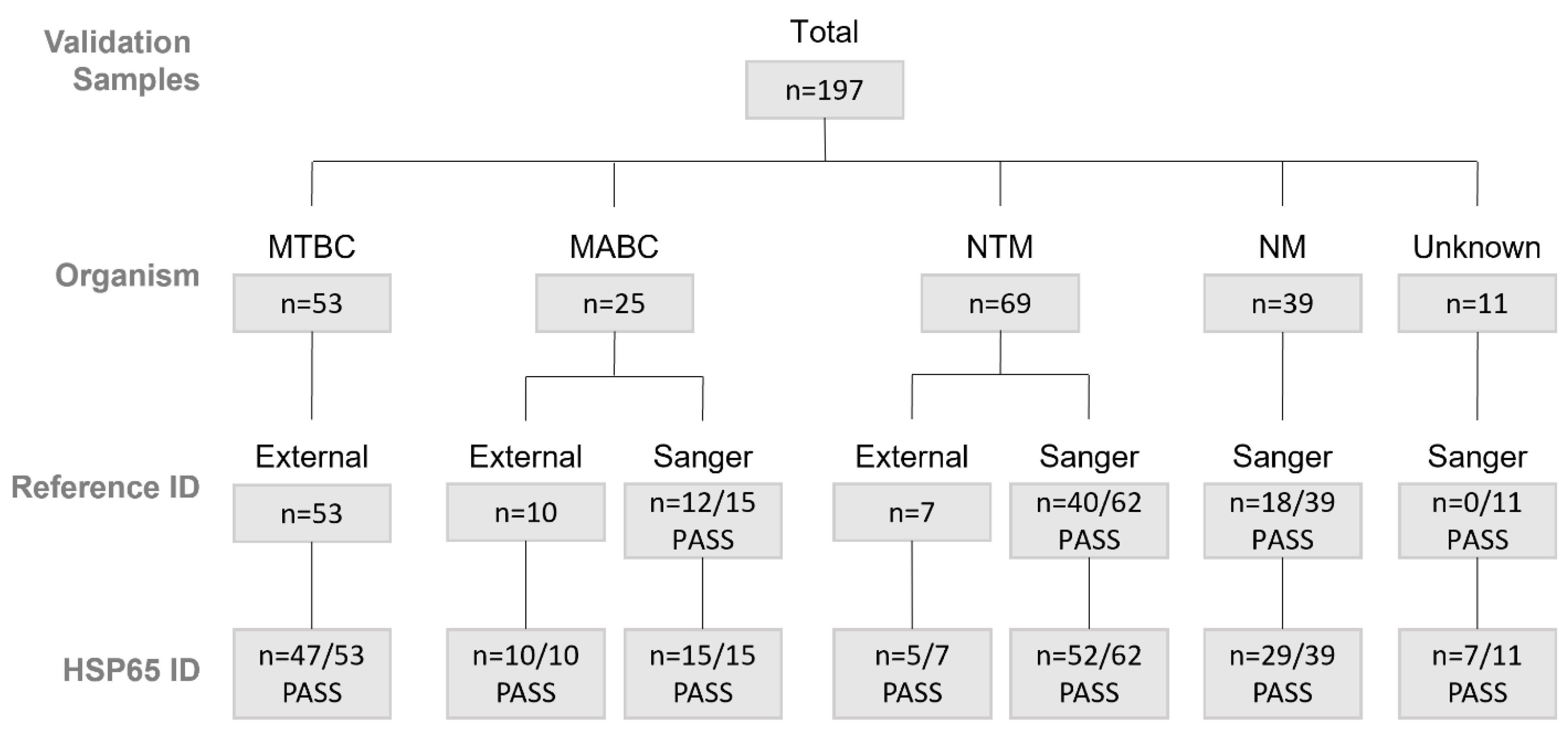
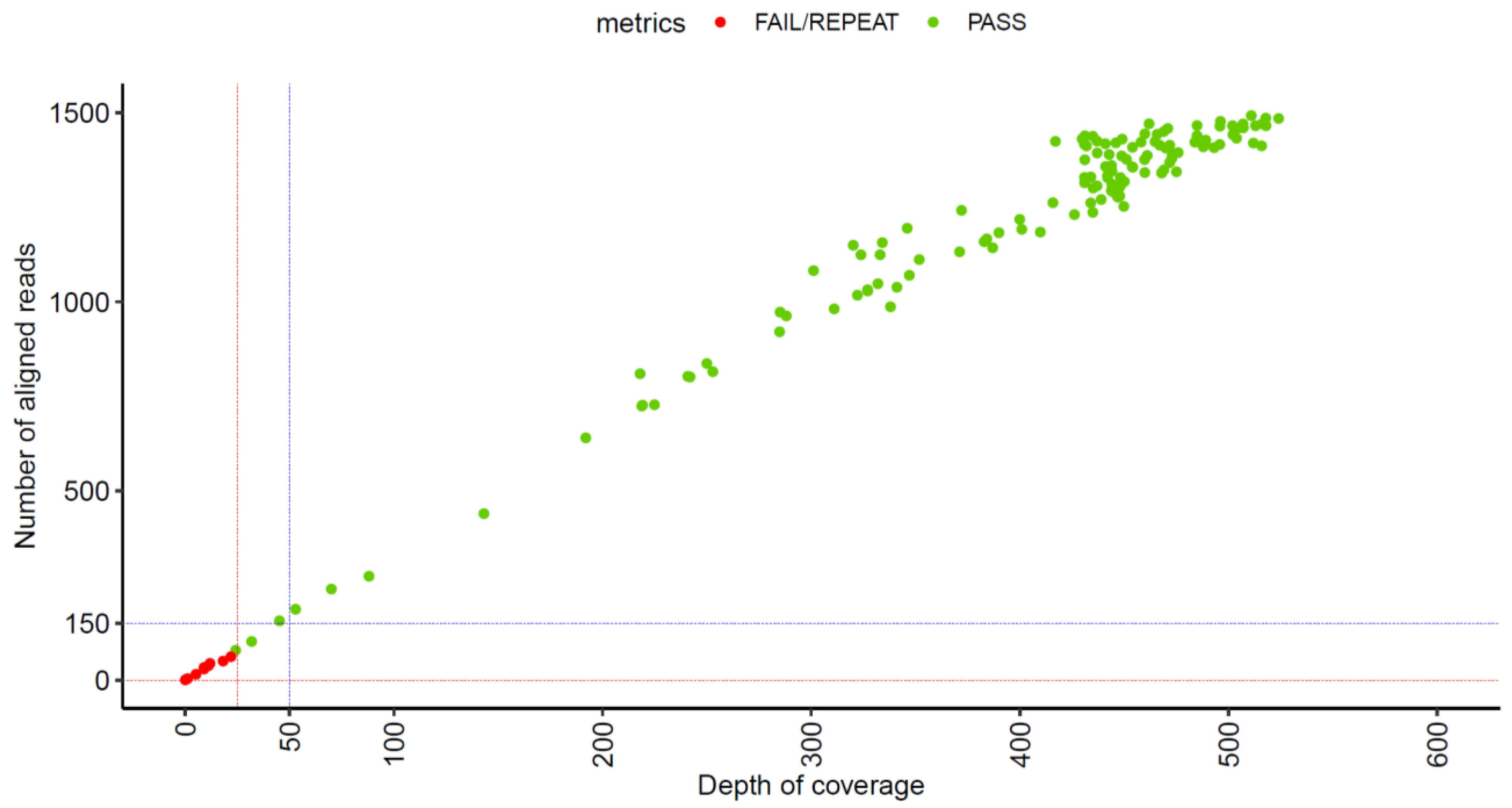
1. Introduction
The
Mycobacterium genus comprises several groups, including the heterogeneous group of non-tuberculous mycobacteria (NTM). NTM are organisms with an environmental reservoir that can be isolated in the clinical laboratory as either an etiological agent of clinically significant infections, a colonizer (in particular in respiratory samples), or an environmental contaminant. They are more likely to be of clinical significance in immunocompromised patients, those with organ damage conducive to infection (e.g., cystic fibrosis or bronchiectasis patients), or post-procedure wounds [
1,
2,
3]. The clinical significance of NTM in recent decades has been increasing, with the expanding use of immunosuppressive medications/transplantation and longer lifespans of patients with congenital immunosuppressive and other genetic conditions. It has been noted that many developed countries now have a higher incidence of NTM vs.
M. tuberculosis infection [
4,
5]. As such, it is crucial for a clinical laboratory to be able to accurately and rapidly identify NTM in clinical specimens to facilitate timely and accurate clinical management where necessary. Moreover, while antimicrobial susceptibility testing is not routinely recommended for all NTM isolated in a clinical laboratory, recent clinical guidelines do recommend that baseline susceptibility testing be performed for some clinically relevant species—for instance, testing for inducible macrolide resistance in
M. abscessus subspecies, either phenotypically via a prolonged incubation (14 days) or sequencing of the
erm(41) gene [
6].
Inducible macrolide resistance in
M. abscessus complex (subspecies
abscessus,
bolletii, and
massiliense) is conferred by a functional
erm(41) gene, which is typically present in
M. abscessus subsp.
abscessus, but not in
M. abscessus subsp.
massiliense organisms [
7]. A wild-type
erm(41) gene produces a functional 23S rRNA methylase, and its expression is induced by exposure to macrolide antibiotics. Macrolides have a lower binding affinity to methylated 23S rRNA, resulting in inducible macrolide resistance [
8]. When the
erm(41) gene is rendered non-functional, this can be via a 276 bp deletion within the gene (truncation mutation) or loss of function of the
erm(41) gene due to a T28C point mutation leading to an amino acid change from Trp to Arg [
9,
10].
The identification of NTM at the species level can be performed using various methods. Commercially available AccuProbe tests (Hologic) have been a commonly used method of
Mycobacterium avium complex (MAC) and
M. gordonae species identification in many clinical laboratories. However, the company discontinued the production of these products in late 2022, forcing clinical laboratories to explore other options for NTM identification. Mass spectrometry methods (MALDI-ToF), targeted PCRs, and sequencing of key housekeeping genes (e.g., 16S rRNA and
hsp65) have also been used [
11,
12]. Each methodology has its pros and cons—for instance, while MALDI-ToF-based identification has been improving with expanded NTM databases, it still requires a pure culture of an organism. For the slowly growing NTM species, this requirement means prolonged delays with identification, as growing a sufficient amount of organism in pure culture might take over a week from the initial growth of the organism from a clinical specimen. Targeted PCRs usually target only a single or a small subset of species—usually at a higher cost than the previously available AccuProbe reagents. The choice of housekeeping gene for sequencing-based NTM identification needs to be carefully considered. For instance, while there are CLSI-based guidelines available for the interpretation of 16S rRNA-based species identification [
13], some key clinically significant NTM complex members have indistinguishable 16S rRNA sequences (e.g., the
M. abscessus complex group), making it an unsuitable target for this purpose. Several other essential genes have also been previously evaluated with respect to their capacity to differentiate
Mycobacterium spp. Combinations of housekeeping genes (e.g.,
gyrB, 16S, and ITS fragment sequences [
14];
rpoB,
argH, and
cya fragment sequences [
15]; or
hsp65,
rpoB, and ITS fragment sequences [
16]),
rpoB sequencing alone [
17], and
gyrB-targeted microarrays [
14,
18] have been demonstrated to have good capacity for resolving NTM organisms. Regardless of the choice of a housekeeping gene used for NTM identification, there must be careful consideration of the selection and curation of a database against which to compare the sequences. In our laboratory, we have previously developed an
hsp65-based sequencing method for the identification of NTM species, including a curated database built for this purpose [
19]. The original method was developed using Sanger sequencing, which was a well-supported technology in our laboratory. However, in recent years, the manufacturer has discontinued technical support for some of their instruments, creating a lack of contingency for our routine laboratory operations and prompting an evaluation of other sequencing platforms. With the decreasing costs and increasing use of next-generation sequencing (NGS) technologies, we have worked to develop and implement an NGS-based method to include the concurrent sequencing of a 401 bp fragment of the
hsp65 gene (similar to our original methodology), as well as introduced an additional
erm(41) target to evaluate inducible macrolide resistance. We describe herein this newly developed methodology.
4. Discussion
The increased clinical significance of NTM warrants clinical laboratories to have on menu rapid and reliable methods for their differentiation from
M. tuberculosis and species/subspecies-level identification. This is particularly true for clinical laboratories in developed countries, where the incidence of NTM disease can be on par with or higher than the incidence of
M. tuberculosis disease. Similarly, with recent recommendations from IDSA guidelines to assess baseline susceptibilities for clinically relevant cases [
6], it is prudent to develop methodologies that can provide preliminary genotypic-based predictions of susceptibilities for these relatively slow-growing organisms.
NGS technologies are continuously evolving, providing laboratories with increasing efficiencies, often at lower costs. This becomes particularly attractive when previously available technologies become outdated or commercially available products are discontinued. Our laboratory successfully introduced an Illumina NGS amplicon sequencing assay that combines NTM identification with inducible macrolide resistance prediction for
M. abscessus complex members and has been accredited by both the Diagnostic Accreditation Program (DAP—our jurisdiction’s regulatory body) and the College of American Pathologists (CAP). We chose Illumina as our sequencing platform, based on it being the most utilized technology in our laboratory, to facilitate multiplexing of different organisms, increasing efficiencies. Depending on individual laboratory preferences, other sequencing platforms, e.g., Nanopore, another platform commonly available in clinical microbiology laboratories, can be chosen for targeted NGS for rapid organism identification and resistance testing [
28]. While Illumina allows for an efficient multiplexing of larger runs, Nanopore would have the potential to offer faster turn-around times for individual samples, though potentially at higher costs. Metagenomics sequencing has the potential to offer even higher resolution and a reliable identification of polymicrobial infections [
29]. However, metagenomics approaches are particularly costly, which would be prohibitive for routine use in most laboratories. For our laboratory, the cost per sample of this amplicon NGS assay is only CAD 17 higher than the combined cost of previously utilized Sanger
hsp65 sequencing and AccuProbe testing, which was our previous NTM identification workflow. The amplicon NGS assay, however, offers both identification and inducible macrolide resistance prediction. The decreased hands-on time required for NGS-based testing versus the previously utilized Sanger testing allowed us to increase the frequency of
hsp65 amplicon sequencing to twice weekly, improving turn-around times and thus optimizing clinical utility. These findings are concordant with recently published assessments of various molecular technologies available for NTM identification [
12]. Our observed discrepancies between the
hsp65 organism ID on direct sample sequencing vs. the organism ultimately isolated in culture, for cases that had documented instances of coinfection with multiple organisms, highlight the need to evaluate for multiple coinfections whenever possible. Additionally, this brings up the possibility of the differential growth of one organism vs. another depending on the clinical context, e.g., concurrent antimicrobial pressures or competitive inhibition from other members of normal respiratory flora. The two organisms that had discrepant genotypic/phenotypic results for inducible macrolide resistance highlight that while the
erm(41) gene has very high predictive potential for inducible macrolide resistance phenotype, phenotypic confirmation remains important. Point mutations in
erm(41) have been described in association with the duration of time to inducible resistance [
30], and perhaps future studies will uncover additional less common genetic loci that increase the reliability of the prediction of this phenotype.
Our assay has undergone optimization over the course of its development and implementation, with the bioinformatics pipeline initially capturing only the top ID choice for the hsp65 sequence and currently optimized to report the top three choices to facilitate the detection of potential mixed infections. Possible future optimizations can be considered, such as incorporating additional target genes for identification purposes, or broader antimicrobial predictions. With both the current design and any future modifications, carefully setting up quality parameters for both the wet lab and bioinformatics portions of the test, as well as using well-curated databases for sequence comparisons, are crucial components of a high-quality clinical sequencing program that would meet the stringent requirements of clinical laboratory accreditation bodies.
Since the introduction of amplicon NGS testing as the primary identification method for NTM in our laboratory, one interesting finding that was observed was that the increased resolution of NTM ID can at times result in increased difficulties for clinical decision making. Previously, the majority of
M. avium complex (MAC) isolates were identified via the Hologic AccuProbe at the complex level only. The current approach provides their ID at the individual species level, and this complex has multiple species assigned to it, with ongoing proposed taxonomic rearrangements [
31,
32]. Individual complex members can have highly related
hsp65 sequences, with sometimes just one nucleotide difference between type species; this high level of similarity can be observed in the phylogenetic tree generated from a subset of our
hsp65 sequences (
Figure 3). Our methodological transition has uncovered interesting insights into the phylogenetic distribution of clinical isolates from this group of organisms (
Table 9), which in the future can be coupled with clinical information to assess their relative clinical significance as causative agents of disease. In particular, we have seen a significant number of
M. timonense isolated in our laboratory, despite the fact that this organism has only very rarely been reported in the literature in association with clinically significant infections [
33,
34,
35]. In addition, since transitioning to our amplicon NGS assay, we have witnessed ID results for organisms isolated from samples of the same patient collected over various periods of time identified as different MAC species members, whereas previously, they would have all been identified as “
Mycobacterium avium complex”. In some cases, this has resulted in clinical conundrums (per consultations received from treating physicians), with clinicians struggling to determine the significance of the isolates and whether what is being observed is a persistent infection or multiple reinfection episodes. Additional genotyping, potentially by whole-genome sequencing, might be necessary to help determine the true relatedness and significance of NTM organisms in this type of situation, and future studies examining this phenomenon might shed light on this clinical conundrum and potentially result in updates to clinical guidelines. The true and full extent of these methodological adaptations on clinical decision making and outcomes is not currently known, as NTM are not a reportable organism in our jurisdiction, making systematic data collection difficult. Future studies, however, can focus on a systematic review of clinical presentations, treatment approaches, and outcomes to gain better insights into the clinical significance of different NTM organisms and their persistence, reinfection, or coinfection. The current literature offers sparse guidance for the clinical management of cases of NTM relapse vs. NTM reinfection, although at least one recent example highlighted that different clinical courses can be entertained (e.g., prolongation of treatment course in cases of relapse [
36]). Relapse vs. reinfection outcomes of NTM treatment are reported separately in the literature [
37,
38], and current international NTM practice guidelines do recommend comparing consecutive patient isolates to determine their relatedness [
6]. However, definitive clinical guidance on approaches to patients, either post-relapse or post-reinfection, has not been published to our knowledge and, variability in methods to classify relapse vs. reinfection cases also further complicates the situation.
Overall, our laboratory has had a very positive experience with transitioning to amplicon NGS testing for NTM identification and inducible macrolide resistance predictions, from both the clinical utility and workflow perspectives. While careful attention to quality metrics is paramount with such transitions, and they have the potential to surface new questions regarding clinical significance interpretations, NGS technologies continue to prove themselves to be tremendously helpful in a microbiological clinical laboratory.