Verheyen, C.C. Porous-coated femoral components with or without hydroxyapatite in primary uncemented total hip arthroplasty: A systematic review of randomized controlled trials. Arch. Orthop. Trauma Surg. 2009, 129, 1165–1169. [Google Scholar] [CrossRef]
- Sato, T.; Nakashima, Y.; Komiyama, K.; Yamamoto, T.; Motomura, G.; Iwamoto, Y. The absence of hydroxyapatite coating on cementless acetabular components does not affect long-term survivorship in total hip arthroplasty. J. Arthroplast. 2016, 31, 1228–1232. [Google Scholar] [CrossRef]
- Damerau, J.M.; Bierbaum, S.; Wiedemeier, D.; Korn, P.; Smeets, R.; Jenny, G.; Nadalini, J.; Stadlinger, B. A systematic review on the effect of inorganic surface coatings in large animal models and meta-analysis on tricalcium phosphate and hydroxyapatite on periimplant bone formation. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110B, 157–175. [Google Scholar] [CrossRef]
- Valancius, K.; Soballe, K.; Nielsen, P.T.; Laursen, M.B. No superior performance of hydroxyapatite-coated acetabular cups over porous-coated cups. Acta Orthop. 2013, 84, 544–548. [Google Scholar] [CrossRef]
- Yoon, K.S.; Kim, H.J.; Lee, J.H.; Kang, S.B.; Seong, N.H.; Koo, K.H. A randomized clinical trial of cementless femoral stems with and without hydroxyapatite/tricalcium-phosphate coating: An 8- to 12-year follow-up study. J. Arthroplast. 2007, 22, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.; Harris, A.H.S.; Giori, N.J. Survival of hydroxyapatite-coated versus non-hydroxyapatite-coated total hip arthroplasty implants in a veteran population. J. Arthroplast. 2022, 37, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Lazarinis, S.; Krrholm, J.; Hailer, N.P. Effects of hydroxyapatite coating on survival of an uncemented femoral stem. Acta Orthop. 2011, 82, 399–404. [Google Scholar] [CrossRef]
- Camazzola, D.; Hammond, T.; Gandhi, R.; Davey, J.R. A randomized trial of hydroxyapatite-coated femoral stems in total hip arthroplasty. A 13-year follow-up. J. Arthroplast. 2009, 24, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lazarinis, S.; Maekelae, K.T.; Eskelinen, A.; Havelin, L.; Hallan, G.; Overgaard, S.; Pedersen, A.B.; Kaerrholm, J.; Hailer, N.P. Does hydroxyapatite coating of uncemented cups improve long-term survival? An analysis of 28,605 primary total hip arthroplasty procedures from the Nordic Arthroplasty Register Association (NARA). Osteoarth. Cartilage 2017, 25, 1980–1987. [Google Scholar] [CrossRef]
- Stilling, M.; Rahbek, O.; Søballe, K. Inferior survival of hydroxyapatite versus titanium-coated cups at 15 years. Clin. Orthop. Rel. Res. 2009, 467, 2872–2879. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.S.; Joo, J.H.; Park, J.W. Is hydroxyapatite coating necessary to improve survivorship of porous-coated titanium femoral stem? J. Arthroplast. 2012, 27, 559–563. [Google Scholar] [CrossRef]
- Lombardi, A.V., Jr.; Berend, K.R.; Mallory, T.H. Hydroxyapatite-coated titanium porous plasma spray tapered stem: Experience at 15 to 18 years. Clin. Orthop. Relat. Res. 2006, 453, 81–85. [Google Scholar] [CrossRef]
- Reikerås, O. Femoral revision surgery using a fully hydroxyapatite-coated stem: A cohort study of twenty two to twenty seven years. Int. Orthop. 2017, 41, 271–275. [Google Scholar] [CrossRef]
- Lee, J.J.; Rouhfar, L.; Beirne, O.R. Survival of hydroxyapatite-coated implants: A meta-analytic review. J. Oral Maxillofac. Surg. 2000, 58, 1372–1379. [Google Scholar] [CrossRef]
- Gandhi, R.; Davey, J.R.; Mahomed, N.N. Hydroxyapatite coated femoral stems in primary total hip arthroplasty. A meta-analysis. J. Arthroplast. 2009, 24, 38–42. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; López-Valverde, A.; Aragoneses, J.M.; de Sousa, B.M.; Rodrigues, M.J.; Ramírez, J.M. Systematic review and meta-analysis of the effectiveness of calcium-phosphate coating on the osseointegration of titanium implants. Materials 2021, 14, 3015. [Google Scholar] [CrossRef] [PubMed]
- Gottlander, M.; Johansson, C.B.; Wennerberg, A.; Albrektsson, T.; Radin, S.; Ducheyne, P. Bone tissue reactions to an electrophoretically applied calcium phosphate coating. Biomaterials 1997, 18, 551–557. [Google Scholar] [CrossRef]
- Pegg, E.C.; Matboli, F.; Marriott, T.; Khan, I.; Scotchford, C.A. Topographical and chemical effects of electrochemically assisted deposited hydroxyapatite coatings on osteoblast-like cells. J. Biomater. Appl. 2014, 28, 946–953. [Google Scholar] [CrossRef]
- Piattelli, A.; Cosci, F.; Scarano, A.; Trisi, P. Localized chronic suppurative bone infection as a sequel of peri-implantitis in a hydroxyapatite-coated dental implant. Biomaterials 1995, 16, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Walschus, U.; Hoene, A.; Neumann, H.G.; Wilhelm, L.; Lucke, S.; Luthen, F.; Rychly, J.; Schlosser, M. Morphometric immunohistochemical examination of the inflammatory tissue reaction after implantation of calcium phosphate-coated titanium plates in rats. Acta Biomater. 2009, 5, 776–784. [Google Scholar] [CrossRef]
- Bloebaum, R.D.; Beeks, D.; Dorr, L.D.; Savory, C.G.; DuPont, J.A.; Hofmann, A.A. Complications with hydroxyapatite particulate separation in total hip arthroplasty. Clin. Orthop. Relat. Res. 1994, 298, 19–26. [Google Scholar] [CrossRef]
- Bauer, T.W. Hydroxyapatite: Coating controversies. Orthopedics 1995, 18, 885–888. [Google Scholar] [CrossRef]
- Morscher, E.W.; Hefti, A.; Aebi, U. Severe osteolysis after third-body wear due to hydroxyapatite particles from acetabular cup coating. J. Bone Jt. Surg. Br. 1998, 80, 267–272. [Google Scholar] [CrossRef]
- Lazarinis, S.; Krärholm, J.; Hailer, N.P. Increased risk of revision of acetabular cups coated with hydroxyapatite: A Swedish Hip Arthroplasty Register study involving 8,043 total hip replacements. Acta Orthop. 2010, 81, 53–59. [Google Scholar] [CrossRef]
- Oosterbos, C.J.M.; Vogely, H.C.; Nijhof, M.W.; Fleer, A.; Verbout, A.J.; Tonino, A.J.; Dhert, W.J.A. Osseointegration of hydroxyapatite-coated and noncoated Ti6Al4V implants in the presence of local infection: A comparative histomorphometrical study in rabbits. J. Biomed. Mater. Res. 2002, 60, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Geesink, R.G.T.; de Groot, K.; Klein, C.P.A.T. Chemical implant fixation using hydroxyl-apatite coatings. The development of a human total hip prosthesis for chemical fixation to bone using hydroxyl-apatite coatings on titanium substrates. Clin. Orthop. Rel. Res. 1987, 225, 147–170. [Google Scholar] [CrossRef]
- Furlong, R.J.; Osborn, J.F. Fixation of hip prostheses by hydroxyapatite ceramic coating. J. Bone Jt. Surg. Br. 1991, 73, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Geesink, R.G.T.; Zimmerman, R.; McMahon, J.T. Hydroxyapatite-coated femoral stems. Histological analysis of components retrieved at autopsy. J. Bone Jt. Surg. Am. 1991, 73, 1439–1452. [Google Scholar] [CrossRef]
- Buma, P.; Gardeniers, J.W. Tissue reactions around a hydroxyapatite-coated hip prostheses: Case report of a retrieveal specimen. J. Arthroplast. 1995, 10, 389–395. [Google Scholar] [CrossRef]
- Thomas, K.A.; Cook, C.D.; Ray, R.J.; Jarcho, M. Biologic response to hydroxylapatite coated titanium hips. J. Arthroplast. 1989, 4, 43–53. [Google Scholar] [CrossRef]
- Park, Y.S.; Yi, K.Y.; Lee, I.S.; Han, C.H.; Jung, Y.C. The effects of ion beam-assisted deposition of hydroxyapatite on the grit-blasted surface of endosseous implants in rabbit tibiae. Int. J. Oral Max. Impl. 2005, 20, 31–38. [Google Scholar]
- Junker, R.; Manders, P.J.D.; Wolke, J.G.C.; Borisov, Y.; Jansen, J.A. Bone-supportive behavior of microplasma-sprayed CaP-coated implants: Mechanical and histological outcome in the goat. Clin. Oral Implant. Res. 2010, 21, 189–200. [Google Scholar] [CrossRef]
- Suzuki, M.; Calasans-Maia, M.D.; Marin, C.; Granato, R.; Gil, J.N.; Granjeiro, J.M.; Coelho, P.G. Effect of surface modifications on early bone healing around plateau root form implants: An experimental study in rabbits. J. Oral Maxillofac. Surg. 2010, 68, 1631–1638. [Google Scholar] [CrossRef]
- Barkarmo, S.; Wennerberg, A.; Hoffman, M.; Kjellin, P.; Breding, K.; Handa, P.; Stenport, V. Nano-hydroxyapatite-coated PEEK implants: A pilot study in rabbit bone. J. Biomed. Mater. Res. A 2013, 101A, 465–471. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; Bosco, R.; van den Beucken, J.J.J.P.; Walboomers, X.F.; Jansen, J.A. Osteogenicity of titanium implants coated with calcium phosphate or collagen type-I in osteoporotic rats. Biomaterials 2013, 34, 3747–3757. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, J.; Wang, L.; Ma, X.; Huang, Y.; Qiu, Z.; Cui, F. An improved biofunction of titanium for keratoprosthesis by hydroxyapatite-coating. J. Biomater. Appl. 2014, 28, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Race, A.; Heffernan, C.D.; Sharkey, P.F. The addition of a hydroxyapatite coating changes the immediate postoperative stability of a plasma-sprayed femoral stem. J. Arthroplast. 2011, 26, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Søballe, K.; Hansen, E.S.; Brockstedt-Rasmussen, H.B.; Bünger, C. Hydroxyapatite coating converts fibrous tissue to bone around loaded implants. J. Bone Jt. Surg. Br. 1993, 75, 270–278. [Google Scholar] [CrossRef]
- Daugaard, H.; Elmengaard, B.; Bechtold, J.E.; Jensen, T.; Soballe, K. The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. J. Biomed. Mater. Res. A 2010, 92A, 913–921. [Google Scholar] [CrossRef]
- Mutsuzaki, H.; Sogo, Y.; Oyane, A.; Ito, A. Improved bonding of partially osteomyelitic bone to titanium pins owing to biomimetic coating of apatite. Int. J. Mol. Sci. 2013, 15, 24366–24379. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Nishiwaki, N.; Ueda, K.; Narushima, T.; Kawamura, H.; Takahashi, T. Evaluation of thin amorphous calcium phosphate coatings on titanium dental implants deposited using magnetron sputtering. Implant. Dent. 2014, 23, 343–350. [Google Scholar] [CrossRef]
- Thorfve, A.; Lindahl, C.; Xia, W.; Igawa, K.; Lindahl, A.; Thomsen, P.; Palmquist, A.; Tengvall, P. Hydroxyapatite coating affects the Wnt signaling pathway during peri-implant healing in vivo. Acta Biomater. 2014, 10, 1451–1462. [Google Scholar] [CrossRef]
- Jimbo, R.; Coelho, P.G.; Bryington, M.; Baldassarri, M.; Tovar, N.; Currie, F.; Hayashi, M.; Janal, M.N.; Andersson, M.; Ono, D.; et al. Nano hydroxyapatite-coated implants improve bone nanomechanical properties. J. Dent. Res. 2012, 91, 1172–1177. [Google Scholar] [CrossRef]
- Granato, R.; Marin, C.; Suzuki, M.; Gil, J.N.; Janal, M.N.; Coelho, P.G. Biomechanical and histomorphometric evaluation of a thin ion beam bioceramic deposition on plateau root form implants: An experimental study in dogs. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90B, 396–403. [Google Scholar] [CrossRef]
- Ozeki, K.; Yuhta, T.; Aoki, H.; Nishimura, I.; Fukui, Y. Push-out strength of hydroxyapatite coated by sputtering technique in bone. Bio-Med. Mater. Eng. 2001, 11, 63–68. [Google Scholar]
- Rahbek, O.; Overgaard, S.; Lind, M.; Bendix, K.; Bunger, C.; Soballe, K. Sealing effect of hydroxyapatite coating on peri-implant migration of particles. An experimental study in dogs. J. Bone Jt. Surg. Br. 2001, 83, 441–447. [Google Scholar] [CrossRef]
- Søballe, K.; Hansen, E.S.; Brockstedt-Rasmussen, H.B.; Hjortdal, V.E.; Juhl, G.I.; Pedersen, C.M.; Hvid, I.; Bünger, C. Gap healing enhanced by hydroxyapatite coatings in dogs. Clin. Orthop. 1991, 272, 300–307. [Google Scholar] [CrossRef]
- Stephenson, P.K.; Freeman, M.A.R.; Revell, P.A.; Germain, J.; Tuke, M.; Pirie, C.J. The effect of hydroxyapatite coating on growth of bone into cavities in an implant. J. Arthroplast. 1991, 6, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ducheyne, P.; Healy, K.E. The effect of plasma-sprayed calcium phosphate ceramic coatings on the metal ion release from porous titanium and cobalt-chromium alloys. J. Biomed. Mater. Res. 1988, 22, 1137–1163. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.R.; Barbosa, M.A. Effect of hydroxyapatite thickness on metal ion release from Ti6Al4V substrates. Biomaterials 1996, 17, 397–404. [Google Scholar] [CrossRef]
- Ozeki, K.; Yuhta, T.; Aoki, H.; Fukui, Y. Inhibition of Ni release from NiTi alloy by hydroxyapatite, alumina, and titanium sputtered coatings. Bio-Med. Mater. Eng. 2003, 13, 271–279. [Google Scholar]
- Cheng, X.; Roscoe, S.G. Corrosion behavior of titanium in the presence of calcium phosphate and serum proteins. Biomaterials 2005, 26, 7350–7356. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xi, X.H.; Jia, H.L.; Dan, Z. Controlling the biodegradation rate of AZ31 with biomimetic apatite coating. Adv. Mater. Res. 2013, 821–822, 1047–1050. [Google Scholar] [CrossRef]
- Cui, W.; Beniash, E.; Gawalt, E.; Xu, Z.; Sfeir, C. Biomimetic coating of magnesium alloy for enhanced corrosion resistance and calcium phosphate deposition. Acta Biomater. 2013, 9, 8650–8659. [Google Scholar] [CrossRef]
- Cook, S.D.; Thomas, K.A.; Dalton, J.E.; Volkman, T.K.; Whitecloud III, T.S.; Kay, J.F. Hydroxylapatite coating of porous implants improves bone ingrowth and interface attachment strength. J. Biomed. Mater. Res. 1992, 26, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gross, K.A.; Anderson, G.I.; Dunstan, C.R.; Carbone, A.; Berger, G.; Ploska, U.; Zreiqat, H. Bone growth is enhanced by novel bioceramic coatings on Ti alloy implants. J. Biomed. Mater. Res. A 2009, 90A, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Barkarmo, S.; Andersson, M.; Currie, F.; Kjellin, P.; Jimbo, R.; Johansson, C.; Stenport, V. Enhanced bone healing around nanohydroxyapatite-coated polyetheretherketone implants: An experimental study in rabbit bone. J. Biomater. Appl. 2014, 29, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Pilliar, R.M.; Deporter, D.A.; Watson, P.A.; Pharoah, M.; Chipman, M.; Valiquette, N.; Carter, S.; de Groot, K. The effect of partial coating with hydroxyapatite on bone remodeling in relation to porous-coated titanium-alloy dental implants in the dog. J. Dent. Res. 1991, 70, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Song, J.E.; Um, Y.J.; Chae, G.J.; Chung, S.M.; Lee, I.S.; Jung, U.W.; Kim, C.S.; Choi, S.H. Effects of calcium phosphate coating to SLA surface implants by the ion-beam-assisted deposition method on self-contained coronal defect healing in dogs. Biomed. Mater. 2009, 4, 044107. [Google Scholar] [CrossRef]
- Bigi, A.; Fini, M.; Bracci, B.; Boanini, E.; Torricelli, P.; Giavaresi, G.; Aldini, N.N.; Facchini, A.; Sbaiz, F.; Giardino, R. The response of bone to nanocrystalline hydroxyapatite-coated Ti13Nb11Zr alloy in an animal model. Biomaterials 2008, 29, 1730–1736. [Google Scholar] [CrossRef]
- Park, D.S.; Kim, I.S.; Kim, H.; Chou, A.H.K.; Hahn, B.D.; Li, L.H.; Hwang, S.J. Improved biocompatibility of hydroxyapatite thin film prepared by aerosol deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94B, 353–358. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; Cuijpers, V.M.J.I.; Wolke, J.G.C.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium-phosphate-coated oral implants promote osseointegration in osteoporosis. J. Dent. Res. 2013, 92, 982–988. [Google Scholar] [CrossRef]
- Deplaine, H.; Lebourg, M.; Ripalda, P.; Vidaurre, A.; Sanz-Ramos, P.; Mora, G.; Prósper, F.; Ochoa, I.; Doblaré, M.; Ribelles, J.L.G.; et al. Biomimetic hydroxyapatite coating on pore walls improves osteointegration of poly(L-lactic acid) scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101B, 173–186. [Google Scholar] [CrossRef]
- Luo, R.; Liu, Z.; Yan, F.; Kong, Y.; Zhang, Y. The biocompatibility of hydroxyapatite film deposition on micro-arc oxidation Ti6Al4V alloy. Appl. Surf. Sci. 2013, 266, 57–61. [Google Scholar] [CrossRef]
- Geesink, R.G.T. Osteoconductive coating for total joint arthroplasty. Clin. Orthop. Rel. Res. 2002, 395, 53–65. [Google Scholar] [CrossRef]
- Wu, J.; Guo, Y.Q.; Yin, G.F.; Chen, H.Q.; Kang, Y. Induction of osteoconductivity by BMP-2 gene modification of mesenchymal stem cells combined with plasma-sprayed hydroxyapatite coating. Appl. Surf. Sci. 2008, 255, 336–339. [Google Scholar] [CrossRef]
- Cao, N.; Dong, J.; Wang, Q.; Ma, Q.; Xue, C.; Li, M. An experimental bone defect healing with hydroxyapatite coating plasma sprayed on carbon/carbon composite implants. Surf. Coat. Tech. 2010, 205, 1150–1156. [Google Scholar] [CrossRef]
- Hirota, M.; Hayakawa, T.; Yoshinari, M.; Ametani, A.; Shima, T.; Monden, Y.; Ozawa, T.; Sato, M.; Koyama, C.; Tamai, N.; et al. Hydroxyapatite coating for titanium fibre mesh scaffold enhances osteoblast activity and bone tissue formation. Int. J. Oral Maxillofac. Surg. 2012, 41, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Roden, L.C.; Renton, L.F. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 2012, 33, 3813–3823. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, W.; Xiong, Z.; Hu, Y.; Xiao, J. Effects of biomimetic hydroxyapatite coatings on osteoimmunomodulation. Biomater. Adv. 2022, 134, 112640. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; van der Valk, C.M.; Dalmeijer, R.A.J.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants. J. Biomed. Mater. Res. A 2003, 66A, 779–788. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wang, B.C.; Chang, W.J.; Chang, E.; Wu, J.D. Mechanical and histological evaluations of cobalt-chromium alloy and hydroxyapatite plasma-sprayed coatings in bone. J. Mater. Sci. Mater. Med. 1996, 7, 167–174. [Google Scholar] [CrossRef]
- Mohammadi, S.; Esposito, M.; Hall, J.; Emanuelsson, L.; Krozer, A.; Thomsen, P. Short-term bone response to titanium implants coated with thin radiofrequent magnetron-sputtered hydroxyapatite in rabbits. Clin. Implant Dent. Rel. Res. 2003, 5, 241–253. [Google Scholar] [CrossRef]
- Vercaigne, S.; Wolke, J.G.C.; Naert, I.; Jansen, J.A. A histological evaluation of TiO2-gritblasted and Ca-P magnetron sputter coated implants placed into the trabecular bone of the goat: Part 2. Clin. Oral Implant. Res. 2000, 11, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Dostálová, T.; Himmlová, L.; Jélinek, M.; Grivas, C. Osseointegration of loaded dental implant with KrF laser hydroxylapatite films on Ti6Al4V alloy by minipigs. J. Biomed. Opt. 2001, 6, 239–243. [Google Scholar] [CrossRef]
- Mathew, D.; Bhardwaj, G.; Wang, Q.; Sun, L.; Ercan, B.; Geetha, M.; Webster, T.J. Decreased Staphylococcus aureus and increased osteoblast density on nanostructured electrophoretic-deposited hydroxyapatite on titanium without the use of pharmaceuticals. Int. J. Nanomed. 2014, 9, 1775–1781. [Google Scholar]
- Hu, J.; Zhou, Y.; Huang, L.; Liu, J.; Lu, H. Effect of nano-hydroxyapatite coating on the osteoinductivity of porous biphasic calcium phosphate ceramics. BMC Musculoskelet. Disord. 2014, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yang, Z.; Zhou, Y.; Liu, Y.; Li, K.; Lu, H. Porous biphasic calcium phosphate ceramics coated with nano-hydroxyapatite and seeded with mesenchymal stem cells for reconstruction of radius segmental defects in rabbits. J. Mater. Sci. Mater. Med. 2015, 26, 257. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.L.; He, F.M.; Hu, J.A.; Wang, X.X.; Zhao, S.F. Biomechanical comparison of biomimetically and electrochemically deposited hydroxyapatite-coated porous titanium implants. J. Oral Maxillofac. Surg. 2010, 68, 420–427. [Google Scholar] [CrossRef]
- Stigter, M.; Bezemer, J.; de Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control. Release 2004, 99, 127–137. [Google Scholar] [CrossRef]
- Alt, V.; Bitschnau, A.; Osterling, J.; Sewing, A.; Meyer, C.; Kraus, R.; Meissner, S.A.; Wenisch, S.; Domann, E.; Schnettler, R. The effects of combined gentamicin-hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials 2006, 27, 4627–4634. [Google Scholar] [CrossRef]
- Luong, L.N.; McFalls, K.M.; Kohn, D.H. Gene delivery via DNA incorporation within a biomimetic apatite coating. Biomaterials 2009, 30, 6996–7004. [Google Scholar] [CrossRef]
- Choi, S.; Murphy, W.L. Sustained plasmid DNA release from dissolving mineral coatings. Acta Biomater. 2010, 6, 3426–3435. [Google Scholar] [CrossRef]
- Saran, N.; Zhang, R.; Turcotte, R.E. Osteogenic protein-1 delivered by hydroxyapatite-coated implants improves bone ingrowth in extracortical bone bridging. Clin. Orthop. Relat. Res. 2011, 469, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Majid, K.; Tseng, M.D.; Baker, K.C.; Reyes-Trocchia, A.; Herkowitz, H.N. Biomimetic calcium phosphate coatings as bone morphogenetic protein delivery systems in spinal fusion. Spine J. 2011, 11, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Bastari, K.; Arshath, M.; Ng, Z.H.M.; Chia, J.H.; Yow, Z.X.D.; Sana, B.; Tan, M.F.C.; Lim, S.; Loo, S.C.J. A controlled release of antibiotics from calcium phosphate-coated poly(lactic-co-glycolic acid) particles and their in vitro efficacy against Staphylococcus aureus biofilm. J. Mater. Sci. Mater. Med. 2014, 25, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Liu, Y.; Jin, X.; Fan, C.; Ye, H.; Ou, M.; Lv, L.; Wu, G.; Zhou, Y. Bi-functionalization of a calcium phosphate-coated titanium surface with slow-release simvastatin and metronidazole to provide antibacterial activities and pro-osteodifferentiation capabilities. PLoS ONE 2014, 9, e97741. [Google Scholar] [CrossRef]
- Lin, X.; Chen, J.; Liao, Y.; Pathak, J.L.; Li, H.; Liu, Y. Biomimetic calcium phosphate coating as a drug delivery vehicle for bone tissue engineering: A mini-review. Coatings 2020, 10, 1118. [Google Scholar] [CrossRef]
- Vidal, E.; Guillem-Marti, J.; Ginebra, M.P.; Combes, C.; Rupérez, E.; Rodriguez, D. Multifunctional homogeneous calcium phosphate coatings: Toward antibacterial and cell adhesive titanium scaffolds. Surf. Coat. Tech. 2021, 405, 126557. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef]
- Saithna, A. The influence of hydroxyapatite coating of external fixator pins on pin loosening and pin track infection: A systematic review. Injury 2010, 41, 128–132. [Google Scholar] [CrossRef]
- Tieanboon, P.; Jaruwangsanti, N.; Kiartmanakul, S. Efficacy of hydroxyapatite in pedicular screw fixation in canine spinal vertebra. Asian Biomed. 2009, 3, 177–181. [Google Scholar]
- Coelho, P.G.; Cardaropoli, G.; Suzuki, M.; Lemons, J.E. Early healing of nanothickness bioceramic coatings on dental implants. An experimental study in dogs. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88B, 387–393. [Google Scholar] [CrossRef]
- Hulshoff, J.E.G.; van Dijk, K.; van Der Waerden, J.P.C.M.; Wolke, J.G.C.; Kalk, W.; Jansen, J.A. Evaluation of plasma-spray and magnetron-sputter Ca-P-coated implants: An in vivo experiment using rabbits. J. Biomed. Mater. Res. 1996, 31, 329–337. [Google Scholar] [CrossRef]
- Hulshoff, J.E.G.; Hayakawa, T.; van Dijk, K.; Leijdekkers-Govers, A.F.M.; van der Waerden, J.P.C.M.; Jansen, J.A. Mechanical and histologic evaluation of Ca-P plasma-spray and magnetron sputter-coated implants in trabecular bone of the goat. J. Biomed. Mater. Res. 1997, 36, 75–83. [Google Scholar] [CrossRef]
- Yang, C.Y.; Yang, C.W.; Chen, L.R.; Wu, M.C.; Lui, T.S.; Kuo, A.; Lee, T.M. Effect of vacuum post-heat treatment of plasma-sprayed hydroxyapatite coatings on their in vitro and in vivo biological responses. J. Med. Biol. Eng. 2009, 29, 296–302. [Google Scholar]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Capello, W.D.; D’Antonio, J.A.; Feinberg, J.R.; Manley, M.T. Hydroxyapatite-coated total hip femoral components in patients less than fifty years old: Clinical and radiographic results after five to eight years of follow-up. J. Bone Jt. Surg. Am. 1997, 79, 1023–1029. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Montfort, M.J.; McLoughlin, S.W. Differential healing response of bone adjacent to porous implants coated with hydroxyapatite and 45S5 bioactive glass. J. Biomed. Mater. Res. 2001, 55, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Mistry, S.; Kundu, D.; Datta, S.; Basu, D. Comparison of bioactive glass coated and hydroxyapatite coated titanium dental implants in the human jaw bone. Aust. Dent. J. 2011, 56, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Geesink, R.G.T. Hydroxyapatite-coated total hip prostheses; two-year clinical and roentgenographic results of 100 cases. Clin. Orthop. Rel. Res. 1990, 261, 39–58. [Google Scholar] [CrossRef]
- Makani, A.; Kim, T.W.B.; Kamath, A.F.; Garino, J.P.; Lee, G.C. Outcomes of long tapered hydroxyapatite-coated stems in revision total hip arthroplasty. J. Arthroplast. 2014, 29, 827–830. [Google Scholar] [CrossRef]
- Geesink, R.G.T.; Hoefnagels, N.H.M. Six-year results of hydroxyapatite-coated total hip replacement. J. Bone Jt. Surg. Br. 1995, 77, 534–547. [Google Scholar] [CrossRef]
- Wheeler, S.L. Eight-year clinical retrospective study of titanium plasma-sprayed and hydroxyapatite-coated cylinder implants. Int. J. Oral Max. Impl. 1996, 11, 340–350. [Google Scholar] [CrossRef]
- Chang, J.K.; Chen, C.H.; Huang, K.Y.; Wang, G.J. Eight-year results of hydroxyapatite-coated hip arthroplasty. J. Arthroplast. 2006, 21, 541–546. [Google Scholar] [CrossRef] [PubMed]
- MaNally, S.A.; Shepperd, H.A.N.; Mann, C.V.; Walczak, J.P. The results at nine to twelve years of the use of a hydroxyapatite-coated femoral stem. J. Bone Jt. Surg. 2000, 82B, 378–382. [Google Scholar] [CrossRef]
- Oosterbos, C.J.M.; Rahmy, A.I.A.; Tonino, A.J.; Witpeerd, W. High survival rate of hydroxyapatite-coated hip prostheses 100 consecutive hips followed for 10 years. Acta Orthop. Scand. 2004, 75, 127–133. [Google Scholar] [CrossRef]
- Trisi, P.; Keith, D.J.; Rocco, S. Human histologic and histomorphometric analyses of hydroxyapatite-coated implants after 10 years of function: A case report. Int. J. Oral Max. Impl. 2005, 20, 124–130. [Google Scholar]
- Lecuire, F.; Berard, J.B.; Martres, S. Minimum 10-year follow-up results of ALPINA cementless hydroxyapatite-coated anatomic unicompartmental knee arthroplasty. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 385–394. [Google Scholar] [CrossRef]
- Griffiths, J.T.; Roumeliotis, L.; Elson, D.W.; Borton, Z.M.; Cheung, S.; Stranks, G.J. Long term performance of an uncemented, proximally hydroxyapatite coated, double tapered, titanium-alloy femoral stem: Results from 1465 hips at 10 years minimum follow-up. J. Arthroplast. 2021, 36, 616–622. [Google Scholar] [CrossRef]
- Muirhead-Allwood, S.K.; Sandiford, N.; Skinner, J.A.; Hua, J.; Kabir, C.; Walker, P.S. Uncemented custom computer-assisted design and manufacture of hydroxyapatite-coated femoral components: Survival at 10 to 17 years. J. Bone Jt. Surg. Br. 2010, 92, 1079–1084. [Google Scholar] [CrossRef]
- Shetty, A.A.; Slack, R.; Tindall, A.; James, K.D.; Rand, C. 1 Results of a hydroxyapatite-coated (Furlong) total hip replacement. A 13- to 15-year follow-up. J. Bone Jt. Surg. Br. 2005, 87, 1050–1054. [Google Scholar] [CrossRef]
- Capello, W.N.; D’Antonio, J.A.; Jaffe, W.L.; Geesink, R.G.; Manley, M.T.; Feinberg, J.R. Hydroxyapatite-coated femoral components: 15-year minimum follow up. Clin. Orthop. Relat. Res. 2006, 453, 75–80. [Google Scholar] [CrossRef]
- Rajaratnam, S.S.; Jack, C.; Tavakkolizadeh, A.; George, M.D.; Fletcher, R.J.; Hankins, M.; Shepperd, J.A.N. Long-term results of a hydroxyapatite-coated femoral component in total hip replacement: A 15- to 21-year follow-up study. J. Bone Jt. Surg. Br. 2008, 90, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.M. 16 year review of hydroxyapatite ceramic coated hip implants—A clinical and histological evaluation. Key Eng. Mater. 2005, 284–286, 1049–1052. [Google Scholar] [CrossRef]
- Buchanan, J.M. 17 year review of hydroxyapatite ceramic coated hip implants—A clinical and histological evaluation. Key Eng. Mater. 2006, 309–311, 1341–1344. [Google Scholar] [CrossRef]
- Syed, M.A.; Hutt, N.J.; Shah, N.; Edge, A.J. Hydroxyapatite ceramic-coated femoral components in young patients followed up for 17 to 25 years: An update of a previous report. Bone Joint J. 2015, 97B, 749–754. [Google Scholar] [CrossRef]
- Batta, V.; Coathup, M.J.; Parratt, M.T.; Pollock, R.C.; Aston, W.J.; Cannon, S.R.; Skinner, J.A.; Briggs, T.W.; Blunn, G.W. Uncemented, custom-made, hydroxyapatite-coated collared distal femoral endoprostheses: Up to 18 years’ follow-up. Bone Joint J. 2014, 96B, 263–269. [Google Scholar] [CrossRef]
- Buchanan, J.M.; Goodfellow, S. Nineteen years review of hydroxyapatite ceramic coated hip implants: A clinical and histological evaluation. Key Eng. Mater. 2008, 361–363, 1315–1318. [Google Scholar]
- Jacquot, L.; Machenaud, A.; Bonnin, M.P.; Chouteau, J.; Ramos-Pascual, S.; Saffarini, M.; Dubreuil, S.; Vidalain, J.P. Survival and clinical outcomes at 30 to 35 years following primary total hip arthroplasty with a cementless femoral stem fully coated with hydroxyapatite. J. Arthroplast. 2023, 38, 880–885. [Google Scholar] [CrossRef]
- Tinsley, D.; Watson, C.J.; Russell, J.L. A comparison of hydroxylapatite coated implant retained fixed and removable mandibular prostheses over 4 to 6 years. Clin. Oral Implant. Res. 2001, 12, 159–166. [Google Scholar] [CrossRef]
- Binahmed, A.; Stoykewych, A.; Hussain, A.; Love, B.; Pruthi, V. Long-term follow-up of hydroxyapatite-coated dental implants—A clinical trial. Int. J. Oral Max. Impl. 2007, 22, 963–968. [Google Scholar]
- Iezzi, G.; Scarano, A.; Petrone, G.; Piattelli, A. Two human hydroxyapatite-coated dental implants retrieved after a 14-year loading period: A histologic and histomorphometric case report. J. Periodontol. 2007, 78, 940–947. [Google Scholar] [CrossRef]
- Kato, E.; Yamada, M.; Sakurai, K. Retrospective clinical outcome of nanopolymorphic crystalline hydroxyapatite-coated and anodic oxidized titanium implants for 10 years. J. Prosthodontic Res. 2015, 59, 62–70. [Google Scholar] [CrossRef]
- van Oirschot, B.A.J.A.; Bronkhorst, E.M.; van den Beucken, J.J.J.P.; Meijer, G.J.; Jansen, J.A.; Junker, R. A systematic review on the long-term success of calcium phosphate plasma-spray-coated dental implants. Odontology 2016, 104, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Sadeghi, H.M.; Ghasemi, K.; Khayati, A.; Jafarian, M. Does biphasic calcium phosphate-coated surface increase the secondary stability in dental implants? A split-mouth study. J. Maxillofac. Oral Surg. 2022, 21, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Mateo, J.; Gil-Albarova, J.; Lobo-Escolar, A.; Ibarz, E.; Gabarre, S.; Más, Y.; Gracia, L. Cementless hydroxyapatite coated hip prostheses. BioMed Res. Int. 2015, 2015, 386461. [Google Scholar] [CrossRef] [PubMed]
- Botterill, J.; Khatkar, H. The role of hydroxyapatite coating in joint replacement surgery—Key considerations. J. Clin. Orthop. Trauma 2022, 29, 101874. [Google Scholar] [CrossRef]
- Xia, J.; Yuan, Z.; Cai, F. Toward a biocompatible and degradable battery using a Mg-Zn-Zr alloy with β-tricalcium phosphate nanocoating as anode. J. Mater. Eng. Perform. 2018, 27, 4005–4009. [Google Scholar] [CrossRef]
- Montone, A.M.I.; Malvano, F.; Taiano, R.; Capparelli, R.; Capuano, F.; Albanese, D. Alginate coating charged by hydroxyapatite complexes with lactoferrin and quercetin enhances the pork meat shelf life. Foods 2023, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.F.; Cardaropoli, G.; van Thompson, P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88B, 579–596. [Google Scholar] [CrossRef]
- Wang, G.; Zreiqat, H. Functional coatings or films for hard-tissue applications. Materials 2010, 3, 3994–4050. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Rohanizadeh, R. A review of chemical surface modification of bioceramics: Effects on protein adsorption and cellular response. Colloid Surf. B 2014, 122, 823–834. [Google Scholar] [CrossRef]
- Sirkiä, S.V.; Qudsia, S.; Siekkinen, M.; Hoepfl, W.; Budde, T.; Smått, J.H.; Peltonen, J.; Hupa, L.; Heino, T.J.; Vallittu, P.K. Physicochemical and biological characterization of functionalized calcium carbonate. Materialia 2023, 28, 101742. [Google Scholar] [CrossRef]
- Choi, S.; Yu, X.; Jongpaiboonkit, L.; Hollister, S.J.; Murphy, W.L. Inorganic coatings for optimized non-viral transfection of stem cells. Sci. Rep. 2013, 3, 1567. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.; Vaquette, C.; Fisher, A.G.; Hamlet, S.M.; Xiao, Y.; Hutmacher, D.W.; Ivanovski, S. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials 2014, 35, 113–122. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, T.; Xu, Y.; Liang, C.; Li, G.; Guo, Y.; Zhang, Z.; Lian, J.; Ren, L. Double-layer calcium phosphate sandwiched siloxane composite coating to enhance corrosion resistance and biocompatibility of magnesium alloys for bone tissue engineering. Prog. Org. Coat. 2023, 177, 107417. [Google Scholar] [CrossRef]
- Fosca, M.; Streza, A.; Antoniac, I.V.; Vadalà, G.; Rau, J.V. Ion-doped calcium phosphate-based coatings with antibacterial properties. J. Funct. Biomater. 2023, 14, 250. [Google Scholar] [CrossRef]
- Liao, Z.; Li, J.; Su, Y.; Miao, F.; Zhang, X.; Gu, Y.; Du, J.; Hang, R.; Yan, W.; Weiyi, C.; et al. Antibacterial hydroxyapatite coatings on titanium dental implants. Front. Mater. Sci. 2023, 17, 230628. [Google Scholar] [CrossRef]
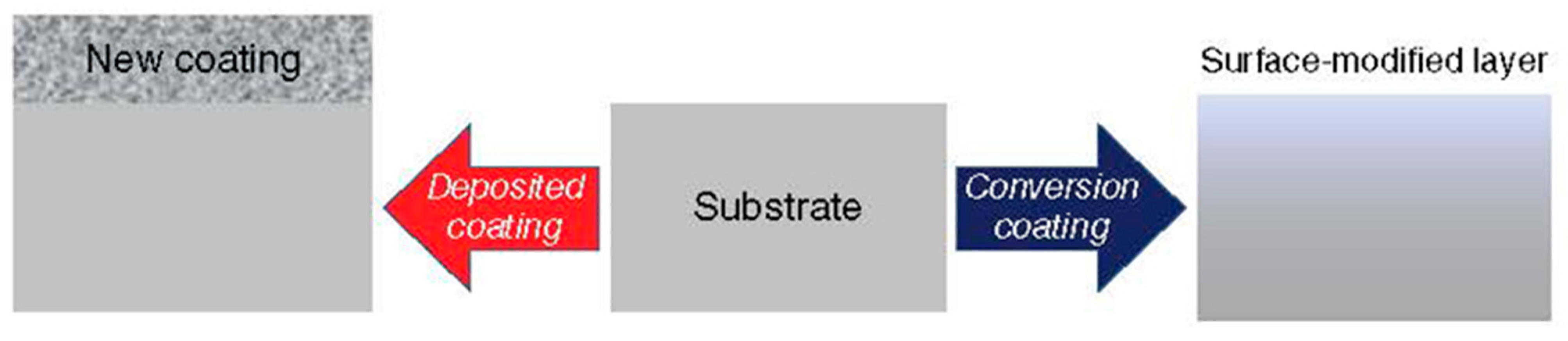


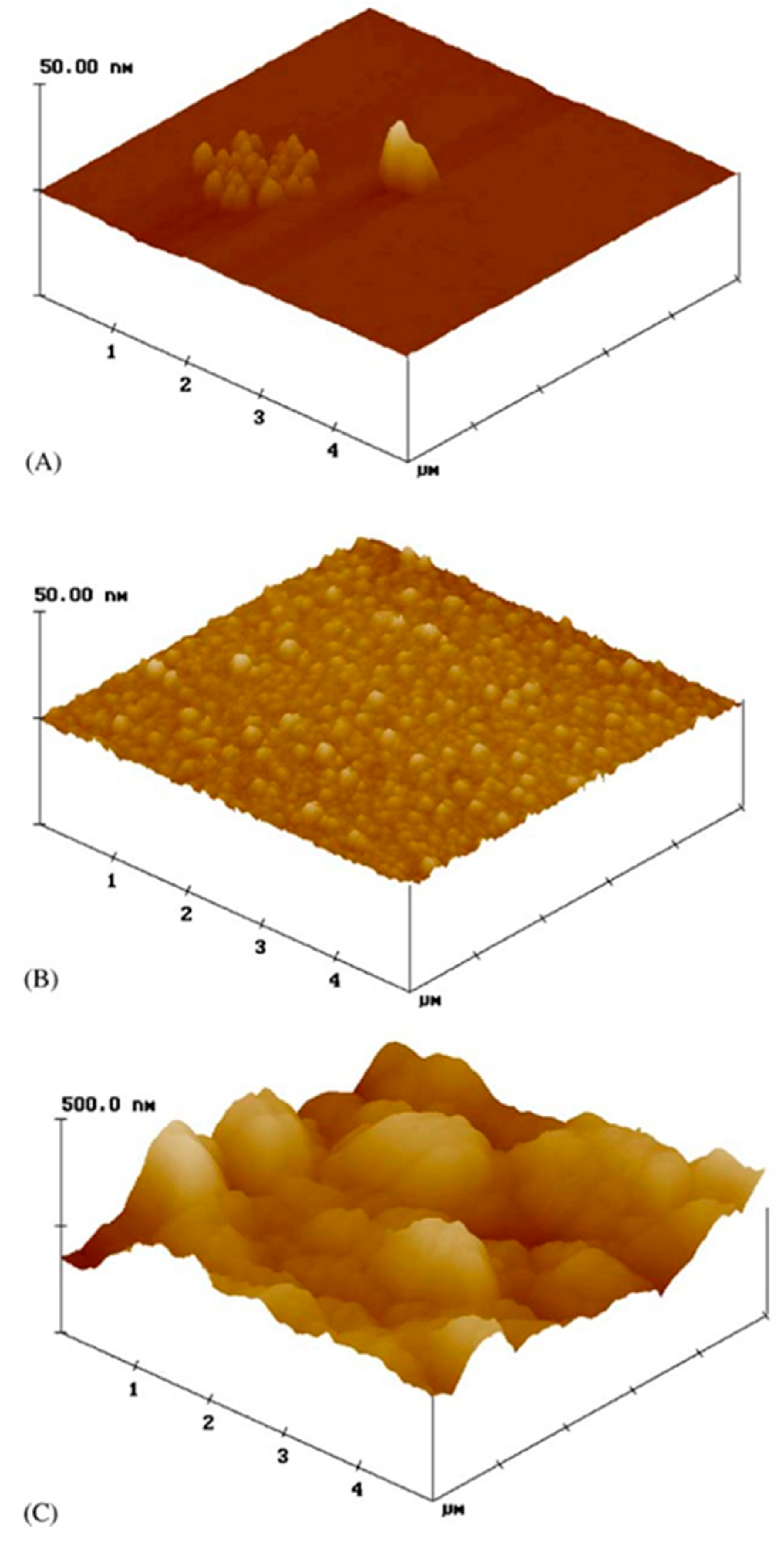

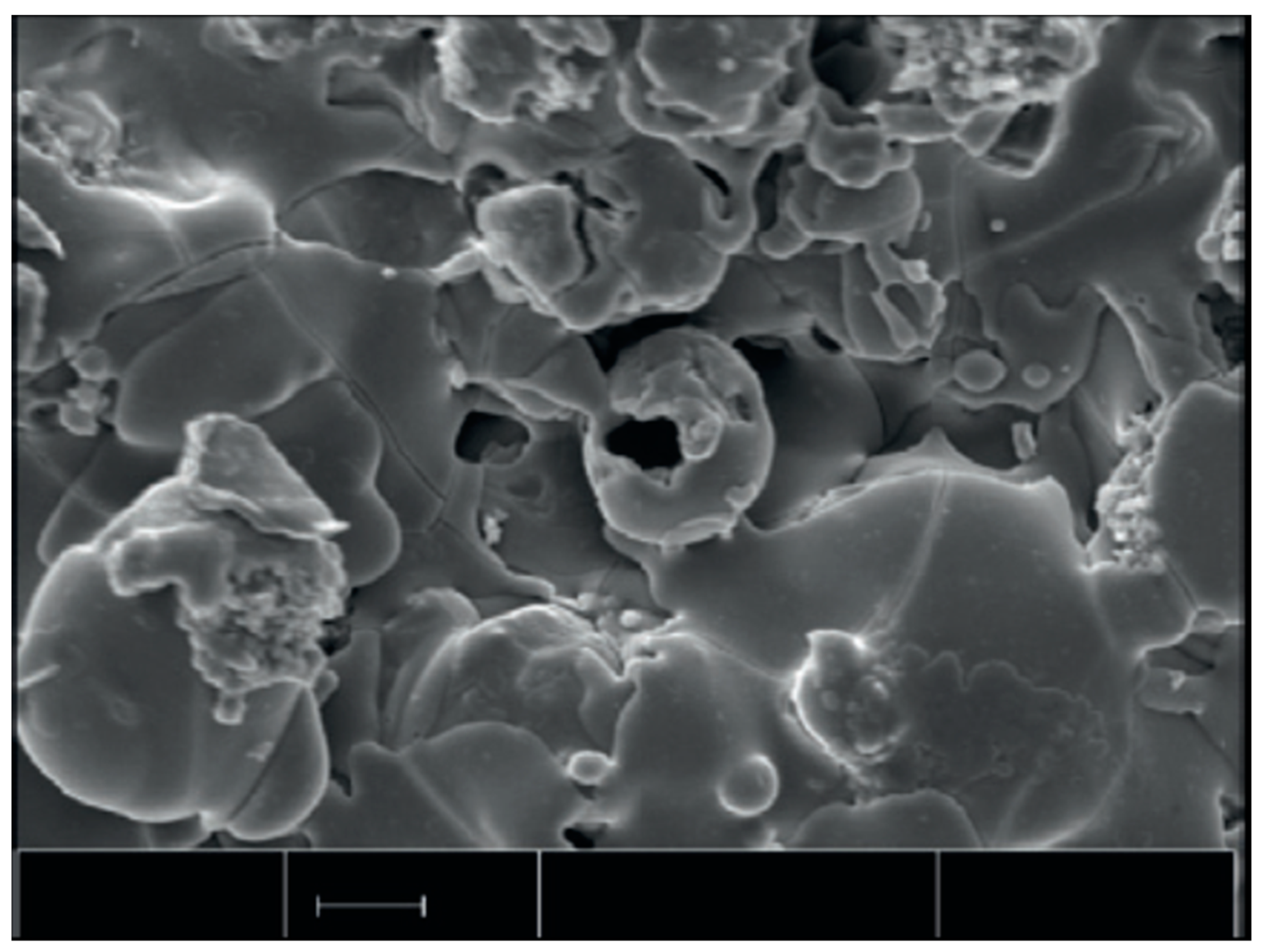
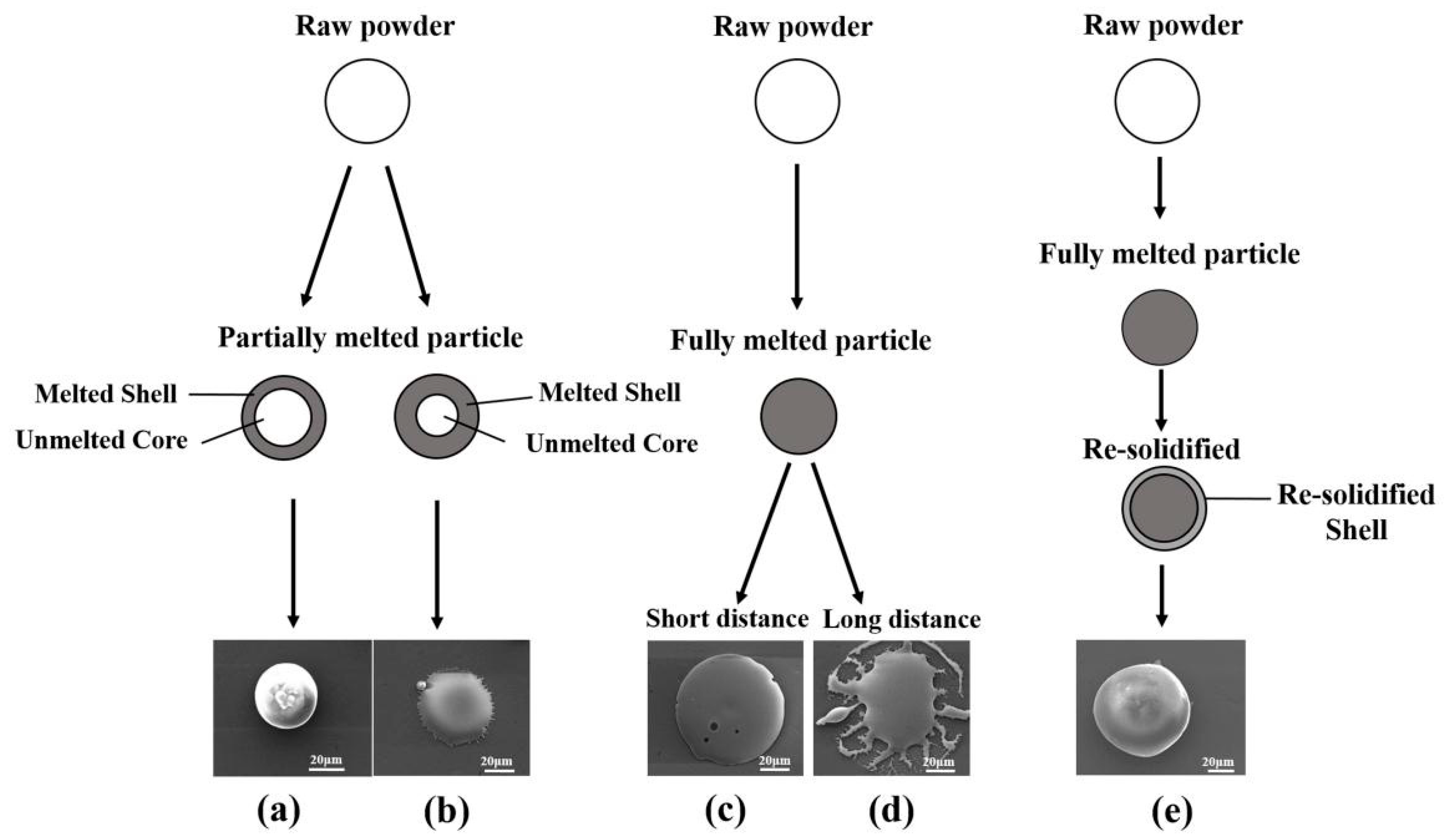


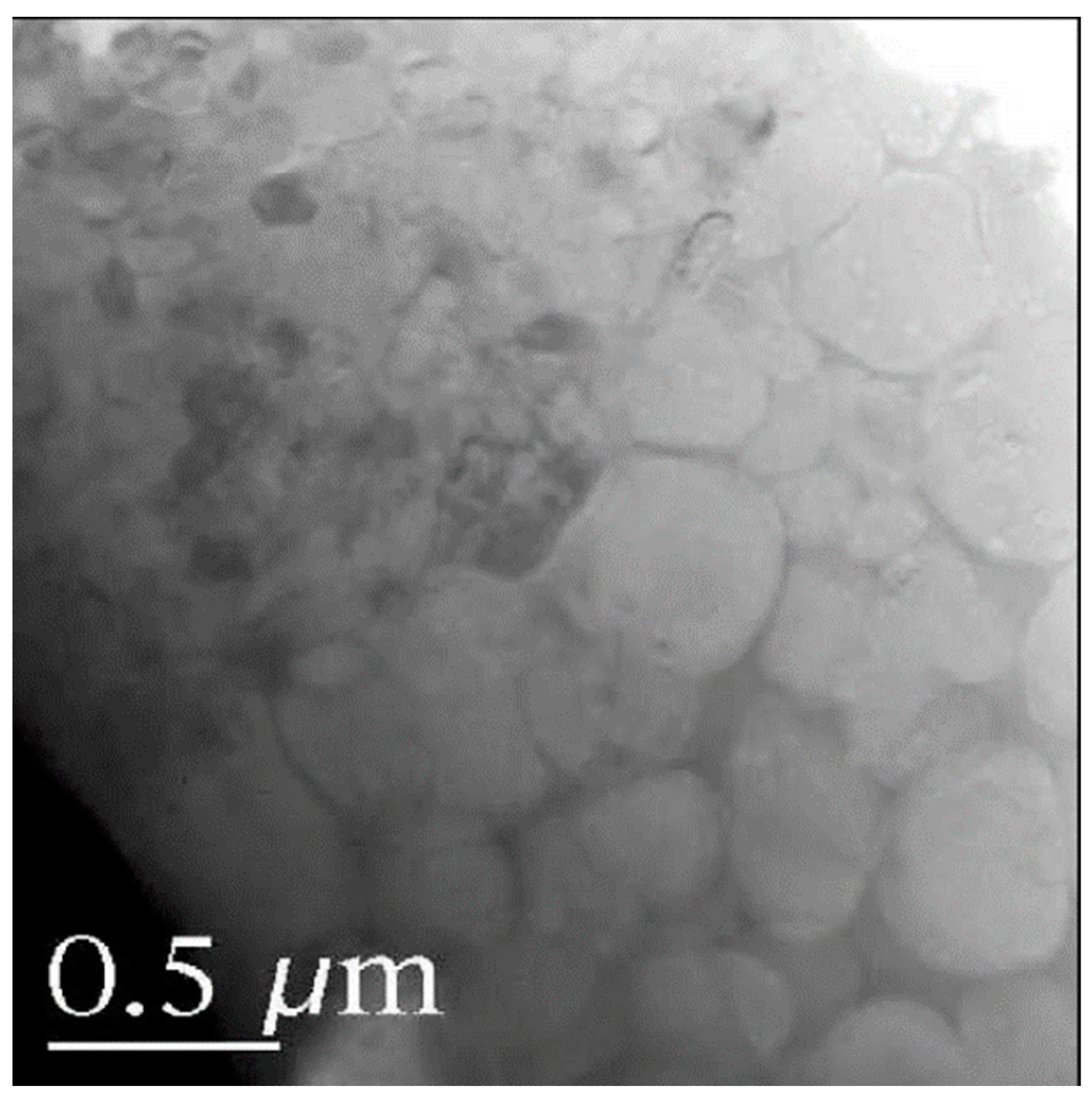
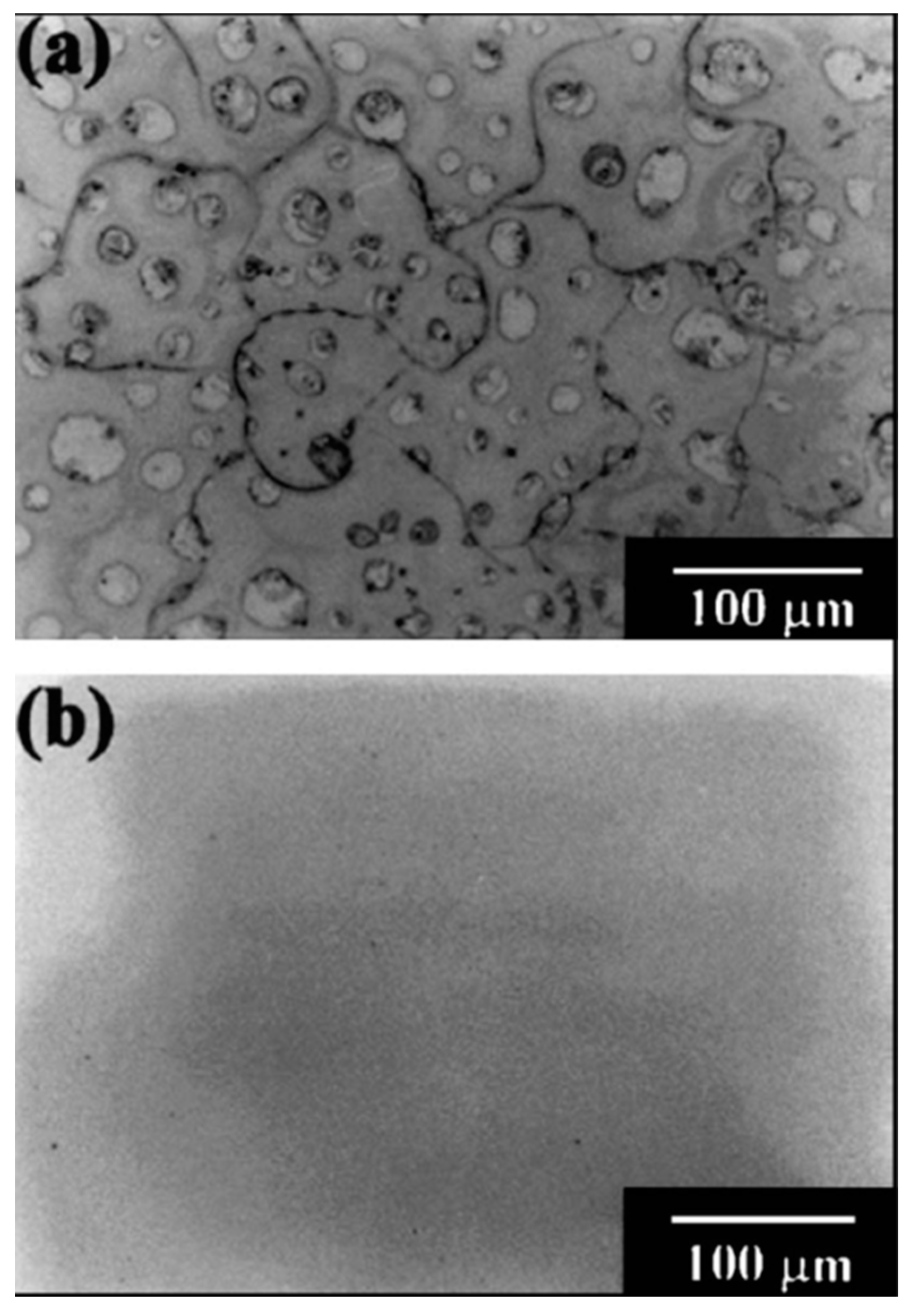

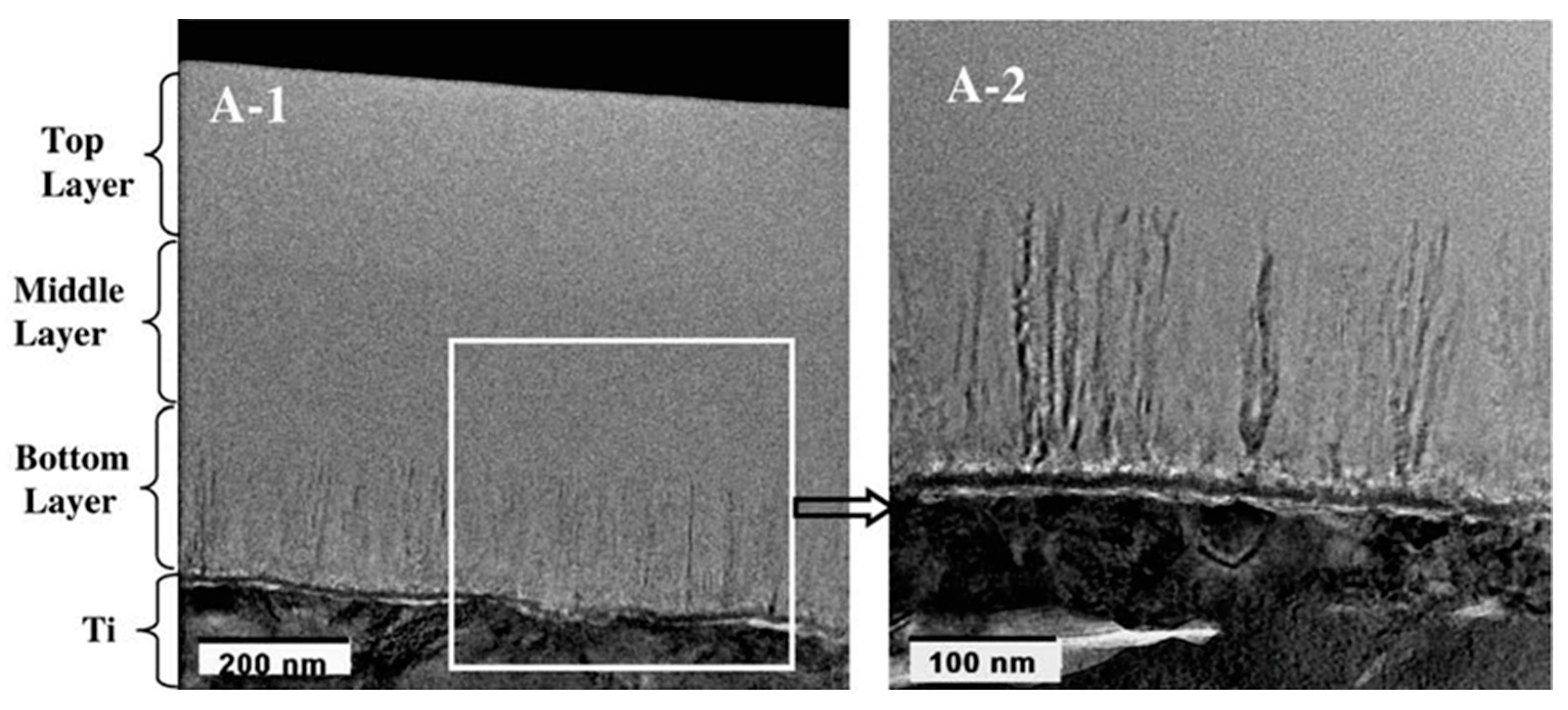

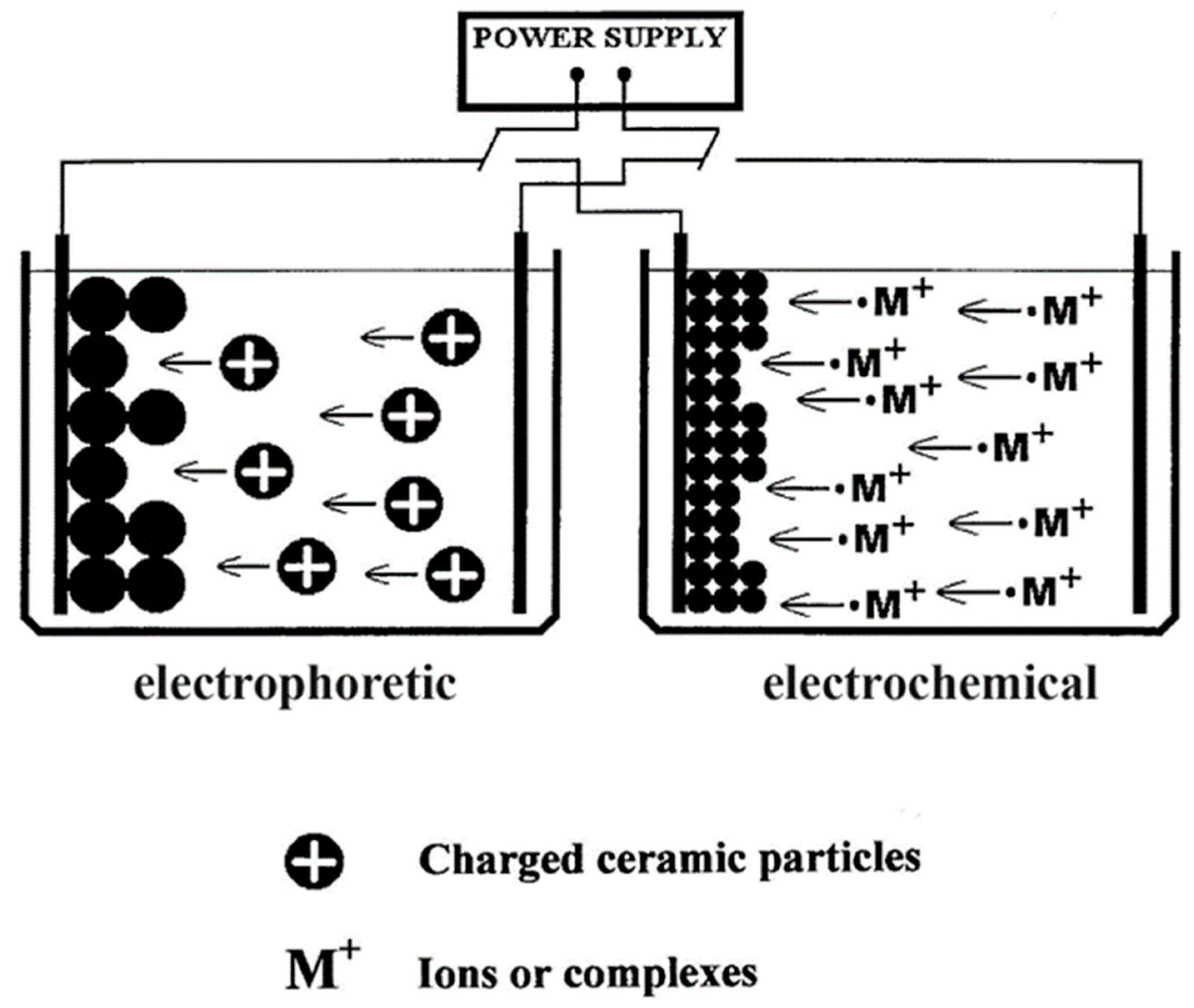
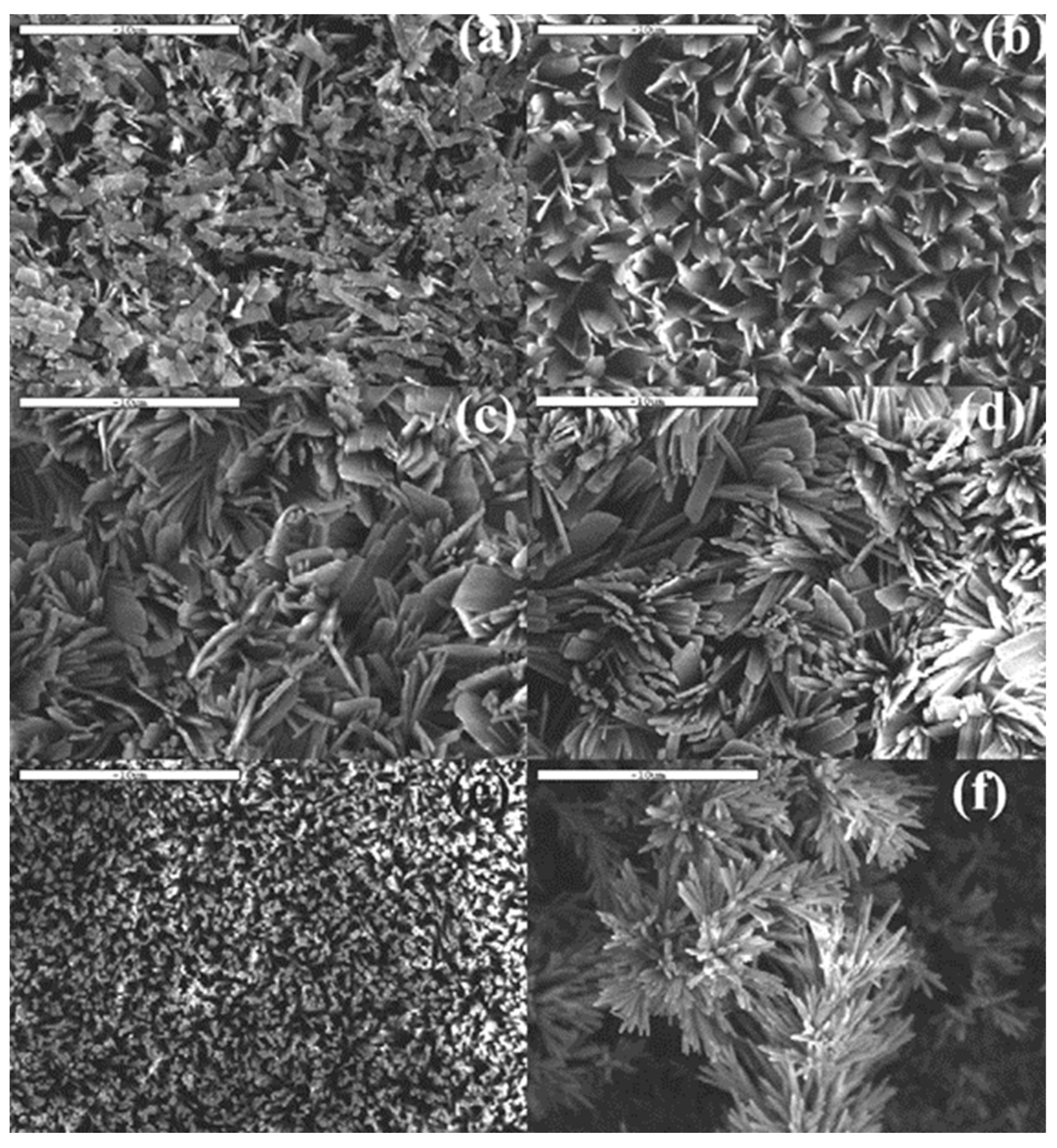
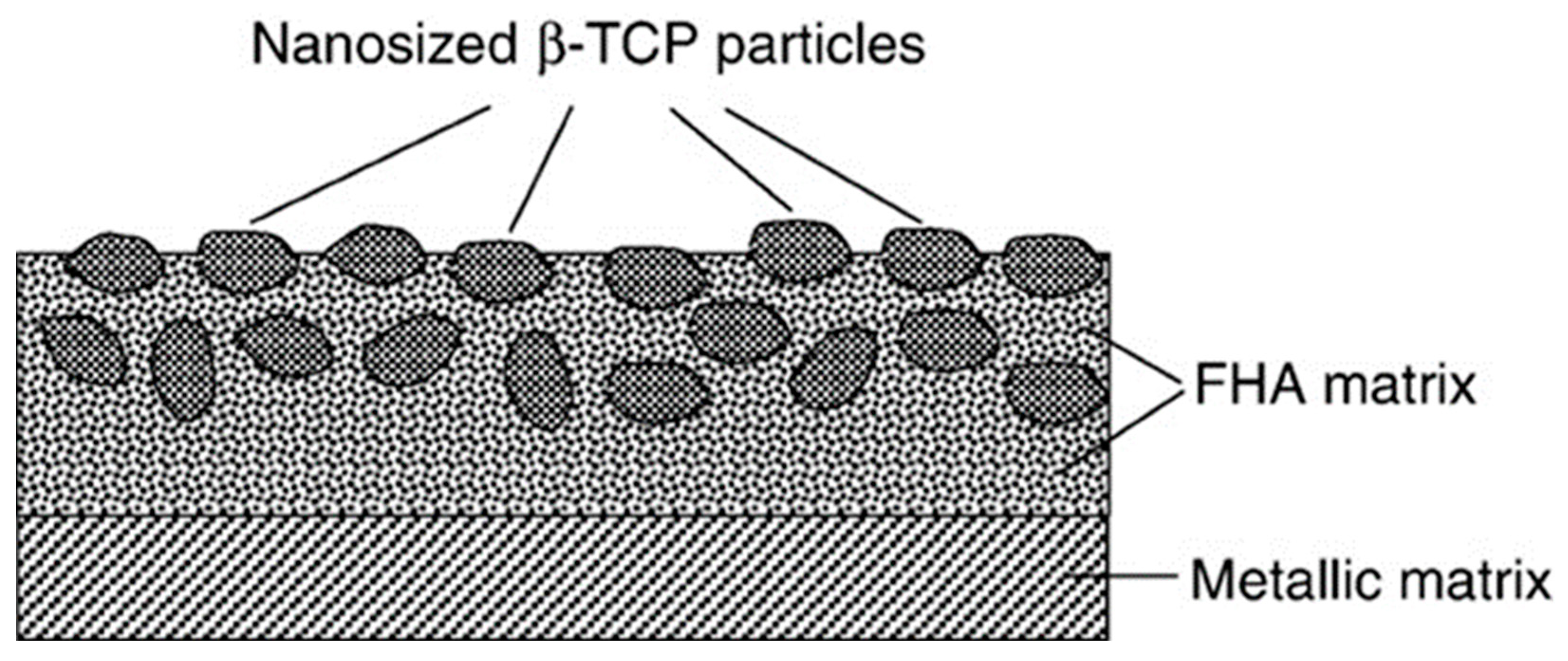




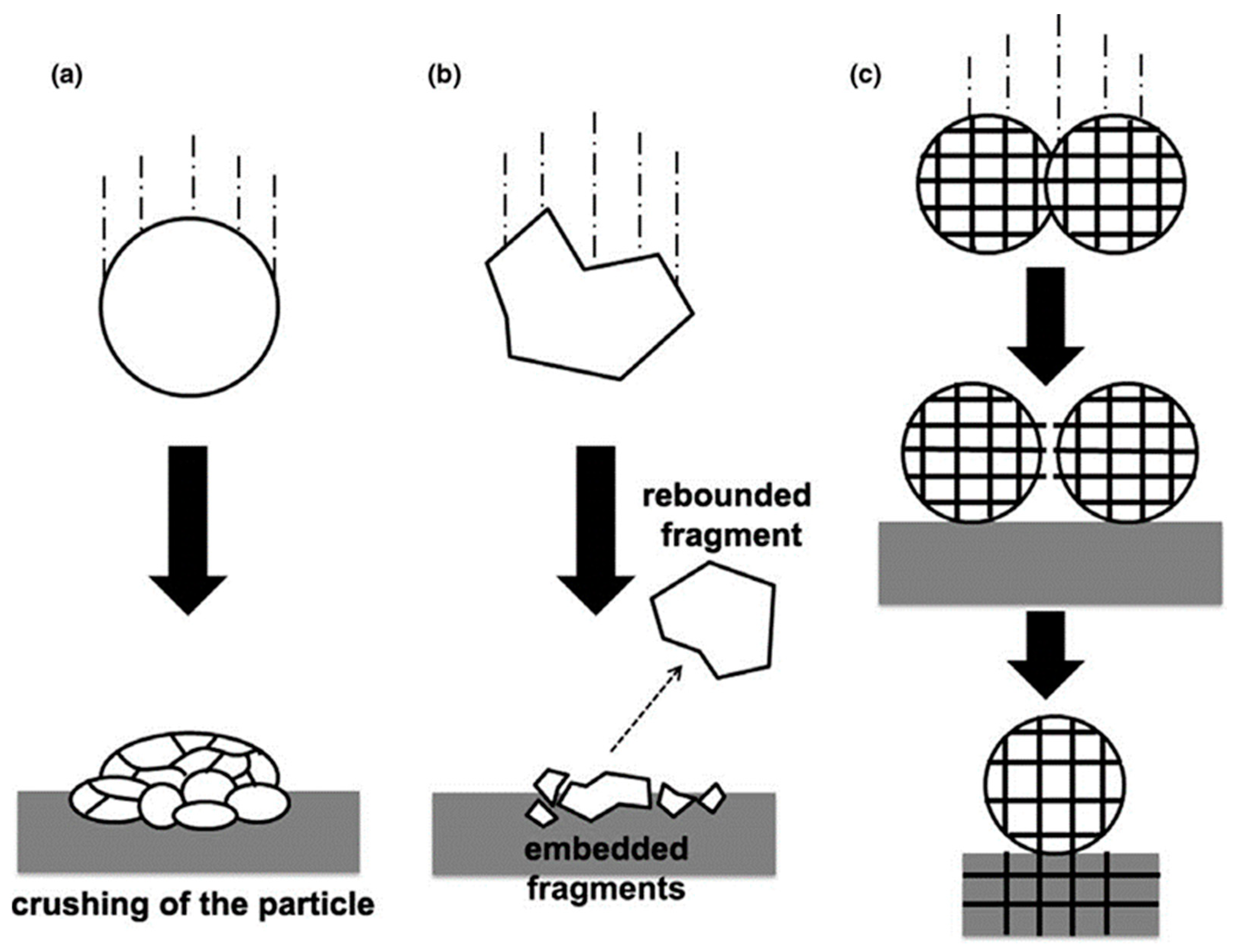

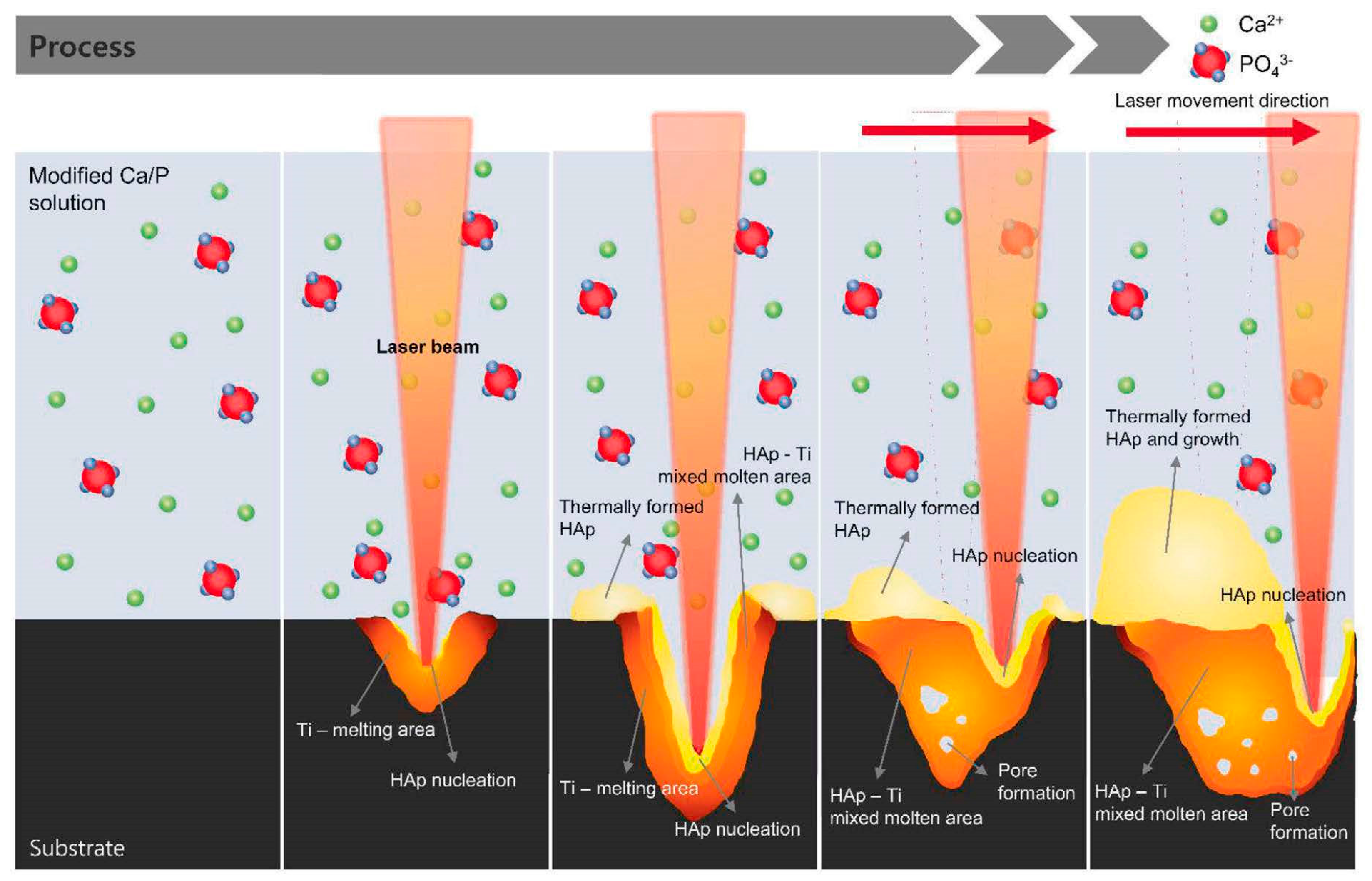
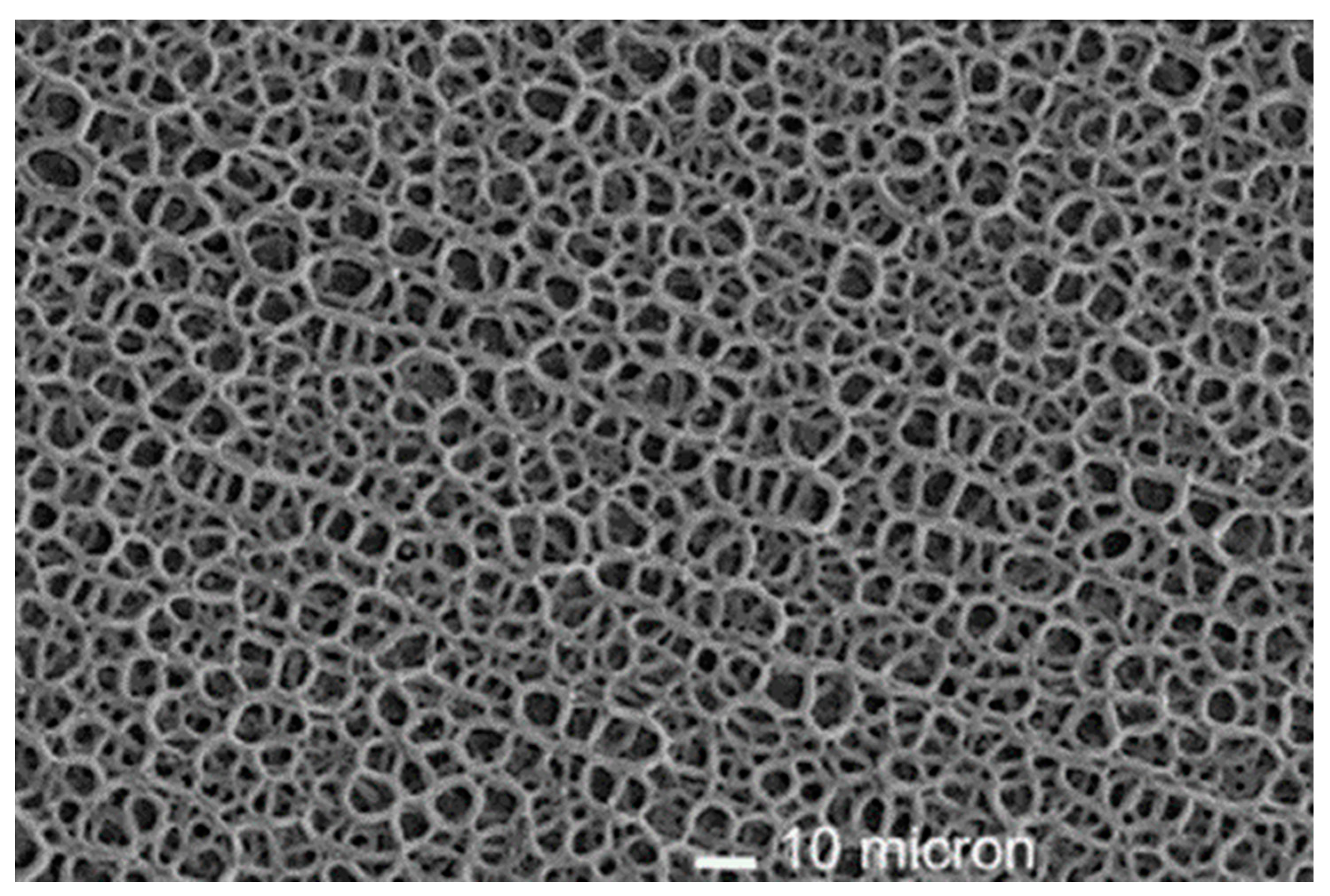

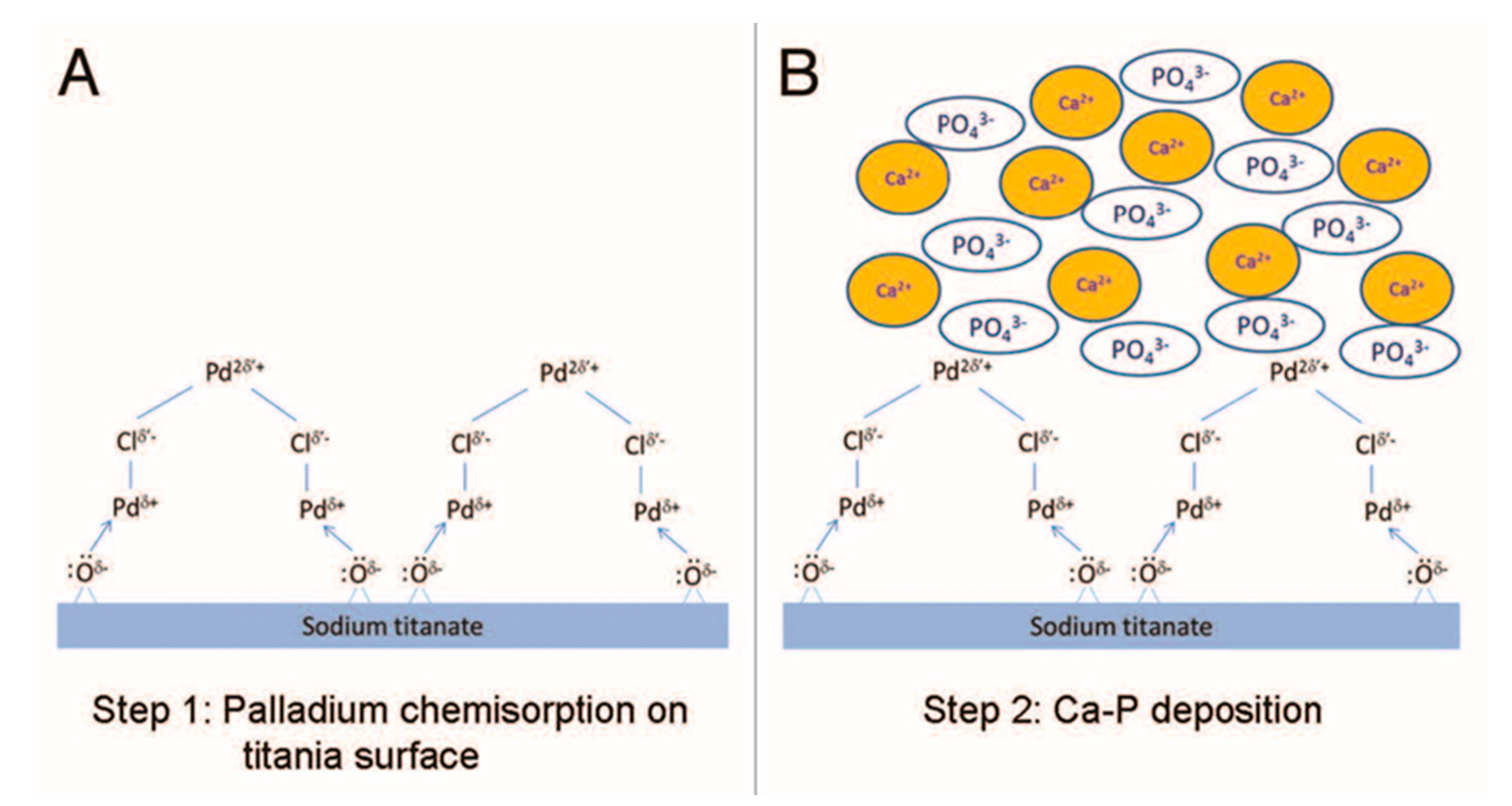



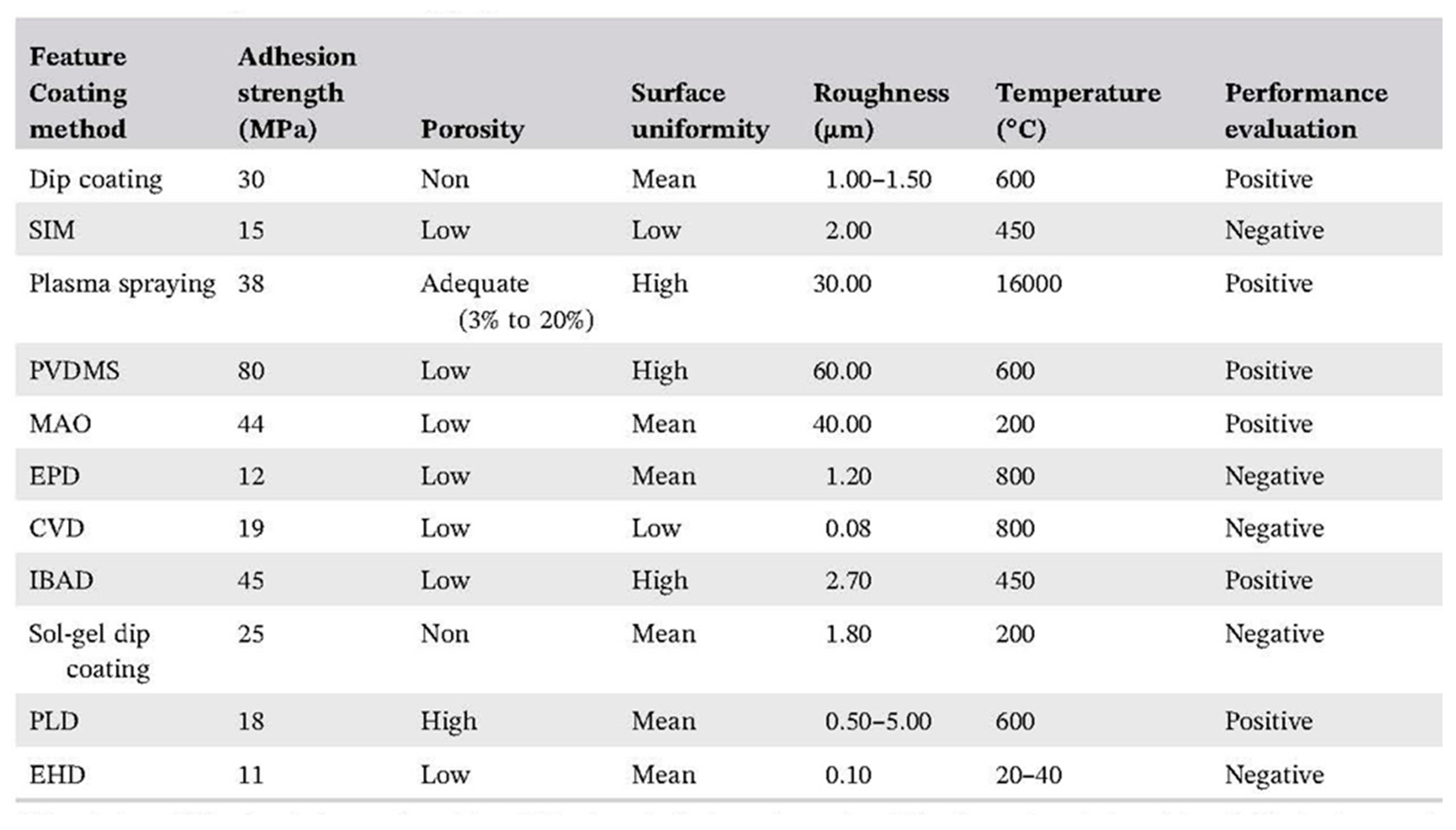

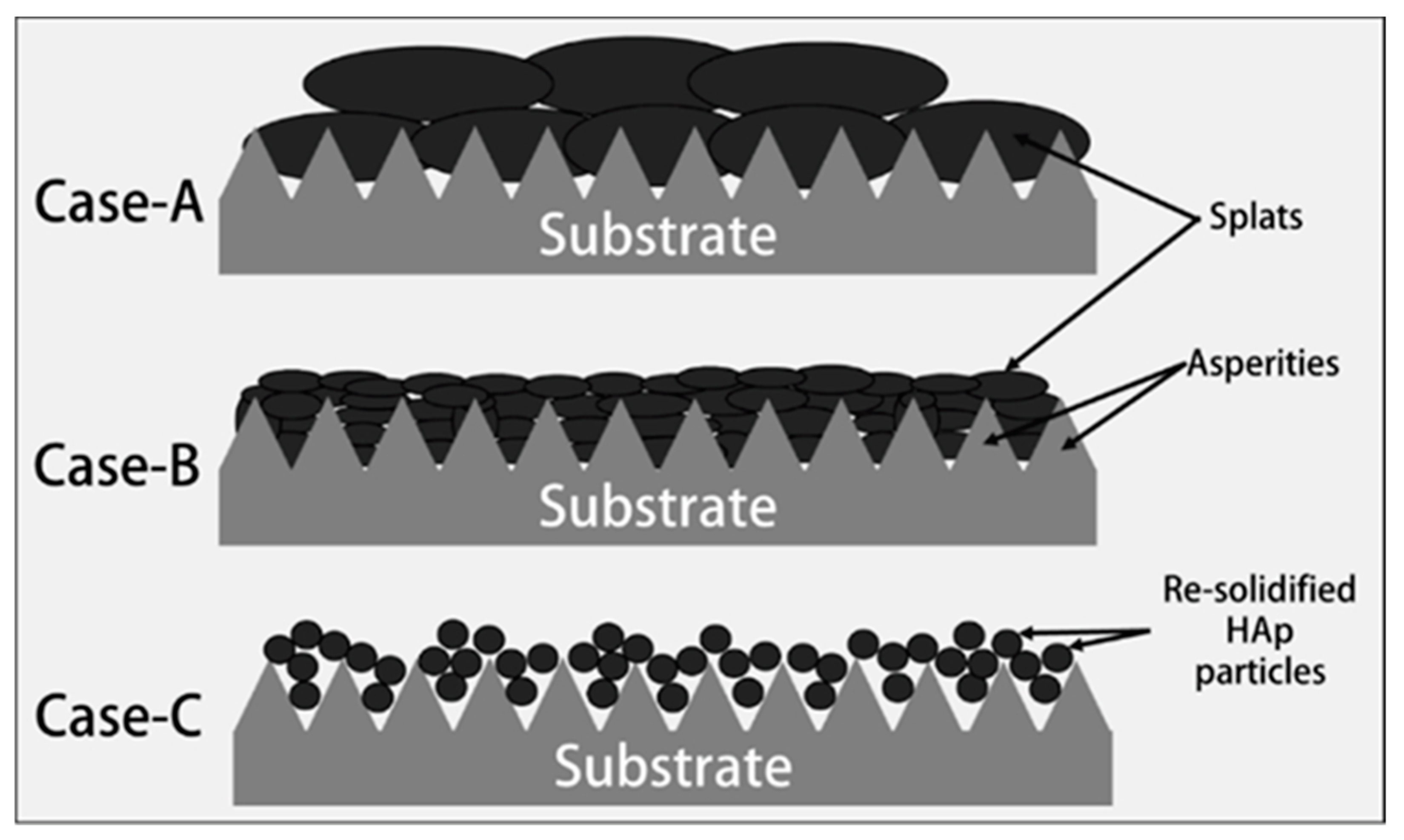

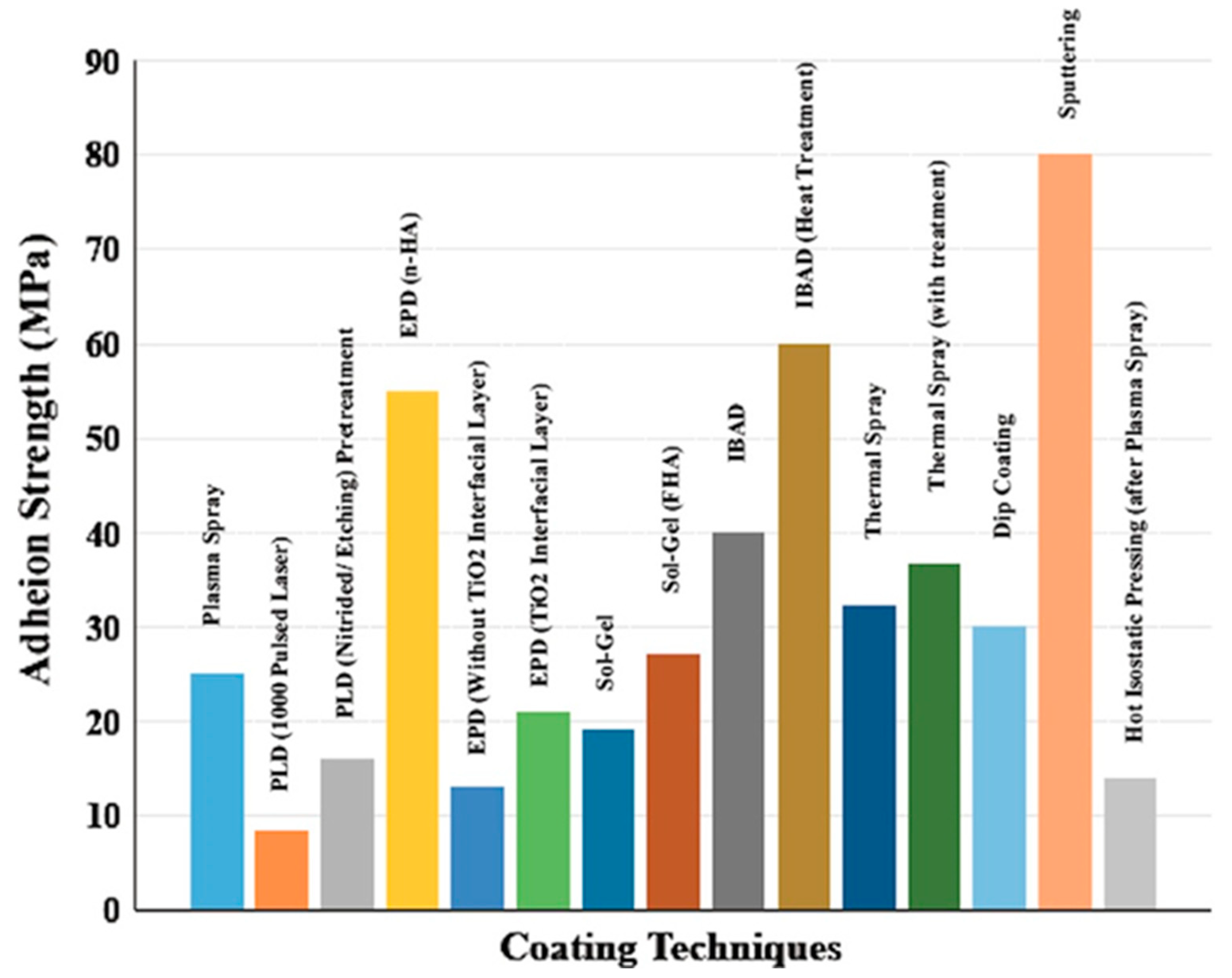

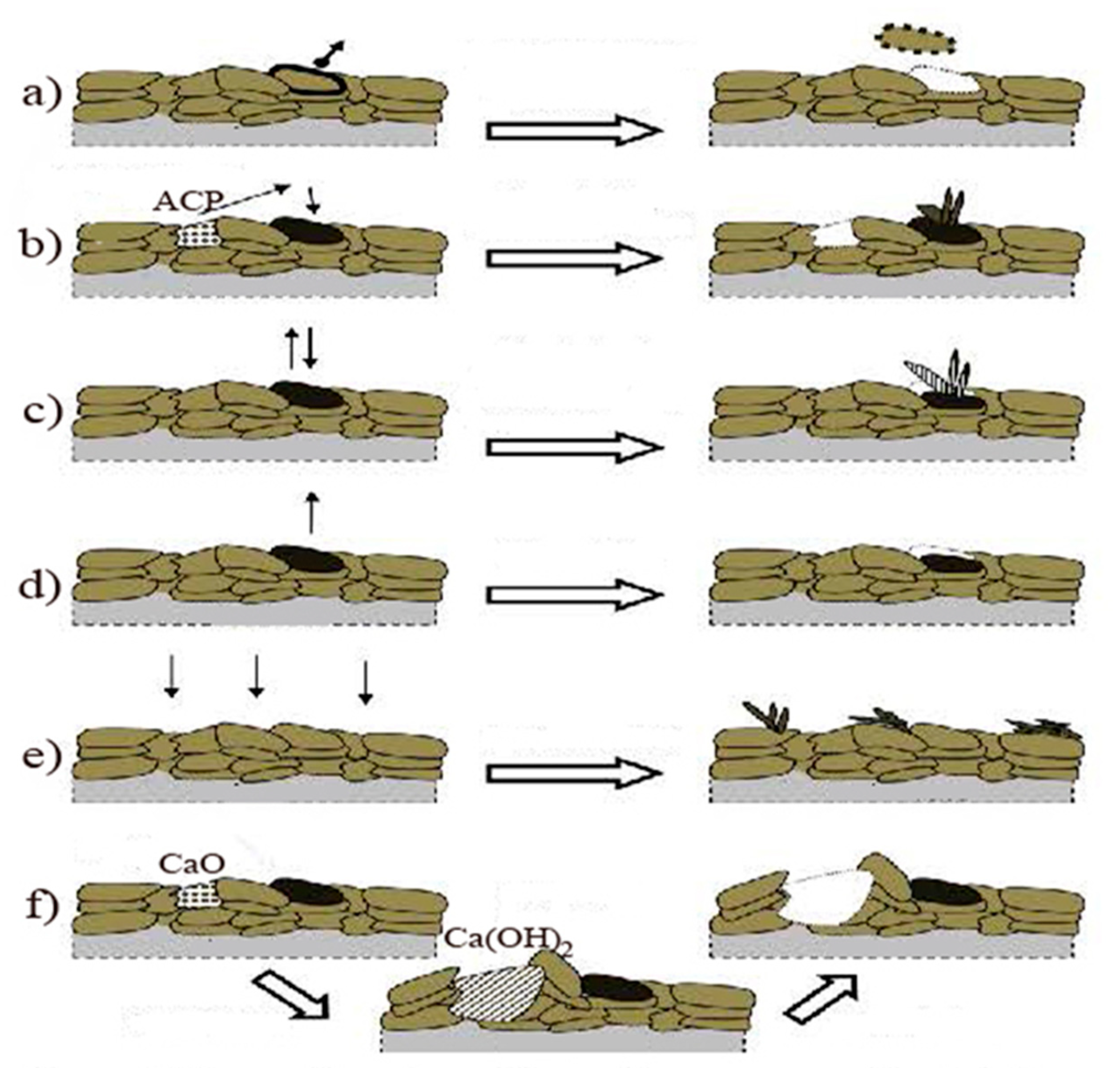
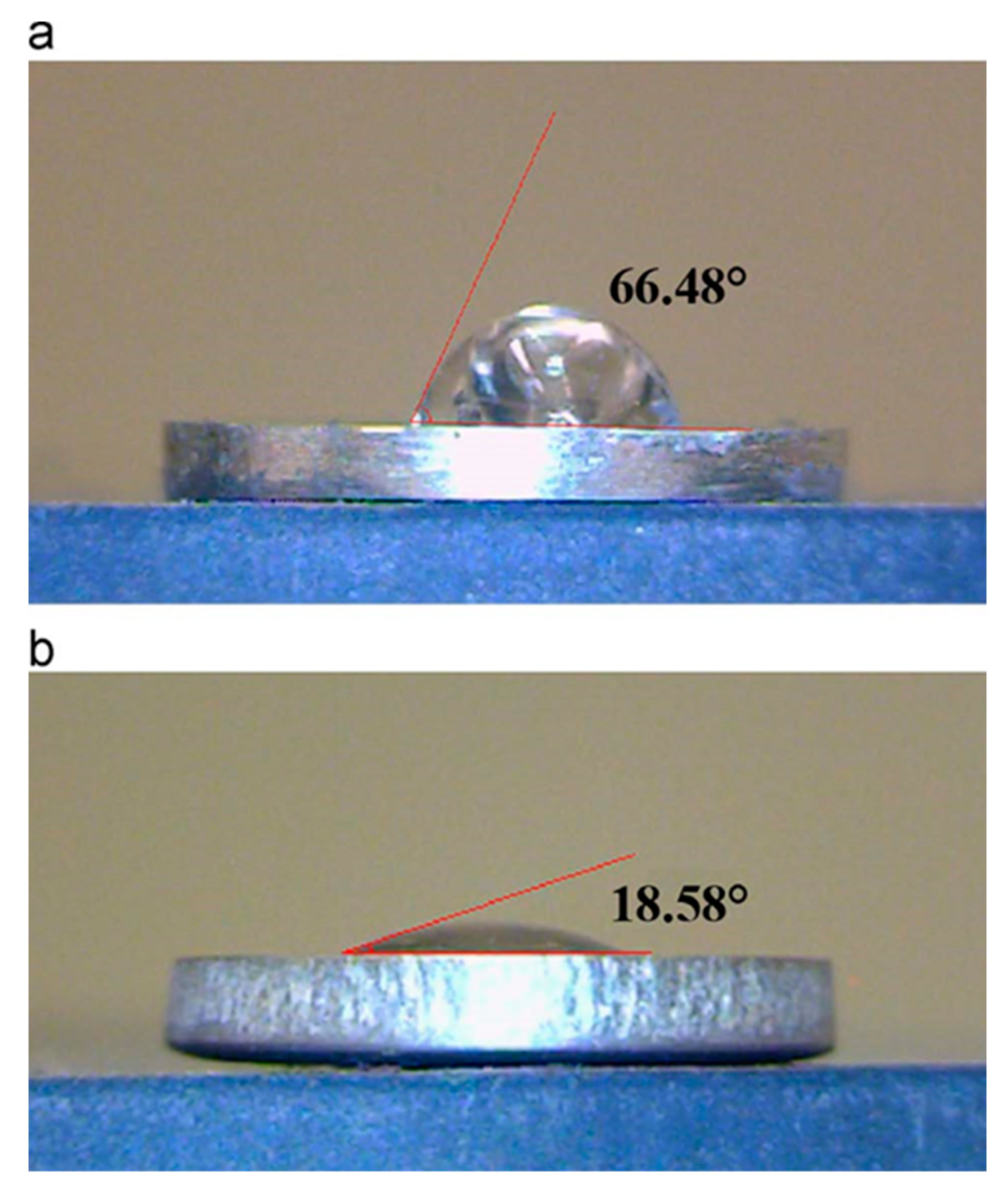

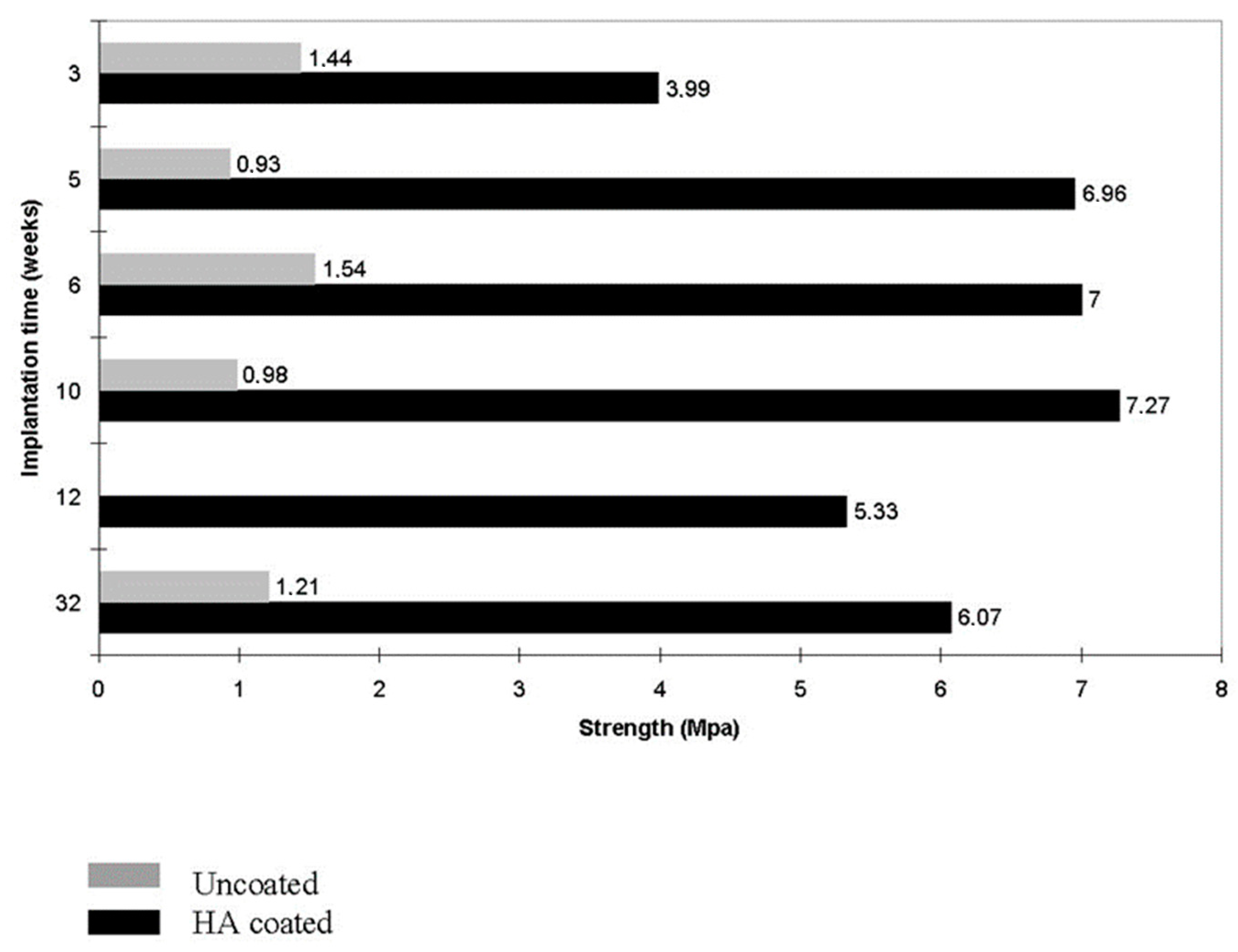
| Ca/P Molar Ratio | Compound | Formula | Solubility at 25 °C, −log(Ks) | Solubility at 25 °C, g/L | pH Stability Range in Aqueous Solutions at 25 °C |
|---|---|---|---|---|---|
| 0.5 | Monocalcium phosphate monohydrate (MCPM) | Ca(H2PO4)2·H2O | 1.14 | ~18 | 0.0–2.0 |
| 0.5 | Monocalcium phosphate anhydrous (MCPA or MCP) | Ca(H2PO4)2 | 1.14 | ~17 | c |
| 1.0 | Dicalcium phosphate dihydrate (DCPD), mineral brushite | CaHPO4·2H2O | 6.59 | ~0.088 | 2.0–6.0 |
| 1.0 | Dicalcium phosphate anhydrous (DCPA or DCP), mineral monetite | CaHPO4 | 6.90 | ~0.048 | c |
| 1.33 | Octacalcium phosphate (OCP) | Ca8(HPO4)2(PO4)4·5H2O | 96.6 | ~0.0081 | 5.5–7.0 |
| 1.5 | α-Tricalcium phosphate (α-TCP) | α-Ca3(PO4)2 | 25.5 | ~0.0025 | a |
| 1.5 | β-Tricalcium phosphate (β-TCP) | β-Ca3(PO4)2 | 28.9 | ~0.0005 | a |
| 1.2–2.2 | Amorphous calcium phosphates (ACP) | CaxHy(PO4)z·nH2O, n = 3–4.5; 15–20% H2O | b | b | ~5–12 d |
| 1.5–1.67 | Calcium-deficient hydroxyapatite (CDHA or Ca-def HA) e | Ca10-x(HPO4)x(PO4)6-x(OH)2-x (0 < x < 1) | ~85 | ~0.0094 | 6.5–9.5 |
| 1.67 | Hydroxyapatite (HA, HAp or OHAp) | Ca10(PO4)6(OH)2 | 116.8 | ~0.0003 | 9.5–12 |
| 1.67 | Fluorapatite (FA or FAp) | Ca10(PO4)6F2 | 120.0 | ~0.0002 | 7–12 |
| 1.67 | Oxyapatite (OA, OAp or OXA) f, mineral voelckerite | Ca10(PO4)6O | ~69 | ~0.087 | a |
| 2.0 | Tetracalcium phosphate (TTCP or TetCP), mineral hilgenstockite | Ca4(PO4)2O | 38–44 | ~0.0007 | a |
| Properties | Specification |
|---|---|
| Thickness | Not specific |
| Crystallinity | 62% minimum |
| Phase purity | 95% minimum |
| Ca/P atomic ratio | 1.67–1.76 |
| Density | 2.98 g/cm3 |
| Heavy metals | <50 ppm |
| Tensile strength | >50.8 MPa |
| Shear strength | >22 MPa |
| Abrasion | Not specific |
| Technique | Thickness | Advantages | Disadvantages |
|---|---|---|---|
| Plasma spraying | 30–200 μm | A simple and flexible technique; uniform and smooth coatings are produced; high deposition rates; low cost | Line-of-sight technique; high temperatures induce partial decomposition and formation of non-stoichiometric and amorphous compounds; expensive equipment; simultaneous incorporation of biological agents is impossible; rapid cooling produces cracks |
| Flame spraying | 100–250 μm | Most economical among all thermal spraying techniques; easily adaptable; porous deposits | Line-of-sight technique; high temperatures induce partial decomposition and formation of non-stoichiometric and amorphous compounds; crack development at lower temperatures, simultaneous incorporation of biological agents is impossible; rapid cooling produces cracks |
| High velocity oxy-fuel spraying | 30–200 µm | High deposition rates; uniform deposition; improved wear and corrosion resistance and biocompatibility; no post treatment required | Line-of-sight technique; high temperatures induce partial decomposition and formation of non-stoichiometric and amorphous compounds; simultaneous incorporation of biological agents is impossible; rapid cooling produces cracks |
| RF magnetron sputtering | 0.5–3 μm | Uniform coating thickness on flat substrates; high purity and adhesion; dense pore-free deposits; excellent coverage of steps and small features; ability to coat heat-sensitive substrates | Line-of-sight technique; expensive; low deposition rates; produces amorphous coatings; high temperatures prevent from simultaneous incorporation of biological agents |
| Pulsed laser deposition (laser ablation) | 0.05–5 μm | Coatings with crystalline and amorphous phases; dense and porous coatings; high adhesive strength; ability to produce wide range of multilayer coatings from different materials | Line-of-sight technique; expensive; high temperatures prevent simultaneous incorporation of biological agents; lack of uniformity |
| Ion beam assisted deposition | 0.05–1 µm | Uniform coating thickness; high reproducibility and reliability; dense; high adhesion; wide atomic intermix zone at the coating/substrate interface | Line-of-sight technique; expensive; produces amorphous coatings |
| Sputtering | 0.5–3 μm | Uniform coating thickness on flat substrates; dense; high adhesion | Line-of-sight technique; expensive equipment; time-consuming; produces amorphous coatings |
| Electrostatic spray deposition | 10 nm–30 μm | Low cost; easy set-up; ambient conditions; a wide choice of both precursors (dissolved salts, suspensions, sols) and substrates | Line-of-sight technique; problems coating large surfaces; low flow rates; requires high temperatures to decompose the precursor solvents and salts |
| Dip coating | 2 μm –5 mm | Easy set-up; low cost; coatings applied quickly; can coat complex substrates | Requires high sintering temperatures; possible thermal expansion mismatch; crack appearance |
| Spin coating | 2 μm–0.5 mm | Easy set-up; low cost; coatings applied quickly | Requires high sintering temperatures; possible thermal expansion mismatch; crack appearance; cannot coat complex substrates |
| Sol–gel technique | <1 μm | Can coat complex shapes; low processing temperatures; thin coatings; inexpensive process; can incorporate biological molecules | Some processes require controlled atmosphere processing; expensive raw materials; high permeability; low wear resistance; hard to control the porosity |
| Electrophoretic deposition | 0.1–2.0 mm | Uniform coating thickness; rapid deposition rates; simple setup; low cost; can coat complex substrates; can incorporate biological molecules | Difficult to produce crack-free coatings; requires post treatment at high temperatures |
| Electrochemical (cathodic) deposition | 0.05–0.5 mm | Good shape conformity; room temperature process; uniform coating thickness; short processing times; can incorporate biological molecules | Sometimes stressed coatings are produced, leading to their poor adhesion with substrate; requires good control of electrolyte parameters |
| Biomimetic process | <30 μm | Low processing temperatures; can form bonelike apatite; can coat complex shapes; can incorporate biological molecules | Very low deposition rates; requires replenishment and a pH constancy of the simulating solutions (HBSS, SBF, etc.) |
| Hydrothermal deposition | 0.2–2.0 μm | Coatings are crystalline; can coat complex shapes | High pressure and temperatures are required |
| Thermal substrate deposition | 0.2–2.0 μm | Deposition is enhanced by heat and current; different CaPO4 phases can be formed | Less common technique; coatings of diverse crystallinities are produced |
| Hot isostatic pressing | 0.2–2.0 μm | Produces dense coatings; homogeneous structure; high uniformity; high precision; no dimensional or shape limitations | Cannot coat complex substrates; high temperature required; thermal expansion mismatch; elastic property differences; expensive; removal/interaction of encapsulation material; high temperatures prevent simultaneous incorporation of biological agents |
| Micro-arc oxidation | 3–30 μm | Simple, economical and environmentally friendly technique, suitable for coating of complex geometries | Unless the proper electrolytes are used, the procedure rather should be considered as a pre-deposition technique onto which CaPO4 are deposited by other methods |
| Dynamic mixing method | 0.05–1.3 μm | High adhesive strength | Line-of-sight technique; expensive; produces amorphous coatings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorozhkin, S.V. There Are over 60 Ways to Produce Biocompatible Calcium Orthophosphate (CaPO4) Deposits on Various Substrates. J. Compos. Sci. 2023, 7, 273. https://doi.org/10.3390/jcs7070273
Dorozhkin SV. There Are over 60 Ways to Produce Biocompatible Calcium Orthophosphate (CaPO4) Deposits on Various Substrates. Journal of Composites Science. 2023; 7(7):273. https://doi.org/10.3390/jcs7070273
Chicago/Turabian StyleDorozhkin, Sergey V. 2023. "There Are over 60 Ways to Produce Biocompatible Calcium Orthophosphate (CaPO4) Deposits on Various Substrates" Journal of Composites Science 7, no. 7: 273. https://doi.org/10.3390/jcs7070273
APA StyleDorozhkin, S. V. (2023). There Are over 60 Ways to Produce Biocompatible Calcium Orthophosphate (CaPO4) Deposits on Various Substrates. Journal of Composites Science, 7(7), 273. https://doi.org/10.3390/jcs7070273






