Abstract
Background: We aimed to describe trends in M. genitalium prevalence and associated resistance in Canada between 1980 and 2022. Methods: Ecological study and a scoping review. We collected publicly available data published by the governments of all Canadian provinces and territories. We also systematically searched PubMed, Medline, Embase, and grey literature using the keywords ‘M. genitalium’, ‘Canada’, and all provinces and territories. We reported M. genitalium prevalence, age, sex, gender, symptoms, coinfections, sample types used for diagnosis, and macrolide and fluoroquinolone resistance rates. Results: National or provincial surveillance systems for M. genitalium are absent. Eight studies reported the epidemiology of M. genitalium. The prevalence ranged between 3% in Quebec and 30.3% in Ontario. Half of the patients reported symptoms. The most collected sample for M. genitalium diagnosis was urine, followed by cervical and urethral swabs. Co-infection with Chlamydia trachomatis was reported in 3.3% to 16.4% of cases and with Neisseria gonorrhoeae in 0.0% to 24.0%. Macrolide resistance ranged between 25% and 82.1%, and fluoroquinolone resistance between 0.0% and 29.1%. Conclusions: M. genitalium prevalence and resistance rates varied by sex, gender, province, and specimen type. In the absence of routine surveillance, incomplete data hinders understanding the bacterium’s natural history, its impact on some key groups, and the tracking of antibiotic resistance.
1. Introduction
Mycoplasma genitalium is a Mollicute class bacterium considered a causative agent of male urethritis, female urethritis, and cervicitis [1]. Long-term consequences, such as infertility, ectopic pregnancy, and pelvic inflammatory disease, have been reported for reproductive women’s health [2]. The impact of chronic infections in males is less clear [2,3,4].
The global epidemiology of M. genitalium infection remains unknown due to the lack of regular testing and bacterial surveillance [5,6,7,8,9]. It is suggested that prevalence tends to be higher than Neisseria gonorrhoeae and similar to Chlamydia trachomatis in several key populations, such as sex workers, men who have sex with men, people who attend care at sexual clinics, and in low- and middle-income countries [1,10,11,12]. In addition, rate variations have been reported according to the samples used for diagnosis in females and males [7,9]. Approximately half of the patients do not exhibit symptoms. Therefore, it is difficult to estimate the true prevalence and the carrier state contributing to transmission [13,14,15].
Unfortunately, rates of antibiotic resistance to traditional empiric therapies have increased in recent years. Macrolides are used for syndromic therapy of urethritis; hence, individuals are frequently exposed to this class prior to treatment of M. genitalium. Macrolides, initially recommended as the first line of treatment, are now threatened due to over 50% macrolide resistance in M. genitalium isolates in Canada [16] and 80% in other countries [17]. In addition, the global resistance to fluoroquinolones in M. genitalium is 13.3% [18], with differing prevalence in some geographic areas (8.4% in Europe vs. 35.6% in the Western Pacific) and in some key populations such as people living with HIV, people with recent sexually transmitted infections (STIs) diagnoses, or people who attend STI clinics [18,19,20,21,22].
M. genitalium infections are not notifiable infectious diseases in Canada [23]. Canadian guidelines do not recommend routine testing for M. genitalium. International guidelines recommend M. genitalium screening only for persons with persistent symptoms or recurrent episodes of urethritis, cervicitis, and pelvic inflammatory disease (PID) when chlamydia and gonorrhea have been ruled out as the etiological cause of these syndromes [6,7,9,24]. Access to testing is limited to cases of recurrent cervicitis or urethritis with negative results for Chlamydia trachomatis and Neisseria gonorrhoeae. Currently, testing for M. genitalium diagnosis is mostly available in provincial laboratories, and the molecular antimicrobial resistance testing is available by referring clinical specimens to the National Microbiology Laboratory in Winnipeg, Canada [23]. Knowledge of the current prevalence of both asymptomatic and symptomatic infections and the rates of antimicrobial resistance is scarce.
Population-based M. genitalium data are needed to understand the prevalence of M. genitalium, risk factors and drivers of development of resistance, and its implications for treatment [17,25]. Reviewing national and regional data is crucial to understanding local variations and geographical distribution and the impact of M. genitalium over time, to identifying which groups are disproportionately affected, and to prioritizing resource allocation.
In the present study, we summarize the publicly available information on M. genitalium infections and resistance patterns in Canada and its provinces. We aimed to describe the rates of infection, macrolide and quinolone resistance in different population groups, and the sample types used for M. genitalium diagnosis.
2. Materials and Methods
2.1. Study Design
Ecological study combined with a scoping review.
2.2. Study Questions
What is the prevalence, incidence, or frequency of M. genitalium in Canada (by provinces and territories, sex and gender, and specimen type)? What are the most common diagnostic tests used to detect M. genitalium? What are the macrolide and fluoroquinolone resistance frequencies?
2.3. Data Collection
We (seven authors and the librarian) systematically searched publicly available data reported between 1980 and 2023 published by the federal Canadian surveillance system and the surveillance systems of the governments of the 10 Canadian provinces and three territories.
In addition, for the scoping review, we systematically searched three major electronic databases (PubMed, MEDLINE, and Embase) and grey literature sources of information published between 1980 and 2023. The search strategy included the following keywords: ‘M. genitalium’, ‘Canada’, and the name of each Canadian province (‘British Columbia’, ‘Alberta’, ‘Saskatchewan’, ‘Manitoba’, ‘Ontario’, ‘Quebec’, ‘Nova Scotia’, ‘Newfoundland and Labrador’, ‘New Brunswick’, and ‘Prince Edward Island’) and territories (‘Yukon’, ‘Nunavut’, ‘Northwest Territories’).
We used the following inclusion criteria: reports or articles documenting the incidence or prevalence of M. genitalium in the Canadian population or its provinces and territories. We also included peer-reviewed manuscripts that reported M. genitalium antibiotic resistance tested among patients or participants.
We excluded studies that focused exclusively on in vitro or diagnostic test development or other laboratory developments, validation, or standardization. Case reports and literature reviews were also excluded, although we reviewed the references of those papers to identify additional publications not retrieved by the search strategies.
Two reviewers independently screened and blinded the titles and abstracts as well as the full-text papers. Discrepancies were resolved by consensus with a third reviewer. The quality of each included study was evaluated by two reviewers in an independent and blinded manner, using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies of the National Institutes of Health (NIH), United States [26].
2.4. Variables
We collected M. genitalium prevalence, incidence, or frequency in general and reported by province/territory, age, sex, gender, and specimen type used for diagnosis. In addition, we extracted information on the year, population, and location where the study was conducted; sexual orientation; ethnicity; symptoms; prevalence or frequency of coinfections with Chlamydia trachomatis and Neisseria gonorrhoeae; the prevalence or frequency of resistance to macrolides and fluoroquinolones; and the most critical genomic mutations conferring the resistance patterns.
2.5. Statistical Analysis
Descriptive statistics were used to summarize sociodemographic characteristics. We reported the prevalence or frequency of M. genitalium infections and molecular antibiotic resistance to macrolides and fluoroquinolone antibiotics. In addition, we reported the proportion of M. genitalium diagnoses by age, sex, gender, sexual orientation, specimen types used for clinical diagnosis, and ethnicity.
3. Results
We did not find any Canadian or provincial/territorial surveillance systems. Between 1980 and 2025, a provincial report of M. genitalium antimicrobial resistance was found [27].
The systematic search identified 65 titles, of which 13 were duplicates. The remaining 52 abstracts were screened, two additional duplicates were removed, and 42 were excluded as described in the flowchart (Figure 1). In the end, eight studies were included. The quality assessment is described in the Supplementary Materials (Table S1).
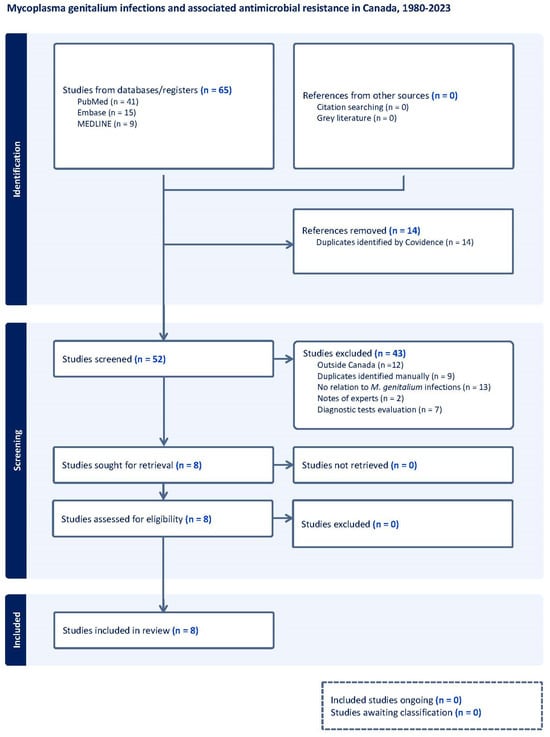
Figure 1.
Flow diagram of studies included in the scoping review of the prevalence/frequency of Mycoplasma genitalium in Canada.
The number of individuals included in these studies ranged from 198 to 2294 [28,29,30,31,32,33,34,35], and the overall prevalence ranged between 3% and 30.3% (Table 1).

Table 1.
Population, province, prevalence/frequency of Mycoplasma genitalium, and diagnostic methods used in the studies conducted in Canada.
Overall, females/women in the research ranged from 16 to 46 years old (median or mean 25–27 years old), and males/men from 16 to 74 years old (median or mean 30–32 years old).
Only three studies disaggregated data by sex, with prevalence between 3.2% [33], 7.2% [29], and 11% [32] in females and 4.5% [33], 5.3% [29], and 6.2% [32] in males. The prevalence/frequency of M. genitalium by gender also varied, ranging from 3%, 5%, 7.4%, 11%, 16%, 20%, 19.7%, and 30.3% in women [30,31,35]; and 15.3% in men [28], and varied between 5.7% [34] and 6.6% [29] in gay, bisexual, and other men who have sex with men (gbMSM).
The prevalence of M. genitalium also varied by the presence or absence of symptoms. Four studies reported that the prevalence in asymptomatic people ranged between 52% [33], 87.3% [31], 86.6% [35], and 91.7% [29], and in symptomatic people between 5% [28], 8.2% [29], 10.6% [35], 12.6% [31], and 48% [33]. The frequency of symptoms varied by sex, from 40% [33] to 44.3% [29] in females and 40.5% [29] to 50% [33] in males. The symptoms reported by women were vaginal discharge, odour or itching, bleeding between periods, painful sex or urination, and for men were pain, dysuria, tingling, or urethral discharge [28,29].
Regarding ethnicity, only one study from Alberta reported the percentage of M. genitalium infections in Indigenous people was 15.2% (26/171), 5.1% (79/1536) in Caucasians, and 7.2% (30/417) in people from other ethnicities [29].
Another key finding was the co-detection of other sexually transmitted bacteria among people with M. genitalium infections. Co-infections with Chlamydia trachomatis varied between 3.27% (22/672), 4% (2/50), 13.4% (53/396), 16.4% (31/189), and 6.7% (4/59) [29,30,32,33,35]; and with Neisseria gonorrhoeae between 0.0% (0/23), 6.8% (13/189), 10% (5/50), 6.7% (4/59), and 24% (12/50) [27,30,31,32,33]. The prevalence of HIV coinfection was 2.0% (2/96) [29].
The detection rate of M. genitalium varied by sample type. Table 2 shows the distribution of M. genitalium in different specimen types. The most common specimen types were urine and cervical or vaginal swabs, followed by urethral, rectal, and pharyngeal swabs (Table 2).

Table 2.
Mycoplasma genitalium positivity rate reported in the studies, based on the specimen type used for diagnosis.
Table 3 reports the macrolide and fluoroquinolone resistance patterns reported in the studies. Macrolide resistance ranges between 25% and 82.1%, and fluoroquinolone resistance ranges between 0.0% and 29.1%. Mutations at position 2058 (Escherichia coli numbering) of 23S rRNA ranged from 2.72% to 57.6% [28,29,30,31,32,34,35], and mutations at position 2059 ranged from 11.6% to 84.7% [28,29,30,31,32,34,35]. Mutations in the parC gene ranged between 1.9 and 29.1% [29,30,32,33]. Mutations in gyrA were not tested or not detected [27,28,32]. Detailed information about the frequency of mutations is described in the Supplementary Materials Table S2.

Table 3.
Mycoplasma genitalium resistance reported in Canada.
4. Discussion
In our study, no surveillance information or data describing national population prevalence of M. genitalium were identified in our systematic search. The scoping review found that M. genitalium prevalence varied by sex, gender, province, presence and absence of symptoms, and specimen used for diagnosis. We also found that the molecular macrolide resistance varies across the provinces from 25% to 82.1%, and fluoroquinolone resistance was lower (0.0–29.1%).
Our study identified different geographic and population prevalence of M. genitalium infections, ranging from 3% in the province of Quebec to 30.3% in Ontario. The variation in the prevalence, or frequency, of M. genitalium in Canada is comparable to the worldwide distribution. Bauman et al. found in a systematic review and meta-analysis an M. genitalium prevalence of 0.80% (95% confidence interval (CI), 0.42–1.57) to 9.10% (95% CI, 6.10–13.50) in the general population, and up to 26.30% (95% CI, 23.30–29.40) in commercial sex workers [1]. The prevalence is also higher in some settings and key populations, e.g., 4.9% in female sex workers in Ecuador [36], 11.5% (21/182) in female sex workers in Burkina Faso [37], and 10.2% in women living with HIV in sub-Saharan Africa [38].
We found that females diagnosed with M. genitalium were younger (median or mean 25–27 years old) than males (median or mean 30–32 years old). This is similar to the findings in a systematic review of population-and clinic-based diagnoses of M. genitalium, which reported less than 2% prevalence among women and men <25 years old [1]. However, the data heterogeneity in age ranges and enrollment procedures limits our ability to draw firm conclusions. Another U.S. study showed that overall, the prevalence was higher in younger ages in both females and males [females < 30 years (19.2%; 95% CI [15.6–23.4]) versus females > 30 years (7.2%; 95%CI [3.8–13.1]); and males < 30 years (22%; 95% CI [17.5–27.4]) versus males > 30 years (9.2%; 95%CI [5.7–14.6])] [10].
The prevalence also varies by sex and gender. Several articles reported data by sex and gender [28,30,31,33], although a few used these terms interchangeably in the same report. M. genitalium prevalence among females ranged between 3.2% and 19.7% (women: 3–30.3%) and among males, between 4.5% and 6.2% (men: 15%). Recently, a systematic review of M. genitalium prevalence across different population groups did not find significant differences between women and men [1], regardless of whether the detection was carried out in the community or clinical settings. Similarly, Getman et al. did not find differences in M. genitalium infection distribution by sex (16.3% in females vs. 17.2% in males) in a multicenter study [10].
Emerging evidence is showing differences in M. genitalium bacterial load between females and males. Munson et al. found differences in median and mean titers of log10 M. genitalium data from males (4 and 3.67, respectively) versus females (3 and 2.98, respectively; p < 0.0001) [39]. In addition, Munson et al. reported that male rectal swabs exhibited a higher rRNA target burden than male first-void urine (p = 0.0002); the same differences were consistent for vaginal and endocervical swabs vs. first-void urine (p = 0.008) [39]. Further studies are needed to understand biological differences in females and males and how those differences can impact diagnostic performance in females with low bacterial density.
Canadian data disaggregated by sexual orientation are scarce. Two studies reported data in gbMSM, and the prevalence was between 5.7% (41/716) and 6.6% (79/1212) [29,34], varying by specimen types used for diagnosis. In global studies, M. genitalium prevalence is higher in gbMSM, and even higher in gbMSM living with HIV (41.5%) [40] when compared to the overall population [1,15]. The reported Canadian prevalence in gbMSM is lower than in other high-income countries: 9.5% (95/1001) in asymptomatic MSM in Australia [11], 10.5% (64/609) in symptomatic and asymptomatic MSM in Sweden [41], and 10.3% (260/2510) in MSM in a community-based health centre in Lisbon [42], and it appears similar to rates reported in MSM attending a sexual clinic in India [7.2% (13/180)] [19] and MSM attending a clinic in South Africa [5.5% (5/91)] [43]. The prevalence in heterosexual men appears similar to gbMSM prevalence (14.6%) [44]. More data are required nationally and internationally.
Canadian trends showed variable reports of symptomatic infections (5–48%) and asymptomatic carriage (52% to 91.7%). Recently, Dumke et al. found asymptomatic M. genitalium carriage among 37.5% of gbMSM and 30.8% of heterosexual individuals. The asymptomatic carriage is highly variable by geographic area: low (0.58%) in France [45] and the United Kingdom (4.5%) [46]; middle in the United States (20.5%) [14]; and higher rates (93%) in Zurich [13]. The true prevalence and incidence of M. genitalium are unknown since current estimates are affected by variable access (e.g., availability or affordability) to testing and treatment in clinical settings as well as included populations in studies.
The clinical guidelines do not recommend M. genitalium screening in asymptomatic people. Ring et al. found that 30% of spontaneous bacterial clearance occurs in asymptomatic and symptomatic patients [13]. However, it is important to investigate the prevalence and risk factors associated with asymptomatic carriage to understand the consequences of asymptomatic infections and transmission as well as the impact of untreated asymptomatic infections.
Canadian data disaggregated by ethnicity is almost nil, with only one Canadian study examining ethnicity and reporting a higher prevalence among Indigenous people than among persons of non-Indigenous ethnicity [29]. Global M. genitalium epidemiology also reports disproportionately high prevalence among Black/African American people [14,15,40,47]. Canadian prevalence studies on other STIs also found that Indigenous and African American persons experience disproportionately higher rates of infection. [48,49]. The province of Manitoba recently reported the highest rates of HIV infection since the first case was reported in 1985 [50] and high rates of co-infection with other STIs. Infections were disproportionately among people who are consistently marginalized by the existing healthcare systems, are poorly housed, and inject drugs. Many self-identify as Indigenous, and many are young women [50]. These data highlight the need to incorporate ethnicity and other equity indicators to identify the most at-risk populations in persons with M. genitalium infection and to understand why.
M. genitalium coinfections are broadly reported with C. trachomatis and N. gonorrhoeae. The prevalence of coinfections is highly variable and also depends on the specimen types and diagnostic tests used [10,28,51]. Higher rates of coinfections with C. trachomatis (28.2%) and N. gonorrhoeae (23.8%) were recently reported, and the C. trachomatis coinfection was significant even after adjusting for specimen type [52]. Parmar et al. found that the prevalence of M. genitalium infection was significantly higher in females with C. trachomatis or C. trachomatis/N. gonorrhoeae coinfection and in males with N. gonorrhoeae [32]. Lê A et al. reported high rates of M. genitalium rectal coinfections in gbMSM in Montreal, 9.1% and 16.7% with C. trachomatis and N. gonorrhoeae, respectively [34]. The pathogenic implications of coinfections need further investigations to assess the potential effect on the reproductive health of females and males. For example, Scoullar et al. found greater reductions (−540.3 g, 95% CI: −859.3 to −221.2, p = 0.001) in the birthweight of newborns from females co-infected with M. genitalium, C. trachomatis, and N. gonorrhoeae compared to uninfected females [53]. In addition, in a systematic review, Frenzer et al. found M. genitalium as a probable risk factor of pre-term birth and spontaneous abortion [54]. Considering the impact of M. genitalium among young females, future studies are required to define the utility of M. genitalium testing in women of reproductive age.
The Canadian STI guidelines recommend M. genitalium testing from first-void urine; cervical, vaginal, urethral, or meatal swabs; and/or endometrial biopsies [23]. International guidelines recommend vaginal swabs for females and first-void urine in males [5,7,9]. Canadian studies comparing specimen types suggest cervicovaginal swabs perform somewhat better than urine to diagnose M. genitalium in women (15.3% vs. 12.6%, p = 0.035, respectively) [28], and more comfort using self-collected samples (urine and vaginal swabs) than those performed in a clinic [31]. Anal samples are only indicated in symptomatic patients, as the evidence shows no clear correlation between carriage and disease [8,55]. Pharyngeal testing is currently not recommended [5,7,9].
Canadian trends confirm the alarming reports of rising rates of antibiotic resistance in M. genitalium. In our study, the rates before 2017 ranged between 25 and 58%; however, after 2019, the macrolide resistance rates ranged between 53.6% and 82.1%, which is similar to other international trends [10,13,52]. These rates were even higher in some key populations [11,12,34,47,56]. The worldwide macrolide resistance has increased dramatically over the last few years. Machaleck et al. described an increase from 10% to 51% from 2010 to 2017, especially in the Western Pacific and the Americas regions [16]. Recently, the same author reported a slight, but not significant, decline in macrolide resistance prevalence in 2018–2021 (42% in 2015–2017 vs. 33% in 2018–2021 [18]). However, macrolide resistance prevalence is variable even between neighbouring countries [Denmark (9.0%), Norway (9.8%), and Sweden (7.2%)] [57].
Fluoroquinolone resistance is less common in Canada (2–29%), similar to other jurisdictions [16,21,47,58,59]. The rates before 2017 ranged from 0.0% to 20.0%, but after 2017 the fluoroquinolone resistance rates have reached up to 29%. Currently the rate of cure has decreased from 100% to 89% after 2010 [16], and some regions, such as the Asia-Pacific region, have a high level of fluoroquinolone resistance mutations in M. genitalium from men with symptomatic urethritis [60]. In addition, alarming data of fluoroquinolone-resistant strains in Berlin and Germany were reported by Glaunsinger and Dumke, showing an increase from 6.8% in 2017 to 38% in 2023 [12].
Macrolides (azithromycin) have been the preferred first-line antimicrobials for the treatment of M. genitalium, with fluoroquinolones, typically moxifloxacin, reserved for the treatment of macrolide-resistant strains. However, with the increasing resistance to first- and second-line antibiotics and the detection of dual resistance, recent guidelines and experts now recommend resistance-guided, sequential antimicrobial therapy whenever possible [7,9]. Data have shown that doxycycline decreases the M. genitalium organism load and, when combined with azithromycin, improves symptoms and results in higher rates of cure when used as part of a course of resistance-guided sequential therapy [61]. Treatment failures have been reported in patients infected with dually resistant strains; no other highly effective treatments are available. Pristinamycin results in about 75% cure and minocycline around 70% cure [62,63]. There is therefore an urgent need to explore additional therapeutic options for treatment and to revise current guidelines.
5. Conclusions
Canada does not conduct routine public health surveillance of M. genitalium. Current data were compiled from several cross-sectional studies in varying settings, populations, and geographic regions in Canada. M. genitalium prevalence and antimicrobial resistance patterns vary by geographic region and key population and show high circulating levels of resistance to macrolides and fluoroquinolones. The absence of population-based estimations of M. genitalium infections limits the understanding of its real impact, natural history and progress, drivers of antibiotic resistance, and disproportionate impact on key populations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/tropicalmed10050139/s1: Table S1. Quality of each study included in this article. Table S2. Frequency of Mycoplama genitalium mutations to macrolides and fluoroquinolones.
Author Contributions
Conceptualization and methodology: A.C., Z.V.R. and Y.K.; writing—original draft preparation: A.C.; formal analysis and data curation: A.C., M.H., C.S., Z.G., M.A.-U., R.K., C.O. and W.R.; writing—review and editing: M.H., C.S.-A., Z.G., M.A.-U., R.K., C.O., A.E.S., S.S., C.S., W.R., L.J.M., K.K., L.I., I.M., J.B., L.L., D.M., M.H.-B., Y.K. and Z.V.R.; funding acquisition and supervision: Z.V.R. and Y.K. All authors have read and agreed to the published version of the manuscript. All authors have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
This research was funded by the Canadian Institutes of Health Research (Grant number: SR7-190790). This research was also supported, in part, by the Canada Research Chairs Programme for ZVR (Award # 950-232963). AC was awarded with The University of Manitoba Graduate Fellowship (UMGF), and an additional studentship from Dr. Rueda’s CRC funds. MH received the CIHR Research Excellence, Diversity, and Independence (REDI) Early Career Transition Award [CIHR: OS3 –190782; ED6-190717].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The epidemiological and laboratory data used for our analysis is publicly available in databases.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
| SD | Standard deviation |
| STI | Sexually transmitted infections |
| NT | Not tested |
| ND | Not detected |
| NR | Not reported |
References
- Baumann, L.; Cina, M.; Egli-Gany, D.; Goutaki, M.; Halbeisen, F.S.; Lohrer, G.-R.; Ali, H.; Scott, P.; Low, N. Prevalence of Mycoplasma Genitalium in Different Population Groups: Systematic Review Andmeta-Analysis. Sex. Transm. Infect. 2018, 94, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chen, X.; Song, Y.; Wang, S.; Pan, Y.; Niu, S.; Wang, R.; Liu, L.; Liu, X. Genital Mycoplasma Infection: A Systematic Review and Meta-Analysis. Reprod. Health 2023, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.C.; Kent, C.K.; Klausner, J.D.; Rauch, L.; Kohn, R.; Hardick, A.; Gaydos, C.A. Prevalence of Rectal Trichomonas Vaginalis and Mycoplasma Genitalium in Male Patients at the San Francisco STD Clinic, 2005–2006. Sex. Transm. Dis. 2008, 35, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Mycoplasma Genitalium in Men Who Have Sex with Men at Male-Only Saunas | Sexually Transmitted Infections. Available online: https://sti-bmj-com.uml.idm.oclc.org/content/85/6/432.long (accessed on 13 March 2025).
- Soni, S.; Horner, P.; Rayment, M.; Pinto-Sander, N.; Naous, N.; Parkhouse, A.; Bancroft, D.; Patterson, C.; Fifer, H. British Association for Sexual Health and HIV National Guideline for the Management of Infection with Mycoplasma Genitalium (2018). Int. J. STD AIDS 2019, 30, 938–950. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Jensen, J.S.; Cusini, M.; Gomberg, M.; Moi, H.; Wilson, J.; Unemo, M. 2021 European Guideline on the Management of Mycoplasma Genitalium Infections. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 641–650. [Google Scholar] [CrossRef]
- Clutterbuck, D.; Asboe, D.; Barber, T.; Emerson, C.; Field, N.; Gibson, S.; Hughes, G.; Jones, R.; Murchie, M.; Nori, A.V.; et al. 2016 United Kingdom National Guideline on the Sexual Health Care of Men Who Have Sex with Men. Int. J. STD AIDS 2018, 1, 956462417746897. [Google Scholar] [CrossRef]
- Mycoplasma Genitalium|STI Guidelines Australia; STI Guidelines: Online, 2024.
- Getman, D.; Jiang, A.; O’Donnell, M.; Cohen, S. Mycoplasma Genitalium Prevalence, Coinfection, and Macrolide Antibiotic Resistance Frequency in a Multicenter Clinical Study Cohort in the United States. J. Clin. Microbiol. 2016, 54, 2278–2283. [Google Scholar] [CrossRef]
- Read, T.R.H.; Murray, G.L.; Danielewski, J.A.; Fairley, C.K.; Doyle, M.; Worthington, K.; Su, J.; Mokany, E.; Tan, L.T.; Lee, D.; et al. Symptoms, Sites, and Significance of Mycoplasma Genitalium in Men Who Have Sex with Men. Emerg. Infect. Dis. 2019, 25, 719–727. [Google Scholar] [CrossRef]
- Dumke, R.; Glaunsinger, T. Trends of Mycoplasma Genitalium Infections in Berlin, Germany, 2017–2023. J. Glob. Antimicrob. Resist. 2025, 41, 29–34. [Google Scholar] [CrossRef]
- Ring, A.; Balakrishna, S.; Imkamp, F.; Burkard, S.; Triet, F.; Brunschweiler, F.; Grube, C.; Bodmer, R.; Kouyos, R.D.; Günthard, H.F.; et al. High Rates of Asymptomatic Mycoplasma Genitalium Infections With High Proportion of Genotypic Resistance to First-Line Macrolide Treatment Among Men Who Have Sex With Men Enrolled in the Zurich Primary HIV Infection Study. Open Forum. Infect. Dis. 2022, 9, ofac217. [Google Scholar] [CrossRef] [PubMed]
- Seña, A.C.; Lee, J.Y.; Schwebke, J.; Philip, S.S.; Wiesenfeld, H.C.; Rompalo, A.M.; Cook, R.L.; Hobbs, M.M. A Silent Epidemic: The Prevalence, Incidence and Persistence of Mycoplasma Genitalium Among Young, Asymptomatic High-Risk Women in the United States. Clin. Infect. Dis. 2018, 67, 73–79. [Google Scholar] [CrossRef]
- Sonnenberg, P.; Ison, C.A.; Clifton, S.; Field, N.; Tanton, C.; Soldan, K.; Beddows, S.; Alexander, S.; Khanom, R.; Saunders, P.; et al. Epidemiology of Mycoplasma Genitalium in British Men and Women Aged 16–44 Years: Evidence from the Third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int. J. Epidemiol. 2015, 44, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Machalek, D.A.; Tao, Y.; Shilling, H.; Jensen, J.S.; Unemo, M.; Murray, G.; Chow, E.P.F.; Low, N.; Garland, S.M.; Vodstrcil, L.A.; et al. Prevalence of Mutations Associated with Resistance to Macrolides and Fluoroquinolones in Mycoplasma Genitalium: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2020, 20, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.S.; Unemo, M. Antimicrobial Treatment and Resistance in Sexually Transmitted Bacterial Infections. Nat. Rev. Microbiol. 2024, 22, 435–450. [Google Scholar] [CrossRef]
- Chua, T.-P.; Vodstrcil, L.A.; Murray, G.L.; Plummer, E.L.; Jensen, J.S.; Unemo, M.; Chow, E.P.F.; Low, N.; Whiley, D.M.; Sweeney, E.L.; et al. Evolving Patterns of Macrolide and Fluoroquinolone Resistance in Mycoplasma Genitalium: An Updated Systematic Review and Meta-Analysis. Lancet Microbe 2025, 0, 101047. [Google Scholar] [CrossRef]
- Biswal, D.; Gupta, S.; Sethi, S.; Singh, S.; Khanna, N.; Dhawan, B. Macrolide and Fluoroquinolone Resistance Associated Mutations in Mycoplasma Genitalium in Men Who Have Sex with Men Attending STI Clinic: A Pilot Study from India. Indian J. Dermatol. Venereol. Leprol. 2024, 90, 632–635. [Google Scholar] [CrossRef]
- García, L.F.; Arnuelos, J.A.; González, É.L.; Bellés-Bellés, A.; Santa, A.M.; Báscones, E.S.; Bayo, S.M.; Sánchez, A.B.; Sánchez, I.P.; Portillo, A.C.; et al. Azithromycin and Moxifloxacin Resistance Determinants in Mycoplasma Genitalium in Lleida, Spain. Rev. Esp. Quimioter. 2024, 37, 270–273. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Sun, H.-Y.; Su, L.-H.; Lin, K.-Y.; Liu, W.-D.; Huang, Y.-S.; Chen, G.-J.; Su, Y.-C.; Liu, W.-C.; Chang, S.-Y.; et al. Mycoplasma Genitalium Infection and Resistance-Associated Mutations to Macrolides and Fluoroquinolones among High-Risk Patients in Taiwan. J. Microbiol. Immunol. Infect. 2024, 57, 629–637. [Google Scholar] [CrossRef]
- Lara, I.; Hernandez-Ruiz, V.; Fernández-Huerta, M.; Rodriguez-Grande, J.; Revillas, F.A.D.L.; Rodriguez-Lozano, J.; Calvo-Montes, J.; Ocampo-Sosa, A.; Fariñas, M.C.; Mesones, M.P.R.; et al. Mycoplasma Genitalium and Antimicrobial Resistance among the General Female and Male Population in Northern Spain. Sex. Transm. Infect. 2025, 12, sextrans-2024-056374. [Google Scholar] [CrossRef]
- Canada, P.H.A. of Mycoplasma Genitalium: Screening and Diagnostic Testing. Available online: https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/mycoplasma-genitalium/screening-diagnostic-testing.html (accessed on 8 November 2024).
- Raya, R.P.; Curtis, H.; Kulasegaram, R.; Cooke, G.S.; Burns, F.; Chadwick, D.; Sabin, C.A. The British HIV Association National Clinical Audit 2021: Management of HIV and Hepatitis C Coinfection. HIV Med. 2023, 24, 471–479. [Google Scholar] [CrossRef]
- Waites, K.B.; Crabb, D.M.; Ratliff, A.E.; Geisler, W.M.; Atkinson, T.P.; Xiao, L. Latest Advances in Laboratory Detection of Mycoplasma Genitalium. J. Clin. Microbiol. 2023, 61, e00790-21. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 16 April 2025).
- Sommaire des Résultats D’antibiorésistance de Mycoplasma genitalium au Québec en 2018–2022|INSPQ. Available online: https://www.inspq.qc.ca/publications/3395 (accessed on 11 May 2025).
- Chernesky, M.; Jang, D.; Smieja, M.; Arias, M.; Martin, I.; Weinbaum, B.; Getman, D. Urinary Meatal Swabbing Detects More Men Infected with Mycoplasma Genitalium and Four Other Sexually Transmitted Infections Than First Catch Urine. Sex. Transm. Dis. 2017, 44, 489. [Google Scholar] [CrossRef] [PubMed]
- Gratrix, J.; Plitt, S.; Turnbull, L.; Smyczek, P.; Brandley, J.; Scarrott, R.; Naidu, P.; Parker, P.; Blore, B.; Bull, A.; et al. Prevalence and Antibiotic Resistance of Mycoplasma Genitalium among STI Clinic Attendees in Western Canada: A Cross-Sectional Analysis. BMJ Open 2017, 7, e016300. [Google Scholar] [CrossRef]
- Chernesky, M.A.; Jang, D.; Martin, I.; Hoang, L.M.N.; Naidu, P.; Levett, P.N.; Wylie, J.; Rebbapragada, A.; Ratnam, S.; Smieja, M.; et al. Mycoplasma Genitalium Antibiotic Resistance–Mediating Mutations in Canadian Women With or Without Chlamydia Trachomatis Infection. Sex. Transm. Dis. 2017, 44, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Chernesky, M.; Jang, D.; Martin, I.; Arias, M.; Shah, A.; Smieja, M.; Ratnam, S.; Getman, D.; Schachter, J. Mycoplasma Genitalium, Chlamydia Trachomatis, and Neisseria Gonorrhoeae Detected With Aptima Assays Performed on Self-Obtained Vaginal Swabs and Urine Collected at Home and in a Clinic. Sex. Transm. Dis. 2019, 46, e87. [Google Scholar] [CrossRef]
- Parmar, N.R.; Mushanski, L.; Wanlin, T.; Lepe, A.; Lang, A.; Minion, J.; Dillon, J.-A.R. High Prevalence of Macrolide and Fluoroquinolone Resistance-Mediating Mutations in Mycoplasma Genitalium-Positive Urine Specimens From Saskatchewan. Sex. Transm. Dis. 2021, 48, 680–684. [Google Scholar] [CrossRef]
- Gesink, D.; Racey, C.S.; Seah, C.; Zittermann, S.; Mitterni, L.; Juzkiw, J.; Jamieson, H.; Greer, J.; Singh, S.; Jensen, J.S.; et al. Mycoplasma Genitalium in Toronto, Ont. Can. Fam. Physician 2016, 62, e96–e101. [Google Scholar]
- Lê, A.-S.; Labbé, A.-C.; Fourmigue, A.; Dvorakova, M.; Cox, J.; Fortin, C.; Martin, I.; Grace, D.; Hart, T.; Moore, D.; et al. Mycoplasma Genitalium Infection among Gay, Bisexual and Other Men Who Have Sex with Men in Montréal, Canada. Can. Commun. Dis. Rep. 2023, 49, 477–486. [Google Scholar] [CrossRef]
- Chernesky, M.; Jang, D.; Martin, I.; Speicher, D.J.; Clavio, A.; Lidder, R.; Ratnam, S.; Smieja, M.; Arias, M.; Shah, A. Comparison of Assays for the Diagnosis of Mycoplasma Genitalium and Macrolide Resistance Mutations in Self-Collected Vaginal Swabs and Urine. Sex. Transm. Dis. 2020, 47, 705. [Google Scholar] [CrossRef]
- Llangarí-Arizo, L.M.; Sadiq, S.T.; Márquez, C.; Cooper, P.; Furegato, M.; Zhou, L.; Aranha, L.; Mateo, M.M.; Romero-Sandoval, N. Sexually Transmitted Infections and Factors Associated with Risky Sexual Practices among Female Sex Workers: A Cross Sectional Study in a Large Andean City. PLoS ONE 2021, 16, e0250117. [Google Scholar] [CrossRef]
- Tovo, S.F.; Zohoncon, T.M.; Dabiré, A.M.; Ilboudo, R.; Tiemtoré, R.Y.; Obiri-Yeboah, D.; Yonli, A.T.; Dovo, E.E.; Ouédraogo, R.A.; Ouattara, A.K.; et al. Molecular Epidemiology of Human Papillomaviruses, Neisseria Gonorrhoeae, Chlamydia Trachomatis and Mycoplasma Genitalium among Female Sex Workers in Burkina Faso: Prevalence, Coinfections and Drug Resistance Genes. Trop. Med. Infect. Dis. 2021, 6, 90. [Google Scholar] [CrossRef]
- Jarolimova, J.; Platt, L.R.; Curtis, M.R.; Philpotts, L.L.; Bekker, L.-G.; Morroni, C.; Shahmanesh, M.; Mussa, A.; Barracks, K.; Ciaranello, A.L.; et al. Curable Sexually Transmitted Infections among Women with HIV in Sub-Saharan Africa. AIDS 2022, 36, 697–709. [Google Scholar] [CrossRef]
- Munson, E.; Moore, J.; Krueger, T.; Zapp, A.; Lavey, S.C.; Munson, K.L.; Stafford, I.A.; Mustanski, B. Mycoplasmoides Genitalium Nucleic Acid Semi-Quantitation and Molecular Macrolide Resistance Detection via Automated Assays: Gender and Specimen Source Considerations. J. Clin. Microbiol. 2024, 62, e0048524. [Google Scholar] [CrossRef] [PubMed]
- Munson, E.; Morgan, E.; Sienkiewicz, L.; Thomas, Y.; Buehler, K.; Ryan, D.; Clifford, A.; Mustanski, B. Molecular Screening in a Longitudinal Cohort of Young Men Who Have Sex with Men and Young Transgender Women: Associations with Focus on the Emerging Sexually Transmitted Pathogen Mycoplasma Genitalium. Sex. Transm. Infect. 2021, 97, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Bjartling, C.; Kertes, R.; Kristiansen, S.; Johnsson, A.; Forslund, O. Prevalence of Mycoplasma Genitalium and Macrolide Resistance in Rectal and Urine Samples among Men Who Have Sex with Men in Sweden. Sex. Transm. Infect. 2024, 100, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Minetti, C.; Rocha, M.; Duque, L.M.; Meireles, P.; Correia, C.; Cordeiro, D.; João, I.; Manita, C.; Soeiro, S.; Santos, J.A.; et al. Orogenital and Anal Infection by Chlamydia Trachomatis, Neisseria Gonorrhoeae, Mycoplasma Genitalium, and Other Sexually Transmitted Infections in Men Who Have Sex with Men in Lisbon. Int. J. STD AIDS 2024, 35, 379–388. [Google Scholar] [CrossRef]
- Dias, B.D.C.; Sekgele, W.; Nhlapo, D.; Mahlangu, M.P.; Venter, J.M.E.; Maseko, D.V.; Müller, E.E.; Greeves, M.; Botha, P.; Radebe, F.; et al. Extragenital Sexually Transmitted Infections Among High-Risk Men Who Have Sex With Men in Johannesburg, South Africa. Sex. Transm. Dis. 2024, 51, 245. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Nguyen, H.D.K.; Pham, M.Q.; Nguyen, C.T.; Adamson, P.C. Clinical Characteristics and Symptoms Associated with Mycoplasma Genitalium Infections among Heterosexual Men in Hanoi, Vietnam. Sex. Transm. Infect. 2025, 0, sextrans-2024-056396. [Google Scholar] [CrossRef]
- Clarivet, B.; Picot, E.; Marchandin, H.; Tribout, V.; Rachedi, N.; Schwartzentruber, E.; Ledésert, B.; Dereure, O.; Guillot, B.; Picot, M.-C. Prevalence of Chlamydia Trachomatis, Neisseria Gonorrhoeae and Mycoplasma Genitalium in Asymptomatic Patients under 30 Years of Age Screened in a French Sexually Transmitted Infections Clinic. Eur. J. Dermatol. 2014, 24, 611–616. [Google Scholar] [CrossRef]
- Ross, J.D.C.; Brown, L.; Saunders, P.; Alexander, S. Mycoplasma Genitalium in Asymptomatic Patients: Implications for Screening. Sex. Transm. Infect. 2009, 85, 436–437. [Google Scholar] [CrossRef]
- Sukhija-Cohen, A.C.; Patani, H.; Robinson, A.C.; Santos, M.R.; Granados, Y. Mycoplasma Genitalium Incidence, Coinfection, and Antibiotic Resistance: A Prospective Study at a Walk-In Clinic in Los Angeles County, CA. Open Forum. Infect. Dis. 2024, 11, ofae419. [Google Scholar] [CrossRef] [PubMed]
- Wenman, W.; Joffres, M.; Tataryn, I. A Prospective Cohort Study of Pregnancy Risk Factors and Birth Outcomes in Aboriginal Women. Can. Med. Assoc. J. 2004, 171, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Remis, R.S.; Liu, J.; Loutfy, M.; Tharao, W.; Rebbapragada, A.; Perusini, S.J.; Chieza, L.; Saunders, M.; Green-Walker, L.; Kaul, R. The Epidemiology of Sexually Transmitted Co-Infections in HIV-Positive and HIV-Negative African-Caribbean Women in Toronto. BMC Infect. Dis. 2013, 13, 550. [Google Scholar] [CrossRef]
- Sharp, A.; Sorokopud-Jones, M.; Haworth-Brockman, M.; Kasper, K.; MacKenzie, L.; Ireland, L.; Gawlik, K.; Lopez, L.; Vanegas, J.M.; Bullard, J.; et al. Sex Differences in Houselessness, Injection Drug Use, and Mental Health Conditions among People Newly Diagnosed with HIV in Manitoba, Canada from 2018 to 2021: A Retrospective Cohort Study. Lancet Reg. Health Am. 2024, 36, 100805. [Google Scholar] [CrossRef]
- Tosh, A.K.; Van Der Pol, B.; Fortenberry, J.D.; Williams, J.A.; Katz, B.P.; Batteiger, B.E.; Orr, D.P. Mycoplasma Genitalium among Adolescent Women and Their Partners. J. Adolesc. Health 2007, 40, 412–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manhart, L.E.; Leipertz, G.; Soge, O.O.; Jordan, S.J.; McNeil, C.; Pathela, P.; Reno, H.; Wendel, K.; Parker, A.; Geisler, W.M.; et al. Mycoplasma Genitalium in the US (MyGeniUS): Surveillance Data From Sexual Health Clinics in 4 US Regions. Clin. Infect. Dis. 2023, 77, 1449–1459. [Google Scholar] [CrossRef]
- Scoullar, M.J.L.; Melepia, P.; Peach, E.; Fidelis, R.; Supsup, H.; Davidson, E.M.; Boeuf, P.; Bradshaw, C.S.; Fehler, G.; Hezeri, P.; et al. Mycoplasma Genitalium in Pregnancy, Including Specific Co-Infections, Is Associated with Lower Birthweight: A Prospective Cohort Study. Med 2024, 5, 1123–1136.e3. [Google Scholar] [CrossRef]
- Frenzer, C.; Egli-Gany, D.; Vallely, L.M.; Vallely, A.J.; Low, N. Adverse Pregnancy and Perinatal Outcomes Associated with Mycoplasma Genitalium: Systematic Review and Meta-Analysis. Sex. Transm. Infect. 2022, 98, 222–227. [Google Scholar] [CrossRef]
- Latimer, R.L.; Shilling, H.S.; Vodstrcil, L.A.; Machalek, D.A.; Fairley, C.K.; Chow, E.P.F.; Read, T.R.; Bradshaw, C.S. Prevalence of Mycoplasma Genitalium by Anatomical Site in Men Who Have Sex with Men: A Systematic Review and Meta-Analysis. Sex. Transm. Infect. 2020, 96, 563–570. [Google Scholar] [CrossRef]
- McIver, R.; Jalocon, D.; McNulty, A.; Jeoffreys, N.J.; Chen, S.C.-A.; Power, M.; Couldwell, D.L. Men Who Have Sex With Men With Mycoplasma Genitalium–Positive Nongonococcal Urethritis Are More Likely to Have Macrolide-Resistant Strains Than Men With Only Female Partners: A Prospective Study. Sex. Transm. Dis. 2019, 46, 513. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Salado-Rasmussen, K.; Hansen, M.; Olsen, A.O.; Falk, M.; Golparian, D.; Aasterød, M.; Ringlander, J.; Nilsson, C.S.; Sundqvist, M.; et al. Clinical and Analytical Evaluation of the New Aptima Mycoplasma Genitalium Assay, with Data on M. Genitalium Prevalence and Antimicrobial Resistance in M. Genitalium in Denmark, Norway and Sweden in 2016. Clin. Microbiol. Infect. 2018, 24, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Hackett, A.; Yossepowitch, O.; Goor, Y.; Sheffer, R.; Schwartz, O.; Sheftel, Y.; Weiss, Y.; Maor, Y. Prevalence and Risk Factors for Antimicrobial Resistance of Mycoplasma Genitalium Infections in a High-Risk Population. J. Clin. Med. 2024, 13, 4924. [Google Scholar] [CrossRef]
- Chua, T.-P.; Danielewski, J.A.; Sweeney, E.L.; Plummer, E.L.; Bradshaw, C.S.; Whiley, D.M.; Machalek, D.A.; Garland, S.M.; Murray, G.L. Diversity of Mycoplasma Genitalium Strains in Australia: Relationship with Sexual Networks and Antimicrobial Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 1167–1175. [Google Scholar] [CrossRef]
- Li, Y.; Su, X.; Le, W.; Li, S.; Yang, Z.; Chaisson, C.; Madico, G.; Gong, X.; Reed, G.W.; Wang, B.; et al. Mycoplasma Genitalium in Symptomatic Male Urethritis: Macrolide Use Is Associated With Increased Resistance. Clin. Infect. Dis. 2020, 70, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Read, T.R.H.; Fairley, C.K.; Murray, G.L.; Jensen, J.S.; Danielewski, J.; Worthington, K.; Doyle, M.; Mokany, E.; Tan, L.; Chow, E.P.F.; et al. Outcomes of Resistance-Guided Sequential Treatment of Mycoplasma Genitalium Infections: A Prospective Evaluation. Clin. Infect. Dis. 2019, 68, 554–560. [Google Scholar] [CrossRef]
- Read, T.R.H.; Jensen, J.S.; Fairley, C.K.; Grant, M.; Danielewski, J.A.; Su, J.; Murray, G.L.; Chow, E.P.F.; Worthington, K.; Garland, S.M.; et al. Use of Pristinamycin for Macrolide-Resistant Mycoplasma Genitalium Infection. Emerg. Infect. Dis. 2018, 24, 328–335. [Google Scholar] [CrossRef]
- Doyle, M.; Vodstrcil, L.A.; Plummer, E.L.; Aguirre, I.; Fairley, C.K.; Bradshaw, C.S. Nonquinolone Options for the Treatment of Mycoplasma Genitalium in the Era of Increased Resistance. Open Forum. Infect. Dis. 2020, 7, ofaa291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).