Influence of the Reaction Injection Moulding Process on the Thermomechanical Behaviour of Fast Curing Polyurethane
Abstract
:1. Introduction
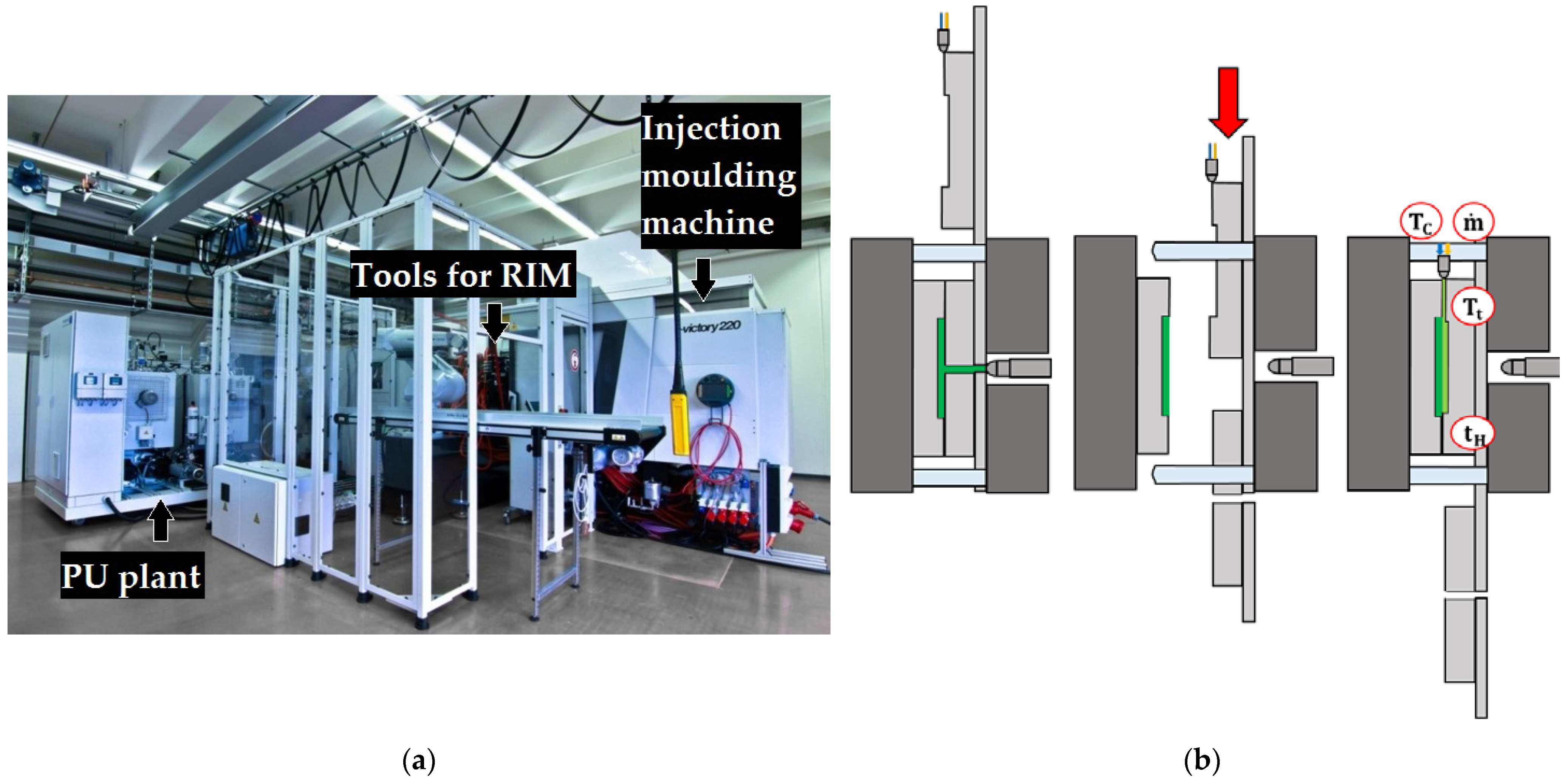
2. Materials and Methods
3. Results and Discussion
3.1. Process Influence at the Mechanical Material Properties
3.2. Explanation of the Mechanical Differences of the Parameter Sets
3.3. Process Influence at the Thermal Material Properties
3.4. Mutual Effects of the Analysed Process Variables
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.-W.; Lee, H.-S. Analysis of impingement mixing for coating in injection mold. J. Korea Soc. Die Mold Eng. 2019, 13, 1–9. [Google Scholar]
- Bürkle, E.; Wobbe, H. Kombinationstechnologien auf Basis des Spritzgießverfahrens; Carl Hanser Verlag GmbH & Co. KG: Munich, Germany, 2016. [Google Scholar]
- Park, J.-L.; Lee, H.-S. Analysis for injection molding and in-mold coating of automotive armrests. J. Korea Soc. Die Mold Eng. 2019, 13, 45–54. [Google Scholar]
- Nummy, A. Direct Coating Technology for Improved Piano Black Appearance and Scratch Resistance. SAE Int. J. Mater. Manuf. 2018, 11, 261–266. [Google Scholar] [CrossRef]
- Yacoub, F.; MacGregor, J. Analysis and optimization of a polyurethane reaction injection molding (RIM) process using multivariate projection methods. Chemom. Intell. Lab. Syst. 2003, 65, 17–33. [Google Scholar] [CrossRef]
- Nishimura, H.; Kojima, H.; Yarita, T.; Noshiro, M. Phase structure of polyetherpolyol-4,4′-diphenylmethane diisocyanate-based reaction injection molded (RIM) polyurethanes. Polym. Eng. Sci. 1986, 26, 585–592. [Google Scholar] [CrossRef]
- Kang, C.; Song, Y.S. Enhancement in Surface Property via In-Mold Coating Process. Macromol. Res. 2021, 29, 185–190. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A. A Brief Review on injection moulding manufacturing process. Mater. Proc. 2017, 4, 1423–1433. [Google Scholar] [CrossRef]
- Dang, X.-P. General frameworks for optimization of plastic injection molding process parameters. Simul. Model. Pract. Theory 2014, 41, 15–27. [Google Scholar] [CrossRef]
- Rahimi, M.; Esfahanian, M.; Moradi, M. Effect of reprocessing on shrinkage and mechanical properties of ABS and investigating the proper blend of virgin and recycled ABS in injection molding. J. Mater. Process. Technol. 2014, 214, 2359–2365. [Google Scholar] [CrossRef]
- Kusić, D.; Kek, T.; Slabe, J.M.; Svečko, R.; Grum, J. The impact of process parameters on test specimen deviations and their correlation with AE signals captured during the injection moulding cycle. Polym. Test. 2013, 32, 583–593. [Google Scholar] [CrossRef]
- Mok, S.; Kwong, C.; Lau, W. A Hybrid Neural Network and Genetic Algorithm Approach to the Determination of Initial Process Parameters for Injection Moulding. Int. J. Adv. Manuf. Technol. 2001, 18, 404–409. [Google Scholar] [CrossRef]
- Kalay, G.; Bevis, M.J. Processing and physical property relationships in injection-molded isotactic polypropylene. 1. mechanical properties. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 241–263. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Esneider-Alcalá, M.A.; Teran, A.T.; Márquez-Lucero, A.; Aguilar-Martínez, J.A. Effect of Molding Parameters on Young’s Modulus of an Injection Molded Low-Density Polyethylene (LDPE). Ind. Eng. Chem. Res. 2013, 52, 5666–5671. [Google Scholar] [CrossRef]
- Gopi, J.; Nando, G.B. Effect of processing parameters on phase morphology and mechanical properties of thermoplastic polyurethane and polydimethylsiloxane rubber blends. Int. J. Plast. Technol. 2015, 19, 288–308. [Google Scholar] [CrossRef]
- Li, D.; Zhai, T.; Gong, Q.; Fei, G.; Xia, H. Effect of processing temperature on structure and properties of microinjection moulded thermoplastic polyurethane/multiwalled carbon nanotube composites. Plast. Rubber Compos. 2015, 44, 197–205. [Google Scholar] [CrossRef] [Green Version]
- El-Shekeil, Y.; Sapuan, S.M.; Zainudin, E.; Khalina, A. Optimizing Processing Parameters and Fiber Size for Kenaf Fiber Reinforced Thermoplastic Polyurethane Composite. Key Eng. Mater. 2011, 471–472, 297–302. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Peng, J.; Turng, L.-S.; Peng, X.-F. Influence and prediction of processing parameters on the properties of microcellular injection molded thermoplastic polyurethane based on an orthogonal array test. J. Cell. Plast. 2013, 49, 439–458. [Google Scholar] [CrossRef]
- Löhner, M.; Drummer, D. Influence of Processing Parameters in Reaction Injection Foam Molding for Multi-Layer Parts on Foam Structure and Mechanical Properties. Appl. Mech. Mater. 2015, 805, 131–138. [Google Scholar] [CrossRef]
- Jackovich, D.; O’Toole, B.; Hawkins, M.C.; Sapochak, L. Temperature and Mold Size Effects on Physical and Mechanical Properties of a Polyurethane Foam. J. Cell. Plast. 2005, 41, 153–168. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Riedel, G.; Trawiel, P. Praxis der Thermischen Analyse von Kunststoffen, 2., völlig überarb. Aufl.; Hanser: Munich, Germany, 2003; ISBN 3446223401. [Google Scholar]
- Puszka, A. Thermal and Mechanical Behavior of New Transparent Thermoplastic Polyurethane Elastomers Derived from Cycloaliphatic Diisocyanate. Polymers 2018, 10, 537. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Boyce, M.C.; Lee, G.F.; Balizer, E. Large deformation rate-dependent stress–strain behavior of polyurea and polyurethanes. Polymer 2006, 47, 319–329. [Google Scholar] [CrossRef]
- Sarva, S.S.; Deschanel, S.; Boyce, M.C.; Chen, W. Stress–strain behavior of a polyurea and a polyurethane from low to high strain rates. Polymer 2007, 48, 2208–2213. [Google Scholar] [CrossRef]
- Dany, R. Untersuchungen zum Mischungsverhalten von Flüssigkristallinen Polyurethanen mit Herkömmlichen Polyurethanen und Mit Vinylchloridpolymeren. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2006. [Google Scholar]
- Schwarzl, F.R. Polymermechanik: Struktur und Mechanisches Verhalten von Polymeren; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 3-540-51965-3. [Google Scholar]
- DIN EN ISO 527; Normenausschuss Kunststoffe. Deutsches Institut für Normung e.V.: Berlin, Germany, 2012.
- Fortman, D.J.; Sheppard, D.T.; Dichtel, W.R. Reprocessing Cross-Linked Polyurethanes by Catalyzing Carbamate Exchange. Macromolecules 2019, 52, 6330–6335. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Mizera, K.; Ryszkowska, J. Thermal properties of polyurethane elastomers from soybean oil-based polyol with a different isocyanate index. J. Elastomers Plast. 2019, 51, 157–174. [Google Scholar] [CrossRef]
- Flory, P.J. Effects of Molecular Structure on Physical Properties of Butyl Rubber. Rubber Chem. Technol. 1946, 19, 552–598. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Ilavský, M.; Dušek, K.; Vidaković, M.; Javni, I.; Banjanin, B. The effect of crosslinking on properties of polyurethane elastomers. J. Appl. Polym. Sci. 1991, 42, 391–398. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Farzaneh, S.; Abdallah-Elhirtsi, S.; Esmaeillou, B.; Nony, F.; Baron, A. Thermal Aging Effect on Mechanical Properties of Polyurethane. Int. J. Polym. Anal. Charact. 2014, 19, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Kästner, S. Eine einfache Theorie des mechanischen und dielektrischen Relaxationsverhaltens während der Bildung eines Polymernetzwerkes aus reaktiven Oligomeren. Acta Polym. 1980, 31, 341–347. [Google Scholar] [CrossRef]
- Li, S.; Vatanparast, R.; Lemmetyinen, H. Cross-linking kinetics and swelling behaviour of aliphatic polyurethane. Polymer 2000, 41, 5571–5576. [Google Scholar] [CrossRef]
- Ionescu, M. Polyols for Polyurethanes: Chemistry and Technology, 3rd ed.; De Gruyter: Berlin, Germany, 2019; ISBN 9783110640335. [Google Scholar]
- Segiet, D.; Raidt, T.; Özdem, H.; Weckes, S.; Tiller, J.C.; Katzenberg, F. Thermo-/moisture-responsive shape-memory effect of poly(2-ethyl-2-oxazoline) networks. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1053–1061. [Google Scholar] [CrossRef]
- Segiet, D.; Jerusalem, R.; Katzenberg, F.; Tiller, J.C. Investigation of the swelling behavior of hydrogels derived from high-molecular-weight poly(2-ethyl-2-oxazoline). J. Appl. Polym. Sci. 2020, 58, 747–755. [Google Scholar] [CrossRef]
- Cluff, E.F.; Gladding, E.K. Relation of structure to properties in polyurethanes. J. Appl. Polym. Sci. 1960, 3, 290–295. [Google Scholar] [CrossRef]
- Fuith, A.; Reinecker, M.; Sánchez-Ferrer, A.; Mezzenga, R.; Mrzel, A.; Knite, M.; Aulika, I.; Dunce, M.; Schranz, W. Dynamic- and Thermo-mechanical Analysis of Inorganic Nanotubes/Elastomer Composites. Sens. Tranducers 2011, 12, 71–79. [Google Scholar]
- Lee, S.H.; Shin, S.-R.; Lee, D.-S. Sorbitol as a Chain Extender of Polyurethane Prepolymers to Prepare Self-Healable and Robust Polyhydroxyurethane Elastomers. Molecules 2018, 23, 2515. [Google Scholar] [CrossRef] [Green Version]
- Szycher, M. (Ed.) Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-3958-4. [Google Scholar]
- Fang, Z.; Zhou, M.; Zhong, J.; Qi, Y.; Li, L.; Dong, Q. Preparation and properties of novel ultraviolet-cured waterborne polyurethanes. High Perform. Polym. 2013, 25, 668–676. [Google Scholar] [CrossRef]
- Stepanski, H.; Leimenstoll, M. Polyurethan-Klebstoffe; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2016; ISBN 978-3-658-12269-0. [Google Scholar]
- Kampouris, E.; Andreopoulos, A. Gel content determination in cross-linked polyethylene. Biomaterials 1989, 10, 206–208. [Google Scholar] [CrossRef]
- DIN EN ISO 11357; German Norm. 1–42. Deutsches Institut für Normung e.V.: Berlin, Germany, 2016.
- Krol, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Sreedhar, B.; Raju, K. The phase mixing studies on moisture cured polyurethane-ureas during cure. Polymer 2006, 47, 3814–3825. [Google Scholar] [CrossRef]
- Long, T.R.; Elder, R.M.; Bain, E.D.; Masser, K.A.; Sirk, T.W.; Yu, J.H.; Knorr, D.B.; Lenhart, J.L. Influence of molecular weight between crosslinks on the mechanical properties of polymers formed via ring-opening metathesis. Soft Matter 2018, 14, 3344–3360. [Google Scholar] [CrossRef]
- Zhou, Y.; Choi, P. Molecular dynamics study of water diffusion in an amphiphilic block copolymer with large difference in the blocks’ glass transition temperatures. Front. Chem. Sci. Eng. 2017, 11, 440–447. [Google Scholar] [CrossRef]
- Olejnik, A.; Gosz, K.; Piszczyk, Ł. Kinetics of cross-linking processes of fast-curing polyurethane system. Thermochim. Acta 2020, 683, 178435. [Google Scholar] [CrossRef]
- Stanko, M.; Stommel, M. Kinetic Prediction of Fast Curing Polyurethane Resins by Model-Free Isoconversional Methods. Polymers 2018, 10, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijkants, R.G.; Van Calck, R.V.; Van Tienen, T.G.; De Groot, J.H.; Buma, P.; Pennings, A.J.; Veth, R.P.; Schouten, A.J. Uncatalyzed synthesis, thermal and mechanical properties of polyurethanes based on poly(ε-caprolactone) and 1,4-butane diisocyanate with uniform hard segment. Biomaterials 2005, 26, 4219–4228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.A.; Lou, C.-W.; Lin, J.-H. The Effects of Thermoplastic Polyurethane on the Structure and Mechanical Properties of Modified Polypropylene Blends. Appl. Sci. 2017, 7, 1254. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.; Bian, J.; Wu, J.; Sun, X.; Wang, Y.; Jia, Z. Prediction of Seasonal Frost Heave Behavior in Unsaturated Soil in Northeastern China Using Interactive Factor Analysis with Split-Plot Experiments and GRNN. Water 2019, 11, 1587. [Google Scholar] [CrossRef] [Green Version]
- Şimşek, B.; Uygunoğlu, T. A full factorial-based desirability function approach to investigate optimal mixture ratio of polymer concrete. Polym. Compos. 2018, 39, 3199–3211. [Google Scholar] [CrossRef]






| Parameter Set | °C | in s | in g/s | °C |
|---|---|---|---|---|
| 1 | 80 | 60 | 16 | 65 |
| 2 | 90 | 60 | 16 | 65 |
| 3 | 100 | 60 | 16 | 65 |
| 4 | 90 | 80 | 16 | 65 |
| 5 | 90 | 100 | 16 | 65 |
| 6 | 90 | 60 | 15 | 65 |
| 7 | 90 | 60 | 17 | 65 |
| 8 | 90 | 60 | 16 | 70 |
| 9 | 90 | 60 | 17 | 70 |
| 10 | 90 | 100 | 16 | 70 |
| Sample Name | |
|---|---|
| 6-1 | 0.271 |
| 6-2 | 0.038 |
| 6-3 | 0.181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmenkühler, P.; Stommel, M. Influence of the Reaction Injection Moulding Process on the Thermomechanical Behaviour of Fast Curing Polyurethane. J. Manuf. Mater. Process. 2022, 6, 53. https://doi.org/10.3390/jmmp6030053
Lehmenkühler P, Stommel M. Influence of the Reaction Injection Moulding Process on the Thermomechanical Behaviour of Fast Curing Polyurethane. Journal of Manufacturing and Materials Processing. 2022; 6(3):53. https://doi.org/10.3390/jmmp6030053
Chicago/Turabian StyleLehmenkühler, Peter, and Markus Stommel. 2022. "Influence of the Reaction Injection Moulding Process on the Thermomechanical Behaviour of Fast Curing Polyurethane" Journal of Manufacturing and Materials Processing 6, no. 3: 53. https://doi.org/10.3390/jmmp6030053
APA StyleLehmenkühler, P., & Stommel, M. (2022). Influence of the Reaction Injection Moulding Process on the Thermomechanical Behaviour of Fast Curing Polyurethane. Journal of Manufacturing and Materials Processing, 6(3), 53. https://doi.org/10.3390/jmmp6030053







