Abstract
This study examined the temporal dynamics of metal(loid) concentrations in agricultural soils, fern Amauropelta rivularioides, and surface waters in a peri-urban region on the Brazil–Paraguay border during 2019–2020. Elevated levels of As, Se, Co, Mn, Cu, and Zn raised concerns about environmental and human health risks, especially when compared to international guidelines. Post-harvest and pre-harvest periods, particularly during corn cultivation, revealed higher concentrations of toxic metals, suggesting cumulative effects of agrochemical use. Principal Component Analysis indicated significant geochemical variation, with particular emphasis on the Collection 1 period (1 June 2019). The fern A. rivularioides demonstrated metal accumulation, especially for As, Pb, Cr, and Ba, reflecting the influence of agrochemical residues and seasonal runoff. Surface waters displayed metal concentrations below detection limits, but phosphorus levels surpassed USEPA thresholds for eutrophication risk. Risk assessments indicated moderate to high contamination in soils, particularly for P, As, Mg, and Se. Hazard Quotient and Hazard Index values suggested chronic health risks, and Incremental Lifetime Cancer Risk values for dermal exposure to As, Pb, and Cr indicated an elevated cancer risk.
1. Introduction
Peri-urban agriculture, practiced in the transitional zones between urban and rural areas, has emerged as a strategy to promote food security, income generation, and environmental sustainability [1]. However, when poorly managed, it can expose communities to environmental risks, such as the improper use of pesticides or contamination by heavy metals [2]. Its proximity to urban zones also makes these areas vulnerable to industrial pollution and the use of wastewater. Therefore, proper management is essential to maximize benefits and minimize risks to public health and the environment [3].
Among the key environmental challenges, elevated concentrations of phosphorus (P) and nitrogen (N) in surface waters—primarily resulting from urban and peri-urban agricultural activities—stand out as major contributors to the eutrophication process [1,2,3]. This process leads to excessive algal blooms, reduced dissolved oxygen levels, and significant alterations in aquatic biodiversity. Additionally, elements such as potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) can indirectly influence eutrophication by altering the physicochemical properties of the water and affecting nutrient cycling dynamics [3].
Heavy metals, in addition to contaminating soil and water, can also be incorporated by biota and, consequently, reach humans through the food chain. Elements such as lead (Pb), arsenic (As), cadmium (Cd), mercury (Hg), copper (Cu), chromium (Cr), nickel (Ni), and zinc (Zn) are frequently detected in urban and peri-urban environments, especially in agricultural areas close to sources of industrial pollution or exposed to the use of wastewater for irrigation [4,5,6]. These elements are highly toxic even at relatively low concentrations and tend to accumulate in living organisms, potentially causing adverse health effects, such as neurological, liver, and kidney dysfunctions and even cancer [7,8,9].
Heavy metals in soil and water are significant risk factors for human health, especially in peri-urban areas with intense agricultural or industrial activity [10]. Several studies have shown that elements such as arsenic (As), cadmium (Cd), lead (Pb), chromium (Cr), nickel (Ni), copper (Cu), mercury (Hg), manganese (Mn), zinc (Zn) and cobalt (Co) are frequently detected in contaminated environments and may come into contact with human skin during daily, agricultural, or recreational activities [11,12,13].
Although the dermal route is generally considered less significant than ingestion or inhalation, cutaneous absorption of metals can be substantial, depending on the duration of exposure, the concentration of the contaminant, the chemical form involved, and the characteristics of the skin [14]. For example, hexavalent chromium (Cr6+) is recognized for its ability to cross the skin barrier and can cause dermatitis and neoplastic lesions with chronic exposure [15]. Nickel (Ni) is a common allergen associated with hypersensitivity reactions and allergic contact dermatitis [16]. Arsenic (As) can lead to chronic skin lesions, such as hyperkeratosis, melanosis, and skin cancer, particularly after prolonged exposure in areas with contaminated water [17,18].
Additionally, metals such as copper (Cu), manganese (Mn), and zinc (Zn) can cause dermal irritation and contribute to metabolic dysfunction when present in high concentrations in surface water [19]. Although dermal absorption of lead (Pb) and cadmium (Cd) is limited, prolonged exposure can lead to bioaccumulation and result in systemic effects, including neurological, hematological, and renal alterations [20,21]. Therefore, dermal exposure to heavy metals in contaminated soils and waters should be accounted for in environmental and public health risk assessments, particularly in regions where peri-urban agricultural practices overlap with urbanized areas and potential sources of pollution. Monitoring measures, environmental education, and the safe use of natural resources are crucial to mitigating such risks. However, there is a lack of studies evaluating dermal health risks from soil and water contamination in peri-urban areas.
The increasing contamination of soils and water bodies by heavy metals has raised environmental and public health concerns due to the toxicity, persistence, and bioaccumulation of these elements in ecosystems [22]. In this context, the use of plants as bioindicators has emerged as an efficient and low-cost strategy for detecting and monitoring heavy metal pollution. Ferns, for example, have been widely studied for their ability to absorb and accumulate metals such as arsenic, cadmium, and lead, making them useful both for phytoremediation and environmental quality monitoring [23,24]. On the other hand, species from the Apiaceae family, known for their broad distribution and adaptation to anthropized environments, have also shown potential for accumulating heavy metals in different plant tissues, which makes them suitable for monitoring soils contaminated by agricultural and industrial activities [25]. Thus, the use of plants as bioindicators for heavy metal contamination has gained increasing attention in environmental monitoring due to their capacity to reflect soil and atmospheric metal burdens.
In a recent study, Cakaj et al. (2024) [26] demonstrated the efficacy of various plant species in accumulating and signaling the presence of metals such as Pb, Cd, Zn, and Ni across diverse environmental settings. Their findings reinforce the importance of selecting appropriate plant taxa based on metal specificity and accumulation potential, highlighting that certain species not only tolerate but also reflect spatial variations in pollution levels [26]. This supports the integration of biomonitoring strategies in peri-urban agricultural landscapes, where continuous exposure to anthropogenic contaminants necessitates cost-effective and ecologically sound surveillance tools [27]. Nevertheless, few studies have quantified heavy metals in soils, river waters, and native plants specifically in peri-urban border regions between Brazil and Paraguay.
Most previous studies have focused on regions with a long history of industrial contamination or on urban areas with wastewater irrigation practices, especially in Asia, Europe, and North America [4,5,6,10,11,12,13,26]. Andrade et al. (2024) [27] investigated native species for urban metal pollution in industrial regions of Brazil, but their focus was on atmospheric deposition rather than peri-urban agricultural systems. Furthermore, many of these studies have prioritized the ingestion and inhalation pathways over dermal exposure, limiting the understanding of cutaneous risk in tropical, peri-urban agricultural settings [14,19]. While some research quantified metal accumulation in vegetables or crops for human consumption [4,5], few have assessed simultaneous contamination levels in soil, water, and local bioindicator plants, especially through temporal monitoring that considers agricultural cycles.
The intensification of modern agriculture, especially in monocultures such as soybean and corn, has significantly contributed to changes in soil and water quality, including the accumulation of heavy metals. These elements, such as cadmium (Cd), lead (Pb), arsenic (As), chromium (Cr), nickel (Ni), zinc (Zn), and copper (Cu), can be introduced into agroecosystems through phosphate fertilizers, soil amendments, pesticides, and irrigation with contaminated water [28,29]. In addition, studies have shown that the concentration of heavy metals in soil can vary over the years depending on planting intensity and frequency, management practices, and local climatic conditions [30].
In soils used for continuous cultivation of soybeans, for instance, a gradual accumulation of metals such as Cd, Cu, Zn, and Pb has been observed, especially in areas with intensive use of agricultural inputs [31]. This accumulation may negatively impact soil microbiota, reduce long-term productivity, and affect groundwater quality through metal leaching [32]. Additionally, plants grown in such environments may absorb these elements through their roots and translocate them to edible parts, increasing the risk of human exposure via the food chain [33]. Previous research on plant-based bioindicators of heavy metal contamination has significantly contributed to identifying hyperaccumulator species [23] and understanding physiological mechanisms of metal uptake and translocation [24,25]. Other studies focused on single-season assessments [5,6,12,33] or used plants mainly for source apportionment in industrial contexts [26,27]. However, most previous studies presented two main limitations: (1) lack of integrated multi-matrix monitoring (soil, water, and plants simultaneously) under real agricultural field conditions; and (2) absence of seasonal or agricultural-cycle-based temporal assessments.
Annual variation in heavy metal concentrations can also be related to factors such as crop rotation, soil type, sampling depth, and precipitation regime, which influence metal mobility within the soil profile [34]. Therefore, continuous monitoring of these contaminants is essential for environmental risk assessment and the development of public policies aimed at sustainable agriculture. Thus, soils, water, and plants due to their great availability close to peri-urban areas avenues with high traffic of vehicles and quantity in the state of Mato Grosso do Sul, Central-West Brazil, can be used in studies to investigate the deposition and accumulation of metal(loid)s in the environment. Another critical knowledge gap lies in the geographic focus. There is a scarcity of research addressing the border regions between Brazil and Paraguay, specifically the peri-urban areas of Central-West Brazil, where agricultural intensification (soybean and corn monocultures) coexists with environmental vulnerability and cross-border pollution potential. To our knowledge, no previous study has assessed the seasonal variation in heavy metal concentrations in soils, water bodies, and native plants in this region, nor evaluated dermal exposure risks from these combined environmental matrices under real agricultural conditions.
Overall, analytical methods as Atomic Absorption Spectroscopy (AAS) [5,6,10,12], Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) [10,26,29,34], and Inductively Coupled Plasma Mass Spectrometry (ICP-MS) [4] were essential for accurately detecting metal concentrations, assessing environmental risks, and evaluating potential human exposure through food and soil contact. However, these studies do not simultaneously consider dermal exposure to metal(loids) in water and soil, calculate the Geoaccumulation Index (Igeo), Biological accumulation coefficient (BAC) of heavy metals, Contamination factor (CF), and Pollution load index (PLI).
Given these research gaps, this study presents a regionally novel, multicomponent approach, simultaneously assessing advances in the field of plant-based heavy metal biomonitoring, providing an integrated and time-resolved assessment of metal contamination in soil, water, and native vegetation matrices in a peri-urban agricultural area located along the Santa Virgem River, in Mato Grosso do Sul (MS), Brazil, close to the Brazil–Paraguay border. The Santa Virgem River represents a critical case study area due to its dual use: agricultural activities are developed along its banks, while downstream segments are frequently used for recreational purposes, such as bathing and fishing, thus increasing human exposure potential to contaminated soils and waters. By incorporating ferns as bioindicators, this study extends the bioaccumulation assessment beyond traditional crop species, addressing specific knowledge gaps in tropical, peri-urban contexts highlighted by FAO (2022) [3] and Gunapala et al. (2025) [1]. Moreover, this work integrates ecological indices (CF, BAC, PLI) with human health risk metrics (Chronic Daily Dose (CDD), Hazard Quotient (HQ), Hazard Index (HI) and Incremental Lifetime Cancer Risk (ILCR)) focused on dermal exposure—an exposure route largely overlooked in previous regional and international studies [8,13].
Therefore, this study focuses on the Santa Virgem River region, a peri-urban area in the state of Mato Grosso do Sul, Brazil, and aims to (1) quantify the concentrations of arsenic (As), barium (Ba), calcium (Ca), cobalt (Co), chromium (Cr), copper (Cu), potassium (K), magnesium (Mg), manganese (Mn), nickel (Ni), phosphorus (P), sulfur (S), selenium (Se), zinc (Zn), and lead (Pb) in soil, surface water, and fern (Amauropelta rivularioides (Fée) Salino & T.E.Almeida) using Inductively Coupled Plasma Optical Emission Epectroscopy (ICP OES); (2) assess temporal variations in metal concentrations across different agricultural cycles (e.g., soybean and corn planting), to understand how seasonal practices and crop rotation influence environmental contamination levels; (3) evaluate the ecological risk through indices such as the Contamination Factor (CF), Pollution Load Index (PLI), and Biological Accumulation Coefficient (BAC); (4) estimate human health risks related to dermal exposure to contaminated water and soil by calculating Chronic Daily Dose (CDD), Hazard Quotient (HQ), Hazard Index (HI), and Incremental Lifetime Cancer Risk (ILCR); and (5) investigate the potential of selected A. rivularioides plant as bioindicators, highlighting their ability to accumulate metal(loid)s.
2. Materials and Methods
2.1. Study Area
The Santa Virgem River extends for 23.5 km and is located 18 km from the district of Nova Itamarati, which currently has 17,000 inhabitants [35]. This district, which belongs to the municipality of Ponta Porã, state of Mato Grosso do Sul (MS), is located on the border between Brazil and Paraguay. The Santa Virgem River is a tributary of the right bank of the Santa Virgínia River; it forms the border between the municipalities of Antônio João and Ponta Porã, Paraná River basin, Brazil [36]. The region where the Santa Virgem River is located is characterized by rivers and streams that play a fundamental role in territorial delimitation and local water supply (Figure 1).
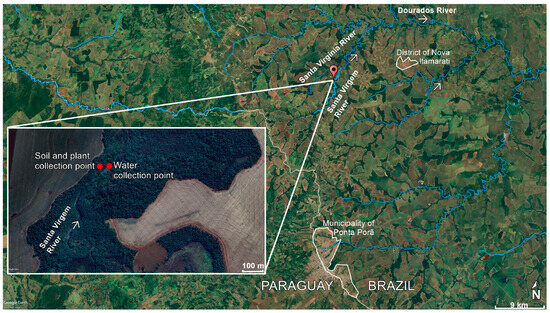
Figure 1.
Soil, plant, and water collection points in the Santa Virgem River, state of Mato Grosso do Sul, Central-West Brazil. The arrows indicate the flow of rivers (Satellite image from Google Earth).
The Paraná River basin, of which the Santa Virgem River is a part, is one of the most important in Brazil, contributing significantly to the hydrography and regional economy. According to IMASUL [36], this region lies within the “Faixa de Fronteira” geo-environment, characterized by undulating planation surfaces underlain by sedimentary rocks of the Paraná Basin, particularly the Serra Geral basalts (underlain by Jurassic–Cretaceous Formation basalts) and overlying Caiuá sandstones. The basaltic flows in the study area weather into nutrient-rich latossolos, which are classified as Ferralsols under the WRB (Working Group WRB) system and are typical of the so-called “red earth” soils. In contrast, the quartz-rich Caiuá arenites produce sandy soils with low natural fertility, corresponding mainly to Acrisols or Arenosols, depending on their textural characteristics and degree of weathering. Regarding vegetation, the region was originally dominated by the Cerrado biome, which has now been almost completely degraded due to extensive agricultural expansion and pasture planting. This area is part of the Semideciduous Seasonal Forest domain, now a mosaic of remnant natural vegetation patches interspersed with croplands and grazing lands. The climate is classified as humid subtropical in the southern part of Mato Grosso do Sul, with temperatures in the coldest months ranging from 14 °C to 15 °C and occasional frost events. The effective humidity index varies annually between 40% and 60%. Average annual rainfall ranges from 1500 to 1700 mm, with a positive water balance of 800 to 1200 mm for five to six months and a water deficit of 350 to 500 mm over four months.
In addition to its geographical importance, the Santa Virgem River and its tributaries play a crucial role in the local ecosystem, sustaining biodiversity and providing essential water resources for the communities and economic activities of the region [36]. Due to the presence of waterfalls and rapids in its bed, this river is used by bathers and fishermen on weekends as a form of leisure for the local population.
The Santa Virgem River, in turn, crosses agricultural and livestock farms. Additionally, according to an on-site survey, there is fish farming in cages and net tanks on its banks, which is one of the most intensive forms of farming currently practiced in Brazil. The Santa Virgem River also serves as a leisure or fishing spot for people living near the Nova Itamarati district.
Due to the great anthropogenic activity carried out on the banks of the Santa Virgem River, we carried out 5 collections of soil, plant, and water samples on the banks of this river between 2019 and 2022. Table 1 shows the dates of collection of the samples carried out during the soybean and corn planting period.

Table 1.
Dates of collection of soil, plant, and water samples.
2.2. Soil Collection Procedures
Twenty-five soil samples were collected, each weighing approximately 300 g, on five different dates, over different years (Table 1). Five soil samples were collected at each sampling site to ensure the representativeness of the points analyzed. The collections took place near the slope of the Santa Virgem River (Latitude 22°13′31″ S; Longitude 55°42′41″ W), approximately 50 m from a corn and soybean plantation (Figure 1). Soil samples were collected either after planting or after harvesting soybeans and corn, specifically during the inter-harvest and post-planting period for both crops (Table 1). To ensure the quality of the samples, the surface layer containing leaves and organic matter was carefully removed with the help of a hoe, avoiding contamination. The samples were extracted from a depth of 20 to 30 cm, using only the central portion (core), with the sides discarded to minimize external interference. All soil collections were carried out strictly following the guidelines in the Embrapa manual for soil collection [37]. After extraction, the samples were properly stored in sterile plastic bags, ensuring the preservation of their characteristics for subsequent analyses.
2.3. Specimen Collection and Plant Identification
During the five different dates over different years—1 June 2019; 24 August 2019; 28 December 2019; 9 April 2020; and 7 August 2020—a total of 25 plants were collected, with five samples of plants obtained during each period. In each phase, samples were obtained from five distinct points within the same representative location. This study focused on plants located near the slope of the Santa Virgem River, in agricultural areas close to the Nova Itamarati district, in the municipality of Ponta Porã (MS), Brazil, near the border with Paraguay. Plant samples were collected either after planting or after harvesting soybeans and corn, specifically during the off-season and the post-planting period for both crops. The plant collected was the fern Amauropelta rivularioides (Fée) Salino & T.E.Almeida. From each plant, 50–150 g of samples such as leaves and stems were collected, which were stored in sterile sample bags. The distance between the sample collection site and the Nova Itamarati district is approximately 7 km (Figure 1).
At each collection site, after drying and preparing the samples, the specimen of the plant was sent to the Herbarium of the Federal University of Mato Grosso do Sul (UFMS), Brazil. The identification revealed that the species corresponds to a fern, belonging to the pteridophyte group (Order Polypodiales, Family Thelypteridaceae), and was cataloged under specimen number CGMS 37791.
2.4. Procedure for Collecting Water
In total, 25 water samples were collected during the same periods as the soil and plant sampling: 1 June 2019; 24 August 2019; 28 December 2019; 9 April 2020; and 7 August 2020—corresponding to off-season and post-planting phases of corn and soybean cultivation. For each sampling period (C1 to C5), five 50 mL water samples were manually collected near the banks of the Santa Virgem River by directly submerging sterile 50 mL Falcon tubes to a depth of 10–20 cm. All tubes were labeled on site, acidified with 1 mL of nitric acid (HNO3, 65%, ultrapure grade, Merck, Darmstadt, Germany) to preserve metal(loid) content, transported in refrigerators, and stored at 4 °C until analysis [38]. Water temperature and pH were measured in situ using a HI98107 pHep® pocket pH meter (Hanna Instruments, Woonsocket, RI, USA), which simultaneously records pH and temperature with an accuracy of ±0.1 pH units and ±0.5 °C. The estimated average water temperatures during the sampling periods were approximately 20 °C in June 2019 (C1), 22 °C in August 2019 (C2 and C5), 27 °C in December 2019 (C3), and 24 °C in April 2020 (C4). The pH of the Santa Virgem River water ranged from 6.7 to 7.2 in C1, 6.6–7.1 in C2, 6.8–7.4 in C3, 6.7–7.3 in C4, and 6.5–7.0 in C5.
2.5. Soil Digestion Procedure
Soil samples were placed in an oven and dried at 50° C for 72 h until reaching a constant weight. Approximately 25 mg of each soil sample from different days and years was weighed directly into a 25 mL beaker. Then, 3 mL of hydrogen peroxide (H2O2, 35%, ultrapure grade, Merck, Darmstadt, Germany), 6 mL of high-purity water (18 MΩ cm, Milli-Q, Millipore, Middlesex, MA, USA), and 3 mL of nitric acid (HNO3, 65%, ultrapure grade, Merck, Darmstadt, Germany) were added for acid digestion. The samples were left in suspension for 20 min until the mixture stabilized, and then they were inserted into an ultrasonic sterilizer for 60 s at 20% power (Sonics & Materials. INC., Newtown, CT, USA, Model VCX 750 Watts, Frequency 20 KHz). The cycle described above should be performed 4 times until the sample becomes homogeneous and clear, a methodology adapted from Ramanathan and Ting (2015) [39]. This wet acid digestion protocol is analogous to the methodology validated by USEPA Method 3050B for sediments and soils, as well as closely matching the non-microwave compared procedures shown to yield accurate and precise trace-element recovery in biological matrices [39,40,41]. The experimental procedure was performed in triplicate.
2.6. Plant Digestion Procedure
The harvested plants (roots, stems, and leaves) were washed with high-purity water (18 MΩcm, Milli-Q Millipore, Middlesex, MA, USA) to remove debris and reduce fine dirt such as mud. After leaf samples from each plant were placed in an oven and subjected to a drying process at 40° C for 24 h until reaching a constant weight. Approximately 250 g of dried leaf samples from each plant were separately ground using a portable stainless steel electric grinder to obtain a very fine powder (Thermomix, São Paulo, SP, Brazil) and then sieved (stainless steel sieve, 200 μm particle size). Then, approximately 0.25 g of each leaf sample from different planting periods was individually placed in the digestion vessels and 3.0 mL of nitric acid (HNO3, 65%, ultrapure grade, Merck, Darmstadt, Germany), 1 mL of high-purity water (18 MΩ cm, Milli-Q, Millipore, Bedford, MA, USA), and 2 mL of hydrogen peroxide (H2O2, 35%, ultrapure grade, Merck, Darmstadt, Germany) were added [39,40]. The sample preparation technique used was Open Acid Digestion (Tecnal, São Paulo, Brazil), according to Junior et al. (2024) [40]. The samples were then stored in airtight bags at 4° C for further analysis.
2.7. Quantification Using ICP OES
The concentration of As, Ba, Ca, Co, Cr, Cu, K, Mg, Mn, Ni, P, S, Se, Zn, and Pb in the soils, plants (roots, stem, leaves), and water collected in different periods of the year were determined by inductively coupled plasma optical emission spectroscopy (ICP OES) (iCAP 6300 Duo, Thermo Fisher Scientific, Bremen, Germany). The ICP OES parameters used are summarized as follows: sample flow rate = 0.35 L mn−1; plasma gas flow rate = 12 L mn−1; power = 1250 W; Integration time = 5 s; pressure of nebulization = 20 psi; plasm view = axial, stabilization time = 20 s; gas view: air. In addition, the following emission wavelengths were set and are used by the ICP OES for analysis of each of the elements: As 193.759 nm, Ca 422.673 nm, Co 228.616 nm, Cr 267.716 nm, Cu 324.754 nm, K 766.490 nm, Mg 279.553 nm, Mn 257.610 nm, Ni 231.604 nm, P 177.495 nm, S 180.731 nm, Se 196.090 nm, Zn 213.856 nm, and Pb 220.353 nm [40,41].
The calibration curves for quantification of the element concentrations were prepared from standard solutions of 100 mg/L (SpecSol, Quinlab, Brazil) using ultrapure water (18 MΩcm, Milli-Q Millipore, Bedford, MA, USA). External calibration curves were built on five different concentrations in the range of 0.005–1 mg/L. The fundamental parameters to ensure the acceptability of the performance of our analytical method validation were the accuracy (recovery test), limit of detection (LOD), and limit of quantification (LOQ). The limits of detection (LODs) and limits of quantification (LOQs) were calculated according to Rosa et al. (2022) [40,41]. Thus, the solution was prepared by adding 1000 ppm (100 mg/L) of an analyte to a sample, and a 0.25 mg/L recovery test was conducted. The values of LOD, LOQ, correlation coefficients (R2), and spike concentration obtained by external calibration are shown in Table 2. The method had a recovery interval of 81–112%. Then, the range of all elements’ LOD was 0.00026–0.20316 mg/L, and the range of all elements LOQ was 0.00088–0.67721 mg/L. The range of the correlation coefficient (R2) was 0.9851–0.9998.

Table 2.
Values of limits of detection (LODs), limits of quantification (LOQs), correlation coefficients (R2), and spike concentration obtained by external calibration.
2.8. Calculation of Geoaccumulation Index (Igeo)
The Geoaccumulation Index (Igeo) was calculated using the equation proposed by Muller (1969) [42]. This index was introduced in the 1960s and is used to quantify metal pollution in soils and aquatic sediments. It is calculated using a specific equation.
where Cn represents the measured metal concentration in the sample, Bn is the background concentration of the corresponding metal, and 1.5 is a correction factor that accounts for natural lithological variations. Muller (1969) [42] categorized Igeo into seven pollution levels: Igeo ≤ 0: practically unpolluted; 0 < Igeo ≤ 1: unpolluted to moderately polluted; 1 < Igeo ≤ 2: moderately polluted; 2 < Igeo ≤ 3: moderately to strongly polluted; 3 < Igeo ≤ 4: strongly polluted; 4 < Igeo ≤ 5: strongly to extremely polluted; Igeo > 5: extremely polluted. Thus, this classification helps evaluate the extent of metal contamination in different environmental settings, aiding in pollution monitoring and risk assessment. In our study, we used as quality reference values (Bn) for the elements As (3.17 mg/kg), Cd (0.07 mg/kg), Co (11.68 mg/kg), Cu (28.49 mg/kg), Cr (30.30 mg/kg), Ni (8.61 mg/kg), Ba (67.70 mg/kg), Zn (16.40 mg/kg), and Pb (11.05 mg/kg) those stipulated for soils in the state of Mato Grosso do Sul, Brazil [43], and for Mn and S, the values 183 mg/kg and 1.700 mg/kg obtained in studies in the state of Rio Grande do Sul, Brazil [44]. The Bn values of Ca, K, and Mg refer to soils from southeast of the state of Pará, Brazil, with minimums of 104.18 mg/kg (Ca and K) and 158.08 mg/kg (Mg) [45]. The minimum Bn values for P and Se were 1.0 mg/kg [46] and 1.61 mg/kg [47].
2.9. Calculation of Biological Accumulation Coefficient (BAC) of Heavy Metals
The biological accumulation coefficient (BAC) measures the ratio of heavy metals in an organism compared to its environment (water or soil). It is used to identify hyperaccumulator plants, assess their phytoremediation potential, and predict chemical interactions with organisms [48]. BAC is calculated as the ratio of metal concentration in the plant to that in the soil, estimating the plant’s ability to accumulate metals; it can be calculated by the following equation:
where Cplant is the concentration of heavy metal(loid)s in the plant; Csoil is the concentration of heavy metals in soil. When BAC ≤ 1, it indicates that the plant can only absorb but not accumulate heavy metals; on the other hand, if BAC > 1, it shows that the plant can accumulate metals [48].
2.10. Calculation of Contamination Factor (CF) and Pollution Load Index (PLI)
The contamination factor (CF) provides the estimation of contamination by considering the quantification of metal(loid) concentrations from soils to their background value concentrations. The equation for contamination factor (CF) is as follows [49].
where Cn is the concentration of the metal(loid)s in soil and Cs is the standard pre-industrial reference level or the background values of metal(loid)s in earth’s crust. When the contamination value is CF < 1, there is low contamination, if 1 < CF < 3—moderate contamination, 3 < CF < 6—considerable contamination, and CF > 6—very high contamination. In our study, we adopted the quality reference values Cs = Bn for each element based on Brazilian soil standards and literature. The Cs = Bn values used were the following: As = 3.17 mg/kg, Cd = 0.07 mg/kg, Co = 11.68 mg/kg, Cu = 28.49 mg/kg, Cr = 30.30 mg/kg, Ni = 8.61 mg/kg, Ba = 67.70 mg/kg, Zn = 16.40 mg/kg, and Pb = 11.05 mg/kg, as stipulated for soils in the state of Mato Grosso do Sul, Brazil [43]. For Mn and S, the reference values were Mn = 183 mg/kg and S = 1.700 mg/kg, based on studies conducted in the state of Rio Grande do Sul, Brazil [44]. The values for Ca = 104.18 mg/kg, k = 104.18 mg/kg, and Mg = 158.08 mg/kg were obtained from soils in southeast of the state of Pará, Brazil [45]. For P and Se, we used P = 1.0 mg/kg [46] and Se = 1.61 mg/kg [47].
The pollution load index (PLI) was obtained according to Belzunce et al. (2004) [50]. In this case, PLI < 1 is considered no pollution load by toxic elements, PLI = 1 indicates that baseline levels of pollutants are present, and PLI > 1 indicates that it is polluted. The PLI was calculated using Equation (3) [50].
where CF is the contamination factor (Equation (2)), and n is the number of metal(loid)s.
2.11. Calculation of Human Health Risk Assessment of Heavy Metals
Dermal exposure can result from skin contact with contaminated environmental media, including water (during bathing, washing, and swimming) or soil (e.g., while wading or fishing). Here, there are two pathways of metal(loid)s exposures associated with soil. Chronic daily dose (CDD: unit mg/kg·day) was calculated for (i) dermal exposures to metal(loid)s in water (Equation (5)) [51]; and (ii) dermal exposure to metal(loid)s in soil (Equation (6)) [52]. It is given by the following equations:
The following exposure conditions in Equation (5) are used to calculate exposure to metal(loid)s in water through showering, assuming non-carcinogenic effects: Cwater is the concentration of metal(loid)s in water (mg/kg) obtained by ICP OES; SA is the surface body area of an adult given as 18,000 cm2; Kp (cm/h) represents the dermal permeability coefficient in water; 0.001 cm/h for As, Cu Se, and Mn, 0.0001 cm/h for Pb, 0.002 cm/h for Cr, and 0.0006 cm/h for Zn, 0.0002 cm/h for Ni [14], and 0.004 for Co [53]. There is no value of the dermal permeability coefficient in water for P, S, K, Mg, and Ca; however, in the present study, considering a default conservative Kp for ionic metals in water with a value of 0.01 cm/h [54]. From the swimming river, the average swim time per day (exposure time) is 7 h (i.e., 7 h/days), EF = exposure frequency (60 days/year), ED = exposure time (30 years), BW = body weight (70 kg), AT = ED × 365 is the averaging time for noncarcinogenic effects (AT = 30 years × 365 days/year = 10,950 days); however, AT for carcinogens (As, Cr, Ni, and Pb) correspond to AT = 70 years/days × 365 days = 25,550 days [14].
Where in Equation (6), Csoil is the concentration of chemical elements in soil samples quantification by ICP OES, SL = soil adherence factor (0.1 mg/cm2·day), SA = skin surface area available for contact with soil, that is, the exposed skin surface was limited to the hands, forearms, feet and lower legs, surface area of adults (6170 cm2); ABS = dermal absorption fraction from soil, 0.03 for As and 0.001 for others elements [14]. The parameters EF, ED, BW, and AT were the same in Equation (5) described above.
As five sampling collections (C1, C2, C3, C4, and C5) were conducted during the years 2019 and 2020 (Table 1), it was considered that, for each collection, the quantified concentration values represent the exposure conditions for that period. These values were used to estimate the Chronic Daily Dose (CDD) for the assessment of human health risks through dermal contact with both water and soil.
2.12. Calculation of Hazard Quotient (HQ) and Hazard Index (HI)
The non-carcinogenic health hazards through dermal exposures to metal(loid)s in water (Equation (5)), and dermal exposure to metal(loid)s in soil (Equation (6)) were evaluated by the target hazard quotient (HQ) using Equation (7) [14].
Here, the CDDdermal/β was obtained in Equations (5) and (6) for each route of exposure, and RfDβ is the reference dose. That is, in Equation (5), the subscript β = water corresponds to dermal exposures to metal(loid)s in water, and β = soil–dermal reference dose to dermal exposure to metal(loid)s in soil (Equation (6)). Therefore, this study considered the reference dose values shown in Table 3. To obtain the values of the dermal reference dose due to water contact with the skin, the equation RfDwater = RfD × ABSd (ABSd = dermal absorption fraction from Soil) [14] was used, and for soil contact with the skin: RfDsoil = RfD × GIABS (where GIABS is the fraction of contaminant absorbed in the gastrointestinal tract, dimensionless) [14]. RfD is the reference dose for oral exposure (mg/kg/day) from EPA/IRIS, ATSDR [14]. This adjustment is necessary because RfD oral assumes 100% absorption, but only a fraction of that amount is likely absorbed through the skin.

Table 3.
Chemical elements, RfDwater corresponds to dermal exposure to metal(loids) in water, and RfDsoil—dermal reference dose to dermal exposure to metal(loids) in soil.
The non-carcinogenic risk was estimated based on the hazard index (HI) as given in Equation (8).
According to Equation (8), the hazard index recorded for adults was obtained as follows: HI = HQAs + HQCa + HQCo + HQCr + HQCu + HQK + HQMg + HQMn + HQNi + HQP + HQS + HQSe + HQZn + HQPb, for each different contact with skin (water + dermal and soil + dermal). If HI < 1, exposures are unlikely to result in non-cancer adverse health effects during the lifetime of exposure; however, when HI > 1, exposure may pose a health risk.
2.13. Calculation of Carcinogenic Risk
The assessment of cancer risk due to dermal contact with water and soil containing metal(loid)s was assessed using the Incremental Lifetime Cancer Risk (ILCR). That is, ILCR represents the incremental (additional) probability that a person will develop cancer as a result of chronic exposure to a carcinogenic agent, typically over a 70-year lifetime. The cancer risk assessment was estimated by use of the following equation [40]:
where the subscript ILCRdermal/β corresponds to risk due to dermal contact with water and soil, and CDDdermal/water and CDDdermal/soil are represented in Equations (5) and (6), CSF = Slope Factor (mg/kg/day)−1—a carcinogenic potency factor determined by regulatory agencies (e.g., USEPA) [14]. The following values were considered for CSF: oral CSF—Cr 0.5, Pb 0.0085, and As 1.5. However, dermal slope factors are not always directly provided in databases. Often, they are derived by adjusting oral slope factors using the dermal absorption fraction (ABS) [14], that is,
CSFdermal = CSForal/ABS
CSFdermal = 50 mg/kg/day for As, CSFdermal = 8.5 mg/kg/day for Pb, and 500 mg/kg/day for Cr. The permissible limits are considered to be 10−6 and <10−4 for a single carcinogenic element and multi-element carcinogens. The carcinogenic risk level was classified as <10−6 estimated as a very low level (considered negligible); 10−6−10−5—estimated as a low level; 10−5−10−4—the medium level; while 10−4−10−3—high level and >10−3—estimated as a very high level are considered unacceptable [55].
The total excess lifetime cancer risk for an individual is finally calculated from the average contribution of the individual heavy metals for all the pathways using the following equation:
For the risk calculation, Equation (11) considers collections C1, C2, C3, C4, and C5.
2.14. Statistical Analysis
Data were processed using the Origin 9.0 software (OriginLab Corporation, Northampton, MA, USA). Concentrations were expressed as mean (±standard deviation). Prior to the statistical analyses, data normality was assessed using the Shapiro–Wilk test with a significance level of α = 0.05. For normally distributed data (p > 0.05), the mean and standard deviation were calculated; for non-normally distributed data (p < 0.05), the median and interquartile range (IQR) were used instead. One-way analysis of variance (ANOVA) was performed to test for differences in element levels in plant and soil samples across the different collection sites. When significant differences were found, complementary pairwise comparisons were conducted using Student’s t-test to identify which groups differed significantly. Principal Component Analysis (PCA) was performed respecting the appropriate ratio between the number of samples and the number of variables, to explore correlations and patterns among the elements.
3. Results
3.1. Results of Concentration of Meta(loid)s in Soil
Table 4 shows the concentrations of metal(loid)s quantified in soil samples collected near the banks of the Santa Virgem River, located close to the Brazil–Paraguay border and influenced by agricultural activities and recreational use, considering the five sampling periods (C1 to C5) conducted between June 2019 and August 2020, each corresponding to specific agricultural periods: post-harvest corn (C1), inter-harvest (C2), post-planting soybean (C3), inter-harvest (C4), and before corn harvest (C5). In addition, the values of concentrations of elements obtained in soil collected near the banks of the Santa Virgem River were compared to threshold values of Brazil National Council of Environment (Conama/Brazil) determined from human health-based risk analysis [56], and compared to the Soil Metal Background Concentrations from Alabama/USA [57], and forested soils of the state of Pará, Brazilian Amazon [58]. The Brazilian resolution establishes the prevention values (PVs), which are established by the Conama Resolution and are valid for the whole country [56]. These values represent the maximum permitted concentrations that guarantee the maintenance of the natural functions of the soil, as well as the protection of human health and the environment. Risk values for Al, Cr, and Fe in soils were not established by Brazilian legislation (Conama). In addition, according to Conama (2009) [56], there are no concentration values for Ca, K, Mg, Mn, and S for soil (Table 3).

Table 4.
Concentration of metal(loid)s (mean ± standard deviation, in mg/kg) quantified in soil samples collected on the banks of the Santa Virgem River compared to Conama, Brazil (2009) [56]; soils with Soil Metal Background Concentrations from Alabama, USA (USEPA) [57]; and Forested Soils of the state of Pará, Brazilian Amazon [58].
Figure 2 shows the Principal Component Analysis (PCA) performed using the soil metal(loid) concentration data presented in Table 4. The analysis aimed to identify patterns and relationships among the quantified elements across the different collection periods along the banks of the Santa Virgem River. The two main components, PC1 and PC2, explain together 81.12% of the total variance (PC1 = 57.13%; PC2 = 23.99%), demonstrating a strong ability of the model to describe the data variability. The distribution of chemical elements across the PC1 and PC2 axes highlights temporal differences associated with the distinct agricultural periods, especially before and after the harvest cycle.

Figure 2.
Loading graphs for PC1 versus PC2 obtained by processing metal(loid)s determination data in soil samples at different collection periods (C1 = 1 June 2019, C2 = 24 August 2019, C3 = 28 December 2019, C4 = 9 April 2020, and C5 = 7 August 2020).
3.2. Results of Concentration of Meta(loid)s in Plant and Water
The results of the quantification of As, Ba, Ca, Co, Cr, Cu, K, Mg, Mn, Ni, P, Pb, S, Se, and Zn in A. rivularioides collected near the banks of the Santa Virgem River, located close to the Brazil–Paraguay border and subject to agricultural activities and recreational use, are presented in Table 5. This table also details the five sampling periods (C1 to C5) carried out between June 2019 and August 2020, covering distinct agricultural periods: post-harvest corn (C1), inter-harvest (C2), post-planting soybean (C3), inter-harvest (C4), and before corn harvest (C5). To explore patterns of metal(loid) accumulation in A. rivularioides across the five sampling periods conducted along the banks of the Santa Virgem River, a Principal Component Analysis (PCA) was performed using the concentration data presented in Table 5. The PCA biplot (Figure 3) demonstrates a clear separation of samples according to both sampling periods (C1 to C5) and the associated metal(loid). These PCA analyses were based on the concentrations of metal(loid)s quantified in the plant collected during the following sampling periods: C1 = 1 June 2019; C2 = 24 August 2019; C3 = 28 December 2019; C4 = 9 April 2020; and C5 = 7 August 2020.

Table 5.
Concentration of metal(loid)s (mean ± standard deviation, in mg/kg) in fern (leaves and stems) collected on the banks of the Santa Virgem River.

Figure 3.
Loading graphs for PC1 versus PC2 obtained by processing metal(loid)s determination data in fern (leaves and stem) at different collection periods (C1 = 1 June 2019; C2 = 24 August 2019; C3 = 28 December 2019; C4 = 9 April 2020; and C5 = 7 August 2020).
Finally, Table 6 presents the concentrations of metal(loid)s (mg/kg) quantified in water samples collected during the five established sampling periods (C1 to C5), following the same temporal framework and agricultural context described previously.

Table 6.
Concentration of metal(loid)s (mean ± standard deviation, in mg/L) in water collected during different periods in the Santa Virgem River.
3.3. Results of Geoaccumulation Index (Igeo)
The Geoaccumulation Index (Igeo), used to assess the degree of metal(loid) contamination in soils along the banks of the Santa Virgem River, was calculated according to Equation (1), using the maximum concentrations of each element (Table 4) and background values for Brazilian soils. The Igeo results (Table 7) show that most elements (Ba, Co, Cr, Cu, K, Pb, S, and Zn) remained within the “unpolluted” category across all sampling periods (C1 to C5). In contrast, Ca, Mn, As, Mg, and Se exhibited variations ranging from “unpolluted” to “moderately polluted.” Particularly noteworthy, phosphorus (P) presented consistently high Igeo values, classifying the soil as “extremely polluted” throughout the study period. Table 7 also details the sampling periods—C1 (1 June 2019), C2 (24 August 2019), C3 (28 December 2019), C4 (9 April 2020), and C5 (7 August 2020)—along with the corresponding pollution levels for each element.
3.4. Results of Biological Accumulation Coefficient (BAC) of Heavy Metals
Biological Accumulation Coefficient (BAC) values for A. rivularioides across the five collection periods (C1 to C5) are shown in Table 8. This study was conducted along the banks of the Santa Virgem River, a watercourse located near the Brazil–Paraguay border. The analysis considered exclusively the highest recorded concentrations of metal(loid)s in both soil and plant samples (Table 4 and Table 5). The BAC was calculated using the maximum concentration approach, defined as the sum of the mean and standard deviation (mean + SD) for each metal(loid) in soil and plant tissues. This methodological choice provided a conservative estimation of the plant’s accumulation potential across temporal and agricultural variations. The BAC results highlight distinct seasonal and land-use influences on the capacity of A. rivularioides to accumulate various metal(loid)s from the surrounding soils.
3.5. Results of Contamination Factor (CF) and Pollution Load Index (PLI)
Table 9 shows the values of the Contamination Factor (CF) and Pollution Load Index (PLI), calculated based on the quantification of metal(loid) concentrations in soil samples relative to their background values. This study was conducted along the Santa Virgem River, located close to the Brazil–Paraguay border, considering the five sampling periods (C1 to C5) conducted between June 2019 and August 2020, each corresponding to specific agricultural periods: post-harvest corn (C1 = 1 June 2019), inter-harvest (C2 = 24 August 2019), post-planting soybean (C3 = 28 December 2019), inter-harvest (C4 = 9 April 2020), and before corn harvest (C5 = 7 August 2020).
According to the CF values, the soil was classified as moderately contaminated by As (C1–C5), Ca (C1–C5), Co (C1 and C4), K (C3), and Mn (C1–C5). Furthermore, contamination was observed for Mg (C1–C5) and Se (C1–C5), with very high contamination levels recorded for P (C1–C5). Conversely, low contamination levels were observed for Ba, Cr, Cu, Ni, Pb, S, Zn, K (C1, C2, C3, and C5), and Co (C2, C3, and C5) (Table 9). The Pollution Load Index (PLI) values exceeded 1 for the periods C1, C4, and C5, indicating that the soil was polluted during these collection intervals.

Table 7.
Values of the Geoaccumulation Index (Igeo) calculated from the metal(loid) concentrations (mg/kg) in soil samples collected in different periods between 2019 and 2020 near the plantation located on the banks of the Santa Virgem River and pollution levels.
Table 7.
Values of the Geoaccumulation Index (Igeo) calculated from the metal(loid) concentrations (mg/kg) in soil samples collected in different periods between 2019 and 2020 near the plantation located on the banks of the Santa Virgem River and pollution levels.
| Element | C1 (Igeo) | Pollution Levels (C1) | C2 (Igeo) | Pollution Levels (C2) | C3 (Igeo) | Pollution Levels (C3) | C4 (Igeo) | Pollution Levels (C4) | C5 (Igeo) | Pollution Levels (C5) |
|---|---|---|---|---|---|---|---|---|---|---|
| As | 0.185 | Unpolluted–Moderate | −0.077 | Unpolluted | 0.081 | Unpolluted–Moderate | 0.142 | Unpolluted–Moderate | 0.233 | Unpolluted–Moderate |
| Ba | −2.055 | Unpolluted | −2.298 | Unpolluted | −2.129 | Unpolluted | −2.037 | Unpolluted | −2.077 | Unpolluted |
| Ca | 0.463 | Unpolluted–Moderate | −0.418 | Unpolluted | −0.017 | Unpolluted | 0.109 | Unpolluted–Moderate | 0.303 | Unpolluted–Moderate |
| Co | −0.228 | Unpolluted | −0.739 | Unpolluted | −1.102 | Unpolluted | −0.859 | Unpolluted | −0.305 | Unpolluted |
| Cr | −1.794 | Unpolluted | −2.190 | Unpolluted | −2.287 | Unpolluted | −2.176 | Unpolluted | −2.067 | Unpolluted |
| Cu | −1.620 | Unpolluted | −1.849 | Unpolluted | −1.869 | Unpolluted | −1.613 | Unpolluted | −1.741 | Unpolluted |
| K | −1.235 | Unpolluted | −1.163 | Unpolluted | −0.849 | Unpolluted | −0.498 | Unpolluted | −2.103 | Unpolluted |
| Mg | 1.133 | Moderately polluted | 1.287 | Moderately polluted | 1.335 | Moderately polluted | 1.311 | Moderately polluted | 1.440 | Moderately polluted |
| Mn | 0.966 | Unpolluted–Moderate | 0.087 | Unpolluted–Moderate | −0.197 | Unpolluted | −0.124 | Unpolluted | 0.201 | Unpolluted–Moderate |
| Ni | −2.142 | Unpolluted | −2.387 | Unpolluted | −2.508 | Unpolluted | −3.193 | Unpolluted | −2.869 | Unpolluted |
| P | 7.641 | Extremely polluted | 7.133 | Extremely polluted | 7.405 | Extremely polluted | 7.702 | Extremely polluted | 7.764 | Extremely polluted |
| Pb | −1,246 | Unpolluted | −1.763 | Unpolluted | −1.545 | Unpolluted | −1.571 | Unpolluted | −1.553 | Unpolluted |
| S | −5.263 | Unpolluted | −5.704 | Unpolluted | −5.462 | Unpolluted | −5.301 | Unpolluted | −5.459 | Unpolluted |
| Se | 1.738 | Moderately polluted | 1.442 | Moderately polluted | 1.637 | Moderately polluted | 1.614 | Moderately polluted | 1.728 | Moderately polluted |
| Zn | −1.208 | Unpolluted | −1.373 | Unpolluted | −1.247 | Unpolluted | −1.396 | Unpolluted | −1.438 | Unpolluted |
C1 = 1 June 2019; C2 = 24 August 2019; C3 = 28 December 2019; C4 = 9 April 2020; and C5 = 7 August 2020.

Table 8.
Biological Accumulation Coefficient (BAC) values for A. rivularioides and metal(loid)s by collection (C1 to C5).
Table 8.
Biological Accumulation Coefficient (BAC) values for A. rivularioides and metal(loid)s by collection (C1 to C5).
| Element | A. rivularioides—C1 | A. rivularioides—C2 | A. rivularioides—C3 | A. rivularioides—C4 | A. rivularioides—C5 |
|---|---|---|---|---|---|
| As | 10.299 | 2.949 | 1.146 | 0.141 | 0.384 |
| Ba | 8.514 | 4.698 | 4.724 | 4.979 | 2.495 |
| Ca | 29.914 | 41.027 | 37.006 | 21.484 | 23.418 |
| Co | 1.656 | 0.213 | 0.520 | 0.350 | 0.056 |
| Cr | 7.480 | 0.711 | 0.309 | 0.424 | 0.251 |
| Cu | 0.248 | 0.079 | 0.144 | 0.107 | 0,060 |
| K | 149.386 | 143.555 | 122.994 | 94.188 | 417.876 |
| Mg | 5.664 | 4.704 | 5.096 | 4.673 | 4.428 |
| Mn | 2.082 | 0.470 | 0.864 | 0.905 | 0.381 |
| Ni | 6.574 | 0.940 | 0.613 | 1.283 | 1.663 |
| P | 19.272 | 20.538 | 28.098 | 20.126 | 29.783 |
| Pb | 10.785 | 2.524 | 0 | 0 | 0 |
| S | 20.019 | 18.659 | 24.170 | 26.860 | 28.633 |
| Se | 10.011 | 2.834 | 0.956 | 0 | 0.144 |
| Zn | 3.674 | 1.172 | 2.160 | 1.703 | 1.546 |
C1 = 1 June 2019, C2 = 24 August 2019, C3 = 28 December 2019, C4 = 9 April 2020, and C5 = 7 August 2020.

Table 9.
Elements and calculations of the Contamination factor (CF) and the Pollution load index (PLI), considering the collections made between C1 and C5.
Table 9.
Elements and calculations of the Contamination factor (CF) and the Pollution load index (PLI), considering the collections made between C1 and C5.
| Element | C1 (CF) | C2 (CF) | C3 (CF) | C4 (CF) | C5 (CF) |
|---|---|---|---|---|---|
| As | 1.705 | 1.420 | 1.587 | 1.653 | 1.762 |
| Ba | 0.361 | 0.305 | 0.342 | 0.365 | 0.355 |
| Ca | 2.068 | 1.121 | 1.485 | 1.619 | 1.851 |
| Co | 1.280 | 0.899 | 0.699 | 0.826 | 1.217 |
| Cr | 0.432 | 0.328 | 0.307 | 0.332 | 0.358 |
| Cu | 0.488 | 0.416 | 0.411 | 0.490 | 0.448 |
| K | 0.638 | 0.670 | 0.833 | 1.063 | 0.349 |
| Mg | 3.296 | 3.664 | 3.785 | 3.723 | 4.070 |
| Mn | 2.931 | 1.596 | 1.309 | 1.379 | 1.725 |
| Ni | 0.339 | 0.287 | 0.263 | 0.164 | 0.205 |
| P | 299.342 | 210.569 | 254.198 | 312.359 | 325.989 |
| Pb | 0.633 | 0.441 | 0.514 | 0.505 | 0.511 |
| S | 0.039 | 0.029 | 0.034 | 0.038 | 0.034 |
| Se | 5.003 | 4.080 | 4.668 | 4.594 | 4.965 |
| Zn | 0.650 | 0.579 | 0.633 | 0.570 | 0.553 |
| PLI | 1.21 | 0.942 | 0.999 | 1.04 | 1.12 |
3.6. Results of Human Health Risk Assessment of Heavy Metals
The chronic daily dose (CDD) values for dermal exposure to metal(loid)s during recreational water activities such as bathing, washing, and swimming (CDDdermal/water, calculated using Equation (5)) and from dermal contact with soil (CDDdermal/soil, calculated using Equation (6)) are presented in Table 10 and Table 11, respectively. The analyses were conducted using water samples from the Santa Virgem River and soil samples collected along its margins, a region located near the Brazil–Paraguay border characterized by agricultural and recreational activities. Sampling was carried out during five distinct periods: C1 = 1 June 2019; C2 = 24 August 2019; C3 = 28 December 2019; C4 = 9 April 2020; and C5 = 7 August 2020. For both water and soil exposure assessments, the CDD calculations were based on the highest recorded concentration of each metal(loid) found in Table 4 (soil) and Table 6 (water. The calculations considered an adult individual with an exposure time of 30 years and a body weight of 70 kg, following the parameters detailed in Section 2.11.

Table 10.
Chronic daily dose (CDD: unit mg/kg·day) was calculated for dermal exposures to metal(loid)s in water (Equation (5)).

Table 11.
Chronic daily dose (CDD: unit mg/kg·day) was calculated for dermal exposures to metal(loid)s in soil (Equation (6)).
3.7. Results of Hazard Quotient (HQ) and Hazard Index (HI)
The hazard quotient (HQ) and hazard index (HI) values for the assessed metal(loid)s are presented in Table 12 and Table 13. The HQ for each metal(loid) was calculated based on the chronic daily dose (CDD) values derived from dermal exposure to metal(loid)s in water (Table 10) and in soil (Table 11). The HI values, representing the cumulative non-carcinogenic risk for adults through dermal contact with metal(loid)s, are also summarized in these tables for the various collection periods (C1 to C5). It is important to note that the non-carcinogenic risks associated with different metal(loid)s within the same exposure medium are assumed to be additive. Reference dose (RfD) values are not available for Ba, Ca, K, Mg, and S, and therefore HQ and HI could not be calculated for these elements.

Table 12.
Hazard Quotient (HQ) and Hazard Index (HI) for dermal exposures to metal(loid)s in water (Equation (5)).

Table 13.
Hazard Quotient (HQ) for dermal exposures to metal(loid)s in soil (Equation (6)).
The hazard quotient (HQ) and hazard index (HI) values for the evaluated metal(loid)s are presented in Table 12 and Table 13. HQ values were calculated for each element using the chronic daily dose (CDD) values obtained from dermal exposure scenarios to metal(loid)s in water (Table 10) and soil (Table 11), as per the methodology detailed in Section 2.11. For water exposure, parameters included an average daily exposure time (ET) of 7 h per day, an exposure frequency (EF) of 60 days per year, and an exposure duration (ED) of 30 years, with a body weight (BW) of 70 kg. The averaging time (AT), representing the exposure period for non-carcinogenic effects, was set at 10,950 days.
The HI values represent the cumulative non-carcinogenic risk, calculated as the sum of individual HQs for all metal(loid)s within the same exposure medium (soil or water) across the different sampling periods (C1 to C5). It is important to note that the risk characterization assumes additive effects for different metal(loid)s within the same medium. Due to the absence of reference dose (RfD) values in regulatory guidelines, HQ and HI could not be calculated for Ba, Ca, K, Mg, and S.
3.8. Results of Carcinogenic Risk
To assess the total cancer risk associated with dermal exposure to arsenic (As), lead (Pb), and chromium (Cr) present in water and soil, the total Incremental Lifetime Cancer Risk (ILCRtotal) was calculated for each sampling period (C1 to C5). The ILCR for each medium was first estimated separately: ILCRdermal/water (sum of ILCR for As, Pb, and Cr from water exposure) and ILCRdermal/soil (sum of ILCR for As, Pb, and Cr from soil exposure), as shown in Equation (8).
For each collection period, ILCRtotal was obtained by summing the ILCR values from both water and soil dermal exposure pathways, reflecting the cumulative carcinogenic risk (Table 14). The calculations considered exposure parameters for an adult individual: average swim time of 7 h per day, exposure frequency of 60 days per year, exposure duration of 30 years, and a body weight of 70 kg.

Table 14.
Total incremental lifetime cancer risk (ILCR) by exposure pathway.
For carcinogenic risk estimation, the averaging time (AT) was set at 25,550 days, corresponding to a 70-year lifetime, as recommended for carcinogen risk assessment. The total excess lifetime cancer risk was determined by summing the individual contributions of As, Pb, and Cr across both exposure pathways (water and soil), as presented in Table 14.
4. Discussion
4.1. Concentration of Meta(loid)s in Soil
The analysis of the maximum concentrations of the elements in the soil throughout the five collection periods (C1 to C5) (Table 4) revealed significant variations between the cycles, reflecting possible influences of agricultural practices, edaphoclimatic characteristics, local geology, and seasonality.
In the C1 sampling period, the elements with the highest concentrations in the soil were the following: Mn > Mg > P > Ca > K > S > Ba > Cu > Co > Cr > Zn > Se > Pb > As > Ni.
In the second sampling period (C2), the descending order of soil concentrations was the following: Mg > Mn > P > Ca > K > S > Ba > Cu > Cr > Zn > Co > Se > Pb > As > Ni.
During the third sampling period (C3), the descending order of concentrations was the following: Mg > Mn > Ca > K > P > S > Ba > Cu > Cr > Zn > Se > Co > Pb > As > Ni.
In sampling period C4, the descending order was the following: Mg > P > Mn > Ca > K > S > Ba > Cu > Cr > Co > Zn > Se > Pb > As > Ni.
The fifth and final sampling cycle (C5) presented the following descending order: Mg > P > Ca > Mn > K > S > Ba > Cu > Cr > Zn > Co > Se > Pb > As > Ni.
The analysis of metal concentrations in the soil samples collected during periods C1 to C5 revealed notable variations and several instances of regulatory exceedances. Particularly concerning are the elevated levels of As, Co, Mn, Se, Cu, and Zn when compared to environmental guidelines.
Arsenic concentrations remained below the Brazilian Conama threshold (15 mg/kg), but exceeded the more restrictive limits set by the USEPA for Alabama city (4.7 mg/kg) in C1 and C5, and were consistently above the threshold of the state of Pará (0.8 mg/kg) across all five periods. This suggests a potential chronic exposure risk to biota and humans in this peri-urban environment, especially given arsenic’s recognized carcinogenicity and mobility in soils [18].
Cobalt levels were significantly higher than both the Alabama city (4.4 mg/kg) and the state of Pará (1.6 mg/kg) limits in all collection periods, though they remained within the Conama guideline (25 mg/kg). These values may reflect contributions from anthropogenic sources such as agrochemicals or vehicular emissions, as cobalt is commonly associated with phosphate fertilizers and combustion byproducts [28].
Manganese concentrations exceeded the state of Pará limit (40.7 mg/kg) and, in the case of C1, also the Alabama city (420 mg/kg). According to Wan et al. (2024) [59], high Mn concentrations in soil are associated with intense agricultural activities.
Selenium was another critical element, with concentrations surpassing both the Conama (5 mg/kg) and the extremely low Alabama city (0.3 mg/kg) in all collection periods. This pattern points to local selenium enrichment, potentially natural in origin (e.g., parent rock), but could also be exacerbated by agricultural runoff, especially from livestock feed supplements and pesticides [60].
Copper concentrations were below the Brazilian guideline (60 mg/kg) but consistently exceeded the thresholds set by the Alabama city (9.6 mg/kg) and the state of Pará (6.0 mg/kg), indicating possible ecotoxicological risks. Copper is frequently enriched in agricultural soils due to its use in fungicides and manure applications [28].
Zinc concentrations remained well below the Conama and USEPA limits but slightly surpassed the state of Pará standard (7.0 mg/kg) in all samples. Although zinc is less toxic at moderate levels, its persistent presence may pose long-term risks to soil health and plant uptake dynamics [59].
Interestingly, Ni also slightly exceeded the state of Pará threshold (1.4 mg/kg) during the first three collection periods, aligning with the transition from the dry to the rainy season, potentially enhancing the mobility and availability of this metal in agricultural soils [29].
The temporal variation in the concentration of metals and metalloids in soil samples collected during different agricultural phases highlights the potential influence of crop cycles, particularly corn and soybean cultivation, on soil contamination dynamics. In collection C1 (1 June 2019—post-harvest corn), elevated concentrations of several elements were observed, including Co (14.93 mg/kg), Cr (12.99 mg/kg), Cu (13.85 mg/kg), Mn (524.56 mg/kg), and Se (7.88 mg/kg). These values suggest a possible cumulative effect from previous fertilization or pesticide applications during corn production, which is consistent with observations in other agricultural areas where residual inputs accumulate post-harvest [61,62].
During C2 (24 August 2019—inter-harvest), lower concentrations were generally recorded for most elements. This reduction may be attributed to decreased anthropogenic inputs during this fallow period and potential dilution or leaching effects due to rainfall, a pattern supported by seasonal studies in tropical agricultural systems [34,63].
In C3 (28 December 2019—post-planting soybean), an increase was observed for elements like Ca (119.14 mg/kg), P (247.41 mg/kg), and Mg (586.24 mg/kg), which may reflect fertilization practices typically associated with soybean sowing. The result indicated that Cd, Pb, and As concentrations increased during the years in the cultivated soils due to fertilizer application [64]. Despite this, potentially toxic elements like As (4.65 mg/kg) and Cr (9.28 mg/kg) remained below the thresholds established by Conama (15 mg/kg and 75 mg/kg, respectively) but approached or exceeded the more restrictive limits of the Alabama city and state of Pará for some elements.
Collection C4 (9 April 2020—inter-harvest) again showed intermediate values for most elements, resembling patterns in C2. For instance, Mn (243.41 mg/kg) and Zn (9.20 mg/kg) showed slightly increased values compared to C3 but remained well within the regulatory limits. These fluctuations further emphasize the role of agricultural downtime in stabilizing metal dynamics in the soil.
In C5 (7 August 2020—pre-harvest corn), several elements exhibited a new increase, particularly Mg (639.18 mg/kg), P (317.08 mg/kg), and As (5.13 mg/kg), with the latter surpassing the USEPA/Alabama city limit (4.7 mg/kg) and significantly exceeding the state of Pará limit (0.8 mg/kg). This pattern may be attributed to the intensive use of agrochemicals during corn and soybean growth stages, as both generally require higher inputs of fertilizers and herbicides, potentially mobilizing As and other elements [65,66].
Importantly, certain elements, such as Se and Pb, displayed levels above recommended thresholds in more than one collection. Se surpassed both the Conama and USEPA limits across all periods, and Pb approached or exceeded the state of Pará standard (10.4 mg/kg) in all collections, indicating a consistent contamination risk. These findings align with concerns raised by Alloway (2013) [28] and Chen et al. (2015) [67] about the persistence of trace elements in soils under intensive agriculture and their long-term ecological risks.
Overall, the variations in metal concentrations observed across the five collections reinforce the impact of seasonal and crop-specific management practices on soil geochemistry. The post-harvest and pre-harvest periods, particularly for corn, were associated with elevated levels of several contaminants, underscoring the need for best management practices (BMPs) to mitigate heavy metal accumulation and preserve soil health in intensively cultivated regions.
A one-way ANOVA was conducted to assess differences in element concentrations across the five sampling periods. Prior to the analysis, data normality was verified using the Shapiro–Wilk test (α = 0.05), confirming that the datasets met the assumptions for parametric tests. The ANOVA results indicated statistically significant differences between the means (p < 0.05). Complementary pairwise t-tests (e.g., comparing C1 and C5) confirmed significant temporal variations for several elements, suggesting seasonal and land-use influences on the distribution of trace elements. In addition, Principal Component Analysis (PCA) was applied to investigate patterns in metal(loid) concentrations in soil samples collected over five distinct periods (C1 to C5). The PCA biplot (Figure 2) reveals clear temporal variability in the chemical composition of the samples. The first two principal components (PC1 and PC2) explained a significant portion of the total variance (81.02%), allowing effective visualization of similarities and differences among the sampling periods. PC1 strongly separated collection period C1 from all others, indicating a distinct geochemical profile characterized by higher concentrations of several metal(loid)s, including Ca, Se, As, Pb, Mn, Zn, Cr, and Co. This suggests that C1 (collected on 1 June 2019—post-harvest corn) was marked by a pronounced enrichment of potentially toxic elements in the soil, which may reflect specific environmental or anthropogenic inputs during this period. In contrast, samples from C2 (24 August 2019—inter-harvest) and C3 (28 December 2019—post-planting soybean) clustered on the opposite side of the PCA space, suggesting lower concentrations or different patterns of metal(loid)s accumulation. These periods may reflect a dilution or attenuation phase, potentially due to seasonal changes or decreased inputs. C4 (9 April 2020—inter-harvest) and C5 (7 August 2020—pre-harvest corn) were grouped together in the lower-left quadrant of the PCA plot, indicating similar compositional profiles and suggesting a relatively stable geochemical regime during these periods. The proximity of their positions implies low variability between them, potentially associated with equilibrium conditions or similar environmental influences. The vector directions and lengths indicate the influence of individual elements on each principal component. For example, Mg showed a loading vector in the opposite direction of most other elements, suggesting an inverse behavior—possibly more concentrated in C2 or C3, where other metals were depleted. Overall, the PCA results provide strong evidence of dynamic changes in soil metal(loid) content over time, reinforcing the need for temporal monitoring and assessment of contamination sources.
These findings are consistent with previous studies that have applied PCA to assess temporal variations in soil metal(loid) concentrations. Similar patterns of seasonal or period-specific enrichment of heavy metals have been reported in agricultural and peri-urban environments, where both natural fluctuations and anthropogenic activities influence the metal load in soils. Morton-Bermea et al. (2021) [68], using principal component analysis (PCA), found it difficult to identify groups of metals associated with specific sources (anthropogenic and geogenic), given the high complexity of the study area and the long period evaluated (during the dry/cold season (October to January) for the 2004–2014 period). However, our PCA result (Figure 2) suggests that C1 (collected on 1 June 2019) was marked by a pronounced enrichment of potentially toxic elements in the soil. In addition, the results obtained in the present study corroborate findings by Cao et al. (2018) [69], reinforcing the effectiveness of PCA as a multivariate tool for detecting temporal trends in soil contamination. These similarities suggest that seasonal environmental factors—such as rainfall patterns, agricultural practices, and runoff—may systematically influence the mobility and accumulation of metal(loid)s in the soil matrix.
The alternation and intensification of soybean and maize cultivation over the years can significantly influence soil contamination by metal(loid)s. These crops are often associated with high agrochemical inputs—such as phosphate fertilizers, which may contain trace metals like Cd, As, and Pb—and repeated soil tillage, which can enhance the mobilization and redistribution of contaminants within the soil matrix. Moreover, the seasonal variation in planting and harvesting cycles can affect the timing of fertilizer and pesticide applications, increasing the likelihood of metal accumulation during specific periods. Studies have shown that crop rotation and intensive monoculture systems can alter the physicochemical properties of soils, including pH, organic matter content, and cation exchange capacity, all of which modulate metal availability and mobility [28,70]. Therefore, long-term changes in soybean and maize planting patterns may act as indirect drivers of metal(loid) enrichment in agricultural soils, particularly in areas subject to seasonal rainfall and surface runoff.
4.2. Concentration of Meta(loid)s in Plant and Water
The metal(loid) concentration analysis in fern samples revealed distinct patterns across the five sampling periods (Table 5). When ranked in descending order of concentration for each collection (C1 to C5), the most abundant elements were the following:
C1 (1 June 2019): K > Ca > P > S > Mg > Mn > Ba > Zn > Pb > Cr > Co > Ni > As > Cu > Se.
C2 (24 August 2019): K > Ca > P > S > Mg > Mn > Ba > Zn > As > Pb > Cr > Co > Cu > Ni > Se.
C3 (28 December 2019): K > P > Ca > S > Mg > Mn > Ba > Zn > Cr > Co > Cu > Ni > Se > As.
C4 (9 April 2020): K > P > S > Ca > Mg > Mn > Ba > Zn > Cr > Co > Cu > Ni > As.
C5 (7 August 2020): K > P > S > Ca > Mg > Mn > Ba > Zn > Ni > Co > Cu > As > Se.
Potassium consistently exhibited the highest concentrations in all periods, especially in C5 (15,130.38 mg/kg), suggesting its strong accumulation capacity in ferns. Phosphorus, sulfur, calcium, and magnesium also showed elevated values, indicating potential uptake from both natural and anthropogenic sources.
In terms of temporal comparison, C1 displayed the highest levels of several toxic elements such as arsenic (53.91 mg/kg), lead (74.79 mg/kg), cobalt (24.56 mg/kg), chromium (97.99 mg/kg), and selenium (80.35 mg/kg), suggesting that this period might have been influenced by runoff or leaching following agricultural practices or seasonal changes such as post-harvest residual inputs. Conversely, C4 and C5 generally exhibited lower concentrations of heavy metals, which may be attributed to reduced input from agricultural or urban sources or higher vegetation cover that limits soil exposure and metal mobilization.
The seasonal variation in concentrations supports the hypothesis that environmental factors such as precipitation, agricultural cycles, and runoff dynamics systematically influence the bioavailability and accumulation of metals in plants. June (C1) and December (C3) correspond to post-planting and late crop-cycle periods in the region, which may contribute to higher exposure to agrochemical residues. April (C4), after the rainy season, may represent a dilution or leaching effect, reflected in the comparatively lower concentrations of several elements.
Metal(loid) dynamics in the soil and their uptake by plants are directly linked to the soil properties, altering their bioavailability. Thus, these findings are in line with previous studies reporting seasonal fluctuations in metal accumulation by plants, particularly in regions with intensive agriculture and peri-urban regions. For instance, studies have shown that ferns accumulate higher levels of Pb and As in areas with recent anthropogenic disturbance [71,72]. The pattern of accumulation observed here also agrees with Alloway (2013) [28], who emphasized the mobility of metals in soils depending on pH, organic matter, and environmental conditions, as well as with Costa and Lia (2023) [73], who demonstrated temporal changes in plant metal uptake in agricultural fields. In fact, the bioavailability of heavy metals in peri-urban agricultural soils is influenced by the interaction between soil pH, organic matter, and metal mobility. Acidic soils increase the solubility and mobility of metals like Cd, Pb, and Zn, while alkaline conditions may reduce their mobility but enhance that of arsenic. In addition, organic matter can immobilize metals through complexation, yet under certain physical–chemical conditions, it may promote their release [28,34,67,70].
The accumulation of metal(loid)s in ferns observed in this study is consistent with the timing and intensity of local agricultural cycles, particularly the cultivation of soybean and maize. The elevated concentrations of As, Pb, and Zn found in the C1 collection (June 2019) likely reflect post-harvest soil conditions following soybean cultivation, which commonly includes the application of phosphate fertilizers known to contain trace metals such as As and Cd. Additionally, seasonal rainfall during this period may have enhanced metal mobility in the soil, facilitating uptake by the ferns. Phosphate fertilizers, commonly applied in large quantities to soybean and corn crops, often contain impurities such as Cd, As, and Pb, which accumulate in the soil over time [74]. In addition, rotation and succession between soybean and corn contribute to increased soil disturbance, increasing the exposure of horizons with metals and facilitating surface transport via runoff and leaching during seasonal rains [75].
The use of agrochemicals, such as pesticides, can leave residues of heavy metals in water and soil, which are subsequently absorbed by bioaccumulating plants such as ferns [76]. The variations observed between collection periods—for example, the high levels of metals such as As, Pb, and Co in C1 (1 June 2019)—may be related to the accumulation of inputs in the soil after the production cycles, as June marks the end of the soybean cycle and the beginning of preparation for the second corn crop in many areas of the Brazilian Midwest. Collections such as C4 (April/2020) and C5 (August/2020) showed lower levels of several metals, which may indicate periods of less direct agricultural activity or dilution of contaminants by rainfall in the previous summer. This pattern aligns with the findings by Xie et al. (2021) [77], who reported the experimental results of the variation in heavy metal concentration in the cultivated land before and after planting different crops (corn, potato, broad beans, oats, beans, and soybeans) and accumulation of heavy metal concentration in the crops. These results support the notion that intensive monoculture systems can significantly alter the elemental composition of soils and increase the exposure of native or spontaneous vegetation, such as ferns, to toxic elements.
Furthermore, studies by Alloway (2013) [28] and Gventsadze et al. (2024) [78] have highlighted that repeated agricultural activities can lead to cumulative metal enrichment in soils, especially under conditions of limited crop rotation and excessive agrochemical input. The presence of elevated P, K, and S levels in the later collection periods (C3–C5) in our study may also be attributed to continuous fertilization during successive maize cycles, consistent with these authors’ conclusions [28,77,78].
The Principal Component Analysis (PCA) performed on the metal(loid) concentration data in fern leaves and stems across different collection periods revealed distinct accumulation patterns (Figure 3). The first principal component (PC1) explained 71.64% of the total variance, while the second principal component (PC2) accounted for 15.73%. Together, these two components captured 87.37% of the total variability, demonstrating a strong summarization of the dataset. The distribution of the collection periods (C1 to C5) in the PCA biplot reflected variations associated with agricultural practices (Figure 3):
C1 (1 June 2019—post-harvest of maize) was positioned distinctly along the positive axis of PC1, indicating higher concentrations of Ba, As, Pb, Cr, Cu, and Zn. This suggests a significant accumulation of contaminants, potentially linked to the residual impact of agrochemical applications after maize harvesting.
C2 (24 August 2019—between crops) and C4 (9 April 2020—between crops) were clustered in the negative quadrant of both PC1 and PC2, characterized by lower overall metal(loid) concentrations. These periods correspond to minimal agricultural activity, potentially promoting greater metal leaching and reducing bioaccumulation.
C3 (28 December 2019—post-planting of soybean) was located near the origin, with a slight positive loading on PC2. This positioning reflects moderate concentrations of metal(loid)s, notably influenced by elements such as P and K, likely due to fertilizer application during soybean cultivation.
C5 (7 August 2020—pre-harvest of maize) appeared in the positive region of PC2, indicating enrichment with nutrients, particularly P and K, which is consistent with pre-harvest fertilization practices.
The PCA loadings demonstrated that Ba, As, Pb, Cr, Cu, and Zn were strongly associated with PC1, highlighting their contribution to differentiating contamination profiles. In contrast, elements such as P and K were more closely aligned with PC2, suggesting their association with nutrient management rather than contamination.
When comparing these findings with the literature, the observed accumulation patterns corroborate previous studies indicating that ferns are efficient bioindicators of environmental contamination by heavy metals and respond sensitively to agricultural management practices. For instance, studies reported that ferns accumulated higher concentrations of As and Pb in areas subjected to intensive agricultural activities [23,79]. Similarly, research by Oluyemi et al. (2008) [80] observed that concentrations of As (8.31mg/kg), Cr (9.00 mg/kg), and Ni (40.00 mg/kg) in Manihot esculenta leaves; Cu (25.0 0 mg/kg) and Fe (176.00 mg/kg) in Xanthosoma mafaffa tuber; Cd (14.50 mg/kg), Co (22.50 mg/kg), Mn (189.50 mg/kg), Pb (680.00 mg/kg), and Zn (440.59 mg/kg) in Talinum triangulare were elevated during periods of seasonal variations. However, in contrast to our findings, some studies in temperate regions showed less seasonal variation in metal accumulation, likely due to differences in agricultural intensity and rainfall patterns affecting metal mobility [81].
Thus, the present results strengthen the evidence that agricultural cycles significantly influence metal(loid) bioavailability and accumulation in ferns, especially in tropical and subtropical regions where climatic conditions exacerbate soil–plant transfer dynamics. Moreover, these findings reinforce the role of ferns as effective bioindicators of soil contamination and support previous research suggesting that seasonal dynamics and agricultural intensity are critical drivers of metal(loid) distribution in agroecosystems.
The temporal monitoring of metal(loid) concentrations in surface waters from June 2019 to August 2020 revealed notable patterns associated with seasonal agricultural activities (Table 6). Overall, most toxic metals, such as As, Pb, Co, Cu, and Se, remained below detection limits throughout the study, indicating a favorable environmental quality within the study area. The descending order of metal(loid) concentrations in water collected near the Santa Virgem River plantation bank was the following:
C1 (1 June 2019—Post-harvest corn): Mg > S > K > P > Ba > Mn > Zn;
C2 (24 August 2019—Inter-harvest): Mg > S > K > P > Ba > Mn;
C3 (28 December 2019—Post-planting soybean): Mg > S > K > P > Ba > Mn;
C4 (9 April 2020—Inter-harvest): Mg > S > K > P > Ba > Cr > Mn > Ni;
C5 (7 August 2020—Before corn harvest): Mg > S > K > P > Ba > Mn.
As shown in Table 6, Ba concentrations, while consistently detected across all periods (0.015–0.020 mg/L), showed a slight decrease during inter-harvest periods (C2 and C4). Furthermore, the concentration of Ba is lower than the maximum values (0.7 mg/L) established by Brazilian legislation in resolution number 357, on 17 March 2005, in Conama [82] (Table 6). These levels were higher than those reported in groundwater in China, where Ba concentrations reached up to 0.010 mg/L [83], suggesting a limited influence of local agricultural practices on Ba mobilization.
Potassium is an essential macronutrient commonly associated with agricultural inputs, especially fertilizers used in row crops such as soybeans and corn. In the present study (Table 6), potassium concentrations in river water samples ranged from 0.457 mg/L in C1 (June 2019, post-harvest of maize) to 0.590 mg/L in C3 (December 2019, post-planting of soybean). The highest concentration coincided with the early rainy season and a period immediately following fertilizer application, indicating a possible link between agricultural activity and nutrient runoff.
These findings in Table 6 for K align with those reported by Pires et al. (2024) [84], who investigated the seasonal dynamics of N, P, and K in surface and groundwater from an agricultural hydrographic basin in the Brazilian Cerrado. In their study, K concentrations in surface waters varied from 0.048 mg/L during the dry season of 2019 to 1.077 mg/L in the dry season of 2020, with a mean value of 0.573 mg/L. This range encompasses the concentrations observed in our study and supports the hypothesis that fertilizer use and seasonal variability strongly influence K levels in aquatic systems within agricultural landscapes. Furthermore, the temporal coincidence between elevated potassium levels and key agricultural periods, such as the post-planting phase observed in both studies, suggests that fertilizer application, followed by rainfall events, facilitates the leaching and transport of K into nearby water bodies. The agreement in concentration magnitude and temporal trends reinforces the importance of agricultural practices as major contributors to potassium dynamics in surface waters of the Cerrado biome.
Magnesium concentrations ranged from 0.996 to 1.248 mg/L, with the lowest value occurring during C4 (inter-harvest) (Table 6). These concentrations in Table 6 are lower than those reported in other studies of river waters in Brazil. For instance, in the Mangueirão and Salso Streams in Caçapava do Sul, state of Rio Grande do Sul, Mg concentrations ranged from 4.6 to 8.97 mg/L, reflecting the influence of local lithology and potential anthropogenic input [85]. The decrease in Mg concentration during the inter-harvest period (C4) in the current study may reflect reduced agricultural runoff, supporting the notion that fertilizer application and subsequent leaching during rainfall events are key contributors to Mg levels in surface waters. This pattern underscores the importance of considering both natural and anthropogenic factors when assessing nutrient dynamics in riverine systems.
Phosphorus concentrations across all collection periods remained significantly above the threshold established by the U.S. Environmental Protection Agency (USEPA) for streams, which is 0.1 mg/L to prevent eutrophication [86]. In the present study (Table 6), P levels were recorded as follows: C1 = 0.332 ± 0.006 mg/L (post-harvest corn, 1 June 2019), C2 = 0.308 ± 0.018 mg/L (inter-harvest, 24 August 2019), C3 = 0.362 ± 0.011 mg/L (post-planting soybean, 28 December 2019), C4 = 0.348 ± 0.005 mg/L (inter-harvest, 9 April 2020), and C5 = 0.309 ± 0.009 mg/L (before corn harvest, 7 August 2020). These values are more than three times higher than the USEPA guideline, indicating a persistent risk of nutrient enrichment and potential eutrophication in the aquatic system. The highest P concentration occurred in C3, during the early stages of soybean development, which may reflect increased fertilizer runoff following planting activities and initial rainfall events [87]. The consistently elevated phosphorus levels throughout both inter-harvest and crop phases underscore the ongoing influence of agricultural practices on stream nutrient dynamics and highlight the need for improved nutrient management strategies to mitigate environmental impacts. Elevated phosphorus levels are commonly associated with agricultural runoff, particularly from corn and soybean crops [87], and indicate a risk of nutrient enrichment in the aquatic ecosystem.
Chromium and Nickel were detected exclusively during collection period C4 (9 April 2020—inter-harvest), at concentrations of 0.013 mg/L and 0.010 mg/L, respectively. When compared to the maximum permissible limits established by the Brazilian National Environmental Council (Conama) under Resolution No. 357/2005—0.05 mg/L for Cr and 0.025 mg/L for Ni—both values remain well below the regulatory thresholds for freshwater bodies classified under Class 2 [82]. In addition, the Ni concentration values in Table 5 are also lower than those obtained in another Brazilian river located in Montes Claros, state of Minas Gerais, where a value of 0.025 mg/L was reported [88]. The presence of these metals exclusively during C4 may be attributed to leaching from residual fertilizers, agrochemicals, or soil particles during the rainy season that characterizes the transitional inter-harvest period. Despite their concentrations being within legal limits, the detection of Cr and Ni indicates episodic inputs of trace metals into the aquatic system, which may accumulate over time and require continuous monitoring to prevent chronic ecological and health risks.
In Table 6, temporal fluctuations in manganese concentrations were also observed, with the highest values recorded during active farming periods (C1 and C3). Manganese concentrations in surface water were assessed over five distinct sampling periods. During the post-harvest corn period (C1), the Mn concentration was 0.020 ± 0.000 mg/L. In the inter-harvest period (C2), the concentration decreased to 0.012 ± 0.000 mg/L. The post-planting soybean period (C3) saw a slight increase in Mn levels, with a concentration of 0.018 ± 0.000 mg/L. During the second inter-harvest period (C4), the Mn concentration was 0.013 ± 0.000 mg/L. The lowest Mn concentration was observed just before the corn harvest (C5), at 0.005 ± 0.000 mg/L. These concentrations were consistently well below the maximum permissible limit of 0.1 mg/L for manganese (Mn) established by the Brazilian National Environmental Council (Conama) under Resolution No. 357/2005 for Class 2 water bodies [82]. This class includes water bodies used for human consumption after conventional treatment, recreational activities, and the protection of aquatic life. The highest concentration recorded was 0.020 mg/L during the post-harvest corn period (C1), while other sampling periods (C2–C5) exhibited even lower values. This suggests that manganese concentrations in the study area are not indicative of significant environmental pollution or water quality concerns. Despite the concentrations remaining well within safe regulatory limits, it is crucial to continue monitoring manganese and other trace metals in water bodies, as changes in agricultural practices or other anthropogenic activities may lead to future increases in metal runoff and potential contamination. The manganese levels observed in the studied water body comply with the established environmental standards. However, regular monitoring is essential to detect any future shifts in water quality.
4.3. Geoaccumulation Index (Igeo)
The analysis of the Igeo values for the chemical elements present in the soils of samples collected during periods C1, C2, C3, C4, and C5 provided important information about the degree of soil pollution in relation to these metals (Table 7). The classification of the chemical elements in the soil revealed that the Igeo analysis revealed significant variations in the pollution levels of different elements in soils collected near the Santa Virgem River between 2019 and 2020. Phosphorus exhibited extremely high Igeo values (>7) across all sampling periods, classifying it as extremely polluted and suggesting intense anthropogenic input, possibly from agricultural fertilizers. Magnesium and Selenium showed moderate pollution levels consistently, indicating a persistent enrichment of these elements. In contrast, elements such as Ba, Co, Cr, Cu, K, Ni, Pb, S, and Zn remained within the unpolluted category throughout the study, suggesting minimal contamination risk. Arsenic, Calcium, and Manganese fluctuated between unpolluted and unpolluted to moderately polluted levels, reflecting minor spatial or temporal variations. Overall, the results highlight the necessity of monitoring and managing phosphorus inputs to prevent further soil and environmental degradation in the region.
These results suggest the presence of a variety of contaminants in the soils, particularly heavy metals like arsenic and selenium, which may pose a high risk to public health and the environment. Additionally, continuous monitoring of soil quality and the adoption of mitigation strategies are crucial for the preservation of ecosystems and the prevention of harm to human health.
This analysis emphasizes the importance of Igeo as an effective tool for evaluating soil quality, providing a clear view of the degree of contamination and the risks associated with the presence of heavy metals in soil, especially in peri-urban regions where anthropogenic impacts are constant and cumulative.
4.4. Biological Accumulation Coefficient (BAC) of Heavy Metals
This study considered exclusively the highest recorded concentrations of metals in soil and plant samples, as detailed in Table 4 and Table 5. To estimate the potential for metal accumulation, we calculated the BAC using an elevated concentration value, obtained by summing the mean and standard deviation (mean + SD) of metal concentrations in soil and plant samples.
4.5. Biological Accumulation Coefficient (BAC) Analysis of Fern Plant and Comparison with Other Studies
According to Table 8, the analysis of the Biological Accumulation Coefficient (BAC) values for As in A. rivularioides over five sampling periods revealed significant variation, with the highest value observed in C1 (10.299), clearly classifying the plant as a hyperaccumulator during this period (BAC > 1). In subsequent collections, the values declined to 2.949 in C2, 1.146 in C3, 0.141 in C4, and 0.384 in C5, indicating a reduction in arsenic bioaccumulation. Our results for As corroborate other studies on Pteris semipinnata fern, which accumulated low As concentrations [89].
These findings on As are consistent with the study by Indriolo et al. (2010) [90], which identified the ACR3 gene in Pteris vittata fern as encoding a vacuolar arsenite transporter protein. The expression of ACR3 is induced by arsenic exposure, especially in roots and gametophytes, suggesting that the hyperaccumulation capacity of P. vittata is directly related to the regulation of this gene.
The decrease in BAC values during periods C4 and C5 may be attributed to environmental factors affecting the bioavailability of arsenic in the soil, such as changes in soil chemistry, agricultural practices, and seasonal conditions. Although the presence of the ACR3 gene equips the plant with the physiological capacity for arsenic hyperaccumulation, the efficiency of this process depends on the environmental availability of the element [90]. Therefore, the variation in BAC values observed in this study likely reflects the interaction between plant physiology and environmental conditions influencing arsenic uptake. Although there are studies considering the various species of ferns, there are still few studies carried out in Brazil considering A. rivularioides.
The BAC for barium decreases gradually but remains above 1 throughout the sampling periods (Table 8). In C1, the BAC is high (8.514), indicating substantial accumulation, and although the values decrease over time, they remain above 1, suggesting that the fern plant is continuously accumulating barium. These findings are consistent with previous studies, such as that by Kamachi et al. (2015) [91], which investigated barium accumulation in the fern Athyrium yokoscense. In that study, fronds were found to accumulate up to 1020 mg/kg of Ba in dry biomass, with an average of 443 mg/kg, reinforcing the species’ ability to take up barium from the environment—even though BAC values were not directly reported by Kamachi et al. (2015) [91].
Furthermore, Fačkovcová et al. (2020) [92] evaluated trace element accumulation in the aquatic fern Azolla filiculoides after the application of pyroligneous acid. Their results indicated significant barium accumulation after three days of incubation, showing that even aquatic ferns can absorb Ba under specific conditions [92]. These findings suggest that several fern species, including A. rivularioides, possess efficient mechanisms for barium uptake and accumulation, even if they are not traditionally classified as hyperaccumulators of this element. This trait may offer valuable applications in phytoremediation strategies for environments contaminated with barium.
The observed high Biological Accumulation Coefficients (BACs) for calcium (Ca), peaking at 41.027 in the second sampling period (C2), underscore the species’ capacity to accumulate this essential nutrient. Studies have shown that ferns typically exhibit lower foliar calcium concentrations compared to angiosperms. This difference is attributed to physiological factors such as lower transpiration rates and variations in cation exchange capacities, which influence calcium uptake and transport within the plant. However, environmental conditions can lead to significant variations in calcium accumulation among fern species [93]. For instance, studies on ferns in karst regions of China, characterized by high soil calcium content, revealed that certain species, including Cyrtomium fortunei, Pteris multifida, and Selaginella moellendorffii, can tolerate and accumulate substantial amounts of calcium in their tissues. These adaptations suggest that environmental calcium availability plays a significant role in influencing calcium accumulation in ferns [94]. Furthermore, studies on Adiantum species have demonstrated that soil type affects calcium accumulation. Species such as Adiantum capillus-veneris f. dissectum and Adiantum malesianum, which prefer calcareous soils, exhibit higher root calcium concentrations when grown in such environments compared to acidic soils. This indicates that soil calcium content and pH can influence calcium uptake and accumulation in ferns [95].
In summary, the high BACs for calcium observed in A. rivularioides (Table 8) align with findings from other studies, suggesting that both physiological traits and environmental factors, such as soil calcium availability and pH, contribute to calcium accumulation in ferns. These insights enhance our understanding of nutrient dynamics in ferns and their potential role in phytoremediation and nutrient cycling in various ecosystems, especially those near urban areas.
In Table 8, Co exhibited BACs above 1 only in C1 (1.656), with markedly lower values in other periods, notably dropping to 0.056 in C5. The BAC for Co in A. rivularioides was above 1 only during the first sampling period (C1), with a value of 1.656. In subsequent periods, BAC values dropped significantly, reaching as low as 0.056 in C5. This trend suggests a limited ability of this fern species to accumulate Co over time, possibly influenced by environmental and physiological factors. Previous studies support this observation. For example, Kříbek et al. (2011) [96] investigated metal accumulation in Pteris vittata and Cyperus involucratus growing on Cu–Co-rich mine tailings in Zambia [96]. The results showed that P. vittata accumulated between 18 and 38 μg/g of Co in its fronds, demonstrating a moderate tolerance and accumulation capacity. Despite the elevated cobalt concentrations in the substrate, the plants maintained relatively stable levels of Co in their tissues, suggesting the presence of exclusion or regulation mechanisms that prevent toxicity [97].
These findings indicate that while some fern species can tolerate and accumulate cobalt to a certain extent, this capacity is limited and highly dependent on environmental conditions and species-specific physiological traits. Factors such as soil cobalt availability, pH, the presence of competing metals, and interactions with soil microorganisms may significantly affect the uptake and accumulation of this element [96,97]. Therefore, the observed decrease in BAC values for cobalt in A. rivularioides over the sampling periods may reflect an adaptive response by the plant to its environment, favoring Co exclusion or regulation rather than accumulation. This strategy is likely common among non-hyperaccumulating plants and highlights the importance of species-specific assessments when evaluating phytoremediation potential for heavy metals [98].
The pattern of Co mirrors that of Cr, which had a BAC of 7.48 in C1, followed by a sharp decline in C2–C5, again suggesting C1 as a critical period of higher environmental availability or plant demand. Despite this reduction, A. rivularioides fern initially exhibits a BAC > 1, indicating Cr accumulation. Similar patterns have been observed in other fern species. For instance, Pteris vittata, a known As hyperaccumulator, has demonstrated the ability to accumulate substantial amounts of Cr, particularly in roots. Studies have reported Cr concentrations up to 5717 mg/kg in roots and 1145 mg/kg in shoots of P. vittata grown in Cr-contaminated soils. However, this accumulation was accompanied by phytotoxic effects, including reduced biomass and structural damage to plant tissues, indicating a threshold beyond which Cr becomes detrimental to plant health [99,100]. Another fern species, Pteridium aquilinum, has been identified as a hyperaccumulator of hexavalent chromium. Research has shown that both gametophyte and sporophyte stages of P. aquilinum can accumulate high levels of chromium, with concentrations reaching up to 11,973.93 mg/kg in dry weight. This remarkable accumulation capacity suggests that P. aquilinum possesses efficient mechanisms for Cr uptake and tolerance [101]. The initial high BAC observed in A. rivularioides during C1, followed by a decline in later periods, may reflect an adaptive response to fluctuating environmental chromium levels or internal regulatory mechanisms limiting chromium uptake to prevent toxicity. This pattern aligns with observations in other fern species, where Cr accumulation is influenced by factors such as Cr speciation, soil pH, and plant physiological status [96,97]. In this sense, A. rivularioides has the ability to accumulate Cr under natural conditions or when exposed to soils close to peri-urban regions. Therefore, its accumulation pattern suggests a regulated absorption mechanism that prevents excessive accumulation and possible toxicity. Understanding these mechanisms is fundamental to evaluating the potential of fern species in phytoremediation strategies aimed at Cr-contaminated environments.
The BAC for Cu in A. rivularioides exhibited a consistent decline across the sampling periods, starting at 0.248 in C1 and decreasing to 0.06 in C5. Throughout all periods, the BAC remained below 1, indicating that while the plant absorbs Cu, it does not accumulate it significantly. This pattern suggests a limited capacity for Cu accumulation in A. rivularioides, potentially due to physiological mechanisms that regulate metal uptake to prevent toxicity.
Comparative studies on other fern species provide additional insights. For instance, Pteris melanocaulon demonstrated a high Bioaccumulation Factor (BF) of 4.04 for Cu, primarily accumulating the metal in its roots and rhizomes, with concentrations reaching up to 4590 µg/g in roots. This accumulation pattern suggests that P. melanocaulon may serve as a potential candidate for phytoremediation in Cu-contaminated sites [102]. In addition, aquatic ferns, such as Salvinia biloba, have also shown significant capabilities in copper accumulation. In controlled experiments, S. biloba accumulated up to 11,861 µg/g of Cu in its biomass over 14 days when exposed to 5 µg/mL of copper in solution. However, high copper concentrations led to toxicity symptoms, indicating a threshold beyond which the plant’s health is adversely affected [103]. Similarly, Salvinia minima was able to accumulate Cu up to 6.96 mg/g dry weight when exposed to 80 µmol/L of CuSO4 over 96 h. Despite this accumulation, copper exposure resulted in more severe physiological stress compared to Zn, affecting growth potential and cell membrane integrity [104].
These studies highlight the variability in Cu accumulation among different fern species. While some, like P. melanocaulon and Salvinia species, exhibit significant accumulation, others like A. rivularioides show limited capacity. This variability underscores the importance of species selection in phytoremediation efforts targeting Cu-contaminated environments [102]. Thus, A. rivularioides demonstrates a limited ability to accumulate Cu, as evidenced by BAC values consistently below 1 across multiple sampling periods. This characteristic suggests that while the species can absorb Cu, it does not do so to a degree that would classify it as a hyperaccumulator, thereby limiting its utility in phytoremediation applications for Cu-contaminated sites.
The BAC for K in A. rivularioides exhibited notably high values across the sampling periods, peaking at 417.876 in C5. This exceptional uptake underscores the plant’s capacity to accumulate K, an essential macronutrient vital for various physiological processes. The observed trend—a decrease in BAC from C1 to C4, followed by a significant surge in C5—suggests that external factors, such as soil potassium availability influenced by fertilization practices, may play a pivotal role in modulating K accumulation. Research on Dicranopteris linearis (common ferns in the humid subtropical and tropical regions) revealed that phytoliths can occlude K, which becomes available upon phytolith dissolution, especially under conditions such as burning, common in slash-and-burn agriculture. This mechanism not only aids in nutrient recycling but also enhances soil fertility in such agricultural systems [105].
The substantial increase in BAC observed in C5 may be attributed to fertilization cycles that elevate soil K levels. Long-term studies on tropical soils have demonstrated that consistent K fertilization can enhance the availability of both exchangeable and non-exchangeable K reserves in the soil, thereby influencing plant uptake [106]. Furthermore, the role of K in plant stress responses cannot be overlooked. Adequate K nutrition has been associated with improved tolerance to abiotic stresses, such as drought and salinity, by modulating stomatal conductance and osmotic balance. In the context of A. rivularioides, the elevated BAC in C5 might also reflect an adaptive response to environmental conditions prevailing during that period. Therefore, the high BAC values for K in A. rivularioides, particularly the peak in C5, highlight the species’ proficiency in accumulating this essential nutrient. This accumulation is likely influenced by a combination of physiological necessity and external factors, such as soil K availability driven by fertilization practices. Understanding these dynamics is crucial for optimizing nutrient management strategies, especially in ecosystems where ferns play a significant ecological role.
The BAC for Mg in A. rivularioides consistently exceeded 1 across all sampling periods, ranging from 4.428 to 5.664. These values underscore the species’ robust capacity to absorb and accumulate Mg, reflecting its essential role in plant physiological processes, notably in photosynthesis and enzymatic activities. Comparative studies on other fern species corroborate these findings. For instance, research on Dryopteris filix-mas revealed Mg concentrations in young fronds ranging from 0.18% to 0.21%, indicating a stable Mg uptake across different populations. Similarly, an investigation into six fern species along a tropical elevational gradient, including Adiantum humile and Maxonia apiifolia, demonstrated significant interspecific and intraspecific variations in foliar Mg content. Notably, Mg concentrations tended to decrease with elevation in certain species, suggesting environmental factors influence Mg accumulation [107,108]. In addition, environmental conditions, such as light intensity, also impact Mg accumulation. A study on four edible fern species (Matteuccia struthiopteris (L.) Todaro (MS), Athyrium multidentatum (Doll.) Ching (AM), Osmunda cinnamomea (L.) var. asiatica Fernald (OCA) and Pteridium aquilinum (L.) Kuhn var. latiusculum (Desy.) Underw. ex Heller (PAL)) found that moderate shading (8% full sunlight) led to the highest Mg content, implying that light availability modulates mineral uptake. Furthermore, soil characteristics, including pH, cation exchange capacity, and organic matter content, significantly affect Mg availability and uptake. For example, acidic soils (pH < 6.0) can reduce Mg solubility, limiting its accessibility to plants [109]. Further evidence from Liao et al. [95] showed differential responses in Mg uptake among three Adiantum species based on their calcicole (calcium-loving) or calcifuge (calcium-avoiding) behavior. Their study indicated that soil chemistry, especially calcium and magnesium availability, directly affects elemental concentrations in fern tissues. This highlights the influence of substrate preference and ecological adaptation in determining Mg accumulation capacity.
The elevated BAC values for Mg in A. rivularioides align with patterns observed in other fern species, highlighting the importance of Mg in fern physiology. Environmental factors, including elevation, light intensity, and soil properties, alongside physiological mechanisms, collectively influence Mg accumulation. Understanding these interactions is crucial for elucidating nutrient dynamics in ferns and their potential applications in ecological and phytoremediation contexts.
The BAC for Mn in A. rivularioides exhibited a notable decline over successive sampling periods. Initially, in C1, the BAC was significantly elevated at 2.082, indicating substantial Mn uptake. However, in subsequent periods, the BAC values decreased to below 1, suggesting a reduced accumulation capacity or bioavailability of Mn over time.
This pattern aligns with findings from Kříbek et al. (2011) [96], who investigated metal uptake in Pteris vittata and Cyperus involucratus growing in Cu- and Co-rich tailings in the Zambian Copperbelt. Their study revealed that, despite high concentrations of metals like Cu and Co in the substrate, the concentrations of Mn in the fronds of P. vittata remained low and comparable to those in common plants. This suggests that P. vittata may possess exclusion or avoidance mechanisms to regulate Mn uptake, especially in environments with high metal concentrations [95].
Similarly, Fačkovcová et al. (2020) [92] examined the uptake of trace elements in the water fern Azolla filiculoides after short-term application of chestnut wood distillate. Their findings indicated that A. filiculoides exhibited limited Mn accumulation, even when exposed to solutions containing various trace elements. This further supports the notion that certain fern species have inherent mechanisms to regulate Mn uptake, preventing excessive accumulation.
In contrast, other studies have demonstrated that some aquatic macrophytes can accumulate significant amounts of Mn. For instance, research on Spirodela polyrhiza showed that this species could accumulate Mn concentrations up to 17.062 mg/g dry weight, indicating a high tolerance and accumulation capacity. However, this accumulation was associated with reductions in chlorophyll content and growth, highlighting potential phytotoxic effects at elevated Mn levels [110].
The observed decline in Mn BAC in A. rivularioides over time may be attributed to several factors. Firstly, the initial high BAC could result from higher Mn availability in the soil during the early sampling period. As Mn is a micronutrient essential for various plant physiological processes, its uptake is tightly regulated. Over time, as the plant’s Mn requirements are met or as soil Mn availability decreases, the uptake may diminish, leading to lower BAC values. Secondly, environmental factors such as soil pH, redox conditions, and organic matter content can influence Mn availability. In acidic soils or under anaerobic conditions, Mn becomes more soluble and available for plant uptake. Therefore, changes in soil conditions over time could affect Mn bioavailability and, consequently, its accumulation in plant tissues.
The temporal decrease in Mn BAC observed in A. rivularioides reflects the complex interplay between plant physiological regulation and environmental factors influencing Mn availability. Understanding these dynamics is crucial for assessing the role of ferns in the biogeochemical cycling of micronutrients and their potential application in phytoremediation strategies.
The BAC for nickel Ni in A. rivularioides exhibited considerable variability across sampling periods, with all values exceeding 1 and peaking at 6.574 in the initial period (C1). This pattern suggests a pronounced capacity for Ni uptake, potentially influenced by natural soil enrichment or anthropogenic inputs.
Comparable studies reinforce these observations. Fačkovcová et al. (2020) [92] investigated the water fern Azolla filiculoides and reported significant Ni accumulation following exposure to chestnut wood distillate (pyroligneous acid) used in agriculture. The study highlighted A. filiculoides’ ability to remove Ni from aqueous solutions, achieving removal efficiencies up to 70% over 10 days. This underscores the species’ potential in phytoremediation applications [92]. In addition, research on Pteridium aquilinum revealed that Ni concentrations in fronds were at least twice as high as those of Cr, indicating a higher translocation of Ni to aboveground tissues. This suggests that P. aquilinum does not employ exclusion mechanisms for Ni, allowing for substantial accumulation in aerial parts [111].
The elevated BAC values observed in A. rivularioides may be attributed to several factors. Firstly, Ni is an essential micronutrient involved in various plant physiological processes, including urease activity. However, excessive Ni can be toxic, necessitating regulated uptake mechanisms. Secondly, environmental factors such as soil pH, organic matter content, and the presence of competing ions can influence Ni availability and uptake. Anthropogenic activities, such as industrial emissions and the application of Ni-containing fertilizers, may also contribute to increased soil Ni levels.
Therefore, the consistent BAC values above 1 for Ni in A. rivularioides, with a notable peak in the initial sampling period, reflect the species’ capacity to accumulate Ni. This accumulation is influenced by both physiological requirements and environmental factors. Understanding these dynamics is crucial for assessing the role of ferns in the biogeochemical cycling of Ni and their potential application in phytoremediation strategies.
The BAC for P in A. rivularioides exhibited a consistent upward trend across sampling periods, increasing from 19.272 in C1 to 29.783 in C5. This pattern indicates an active and sustained accumulation of phosphorus by the plant over time. Comparative studies on other fern species provide insights into this accumulation behavior. Liao et al. (2020) [95] investigated the growth performance and element concentrations of three Adiantum species—A. capillus-veneris f. dissectum, A. malesianum, and A. flabellulatum—on calcareous, acidic, and mixed soils. Their findings revealed that the calcicole species (A. capillus-veneris f. dissectum and A. malesianum) exhibited higher leaf P concentrations and relative growth rates when grown on calcareous soils compared to acidic soils. This suggests that these species have adapted mechanisms to effectively uptake and accumulate P in environments where it is more available [95].
The increasing BAC values for P in A. rivularioides (Table 7) may be attributed to several factors. Firstly, P is an essential macronutrient involved in key plant functions such as energy transfer, signal transduction, and the synthesis of nucleic acids. As the plant grows, its demand for phosphorus increases, potentially leading to enhanced uptake mechanisms [95]. Secondly, environmental factors such as soil P availability, pH, and microbial activity can influence P uptake. For instance, in calcareous soils with higher pH, P availability can be limited due to precipitation with Ca; however, certain plant species have developed strategies to mobilize and absorb P under such conditions.
The observed increase in P BAC in A. rivularioides over time reflects the plant’s active accumulation strategy to meet its physiological needs. This behavior is consistent with findings in other fern species and underscores the importance of P in fern growth and development. Understanding these accumulation patterns can inform ecological studies and potential applications in phytoremediation and soil fertility management.
The BAC for Pb in A. rivularioides demonstrated a pronounced peak during the initial sampling period (C1), reaching a value of 10.785. This indicates significant Pb accumulation at this stage. However, in subsequent periods (C3, C4, and C5), the BAC values dropped to zero, suggesting that either lead was no longer available for uptake or environmental conditions—such as soil pH or metal mobility—had changed. This pattern implies that the plant accumulated lead during C1 but ceased accumulation or possibly released it in the following periods, aligning with observations from other studies on lead bioaccumulation in plants. Supporting this, Fačkovcová et al. (2020) [92] investigated the uptake of trace elements in the water fern Azolla filiculoides after short-term application of chestnut wood distillate. Their findings revealed that A. filiculoides exhibited significant Pb accumulation, with removal efficiencies increasing over time, reaching up to 70% after 10 days of exposure [92]. This study underscores the capability of certain fern species to accumulate Pb under specific conditions. Additionally, research on Athyrium yokoscense has demonstrated its remarkable ability to tolerate and accumulate lead. Kamachi et al. (2005) [91] reported that gametophytes of A. yokoscense accumulated Pb concentrations up to 23,000 mg/kg when grown in solutions containing 2 mg/L of lead acetate over three weeks. This accumulation was primarily localized in the cell walls, suggesting a mechanism of sequestration that prevents Pb from interfering with cellular metabolism [112].
The observed decline in Pb BAC in A. rivularioides (Table 8) over time may be attributed to several factors. Firstly, the initial high BAC could result from elevated Pb availability in the soil during the early sampling period, possibly due to recent anthropogenic inputs or environmental disturbances. As time progresses, Pb may become less bioavailable due to processes such as adsorption to soil particles, precipitation, or changes in soil pH, which can reduce Pb solubility. Secondly, the plant may activate physiological mechanisms to limit Pb uptake or translocate accumulated Pb to older tissues, thereby reducing its concentration in actively growing parts.
The temporal pattern of Pb accumulation in A. rivularioides, characterized by an initial high BAC followed by a decline to zero, reflects the complex interplay between environmental Pb availability and plant physiological responses. Understanding these dynamics is crucial for assessing the potential of ferns in phytoremediation strategies aimed at mitigating Pb contamination in various ecosystems.
The BAC values for S in the studied fern species exhibited consistently high levels across all sampling periods, ranging from 18.659 in C2 to 28.633 in C5. This persistent elevation indicates a substantial and sustained accumulation of sulfur by the fern, suggesting both physiological necessity and environmental availability.
Sulfur is an essential macronutrient involved in critical plant functions, including the synthesis of amino acids (such as cysteine and methionine), vitamins, and cofactors. It also plays a pivotal role in the formation of glutathione, a key antioxidant that protects plants against oxidative stress. The high BAC values observed may reflect the fern’s metabolic demand for sulfur, particularly under stress conditions or in environments with fluctuating sulfur availability.
Studies have shown that certain fern species, like Pteris vittata and Adiantum capillus-veneris, enhance sulfur assimilation when exposed to arsenic, leading to increased production of sulfhydryl groups (-SH) that aid in detoxification processes. This adaptive mechanism underscores the importance of sulfur in the stress response and detoxification pathways of ferns [113].
Furthermore, research indicates that ferns may accumulate higher concentrations of sulfate (SO42−) compared to angiosperms, potentially due to differences in nitrogen utilization and protein synthesis capacities. This accumulation could be a strategy to store sulfur for future metabolic needs or to cope with environmental stressors [114]
Therefore, the consistently high BAC values for sulfur in the studied A. rivularioides fern species highlight the plant’s efficient sulfur uptake and assimilation mechanisms. These findings align with existing literature on sulfur metabolism in ferns and emphasize the significance of sulfur in their physiological and stress response processes.
Initially, in C1, the high BAC value (>10) indicates significant Se accumulation by the fern species. However, from C2 onward, a progressive decrease is observed, reaching zero in C4 and only a slight increase in C5. This dynamic suggests that, after an initial period of high Se availability or physiological demand, there was a decline in Se uptake or environmental availability. Studies on Pteris vittata, a well-known selenium-accumulating fern, support this observation. In hydroponic experiments, P. vittata was shown to accumulate up to 1573 mg/kg of Se in its roots, with lower concentrations found in leaves. Under soil-based conditions, Se levels were significantly reduced, with maximum values of 81 mg/kg in leaves and 233 mg/kg in roots, indicating that selenium accumulation is strongly influenced by its environmental availability [115]. Moreover, the chemical form of selenium in the soil plays a critical role in plant uptake. Selenate (SeO42−) is more soluble and thus more bioavailable than selenite (SeO32−). Changes in soil conditions, such as pH and redox potential, can shift the predominance of these forms, thereby affecting selenium uptake by plants [116].
The decline in BAC values over time may also reflect the plant’s physiological mechanisms to avoid selenium toxicity. In P. vittata, increasing Se exposure induces antioxidant responses, such as heightened catalase and peroxidase enzyme activities, up to a threshold, after which these activities decrease, indicating a limit in the plant’s tolerance capacity [117]. Moreover, recent studies have shown that Se can interact synergistically with arsenic accumulation in P. vittata. For instance, Li et al. (2024) [117] demonstrated that foliar application of selenium enhanced both plant growth and arsenic accumulation by modulating glutathione homeostasis and activating arsenite antiporters such as PvACR3. Similarly, Dai et al. (2022) [118] observed that selenium supplementation upregulated genes responsible for arsenic reduction, transport, and sequestration, enhancing arsenic uptake in P. vittata. Although these studies focus on arsenic, they provide strong evidence of selenium’s influence on broader physiological and metabolic processes, which could affect its accumulation and that of other elements.
According to the results above, the data indicate that ferns are capable of accumulating selenium, particularly when it is readily available in the environment. However, this accumulation is regulated by environmental factors (e.g., Se speciation, soil conditions) as well as internal plant mechanisms to maintain homeostasis and avoid toxicity. These traits make ferns suitable candidates for phytoremediation in selenium-contaminated environments, provided that environmental conditions are favorable for uptake and controlled accumulation.
Finally, the BAC for Zn decreases over time, from 3674 in C1 to 1546 in C5, and maintains values above 1 in all periods, suggesting that the plant initially accumulates zinc, but the accumulation capacity decreases over time, likely due to soil depletion or changes in metal availability. These values indicate that ferns initially accumulate significant amounts of zinc, with BAC values well above 1, suggesting active uptake. However, over time, the accumulation capacity diminishes, possibly due to soil zinc depletion or changes in metal availability.
Studies on Dryopteris filix-mas have shown that this species contains substantial amounts of essential minerals, including zinc, which are influenced by ecological factors such as soil composition and environmental conditions. Similarly, research on Pteris vittata has demonstrated its ability to accumulate zinc in its fronds, with concentrations reaching up to 737 mg/kg under field conditions [107,119]
The observed decrease in BAC values over time may also be attributed to the plant’s physiological regulation mechanisms to prevent metal toxicity. As Zn is an essential micronutrient, plants have evolved strategies to maintain homeostasis, adjusting uptake and storage based on internal and external cues. While the study by Montanari et al. (2023) [116] primarily focuses on Se accumulation, it highlights the concept of nutrient foraging and accumulation strategies in plants, which could be analogous to zinc uptake in ferns.
The declining BAC values for zinc in A. rivularioides over time suggest an initial phase of active accumulation followed by a reduction, potentially due to environmental factors and internal regulatory mechanisms. Understanding these patterns is crucial for assessing the role of ferns in phytoremediation and their adaptability to varying soil metal concentrations.
4.6. Contamination Factor (CF) and Pollution Load Index (PLI)
The temporal evaluation of soil contamination in the study area, based on Contamination Factor (CF) and Pollution Load Index (PLI) values (Table 9), reveals distinct variations linked to agricultural activities and seasonal patterns across the five sampling periods (C1 to C5).
The soil exhibited moderate contamination for arsenic (As) across all periods (C1–C5), with CF values ranging from 1.420 to 1.762. Similarly, calcium (Ca), manganese (Mn), and magnesium (Mg) consistently showed moderate to high contamination, with Mg displaying notably elevated CF values (from 3.296 in C1 to 4.070 in C5), suggesting an accumulation trend over time. Selenium (Se) also remained at consistently high contamination levels (CF > 4 in all periods), and phosphorus (P) presented extremely high contamination, with CF values exceeding 200 in every collection, peaking at 325.989 in C5. In contrast, elements like barium (Ba), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), sulfur (S), and zinc (Zn) generally exhibited low contamination levels (CF < 1), with minor fluctuations between sampling periods.
A clear temporal pattern is observed, correlating contamination levels with specific phases of the agricultural calendar. The highest CF values for several elements (e.g., P, Mg, Se) were recorded during C3 (post-soybean planting: 28 December 2019), C4 (between crops: 9 April 2020), and C5 (pre-maize harvest: 7 August 2020). These peaks likely reflect agricultural inputs, including fertilizers and soil amendments. Conversely, lower CF values were noted during C2 (inter-harvest: 24 August 2019), suggesting reduced agrochemical inputs during fallow or between-crop periods.
The PLI values provide an integrated measure of overall soil pollution across all analyzed metals. Periods C1 (1.21), C4 (1.04), and C5 (1.12) presented PLI > 1, indicating polluted soil conditions. The elevated PLI in C1 likely reflects residual contamination from previous cropping cycles or baseline pollution levels. In contrast, PLI values for C2 (0.942) and C3 (0.999) remained below 1, suggesting that during the inter-harvest period (C2) and immediately post-soybean planting (C3), the cumulative pollution load was lower. This aligns with expectations, as agrochemical applications typically intensify post-planting and towards the pre-harvest phase, which is reflected in the increasing PLI observed in C4 and C5.
These findings are consistent with previous reports linking agricultural cycles and fertilizer use to temporal variations in soil metal concentrations [1,4,31]. Notably, Hu et al. (2023) [4], in a meta-analysis of peri-urban agricultural soils in China, found that intensive fertilizer application was responsible for seasonal spikes in heavy metal levels, especially following planting and fertilization phases, with over 30% of total metal accumulation attributed to agricultural inputs. Likewise, Alengebawy et al. (2021) [31] reported that repeated fertilizer and pesticide applications significantly increase the availability and mobility of metals, posing long-term ecological risks due to cumulative contamination across multiple cropping cycles. Therefore, many fertilizers [1,4,31,74,75] contain heavy metals such as cadmium (Cd), lead (Pb), and arsenic (As) as impurities. When these fertilizers are applied repeatedly across seasons, especially without alternating with low-input crops, metals can progressively build up in the soil. Over time, this accumulation may exceed background levels and pose environmental and health risks.
4.7. Hazard Quotient (HQ) and Hazard Index (HI)
Dermal Exposure to Water
Table 12 presents the Hazard Quotient (HQ) and Hazard Index (HI) values for dermal exposure to metal(loid)s in water across five collection periods. The results reveal extremely high non-carcinogenic risks, primarily driven by P, followed by Mn, Cr, Ni, and Zn in specific periods.
Phosphorus exhibited the highest HQ values, ranging from 4.70 × 106 (C5) to 5.52 × 106 (C3). These values greatly exceed the acceptable risk threshold (HQ = 1), indicating a disproportionate exposure or a potential inadequacy in the reference dose (RfD) applied to P in dermal exposure assessments. It is important to note that the absence of RfD values for certain elements (e.g., Ba, Ca, K, Mg, and S) is denoted by “—“ in the table, indicating that HQ could not be calculated for these substances due to a lack of toxicological reference data.
Manganese also showed elevated HQ values across all periods, ranging from 61.64 (C5—before corn harvest) to 246.58 (C1—post-harvest corn), representing the second most significant contributor to the overall risk. In C4 (inter-harvest), both Cr and Ni showed high HQs of 51.29 and 29.59, respectively, suggesting possible seasonal or activity-related contamination events.
Zinc also contributed to the risk in C1, with HQ of 4.73. In contrast, As, Co, Cu, Pb, and Se displayed HQ values of 0 in all periods, indicating no significant dermal risk under current exposure assumptions.
The cumulative risk, represented by the Hazard Index (HI), was high in all periods, ranging from 4.70 × 106 (C5) to 5.52 × 106 (C3). Since HI values above 1 indicate a potential for adverse non-carcinogenic health effects, the extremely elevated indices observed across all periods strongly suggest a significant risk from dermal contact with contaminated water. However, it is important to note that regular phosphorus forms found naturally in water and soil, such as orthophosphates, are generally considered safe for skin contact at typical environmental concentrations. To date, no direct studies have linked naturally occurring aquatic phosphorus to dermal irritation in humans [14]. Nevertheless, acidic or industrial phosphorus compounds, like phosphoric acid, are well-documented skin irritants in occupational settings and at high concentrations [21]. Therefore, while phosphorus in its environmental forms poses minimal dermal risk, site-specific water chemistry parameters (e.g., pH and phosphorus speciation) should still be evaluated in risk assessments to rule out localized hazards.
Similarly, although several forms of manganese are water-soluble, current evidence indicates that dermal absorption of manganese is minimal. As a result, skin contact with manganese is not considered a significant exposure pathway for the general population. Moreover, available toxicological data do not report notable dermal irritation or adverse skin effects associated with environmental exposure to manganese [120].
Regarding chromium, Georgaki et al. (2023) [121] reported that dermal exposure to chromium through water contact poses a comparatively low health risk. Their study emphasizes that while ingestion remains the primary route of concern, the limited permeability of chromium species through human skin reduces the likelihood of significant dermal absorption and associated toxicity under typical environmental conditions. Nonetheless, chromium has the ability to penetrate human skin to a certain extent, particularly when the skin barrier is compromised or damaged. Once absorbed, chromium can interact with dermal immune cells, potentially triggering local inflammatory responses and dermal toxicity [122].
In contrast, nickel (Ni) plays a more significant role in dermal toxicity due to its higher capacity to permeate human skin, especially in the form of soluble nickel salts. Hagvall et al. (2021) demonstrated, through mass spectrometry imaging, that nickel ions can penetrate both the stratum corneum and deeper epidermal layers in ex vivo human skin models. This permeation was notably more pronounced in damaged or compromised skin. Once inside the skin, nickel ions can bind to skin proteins and immune cells, triggering sensitization processes that lead to allergic contact dermatitis, one of the most common forms of metal-induced skin disease [122]. Furthermore, the study highlighted that the extent of dermal absorption depends on the chemical form of nickel, exposure duration, and skin integrity. These findings underscore the importance of considering dermal exposure as a relevant pathway in human health risk assessments for nickel-contaminated environments.
Finally, according to ATSDR (2005) [123], environmental water and soil, which primarily contain common zinc forms such as Zn2+ ions and zinc oxide (ZnO), pose minimal dermal risk under typical exposure scenarios.
Dermal Exposure to Soil
Table 13 presents the Hazard Quotient (HQ) values for dermal exposure to various metal(loid)s in soil across five sampling periods, along with the resulting Hazard Index (HI). The periods correspond to different agricultural phases: C1 (post-harvest corn), C2 (inter-harvest), C3 (post-planting soybean), C4 (inter-harvest), and C5 (before corn harvest).
The results show exceedingly high HQ values for As, especially in C1 (153,200) and C2 (146,933), indicating critical non-carcinogenic risks from dermal contact with soil during those periods. Even in the lowest-risk collection, C3 (post-planting soybean), As still presents an HQ of 16,423, far above the threshold of 1, which indicates unacceptable risk. These findings underscore arsenic as the primary contributor to the overall hazard index.
Manganese also showed consistently elevated HQs in all sampling periods, ranging from 129.83 (C3) to 301.11 (C1). Similarly, cobalt presented high HQs, with values between 73.68 (C3) and 150.00 (C1), further contributing significantly to the total non-carcinogenic risk. These metals, though essential in trace amounts, are known to pose dermal toxicity risks at elevated concentrations.
Nickel HQ values also exceeded safe thresholds, particularly in C1 (58.75) and C2 (49.11), while phosphorus ranged from 1053 (C2) to 2100 (C1). The application of RfD values to phosphorus in dermal exposure should be viewed cautiously, given the scarcity of toxicological benchmarks for this pathway.
In contrast, Zn, Pb, Se, Cu, and Cr presented relatively low HQ values, all below or near 10, indicating minor contributions to the overall hazard. However, Pb exceeded the safety threshold slightly in all periods (HQ between 1.25 and 1.78), suggesting potential concern due to chronic exposure. Elements such as Ba, Ca, K, Mg, and S were not included in the HQ calculations, denoted by “—”, indicating the absence of dermal RfD values, which prevented the estimation of their associated risks.
The Hazard Index (HI), representing the sum of all HQs, was high in C1 (157,837.42) and C2 (148,820.14), driven primarily by the extremely high levels of As and Mn. Even in the lower-risk periods, such as C3 (16,858.57) and C4 (19,275.72), the HI far exceeds the acceptable threshold of 1, signifying a consistent risk of non-carcinogenic effects across all sampling events. In contrast, the study by Alharbi et al. (2025) [124], conducted on agricultural soils in the central region of Saudi Arabia, reported total HI values below 1 for all age groups and all evaluated elements, indicating no significant non-carcinogenic risk. The discrepancy can be attributed to local factors such as soil type, agricultural intensity, use of agrochemicals, and climatological characteristics.
Several heavy metals found in soils—including chromium (Cr), arsenic (As), lead (Pb), and copper (Cu)—pose potential dermal health risks, particularly in contaminated agricultural or industrial areas. Among these, arsenic (As) is notably hazardous due to its ability to penetrate the skin and accumulate in tissues. Dermal exposure has been linked to hyperkeratosis, pigmentation disorders, and carcinogenic effects with prolonged exposure, as reported by the Agency for Toxic Substances and Disease Registry [125].
Chromium (Cr), especially in its hexavalent form (Cr VI), can permeate the skin, particularly when the barrier is damaged. Once absorbed, Cr causes allergic contact dermatitis. Georgaki et al. (2023) emphasized that although the risk via dermal exposure is relatively lower than ingestion, it is not negligible [121]. Hagvall et al. (2021) also confirmed chromium penetration into human skin using mass spectrometry imaging [122]. According to studies, when the human skin comes in contact with chromium, small amounts of chromium will enter into body [126].
Lead (Pb) dermal absorption is generally low but increases with damaged skin or prolonged exposure. Inorganic lead (Pb) can be absorbed through dermal contact, though this route is considerably less efficient than inhalation or ingestion—except in cases involving hand-to-mouth behavior [20]. While acute dermal toxicity is rare, repeated exposure may lead to skin irritation or allergic reactions in sensitive individuals. Therefore, in environments with elevated soil Pb levels, protective measures and hygiene practices are crucial to reduce the risk of lead-related health effects via dermal pathways [13,20].
Repeated contact with copper-contaminated soils has been shown to elicit skin irritation and allergic responses. Carrillo-Niquete et al. (2022) [104] and ATSDR (2004) both highlight Cu’s potential for inducing localized effects when dermal exposure is significant [104,127]. Occupational exposure to copper has been associated with documented cases of dermal irritation, including hand eczema and contact dermatitis, particularly among individuals handling copper-based fertilizers, fungicides, coins, and cleaning agents. Reported symptoms commonly include erythema, vesiculation, and pruritus, highlighting copper’s potential as a cutaneous irritant in an occupational setting [128].
Cobalt in soil poses potential dermal risks, particularly in occupational or contaminated environments. Although cobalt is an essential trace element, prolonged skin contact—especially with soluble cobalt compounds—can lead to dermatological effects such as allergic contact dermatitis and irritant reactions. Dermal contact with cobalt-contaminated soils can occur during agricultural work, recreational activities, or industrial handling [11,13,129]. Although the percutaneous absorption of cobalt is generally low, studies have shown that soluble cobalt salts, such as cobalt chloride, can penetrate the skin barrier, especially when the skin is damaged or occluded [130]. Once absorbed, cobalt ions may interact with skin proteins, triggering allergic contact dermatitis in sensitized individuals [131].
Although there is limited epidemiological evidence linking manganese in soil directly to widespread dermal disease in the general population, the potential for skin irritation and sensitization should not be underestimated in high-exposure settings, particularly among vulnerable populations or individuals with pre-existing skin conditions. According to the Agency for Toxic Substances and Disease Registry (ATSDR), manganese exhibits low dermal permeability, and there is limited evidence supporting significant absorption through intact human skin. As a result, dermal contact with manganese is generally not considered a major route of exposure for the general population under typical environmental conditions [132].
According to Ahlström et al. (2019), repeated or prolonged skin contact with nickel—particularly in its soluble forms—can trigger sensitization and elicit immunological reactions characterized by pruritus, erythema, and eczema [132]. The review highlights that even low-dose exposures, such as those encountered through soil, jewelry, or occupational tools, are sufficient to induce or exacerbate allergic responses in sensitized individuals. Although only a small fraction of nickel is dermally absorbed, prolonged exposure is associated with an increase in some types of cancer [133].
Among the various forms of phosphorus, only white phosphorus is classified as acutely toxic to humans. It can cause severe dermal burns and systemic toxicity upon skin contact or ingestion [134]. In contrast, common phosphate compounds used in agriculture—such as monoammonium phosphate (MAP)—are not associated with dermal toxicity under normal environmental conditions [134].
Dermal exposure assessment should distinguish between compound-specific effects. While selenium compounds such as sodium selenite can lead to allergic dermatitis in exposed individuals—confirmed by clinical patch testing [135]—these effects are typically associated with chronic or occupational exposures rather than routine contact with selenium-containing soils. Similarly, zinc in its common soil form (zinc oxide) is considered safe and non-irritant to the skin [123]. However, zinc chloride, a soluble and caustic compound, clearly induces severe skin irritation and histopathological changes in animal models, warranting caution in environments where such forms may be present. This underscores the importance of considering both the chemical speciation of soil-bound elements and the exposure context—especially in agricultural or industrial zones—when evaluating dermal risk [123].
These results point to an urgent need for remediation strategies and stricter regulation of soil contamination in the region, especially considering the close linkage between soil quality and agricultural activities. Moreover, the absence of dermal RfD values for several elements highlights a gap in toxicological data, warranting further research to enable comprehensive risk assessments.
4.8. Carcinogenic Risk
The results for carcinogenic risk obtained reveal variations across the sampling periods (Table 14), reflecting fluctuations in the concentrations of the analyzed elements and their respective contributions to the potential lifetime cancer risk. This approach enables a more realistic estimation of human health risks resulting from simultaneous exposure to multiple environmental matrices, particularly in mixed-use areas such as peri-urban regions. These values represent the Incremental Lifetime Cancer Risk (ILCR) for each element in each collection period. According to USEPA guidelines, ILCR values above 1 × 10−4 (0.0001) are considered a public health concern. However, as shown in Table 13, the total ILCR (ILCRtotal) values—considering dermal exposure to As, Pb, and Cr in both soil and water—exceed 1 × 10−3, indicating a very high and unacceptable level of cancer risk.
In the agriculturally intensive region surrounding the Santa Virgem River, Brazil, mitigating dermal exposure to arsenic (As), lead (Pb), and chromium (Cr) is vital due to elevated hazard indices (HI) identified in soil and water. These elements, often introduced through long-term agrochemical use, pose chronic health risks via skin contact. Therefore, effective mitigation strategies include phytoremediation with hyperaccumulator species (e.g., Pteris vittata, Amaranthus spp.), soil amendments like biochar, and the consistent use of personal protective equipment (PPE) during fieldwork, or avoiding bathing and contact with soil from contaminated areas. Public health campaigns should promote hygiene practices to reduce secondary exposure. Additionally, regular monitoring and treatment of irrigation water—especially when contaminant levels exceed health standards—are essential. Finally, implementing regulatory controls and soil quality guidelines can limit future contamination and protect rural populations. These measures are critical for integrating public health protection into sustainable agricultural practices in the region.
5. Conclusions
The conclusions should be rewritten, avoiding limiting themselves to a simple summary of the previous paragraphs. It is recommended to provide a critical synthesis of the results obtained, underlining their importance and limitations, and proposing possible future research perspectives.
This study demonstrated the temporal dynamics of metal(loid) concentrations in agricultural soils, ferns, and surface waters within a peri-urban region on the Brazil–Paraguay border during 2019–2020. The consistently elevated levels of As, Se, Co, Mn, Cu, and Zn raise justified concerns about environmental and human health risks, especially when benchmarked against international regulatory standards. The highest concentrations were observed during pre- and post-harvest periods, notably in corn cultivation phases, reflecting the cumulative effects of agrochemical inputs and highlighting the strong link between land-use practices and trace element enrichment.
The Principal Component Analysis clarified distinct accumulation profiles for each sampling period, underlining the effectiveness of the fern A. rivularioides as a bioindicator of local contamination. While toxic metal concentrations in surface waters mostly remained below detection thresholds, nutrient elements such as K, Mg, and P showed seasonal enrichment patterns that may contribute to eutrophication risks, as evidenced by P concentrations exceeding USEPA thresholds.
The combined indices (Igeo, BAC, CF, PLI) confirmed that soils in the studied area face moderate to severe contamination by specific elements, notably P, Mg, Se, and As. Risk assessments (HQ, HI, ILCR) indicate significant non-carcinogenic and carcinogenic health hazards, with ILCR values well above acceptable thresholds, particularly due to dermal exposure to As, Pb, and Cr. These findings reinforce the urgent need for integrated management and monitoring strategies.
However, this study also has limitations that should be acknowledged. The monitoring period was limited to a two-year window, which may not capture longer-term trends or interannual variability. The sampling design, while robust for a preliminary survey, could benefit from broader spatial replication and finer temporal resolution to better capture seasonal dynamics. Furthermore, the risk assessments relied on standard exposure models, which may not fully reflect local population behaviors and bioavailability nuances in tropical soils.
Future research should address these limitations by expanding sampling campaigns over multiple crop cycles and diverse land-use contexts. Integrating advanced speciation analyses and bioavailability studies would refine risk estimates, while long-term ecotoxicological monitoring could help validate the bioindicator role of A. rivularioides. Finally, socio-environmental studies involving local communities would be valuable for developing targeted mitigation strategies and sustainable agricultural practices that balance productivity and environmental safety.
Overall, this work provides a critical basis for environmental authorities to implement evidence-based regulations, prioritize contaminated sites, and promote more sustainable agricultural management in sensitive peri-urban regions along the Brazil–Paraguay frontier.
Author Contributions
Conceptualization, P.R.E., E.S.d.P.M. and V.A.d.N.; methodology, P.R.E.; validation, P.R.E., E.S.d.P.M., M.A.P.A., D.A.L.F.E. and A.P.; formal analysis, E.S.d.P.M. and M.A.P.A.; data curation, P.R.E. and E.S.d.P.M.; writing—original draft preparation, V.A.d.N.; writing—review and editing, P.R.E., M.A.P.A., D.A.Z.G. and V.A.d.N.; visualization, M.A.P.A. and V.A.d.N.; supervision, V.A.d.N.; project administration, P.R.E.; funding acquisition, V.A.d.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Brazilian Research Council (CNPq) (CNPq: Process No 314551/2023-9) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors thank the Federal University of Mato Grosso do Sul, Faculty of Medicine, for their scientific support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gunapala, R.; Gangahagedara, R.; Wanasinghe, W.C.S.; Samaraweera, A.U.; Gamage, A.; Rathnayaka, C.; Hameed, Z.; Baki, Z.A.; Madhujith, T.; Merah, O. Urban agriculture: A strategic pathway to building resilience and ensuring sustainable food security in cities. Farming Syst. 2025, 3, 100150. [Google Scholar] [CrossRef]
- Fei, S.; Wu, R.; Liu, H.; Yang, F.; Wang, N. Technological innovations in urban and peri-urban agriculture: Pathways to sustainable food systems in metropolises. Horticulturae 2025, 11, 212. [Google Scholar] [CrossRef]
- FAO; Rikolto and RUAF. Urban and Peri-Urban Agriculture Sourcebook—From Production to Food Systems; FAO and Rikolto: Rome, Italy, 2022. [Google Scholar]
- Hu, N.-W.; Yu, H.-W.; Deng, B.-L.; Hu, B.; Zhu, G.-P.; Yang, X.-T.; Wang, T.-Y.; Zeng, Y.; Wang, Q.-Y. Levels of heavy metal in soil and vegetable and associated health risk in peri-urban areas across China. Ecotoxicol. Enviro. Saf. 2023, 259, 115037. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Khan, D.K.; Santra, S.C. An assessment of heavy metal contamination in vegetables grown in wastewater-irrigated areas of Titagarh, West Bengal, India. Bull. Environ. Contam. Toxicol. 2008, 80, 115–118. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M.; Marshall, F.M. Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food Chem. Toxicol. 2009, 47, 583–591. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. [Google Scholar] [CrossRef]
- WHO. Preventing Disease Through Healthy Environments: Exposure to Cadmium: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.who.int/publications/i/item/WHO-CED-PHE-EPE-19-4-3 (accessed on 29 April 2025).
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Oubane, M.; Khadra, A.; Ezzariai, A.; Kouisni, L.; Hafidi, M. Heavy metal accumulation and genotoxic effect of long-term wastewater irrigated peri-urban agricultural soils in semiarid climate. Sci. Total Environ. 2021, 794, 148611. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M.; Marshall, F. Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull. Environ. Contam. Toxicol. 2006, 77, 312–318. [Google Scholar] [CrossRef]
- Wang, J.; Gao, P.; Li, M.Y.; Ma, J.Y.; Li, J.Y.; Yang, D.L.; Cui, D.L.; Xiang, P. Dermal bioaccessibility and cytotoxicity of heavy metals in urban soils from a typical plateau city: Implication for human health. Sci. Total Environ. 2022, 835, 155544. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment), Washington, DC, USA, 2004. Available online: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part-e (accessed on 29 April 2025).
- Shelnutt, S.R.; Goad, P.; Belsito, D.V. Dermatological toxicity of hexavalent chromium. Crit. Rev. Toxicol. 2007, 37, 375–387. [Google Scholar] [CrossRef]
- Silverberg, N.B.; Pelletier, J.L.; Jacob, S.E.; Schneider, L.C. Section on dermatology, section on allergy and immunology. Nickel Allergic Contact Dermatitis: Identification, Treatment, and Prevention. Pediatrics 2020, 145, e20200628. [Google Scholar] [CrossRef]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human health effects from chronic arsenic poisoning—A review. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2006, 41, 2399–2428. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2020. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 29 April 2025).
- ATSDR. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=48&tid=15 (accessed on 29 April 2025).
- Angon, P.B.; Islam, M.S.; Shreejana, K.C.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant. Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Cakaj, A.; Drzewiecka, K.; Hanć, A.; Lisiak-Zielińska, M.; Ciszewska, L.; Drapikowska, M. Plants as effective bioindicators for heavy metal pollution monitoring. Environ. Res. 2024, 256, 119222. [Google Scholar] [CrossRef]
- Andrade, G.C.; Santana, B.V.N.; Rinaldi, M.C.S.; Ferreira, S.O.; da Silva, R.C.; da Silva, L.C. Using native plants to evaluate urban metal pollution and appoint emission sources in the Brazilian Steel Valley region. Environ. Sci. Pollut. Res. 2024, 31, 30427–30439. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hajyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Beni, A.A. Comprehensive analysis of heavy metal soil contamination in mining environments: Impacts, monitoring techniques, and remediation strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Sazykin, I.; Khmelevtsova, L.; Azhogina, T.; Sazykina, M. Heavy metals influence on the bacterial community of soils: A review. Agriculture 2023, 13, 653. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M.; Marshall, F.M. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef]
- Liu, H.; Probst, A.; Liao, B. Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci. Total Environ. 2005, 1, 153–166. [Google Scholar] [CrossRef]
- National Institute of Colonization and Agrarian Reform (INCRA)—Brazil. Available online: http://www.gov.br/incra/pt-br/assuntos/noticias/mutirao-leva-atendimento-a-familias-do-assentamento-itamarati (accessed on 24 April 2025).
- Governo do Estado de Mato Grosso do Sul, Secretaria de Estado de Meio Ambiente e Desenvolvimento Econômico—SEMADE—Geoambientes da Faixa de Fronteira, GTNS/MS, Instituto do Meio Ambiente de Mato Grosso do Sul, Brasil. 2016. Available online: https://www.imasul.ms.gov.br/wp-content/uploads/2016/02/Geoambientes-da-Faixa-de-Fronteira-Versao-2016.pdf (accessed on 24 April 2025).
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelhor, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; 356p. [Google Scholar]
- Fernandes, M.R.; Melo, E.S.d.P.; Ancel, M.A.P.; Guimarães, R.d.C.A.; Hiane, P.A.; Geilow, K.d.C.F.; Bogo, D.; Tschinkel, P.F.S.; Rosa, A.C.G.; Medeiros, C.S.d.A.; et al. Assessment of the risk to human health and pollution levels due to the presence of metal(loid)s in sediments, water, and fishes in urban rivers in the state of Mato Grosso do Sul, Brazil. Urban Sci. 2025, 9, 114. [Google Scholar] [CrossRef]
- Ramanathan, T.; Ting, Y.-P. Selection of wet digestion methods for metal quantification in hazardous solid wastes. J. Environ. Chem. Eng. 2015, 3, 1459–1467. [Google Scholar] [CrossRef]
- Junior, A.d.S.A.; Ancel, M.A.P.; Garcia, D.A.Z.; Melo, E.S.d.P.; Guimarães, R.d.C.A.; Freitas, K.d.C.; Bogo, D.; Hiane, P.A.; Vilela, M.L.B.; Nascimento, V.A.d. Monitoring of metal(loid)s using Brachiaria decumbens Stapf leaves along a highway located close to an urban region: Health risks for tollbooth workers. Urban Sci. 2024, 8, 128. [Google Scholar] [CrossRef]
- Rosa, A.C.G.; Melo, E.S.P.; Junior, A.S.A.; Gondim, J.M.S.; de Sousa, A.G.; Cardoso, C.A.L.; Viana, L.F.; Carvalho, A.M.A.; Machate, D.J.; do Nascimento, V.A. Transfer of metal(loid)s from soil to leaves and trunk xylem sap of medicinal plants and possible health risk assessment. Int. J. Environ. Res. Public Health 2022, 19, 660. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Perez, D.V.; Pereira, N.R.; Carvalho Junior, W.d.; Calderano Filho, B.; Bhering, S.B.; Chagas, C.d.S.; Silva, E.F.d.; Macedo, J.R.d. Determinação de Valores de Referência de Qualidade Para Solos do Estado do Mato Grosso do Sul; Embrapa Solos; Boletim de Pesquisa e Desenvolvimento: Rio de Janeiro, Brazil, 2022; p. 283. [Google Scholar]
- Senger, C.C.D.; Sanchez, L.M.B.; Pires, M.B.G.; Kaminski, J. Teores minerais em pastagens do Rio Grande do Sul. II. Sódio, enxofre, zinco, cobre, ferro e manganês. Pesqui. Agropecuária Bras. 1997, 32, 101–108. [Google Scholar]
- Da Silva, L.C. Teores Totais de Potássio, Cálcio, Magnésio e Sódio em solos do Sudeste da Província Mineral de Carajás; Final Project (Graduation); Curso de Engenharia Ambiental e Energias Renováveis, Universidade Federal Rural da Amazônia: Belém, Brazil, 2019. [Google Scholar]
- Tokura, L.K.; Nogueira, M.A.; Costa, A.C.S. Estoques e Frações de Fósforo no solo sob Plantio Direto com Diferentes Espécies de Cobertura e Sistemas De Rotação; Technical Report. Documentos 231; Embrapa Soja: Londrina, Brazil, 2002. [Google Scholar]
- Gabos, M.B. Background Concentrations and Adsorption of Selenium in Tropical Soils. Ph.D. Thesis, Escola Superior de Agricultura Luiz de Queiroz, Piracicaba, Brazil, 2012. [Google Scholar][Green Version]
- Sabir, M.; Baltrėnaitė-Gedienė, E.; Ditta, A.; Ullah, H.; Kanwal, A.; Ullah, S.; Faraj, T.K. Bioaccumulation of heavy metals in a soil–plant system from an open dumpsite and the associated health risks through multiple routes. Sustainability 2022, 14, 13223. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control: A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Belzunce, M.J.; Solaun, O.; Oreja, J.A.G.; Millán, E.; Víctor Pérez, V. Chapter 12—Contaminants in sediments. In Oceanography and Marine Environment of the Basque Country; Borja, Á., Collins, M., Eds.; Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 2004; Volume 70, pp. 283–315. [Google Scholar] [CrossRef]
- Fahimah, N.; Salami, I.R.S.; Oginawati, K.; Mubiarto, H. Appraisal of pollution levels and non-carcinogenic health risks associated with the emergence of heavy metals in Indonesian community water for sanitation, hygiene, and consumption. Emerg. Contam. 2024, 10, 100313. [Google Scholar] [CrossRef]
- Miletić, A.; Lučić, M.; Onjia, A. Exposure factors in health risk assessment of heavy metal(loid)s in soil and sediment. Metals 2023, 13, 1266. [Google Scholar] [CrossRef]
- Didier, J.; Ewodo Mboudou, G.; Fantong, W.; Ombolo, A.; Chounna Yemele, G.; Jokam Nenkam, T.; Messi, G. Potentially toxic elements contamination in groundwater and human health risk assessment in the Mayo Bocki Watershed, North Cameroon. Arab. J. Geosci. 2023, 16, 479. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Dermal Exposure Assessment: A Summary of EPA Approaches; National Center for Environmental Assessment: Washington, DC, USA, 2007. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Risk Assessment Guidance for Superfund Human—Health Evaluation Manual Part A; USEPA: Washington, DC, USA, 1989. Available online: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part (accessed on 30 April 2025).
- Ministério do Meio Ambiente. Conselho Nacional do Meio Ambiente (Conama). Resolução No 420, de 28 de Dezembro de 2009, Brasil. Available online: http://hab.eng.br/wp-content/uploads/2017/09/resolucao-conama-420-2009-gerenciamento-de-acs.pdf (accessed on 3 June 2024).
- USEPA (United States Environmental Protection Agency). Guidance for Developing Ecological Soil Screening Levels; USEPA: Washington, DC, USA, 2003. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/ecossl_guidance_chapters.pdf (accessed on 1 April 2025).
- Gonçalves, D.A.M.; Pereira, W.V.d.S.; Johannesson, K.H.; Pérez, D.V.; Guilherme, L.R.G.; Fernandes, A.R. Geochemical background for potentially toxic elements in forested soils of the state of Pará, Brazilian Amazon. Minerals 2022, 12, 674. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy metals in agricultural soils: Sources, influencing factors, and remediation strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Shalaby, T.A.; Prokisch, J.; Fári, M. Selenium in agriculture: Water, air, soil, plants, food, animals and nanoselenium. In Co2 Sequestration, Biofuels and Depollution; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Cham, Switzerland, 2015; Volume 5. [Google Scholar] [CrossRef]
- Li, W.; Zhu, T.; Yang, H.; Zhang, C.; Zou, X. Distribution characteristics and risk assessment of heavy metals in soils of the typical karst and non-karst aeas. Land 2022, 11, 1346. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Deng, M.; Japenga, J.; Li, T.; Yang, X.; He, Z. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J. Environ. Manag. 2018, 207, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wysokinski, A.; Kuziemska, B.; Lozak, I. Heavy metal allocation to pea plant organs (Pisum sativum L.) from soil during different development stages and years. Agronomy 2023, 13, 673. [Google Scholar] [CrossRef]
- Atafar, Z.; Mesdaghinia, A.; Nouri, J.; Homaee, M.; Ahmadimoghaddam, M.; Mahvi, A.H. Effect of fertilizer application on soil heavy metal concentration. Environ. Monit. Assess. 2009, 160, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef]
- Morton-Bermea, O.; Hernández-Alvarez, E.; Almorín-Ávila, M.A.; Ordoñez-Godínez, S.; Bermendi-Orosco, L.; Retama, A. Historical trends of metals concentration in PM10 collected in the Mexico City metropolitan area between 2004 and 2014. Environ. Geochem. Health 2021, 43, 2781–2798. [Google Scholar] [CrossRef]
- Cao, C.; Wang, L.; Li, H.; Wei, B.; Yang, L. Temporal variation and ecological risk assessment of metals in soil nearby a Pb–Zn mine in Southern China. Int. J. Environ. Res. Public Health 2018, 15, 940. [Google Scholar] [CrossRef]
- Haruna, S.I.; Nkongolo, N.V. Tillage, cover crop and crop rotation effects on selected soil chemical properties. Sustainability 2019, 11, 2770. [Google Scholar] [CrossRef]
- Jin-Soo, C.; In-Ho, Y.; Kyoung-Woong, K. Heavy metal and arsenic accumulating fern species as potential ecological indicators in As-contaminated abandoned mines. Ecol. Indic. 2009, 9, 1275–1279. [Google Scholar] [CrossRef]
- Leal-Alvarado, D.A.; Martínez-Hernández, A.; Calderón-Vázquez, C.L.; Uh-Ramos, D.; Fuentes, G.; Ramírez-Prado, J.H.; Sáenz-Carbonell, L.; Santamaría, J.M. Identification of up-regulated genes from the metal-hyperaccumulator aquatic fern Salvinia minima Baker, in response to lead exposure. Aquat. Toxicol. 2017, 193, 86–96. [Google Scholar] [CrossRef]
- Costa, C.; Lia, F. Temporal variations of heavy metal sources in agricultural soils in Malta. Appl. Sci. 2022, 12, 3120. [Google Scholar] [CrossRef]
- Guilherme, L.R.G.; Corguinha, A.P.B.; Ribeiro do Valle, L.A.; Marchi, G. Heavy metals in P fertilizers marketed in Brazil: Is this a concern in our agroecosystems? In Proceedings of the SYMPHOS 2019—5th International Symposium on Innovation & Technology in the Phosphate Industry. Benguerir, Morocco, 7–9 October 2019; Available online: http://dx.doi.org/10.2139/ssrn.3627425 (accessed on 29 April 2025). [CrossRef]
- Pham, V.-D.; Fatimah, M.-S.; Sasaki, A.; Duong, V.-H.; Pham, K.-L.; Susan, P.; Watanabe, T. Seasonal variation and source identification of heavy metal(loid) contamination in peri-urban farms of Hue city, Vietnam. Environ. Polluti. 2021, 278, 116813. [Google Scholar] [CrossRef]
- Iksan, M.; Aba Kusrini, L. The ability of ferns to accumulate heavy metals (Hg, Pb And Cd) in the waters of the Gorontalo River. Int. J. App. Biol. 2019, 3, 36–44. [Google Scholar] [CrossRef]
- Xie, N.; Kang, C.; Ren, D.; Zhang, L. Assessment of the variation of heavy metal pollutants in soil and crop plants through field and laboratory tests. Sci. Total Environ. 2022, 811, 152343. [Google Scholar] [CrossRef]
- Gventsadze, G.; Ghambashidze, G.; Chankseliani, Z.; Sarjveladze, I.; Blum, W.E.H. Impacts of crop-specific agricultural practices on the accumulation of heavy metals in soil in Kvemo Kartli Region (Georgia): A preliminary assessment. Sustainability 2024, 16, 4244. [Google Scholar] [CrossRef]
- Rai, P.K. An eco-sustainable green approach for heavy metals management: Two case studies of developing industrial region. Environ. Monit. Assess. 2012, 184, 421–448. [Google Scholar] [CrossRef]
- Oluyemi, E.A.; Feuyit, G.; Oyekunle, J.A.O.; Ogunfowokan, A.O. Seasonal variations in heavy metal concentrations in soil and some selected crops at a landfill in Nigeria. Afr. J. Environ. Sci. Technol. 2008, 2, 89–96. [Google Scholar]
- Saarinen, T.S.; Kløve, B. Past and future seasonal variation in pH and metal concentrations in runoff from river basins on acid sulphate soils in Western Finland. J. Environ. Sci. Health Part A 2012, 47, 1614–1625. [Google Scholar] [CrossRef]
- Ministério do Meio Ambiente. Conselho Nacional do Meio Ambiente (Conama). Resolução Nº 357, de 17 de Novembro de 2005, Brasil. Available online: https://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=450 (accessed on 3 June 2024).
- Zhang, H.; Han, X.; Wang, G.; Mao, H.; Chen, X.; Zhou, L.; Huang, D.; Zhang, F.; Yan, X. Spatial distribution and driving factors of groundwater chemistry and pollution in an oil production region in the Northwest China. Sci. Total Environ. 2023, 875, 162635. [Google Scholar] [CrossRef]
- Pires, N.L.; Muniz, D.H.d.F.; Araújo, L.S.d.; Lima, J.E.F.W.; Gomes, R.A.T.; Caldas, E.D.; Oliveira-Filho, E.C. The seasonal characterization and temporal evolution of nitrogen, phosphorus and potassium in the surface and groundwater of an agricultural hydrographic basin in the Midwestern Brazilian Savanna. Sustainability 2024, 16, 7659. [Google Scholar] [CrossRef]
- Costa, L.A.; Steffen, J.L.; Oliveira, M.L.; Heemann, R.; Tassi, R.; Machado, C.B. Geochemical analyses of water and public health of the Mangueirão and Salso Streams in Caçapava do Sul, RS, Brazil. Rev. Ambient. Água. 2017, 12, 752–765. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Ambient Water Quality Criteria to Address Nutrient Pollution in Lakes and Reservoirs. EPA 822-R-21-005, 2021. Available online: https://www.epa.gov/system/files/documents/2021-08/nutrient-lakes-reservoirs-report-final.pdf (accessed on 14 August 2025).
- Sharpley, A.N.; Jarvie, H.P.; Buda, A.R.; May, L.; Spears, B.; Kleinman, P.J.A. Phosphorus legacy: Overcoming the effects of past management practices to mitigate future water quality impairment. J. Environ. Qual. 2013, 42, 1308–1326. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.M.; Santos, L.D.T.; Silva, A.J.; Pinho, G.P.; Montes, W.G. Metal Contamination of water and sediments of the Vieira River, Montes Claros, Brazil. Arch. Environ. Contam. Toxicol. 2019, 77, 527–536. [Google Scholar] [CrossRef]
- Wang, H.B.; Ye, Z.H.; Shu, W.S.; Li, W.C.; Wong, M.H.; Lan, C.Y. Arsenic uptake and accumulation in fern species growing at arsenic-contaminated sites of Southern China: Field surveys. Int. J. Phytoremediation 2006, 8, 1–11. [Google Scholar] [CrossRef]
- Indriolo, E.; Na, G.; Ellis, D.; Salt, D.E.; Banks, J.A. A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell. 2010, 22, 2045–2057. [Google Scholar] [CrossRef]
- Kamachi, H.; Kitamura, N.; Sakatoku, A.; Tanaka, D.; Nakamura, S. Barium accumulation in the metalliferous fern Athyrium yokoscense. Theor. Exp. Plant Physiol. 2015, 27, 99–107. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Uptake of trace elements in the water fern Azolla filiculoides after short-term application of chestnut wood distillate (Pyroligneous Acid). Plants 2020, 11, 1179. [Google Scholar] [CrossRef]
- Funk, J.L.; Amatangelo, K.L. Physiological mechanisms drive differing foliar calcium content in ferns and angiosperms. Oecologia 2013, 173, 23–32. [Google Scholar] [CrossRef]
- Li, W.; Xu, F.; Chen, S.; Zhang, Z.; Zhao, Y.; Jin, Y.; Li, M.; Zhu, Y.; Liu, Y.; Yang, Y.; et al. A comparative study on Ca content and distribution in two Gesneriaceae species reveals distinctive mechanisms to cope with high rhizospheric soluble calcium. Front. Plant Sci. 2014, 5, 647. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.X.; Liang, D.Y.; Jiang, Q.W.; Mo, L.; Pu, G.Z.; Zhang, D. Growth performance and element concentrations reveal the calcicole-calcifuge behavior of three Adiantum species. BMC Plant Biol. 2020, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Kříbek, B.; Mihaljevič, M.; Sracek, O.; Knésl, I.; Ettler, V.; Nyambe, I. The extent of arsenic and of metal uptake by aboveground tissues of Pteris vittata and Cyperus involucratus growing in copper- and cobalt-rich tailings of the Zambian Copperbelt. Arch. Environ. Contam. Toxicol. 2011, 61, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Lwalaba, J.L.W.; Louis, L.T.; Zvobgo, G.; Richmond, M.E.A.; Fu, L.; Naz, S.; Mwamba, M.; Mundende, R.P.M.; Zhang, G. Physiological and molecular mechanisms of cobalt and copper interaction in causing phyto-toxicity to two barley genotypes differing in Co tolerance. Ecotoxicol. Environ. Saf. 2020, 187, 109866. [Google Scholar] [CrossRef]
- Souza, L.A.; Piotto, F.A.; Nogueirol, R.C.; Azevedo, R. Use of non-hyperaccumulator plant species for the phytoextraction of heavy metals using chelating agents. Sci. Agric. 2013, 70, 290–295. [Google Scholar] [CrossRef]
- Sridhar, B.B.M.; Han, F.X.; Diehl, S.V.; Monts, D.L.; Su, Y. Effect of phytoaccumulation of arsenic and chromium on structural and ultrastructural changes of brake fern (Pteris vittata). Braz. J. Plant Physiol. 2011, 23, 285–293. [Google Scholar] [CrossRef]
- Su, Y.; Han, F.X.; Maruthi Sridhar, B.B.; Monts, D.L. Phytotoxicity and phytoaccumulation of trivalent and hexavalent chromium in brake fern. Environ. Toxicol. Chem. 2005, 24, 2019–2026. [Google Scholar] [CrossRef]
- Eslava-Silva, F.d.J.; Muñíz-Díaz de León, M.E.; Jiménez-Estrada, M. Pteridium aquilinum (Dennstaedtiaceae), a novel hyperaccumulator species of hexavalent chromium. Appl. Sci. 2023, 13, 5621. [Google Scholar] [CrossRef]
- De la Torre, J.B.; Claveria, R.J.; Perez, R.E.; Perez, T.R.; Doronila, A.I. Copper uptake by Pteris melanocaulon Fée from a copper-gold mine in Surigao del Norte, Philippines. Int. J. Phytoremediation 2016, 18, 435–441. [Google Scholar] [CrossRef]
- Freitas, F.; Lunardib, S.; Souza, L.B.; von der Ostena, J.S.C.; Arrudaa, R.; Andradec, R.L.T.; Battirola, L.D. Accumulation of copper by the aquatic macrophyte Salvinia biloba Raddi (Salviniaceae). Braz. J. Biol. 2018, 78, 133–139. [Google Scholar] [CrossRef]
- Carrillo-Niquete, G.; Andrade, J.L.; Hernández-Terrones, L.; Cobos-Gasca, V.; Fuentes, G.; Santamaría, J.M. Copper accumulation in the aquatic fern Salvinia minima causes more severe physiological stress than zinc. Biometals 2022, 35, 1043–1057. [Google Scholar] [CrossRef]
- Tran, C.T.; Mai, N.T.; Nguyen, V.T.; Nguyen, H.X.; Meharg, A.; Carey, M.; Dultz, S.; Marone, F.; Cichy, S.B.; Nguyen, M.N. Phytolith-associated potassium in fern: Characterization, dissolution properties and implications for slash-and-burn agriculture. Soil Use Manag. 2018, 34, 28–36. [Google Scholar] [CrossRef]
- Firmano, R.F.; Melo, V.F.; Montes, C.R.; de Oliveira, A.; de Castro, C.; Alleoni, L.R.F. Potassium reserves in the clay fraction of a tropical soil fertilized for three decades. Clays Clay Miner. 2020, 68, 237–249. [Google Scholar] [CrossRef]
- Khan, N.; Ullah, R.; Okla, M.K.; Abdel-Maksoud, M.A.; Saleh, I.A.; Abu-Harirah, H.A.; AlRamadneh, T.N.; AbdElgawad, H. Ecological factors affecting minerals and nutritional quality of “Dryopteris filix-mas (L.) Schott”: An underutilized wild leafy vegetable in rural communities. Front. Nutr. 2024, 11, 1276307. [Google Scholar] [CrossRef] [PubMed]
- Salazar, L.; Páez-Vacas, M.; Kessler, M.; Kluge, J.; Homeier, J. Variation of foliar calcium and magnesium in six fern species at different elevations. IOP Conf. Ser. Earth Environ. Sci. 2021, 690, 012056. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Zhang, Y.; Chen, W. Response of total phenols, flavonoids, minerals, and amino acids of four edible fern species to four shading treatments. PeerJ 2020, 13, e8354. [Google Scholar] [CrossRef]
- Lizieri, C.; Aguiar, R.; Kuki, K.N. Manganese accumulation and its effects on three tropical aquatic macrophytes: Azolla caroliniana, Salvinia minima and Spirodela polyrhiza. Rodriguésia 2011, 62, 909–917. [Google Scholar] [CrossRef]
- Kubicka, K.; Samecka-Cymerman, A.; Kolon, K.; Kosiba, P.; Kempers, A.J. Chromium and nickel in Pteridium aquilinum from environments with various levels of these metals. Environ. Sci. Pollut. Res. 2015, 22, 527–534. [Google Scholar] [CrossRef]
- Kamachi, H.; Komori, I.; Tamura, H.; Sawa, Y.; Karahara, I.; Honma, Y.; Wada, N.; Kawabata, T.; Matsuda, K.; Ikeno, S.; et al. Lead tolerance and accumulation in the gametophytes of the fern Athyrium yokoscense. J. Plant Res. 2005, 118, 137–145. [Google Scholar] [CrossRef]
- Li, X.-W.; Lei, M.; Chen, T.-B.; Wan, X.-M. Roles of sulfur in the arsenic tolerant plant adiantum capillus-veneris and the hyperaccumulator Pteris vittata. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 498–502. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Azuma, T.; Maruyama, H.; Shinano, T.; Watanabe, T. Different nitrogen acquirement and utilization strategies might determine the ecological competition between ferns and angiosperms. Ann. Bot. 2023, 131, 1097–1106. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S.; Wu, F. Effects of Se on the uptake of essential elements in Pteris vittata L. Plant Soil. 2009, 325, 123–132. [Google Scholar] [CrossRef]
- Montanari, S.; Salinitro, M.; Simoni, A.; Ciavatta, C.; Tassoni, A. Foraging for selenium: A comparison between hyperaccumulator and non-accumulator plant species. Sci. Rep. 2023, 13, 10661. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, S.-X.; Zhou, Q.-Y.; Dai, Z.-H.; Liu, C.-J.; Xiao, S.-F.; Deng, S.-G.; Ma, L.Q. Foliar-selenium enhances plant growth and arsenic accumulation in As-hyperaccumulator Pteris vittata: Critical roles of GSH-GSSG cycle and arsenite antiporters PvACR3. J. Hazard Mater. 2024, 476, 135154. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.-H.; Peng, Y.-J.; Ding, S.; Chen, J.-Y.; He, S.-X.; Hu, C.-Y.; Cao, Y.; Guan, D.-X.; Ma, L.Q. Selenium increased arsenic accumulation by upregulating the expression of genes responsible for arsenic reduction, translocation, and sequestration in arsenic hyperaccumulator Pteris vittata. Environ. Sci. Technol. 2022, 56, 14146–14153. [Google Scholar] [CrossRef] [PubMed]
- An, Z.-Z.; Huang, Z.-C.; Lei, M.; Liao, X.-Y.; Zheng, Y.-M.; Chen, T.-B. Zinc tolerance and accumulation in Pteris vittata L. and its potential for phytoremediation of Zn- and As-contaminated soil. Chemosphere 2006, 62, 796–802. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for manganese. In Chapter 2: Relevance to Public Health—Background and Environmental Exposures to Manganese in the United States; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2012. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp151-c2.pdf (accessed on 24 June 2025).
- Georgaki, M.-N.; Charalambous, M.; Kazakis, N.; Talias, M.A.; Georgakis, C.; Papamitsou, T.; Mytiglaki, C. Chromium in Water and Carcinogenic Human Health Risk. Environments 2023, 10, 33. [Google Scholar] [CrossRef]
- Hagvall, L.; Pour, M.D.; Feng, J.; Karma, M.; Hedberg, Y.; Malmberg, P. Skin permeation of nickel, cobalt and chromium salts in ex vivo human skin, visualized using mass spectrometry imaging. Toxicol Vitr. 2021, 76, 105232. [Google Scholar] [CrossRef]
- ATSDR—Agency for Toxic Substances and Disease Registry (2005). Toxicological Profile for Zinc. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp60.pdf (accessed on 24 June 2025).
- Alharbi, T.; Nour, H.E.; El-Sorogy, A.S.; Al-Kahtany, K.; Giacobbe, S.; Alarif, S.S. Evaluation of health risks and heavy metals toxicity in agricultural soils in Central Saudi Arabia. Environ. Monit. Assess. 2025, 197, 419. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Arsenic; U.S. Department of Health and Human Services: Research Triangle Park, NC, USA, 2007. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf (accessed on 24 June 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Chromium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=62&tid=17 (accessed on 24 June 2025).
- ATSDR. Toxicological Profile for Copper. 2004. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp132.pdf (accessed on 24 June 2025).
- Li, H.; Toh, P.Z.; Tan, J.Y.; Zin, M.T.; Lee, C.-Y.; Li, B.; Leolukman, M.; Bao, H.; Kang, L. Selected Biomarkers Revealed Potential Skin Toxicity Caused by Certain Copper Compounds. Sci. Rep. 2016, 6, 37664. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). ToxGuide for Cobalt; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2004. Available online: https://www.atsdr.cdc.gov/toxguides/toxguide-33.pdf (accessed on 24 June 2025).
- Anderson, S.E.; Meade, B.J. Potential health effects associated with dermal exposure to occupational chemicals. Environ. Health Insights 2014, 8 (Suppl. S1), 51–62. [Google Scholar] [CrossRef]
- Lidén, C.; Bruze, M.; Menne, T. Metals. In Contact Dermatitis; Springer: Berlin/Heidelberg, Germany, 2011; pp. 643–674. Available online: https://link.springer.com/book/10.1007/978-3-642-03827-3 (accessed on 24 June 2025).
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]
- Chiou, Y.H.; Wong, R.H.; Chao, M.R.; Chen, C.Y.; Liou, S.H.; Lee, H. Nickel accumulation in lung tissues is associated with increased risk of p53 mutation in lung cancer patients. Environ. Mol. Mutagen. 2014, 55, 624–632. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Phosphorus; ATSDR: Washington, DC, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK598124/ (accessed on 24 June 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Selenium; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2003. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp92-c3.pdf (accessed on 24 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).