Adaptive Response of Petunia × hybrida Plants to Water-Scarce Urban Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Materials
2.2. Visual Assessment and Mortality Rate Calculation
2.3. Morphological Parameters
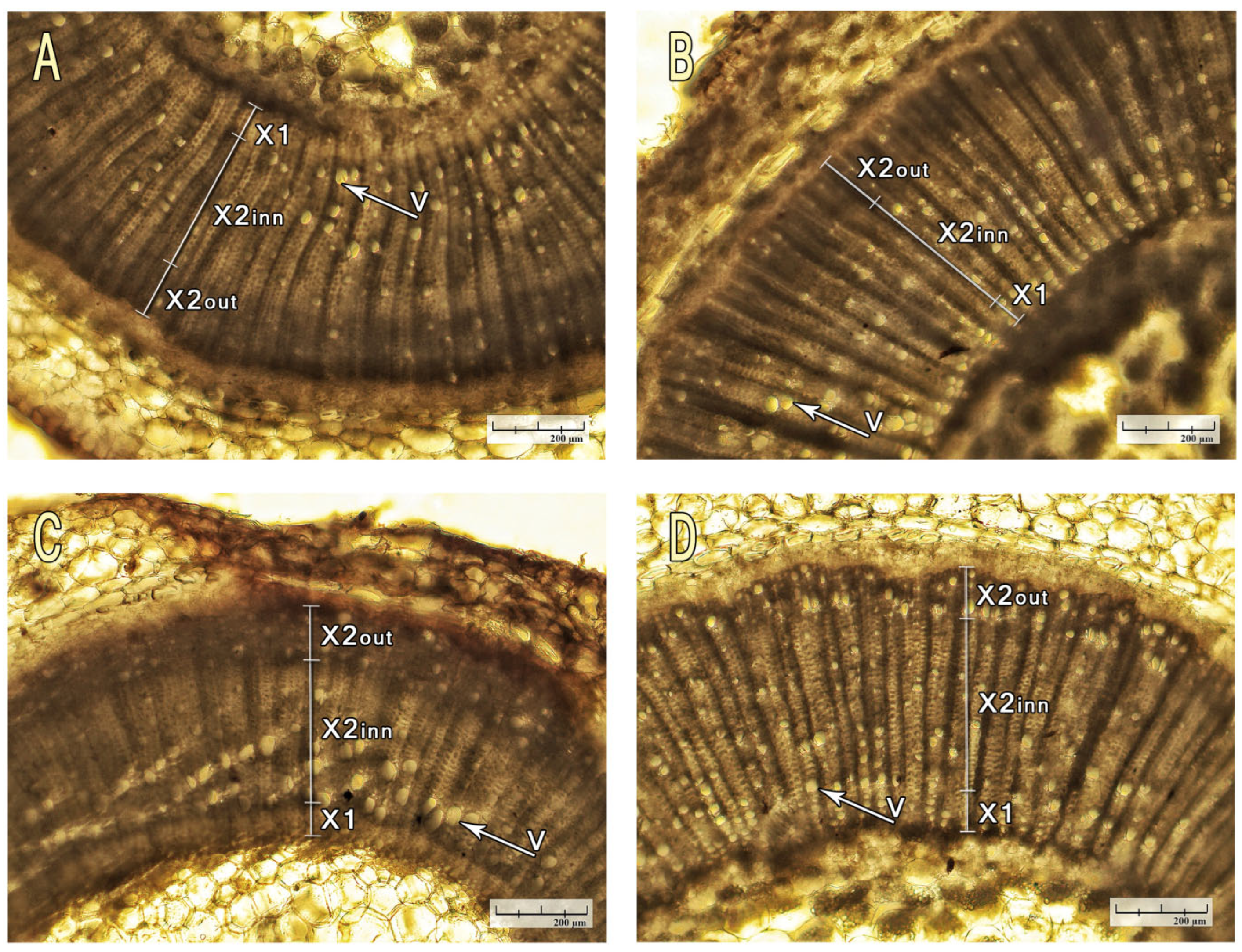
2.4. Anatomical Traits
2.5. Statistical Analysis
3. Results
3.1. Visual Assessment and Mortality Rate
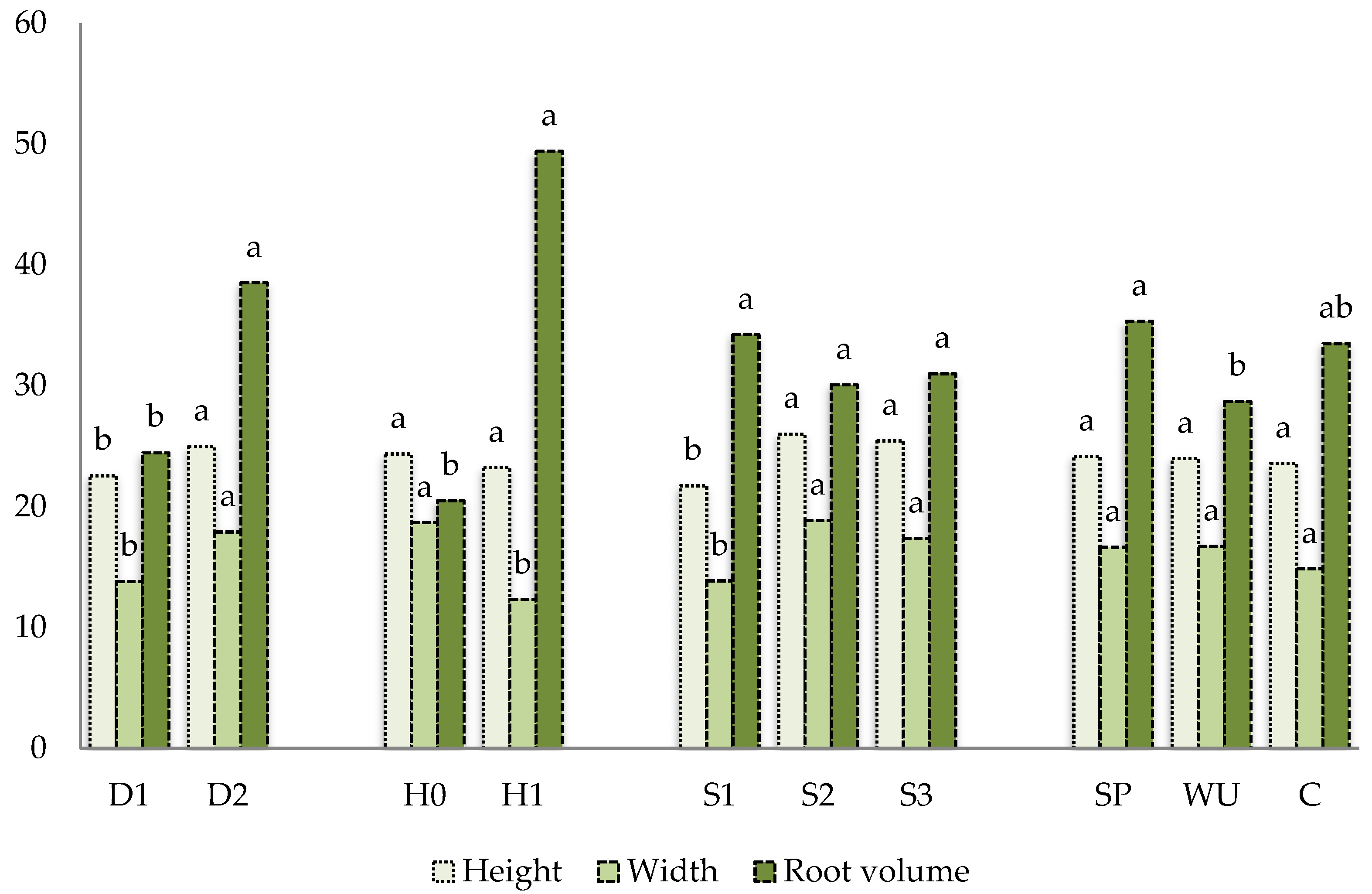
3.2. Vegetative Growth
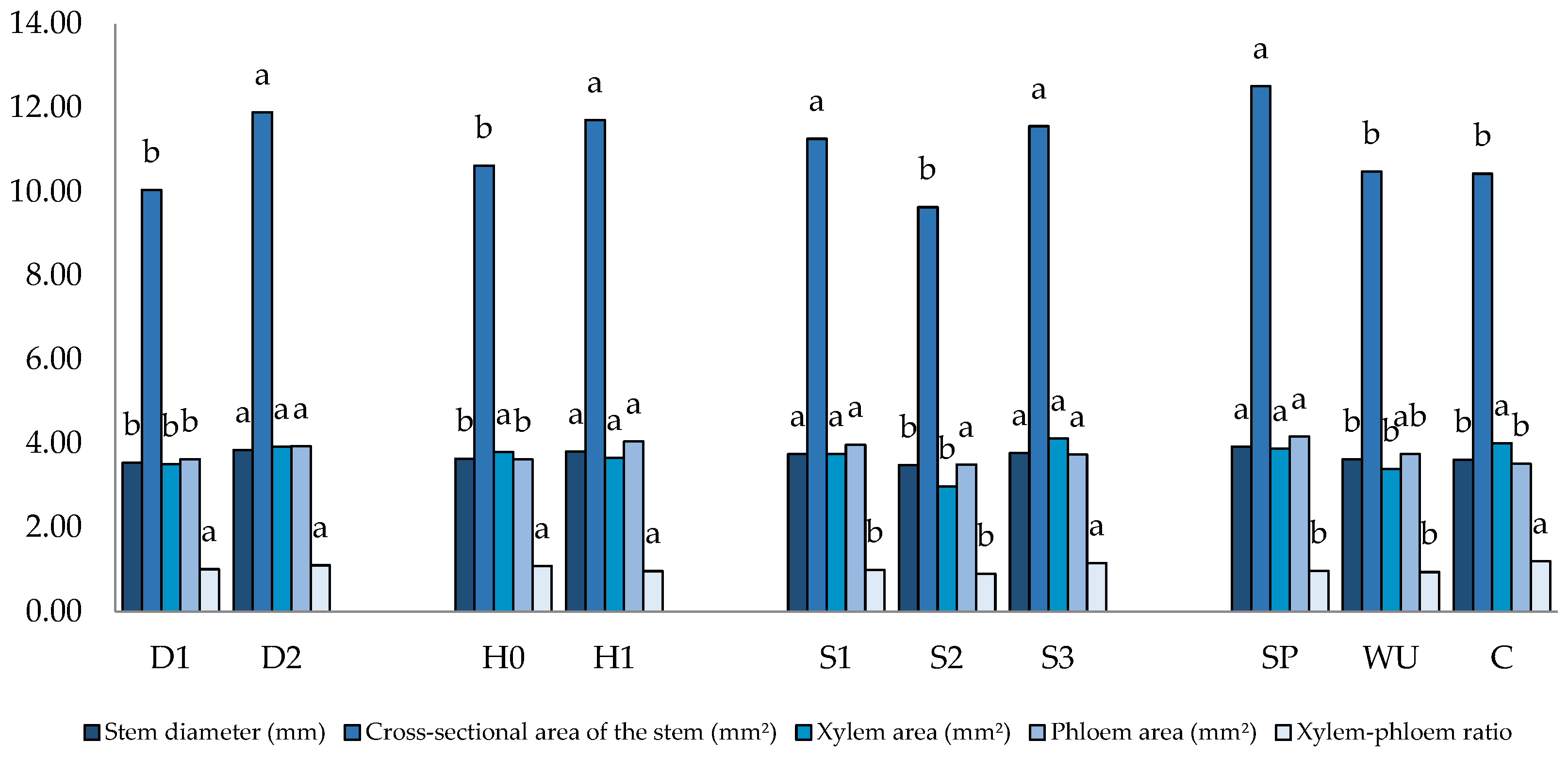
3.3. Macro Anatomical Characterization
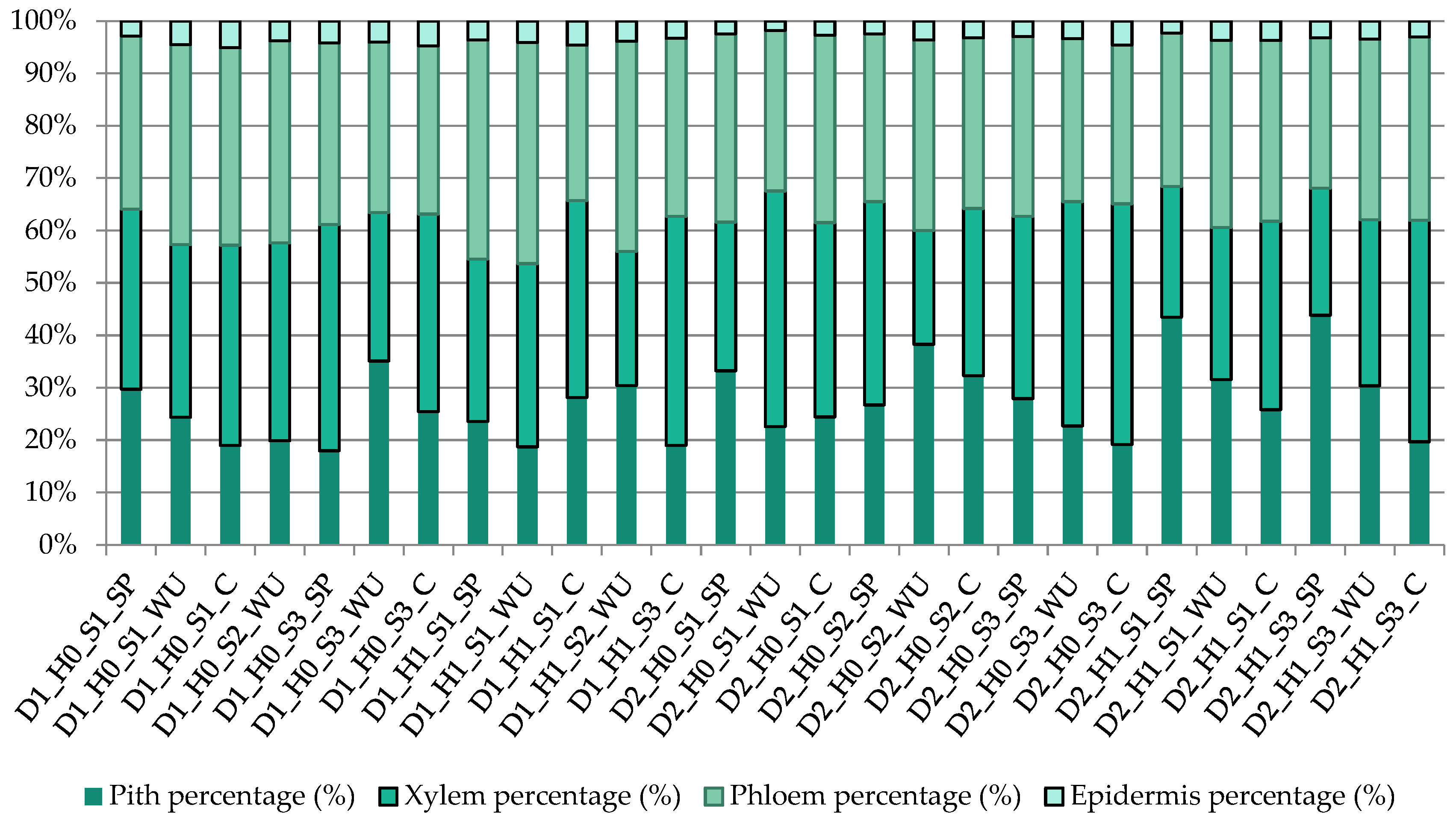
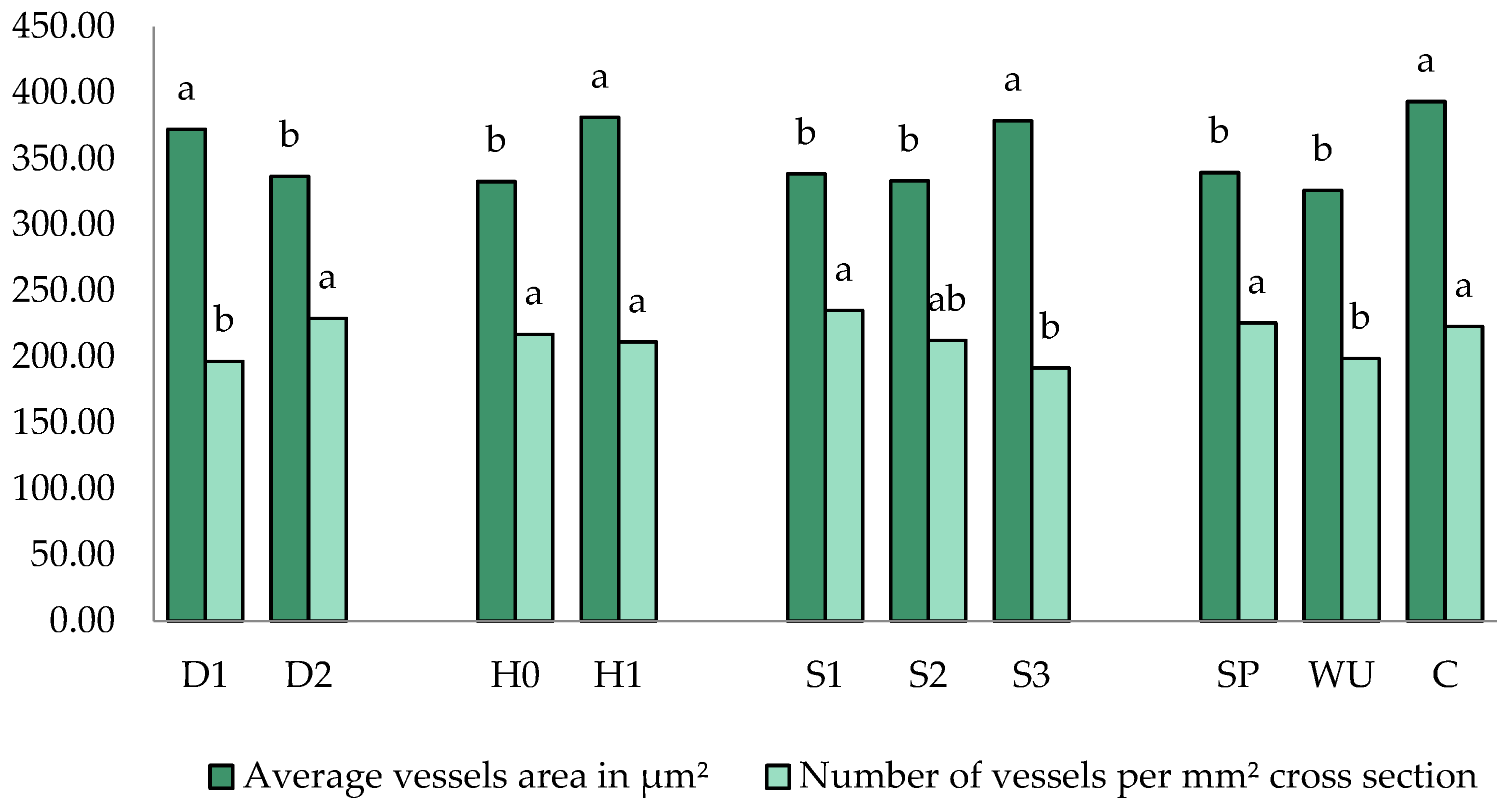
3.4. Micro Anatomical Characterization
3.5. Correlation Analysis
4. Discussion
4.1. Factors Influencing Petunia × hybrida Survival and Ornamental Value in Container-Based Planting Systems
4.2. Deciphering Petunia × hybrida Adaptive Anatomical Responses in Water-Scarce Environment
4.3. Integration of Obtained Results into Practice
4.4. Aligning Research on Petunia × hybrida with the Sustainable Development Goals (SDGs)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SP | Stim pure AA Liquid (SP) (Van Iperen International, Westmaas, The Netherlands) |
| WU | WAKE-up (WU) Liquid (Van Iperen International, Westmaas, The Netherlands) |
| C | Control plants—without biostimulant application |
| D1 | Substrate depth (7 cm) |
| D2 | Substrate depth (10 cm) |
| H0 | Without the hydrogel application |
| H1 | With the hydrogel application |
| S1 | Perlite substrate |
| S2 | Peat substrate |
| S3 | Mixture of peat and perlite substrate |
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C: Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC, Ed.; World Meteorological Organization: Geneva, Switzerland, 2018; pp. 1–32. [Google Scholar]
- Naumann, G.; Alfieri, L.; Wyser, K.; Mentaschi, L.; Betts, R.A.; Carrao, H.; Spinoni, J.; Vogt, J.; Feyen, L. Global changes in drought conditions under different levels of warming. Geophys. Res. Lett. 2018, 45, 3285–3296. [Google Scholar] [CrossRef]
- Ionita, M.; Nagavciuc, V.; Scholz, P.; Dima, M. Long-term drought intensification over Europe driven by the weakening trend of the Atlantic Meridional Overturning Circulation. J. Hydrol. Reg. Stud. 2022, 42, 101176. [Google Scholar] [CrossRef]
- Poje, M.; Židovec, V.; Prebeg, T.; Kušen, M. Does the Use of Perennials in Flower Beds Necessarily Imply Sustainability? Plants 2023, 12, 4113. [Google Scholar] [CrossRef]
- Grant, G.; Engleback, L.; Nicholson, B.; Dusty, G.; Frith, M.; Harvey, P. Green Roofs: Their Existing Status and Potential for Conserving Biodiversity in Urban Areas; English Nature: Peterborough, UK, 2003; p. 498.
- Dunnett, N.; Kingbury, N. Planting Green Roofs and Living Walls; Timber Press Inc.: Portland, OR, USA, 2008; p. 328. [Google Scholar]
- Alim, M.A.; Rahman, A.; Tao, Z.; Garner, B.; Griffith, R.; Liebman, M. Green roof as an effective tool for sustainable urban development: An Australian perspective in relation to stormwater and building energy management. J. Clean. Prod. 2022, 362, 132561. [Google Scholar] [CrossRef]
- Mihalakakou, G.; Souliotis, M.; Papadaki, M.; Menounou, P.; Dimopoulos, P.; Kolokotsa, D.; Paravantis, J.A.; Tsangrassoulis, A.; Panaras, G.; Giannakopoulos, E.; et al. Green roofs as a nature-based solution for improving urban sustainability: Progress and perspectives. Renew. Sustain. Energy Rev. 2023, 180, 113306. [Google Scholar] [CrossRef]
- Panda, P.; Rao, G.P.; Sipahioğlu, H.M.; Hemmati, C.; Madhupriya; Kalita, M.K.; Oksal, H.D.; Usta, M.; Rastgou, M.; Alp, Ş.; et al. An update on phytoplasma diseases associated with ornamentals in Asia. Phytoplasma Dis. Major Crops Trees Weeds 2023, 2, 167–214. [Google Scholar]
- Adams, S.R.; Hadley, P.; Pearson, S. The effects of temperature, photoperiod, and photosynthetic photon flux on the time to flowering of petunia ‘Express Blush Pink’. J. Amer. Soc. Hort. Sci. 1998, 123, 577–580. [Google Scholar] [CrossRef]
- Verdonck, O.; Demeyer, P. The influence of the particle size on physical properties of growing media. Acta Hortic. 2004, 644, 99–101. [Google Scholar] [CrossRef]
- Dzięcioł, J.; Szlachetka, O.; Rodrigues Tavares, J.M. From Volcanic Popcorn to the Material of the Future: A Critical Review of Expanded Perlite Applications and Environmental Impacts. Sustainability 2025, 17, 1454. [Google Scholar] [CrossRef]
- Akhter, J.; Mahmood, K.; Malik, K.A.; Mardan, A.; Ahmad, M.; Iqbal, M.M. Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant Soil Environ. 2004, 50, 463–469. [Google Scholar] [CrossRef]
- Arbona, V.; Iglesias, D.J.; Jacas, J.; Primo-Millo, E.; Talon, M.; Gómez-Cadenas, A. Hydrogel substrate amendment alleviates drought effects on young citrus plants. Plant Soil 2005, 270, 73–82. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Shao, J.; Deng, S.; Wang, R.; Li, N.; Sun, J.; Zhang, H.; Zhu, H.; Zhang, Y.; et al. Effects of Stockosorb and Luquasorb polymers on salt and drought tolerance of Populus popularis. Sci. Hortic. 2010, 124, 268–273. [Google Scholar] [CrossRef]
- Chirino, E.; Vilagrosa, A.; Vallejo, V.R. Using hydrogel and clay to improve the water status of seedlings for dryland restoration. Plant Soil 2011, 344, 99–110. [Google Scholar] [CrossRef]
- Ljubojević, M.; Ognjanov, V.; Maksimović, I.; Čukanović, J.; Dulić, J.; Szabò, Z.; Szabò, E. Effects of hydrogel on growth and visual damage of ornamental Salvia species exposed to salinity. CLEAN–Soil Air Water 2017, 45, 1600128. [Google Scholar] [CrossRef]
- Ma, L.; Chai, C.; Wu, W.; Qi, P.; Liu, X.; Hao, J. Hydrogels as the plant culture substrates: A review. Carbohydr. Polym. 2023, 305, 120544. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Sindhu, S.S.; Anand, P.; Singh, A.; Chauhan, V.B.S.; Verma, S.K. Vermi products and biodegradable superabsorbent polymer improve physiological activities and leaf nutrient contents of gerbera. Res. J. Biotechnol. 2018, 13, 8–18. [Google Scholar]
- M’barki, N.; Chehab, H.; Aissaoui, F.; Dabbaghi, O.; Attia, F.; Mahjoub, Z.; Laamari, S.; Chihaoui, B.; del Giudice, T.; Jemai, A.; et al. Effects of mycorrhizal fungi inoculation and soil amendment with hydrogel on leaf anatomy, growth and physiology performance of olive plantlets under two contrasting water regimes. Acta Physiol. Plant. 2018, 40, 116. [Google Scholar] [CrossRef]
- Yavas, I.; Jamal, M.A.; Ul Din, K.; Ali, S.; Hussain, S.; Farooq, M. Drought-Induced Changes in Leaf Morphology and Anatomy: Overview, Implications and Perspectives. Pol. J. Environ. Stud. 2024, 33, 1517–1530. [Google Scholar] [CrossRef]
- Wang, Z.; Geng, Y.; Liang, T. Optimization of reduced chemical fertilizer use in tea gardens based on the assessment of related environmental and economic benefits. Sci. Total Environ. 2020, 713, 136439. [Google Scholar] [CrossRef]
- Newman, S.E.; Davies, F.T., Jr. High soil temperature and water relations of endomycorrhizal nursery crops. J. Environ. Hortic. 1987, 5, 93–96. [Google Scholar] [CrossRef]
- De Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and their role in improving plant growth under abiotic stresses. In Biostimulants in Plant Science; Mirmajlessi, S.M., Radhakrishnan, R., Eds.; IntechOpen Limited: London, UK, 2020; pp. 3–16. [Google Scholar]
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.-J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Russo, R.O.; Berlyn, G.P. The use of organic biostimulants to help low-input sustainable agriculture. J. Sustain. Agric. 1990, 1, 19–42. [Google Scholar] [CrossRef]
- Lana, A.M.Q. Aplicação de reguladores de crescimentonacultura do feijoeiro. Biosci. J. 2009, 25, 13–20. [Google Scholar]
- Tütüncü, M.; DaldaŞekerci, A.; Dönmez, D.; İzgü, T.; Isak, M.A.; Şimşek, Ö. Plant biostimulants in ornamentals: Enhancing growth and stress tolerance. Adv. Hortic. Sci. 2024, 38, 211–222. [Google Scholar] [CrossRef]
- RHSS Republic Hydrometeorological Service of Serbia. Available online: https://www.hidmet.gov.rs/ (accessed on 25 October 2022).
- Osmocot Supplement for Balcony Flower Plants. Available online: https://www.floraekspres.rs/proizvod/osmokot-prihrana-za-balkonske-cvetnice-80340 (accessed on 15 April 2021).
- Anastasijević, N. Podizanje i Negovanje Zelenih Površina; Šumarski fakultet Univerziteta u Beogradu: Beograd, Srbija, 2007; pp. 227–234. [Google Scholar]
- Harrington, J.T.; Mexal, J.G.; Fisher, J.T. Volume displacement provides a quick and accurate way to quantify new root production. Seedling 1994, 121, 124. [Google Scholar]
- Narandžić, T.; Ljubojević, M.; Ostojić, J.; Barać, G.; Ognjanov, V. Investigation of stem anatomy in relation to hydraulic conductance, vegetative growth and yielding potential of ‘Summit’ cherry trees grafted on different rootstock candidates. Folia Hortic. 2021, 33, 248–264. [Google Scholar] [CrossRef]
- Bombarely, A.; Moser, M.; Amrad, A.; Bao, M.; Bapaume, L.; Barry, C.S.; Bliek, M.; Boersma, M.R.; Borghi, L.; Bruggmann, R.; et al. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nat. Plants 2016, 2, 16074. [Google Scholar] [CrossRef]
- Van Iersel, M.W. Short-term temperature change affects the carbon exchange characteristics and growth of four bedding plant species. J. Am. Soc. Hortic. Sci. 2003, 128, 100–106. [Google Scholar] [CrossRef]
- Henson, D.Y.; Newman, S.E.; Hartley, D.E. Performance of selected herbaceous annual ornamentals grown at decreasing levels of irrigation. HortScience 2006, 41, 1481–1486. [Google Scholar] [CrossRef]
- Wang, R.; Yang, C.; Ni, L.; Yao, Y. Experimental Study on Heat Transfer of Soil with Different Moisture Contents and Seepage for Ground Source Heat Pump. Indoor Built Environ. 2020, 29, 1238–1248. [Google Scholar] [CrossRef]
- Ghobashy, M.M. The Application of Natural Polymer-Based Hydrogels for Agriculture. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–356. [Google Scholar]
- Obede da Silva Aragão, O.; de Almeida Leite, R.; Araújo, A.P.; da Conceição Jesus, E. Effect of pot size on the growth of common bean in experiments with rhizobium. J. Soil Sci. Plant Nutr. 2020, 20, 865–871. [Google Scholar] [CrossRef]
- de Mello, B.F.F.R.; Trevisan, M.V.; Steiner, F. Quality of cucumber seedlings grown in different containers. Rev. Agric. Neotrop. 2016, 3, 33–38. [Google Scholar] [CrossRef]
- Haase, D.L. Understanding forest seedling quality: Measurements and interpretation. Tree Plant. Notes 2008, 52, 24–30. [Google Scholar]
- Moreira, M.A.; Carvalho, J.G.; Pasqual, M.; Fráguas, C.B.; Silva, A.B. Acclimatization of micropropagated pineapple plants cv. “Pérola”: Substrate effect. Ciênc. Agrotec. 2005, 30, 875–879. [Google Scholar] [CrossRef]
- Witcher, A.L.; Pickens, J.M.; Blythe, E.K. Container color and compost substrate affect root zone temperature and growth of “green Giant” arborvitae. Agronomy 2020, 10, 484. [Google Scholar] [CrossRef]
- Markoska, V.; Spalevic, V.; Gulaboski, R. A research on the influence of porosity on perlite substrate and its interaction on porosity of two types of soil and peat substrate. Poljopr. Šumarstv 2018, 64, 15–29. [Google Scholar] [CrossRef]
- Al-Shammari, A.M.A.; Abood, M.A.; Hamdi, G.J. Perlite affects some plant indicators and reduces water deficit in tomato. Int. J. Veg. Sci. 2018, 24, 490–500. [Google Scholar] [CrossRef]
- Al-Wazzan, F.A.; Abdulrahman, M.K. Effect of soil amendment (Perlite) on some physical characteristics and Zea mays L productivity. Membr. Technol. 2025, 1, 1873–4049. [Google Scholar]
- Salighehdar, F.; Safari, A.R.; Nalousi, A.M.; Avestan, S. The effect of different ratios of peat and perlite on quantitative and qualitative characteristics of Aloe vera grown in hydroponic system. J. Sci. Technol. Greenh. Cult. 2016, 7, 113–123. [Google Scholar] [CrossRef]
- Kostas, S.; Kaplani, A.; Koulaouzidou, E.; Kotoula, A.-A.; Gklavakis, E.; Tsoulpha, P.; Hatzilazarou, S.; Nianiou-Obeidat, I.; Kanellis, A.K.; Economou, A. Sustainable exploitation of greek Rosmarinus officinalis L. populations for ornamental use through propagation by shoot cuttings and in vitro cultures. Sustainability 2022, 14, 4059. [Google Scholar] [CrossRef]
- Ma, D.; Teng, W.; Mo, Y.T.; Yi, B.; Chen, W.L.; Pang, Y.P.; Wang, L. Effects of nitrogen, phosphorus, and potassium fertilization on plant growth, element levels in plants and soil, and the relationships among nutrient concentrations, plant yield, and nutrient status in Erythropalum scandens (Blume). J. Plant Nutr. 2024, 47, 82–96. [Google Scholar] [CrossRef]
- Soliman, D.M.; Elkaramany, M.F.; El-sayed, I.M. Using hydrogel polymers to mitigate the negative impact of salinity stress on Calendula officinalis plants. Egypt. J. Chem. 2024, 67, 57–77. [Google Scholar] [CrossRef]
- Sharbazhery, A.O.; Abu-Bakr, L.A. Effect of plant gel on the flowers of balconies garden (Verbena canadiensis) under Sulaymani city conditions. J. Duhok Univ. 2018, 20, 8–12. [Google Scholar] [CrossRef]
- Kumar, A.T.; Kameswari, P.L.; Girwani, A. Impact of Pusa Hydrogel incorporated growing media on floral characters and yield of pot mums (Dendranthema grandiflora L.) under various irrigation regimes. Int. J. Agric. Sci. Res. 2016, 6, 195–200. [Google Scholar]
- Heidari, H.; Hosseini, A. Hydrogel polymer improves plant biomass and leaf relative water content in dill and fenugreek. Ratar. Povrt. 2024, 61, 25–32. [Google Scholar] [CrossRef]
- Huchthausen, J.; Escher, B.I.; Grasse, N. Reactivity of acrylamides causes cytotoxicity and activates oxidative stress response. Chem. Res. Toxicol. 2023, 36, 1374–1385. [Google Scholar] [CrossRef]
- Pontes Filho, R.A.; Gondim, F.A.; Costa, M.C.G. Seedling growth of tree species under doses of hydrogel and two levels of luminosity. Rev. Árvore 2018, 42, e420112. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Grammenou, A.; Petropoulos, S.A.; Thalassinos, G.; Khah, E.; Barouchas, P.E.; Bilalis, D. Biostimulants in the Soil–Plant Interface: Agro-Environmental Implications—A Review. Earth Syst. Environ. 2023, 7, 583–600. [Google Scholar] [CrossRef]
- Mandal, S.; Anand, U.; López-Bucio, J.; Radha; Kumar, M.; Lal, M.K.; Tiwari, R.K.; Dey, A. Biostimulants and environmental stress mitigation in crops: A novel and emerging approach for agricultural sustainability under climate change. Environ. Res. 2023, 233, 116357. [Google Scholar] [CrossRef]
- Gaberščik, A.; Grašič, M.; Vogel-Mikuš, K.; Germ, M.; Golob, A. Water shortage strongly alters formation of calcium oxalate druse crystals and leaf traits in Fagopyrum esculentum. Plants 2020, 9, 917. [Google Scholar] [CrossRef]
- Franceschi, V.R. Calcium oxalate in plants. Trends Plant Sci. 2001, 6, 331. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Horner, H.T. Calcium oxalate crystals in plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium Oxalate in Plants: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Iwano, M.; Entani, T.; Shiba, H.; Takayama, S.; Isogai, A. Calcium Crystals in the anther of Petunia: The existence and biological significance in the pollination process. Plant Cell Physiol. 2004, 45, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.L.; Tian, H.Q.; Russell, S.D. Calcium function and distribution during fertilization in Angiosperms. Am. J. Bot. 2007, 94, 1046–1060. [Google Scholar] [CrossRef]
- Horner, H.T.; Wagner, B.L. The Association of druse crystals with the developing stomium of Capsicum annuum (Solanaceae) anthers. Am. J. Bot. 1980, 67, 1347–1360. [Google Scholar] [CrossRef]
- Horner, H.T.; Wagner, B.L. Association of four different calcium crystals in the anther connective tissue and hypodermal stomium of Capsicum annuum (Solanaceae) during microsporogenesis. Am. J. Bot. 1992, 79, 531–541. [Google Scholar] [CrossRef]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. “Alarm Photosynthesis”: Calcium oxalate crystals as an internal CO2 source in plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Horner, H.T.; Bresta, P.; Nikolopoulos, D.; Liakopoulos, G. New Insights into the functions of carbon–calcium inclusions in plants. New Phytol. 2020, 226, 1040–1047. [Google Scholar] [CrossRef]
- Kisvarga, S.; Horotán, K.; Wani, M.A.; Orlóci, L. Plant responses to global climate change and urbanization: Implications for sustainable urban landscapes. Horticulturae 2023, 9, 1051. [Google Scholar] [CrossRef]
- Vannucchi, F.; Bretzel, F.; Pini, R.; Rumble, H. Less is more: Soil and substrate quality as an opportunity for urban greening and biodiversity conservation. In Urban Services to Ecosystems: Green Infrastructure Benefits from the Landscape to the Urban Scale; Springer International Publishing: Cham, Switzerland, 2021; pp. 207–224. [Google Scholar]
- Young, T.M.; Cameron, D.D.; Phoenix, G.K. Increasing green roof plant drought tolerance through substrate modification and the use of water retention gels. Urban Water J. 2017, 14, 551–560. [Google Scholar] [CrossRef]
- Cuce, P.M.; Cuce, E.; Santamouris, M. Towards sustainable and climate-resilient cities: Mitigating urban heat islands through green infrastructure. Sustainability 2025, 17, 1303. [Google Scholar] [CrossRef]
- Priya, U.K.; Senthil, R. Framework for enhancing urban living through sustainable plant selection in residential green spaces. Urban Sci. 2024, 8, 235. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Syed, T.N.; Zhang, C.; Shaikh, S.A.; Rakibuzzaman, M.; Vistro, R.B. Soilless agricultural systems: Opportunities, challenges, and applications for enhancing horticultural resilience to climate change and urbanization. Horticulturae 2025, 11, 568. [Google Scholar] [CrossRef]
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The contribution of ornamental plants to urban ecosystem services. Earth 2022, 3, 1258–1274. [Google Scholar] [CrossRef]
- Oikonomaki, E.; Papadaki, I.; Kakderi, C. Promoting green transformations through smart engagement: An assessment of 100 citizen-led urban greening projects. Land 2024, 13, 556. [Google Scholar] [CrossRef]
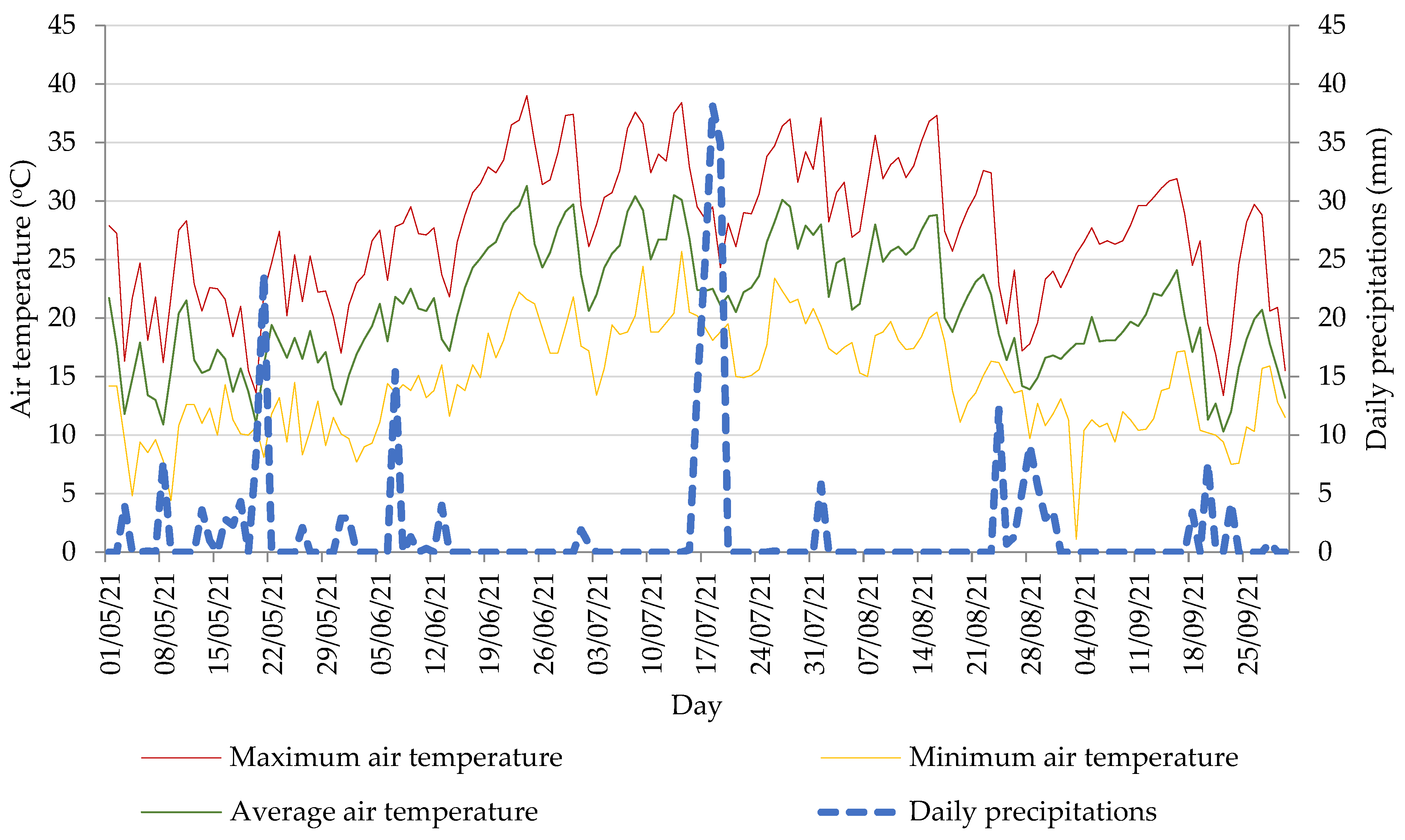
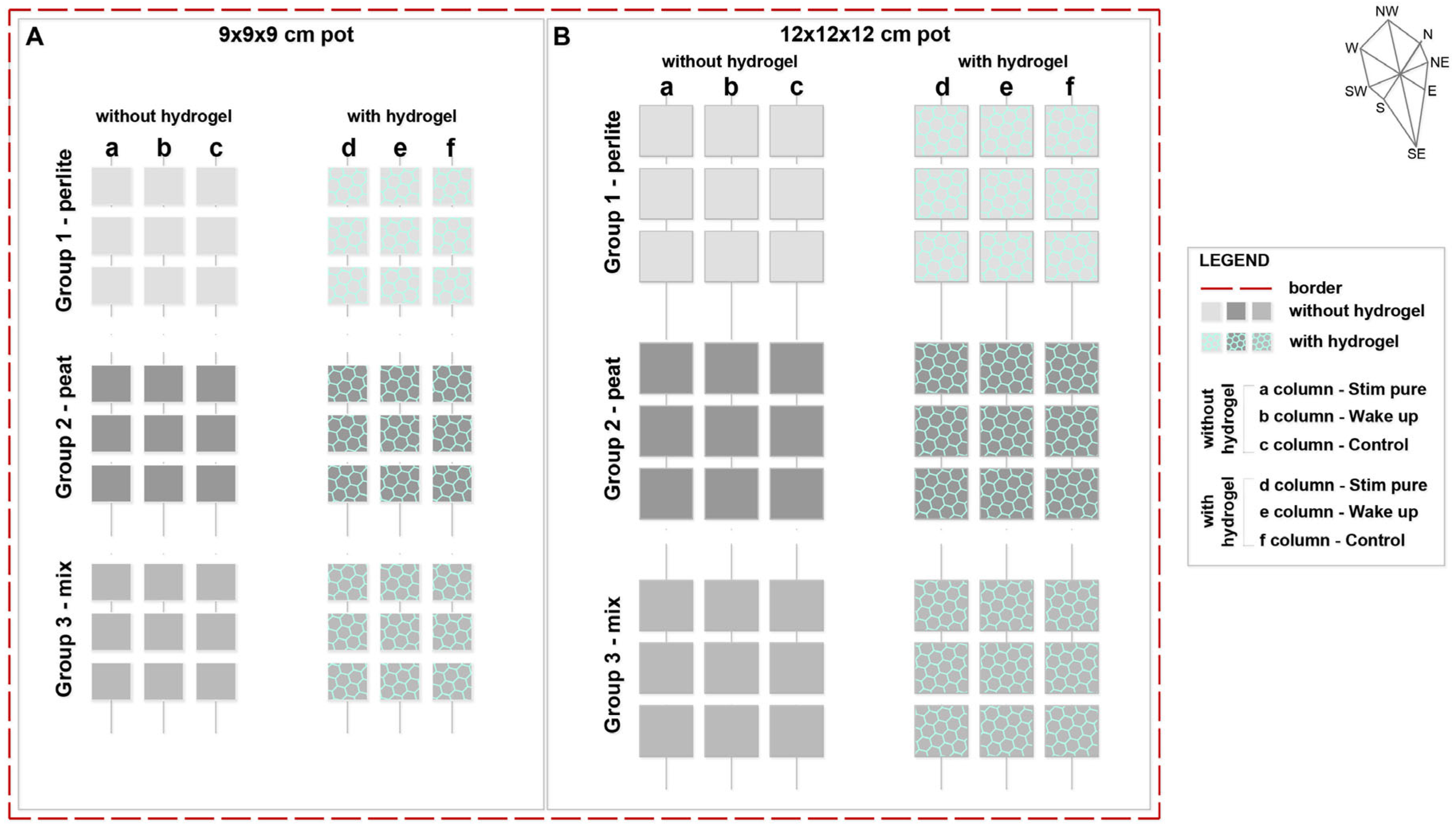


| Substrate Treatment | Frequency of Irrigation | |
|---|---|---|
| T1 | Perlite without hydrogel | 24 h |
| T2 | Perlite with hydrogel | 48 h |
| T3 | Peat and a mixture of perlite and peat without hydrogel | 72 h |
| T4 | Peat and a mixture of perlite and peat with hydrogel | 96 h |
| Treatments | Survival Rate > 50% | Moderate Vitality | Good Vitality | High Decorativeness | 100% Regenerative Potential |
|---|---|---|---|---|---|
| D1_H0_S1_SP | ☑ | ☑ | ☑ | ☑ | ☑ |
| D1_H0_S1_WU | ☑ | ☑ | ☑ | ☑ | ☑ |
| D1_H0_S1_C | ☑ | ☑ | ☑ | ☑ | ☑ |
| D1_H0_S2_SP | ☑ | ||||
| D1_H0_S2_WU | ☑ | ☑ | ☑ | ||
| D1_H0_S2_C | ☑ | ||||
| D1_H0_S3_SP | ☑ | ☑ | ☑ | ☑ | |
| D1_H0_S3_WU | ☑ | ☑ | ☑ | ☑ | |
| D1_H0_S3_C | ☑ | ☑ | ☑ | ☑ | |
| D1_H1_S1_SP | ☑ | ☑ | ☑ | ☑ | |
| D1_H1_S1_WU | ☑ | ☑ | ☑ | ☑ | |
| D1_H1_S1_C | ☑ | ☑ | ☑ | ☑ | ☑ |
| D1_H1_S2_SP | ☑ | ||||
| D1_H1_S2_WU | ☑ | ☑ | ☑ | ||
| D1_H1_S2_C | ☑ | ||||
| D1_H1_S3_SP | ☑ | ||||
| D1_H1_S3_WU | ☑ | ☑ | |||
| D1_H1_S3_C | ☑ | ☑ | ☑ | ||
| D2_H0_S1_SP | ☑ | ☑ | ☑ | ☑ | ☑ |
| D2_ H0_S1_WU | ☑ | ☑ | ☑ | ☑ | |
| D2_ H0_S1_C | ☑ | ☑ | ☑ | ☑ | ☑ |
| D2_ H0_S2_SP | ☑ | ☑ | ☑ | ||
| D2_ H0_S2_WU | ☑ | ☑ | ☑ | ☑ | |
| D2_ H0_S2_C | ☑ | ☑ | ☑ | ☑ | |
| D2_ H0_S3_SP | ☑ | ☑ | ☑ | ☑ | |
| D2_ H0_S3_WU | ☑ | ☑ | ☑ | ||
| D2_ H0_S3_C | ☑ | ☑ | ☑ | ☑ | ☑ |
| D2_H1_S1_SP | ☑ | ☑ | ☑ | ☑ | |
| D2_ H1_S1_WU | ☑ | ☑ | ☑ | ☑ | |
| D2_ H1_S1_C | ☑ | ☑ | ☑ | ☑ | |
| D2_ H1_S2_SP | ☑ | ☑ | |||
| D2_ H1_S2_WU | ☑ | ||||
| D2_ H1_S2_C | ☑ | ||||
| D2_ H1_S3_SP | ☑ | ☑ | ☑ | ☑ | ☑ |
| D2_ H1_S3_WU | ☑ | ☑ | ☑ | ☑ | |
| D2_ H1_S3_C | ☑ | ☑ | ☑ | ☑ |
| Treatments | Vessel Area on the Whole Xylem (mm2) | Ray Area on the Whole Xylem (mm2) | Xylem Porosity on the Stem Cross-Section (%) | Surface Area of Calcium Oxalate (CaOx) Crystals in the Field of Vision (µm2) |
|---|---|---|---|---|
| D1_H0_S1_SP | 0.26 ± 0.09 b–h | 0.56 ± 0.20 a–f | 7.10 ± 0.70 b–f | 65.12 ± 55.73 a–c |
| D1_H0_S1_WU | 0.19 ± 0.02 e–h | 0.45 ± 0.03 c–f | 6.04 ± 0.35 c–f | 52.26 ± 2.22 a–c |
| D1_H0_S1_C | 0.24 ± 0.05 b–h | 0.53 ± 0.02 a–f | 6.58 ± 1.39 c–f | 41.07 ± 9.28 a–c |
| D1_H0_S2_SP | / | / | / | / |
| D1_H0_S2_WU | 0.30 ± 0.13 a–g | 0.47 ± 0.08 b–f | 8.17 ± 2.29 b–f | 45.60 ± 27.29 a–c |
| D1_H0_S2_C | / | / | / | / |
| D1_H0_S3_SP | 0.19 ± 0.02 d–h | 0.38 ± 0.06 c–f | 6.69 ± 0.28 c–f | 117.49 ± 33.41 ab |
| D1_H0_S3_WU | 0.16 ± 0.14 f–h | 0.37 ± 0.10 c–f | 4.98 ± 2.66 f | 31.24 ± 13.55 bc |
| D1_H0_S3_C | 0.37 ± 0.03 a–e | 0.95 ± 0.13 ab | 5.85 ± 0.12 d–f | 47.22 ± 3.86 a–c |
| 0.24 | 0.53 | 6.49 | 57.14 | |
| D1_H1_S1_SP | 0.30 ± 0.11 a–g | 0.70 ± 0.34 a–e | 7.35 ± 0.60 b–f | 45.35 ± 5.21 a–c |
| D1_H1_S1_WU | 0.19 ± 0.04 e–h | 0.34 ± 0.07 c–f | 7.13 ± 1.36 b–f | 70.68 ± 25.75 a–c |
| D1_H1_S1_C | 0.32 ± 0.03 a–g | 0.29 ± 0.01 c–f | 11.54 ± 1.94 ab | 31.03 ± 0.96 bc |
| D1_H1_S2_SP | / | / | / | / |
| D1_H1_S2_WU | 0.22 ± 0.03 c–h | 0.29 ± 0.08 c–f | 8.25 ± 0.64 b–f | 40.54 ± 3.69 a–c |
| D1_H1_S2_C | / | / | / | / |
| D1_H1_S3_SP | / | / | / | / |
| D1_H1_S3_WU | / | / | / | / |
| D1_H1_S3_C | 0.40 ± 0.07 a–d | 0.51 ± 0.09 a–f | 10.36 ± 1.86 a–d | 30.19 ± 13.42 bc |
| 0.29 | 0.43 | 8.93 | 43.56 | |
| D2_H0_S1_SP | 0.13 ± 0.05 gh | 0.22 ± 0.09 ef | 7.84 ± 2.32 b–f | 44.41 ± 4.13 a–c |
| D2_ H0_S1_WU | 0.26 ± 0.03 b–h | 0.58 ± 0.07 a–f | 6.14 ± 0.76 c–f | 19.35 ± 1.17 c |
| D2_ H0_S1_C | 0.36 ± 0.09 a–f | 0.75 ± 0.41 a–c | 13.23 ± 1.62 a | 125.69 ± 29.09 a |
| D2_ H0_S2_SP | 0.20 ± 0.12 d–h | 0.49 ± 0.29 b–f | 5.60 ± 0.36 ef | 31.87 ± 11.09 bc |
| D2_ H0_S2_WU | 0.09 ± 0.03 h | 0.13 ± 0.04 f | 7.09 ± 1.59 b–f | 31.20 ± 7.34 bc |
| D2_ H0_S2_C | 0.18 ± 0.03 e–h | 0.27 ± 0.03 c–f | 6.40 ± 0.75 c–f | 27.50 ± 6.34 c |
| D2_ H0_S3_SP | 0.49 ± 0.11 a | 0.74 ± 0.22 a–d | 9.92 ± 2.26 a–e | 73.40 ± 55.59 a–c |
| D2_ H0_S3_WU | 0.20 ± 0.02 d–h | 0.47 ± 0.18 b–f | 5.16 ± 0.48 f | 91.96 ± 22.34 a–c |
| D2_ H0_S3_C | 0.43 ± 0.03 ab | 0.50 ± 0.16 a–f | 10.45 ± 0.63 a–c | 79.60 ± 43.68 a–c |
| 0.26 | 0.46 | 7.98 | 58.33 | |
| D2_H1_S1_SP | 0.17 ± 0.02 e–h | 0.27 ± 0.03 c–f | 8.77 ± 1.23 a–f | 81.45 ± 39.04 a–c |
| D2_ H1_S1_WU | 0.18 ± 0.02 e–h | 0.38 ± 0.06 c–f | 8.22 ± 0.64 b–f | 63.04 ± 27.25 a–c |
| D2_ H1_S1_C | 0.14 ± 0.05 gh | 0.26 ± 0.06 d–f | 7.21 ± 2.02 b–f | 73.75 ± 50.78 a–c |
| D2_ H1_S2_SP | / | / | / | / |
| D2_ H1_S2_WU | / | / | / | / |
| D2_ H1_S2_C | / | / | / | / |
| D2_ H1_S3_SP | 0.41 ± 0.09 a–c | 0.65 ± 0.15 a–e | 8.98 ± 1.82 a–f | 55.23 ± 16.50 a–c |
| D2_ H1_S3_WU | 0.18 ± 0.02 e–h | 0.99 ± 0.08 a | 4.50 ± 0.00 f | 39.45 ± 10.17 a–c |
| D2_ H1_S3_C | 0.25 ± 0.05 b–h | 0.33 ± 0.08 c–f | 7.36 ± 1.99 b–f | 56.98 ± 48.85 a–c |
| 0.22 | 0.48 | 7.50 | 61.65 |
| Treatments | Percentage of Total Vessel Area on the Stem Cross-Section (%) | Percentage of Total Ray Area on Xylem (%) | Percentage of the Total Ray Area on the Stem Cross-Section (%) | Percentage of Xylem Area on the Stem Cross-Section (%) |
|---|---|---|---|---|
| D1_H0_S1_SP | 2.43 ± 0.07 d–i | 15.07 ± 0.89 bc | 5.18 ± 0.51 a–d | 26.77 ± 2.42 a–h |
| D1_H0_S1_WU | 1.99 ± 0.19 d–i | 14.55 ± 0.12 bc | 4.79 ± 0.15 a–d | 26.15 ± 0.97 a–h |
| D1_H0_S1_C | 2.51 ± 0.51 c–h | 14.70 ± 0.41 bc | 5.62 ± 0.07 ab | 30.09 ± 1.05 a–h |
| D1_H0_S2_SP | / | / | / | / |
| D1_H0_S2_WU | 3.16 ± 1.20 a–e | 13.31 ± 2.21 b–d | 4.96 ± 0.62 a–d | 29.63 ± 4.63 a–h |
| D1_H0_S2_C | / | / | / | / |
| D1_H0_S3_SP | 2.89 ± 0.17 b–g | 13.43 ± 2.33 b–d | 5.79 ± 0.96 ab | 34.50 ± 1.35 a–d |
| D1_H0_S3_WU | 1.41 ± 0.75 f–i | 12.73 ± 2.11 b–d | 3.62 ± 0.70 b–e | 23.31 ± 0.71 c–h |
| D1_H0_S3_C | 2.20 ± 0.16 d–i | 14.70 ± 0.72 bc | 5.55 ± 0.65 ab | 29.96 ± 2.64 a–h |
| 2.37 | 14.07 | 5.07 | 28.63 | |
| D1_H1_S1_SP | 2.29 ± 0.39 d–i | 17.14 ± 5.18 ab | 5.32 ± 1.79 a–c | 23.37 ± 3.17 b–h |
| D1_H1_S1_WU | 2.49 ± 0.45 c–i | 12.66 ± 2.09 b–d | 4.42 ± 0.69 a–e | 28.06 ± 1.79 a–h |
| D1_H1_S1_C | 4.22 ± 1.00 a–c | 10.36 ± 1.23 b–d | 3.80 ± 0.83 b–e | 29.57 ± 10.00 a–h |
| D1_H1_S2_SP | / | / | / | / |
| D1_H1_S2_WU | 2.12 ± 0.22 d–i | 10.85 ± 2.41 b–d | 2.80 ± 0.78 b–e | 20.70 ± 0.44 f–h |
| D1_H1_S2_C | / | / | / | / |
| D1_H1_S3_SP | / | / | / | / |
| D1_H1_S3_WU | / | / | / | / |
| D1_H1_S3_C | 4.52 ± 0.71 ab | 13.16 ± 2.24 b–d | 5.75 ± 0.96 ab | 33.45 ± 2.33 a–e |
| 3.12 | 12.84 | 4.42 | 27.03 | |
| D2_H0_S1_SP | 1.12 ± 0.37 hi | 6.46 ± 2.28 cd | 1.84 ± 0.72 c–e | 25.41 ± 1.43 a–h |
| D2_H0_S1_WU | 2.76 ± 0.32 c–h | 13.48 ± 1.93 b–d | 6.04 ± 0.64 ab | 36.17 ± 2.32 a |
| D2_ H0_S1_C | 2.46 ± 0.42 d–i | 13.74 ± 5.91 b–d | 5.14 ± 2.35 a–d | 29.50 ± 1.94 a–h |
| D2_ H0_S2_SP | 2.19 ± 0.70 d–i | 14.67 ± 3.86 bc | 5.55 ± 1.81 ab | 31.09 ± 9.52 a–g |
| D2_H0_S2_WU | 0.76 ± 0.12 i | 5.27 ± 1.20 d | 1.13 ± 0.18 e | 19.87 ± 2.03 gh |
| D2_ H0_S2_C | 2.06 ± 0.54 d–i | 9.86 ± 0.69 b–d | 3.15 ± 0.71 b–e | 26.72 ± 5.16 a–h |
| D2_ H0_S3_SP | 3.43 ± 0.99 a–d | 15.16 ± 5.42 bc | 5.18 ± 1.77 a–d | 26.17 ± 6.66 a–h |
| D2_H0_S3_WU | 2.22 ± 0.33 d–i | 12.19 ± 4.28 b–d | 5.30 ± 2.23 a–c | 35.33 ± 1.58 ab |
| D2_ H0_S3_C | 4.80 ± 0.29 a | 12.14 ± 4.08 b–d | 5.58 ± 1.86 ab | 35.59 ± 1.75 a |
| 2.42 | 11.44 | 4.32 | 29.54 | |
| D2_H1_S1_SP | 1.09 ± 0.16 hi | 6.89 ± 1.61 cd | 1.70 ± 0.28 de | 22.16 ± 1.82 e–h |
| D2_H1_S1_WU | 1.19 ± 0.10 g–i | 8.59 ± 0.95 b–d | 2.49 ± 0.33 b–e | 25.38 ± 3.22 a–h |
| D2_ H1_S1_C | 1.33 ± 0.57 g–i | 7.07 ± 0.71 cd | 2.57 ± 0.65 b–e | 32.10 ± 4.26 a–f |
| D2_ H1_S2_SP | / | / | / | / |
| D2_H1_S2_WU | / | / | / | / |
| D2_ H1_S2_C | / | / | / | / |
| D2_ H1_S3_SP | 2.16 ± 0.40 d–i | 14.07 ± 3.19 b–d | 3.39 ± 0.71 b–e | 18.69 ± 2.34 h |
| D2_H1_S3_WU | 1.42 ± 0.14 e–i | 24.29 ± 0.00 a | 7.70 ± 0.75 a | 22.56 ± 2.20 d–h |
| D2_ H1_S3_C | 3.13 ± 0.94 a–f | 9.91 ± 3.17 b–d | 4.22 ± 1.48 a–e | 34.97 ± 1.24 a–c |
| 1.72 | 11.80 | 3.68 | 25.98 |
| Variable | Stem Diameter | Cross Section | Phloem Area | Xylem Area | Xylem/Phloem Ratio | Average Vessel Area | Number of Vessels | Pith Percentage (%) | Xylem Percentage (%) | Phloem Percentage (%) | Epidermis Percentage (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | 0.90 | 0.12 | −0.05 | −0.11 | 0.66 | −0.02 | 0.38 | 0.53 | −0.10 | −0.60 | −0.66 |
| Width | 0.89 | 0.13 | −0.05 | −0.12 | 0.64 | 0.02 | 0.35 | 0.51 | −0.08 | −0.61 | −0.64 |
| Root volume | 0.33 | 0.81 | −0.12 | −0.11 | 0.27 | 0.42 | 0.22 | −0.24 | 0.70 | −0.85 | −0.06 |
| Variable | Vessel area on the whole xylem (mm2) | Ray area on the whole xylem (mm2) | Xylem porosity on the stem cross-section (%) | Surface area of calcium oxalate (CaOx) | Percentage of total vessel area on the stem cross-section (%) | Percentage of total ray area on xylem (%) | Percentage of the total ray area on the stem cross-section (%) | Percentage of xylem area on the stem cross-section (%) | |||
| Height | 0.34 | 0.43 | 0.31 | 0.79 | −0.14 | 0.20 | 0.02 | −0.12 | |||
| Width | 0.37 | 0.46 | 0.32 | 0.80 | −0.11 | 0.23 | 0.06 | −0.10 | |||
| Root volume | 0.83 | 0.83 | 0.56 | 0.90 | 0.56 | 0.41 | 0.62 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grubač, M.; Narandžić, T.; Pušić Devai, M.; Ostojić, J.; Bijelić, S.; Čukanović, J.; Vujović, A.; Ljubojević, M. Adaptive Response of Petunia × hybrida Plants to Water-Scarce Urban Substrates. Urban Sci. 2025, 9, 325. https://doi.org/10.3390/urbansci9080325
Grubač M, Narandžić T, Pušić Devai M, Ostojić J, Bijelić S, Čukanović J, Vujović A, Ljubojević M. Adaptive Response of Petunia × hybrida Plants to Water-Scarce Urban Substrates. Urban Science. 2025; 9(8):325. https://doi.org/10.3390/urbansci9080325
Chicago/Turabian StyleGrubač, Milica, Tijana Narandžić, Magdalena Pušić Devai, Jovana Ostojić, Sandra Bijelić, Jelena Čukanović, Anastasija Vujović, and Mirjana Ljubojević. 2025. "Adaptive Response of Petunia × hybrida Plants to Water-Scarce Urban Substrates" Urban Science 9, no. 8: 325. https://doi.org/10.3390/urbansci9080325
APA StyleGrubač, M., Narandžić, T., Pušić Devai, M., Ostojić, J., Bijelić, S., Čukanović, J., Vujović, A., & Ljubojević, M. (2025). Adaptive Response of Petunia × hybrida Plants to Water-Scarce Urban Substrates. Urban Science, 9(8), 325. https://doi.org/10.3390/urbansci9080325











