1. Introduction
Consider a semi-infinite solid slab, initially at temperature
. At time
, a continuous line heat source
is activated along the line
. The temperature distribution in the semi-infinite medium is found to be [
1] (pg. 261, Equation (5))
where
is the thermal conductivity of the solid, and
is the exponential integral defined as
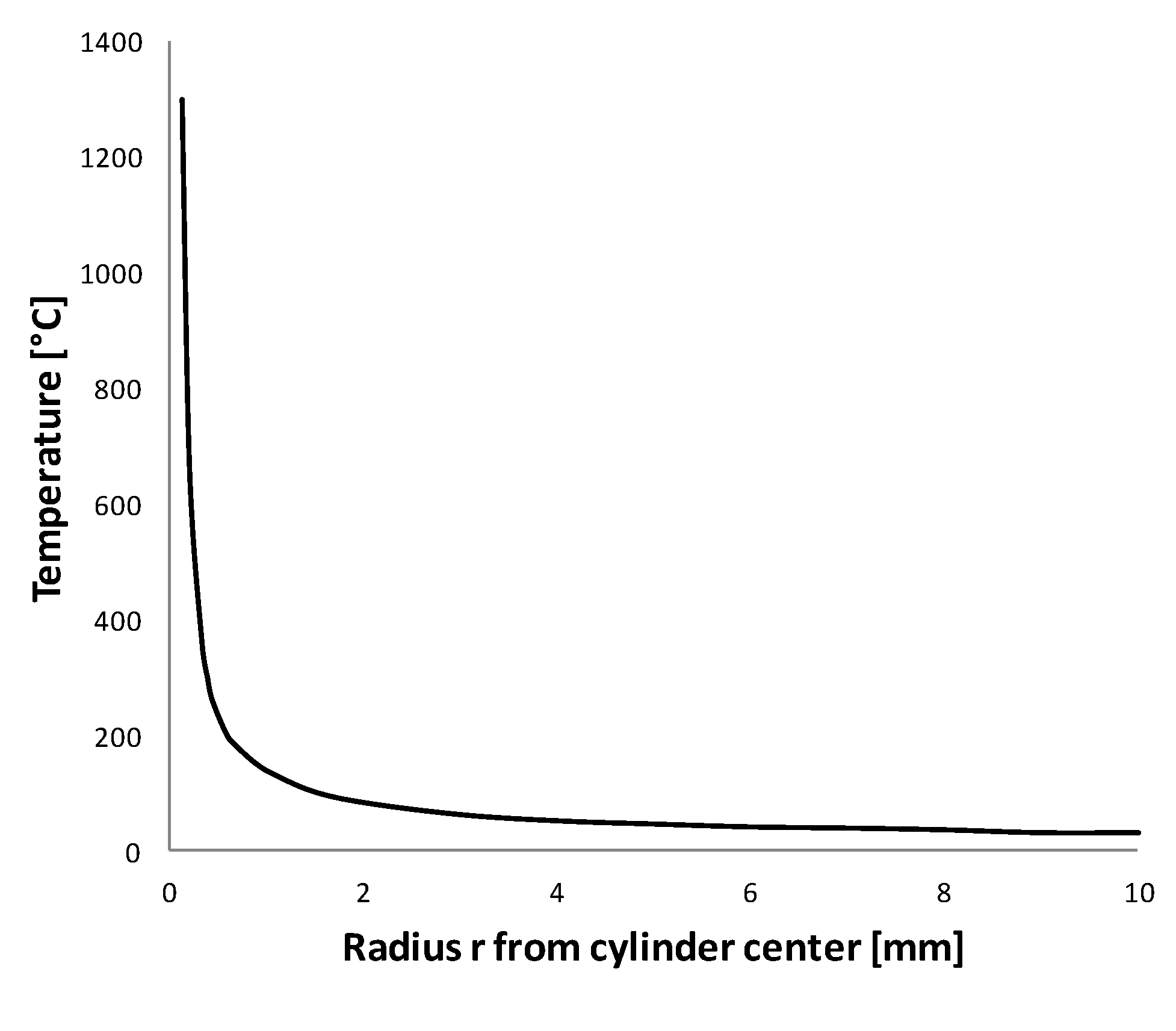
. Similar to 1D Cartesian coordinates, there is no steady-state solution in 1D cylindrical coordinates. The temperature field is cylindrically symmetric, ranging from infinite at
to
at the far field. In reality though, if
is less than the melting temperature
of the material, the high temperature developed around
would lead to melting of the material and a cylindrical interface would emerge separating the solid phase (metal powder) from the liquid phase (molten material). Hence, one has to take into consideration the different properties between the liquid and the solid phases and, in addition, the latent heat of melting
L per unit mass at the interface. For a pure substance the interface is sharp at the melting temperature
of the material, and it moves cylindrically outwards separating the two phases, solid and liquid. Similar arguments apply for the solidification process.
For the case of melting or solidification of a material due to a line source/sink, with constant but different properties between the liquid and solid phases but equal densities, an analytical solution has been obtained by Patterson [
2], who combined two expressions similar to Equation (1). The boundary condition for the energy balance leads to an algebraic equation (characteristic equation) where the unknown, which can be considered to be an eigenvalue, is proportional to the position of the liquid-solid interface (i.e., the speed of melting of the material). The characteristic equation is monotonic with respect to the eigenvalue so there is only one single solution. The analysis is valid when the two phases have the same density. A review is given by Hu and Argyropoulos [
3] and Alexiades and Solomon [
4], where they present the major methods of mathematical modeling of solidification and melting. For the case where the two phases have different densities, the energy balance equation is different [
4,
5], and furthermore a convection term must be included in the heat equation of the liquid phase. The convection term can be obtained from the radially-symmetric continuity equation in cylindrical coordinates with constant density [
4]. A similar approach is used for the analysis of melting of nanoparticles [
6] and bubble growth and oscillations [
7,
8,
9,
10,
11].
The major difference between bubble growth/oscillations and melting/solidification is that, for the former case, the bubble interface is set in motion by the pressure field, hence one has to solve the momentum equation. On the contrary, for the latter case the interface between the liquid and the solid phase is controlled by the conduction process; hence, the heat equation is decoupled from the momentum equation.
In this work, similar to Font et al. [
6], we first solve the continuity equation in the liquid phase to find an expression for the velocity field using mass conservation. Subsequently we solve the heat equation in the two phases using a similarity transformation of the form
, and through the energy boundary condition on the interface we obtain an algebraic (characteristic) equation for the eigenvalue
, which is proportional to the location of the interface. Unlike Font et al. [
6], we have neglected the kinetic energy term in the energy equation because it is small compared to other terms (see
Section 2.2). Furthermore, if needed, an expression for the pressure field in the liquid phase can be obtained by substituting the expression of the velocity field in the radial momentum equation.
In this work, we apply the melting process described above, as a simplified model to describe the dynamics of selective laser melting (SLM) processes. Of course, for the prediction of the melt pool when a particular SLM or selective laser sintering (SLS) process is concerned, different mathematical approaches have been introduced and studied in the literature. Cheng and Chou [
12] described an unsteady temperature simulation based on the finite element method (FEM) of the alloy IN718 [
13], concentrating on the effect of varying scan length on the melt pool size. Polivnikova [
14] studied the melt pool dynamics by means of a finite-element simulation for an SLS/SLM process. The numerical model considered the interaction between laser beam and powder material and phase transformations, while sub-models were developed to describe the capillary phenomena in the powder bed during SLS/SLM processing. Li et al. [
15] investigated the heat transfer and phase transition during an SLM process with a moving volumetric heat source using the finite difference method. They proposed a model incorporating a phase function to differentiate the powder phase, melting liquid phase, dense solid phase and vaporized gas phase that also includes the volume shrinkage induced by the density change during the melting process. Letenneur et al. proposed a three dimensional analytical model which enables the calculation of the temperature distribution in powder for a Gaussian laser heat source [
16]. In [
17], Li et al. enhanced their proposed approach by including the residual stress field analysis. A similar numerical approach was presented by Tan et al. [
18] based on a model addressing thermal, metallurgical, and mechanical effects for selective laser melting of titanium alloy. The aforementioned numerical models, although they consider all physical phenomena and elucidate the physical processes involved in the melt pool formation, are computationally intensive and cannot be used for real-time process control and optimization.
Thus, for the design of a thermal control system, the development of an efficient model is inevitable, and an analytical or semi-analytical model is necessary. On one hand, it should contain all the necessary physics, and on the other hand it should be characterized by the necessary computational efficiency, so that it can be used as an in-process reference for a control algorithm. Examples are available in the literature for pure heat transfer operations, such as scanned thermal processing [
19,
20] and for serial thermal processing methods such as arc welding [
21] and SLM and laser cutting [
22]. The model can be validated through an experimental apparatus via infrared camera and laser profilometry. This kind of sensor was used for output feedback in a closed loop geometry control system in a gas metal arc welding (GMAW) process [
23]. Besides control, a simplified model can also be used to compute the structural shape and residual stresses [
24]. In what follows, we develop a simple model based on the melting achieved due to a line heat source.
2. Mathematical Modeling
Our model is motivated by the melting achieved by a rapidly scanning laser beam moving along the span of a powder-bed [
25].
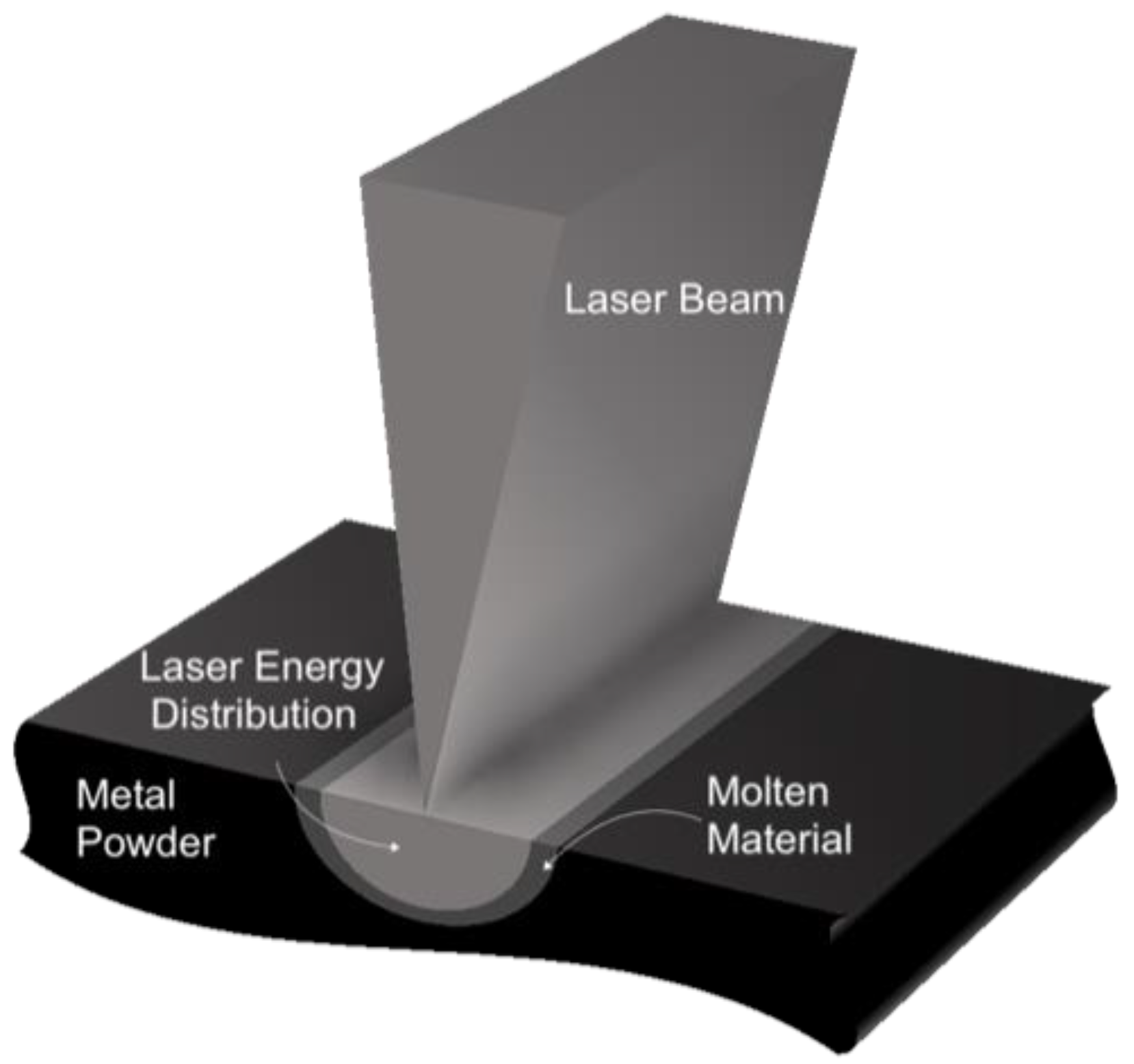
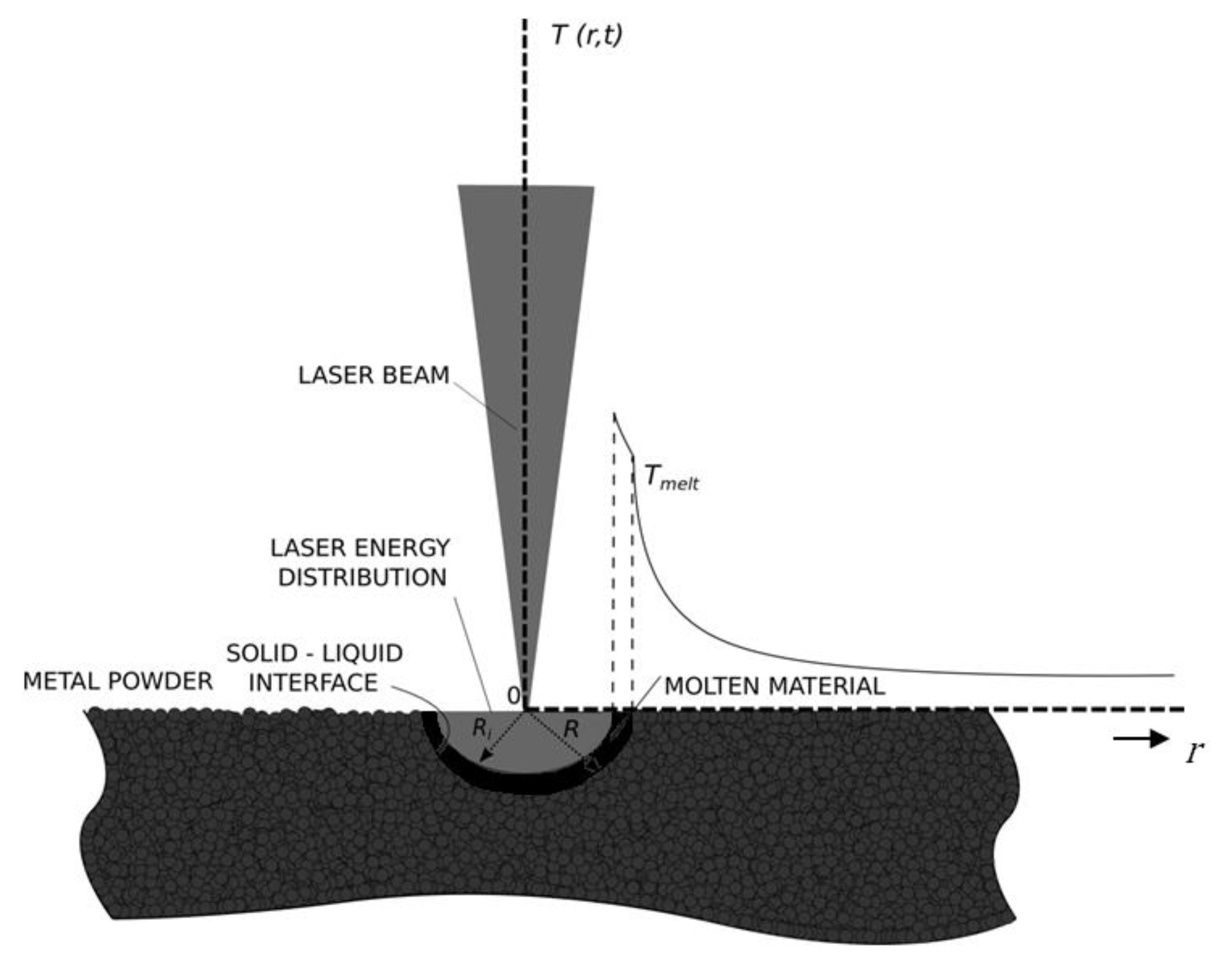
Figure 1 and
Figure 2 provide a detailed description of the model.
Initially, the semi-infinite bed of the fusible material (metal powder, powder-bed) is at the temperature
, lower than the melting temperature
of the powder. A line heat source of strength
is distributed initially along
and is activated for a brief period of time
. The release of energy causes the temperature to increase higher than
, hence the melting commences at the origin. Because the density of the liquid phase is higher than the density of the solid phase (powder), an inner interface is created with radius
(liquid-air interface,
Figure 2). Hence, an annulus is created with an inner surface of radius
, and an outer surface of radius
. The latter separates the liquid (melted material) from the solid (powder) (i.e.,
;
Figure 2); assuming a pure substance, and by ignoring the kinetics of phase change in melting, the interface (
) is sharp. Both the inner
and the outer
interfaces move in the positive r-direction with cylindrical symmetry, as shown in
Figure 1 and
Figure 2. During the time period
, we assume that the laser beam is distributed evenly over the interface
due to irradiation and reflections of the laser beam, whereas at
the laser beam is a line heat source distributed along
. For small times we can neglect gravity and Marangoni effects, hence the process is cylindrically symmetric, and, in addition, a radially-symmetric convection current
is developed in the annulus.
In order to apply this model to SLM of metallic powders a number of assumptions/parameters were adopted as described in detail in the following sections. The metal powder, which rests on a metal substrate, is molten by the scanning laser beam and solidifies into a metal deposit fused to the substrate. The density and thermal properties of the deposit are significantly different from the metal powder (see beginning of
Section 3). We assume that during the initial stages of melting, the gravity and Marangoni effects can be neglected, hence the process is cylindrically symmetric. Furthermore, because the conductivity of the metal powder is on the order of 100 times smaller than that of the metal substrate, we assume that the heat transfer process is controlled by the powder bed, and the heat transfer process is prevalent in the molten pool [
1]. Hence, during the initial melting of the powder, we neglect the presence of the metal substrate and assume a semi-infinite layer of metal powder.
The analysis that follows proceeds along the same lines as the analysis by Font et al. [
6] for melting of a nanoparticle, and the analysis by Scriven [
9] for bubble growth and oscillations [
7,
8,
9,
10,
11], where an explicit expression for the convection term is obtained from the continuity equation and mass conservation.
2.1. Flow Field
Assuming constant density in the liquid phase (i.e., zero thermal expansion coefficient), the equation of continuity in cylindrical coordinates [
26] takes the form
. Integrating with respect to
, and enforcing the conditions at
or
we obtain:
where
is the velocity of the fluid at radius
r and time
t and
is the velocity of the fluid perpendicular to the interface
. Similar expressions are obtained in the case of a moving spherical interface [
6,
7,
8,
9,
10,
11]. We should point out that, while the velocity of the fluid (molten material) is
at
, its velocity is not
at
. In order to find a relation for
we use mass conservation in a frame moving with the interface to obtain
where
is the density of the solid and
is the density of the liquid at the melting temperature
, which can be is resolved to
This is the equation obtained by Özişik [
5] (pg. 403, Equation (10-9b)). To relate
to
, we use mass conservation [
6] on a unit span of the annulus of the molten pool to obtain
Integrating above expression we obtain
Substituting Equation (3) or Equation (4) in Equation (2), we obtain an equation for the velocity field in terms of the location
and velocity
of the interface:
2.2. Governing Equations
Assuming that the properties of the powder and the molten material are constant but different, the mathematical formulation of this problem is given by
where, as mentioned earlier (Equation (5)),
with the initial condition
and the boundary conditions
In the above equations,
represents the density,
the specific heat, and
the conductivity, while the subscripts
and
represent the liquid and the solid phases, respectively. As shown in Özişik [
5] (pg. 403, Equation (10-10a)), the third boundary condition is developed by performing an energy balance across the melting interface at
. In a coordinate system moving with the interface the energy balance takes the form:
If we substitute Equation (3) for
we obtain
which is finally simplified to
where
is the latent heat,
.
A common mistake that appears in phase change problems with density variations is the exclusion of the kinetic energy term [
4,
6]. This term is the consequence of the density change which forces the fluid to move, and results in a kinetic energy deficit or surplus. This term is equal to
and it is usually excluded if it is small compared to the term related to the latent heat
. It is important only at very small times and when the value of the latent heat
is small [
4]; an exception is the melting of nanoparticles [
6]. In our simulation the latent heat is of the order
(J/kg) and the smaller value of the activation time
0.0001 (s). As we will show later
, hence the kinetic energy term is of the order
(kg/s
3) and it does not introduce any significant error. The important advantage of neglecting this term is that it allows for a similarity solution for the system of equations [
2,
4,
5] in the form
. The partial derivatives transform as follows:
Substituting in the partial differential Equations (6) and the boundary conditions (7), we obtain the following system of ordinary differential equations, which we assume that they are independent of time (
):
with boundary conditions
Note that the last equation also describes the initial condition. Multiplying by
and substituting the expression for
(Equation (4)) and
(Equation (4)), the above system simplifies to
with boundary conditions
Above equations are independent of time only if
(i.e.,
, where
is a constant to be determined). We finally obtain:
with boundary conditions
where the square brackets [
T] indicate a temperature dependency of the thermal conductivity
k and the density
ρ. The above system can be brought in the following form:
with boundary conditions
where
is the thermal diffusivity and
. Note that if we set
, the effect of convection is “switched off”; however, the density difference is still included in the energy balance equation (Equation (9), third equation; i.e., we obtain a result similar to Özişik [
5] (pg. 415).
2.3. Characteristic Equation
The system of ordinary differential equations (ODEs) (8) can be integrated once to obtain
Using the first boundary condition from Equations (9) we obtain that
An expression for
can be obtained in the form of the incomplete gamma function and the second boundary condition (Equation (9)); however, it is not required in order to obtain an expression for the eigenvalue
. An expression for
can be obtained in the form of the exponential integral
using the substitution
:
Employing the second and fourth boundary conditions we can obtain an expression for
:
Substituting (10)–(12) in the third boundary condition of Equation (9), we obtain an algebraic equation (i.e., the characteristic equation) for
:
If we replace
with
, we obtain a result similar to Carslaw and Jaeger [
26] (pg. 296):
The factor 1/2, instead of 1/4, is due to the fact that we have considered a semi-infinite domain instead of an infinite domain. We can reproduce the result by Carslaw and Jaeger [
1] if we neglect the convection term (i.e., if we set
, and replace
with
. If we know the properties of the material and the power
per unit length of the heat source, the above equation can be solved to find
; hence the location of the liquid-solid interface
can be obtained for the time period
that the heat source is activated.
4. Application of the Model to an SLM Process
Selective laser melting (SLM) is an additive rapid manufacturing technique where a laser is used to fuse metal powder that consists of micro- and nano-particles, into a specified three-dimensional geometry. In this section, we will apply the results of
Section 2 (Equation (13)) to experimental data obtained in an SLM (selective laser melting) manufacturing process.
In
Table 1 and
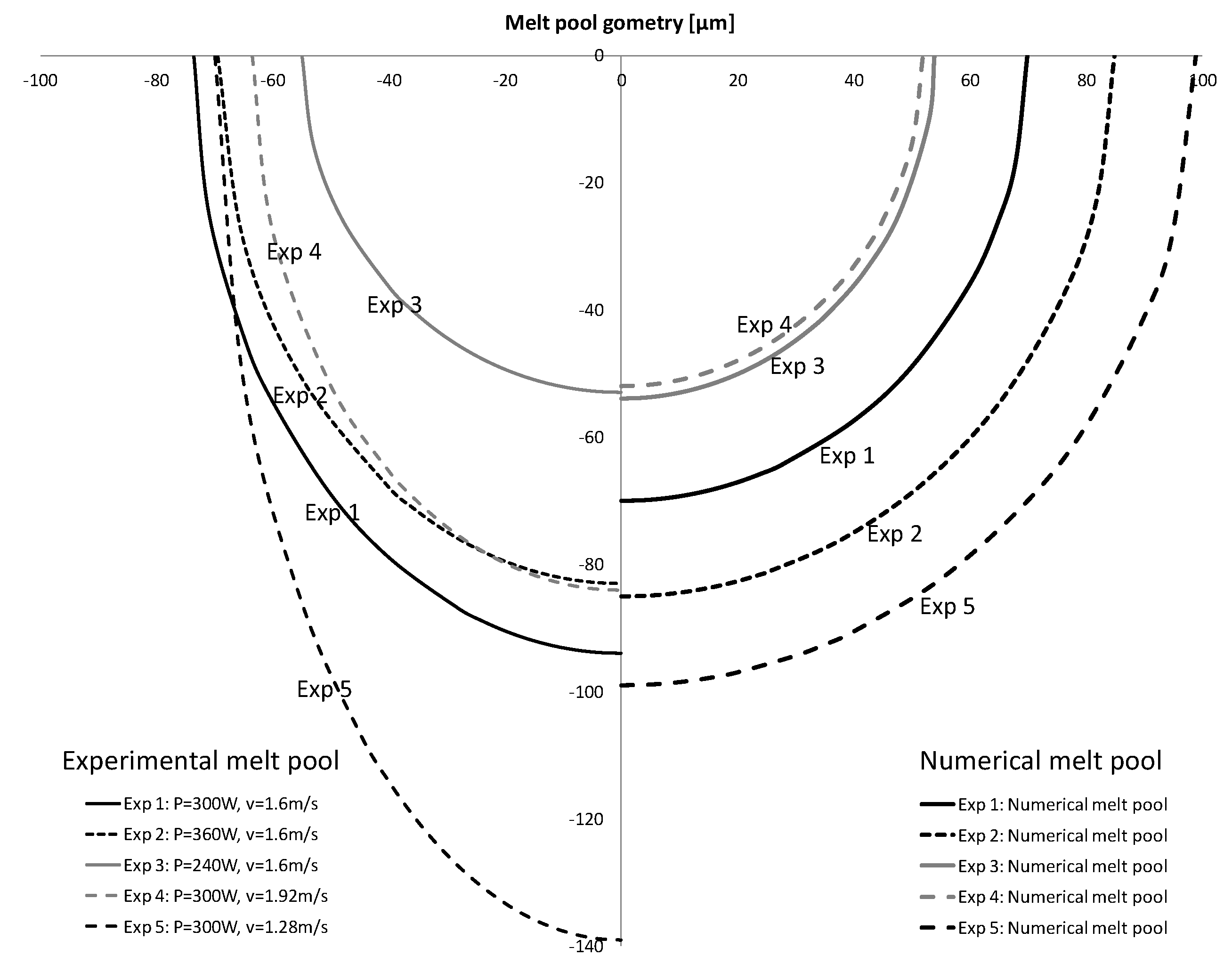
Table 2, we show the results of five experiments for the investigated IN718 alloy, based on the macrographs of a real SLM process, which are shown in
Figure 7. The experiments were performed in the context of the MERLIN project on an SLM 280 HL machine (SLM solutions) with varying scan speed and laser powder [
13]. Further process parameters were kept constant: laser focus diameter
df = 90 μm (Gauss), hatch distance
Δy = 80 μm, and layer thickness
D = 30 μm. The weld pool geometry that resulted in each experiment approximates a cylindrical shape with a varying degree of deviation to the cylindrical shape because of surface (radiation, gas convection, Marangoni effect) and depth effects (conduction to solid substrate, gravity etc). Whereas experiment 1, 2, and 4 proved a marginal deviation from the cylindrical shape, a higher deviation was noticed for higher energy per unit length, i.e., experiment 5, due to the higher laser power and lower scan speed. This phenomenon is known as the keyhole effect during which the laser power density is so high that the metallic material reaches temperatures beyond melting, i.e., it vaporizes. The vaporizing metal reaching the gas state expands creating a keyhole or a capillary penetrating from the surface down to weld depth. As the laser beam moves across the surface, the keyhole follows and creates a typically deep and narrow weld. As long as the laser power is great enough and the travel speed is not excessive, this keyhole will remain open. Process parameter combinations which lead to higher energy per unit length values and, thus, higher weld pool penetration, apart from being energy costly and/or slow, are prone to higher sensitivity to porosity [
29]. In order to achieve process feasibility in SLM, keyhole effects in melt pools are to be avoided, so that overlap with underlying layers and adjacent scan vectors during processing can be attained without failures. For this reason, a cylindrical approach proves to be consistent for modeling melt pool geometries for SLM applications. As the numerical/analytical results (Equation (13)) were obtained under the assumption of cylindrical symmetry (i.e., the cross-section of the melt pool is a semi-circle), for comparison with the experimental results we introduced the equivalent radius
(
Table 1 and
Table 2) (i.e., the radius of the semicircle with an area equal to the area of experimental melt pool;
Figure 7).
An upper bound of the radius of the melt pool was obtained by assuming no conduction, and that all the energy per meter
delivered by the laser was used to melt the powder at the melting temperature
:
where
represents the equivalent radius,
is the laser power and
is the velocity of the laser beam. In
Table 1, we show the experimental data associated with the five experiments, the energy per unit meter delivered by the laser
, the equivalent radius, and the maximum possible (upper bound) radius of molten material that can be achieved, as described by Equation (15). It is easily concluded that only a small fraction of the available laser energy is responsible for the melting of the material. Hence, it is expected that a model that includes conduction would provide better results. Such a model is the model developed in
Section 2 of the paper.
In order to compare the experimental data (
Figure 7) with the results of
Section 2, a number of assumptions/parameters were adopted. As already described in
Section 2, the metal powder, which rests on a metal substrate, is molten by the scanning laser beam and solidifies into a metal deposit fused to the substrate. The density and the thermal material properties of the molten material during the fusion process are significantly different from the metal powder, as presented in
Section 3. Furthermore, we assumed that during the initial stages of melting, the gravity and Marangoni effects can be neglected, hence the process is cylindrically symmetric. Since the conductivity of the metal powder is in the order of 100 times smaller than that of the metal substrate, we assumed that the heat transfer process is controlled by the powder bed, and the heat transfer process is prevalent in the molten pool [
1]. Hence, during the initial melting of the powder, we neglected the presence of the metal substrate and assumed a semi-infinite layer of metal powder. The melting is achieved by a rapidly scanning laser beam of power
moving with velocity
) along the span of a powder-bed, and delivering an amount of energy
per unit length/span [
24]. The energy is conducted through the powder-bed, which was assumed to be a continuum medium. Since the optical or mechanical scanning of the laser beam is much faster than the thermal dynamics in the material, the process can be modeled as a heat source of power
per unit length, distributed simultaneously and evenly along the inner surface
of the molten material (Figures 1 and 2 [
24]), due to irradiation and reflections. As the heat is conducted through, a phase-change takes place. In particular, an interface
is developed at temperature
that separates the molten material (liquid) from the solid material (powder), where
is the melting temperature of the material. Assuming a pure substance, and by ignoring the kinetics of phase change in melting (i.e., the latent heat of fusion
is provided instantaneously), the interface is sharp (
). Furthermore, in order to relate the experimentally obtained
with the
of the numerical model, we defined the characteristic time
and length
of the process. The characteristic parameters are process parameters that were obtained experimentally for a set of experiments performed under similar conditions. They can be obtained by fitting the numerical model (Equation (13)) to the available experimental data (
Table 2), and can be continuously updated in-process to obtain improved values. In order to find an equivalent power per unit length (
) to use in the numerical simulations (Equation (13)) and an activation time (
), we used the characteristic parameters as follows:
where the energy delivered to the powder bed per unit length is equal to
Hence, in the characteristic equation for the eigenvalue
and radius
(Equation (13)), we replaced
with
and
with
. Furthermore, the experimentally-determined melt pool is not circular, hence, for comparison with the numerical results, we used the equivalent radius, described earlier.
Using the data of the experiments and Equation (13) we performed a best fit, and estimated a characteristic time
and length
. The numerically calculated radius is shown in the second to last row of
Table 2. Out of the five sets of data, we obtained an excellent fit for two sets, a reasonable fit for the other two sets, and a poor fit for one data set among experimental and numerical results as illustrated in
Figure 8 and
Figure 9. The fact that our model required two fitted parameters, it was expected to fit at least two sets of data well. Although the fitting (
Table 2) looks promising, a larger amount of experimental data is necessary in order to justify its applicability to an SLM process. For example, we used a far field temperature of
. However, temperature changed during the course of the experiment due to pre-heating, which occurred in the processed layer due the conduction during laser scanning of the adjacent powder (i.e., previously deposited and underlying beads). This was because in actual SLM there is no time for previous deposits and the substrate to cool down to room temperature before the current new bead is processed. Furthermore, the deviation from the circular shape, although it should be avoided for an SLM process, is a dominant effect in the experimental data. Finally, although the power of the laser was known, the total energy delivered to the melt pool was unknown (i.e., the duration of the laser scanning was not reported). Hence, although the results shown in
Table 1 prove that the aforementioned model and assumptions could provide reliable results in a very efficient manner in terms of computation time, further controlled experiments are necessary in order to improve, justify, and extend the applicability of the model.
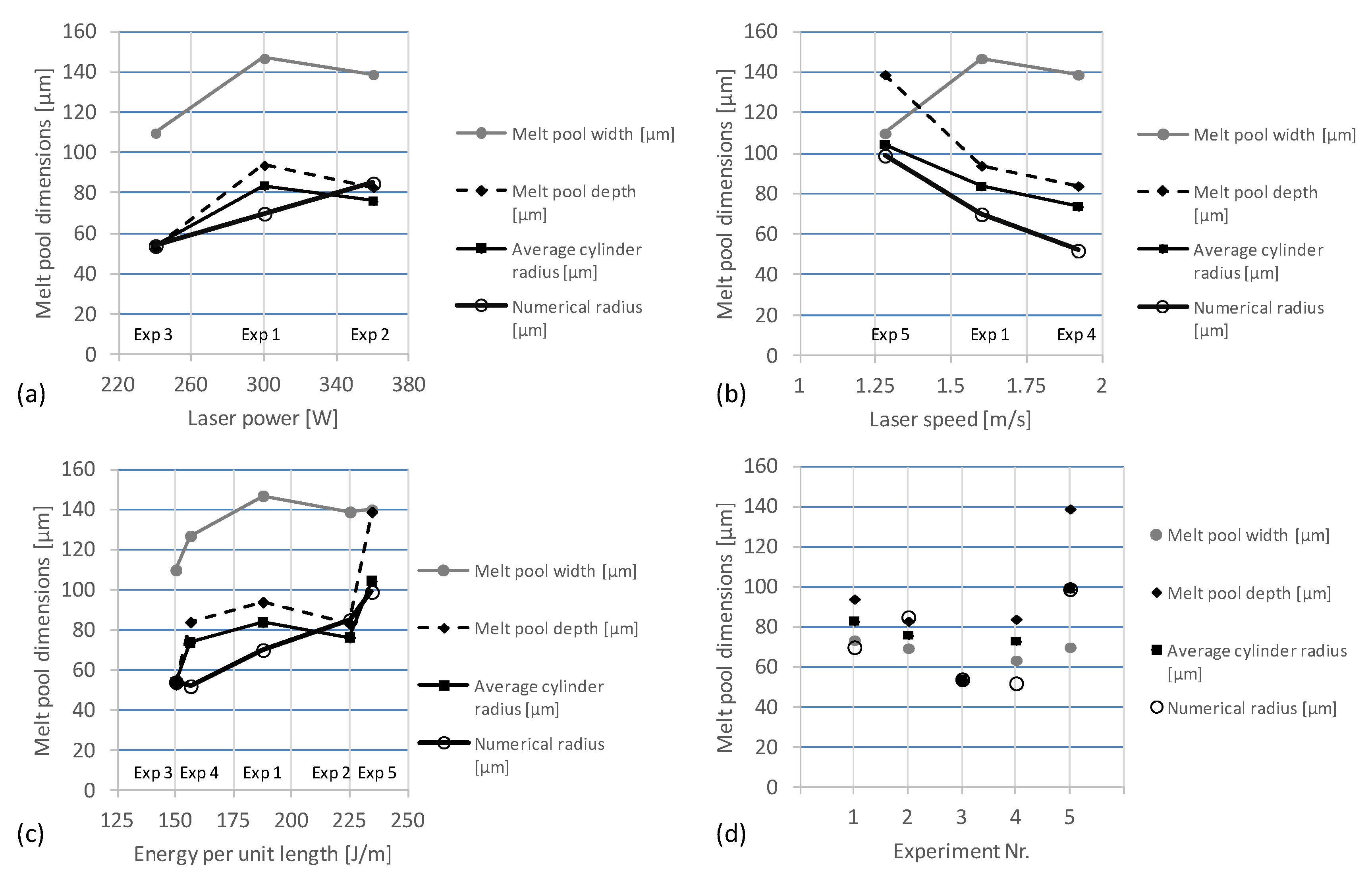
A comprehensive illustration of the results in
Table 2 is provided in
Figure 8. The numerical results of the melt pool cylindrical shape on the right of the figure are compared with the experimental measurements of the melt pool on the left of the figure for all five experiments.
Figure 9 summarizes the numerical and experimental results of the melt pool dimensions in terms of the variation of (a) the laser power, (b) the laser speed, and (c) the energy per unit length. The results for all five experiments (d) demonstrate the deviation of the proposed analytical model compared to real melt pool dimensions.