Properties of Ni-B/B Composite Coatings Produced by Chemical Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
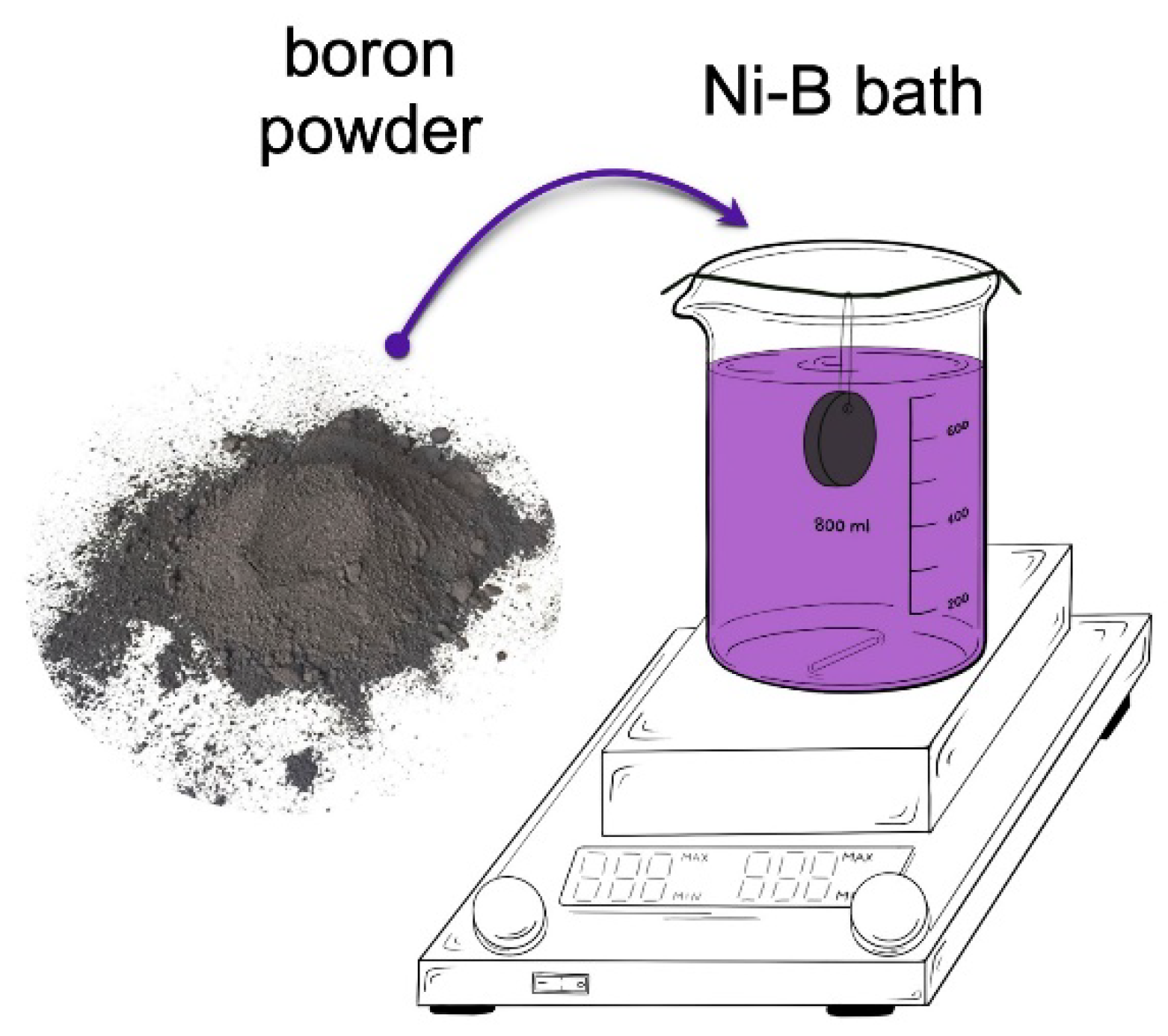
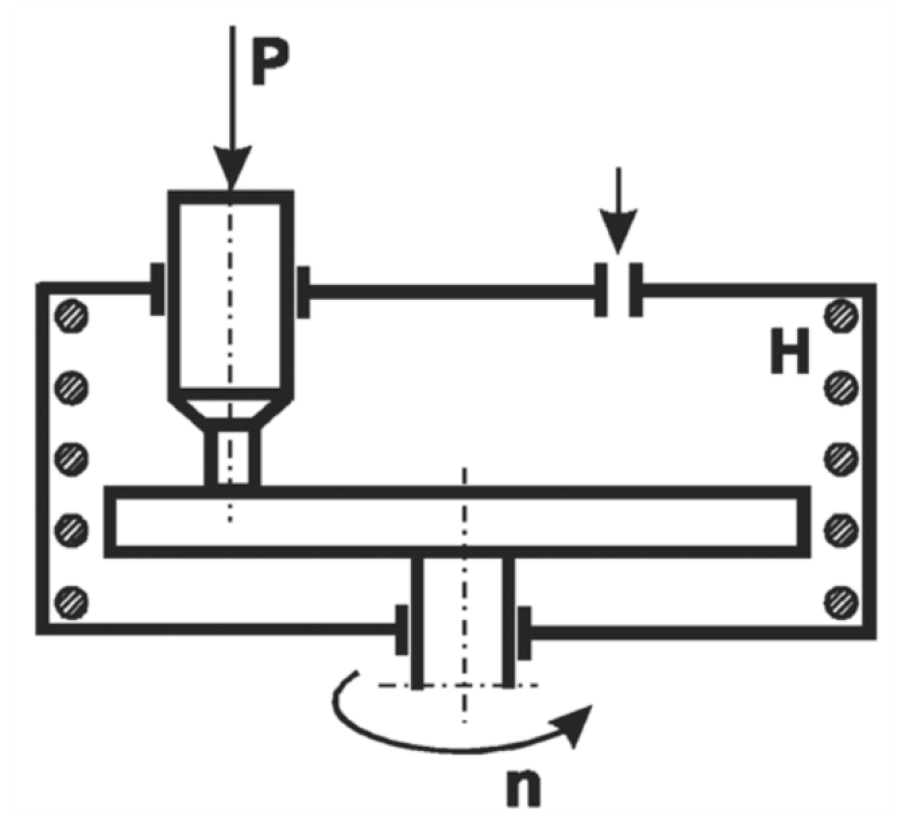
2.2. Methods
3. Results and Discussion
3.1. Characteristics of Boron Powder
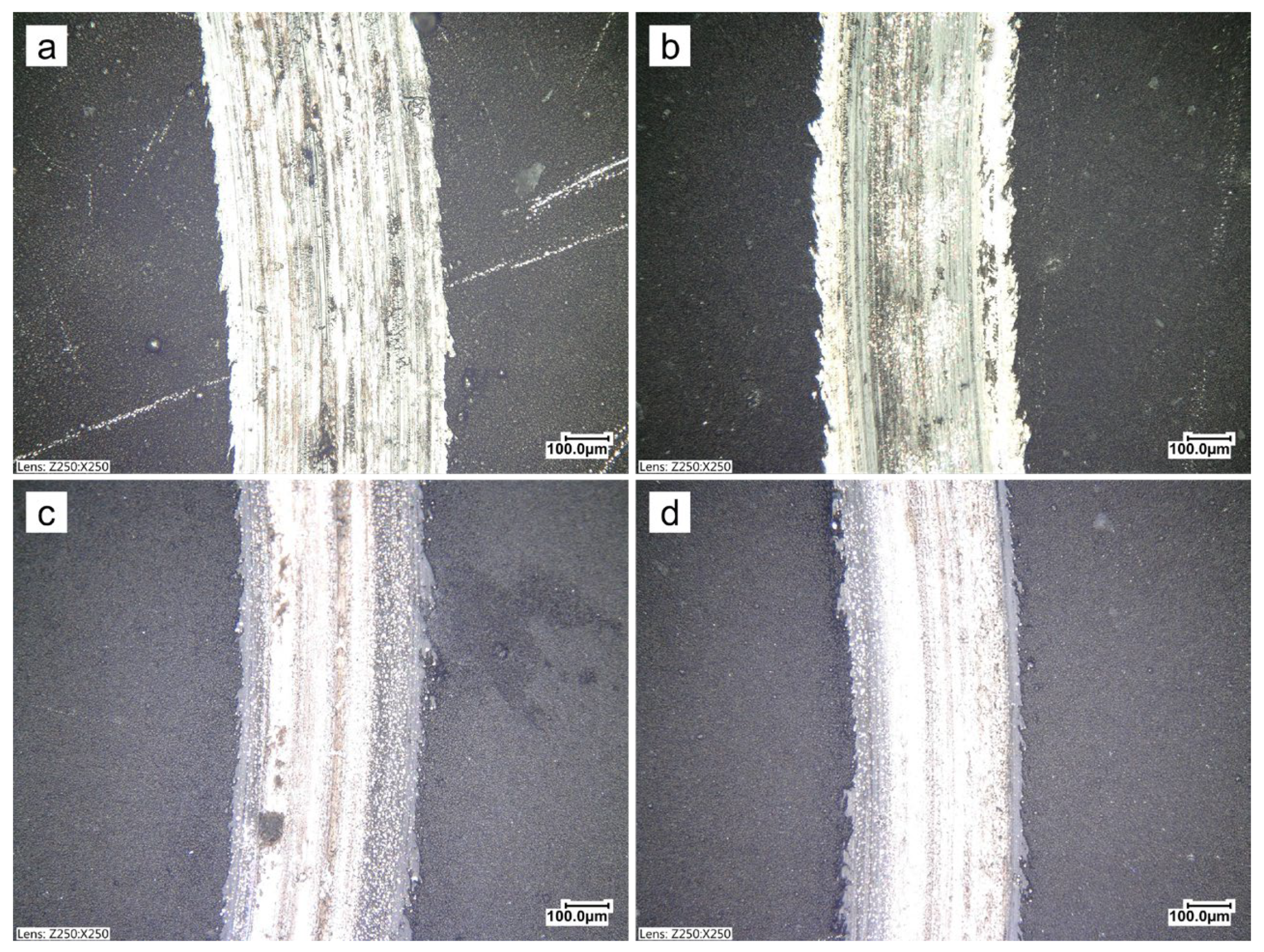
3.2. Structure of the Produced Coatings
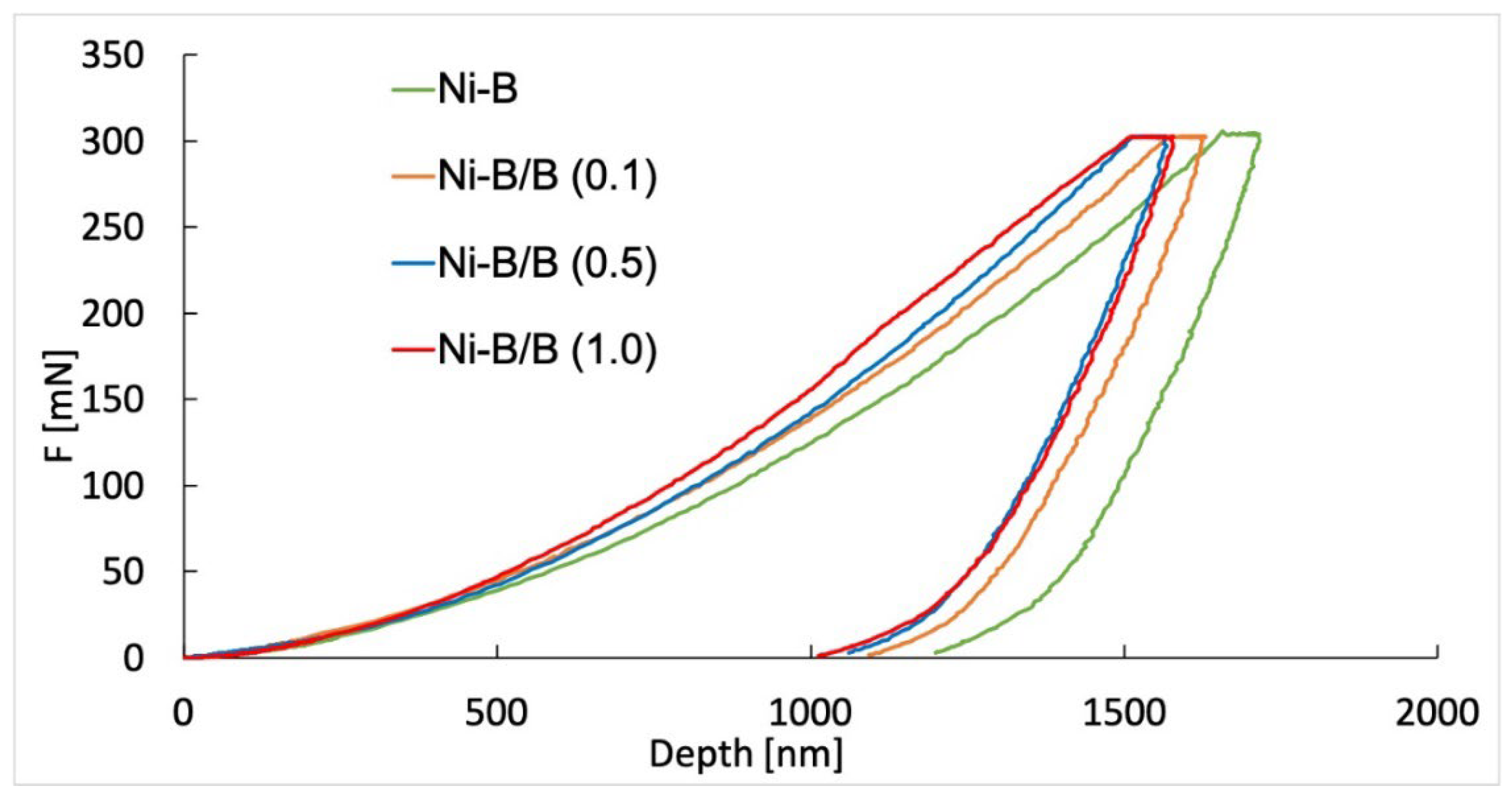
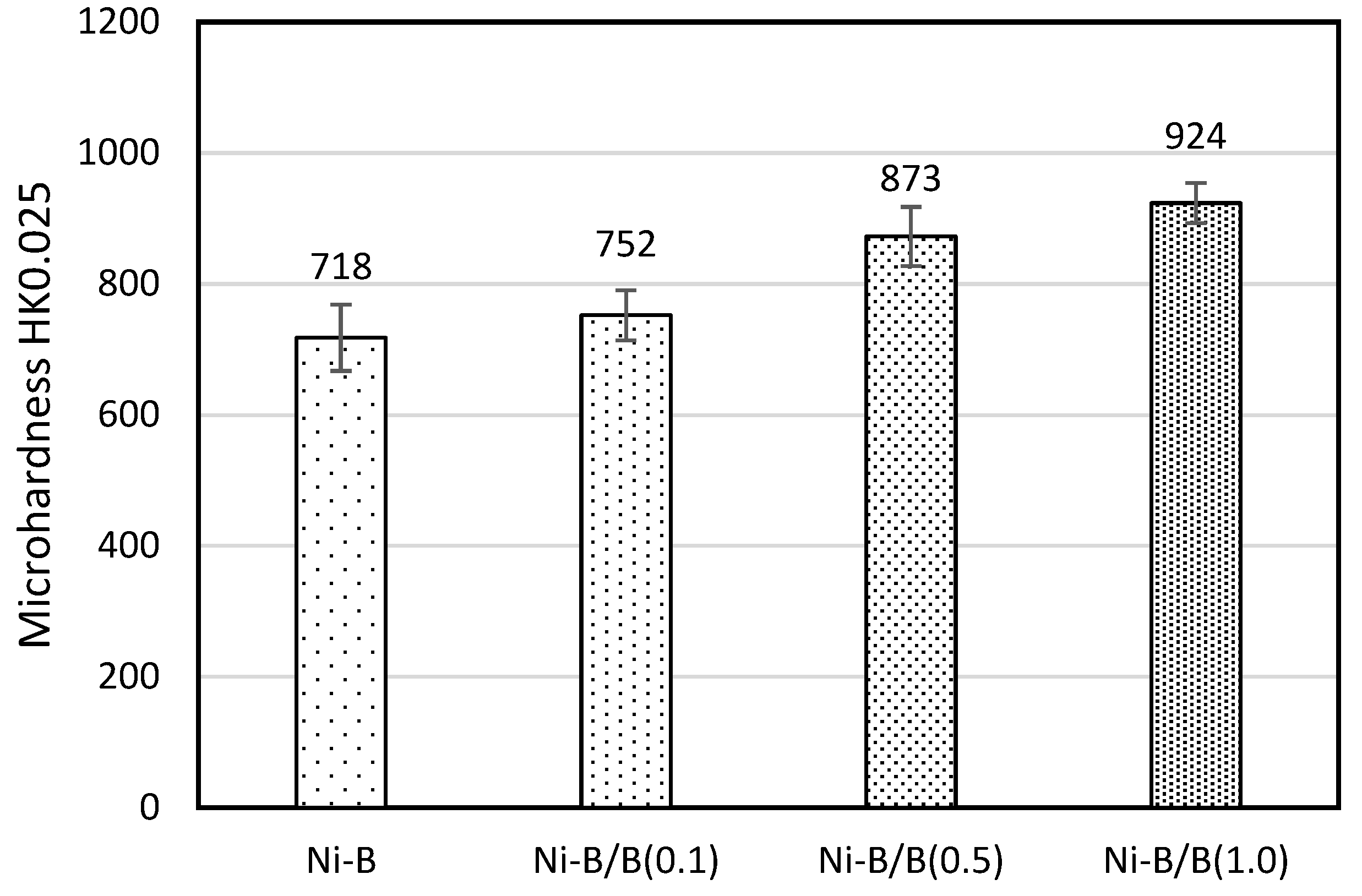
3.3. Mechanical Properties of the Produced Coatings
3.4. Tribological Properties of the Produced Coatings
3.5. Combination of the Produced Coatings with the Substrate Materials
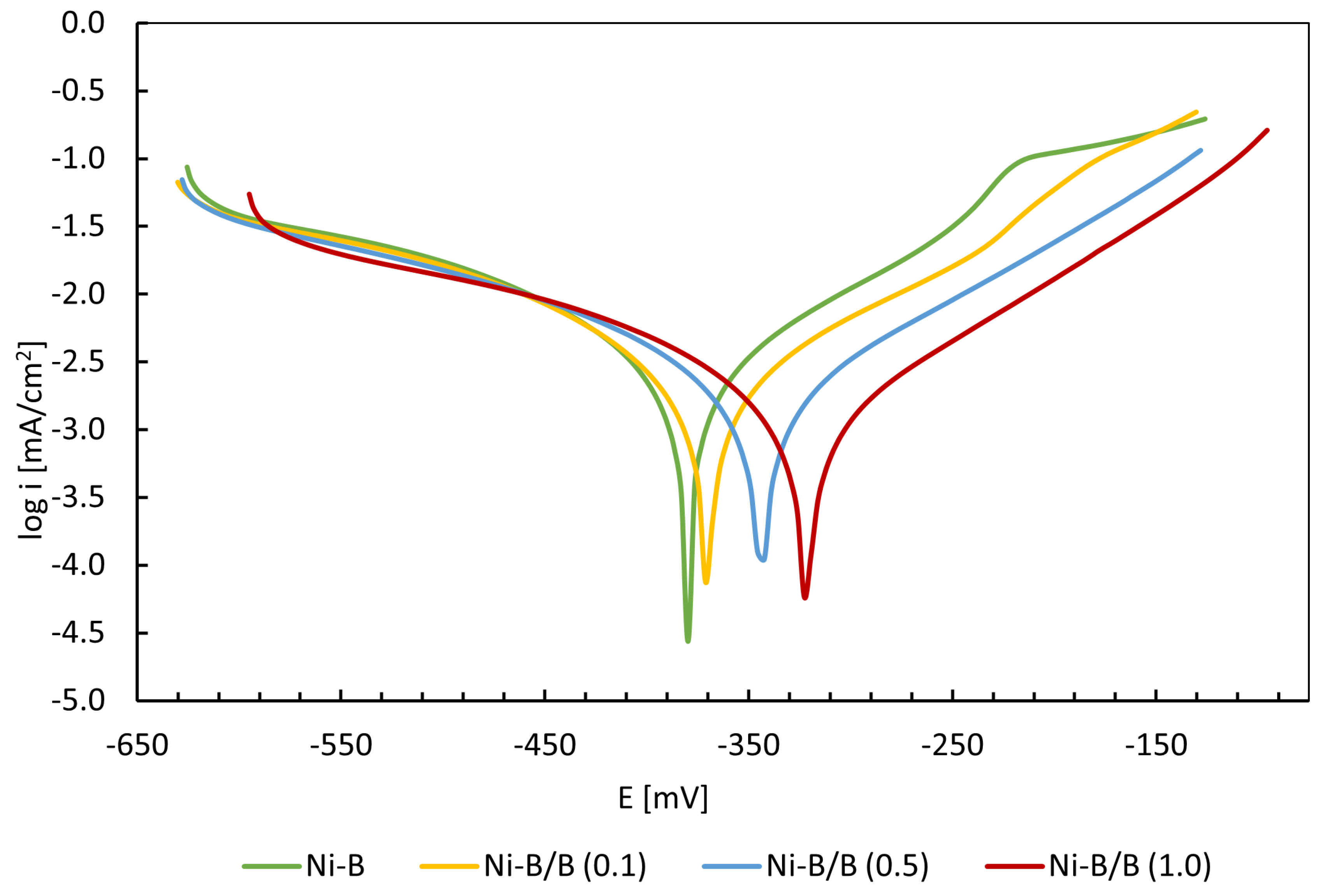
3.6. Corrosion Properties of the Produced Coatings
4. Conclusions
- The average coefficient of friction of the Ni/B-B coating (1.0) is 17% lower compared to the Ni-B coating without embedded particles; for all tested coatings the main failure mechanisms are adhesive wear and abrasive wear.
- The hardness of the Ni-B-B (1.0) coating is higher than the hardness of the Ni-B coating without embedded particles: HK0.025 by 28%, HIT by 17%, and HM by 15%.
- The higher corrosion resistance of the Ni-B/B (1.0) coating compared to the Ni-B coating without embedded particles is visible in the lower values of the corrosion potential (−321 mV) and the corrosion current density (0.85 µA/cm2).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solimani, A.; Meiβner, T.M.; Oskay, C.; Galetz, M.C. Electroless Ni–P coatings on low-Cr steels: A cost-efficient solution for solar thermal applications. Sol. Energy Mater. Sol. Cells 2021, 231, 111312. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.-w.; Wen, C.; Li, S.-z.; Li, J.-f.; Cheng, D.; Bai, J.-y.; Cui, Q.-x.; Zhang, L.-g. Effect of initial deposition behavior on properties of electroless Ni−P coating on ZK60 and ME20 magnesium alloys. Trans. Nonferrous Met. Soc. China 2021, 31, 2307–2322. [Google Scholar] [CrossRef]
- Wojewoda-Budka, J.; Wierzbicka-Miernik, A.; Kwiecień, I.; Valenza, F.; Korneva, A.; Janusz-Skuza, M.; Stan-Glowinska, K.; Guspiel, J.; Bugajska, M. Reactivity with tin and corrosion resistance of electroless Ni-P and Ni-P-Re coatings plated on copper. Electrochim. Acta 2022, 406, 139850. [Google Scholar] [CrossRef]
- Chinchu, K.S.; Riyas, A.H.; Ameen Sha, M.; Geethanjali, C.V.; Saji, V.S.; Shibli, S.M.A. ZrO2–CeO2 assimilated electroless Ni–P anti-corrosion coatings. Surf. Interfaces 2020, 21, 100704. [Google Scholar] [CrossRef]
- Wojewoda-Budka, J.; Wierzbicka-Miernik, A.; Szczerba, M.; Kazimierczak, H.; Kwiecień, I.; Morgiel, J.; Stan-Glowinska, K.; Valenza, F. The effect of Re addition on the thermal stability and structure of Ni–P electroless coatings. Mater. Charact. 2021, 171, 110811. [Google Scholar] [CrossRef]
- Rana, A.R.K.; Farhat, Z. Preparation and tribological characterization of graphene incorporated electroless Ni-P composite coatings. Surf. Coat. Technol. 2019, 369, 334–346. [Google Scholar] [CrossRef]
- Barati, Q.; Hadavi, S.M.M. Electroless Ni-B and composite coatings: A critical review on formation mechanism, properties, applications and future trends. Surf. Interfaces 2020, 21, 10070. [Google Scholar] [CrossRef]
- Bülbül, F. Antibacterial activity of electroless Ni–B coating. Mater. Sci. Technol. 2011, 27, 1540–1546. [Google Scholar] [CrossRef]
- Jończyk, S.; Mazurek, A.; Cieślak, G.; Szawłowski, J.; Trzaska, M. Diffusion boronizing of steel by using the Ni-B alloy coating as a source of boron. Przem. Chem. 2019, 98, 604–609. [Google Scholar]
- Trzaska, M.; Cieślak, G.; Mazurek, A. Corrosion properties of Ni-P and Ni-B alloy coatings produced by chemical method. Ochrona Przed Korozją 2015, 11, 426–429. [Google Scholar] [CrossRef]
- Mazurek, A.; Bartoszek, W.; Cieślak, G.; Gajewska-Midziałek, A.; Oleszak, D.; Trzaska, M. Influence of heat treatment on properties of Ni-B/B composite coatings. Arch. Metall. Mater. 2020, 5, 839–844. [Google Scholar]
- Skowron, J.; Konieczka, K. Occupational Exposure to Chromium (VI) Compounds. Med. Pr. 2015, 66, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Georgiza, E.; Gouda, V.; Vassiliou, P. Production and properties of composite electroless Ni-B-SiC coatings. Surf. Coat. Technol. 2017, 325, 46–61. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Y.; Liu, C.; Gao, W. Microstructure and properties of Ni–B–TiO2 nanocomposite coatings fabricated by electroless plating. Mater. Technol. 2015, 30, A41–A45. [Google Scholar] [CrossRef]
- Niksefat, V.; Ghorbani, M. Mechanical and electrochemical properties of ultrasonic-assisted electroless deposition of Ni–B–TiO2 composite coatings. J. Alloys Compd. 2015, 633, 127–136. [Google Scholar] [CrossRef]
- Cieślak, G.; Trzaska, M. Structure and Properties of Ni-B/Graphene Oxide Composite Coatings Produced by Chemical Reduction Method. J. Mater. Eng. Perform. 2020, 29, 1550–1557. [Google Scholar] [CrossRef]
- Gültekin, D.; Duru, E.; Akbulut, H. Improved wear behaviors of lead-free electroless Ni–B and Ni-B/CeO2 composite coatings. Surf. Coat. Technol. 2021, 422, 12752. [Google Scholar] [CrossRef]
- Mazurek, A.; Cieślak, G.; Bartoszek, W.; Trzaska, M. Abrasion resistance of Ni-B/Si3N4 composite layers produced by electroless method. Arch. Mater. Sci. Eng. 2017, 87, 21–26. [Google Scholar] [CrossRef]
- Cieślak, G.; Mazurek, A.; Bartoszek, W.; Trzaska, M. Corrosion properties of Ni-B/Al2O3 composites layers produced by electroless method. Ochrona Przed Korozją 2017, 60, 215–217. [Google Scholar] [CrossRef]
- Gajewska-Midziałek, A. Wpływ struktury nanokrystalicznych elektrochemicznych powłok kompozytowych Ni-B na wybrane właściwości użytkowe. Ph.D. Thesis, Institute of Precision Mechanics, Warsaw, Poland, 2018. [Google Scholar]
- Das, S.K.; Sahoo, P. Electrochemical impedance spectroscopy of Ni-B coatings and optimization by Taguchi method and Grey relational analysis. Port. Electrochim. Acta 2011, 29, 211–231. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indention experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Cieślak, G.; Trzaska, M.; Betiuk, M. Structure and mechanical properties of nanocrystalline Ni/Cu multilayer coatings produced by the electrocrystallization method. Inz. Pow. 2019, 24, 3–10. [Google Scholar] [CrossRef]
- T-11 Elevated Temperature Pin-on-Disk Tribostestr. Available online: https://www.itee.lukasiewicz.gov.pl/images/karty/tribologia/Karta_T-11_ang.pdf (accessed on 2 August 2023).
- T-11 Elevated Temperature Pin-on-Disk Testing Machine for Tribotesting of Lubricants and Engineering Materials. Available online: https://www.tribologia.eu/ptt/inst/rad/T-11_en.pdf (accessed on 2 August 2023).
- Vitry, V.; Hastir, J.; Megret, A.; Yazdani, S.; Yunacti, M.; Bonin, L. Recent advances in electroless nickel-boron coatings. Surf. Coat. Technol. 2022, 429, 12793. [Google Scholar] [CrossRef]
- Algul, H.; Uysal, M.; Alp, A. A comparative study on morphological, mechanical and tribological properties of electroless NiP, NiB and NiBP coatings. Appl. Surf. Sci. Adv. 2021, 4, 100089. [Google Scholar] [CrossRef]
- Ürdem, S.; Duru, E.; Algül, H.; Uysal, M.; Akbulut, H. Evaluation of high temperature tribological behavior of electroless deposited NiB–Al2O3 coating. Wear 2021, 482–483, 20396. [Google Scholar] [CrossRef]
- Nemane, V.; Chatterjee, S. Evaluation of microstructural, mechanical, and tribological characteristics of Ni-B-W-SiC electroless composite coatings involving multi-pass scratch test. Mater. Charact. 2021, 180, 11141. [Google Scholar] [CrossRef]
- Baskaran, I.; Sakthi Kumar, R.; Sankara Narayanan, T.S.N.; Stephen, A. Formation of electroless Ni–B coatings using low temperature bath and evaluation of their characteristic properties. Surf. Coat. Technol. 2006, 200, 6888–6894. [Google Scholar] [CrossRef]
- Vitry, V.; Bonin, L. Increase of boron content in electroless nickel-boron coating by modification of plating conditions. Surf. Coat. Technol. 2017, 311, 164–171. [Google Scholar] [CrossRef]
- Khodaei, M.; Mohammad Gholizadeh, A. SiC nanoparticles incorporation in electroless NiP-Graphene oxide nanocomposite coatings. Ceram. Int. 2021, 47, 25287–25295. [Google Scholar] [CrossRef]
- Dilek, S.; Algul, H.; Akyol, A.; Alp, A.; Akbulut, H.; Uysal, M. Pulse electro co-deposition of submicron-sized TiC reinforced Ni–W coatings: Tribological and corrosion properties. J. Asian Ceram. Soc. 2021, 9, 673–685. [Google Scholar] [CrossRef]
- Eranegh, F.A.; Azadi, M.; Tavakoli, H. Effect of SiO2 Nanoparticles Addition on Tribological and Electrochemical Behaviors of Ni-P-MoS2 Multi-Component Coatings after Heat Treatment. Surf. Eng. Appl. Elect. 2020, 56, 171–183. [Google Scholar] [CrossRef]
- Natarajan, S.; Narayanasamy, R.; Kumaresh Babu, S.P.; Dinesh, G.; Anil Kumar, B.; Sivaprasad, K. Sliding wear behaviour of Al 6063/TiB2 in situ composites at elevated temperatures. Mater. Des. 2009, 30, 2521–2531. [Google Scholar] [CrossRef]
- Chronowska-Przywara, K.; Kot, M. Wpływ parametrów badań na deformacje i pękanie układu powłoka-podłoże w wyniku próby zarysowania. Tribologia 2014, 2, 19–26. [Google Scholar]
- Li, C.; Piao, Y.; Zhang, F.; Zhang, Y.; Hu, Y.; Wang, Y. Understand anisotropy dependence of damage evolution and material removal during nanoscratch of MgF2 single crystals. Int. J. Extrem. Manuf. 2023, 5, 015101. [Google Scholar] [CrossRef]





| Element | Mass (%) | Atom (%) |
|---|---|---|
| B | 97.77 | 98.48 |
| O | 2.23 | 1.52 |
| Concentration of Boron Particles in the Bath (g/dm3) | Weight of Ni-B/B Coating (g) | Mass of Embedded B Particles in Ni-B/B Coating (g) | Content in the Coating (%) |
|---|---|---|---|
| 0 | 0.3345 | 0 | 0 |
| 0.1 | 0.3744 | 0.0026 | 0.69 |
| 0.5 | 0.2689 | 0.0052 | 1.93 |
| 1.0 | 0.2687 | 0.0101 | 3.76 |
| Coating | Surface Roughness Ra (µm) |
|---|---|
| Ni-B | 0.707 |
| Ni-B/B (0.1) | 0.700 |
| Ni-B/B (0.5) | 0.591 |
| Ni-B/B (1.0) | 0.460 |
| Element | Result (ppm) | SD (ppm) | RSD (%) |
|---|---|---|---|
| B | 71,536.67 | 895.40 | 1.25 |
| Pb | 9090.33 | 376.72 | 4.14 |
| Ni | 932,400.00 | 3500.00 | 0.38 |
| Coating | Microhardness | Depth (nm) | Elastic Modulus Eit (GPa) | KH (%) | |
|---|---|---|---|---|---|
| HIT (MPa) | HM (MPa) | ||||
| Ni-B | 5454 (±47) | 3919 (±47) | 1711 (±11.1) | 130 (±6.1) | 30 |
| Ni-B/B (0.1) | 6194 (±105) | 4289 (±58) | 1636 (±12.9) | 128 (±1.55) | 33 |
| Ni-B/B (0.5) | 6869 (±94) | 4812 (±82) | 1544 (±12.8) | 150 (±4.4) | 32 |
| Ni-B/B (1.0) | 6408 (±195) | 4496 (±103) | 1595 (±19.2) | 140 (±4.6) | 36 |
| Coating | Average Friction Coefficient |
|---|---|
| Ni-B | 0.8277 ± 0.2019 |
| Ni-B/B (0.1) | 0.7618 ± 0.1037 |
| Ni-B/B (0.5) | 0.7203 ± 0.1244 |
| Ni-B/B (1.0) | 0.6879 ± 0.1250 |
| Coating | Ecor (mV) | Icor (µA/cm2) |
|---|---|---|
| Ni-B | −380 | 2.2 |
| Ni-B/B (0.1) | −370 | 1.85 |
| Ni-B/B (0.5) | −345 | 1.42 |
| Ni-B/B (1.0) | −321 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewska-Midziałek, A.; Cieślak, G.; Gostomska, M.; Ciciszwili, T.; Skroban, K.; Dąbrowski, A.; Pęśko, E.; Wojda, E.; Głowacki, M.; Kapuścińska, A.; et al. Properties of Ni-B/B Composite Coatings Produced by Chemical Reduction. Coatings 2023, 13, 1535. https://doi.org/10.3390/coatings13091535
Gajewska-Midziałek A, Cieślak G, Gostomska M, Ciciszwili T, Skroban K, Dąbrowski A, Pęśko E, Wojda E, Głowacki M, Kapuścińska A, et al. Properties of Ni-B/B Composite Coatings Produced by Chemical Reduction. Coatings. 2023; 13(9):1535. https://doi.org/10.3390/coatings13091535
Chicago/Turabian StyleGajewska-Midziałek, Anna, Grzegorz Cieślak, Marta Gostomska, Tinatin Ciciszwili, Katarzyna Skroban, Adrian Dąbrowski, Edyta Pęśko, Edyta Wojda, Michał Głowacki, Anna Kapuścińska, and et al. 2023. "Properties of Ni-B/B Composite Coatings Produced by Chemical Reduction" Coatings 13, no. 9: 1535. https://doi.org/10.3390/coatings13091535
APA StyleGajewska-Midziałek, A., Cieślak, G., Gostomska, M., Ciciszwili, T., Skroban, K., Dąbrowski, A., Pęśko, E., Wojda, E., Głowacki, M., Kapuścińska, A., & Trzaska, M. (2023). Properties of Ni-B/B Composite Coatings Produced by Chemical Reduction. Coatings, 13(9), 1535. https://doi.org/10.3390/coatings13091535





