Assessing the Physical Stability of Soil Organic Carbon in Roadside Ecosystems
Abstract
1. Introduction
2. Materials and Methods
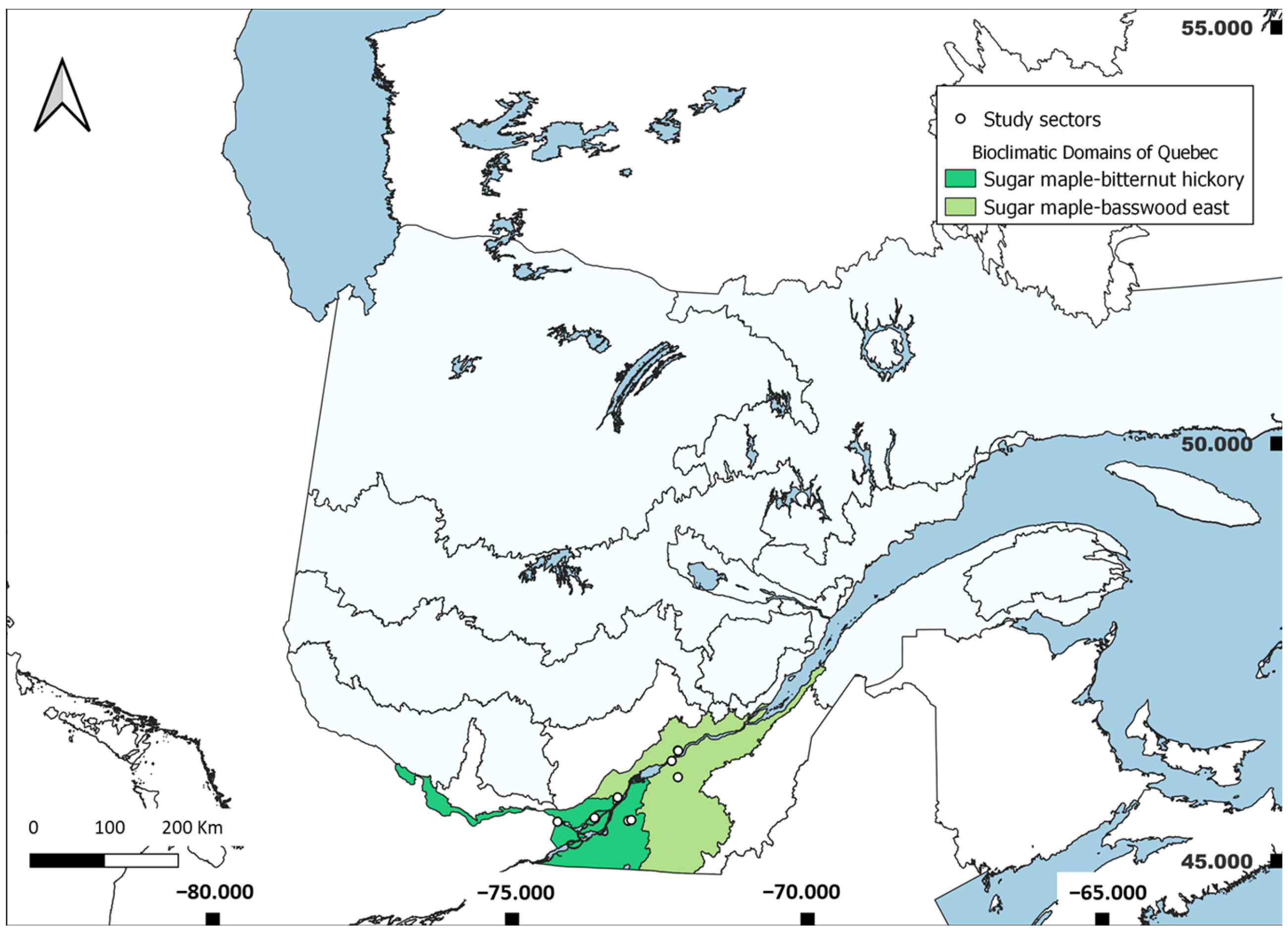
2.1. Study Sites
2.2. Sampling Procedure
2.3. Soil Physical Fractionation
2.4. Analysis of Physicochemical Soil Properties
2.5. Data Analyses
3. Results
3.1. Roadside Characteristics
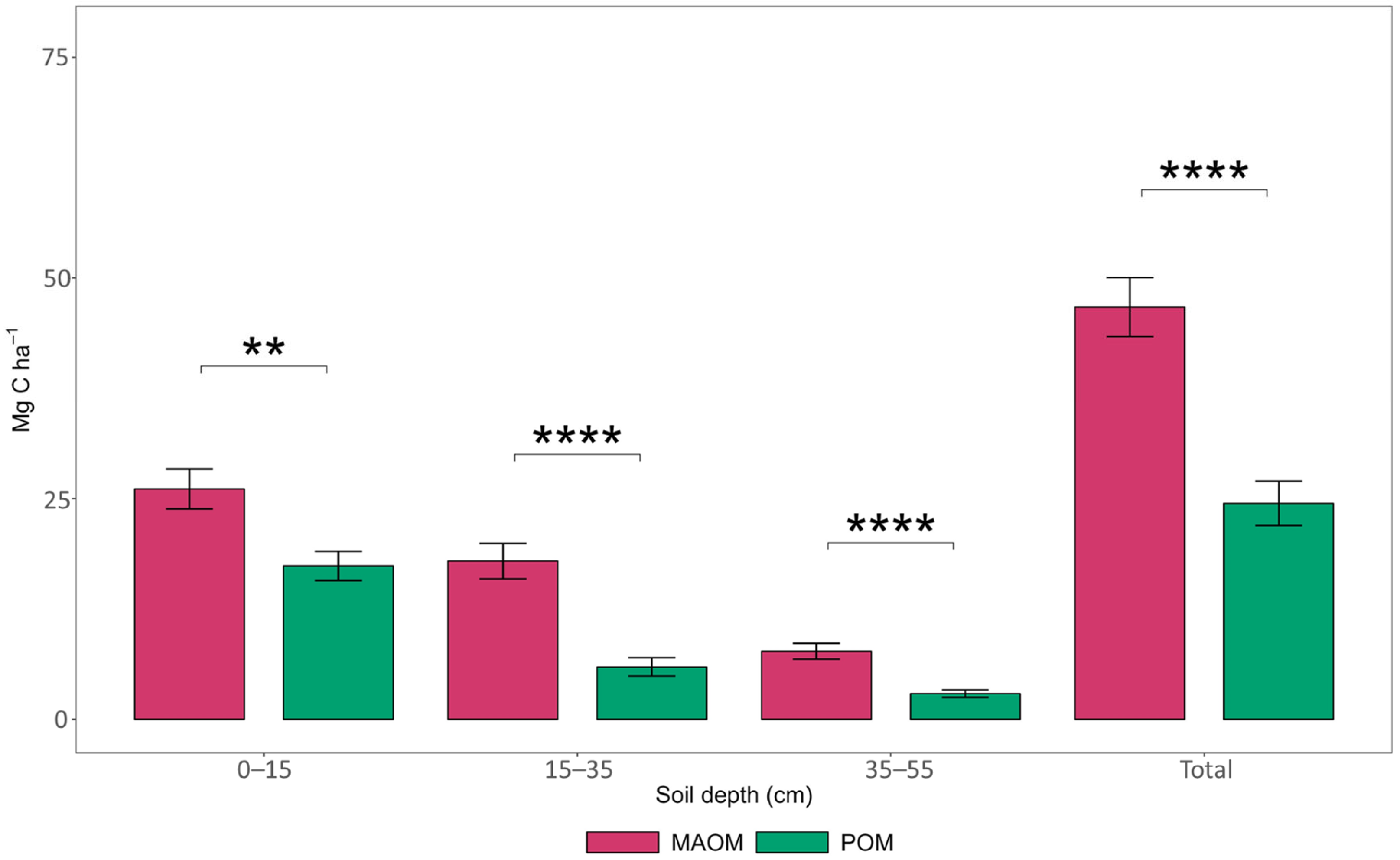
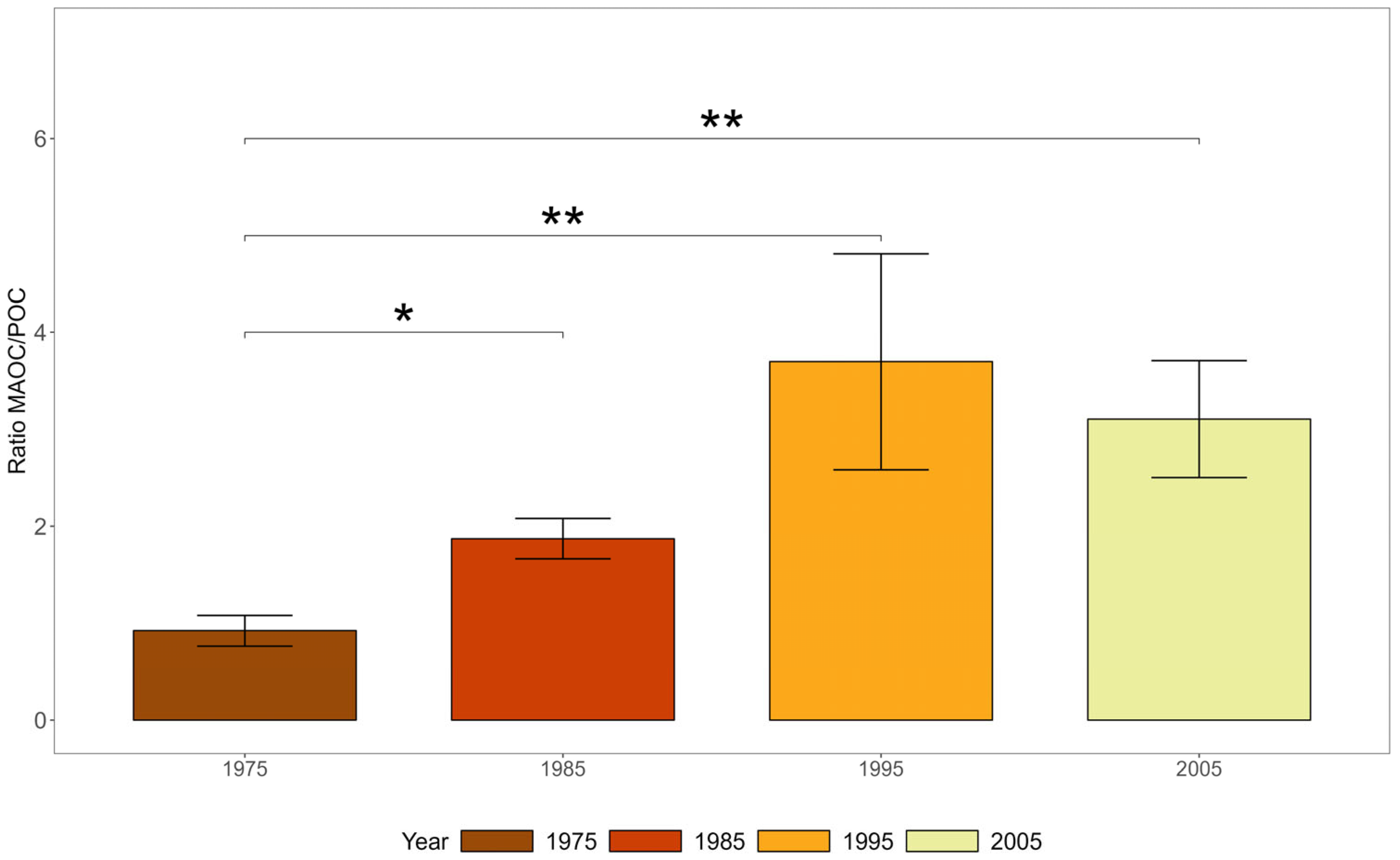
3.2. Carbon Storage in Soil Fractions
3.3. Soil Chemical and Physical Properties
3.4. Correlation Between Road Density and Soil Properties
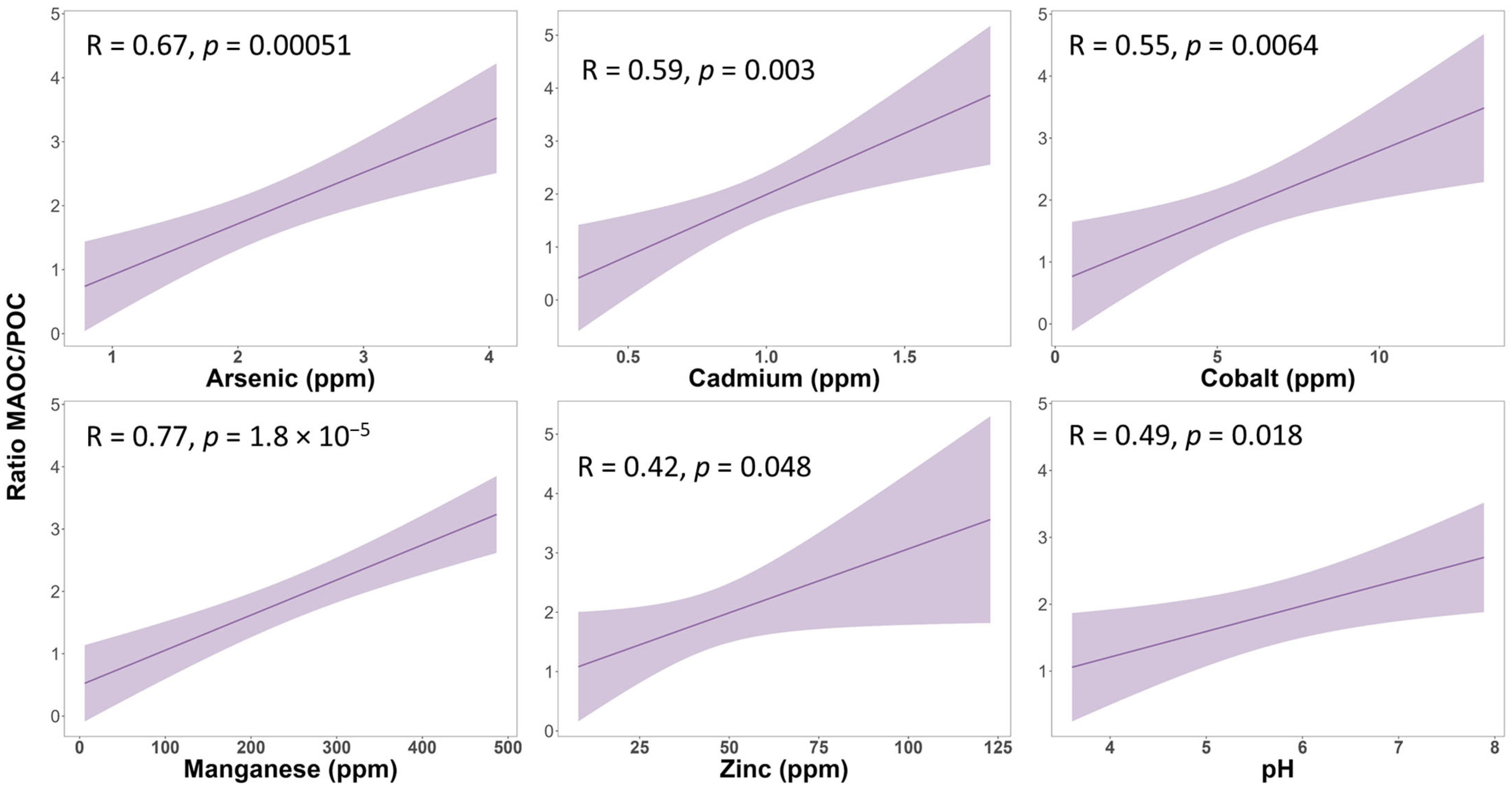
3.5. Relationship Between Carbon Stocks in Soil Fractions and Physicochemical Soil Properties
4. Discussion
4.1. Carbon Storage in Soil Physical Fractions Across Roadside Land Uses
4.2. Relationships Between Road Density and Physicochemical Soil Properties
4.3. Relationship Between Carbon Stock in Soil Fractions and Physicochemical Soil Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Yesilonis, I.D.; Nowak, D.J. Carbon Storage by Urban Soils in the United States. J. Environ. Qual. 2006, 35, 1566–1575. [Google Scholar] [CrossRef]
- Rupprecht, C.D.D.; Byrne, J.A. Informal Urban Green-Space: Comparison of Quantity and Characteristics in Brisbane, Australia and Sapporo, Japan. PLoS ONE 2014, 9, e99784. [Google Scholar] [CrossRef]
- Skaldina, O.; Nylund, A.; Ramula, S. Neglected Puzzle Pieces of Urban Green Infrastructure: Richness, Cover, and Composition of Insect-Pollinated Plants in Traffic-Related Green Spaces. Landsc. Ecol. 2024, 39, 80. [Google Scholar] [CrossRef]
- Säumel, I.; Weber, F.; Kowarik, I. Toward Livable and Healthy Urban Streets: Roadside Vegetation Provides Ecosystem Services Where People Live and Move. Environ. Sci. Policy 2016, 62, 24–33. [Google Scholar] [CrossRef]
- Steinfeld, D.E.; Riley, S.A.; Wilkinson, K.M.; Landis, T.D.; Riley, L.E. A Manager’s Guide to Roadside Revegetation Using Native Plants; Umatilla National Forest (Agency: U.S.), Ed.; Federal Highway Administration, Western Federal Lands Highway Division: Washington, DC, USA, 2007.
- Srour, N.; Thiffault, E.; Boucher, J.-F. Quantifying Carbon Stocks and Functional Diversity of Roadside Ecosystems—A Case Study in Quebec, Canada. Urban For. Urban Green. 2024, 91, 128163. [Google Scholar] [CrossRef]
- Gaston, K.J.; Ávila-Jiménez, M.L.; Edmondson, J.L. REVIEW: Managing Urban Ecosystems for Goods and Services. J. Appl. Ecol. 2013, 50, 830–840. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms Controlling Soil Carbon Turnover and Their Potential Application for Enhancing Carbon Sequestration. Clim. Chang. 2007, 80, 5–23. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I.; Ludwig, B.; Matzner, E.; Flessa, H.; Ekschmitt, K.; Guggenberger, G.; Marschner, B.; Kalbitz, K. Stabilization Mechanisms of Organic Matter in Four Temperate Soils: Development and Application of a Conceptual Model. J. Plant Nutr. Soil Sci. 2008, 171, 111–124. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, C.W.; Prater, I.; Angst, Š.; Frouz, J.; Jílková, V.; Peterse, F.; Nierop, K.G.J. Earthworms Act as Biochemical Reactors to Convert Labile Plant Compounds into Stabilized Soil Microbial Necromass. Commun. Biol. 2019, 2, 441. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing Soil Organic Matter into Particulate and Mineral-Associated Forms to Address Global Change in the 21st Century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.; Angers, D.; Gregorich, E.; Bolinder, M. Characterizing Organic Matter Retention for Surface Soils in Eastern Canada Using Density and Particle Size Fractions. Can. J. Soil Sci. 2003, 83, 11–23. [Google Scholar] [CrossRef]
- Saidy, A.R.; Smernik, R.J.; Baldock, J.A.; Kaiser, K.; Sanderman, J.; Macdonald, L.M. Effects of Clay Mineralogy and Hydrous Iron Oxides on Labile Organic Carbon Stabilisation. Geoderma 2012, 173–174, 104–110. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A History of Research on the Link between (Micro)Aggregates, Soil Biota, and Soil Organic Matter Dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Erktan, A.; Cécillon, L.; Graf, F.; Roumet, C.; Legout, C.; Rey, F. Increase in Soil Aggregate Stability along a Mediterranean Successional Gradient in Severely Eroded Gully Bed Ecosystems: Combined Effects of Soil, Root Traits and Plant Community Characteristics. Plant Soil 2016, 398, 121–137. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Lal, R. Forest Soils and Carbon Sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of Heavy Metals in Soils and Their Immobilization at Micro-Scale Interfaces among Diverse Soil Components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Sieghardt, M.; Mursch-Radlgruber, E.; Paoletti, E.; Couenberg, E.; Dimitrakopoulus, A.; Rego, F.; Hatzistathis, A.; Randrup, T.B. The Abiotic Urban Environment: Impact of Urban Growing Conditions on Urban Vegetation. In Urban Forests and Trees: A Reference Book; Konijnendijk, C., Nilsson, K., Randrup, T., Schipperijn, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 281–323. ISBN 978-3-540-27684-5. [Google Scholar]
- Lasota, J.; Błońska, E.; Łyszczarz, S.; Tibbett, M. Forest Humus Type Governs Heavy Metal Accumulation in Specific Organic Matter Fractions. Water Air Soil Pollut. 2020, 231, 80. [Google Scholar] [CrossRef]
- Quenea, K.; Lamy, I.; Winterton, P.; Bermond, A.; Dumat, C. Interactions between Metals and Soil Organic Matter in Various Particle Size Fractions of Soil Contaminated with Waste Water. Geoderma 2009, 149, 217–223. [Google Scholar] [CrossRef]
- Enya, O.; Heaney, N.; Iniama, G.; Lin, C. Effects of Heavy Metals on Organic Matter Decomposition in Inundated Soils: Microcosm Experiment and Field Examination. Sci. Total Environ. 2020, 724, 138223. [Google Scholar] [CrossRef] [PubMed]
- Green, S.-M.; Machin, R.; Cresser, M. Effect of Long-Term Changes in Soil Chemistry Induced by Road Salt Applications on N-Transformations in Roadside Soils. Environ. Pollut. 2008, 152, 20–31. [Google Scholar] [CrossRef]
- Equiza, M.A.; Calvo-Polanco, M.; Cirelli, D.; Señorans, J.; Wartenbe, M.; Saunders, C.; Zwiazek, J.J. Long-Term Impact of Road Salt (NaCl) on Soil and Urban Trees in Edmonton, Canada. Urban For. Urban Green. 2017, 21, 16–28. [Google Scholar] [CrossRef]
- She, R.; Yu, Y.; Ge, C.; Yao, H. Soil Texture Alters the Impact of Salinity on Carbon Mineralization. Agronomy 2021, 11, 128. [Google Scholar] [CrossRef]
- Cartographie du 5e Inventaire Écoforestier du Québec Méridional—Méthodes et Données Associées. Available online: https://mffp.gouv.qc.ca/nos-publications/cartographie-5e-inventaire-methodes-donnees-associees/ (accessed on 29 May 2023).
- National Forest Inventory. In Canada’s National Forest Inventory Ground Sampling Guidelines; Canadian National Forest Inventory Task Force: Ottawa, ON, Canada, 2008.
- Centre D’expertise en Analyse Environnementale du Québec, 2014 Centre D’expertise En Analyse Environnementale Du Québec. MA. 100 Ph 1.1. Détermination Du Ph: Méthode Électrométrique. Édition—PDF Free Download. Available online: https://docplayer.fr/32257992-Centre-d-expertise-en-analyse-environnementale-du-quebec-ma-100-ph-1-1-determination-du-ph-methode-electrometrique-edition.html (accessed on 18 June 2023).
- Carter, M.R.; Gregorich, E.G. (Eds.) Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-12622-2. [Google Scholar]
- Beaulieu, M. 2021 Guide d’intervention—Protection des sols et Réhabilitation des Terrains Contaminés; Ministère de l’Environnement et de la Lutte Contre les Changements Climatiques: Quebec City, QC, Canada, 2021.
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://cir.nii.ac.jp/crid/1570854175843385600 (accessed on 25 June 2023).
- Wang, N.; Guan, Q.; Sun, Y.; Wang, B.; Ma, Y.; Shao, W.; Li, H. Predicting the Spatial Pollution of Soil Heavy Metals by Using the Distance Determination Coefficient Method. Sci. Total Environ. 2021, 799, 149452. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Qiao, Y.; Yin, C.; Yang, X.; Li, N. Characterizing the Physical and Demographic Variables Associated with Heavy Metal Distribution along Urban-Rural Gradient. Environ. Monit. Assess. 2015, 187, 570. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer Texts in Statistics; Springer: New York, NY, USA, 2021; ISBN 978-1-07-161417-4. [Google Scholar]
- O’Sullivan, O.S.; Holt, A.R.; Warren, P.H.; Evans, K.L. Optimising UK Urban Road Verge Contributions to Biodiversity and Ecosystem Services with Cost-Effective Management. J. Environ. Manag. 2017, 191, 162–171. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Sensitivity of Soil Organic Carbon Stocks and Fractions to Different Land-Use Changes across Europe. Geoderma 2013, 192, 189–201. [Google Scholar] [CrossRef]
- Sainepo, B.M.; Gachene, C.K.; Karuma, A. Assessment of Soil Organic Carbon Fractions and Carbon Management Index under Different Land Use Types in Olesharo Catchment, Narok County, Kenya. Carbon Balance Manag. 2018, 13, 4. [Google Scholar] [CrossRef]
- Laik, R.; Kumar, K.; Das, D.K.; Chaturvedi, O.P. Labile Soil Organic Matter Pools in a Calciorthent after 18 Years of Afforestation by Different Plantations. Appl. Soil Ecol. 2009, 42, 71–78. [Google Scholar] [CrossRef]
- Srour, N.; Thiffault, E.; Boucher, J.-F. Exploring the Potential of Roadside Plantation for Carbon Sequestration Using Simulation in Southern Quebec, Canada. Forests 2024, 15, 264. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Yao, S.; Ye, Y.; Zhang, B. Responses of Soil Mineral-Associated and Particulate Organic Carbon to Carbon Input: A Meta-Analysis. Sci. Total Environ. 2022, 829, 154626. [Google Scholar] [CrossRef]
- Schrumpf, M.; Kaiser, K.; Guggenberger, G.; Persson, T.; Kögel-Knabner, I.; Schulze, E.-D. Storage and Stability of Organic Carbon in Soils as Related to Depth, Occlusion within Aggregates, and Attachment to Minerals. Biogeosciences 2013, 10, 1675–1691. [Google Scholar] [CrossRef]
- Papa, S.; Bartoli, G.; Pellegrino, A.; Fioretto, A. Microbial Activities and Trace Element Contents in an Urban Soil. Environ. Monit. Assess. 2010, 165, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, J.; Ziyath, A.M.; Egodawatta, P.; Ayoko, G.A.; Goonetilleke, A. Sources and Transport Pathways of Common Heavy Metals to Urban Road Surfaces. Ecol. Eng. 2015, 77, 98–102. [Google Scholar] [CrossRef]
- Das, R.S.; Rahman, M.; Sufian, N.P.; Rahman, S.M.A.; Siddique, M.A.M. Assessment of Soil Salinity in the Accreted and Non-Accreted Land and Its Implication on the Agricultural Aspects of the Noakhali Coastal Region, Bangladesh. Heliyon 2020, 6, e04926. [Google Scholar] [CrossRef] [PubMed]
- Shannon, T.P.; Ahler, S.J.; Mathers, A.; Ziter, C.D.; Dugan, H.A. Road Salt Impact on Soil Electrical Conductivity across an Urban Landscape. J. Urban Ecol. 2020, 6, juaa006. [Google Scholar] [CrossRef]
- Zeng, S.-L.; Zhang, T.-T.; Gao, Y.; Li, B.; Fang, C.-M.; Flory, S.L.; Zhao, B. Road Effects on Vegetation Composition in a Saline Environment. J. Plant Ecol. 2012, 5, 206–218. [Google Scholar] [CrossRef]
- Gong, C.; Ma, L.; Cheng, H.; Liu, Y.; Xu, D.; Li, B.; Liu, F.; Ren, Y.; Liu, Z.; Zhao, C.; et al. Characterization of the Particle Size Fraction Associated Heavy Metals in Tropical Arable Soils from Hainan Island, China. J. Geochem. Explor. 2014, 139, 109–114. [Google Scholar] [CrossRef]
- Huang, B.; Li, Z.; Huang, J.; Guo, L.; Nie, X.; Wang, Y.; Zhang, Y.; Zeng, G. Adsorption Characteristics of Cu and Zn onto Various Size Fractions of Aggregates from Red Paddy Soil. J. Hazard. Mater. 2014, 264, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhang, M.; Yao, X.; Ma, Z.; Yu, F.; Bai, J. Heavy Metal Distribution in Different Soil Aggregate Size Classes from Restored Brackish Marsh, Oil Exploitation Zone, and Tidal Mud Flat of the Yellow River Delta. J. Soils Sediments 2016, 16, 821–830. [Google Scholar] [CrossRef]
- Xu, L.; Cui, H.; Zheng, X.; Zhou, J.; Zhang, W.; Liang, J.; Zhou, J. Changes in the Heavy Metal Distributions in Whole Soil and Aggregates Affected by the Application of Alkaline Materials and Phytoremediation. RSC Adv. 2017, 7, 41033–41042. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of Heavy Metal(Loid)s Contaminated Soils--to Mobilize or to Immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Zubek, S.; Stanek, M.; Woch, M.W. Soil Organic Matter Prevails over Heavy Metal Pollution and Vegetation as a Factor Shaping Soil Microbial Communities at Historical Zn–Pb Mining Sites. Chemosphere 2020, 240, 124922. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt Effects on the Soil Microbial Decomposer Community and Their Role in Organic Carbon Cycling: A Review. Soil Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Setia, R.; Marschner, P.; Baldock, J.; Chittleborough, D.; Verma, V. Relationships between Carbon Dioxide Emission and Soil Properties in Salt-Affected Landscapes. Soil Biol. Biochem. 2011, 43, 667–674. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Wang, B.; Shao, H.; Zhang, L.; Qin, X. Co-Effects of Salinity and Moisture on CO2 and N2O Emissions of Laboratory-Incubated Salt-Affected Soils from Different Vegetation Types. Geoderma 2018, 332, 109–120. [Google Scholar] [CrossRef]
- Caravaca, F.; Lozano, Z.; Rodríguez-Caballero, G.; Roldán, A. Spatial Shifts in Soil Microbial Activity and Degradation of Pasture Cover Caused by Prolonged Exposure to Cement Dust. Land Degrad. Dev. 2017, 28, 1329–1335. [Google Scholar] [CrossRef]
- Kupka, D.; Gruba, P. Effect of PH on the Sorption of Dissolved Organic Carbon Derived from Six Tree Species in Forest Soils. Ecol. Indic. 2022, 140, 108975. [Google Scholar] [CrossRef]
- Chen, Y.; Day, S.D.; Wick, A.F.; McGuire, K.J. Influence of Urban Land Development and Subsequent Soil Rehabilitation on Soil Aggregates, Carbon, and Hydraulic Conductivity. Sci. Total Environ. 2014, 494–495, 329–336. [Google Scholar] [CrossRef]
- Bailey, V.L.; Pries, C.H.; Lajtha, K. What Do We Know about Soil Carbon Destabilization? Environ. Res. Lett. 2019, 14, 083004. [Google Scholar] [CrossRef]




| Soil Parameters | AAL (n = 9) | AL (n = 2) | Forest (n = 30) | ROW (n = 9) | p-Value (Kruskal–Wallis) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| As 0–15 cm (ppm) | 3.09 | 0.36 | 2.07 | 0.34 | 2.06 | 0.25 | 2.28 | 0.32 | 0.03 * |
| Cd 0–15 cm (ppm) | 1.23 | 0.10 | 0.77 | 0.19 | 1.00 | 0.11 | 0.93 | 0.02 | 0.12 |
| Co 0–15 cm (ppm) | 8.32 | 0.69 | 4.69 | 1.69 | 5.57 | 1.11 | 6.22 | 0.30 | 0.05 |
| Cr 0–15 cm (ppm) | 21.91 | 1.37 | 15.83 | 8.04 | 17.24 | 2.88 | 19.00 | 1.44 | 0.19 |
| Cu 0–15 cm (ppm) | 14.48 | 1.55 | 8.00 | 3.44 | 9.86 | 1.33 | 16.42 | 1.27 | 0.01 ** |
| Mn 0–15 cm (ppm) | 380.14 | 44.17 | 154.48 | 63.06 | 305.36 | 90.64 | 332.71 | 43.21 | 0.06 |
| Pb 0–15 cm (ppm) | 12.81 | 1.37 | 4.98 | 0.71 | 20.55 | 2.48 | 14.09 | 1.19 | 0.04 * |
| Zn 0–15 cm (ppm) | 52.65 | 3.41 | 26.16 | 7.52 | 39.72 | 5.39 | 47.23 | 3.40 | 0.11 |
| Conductivity 0–15 cm (mS cm−1) | 0.84 | 0.07 | 0.55 | 0.08 | 0.64 | 0.06 | 0.92 | 0.09 | 0.03 * |
| Conductivity 15–35 cm (mS cm−1) | 0.49 | 0.08 | 0.17 | 0.04 | 0.37 | 0.08 | 0.80 | 0.10 | 0.00 ** |
| Conductivity 35–55 cm (mS cm−1) | 0.36 | 0.04 | 0.20 | 0.09 | 0.28 | 0.04 | 0.46 | 0.18 | 0.30 |
| pHwater 0–15 cm | 5.16 | 0.17 | 5.13 | 0.02 | 4.60 | 0.16 | 6.52 | 0.53 | 0.01 ** |
| pHwater 15–35 cm | 5.47 | 0.18 | 5.16 | 0.23 | 5.16 | 0.15 | 7.18 | 0.34 | 0.00 ** |
| pHwater 35–55 cm | 5.70 | 0.23 | 5.48 | 0.10 | 5.43 | 0.13 | 6.17 | 0.47 | 0.40 |
| Soil Parameters | Pearson Correlation Coefficient |
|---|---|
| As (0–15 cm) | 0.13 |
| Cd (0–15 cm) | −0.15 |
| Co (0–15 cm) | 0.02 |
| Cr (0–15 cm) | 0.05 |
| Cu (0–15 cm) | 0.33 * |
| Mn (0–15 cm) | 0.048 |
| Pb (0–15 cm) | −0.10 |
| Zn (0–15 cm) | 0.07 |
| Conductivity (0–15 cm) | 0.27 |
| Conductivity (15–35 cm) | 0.51 ** |
| Conductivity (35–55 cm) | 0.10 |
| pH (0–15 cm) | 0.69 **** |
| pH (15–35 cm) | 0.76 **** |
| pH (35–55 cm) | 0.27 |
| MAOC Model | POC Model | Ratio MAOC/POC Model | |||
|---|---|---|---|---|---|
| (Intercept) | 26.90 *** | (Intercept) | 17.38 *** | (Intercept) | 1.87 *** |
| (1.90) | (1.34) | (0.16) | |||
| Road density | 5.53 * | Cd | −1.12 | As | 0.32 |
| (2.55) | (1.84) | (0.23) | |||
| Cu | −1.15 | Mn | −2.90 | Mn | 1.09 ** |
| (3.71) | (1.97) | (0.36) | |||
| Zn | 3.40 | Pb | 2.49 | Zn | −0.16 |
| (23.12) | (1.50) | (0.23) | |||
| Mn | −0.25 | pH | −0.36 | ||
| (3.69) | (0.26) | ||||
| Conductivity | 2.36 | ||||
| (2.64) | |||||
| n | 23 | n | 23 | n | 23 |
| R2 | 0.45 | R2 | 0.43 | R2 | 0.67 |
| Adjusted R2 | 0.29 | Adjusted R2 | 0.34 | Adjusted R2 | 0.60 |
| MAOC Simplified Model | Ratio MAOC/POC Simplified Model | ||
|---|---|---|---|
| (Intercept) | 26.09 *** | (Intercept) | 1.87 *** |
| (1.93) | (0.16) | ||
| Road density | 5.98 ** | Mn | 0.93 *** |
| (1.97) | (0.17) | ||
| n | 23 | n | 23 |
| R2 | 0.30 | R2 | 0.59 |
| Adjusted R2 | 0.27 | Adjusted R2 | 0.57 |
| Mean | SD | |
|---|---|---|
| As (ppm) | 3.37 | 2.77 |
| Cd (ppm) | 0.85 | 0.34 |
| Co (ppm) | 2.73 | 1.96 |
| Cr (ppm) | 23.28 | 19.43 |
| Cu (ppm) | 6.27 | 4.75 |
| Mn (ppm) | 165.97 | 182.9 |
| Pb (ppm) | 22.99 | 8.71 |
| Zn (ppm) | 23.22 | 17.18 |
| Conductivity (mS cm−1) | 0.08 | 0.03 |
| pHwater | 4.07 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srour, N.; Thiffault, E.; Boucher, J.-F. Assessing the Physical Stability of Soil Organic Carbon in Roadside Ecosystems. Urban Sci. 2025, 9, 90. https://doi.org/10.3390/urbansci9040090
Srour N, Thiffault E, Boucher J-F. Assessing the Physical Stability of Soil Organic Carbon in Roadside Ecosystems. Urban Science. 2025; 9(4):90. https://doi.org/10.3390/urbansci9040090
Chicago/Turabian StyleSrour, Nour, Evelyne Thiffault, and Jean-François Boucher. 2025. "Assessing the Physical Stability of Soil Organic Carbon in Roadside Ecosystems" Urban Science 9, no. 4: 90. https://doi.org/10.3390/urbansci9040090
APA StyleSrour, N., Thiffault, E., & Boucher, J.-F. (2025). Assessing the Physical Stability of Soil Organic Carbon in Roadside Ecosystems. Urban Science, 9(4), 90. https://doi.org/10.3390/urbansci9040090






