Diagnosis and Treatment Pathway of MDR/RR-TB in Taizhou, Zhejiang Province, China
Abstract
1. Introduction
2. Materials and Methods
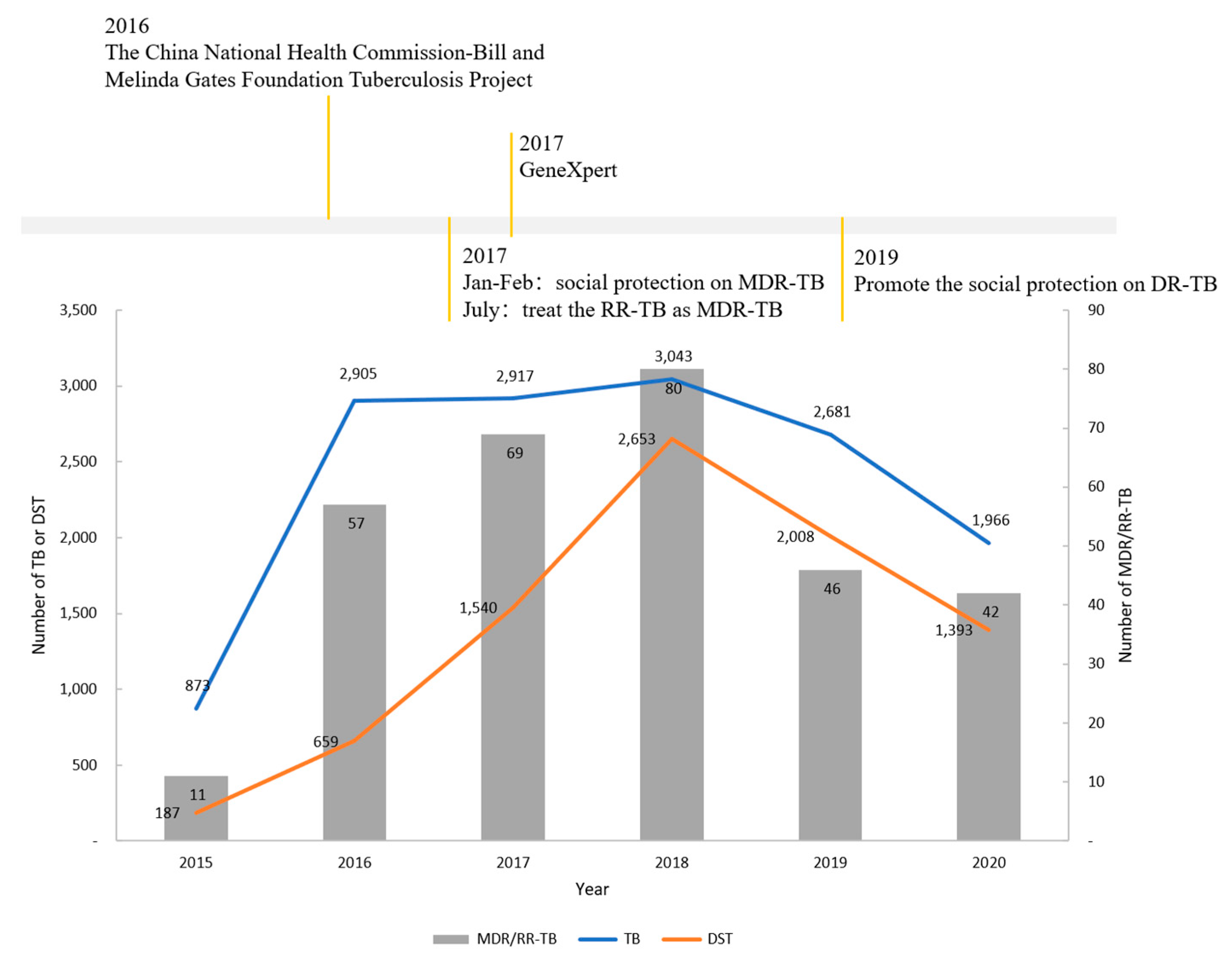
2.1. Study Setting
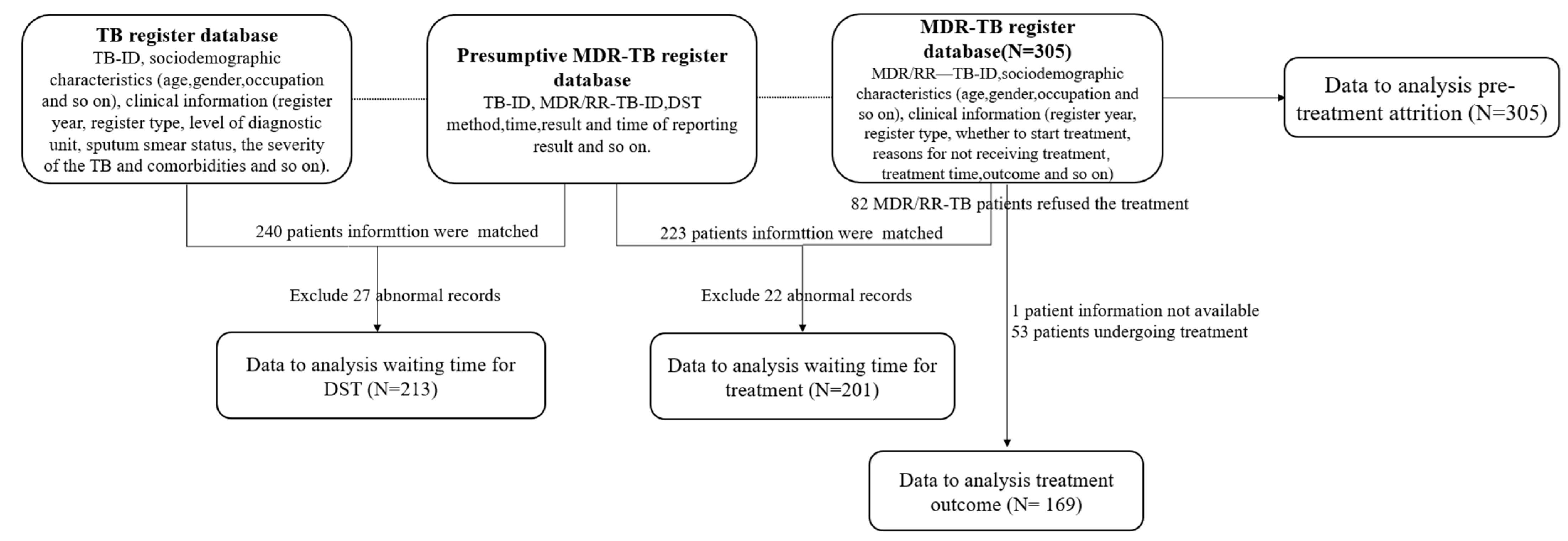
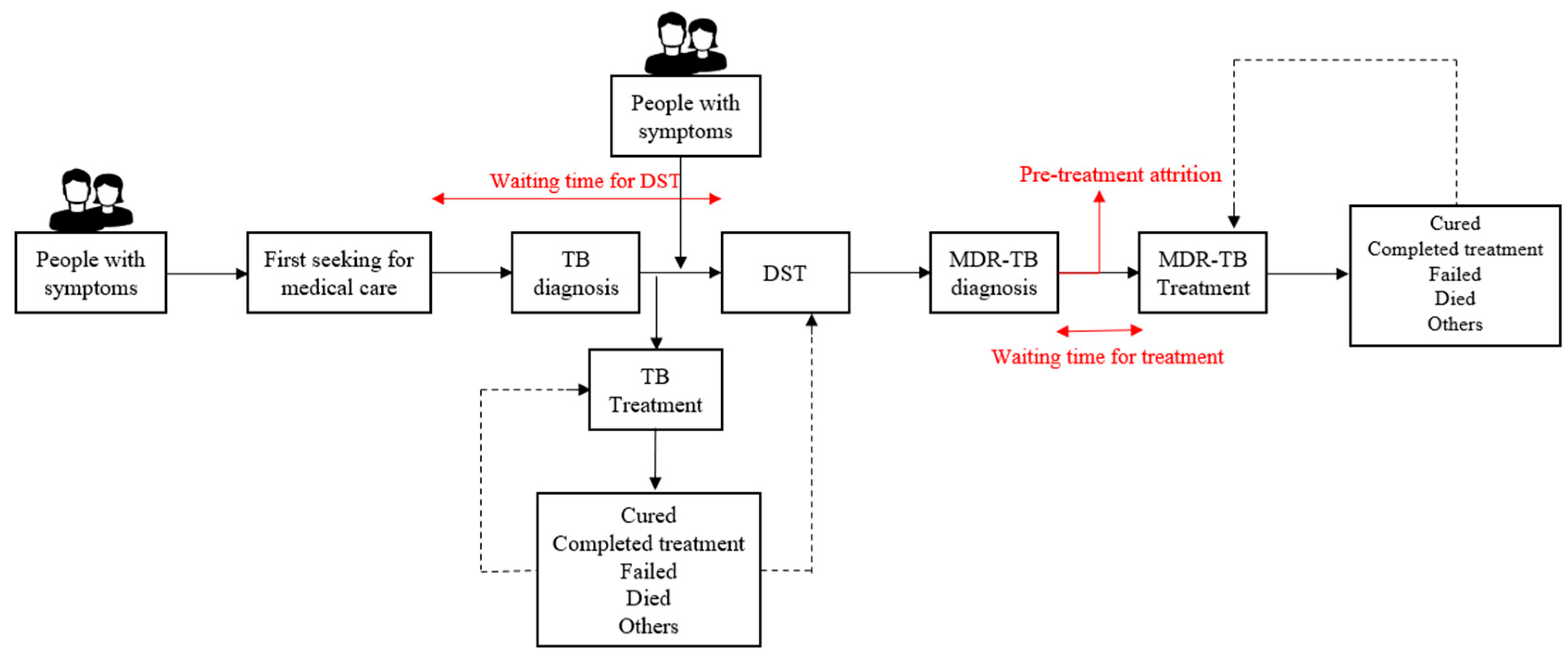
2.2. Data Collection and Definition
2.3. Statistical Analysis
3. Results
3.1. Factors Associated with Waiting Time for DST
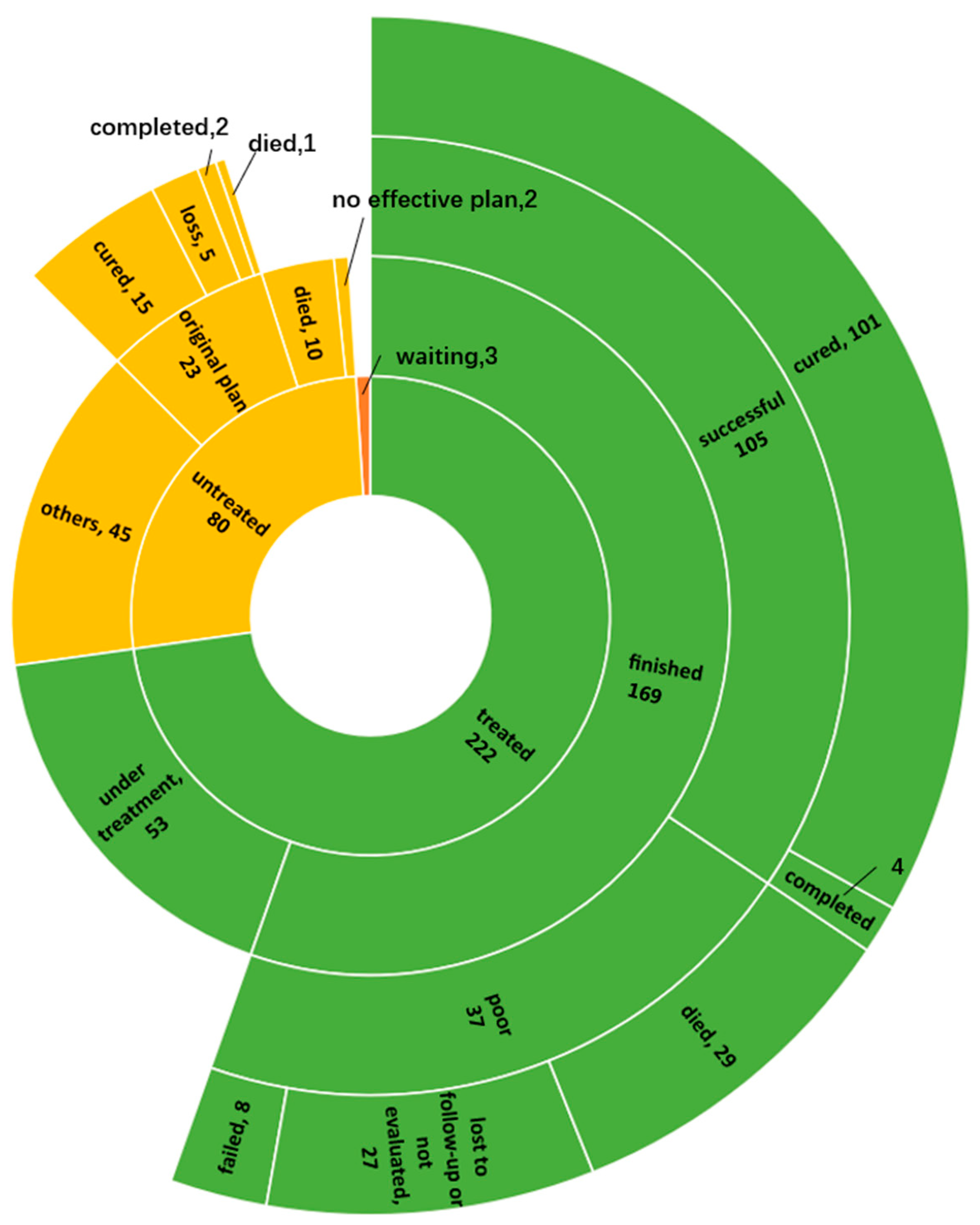
3.2. Factors Associated with Pre-Treatment Attrition
3.3. Factors Associated with Time of Waiting for Treatment
3.4. Treatment Outcome and Associated Factors
4. Discussion
4.1. Waiting Time for DST was Still Long
4.2. Pre-Treatment Attrition was Still High
4.3. Treatment Process Needs to Be Optimized
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2022; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 9 January 2023).
- World Health Organization. Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf?ua=1 (accessed on 20 November 2020).
- Xu, C.; Li, R.; Shewade, H.D.; Jeyashree, K.; Ruan, Y.; Zhang, C.; Wang, L.; Zhang, H. Attrition and delays before treatment initiation among patients with MDR-TB in China (2006-13): Magnitude and risk factors. PLoS ONE 2019, 14, e0214943. [Google Scholar] [CrossRef] [PubMed]
- Chadha, S.S.; Bn, S.; Reddy, K.; Jaju, J.; Ph, V.; Rao, S.; Parmar, M.; Satyanarayana, S.; Sachdeva, K.S.; Wilson, N.; et al. Operational Challenges in Diagnosing Multi-Drug Resistant TB and Initiating Treatment in Andhra Pradesh, India. PLoS ONE 2011, 6, e26659. [Google Scholar] [CrossRef] [PubMed]
- Wakjira, M.K.; Sandy, P.T.; Mavhandu-Mudzusi, A.H. Treatment outcomes of patients with MDR-TB and its determinants at referral hospitals in Ethiopia. PLoS ONE 2022, 17, e0262318. [Google Scholar] [CrossRef] [PubMed]
- Shewade, H.D.; Nair, D.; Klinton, J.S.; Parmar, M.; Lavanya, J.; Murali, L.; Gupta, V.; Tripathy, J.P.; Swaminathan, S.; Kumar, A.M. Low pre-diagnosis attrition but high pre-treatment attrition among patients with MDR-TB: An operational research from Chennai, India. J. Epidemiol. Glob. Health 2017, 7, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Li, H.; Li, S.; Walley, J.; Zou, G.; Zhang, Z.; Wei, X. Diagnostic and treatment delays of multidrug-resistant tuberculosis before initiating treatment: A cross-sectional study. Trop. Med. Int. Health 2015, 20, 1431–1437. [Google Scholar] [CrossRef]
- Htun, Y.M.; Khaing, T.M.M.; Aung, N.M.; Yin, Y.; Myint, Z.; Aung, S.T.; Soonthornworasiri, N.; Silachamroon, U.; Kasetjaroen, Y.; Kaewkungwal, J. Delay in treatment initiation and treatment outcomes among adult patients with multidrug-resistant tuberculosis at Yangon Regional Tuberculosis Centre, Myanmar: A retrospective study. PLoS ONE 2018, 13, e0209932. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Z.; Shen, X.; Wu, J.; Wu, Z.; Xu, B. Time to Multidrug-Resistant Tuberculosis Treatment Initiation in Association with Treatment Outcomes in Shanghai, China. Antimicrob. Agents Chemother. 2018, 62, 4. [Google Scholar] [CrossRef]
- Hoang, T.T.T.; Nguyen, N.V.; Dinh, S.N.; Nguyen, H.B.; Cobelens, F.; Thwaites, G.; Nguyen, H.T.; Nguyen, A.T.; Wright, P.; Wertheim, H.F.L. Challenges in detection and treatment of multidrug resistant tuberculosis patients in Vietnam. BMC Public Health 2015, 15, 980. [Google Scholar] [CrossRef]
- Eliseev, P.; Balantcev, G.; Nikishova, E.; Gaida, A.; Bogdanova, E.; Enarson, N.; Ornstein, T.; Detjen, A.; Dacombe, R.; Gospodarevskaya, E.; et al. The Impact of a Line Probe Assay Based Diagnostic Algorithm on Time to Treatment Initiation and Treatment Outcomes for Multidrug Resistant TB Patients in Arkhangelsk Region, Russia. PLoS ONE 2016, 11, e0152761. [Google Scholar] [CrossRef]
- Long, Q.; Guo, L.; Jiang, W.; Huan, S.; Tang, S. Ending tuberculosis in China: Health system challenges. Lancet Public Health 2021, 6, e948–e953. [Google Scholar] [CrossRef]
- Jiang, W.; Peng, Y.; Wang, X.; Elbers, C.; Tang, S.; Huang, F.; Chen, B.; Cobelens, F. Policy changes and the screening, diagnosis and treatment of drug-resistant tuberculosis patients from 2015 to 2018 in Zhejiang Province, China: A retrospective cohort study. BMJ Open 2021, 11, e047023. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Xia, H.; Zhang, Z.; Li, J.; Dong, Y.; Li, Q.; Ou, X.; Song, Y.; Wang, Y.; O’Brien, R.; et al. Multicenter Evaluation of Genechip for Detection of Multidrug-Resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 2013, 51, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Yagui, M.; Perales, M.T.; Asencios, L.; Vergara, L.; Suarez, C.; Yale, G.; Salazar, C.; Saavedra, M.; Shin, S.; Ferrousier, O.; et al. Timely diagnosis of MDR-TB under program conditions: Is rapid drug susceptibility testing sufficient? Int. J. Tuberc. Lung Dis. 2006, 10, 838–843. [Google Scholar] [PubMed]
- Boehme, C.C.; Nicol, M.P.; Nabeta, P.; Michael, J.S.; Gotuzzo, E.; Tahirli, R.; Gler, M.T.; Blakemore, R.; Worodria, W.; Gray, C.; et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet 2011, 377, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.; Kang, H.; Kwon, Y.-S.; Yim, J.-J.; Shim, T.S. Impact of Molecular Drug Susceptibility Testing on the Time to Multidrug-resistant Tuberculosis Treatment Initiation. J. Korean Med. Sci. 2020, 35, e284. [Google Scholar] [CrossRef] [PubMed]
- Cox, H.; Dickson-Hall, L.; Ndjeka, N.; Hoog, A.V.; Grant, A.; Cobelens, F.; Stevens, W.; Nicol, M. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: A retrospective cohort study. PLOS Med. 2017, 14, e1002238. [Google Scholar] [CrossRef] [PubMed]
- Letter from Taizhou Center for Disease Control and Prevention on Feedback on the Tuberculosis Prevention and Control Work of the City in 2021; Centers for Disease Control and Prevention: Taizhou, China, 2022.
- Chinese Society of Tuberculosis of Chinese Medical Association. Chinese expert consensus on multidrug-resistant tuberculosis and Rifampicin-resistant tuberculosis treatment. Chin. J. Tuberc. Respir. Dis. 2019, 42, 733–749. [Google Scholar]
- Zhang, H.; Cheng, J.; Qu, Y.; Huang, F.; Wang, N.; Huan, S. Validation, demonstration, and promotion of the “Three Technical Innovations Plus Health System Strengthening” comprehensive tuberculosis prevention and control model: The China National Health Commission-Bill and Melinda Gates Foundation Tuberculosis Project. Chin. J. Tuberc. 2021, 43, 757–760. [Google Scholar]
- World Health Organization. Definitions and Reporting Framework for Tuberculosis; WHO: Geneva, Switzerland, 2021; Available online: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf?ua1 (accessed on 16 January 2022).
- Kipiani, M.; Graciaa, D.S.; Buziashvili, M.; Darchia, L.; Avaliani, Z.; Tabagari, N.; Mirtskhulava, V.; Kempker, R.R. Xpert MTB/RIF use is associated with earlier treatment initiation and culture conversion among patients with sputum smear-negative multidrug-resistant tuberculosis. Open Forum Infect. Dis. 2021, 8, ofab551. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, C.-F.; Wang, J.; Zheng, W.-B.; Lu, L.-Z.; Liu, S.-J.; Bao, J. Direct tuberculosis drug susceptibility testing: Time-saving and cost-effective in detecting MDR-TB. Int. J. Tuberc. Lung Dis. 2016, 20, 323–328. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Petrosyan, D.; Peng, C.-T. Cross-countries migratory workers and tuberculosis: Lessons from Armenia. J. Formos. Med. Assoc. 2017, 116, 823–824. [Google Scholar] [CrossRef] [PubMed]
- The People’s Republic of China National Health and Family Planning Commission. Diagnosis of Pulmonary Tuberculosis. Beijing, China, 2017. Available online: www.nhc.gov.cn/ewebeditor/uploadfile/2017/12/20171212154852389.pdf (accessed on 16 January 2022).
- Azeez, A.; Ndege, J.; Mutambayi, R. Associated factors with unsuccessful tuberculosis treatment outcomes among tuberculosis/HIV coinfected patients with drug-resistant tuberculosis. Int. J. Mycobacteriology 2018, 7, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K. Clinical observation of 24 cases of pneumoconiosis complicated with tuberculosis under initial treatment. J. Clin. Med. Liter. 2017, 4, 4777–4780. [Google Scholar] [CrossRef]
- Liao, X. Influencing factors and intervention measures of treatment compliance in patients with multi-drug resistant pulmonary tuberculosis. J. Integr. Chin. Western Med. 2019, 29, 141–142. [Google Scholar] [CrossRef]
- Xu, X. Health Economics Evaluation of Drug-Resistant Tuberculosis; Zhejiang University: Zhejiang, China, 1 May 2006. [Google Scholar]
- Shewade, H.D.; Shringarpure, K.S.; Parmar, M.; Patel, N.; Kuriya, S.; Shihora, S.; Ninama, N.; Gosai, N.; Khokhariya, R.; Popat, C.; et al. Delay and attrition before treatment initiation among MDR-TB patients in five districts of Gujarat, India. Public Health Action 2018, 8, 59–65. [Google Scholar] [CrossRef]
- Sidamo, T.; Shibeshi, W.; Yimer, G.; Aklillu, E.; Engidawork, E. Explorative Analysis of Treatment Outcomes of Levofloxacin- and Moxifloxacin-Based Regimens and Outcome Predictors in Ethiopian MDR-TB Patients: A Prospective Observational Cohort Study. Infect. Drug Resist. 2021, 14, 5473–5489. [Google Scholar] [CrossRef]
- Riccardi, N.; Saderi, L.; Borroni, E.; Tagliani, E.; Cirillo, D.M.; Marchese, V.; Matteelli, A.; Piana, A.; Castellotti, P.; Ferrarese, M.; et al. Therapeutic strategies and outcomes of MDR and pre-XDR-TB in Italy: A nationwide study. Int. J. Tuberc. Lung Dis. 2021, 25, 395–399. [Google Scholar] [CrossRef]
- Feng, Y. Health Economics Evaluation of Drug Resistant Tuberculosis Evaluation of Rapid Diagnosis of Drug Resistant Tuberculosis and Analysis of Therapeutic Effect; Nanjing Meidical University: Nanjing, China, 1 May 2014. [Google Scholar]
- Alsultan, A.; Peloquin, C.A. Therapeutic Drug Monitoring in the Treatment of Tuberculosis: An Update. Drugs 2014, 74, 839–8554. [Google Scholar] [CrossRef]
- Lv, L.; Li, T.; Xu, K.; Shi, P.; He, B.; Kong, W.; Wang, J.; Sun, J. Sputum bacteriology conversion and treatment outcome of patients with multidrug-resistant tuberculosis. Infect. Drug Resist. 2018, 11, 147–154. [Google Scholar] [CrossRef]
- Huang, F.; Xia, Y.; Chen, H.; Wang, N.; Du, X.; Chen, W.; Li, T.; Huan, S.; Sun, M.; Chen, M.; et al. The impact of the COVID-19 epidemic on tuberculosis control in China. Lancet Reg. Health West Pac. 2020, 3, 100032. [Google Scholar]
| Variable | No. | % | |
|---|---|---|---|
| Age(years) | ≤44 | 101 | 33.1 |
| 45~59 | 100 | 32.8 | |
| ≥60 | 104 | 34.1 | |
| Gender | Male | 218 | 71.5 |
| Female | 87 | 28.5 | |
| Occupation | Worker | 38 | 12.5 |
| Housekeeping or unemployment | 53 | 17.4 | |
| Farmer/fisherman | 182 | 59.7 | |
| Others | 32 | 10.5 | |
| Residency status | Residents | 219 | 71.8 |
| Non-residents | 86 | 28.2 | |
| Distance to the hospital | Near | 96 | 31.5 |
| Moderate | 107 | 35.1 | |
| Remote | 102 | 33.4 | |
| Register year | After 2018 | 168 | 55.1 |
| Before 2018 | 137 | 44.9 | |
| Patient types | New case | 169 | 55.4 |
| Recurrent | 109 | 35.7 | |
| Others | 27 | 8.9 | |
| Level of diagnostic unit | Municipal | 18 | 5.9 |
| County-level | 222 | 72.8 | |
| NA | 65 | 21.3 | |
| Baseline sputum smear | Positive | 251 | 82.3 |
| Negative | 54 | 17.7 | |
| Drug-resistant test | Traditional | 172 | 56.4 |
| Xpert | 133 | 43.6 | |
| Drug susceptibility | MDR | 216 | 70.8 |
| RR | 76 | 24.9 | |
| Others | 13 | 4.3 | |
| Severity | No | 195 | 63.9 |
| Yes | 45 | 14.8 | |
| NA | 65 | 21.3 | |
| Comorbidity | No | 183 | 60.0 |
| Pneumonoconiosis | 11 | 3.6 | |
| Diabetes | 29 | 9.5 | |
| Others | 17 | 5.6 | |
| NA | 65 | 21.3 | |
| Waiting Time (Median [IQR]) | Long Waiting Time a (N (%)) | cOR (90%CI) | aOR (95%CI) | |
|---|---|---|---|---|
| Age (years) | ||||
| ≤44 | 23.00 [6.00, 54.00] | 39 (48.1) | 1.00 | 1.00 |
| 45~59 | 38.00 [8.00, 82.75] | 33 (51.6) | 1.15 (0.66–1.99) | 0.65 (0.25–1.69) |
| ≥60 | 26.00 [9.25, 50.25] | 34 (50.0) | 1.08 (0.63–1.85) | 1.68 (0.63–4.60) |
| Gender | ||||
| Male | 23.50 [8.00, 65.25] | 74 (49.3) | 1.00 | 1.00 |
| Female | 28.00 [6.50, 66.50] | 32 (50.8) | 1.06 (0.65–1.74) | 0.9 (0.41–1.95) |
| Occupation | ||||
| Worker | 29.00 [11.00, 55.00] | 18 (54.5) | 1.00 | 1.00 |
| Housekeeping/ unemployment | 14.00 [8.50, 48.00] | 14 (35.9) | 0.47 (0.21–1.03) | 0.7 (0.18–2.81) |
| Farmer/fisherman | 28.00 [5.75, 66.25] | 61 (52.6) | 0.92 (0.48–1.77) | 2 (0.58–7.44) |
| Others | 28.00 [10.00, 166.00] | 13 (52.0) | 0.9 (0.38–2.17) | 1.3 (0.28–6.23) |
| Residency status | ||||
| Residents | 23.00 [7.00, 72.25] | 78 (48.1) | 1.00 | 1.00 |
| Non-residents | 29.00 [8.00, 54.00] | 28 (54.9) | 1.31 (0.77–2.24) | 2.89 (1.14–7.70) ** |
| Distance to the hospital | ||||
| Near | 35.00 [12.00, 73.00] | 39 (60.0) | 1.00 | 1.00 |
| Moderate | 14.00 [4.00, 51.50] | 33 (41.8) | 0.48 (0.27–0.83) ** | 0.44 (0.16–1.14) |
| Remote | 24.00 [7.00, 62.00] | 34 (49.3) | 0.65 (0.36–1.15) | 0.46 (0.16–1.25) |
| Register year | ||||
| After 2018 | 10.00 [3.00, 24.50] | 28 (25.2) | 1.00 | 1.00 |
| Before 2018 | 50.00 [28.00, 134.75] | 78 (76.5) | 9.63 (5.76–16.51) * | 19.93 (8.99–48.51) ** |
| Patient types | ||||
| New case | 24.00 [6.00, 62.50] | 61 (49.6) | 1.00 | 1.00 |
| Recurrent | 19.00 [9.00, 66.00] | 33 (45.2) | 0.84 (0.51–1.36) | 0.67 (0.32–1.40) |
| Others | 41.00 [14.00, 135.00] | 12 (70.6) | 2.44 (1.00–6.54) | 1.10 (0.25–5.17) |
| Level of diagnostic unit | ||||
| Municipal | 10.00 [2.75, 34.75] | 6 (37.5) | 1.00 | 1.00 |
| County-level | 25.00 [7.00, 73.00] | 100 (50.8) | 1.72 (0.72–4.32) | 4.65 (1.08–21.67) ** |
| Baseline sputum smear | ||||
| Positive | 24.00 [7.00, 68.00] | 90 (49.2) | 1.00 | 1.00 |
| Negative | 27.50 [7.00, 41.00] | 16 (53.3) | 1.18 (0.62–2.28) | 3.54 (1.28–10.16) ** |
| Severity | ||||
| No | 21.00 [5.75, 57.25] | 79 (45.9) | 1.00 | 1.00 |
| Yes | 42.00 [13.00, 77.00] | 27 (65.9) | 2.27 (1.26–4.19) ** | 1.29 (0.46–3.68) |
| Comorbidity | ||||
| No | 24.00 [7.00, 66.50] | 80 (49.1) | 1.00 | 1.00 |
| Pneumonoconiosis | 36.00 [12.00, 50.00] | 6 (66.7) | 2.07 (0.66–7.60) | 7.10 (1.23–47.98) ** |
| Diabetes | 15.00 [10.00, 56.00] | 11 (44.0) | 0.82 (0.40–1.66) | 0.98 (0.30–3.12) |
| Others | 31.50 [4.75, 77.00] | 9 (56.2) | 1.33 (0.56–3.25) | 0.30 (0.08–1.16) |
| Variable | No (N (%)) | Yes (N (%)) | cOR (90%CI) | aOR (95%CI) |
|---|---|---|---|---|
| Age (years) | ||||
| ≤44 | 70 (69.3) | 31 (30.7) | 1.00 | 1.00 |
| 45~59 | 74 (74.0) | 26 (26.0) | 0.79 (0.47–1.33) | 1.84 (0.84–4.11) |
| ≥60 | 79 (76.0) | 25 (24.0) | 0.71 (0.42–1.20) | 2.67 (1.16–6.35) ** |
| Gender | ||||
| Male | 167 (76.6) | 51 (23.4) | 1.00 | 1.00 |
| Female | 56 (64.4) | 31 (35.6) | 1.81 (1.15–2.85) ** | 1.70 (0.92–3.13) |
| Occupation | ||||
| Worker | 17 (44.7) | 21 (55.3) | 1.00 | 1.00 |
| Housekeeping/unemployment | 39 (73.6) | 14 (26.4) | 0.29 (0.14–0.60) ** | 0.31 (0.10–0.92) ** |
| Farmer/fisherman | 147 (80.8) | 35 (19.2) | 0.19 (0.10–0.36) ** | 0.20 (0.08–0.53) ** |
| Others | 20 (62.5) | 12 (37.5) | 0.49 (0.21–1.08) | 0.48 (0.15–1.50) |
| Residency status | ||||
| Residents | 172 (78.5) | 47 (21.5) | 1.00 | 1.00 |
| Non-residents | 51 (59.3) | 35 (40.7) | 2.51 (1.60–3.95) ** | 3.10 (1.55–6.30) ** |
| Distance to the hospital | ||||
| Near | 64 (66.7) | 32 (33.3) | 1.00 | 1.00 |
| Moderate | 79 (73.8) | 28 (26.2) | 0.71 (0.43–1.18) | 0.72 (0.35–1.48) |
| Remote | 80 (78.4) | 22 (21.6) | 0.55 (0.32–0.93) * | 0.68 (0.33–1.40) |
| Register year | ||||
| After 2018 | 133 (79.2) | 35 (20.8) | 1.00 | 1.00 |
| Before 2018 | 90 (65.7) | 47 (34.3) | 1.98 (1.29–3.06) ** | 2.17 (1.19–4.00) ** |
| Patient types | ||||
| New case | 118 (69.8) | 51 (30.2) | 1.00 | 1.00 |
| Recurrent | 90 (82.6) | 19 (17.4) | 0.49 (0.29–0.80) ** | 0.43 (0.22–0.84) ** |
| Others | 15 (55.6) | 12 (44.4) | 1.85 (0.92–3.70) | 1.15 (0.40–3.22) |
| Level of diagnostic unit | ||||
| Municipal | 12 (66.7) | 6 (33.3) | 1.00 | |
| County-level | 157 (70.7) | 65 (29.3) | 0.83 (0.36–2.04) | - |
| NA | 54 (83.1) | 11 (16.9) | 0.41 (0.15–1.12) | - |
| Baseline sputum smear | ||||
| Positive | 181 (72.1) | 70 (27.9) | 1.00 | |
| Negative | 42 (77.8) | 12 (22.2) | 0.74 (0.40–1.30) | - |
| Drug-resistant test | ||||
| Traditional | 112 (65.1) | 60 (34.9) | 1.00 | |
| Xpert | 111 (83.5) | 22 (16.5) | 0.37 (0.23–0.58) $ | - |
| Drug susceptibility | ||||
| MDR | 160 (74,1) | 56 (25.9) | 1.00 | |
| RR | 51 (67.1) | 25 (32.9) | 1.4 (0.86–2.25) | - |
| Others | 12 (92.3) | 1 (7.7) | 0.24 (0.02–1.00) | - |
| Severity | ||||
| No | 141 (72.3) | 54 (27.7) | 1.00 | |
| Yes | 28 (62.2) | 17 (37.8) | 1.59 (0.89–2.79) | - |
| NA | 54 (83.1) | 11 (16.9) | 0.53 (0.28–0.95) $ | - |
| Comorbidity | ||||
| No | 122 (66.7) | 61 (33.3) | 1.00 | 1.00 |
| Pneumonoconiosis | 11 (100.0) | 0 (0.0) | 0 (NA-infinite) | 0 (NA-infinite) |
| Diabetes | 24 (82.8) | 5 (17.2) | 0.42 (0.16–0.92) * | 0.49 (0.14–1.46) |
| Others | 12 (70.6) | 5 (29.4) | 0.83 (0.31–2.00) | 1.10 (0.31–3.49) |
| NA | 54 (83.1) | 11 (16.9) | 0.41 (0.22–0.73) ** | 0.33 (0.13–0.74) |
| Variable | Waiting Time (Median [IQR]) | Long (N (%)) | cOR (90%CI) | aOR (95%CI) |
|---|---|---|---|---|
| Age (years) | ||||
| ≤44 | 22.00 [9.50, 55.50] | 27 (42.9) | 1.00 | 1.00 |
| 45~59 | 30.50 [12.25, 94.00] | 33 (50.0) | 1.33 (0.75–2.40) | 1.46 (0.63–3.41) |
| ≥60 | 34.00 [8.00, 104.50] | 37 (51.4) | 1.41 (0.80–2.50) | 1.7 (0.74–3.94) |
| Gender | ||||
| Male | 33.00 [10.00, 98.00] | 78 (52.3) | 1.00 | 1.00 |
| Female | 20.50 [9.75, 57.75] | 19 (36.5) | 0.52 (0.30–0.90) | 0.52 (0.25–1.05) |
| Occupation | ||||
| Worker | 55.00 [30.00, 123.00] | 12 (70.6) | 1.00 | 1.00 |
| Housekeeping/unemployment | 107.50 [21.00, 188.50] | 23 (67.6) | 0.87 (0.29–2.48) | 0.65 (0.15–2.55) |
| Farmer/fisherman | 23.00 [9.00, 76.00] | 58 (43.6) | 0.32 (0.12–0.78) ** | 0.37 (0.10–1.23) |
| Others | 17.00 [6.00, 24.00] | 4 (23.5) | 0.13 (0.03–0.44) ** | 0.11 (0.02–0.52) |
| Residency status | ||||
| Residents | 32.00 [10.00, 88.00] | 80 (51.3) | 1.00 | |
| Non-residents | 18.00 [9.00, 82.00] | 17 (37.8) | 0.58 (0.32–1.01) | - |
| Distance to the hospital | ||||
| Near | 18.00 [5.75, 70.75] | 23 (41.1) | 1.00 | |
| Moderate | 48.00 [8.00, 128.00] | 39 (54.2) | 1.7 (0.94–3.08) | - |
| Remote | 30.00 [14.00, 82.00] | 35 (47.9) | 1.32 (0.73–2.39) | - |
| Register year | ||||
| After 2018 | 32.00 [10.00, 96.75] | 63 (51.6) | 1.00 | |
| Before 2018 | 24.00 [8.50, 74.00] | 34 (43.0) | 0.71 (0.44–1.14) | - |
| Patient types | ||||
| New case | 23.00 [8.00, 80.00] | 48 (44.0) | 1.00 | |
| Recurrent | 42.00 [13.00, 127.00] | 45 (55.6) | 1.59 (0.98–2.59) | - |
| Others | 17.00 [7.00, 59.50] | 4 (36.4) | 0.73 (0.23–2.08) | - |
| Level of diagnostic unit | ||||
| Municipal | 5.50 [2.00, 18.50] | 3 (25.0) | 1.00 | 1.00 |
| County-level | 42.00 [13.00, 98.00] | 82 (56.6) | 3.9 (1.35–13.72) ** | 4.53 (1.19–22.59) ** |
| NA | 14.50 [7.50, 48.25] | 12 (27.3) | 1.12 (0.35–4.27) | 1.09 (0.25–5.82) |
| Baseline sputum smear | ||||
| Positive | 27.00 [10.00, 87.00] | 77 (46.7) | 1.00 | 1.00 |
| Negative | 47.00 [8.00, 100.75] | 20 (55.6) | 1.43 (0.78–2.64) | 2.66 (1.12–6.59) ** |
| Drug-resistant test | ||||
| Traditional | 31.00 [12.00, 95.00] | 50 (51.5) | 1.00 | |
| Xpert | 23.50 [9.00, 85.25] | 47 (45.2) | 0.78 (0.49–1.23) | - |
| Drug susceptibility | ||||
| MDR | 31.00 [10.00, 87.00] | 74 (51.0) | 1.00 | |
| RR | 20.50 [9.25, 75.50] | 19 (41.3) | 0.68 (0.34–1.31) | - |
| Others | 27.50 [23.25, 114.00] | 4 (40.0) | 0.64 (0.16–2.33) | - |
| Severity | ||||
| No | 34.50 [11.00, 105.75] | 72 (54.5) | 1.00 | |
| Yes | 33.00 [17.00, 76.00] | 13 (52.0) | 0.9 (0.44–1.86) | - |
| NA | 14.50 [7.50, 48.25] | 12 (27.3) | 0.31 (0.16–0.58) $ | - |
| Comorbidity | ||||
| No | 31.00 [9.00, 97.00] | 58 (51.3) | 1.00 | |
| Pneumonoconiosis | 111.00 [31.50, 252.75] | 7 (70.0) | 2.21 (0.72–8.04) | - |
| Diabetes | 61.00 [25.25, 93.00] | 15 (68.2) | 2.03 (0.92–4.76) | - |
| Others | 26.50 [13.50, 46.00] | 5 (41.7) | 0.68 (0.24–1.85) | - |
| NA | 14.50 [7.50, 48.25] | 12 (27.3) | 0.36 (0.18–0.66) $ | - |
| Variable | Successful (N(%)) | Poor (N(%)) | cOR (90%CI) | aOR (95%CI) |
|---|---|---|---|---|
| Age (years) | ||||
| ≤44 | 43 (76.8) | 13 (23.2) | 1.00 | 1.00 |
| 45~59 | 34 (64.2) | 19 (35.8) | 1.85 (0.92–3.77) | 2.02 (0.57–7.73) |
| ≥60 | 28 (46.7) | 32 (53.3) | 3.78 (1.96–7.54) ** | 5.18 (1.53–19.98) ** |
| Gender | ||||
| Male | 75 (60.0) | 50 (40.0) | 1.00 | 1.00 |
| Female | 30 (68.2) | 14 (31.8) | 0.70 (0.37–1.28) | 0.40 (0.13–1.15) |
| Occupation | ||||
| Worker | 13 (81.2) | 3 (18.8) | 1.00 | 1.00 |
| Housekeeping / unemployment | 20 (62.5) | 12 (37.5) | 2.60 (0.82–9.78) | 1.04 (0.15–8.00) |
| Farmer/fisherman | 59 (55.7) | 47 (44.3) | 3.45 (1.25–11.89) * | 0.78 (0.14–4.81) |
| Others | 13 ((86.7) | 2 (13.3) | 0.67 (0.11–3.38) | 0 (0-infinite) |
| Residency status | ||||
| Residents | 84 (62.7) | 50 (37.3) | 1.00 | |
| Non-residents | 21 (60.0) | 14 (40.0) | 1.12 (0.58–2.11) | - |
| Distance to the hospital | ||||
| Near | 30 (63.8) | 17 (36.2) | 1.00 | |
| Moderate | 41 (67.2) | 20 (32.8) | 0.86 (0.44–1.69) | - |
| Remote | 34 (55.7) | 27 (44.3) | 1.40 (0.73–2.72) | - |
| Register year | ||||
| After 2018 | 59 (69.4) | 26 (30.6) | 1.00 | 1.00 |
| Before 2018 | 46 (54.8) | 38 (45.2) | 1.87 (1.11–3.20) * | 2.06 (0.81–5.35) |
| Patients types | ||||
| New case | 59 (65.6) | 31 (34.4) | 1.00 | |
| Recurrent | 37 (55.2) | 30 (44.8) | 0.9 (0.47–1.81) | - |
| Others | 9 (75.0) | 3 (25.0) | 1.17 (0.41–3.19) | - |
| Level of diagnostic unit | ||||
| Municipal | 5 (50.0) | 5 (50.0) | 1.00 | |
| County-level | 78 (65.0) | 42 (35.0) | 0.54 (0.18–1.63) | - |
| NA | 22 (56.4) | 17 (43.6) | 0.77 (0.24–2.53) | - |
| Baseline sputum smear | ||||
| Positive | 83 (59.3) | 57 (40.7) | 1.00 | 1.00 |
| Negative | 22 (75.9) | 7 (24.1) | 0.46 (0.21–0.97) * | 0.76 (0.15–3.31) |
| Drug-resistant test | ||||
| Traditional | 57 (57.6) | 42 (42.4) | 1.00 | |
| Xpert | 48 (68.6) | 22 (31.4) | 0.62 (0.36–1.06) | - |
| Drug susceptibility | ||||
| MDR | 77 (73.2) | 47 (37.9) | 1.00 | |
| RR | 21 (77.8) | 12 (36.4) | 0.94 (0.47–1.81) | - |
| Others | 7 (63.6) | 5 (41.7) | 1.17 (0.41–3.19) | - |
| Severity | ||||
| No | 69 (65.1) | 37 (34.9) | 1.00 | |
| Yes | 14 (58.3) | 10 (41.7) | 1.33 (0.61–2.83) | - |
| NA | 22 (56.4) | 17 (43.6) | 1.44 (0.77–2.70) | - |
| Comorbidity | ||||
| No | 65 (69.9) | 28 (30.1) | 1.00 | 1.00 |
| Pneumonoconiosis | 6 (66.7) | 3 (33.3) | 1.16 (0.31–3.78) | 0.81 (0.10–5.04) |
| Diabetes | 8 (50.0) | 8 (50.0) | 2.32 (0.93–5.78) | 5.48 (1.21–28.23) ** |
| Others | 4 (33.3) | 8 (66.7) | 4.64 (1.64–14.59) ** | 3.19 (0.61–18.81) |
| NA | 22 (56.4) | 17 (43.6) | 1.79 (0.93–3.43) | 2.90 (0.89–10.28) |
| Waiting for treatment # | ||||
| Short | 42(56.8) | 32 (43.2) | 1.00 | 1.00 |
| Long | 46(62.2) | 28 (37.8) | 0.80 (0.46–1.39) | 0.48 (0.18–1.21) |
| Time for sputum smear conversion § | ||||
| ≤1 month | 65(70.7) | 27 (29.3) | 1.00 | 1.00 |
| >1 month | 31(58.5) | 22 (41.5) | 1.71 (0.94–3.10) | 3.50 (1.36–9.70) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Xu, Y.; Li, Z.; Chen, X.; Lin, H.; Zhao, Q. Diagnosis and Treatment Pathway of MDR/RR-TB in Taizhou, Zhejiang Province, China. Trop. Med. Infect. Dis. 2023, 8, 79. https://doi.org/10.3390/tropicalmed8020079
Lu J, Xu Y, Li Z, Chen X, Lin H, Zhao Q. Diagnosis and Treatment Pathway of MDR/RR-TB in Taizhou, Zhejiang Province, China. Tropical Medicine and Infectious Disease. 2023; 8(2):79. https://doi.org/10.3390/tropicalmed8020079
Chicago/Turabian StyleLu, Jingting, Yuanyuan Xu, Zhipeng Li, Xiaoxiao Chen, Haijiang Lin, and Qi Zhao. 2023. "Diagnosis and Treatment Pathway of MDR/RR-TB in Taizhou, Zhejiang Province, China" Tropical Medicine and Infectious Disease 8, no. 2: 79. https://doi.org/10.3390/tropicalmed8020079
APA StyleLu, J., Xu, Y., Li, Z., Chen, X., Lin, H., & Zhao, Q. (2023). Diagnosis and Treatment Pathway of MDR/RR-TB in Taizhou, Zhejiang Province, China. Tropical Medicine and Infectious Disease, 8(2), 79. https://doi.org/10.3390/tropicalmed8020079







