Stimulus Onset Modulates Auditory and Visual Dominance
Abstract
1. Introduction
2. Experiment 1
2.1. Materials and Methods
2.1.1. Participants
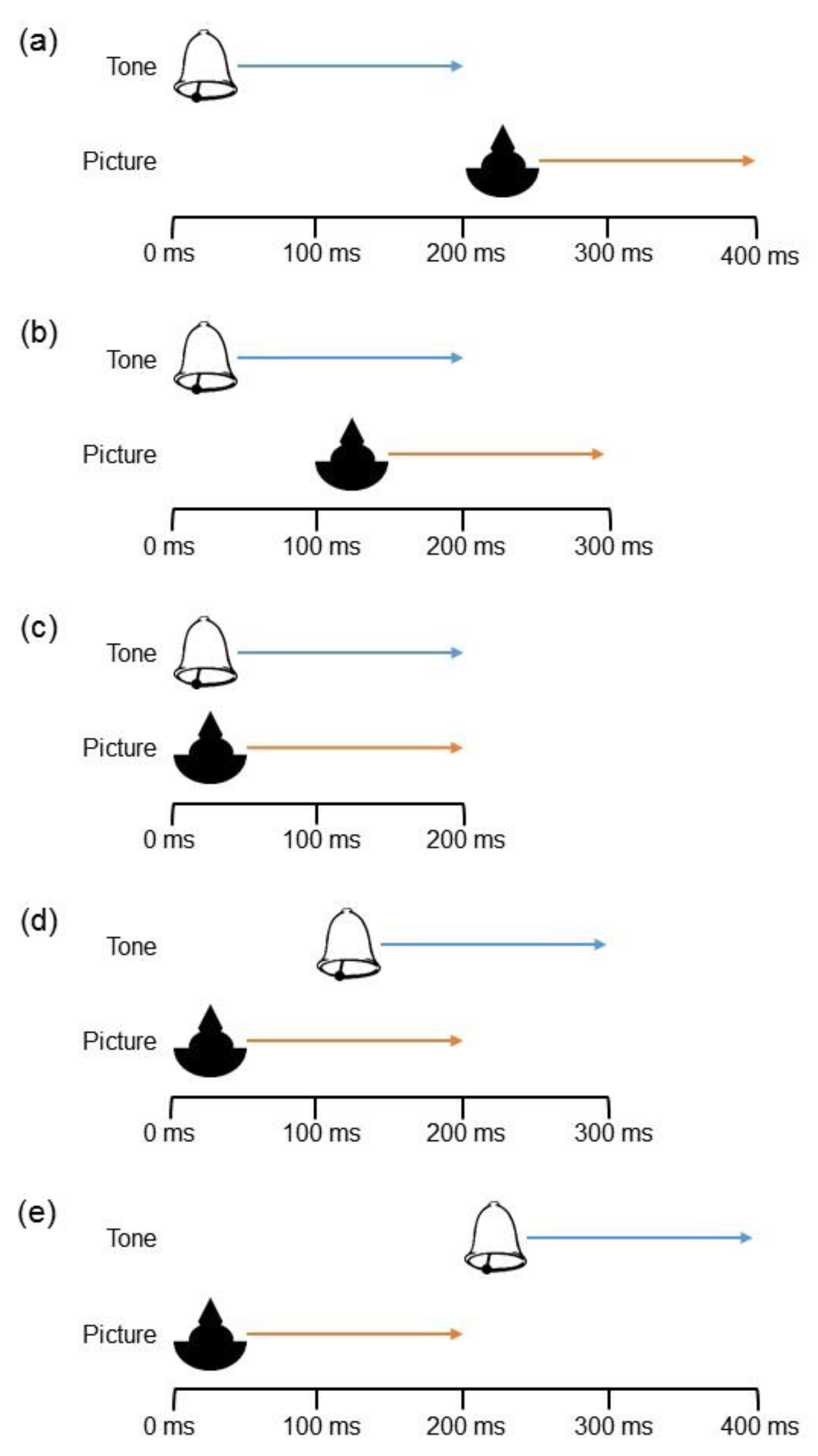
2.1.2. Stimuli and Apparatus
2.1.3. Procedure
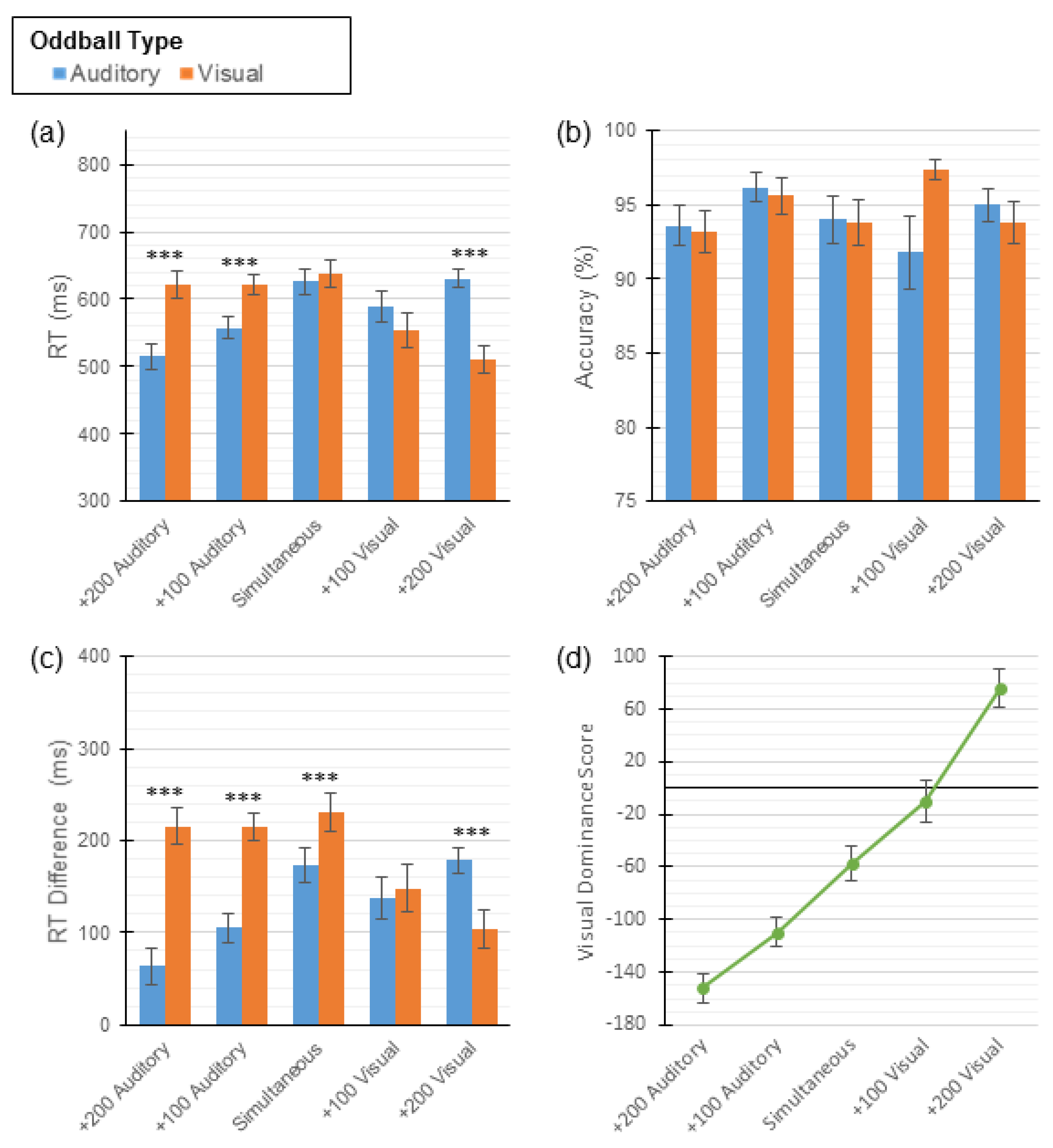
2.2. Results
3. Experiment 2
3.1. Materials and Methods
3.1.1. Participants
3.1.2. Stimuli and Apparatus
3.1.3. Procedure
3.2. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A. Unimodal Control Methodology
Appendix A.1. Participants
Appendix A.2. Stimuli and Procedure
References
- Shimojo, S.; Shams, L. Sensory modalities are not separate modalities: Plasticity and interactions. Curr. Opin. Neurobiol. 2001, 11, 505–509. [Google Scholar] [CrossRef]
- Tang, X.; Wu, J.; Shen, Y. The interactions of multisensory integration with endogenous and exogenous attention. Neurosci. Biobehav. Rev. 2016, 61, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Colavita, F.B. Human sensory dominance. Atten. Percept. Psychophys. 1974, 16, 409–412. [Google Scholar] [CrossRef]
- McGurk, H.; MacDonald, J. Hearing lips and seeing voices. Nature 1976, 264, 746–748. [Google Scholar] [CrossRef]
- Hartcher-O’Brien, J.; Gallace, A.; Krings, B.; Koppen, C.; Spence, C. When vision ‘extinguishes’ touch in neurologically-normal people: Extending the Colavita visual dominance effect. Exp. Brain Res. 2008, 186, 643–658. [Google Scholar] [CrossRef]
- Hecht, D.; Reiner, M. Sensory dominance in combinations of audio, visual and haptic stimuli. Exp. Brain Res. 2009, 193, 307–314. [Google Scholar] [CrossRef]
- Spence, C.; Parise, C.; Chen, Y.C. The Colavita visual dominance effect. In The Neural Bases of Multisensory Processes; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012; pp. 529–556. [Google Scholar]
- Sinnett, S.; Soto-Faraco, S.; Spence, C. The co-occurrence of multisensory competition and facilitation. Acta Psychol. 2008, 128, 153–161. [Google Scholar] [CrossRef]
- Sinnett, S.; Spence, C.; Soto-Faraco, S. Visual dominance and attention: The Colavita effect revisited. Percept. Psychophys. 2007, 69, 673–686. [Google Scholar] [CrossRef]
- Colavita, F.B. Visual dominance and attention in space. Bull. Psychon. Soc. 1982, 19, 261–262. [Google Scholar] [CrossRef]
- Spence, C.; Shore, D.I.; Klein, R.M. Multisensory prior entry. J. Exp. Psychol. Gen. 2001, 130, 799–832. [Google Scholar] [CrossRef]
- Titchener, E.B. The psychology of feeling and attention. Psychol. Bull. 1908, 5, 404. [Google Scholar] [CrossRef]
- Robinson, C.W.; Sloutsky, V.M. Development of cross-modal processing. Wiley Interdiscip. Rev. Cogn. Sci. 2010, 1, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Koppen, C.; Spence, C. Audiovisual asynchrony modulates the Colavita visual dominance effect. Brain Res. 2007, 1186, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.C.; Sloutsky, V.M. Is a picture worth a thousand words? The flexible nature of modality dominance in young children. Child. Dev. 2004, 75, 1850–1870. [Google Scholar] [CrossRef] [PubMed]
- Nava, E.; Pavani, F. Changes in sensory dominance during childhood: Converging evidence from the Colavita effect and the sound-induced flash illusion. Child. Dev. 2013, 84, 604–616. [Google Scholar] [CrossRef]
- Robinson, C.W.; Sloutsky, V.M. Auditory dominance and its change in the course of development. Child. Dev. 2004, 75, 1387–1401. [Google Scholar] [CrossRef]
- Sloutsky, V.M.; Napolitano, A.C. Is a picture worth a thousand words? Preference for auditory modality in young children. Child. Dev. 2003, 74, 822–833. [Google Scholar] [CrossRef]
- Ernst, M.O. Multisensory integration: A late bloomer. Curr. Biol. 2008, 18, R519–R521. [Google Scholar] [CrossRef]
- Ngo, M.K.; Cadieux, M.L.; Sinnett, S.; Soto-Faraco, S.; Spence, C. Reversing the Colavita visual dominance effect. Exp. Brain Res. 2011, 214, 607–618. [Google Scholar] [CrossRef]
- Robinson, C.W.; Chandra, M.; Sinnett, S. Existence of competing modality dominances. Atten. Percept. Psychophys. 2016, 78, 1104–1114. [Google Scholar] [CrossRef]
- Robinson, C.W.; Sloutsky, V.M. When audition dominates vision. Exp. Psychol. 2013, 60, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Shams, L.; Kamitani, Y.; Shimojo, S. Illusions: What you see is what you hear. Nature 2000, 408, 788. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, W.R.; Rivera, S.; Robinson, C.W. Different patterns of modality dominance across development. Acta Psychol. 2018, 182, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Dunifon, C.M.; Rivera, S.; Robinson, C.W. Auditory stimuli automatically grab attention: Evidence from eye tracking and attentional manipulations. J. Exp. Psychol. Hum. Percept. Perform. 2016, 42, 1947–1958. [Google Scholar] [CrossRef]
- Robinson, C.W.; Ahmar, N.; Sloutsky, V.M. Evidence for Auditory Dominance in a Passive Oddball Task. In Proceedings of the 32nd Annual Conference of the Cognitive Science Society, Portland, OR, USA, 11–14 August 2010; Ohlsson, S., Catrambone, R., Eds.; Cognitive Science Society: Austin, TX, USA, 2010; pp. 2644–2649. [Google Scholar]
- Posner, M.I.; Nissen, M.J.; Klein, R.M. Visual dominance: An information-processing account of its origins and significance. Psychol. Rev. 1976, 83, 157–171. [Google Scholar] [CrossRef]
- Wodka, E.L.; Simmonds, D.J.; Mahone, E.M.; Mostofsky, S.H. Moderate variability in stimulus presentation improves motor response control. J. Clin. Exp. Neuropsychol. 2009, 31, 483–488. [Google Scholar] [CrossRef]
- Robinson, C.W.; Chadwick, K.R.; Parker, J.L.; Sinnett, S. Examining Cardiac and Behavioral Responses in a Modality Dominance Task. In Proceedings of the 38th Annual Conference of the Cognitive Science Society, Philadelphia, PA, USA, 10–13 August 2016; Papafragou, A., Grodner, D., Mirman, D., Trueswell, J.C., Eds.; Cognitive Science Society: Austin, TX, USA, 2016; pp. 2201–2206. [Google Scholar]
- Recanzone, G.H. Interactions of auditory and visual stimuli in space and time. Hear. Res. 2009, 258, 89–99. [Google Scholar] [CrossRef]
- Chandra, M.; Robinson, C.; Sinnett, S. Coexistence of Multiple Modal Dominances. In Proceedings of the 33rd Annual Conference of the Cognitive Science Society, Boston, CA, USA, 20–23 July 2011; Carlson, L., Hoelscher, C., Shipley, T.F., Eds.; Cognitive Science Society: Austin, TX, USA, 2011; pp. 2604–2609. [Google Scholar]
- Calvert, G.A.; Campbell, R.; Brammer, M.J. Evidence from functional magnetic resonance imaging of crossmodal binding in the human heteromodal cortex. Curr. Biol. 2000, 10, 649–657. [Google Scholar] [CrossRef]
- Calvert, G.A.; Thesen, T. Multisensory integration: Methodological approaches and emerging principles in the human brain. J. Physiol. Paris 2004, 98, 191–205. [Google Scholar] [CrossRef]
- Dixon, N.; Spitz, L. The detection of auditory visual desynchrony. Perception 1980, 9, 719–721. [Google Scholar] [CrossRef]
- Fujisaki, W.; Shimojo, S.; Kashino, M.; Nishida, S.Y. Recalibration of audiovisual simultaneity. Nat. Neurosci. 2004, 7, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Navarra, J.; Vatakis, A.; Zampini, M.; Soto-Faraco, S.; Humphreys, W.; Spence, C. Exposure to asynchronous audiovisual speech extends the temporal window for audiovisual integration. Cogn. Brain Res. 2005, 25, 499–507. [Google Scholar] [CrossRef] [PubMed]



| +200 Auditory | +100 Auditory | Simultaneous | +100 Visual | +200 Visual | |
|---|---|---|---|---|---|
| Version 1 | A1, V1 | A3, V4 | A2, V5 | A4, V2 | A5, V3 |
| Version 2 | A2, V5 | A1, V1 | A5, V3 | A3, V4 | A4, V2 |
| Version 3 | A5, V3 | A2, V5 | A4, V2 | A1, V1 | A3, V4 |
| Version 4 | A4, V2 | A5, V3 | A3, V4 | A2, V5 | A1, V1 |
| Version 5 | A3, V4 | A4, V2 | A1, V1 | A5, V3 | A2, V5 |
| RT (ms) | Accuracy (%) | |||||
|---|---|---|---|---|---|---|
| SOA Condition | Auditory M (SD) | Visual M (SD) | t Score | Auditory M (SD) | Visual M (SD) | t Score |
| +200 Auditory | 515 (97.26) | 622 (99.54) | −9.31 *** | 93.6 (6.70) | 93.2 (6.90) | 0.32 |
| +100 Auditory | 557 (79.95) | 622 (77.06) | −5.82 *** | 96.2 (4.85) | 95.6 (6.18) | 0.53 |
| Simultaneous | 626 (95.05) | 638 (103.96) | −0.96 | 94.0 (8.04) | 93.8 (7.54) | 0.10 |
| +100 Visual | 590 (117.78) | 555 (130.69) | 2.15 | 91.8 (12.15) | 97.4 (3.26) | 2.54 |
| +200 Visual | 631 (67.53) | 510 (103.33) | 8.32 *** | 95.0 (5.40) | 93.8 (7.11) | 0.77 |
| Slowdown (ms) | Dominance | |||
|---|---|---|---|---|
| SOA Condition | Auditory M (SD) | Visual M (SD) | t Score | M (SD) |
| +200 Auditory | 63 (97.26) | 215 (99.54) | −13.22 *** | −152 (57.51) |
| +100 Auditory | 105 (79.95) | 215 (77.06) | −9.88 *** | −110 (55.40) |
| Simultaneous | 174 (95.05) | 231 (103.69) | −4.52 *** | −57 (63.29) |
| +100 Visual | 138 (117.78) | 148 (130.69) | −0.63 | −10 (80.93) |
| +200 Visual | 179 (67.53) | 103 (103.33) | 5.21 *** | 75 (72.42) |
| RT (ms) | Accuracy (%) | ||||
|---|---|---|---|---|---|
| SOA Condition | Auditory M (SD) | Visual M (SD) | t Score | Auditory M (SD) | Visual M (SD) |
| +200 Auditory | 647 (104.11) | 730 (88.74) | −3.87 ** | 81.1 (19.42) | 80.9 (19.40) |
| +100 Auditory | 680 (80.47) | 749 (94.14) | −3.78 ** | 85.0 (13.14) | 84.1 (14.27) |
| Simultaneous | 730 (76.95) | 768 (90.36) | −2.61 | 77.4 (22.20) | 77.0 (19.93) |
| +100 Visual | 722 (82.20) | 698 (82.07) | 1.43 | 82.2 (22.60) | 82.8 (16.36) |
| +200 Visual | 771 (114.65) | 638 (101.05) | 5.34 *** | 82.0 (17.56) | 89.8 (8.32) |
| Slowdown (ms) | Difference (ms) | |||
|---|---|---|---|---|
| SOA Condition | Auditory M (SD) | Visual M (SD) | t Score | M (SD) |
| +200 Auditory | 195 (104.11) | 323 (88.74) | −5.96 *** | −128 (103.33) |
| +100 Auditory | 228 (80.47) | 342 (94.14) | −6.25 *** | −114 (87.37) |
| Simultaneous | 278 (76.95) | 361 (90.36) | −5.71 *** | −83 (69.64) |
| +100 Visual | 270 (82.20) | 291 (82.07) | −1.23 | −21 (80.94) |
| +200 Visual | 319 (114.65) | 231 (101.05) | 3.28 ** | 88 (119.01) |
| Error Rate (%) | Difference (%) | |||
|---|---|---|---|---|
| SOA Condition | Auditory M (SD) | Visual M (SD) | t Score | M (SD) |
| +200 Auditory | 41.30 (44.63) | 32.61(41.89) | 0.56 | 8.70 (74.00) |
| +100 Auditory | 32.61 (36.83) | 54.35 (40.66) | −1.50 | −21.74 (69.53) |
| Simultaneous | 19.28(30.71) | 76.38 (34.68) | −4.41 ** | −57.10 (62.10) |
| +100 Visual | 10.04 (21.73) | 55.18 (45.97) | −4.09 ** | −45.13 (52.91) |
| +200 Visual | 12.68 (27.28) | 61.23 (45.48) | −3.88 ** | −48.55 (60.07) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciraolo, M.F.; O’Hanlon, S.M.; Robinson, C.W.; Sinnett, S. Stimulus Onset Modulates Auditory and Visual Dominance. Vision 2020, 4, 14. https://doi.org/10.3390/vision4010014
Ciraolo MF, O’Hanlon SM, Robinson CW, Sinnett S. Stimulus Onset Modulates Auditory and Visual Dominance. Vision. 2020; 4(1):14. https://doi.org/10.3390/vision4010014
Chicago/Turabian StyleCiraolo, Margeaux F., Samantha M. O’Hanlon, Christopher W. Robinson, and Scott Sinnett. 2020. "Stimulus Onset Modulates Auditory and Visual Dominance" Vision 4, no. 1: 14. https://doi.org/10.3390/vision4010014
APA StyleCiraolo, M. F., O’Hanlon, S. M., Robinson, C. W., & Sinnett, S. (2020). Stimulus Onset Modulates Auditory and Visual Dominance. Vision, 4(1), 14. https://doi.org/10.3390/vision4010014





